Abstract
Several lines of evidence have shown that defects in the endo-lysosomal autophagy degradation pathway and the ubiquitin-proteasome system play a role in Alzheimer’s Disease (AD) pathogenesis and pathophysiology. Early pathological changes, such as marked enlargement of endosomal compartments, gradual accumulation of autophagic vacuoles (AVs) and lysosome dyshomeostasis, are well-recognized in AD. In addition to these pathological indicators, many genetic variants of key regulators in the endo-lysosomal autophagy networks and the ubiquitin-proteasome system have been found to be associated with AD. Furthermore, altered expression levels of key proteins in these pathways have been found in AD human brain tissues, primary cells and AD mouse models.
In this review, we discuss potential disease mechanisms underlying the dysregulation of protein homeostasis governing systems. While the importance of two major protein degradation pathways in AD pathogenesis has been highlighted, targeted therapy at key components of these pathways has great potential in developing novel therapeutic interventions for AD. Future investigations are needed to define molecular mechanisms by which these complex regulatory systems become malfunctional at specific stages of AD development and progression, which will facilitate future development of novel therapeutic interventions. It is also critical to investigate all key components of the protein degradation pathways, both upstream and downstream, to improve our abilities to manipulate transport pathways with higher efficacy and less side effects.
Keywords: Alzheimer’s disease, Pathogenesis, Endo-lysosomal dysregulation, Impaired autophagy, Ubiquitin-proteasome system, Proteostasis
1. Introduction
Alzheimer’s disease (AD) is the most common progressive cause of dementia in elderly among other neurodegenerative diseases. The main characteristics of AD involve progressive memory loss, behavioral changes and cognitive impairment. The histopathological hallmarks of AD include the deposition of amyloid plaques and the formation of neurofibrillary tangles [1,2]. The amyloid-beta (Aβ) peptide is proteolytically derived from amyloid precursor protein (APP) within the secretory pathway by distinct enzymatic activities known as β-secretase (or BACE) and γ-secretase [3,4]. It is notable that APP and the secretases are dynamically sorted through the plasma membrane and the membranes of intracellular organelles. Generation of the toxic Aβ peptide is critically dependent upon membrane traffic, because APP undergoes a series of post-translational modifications and cleavages that are dependent of its intracellular journey [3]. Specifically, the trafficking of APP and BACE among the trans-Golgi-network (TGN), cell surface and endosome is predicted to be the sorting pathways most relevant to AD [5]. On the other hand, neurofibrillary tangles are insoluble fibers that are mainly made up of hyper-phosphorylated tau proteins. During AD progression, hyper-phosphorylated tau aggregation leads to collapse of microtubule structures followed by neuronal death [6]. The clearance of physiological and pathological tau is primarily mediated by ubiquitin proteasome system and autophagy degradation pathway [7–11]. The compromised degradation pathways contributing to amyloid and tau pathologies have been implicated in AD pathogenesis.
Several lines of evidence demonstrate the involvement of endo-lysosomal autophagy degradation pathway defects and ubiquitin-proteasome system failure in AD pathogenesis and pathophysiology. For example, early pathological changes are well-recognized in AD including marked enlargement of endosomal compartments, gradual accumulation of autophagic vacuoles (AVs) and lysosome dyshomeostasis [12,13]. In addition, many genetic variants of key regulators in the endo-lysosomal autophagy networks and ubiquitin-proteasome system (UPS) have been associated with AD [14–16]. Altered expression levels of key proteins in these pathways have been reported in AD human brain tissues and cells derived from AD mouse models [16–18]. These findings directly link the endosomal-lysosomal dysregulation and ubiquitin-proteasome defects to AD. In this review, we will discuss proposed mechanisms behind the defects in the endo-lysosomal autophagy pathway and the UPS which contribute to development of AD-related pathological changes and disease pathogenesis. Moreover, current therapeutic strategies targeting failures of the endo-lysosomal autophagy pathway and the UPS will be discussed.
2. Endo-lysosomal autophagy pathway failure
The endo-lysosomal and autophagy system is an important apparatus that maintains protein homeostasis in the cells, especially in neurons. It consists of endosomes, retromers, autophagosomes, and lysosomes. Extracellular and plasma membrane proteins are internalized through clathrin-mediated and clathrin-independent endocytosis, followed by cargo sorting through recycling endosomes and retromers, delivery to late endosomes (LE) and lysosomes for degradation [19–22]. During maturation of LE, multi-vesicular bodies (MVBs) are formed to provide access to various hydrolases, and to generate exosomes that are released extracellularly. In parallel, autophagy traffic to lysosomes through macroautophagy, chaperonemediated autophagy, and microautophagy processes [23]. There is functional decline of protein degradation system during aging [24]. However, recent large-scale genome-wide association studies have identified AD risk loci with functional roles implicated in endocytic trafficking, autophagy transport, and lysosomal clearance [25]. Within AD vulnerable brain regions (e.g. hippocampus), abnormalities such as increased numbers of and enlargement of endosomal compartments, abnormal accumulation of autophagic vacuoles, and altered expression levels of protein degradation key regulators have been reported [26–32]. It has been proposed that a number of misfolded proteins of AD are not amenable for degradation. Subsequently, these pathogenic proteins may directly disrupt the endo-lysosomal and autophagy machinery, leading to exacerbated protein aggregation and accumulation [33,34].
2.1. Endosomes
The endosome is the major sorting compartment that transports proteins to and from the Golgi apparatus. It is also responsible for internalizing proteins from the plasma membrane and recycling it back, as well as delivering proteins to lysosomes for hydrolysis [35]. There are approximately 70 Rab superfamily members involved in endocytic trafficking. Rab5 regulates the important steps of endocytosis, ensuring cargo uptake, as well as the sorting and fusion of early endosomes [13]. Abnormal enlargement of Rab5+ endosomes has been observed in pyramidal neurons of the hippocampus and the frontal cortex from both sporadic and familial AD cases [26,36], which is considered as a signature neuropathological feature of AD distinct from normal aging or other neurodegenerative disorders [36–38]. It is known that APP produces toxic C-terminal fragments (CTFs) and Aβ species following β- and γ-secretase cleavages, and the Rab5+ endosomes are the major site for β-secretase [39]. Sustained activation of Rab5 increases cleavage of APP and overproduces toxic beta-CTFs (βCTFs) and Aβ species. In turn, excessive βCTFs and Aβ also increase active forms of Rab5, and lead to enlarged endosomes and accelerated amyloidogenic processing of APP [38]. Evidence also suggests that pathological Rab5 activation can directly impair AMPA receptor recycling, resulting in synaptic dysfunction and cognitive impairment through Aβ-independent mechanisms [38,40–42]. Moreover, hyperactive Rab5 endosome signaling disrupts neurotrophic support of basal forebrain cholinergic neurons leading to neurodegeneration [32,43–45].
In addition to Rab5, other Rab family members act as Rab5 stimulators and/or effectors regulating the function of endocytosis, such as Rab7, Ras and Rab interactor 3 (RIN3) and phosphor-tyrosine interacting with PH domain and leucine zipper 1 [32,46]. Many recently identified AD risk genes, such as bridge integrator 1 (BIN1) and phosphatidylinositol binding clathrin assembly protein (PICALM), have physiological functions in endo-lysosomal trafficking with potential mechanisms implicated in AD pathogenesis [25]. For example, knockdown of PICALM lowered Aβ production through reduction of clathrinmediated endocytosis of APP in N2a cells and APP/Presenilin 1 (PS1) mouse brains [47], or of γ-secretase in cultured cell lines and PICALM+/− mouse brains [48]. On the other hand, studies demonstrated that over-expression of PICALM protects cortical neurons against Aβ oligomer-induced toxicities [49], and that the AP2/PICALM complex facilitates trafficking of CTFs of APP through endocytic pathway to LC3+ autophagic degradation and thereby reduces Aβ production [50]. More recently, a sophisticated study implicated a role for PICALM in regulating Aβ clearance across the blood-brain barrier (BBB) through modulation of clathrin-dependent endocytosis of Aβ-lipoprotein receptor-related protein 1 complex in brain endothelial cells [51].
In general, it is speculated that in pathological conditions, dysfunction of AD risk genes exacerbates accumulation of toxic waste such as Aβ and phosphorylated-tau (pTau), partially through impaired endocytic trafficking pathways. The impaired endocytic proteins interplay with toxic Aβ and pTau aggregates contributing to endosome swelling and dysfunction [26–28,30]. The vicious cycles of impaired endocytic trafficking, sorting and clearance of protein aggregates result in neuronal degeneration. However, the precise molecular mechanism by which individual AD risk genes interact with protein aggregates and impair functions of endosomal machinery are yet to be fully elucidated.
2.2. Retromer
Retromer is a complex of proteins that act as a master conductor mediating endosomal sorting and facilitating cargo protein transport from endosomes back to the TGN or recycling to cell surface [52]. The retrograde transport between endosomes and TGN has important physiological functions including delivery of hydrolases and proteases to the endo-lysosomal protein degradation system, whereas the recycling transport to cell surface is particularly crucial for delivery of receptors for cellular functions such as facilitation of synaptic plasticity [53–56]. Retromer is composed of heterotrimers containing sorting nexin family (SNX) as the tubulation module, and vacuolar protein sorting-associated proteins (VPS) as cargo-recognition module [52].
Retromer deficiency has been linked with AD pathogenesis. Several risk loci have been identified in retromer complex proteins such as Vps35 and Vps26, as well as in their associated proteins including SorLA, SorCS1, SNX1, and SNX3 [57]. Retromer deficiency such as Vps35 and SorLA elevates βCTF and Aβ production through increased retention time of APP in the endosomes promoting amyloidogenic process and leading to memory deficits in mouse models of AD [58–60]. The interaction between SorLA and SNX27 was shown to facilitate APP trafficking to cell surface and attenuate Aβ production using HEK293 cells stably transfected with Swedish APP or mouse primary neurons [61]. Some subunits of retromer or retromer associated molecules directly regulate BACE trafficking or γ-secretase processing of APP. For example, reduction of Vps35 expression in cultured primary cortical neurons or in an AD animal model (Tg2576+/− Vsp35+/−) increased endosomal BACE1 distribution and BACE1-mediated APP processing [62,63]. SorCS1 that genetically linked to diabetes mellitus is found to regulated γ-secretase processing of APP and Aβ production using HEK293t cells and SorCS1−/− mouse brains for studies [64]. Moreover, a role for SorLA to prevent Aβ-induced synaptic toxicity and neurodegeneration through modulation of EphA4 signaling has been suggested using cultured primary hippocampal neurons and mice of wildtype, SorLA transgenic and knockout [65].
Recent studies also suggest the involvement of retromer in AD microglial abnormalities through impaired surface receptor recycling. For example, microglia collected from the brains of AD patients exhibited significantly lower protein levels of Vps35 and an autophagy molecule beclin 1 [66]. Later, it was found that triggering receptor expressed on myeloid cells 2 (TREM2) is recycled back to cell surface through Vps35 and that Vps35 deficiency impaired TREM2 degradation through lysosomal pathway with increased inflammatory responses. An AD-linked TREM2 mutant R47H [67] failed to interact with Vps35 in cultured HeLa cells with over-expressing TREM2 constructs [68]. Retromer dysfunction may contribute to tau-related toxicities. It is shown that retromer is involved in delivery of cathepsin D and other proteases from endosomes to the TGN, and that Cathepsin D deficiency enhances tauinduced neurotoxicity because of retromer and lysosomal dysfunction [69]. However, a direct link between retromer dysfunction and development of tau pathologies is yet to be established.
2.3. Multi-vesicular bodies and exosomes
Intracellular trafficking and recycling play a critical role in maintaining functional homeostasis, and various intracellular dyshomeostasis is one of proposed mechanisms for AD pathogenesis. An important biological mechanism for maintaining intracellular homeostasis is mediated by the generation of exosomes and through the crosstalk between exosome secretion and cellular degradation machineries [70]. Exosomes are extracellular vesicles that have formed following the merging between the MVB and the plasma membrane [71]. The meeting of the MVB and the plasma membrane allows for the release of intraluminal vesicles (ILVs) which are exosomes [71]. However, it is important to note that not all ILVs end up as exosomes [72]. For several decades, interest in exosomes was lacking and they were simply dismissed as a disposal system. It was only recently that exosomes became of interest due to their composition and their ability for intercellular communication through the exchange of their contents [73,74].
Early exosome research demonstrated that exosomes carry the major histocompatibility complex peptide complexes recognizable by T lymphocytes [75], supporting the idea that exosomes are used for intercellular communication. Building upon this finding, an investigation established that the secretion of exosomes can allow for the eradication or suppressed growth of established murine tumors in vivo [76]. With this knowledge, exosomes are now being investigated as potential anticancer clinical therapies [77]. Because exosomes are comprised of cellular membranes, there is no risk of a fatal immune response in patients.
In addition to their composition and their role in intercellular communication, exosomes have another interesting characteristic relevant to neurodegenerative disorders, which is the “prion-like” feature [78]. The proposed mechanism is described as pathologically misfolded proteins could transfer its conformation to the same types of proteins with normal folding through exosomes [79], resulting in the spread of the pathologically misfolded proteins such as tau spread [78].
Several studies have been conducted to better understand the relationship between exosomes and AD. It was found that β-cleavage of APP occurs in early endosomes followed by intracellular Aβ accumulation near MVBs detected by immunogold labeling studies [79], consistent with other reports [80,81]. A fraction of Aβ within MVBs is released into the extracellular milieu through exosomes [79]. Immunohistochemistry analysis performed on postmortem human brain sections of AD patients showed accumulation of Alix, an exosomal marker, around amyloid plaques, further supporting the above findings and the hypothesis that Aβ can be released through exosomes from MVBs [79]. Another study also supported the role of exosomes in Aβ aggregation [82]. It was found that following intraperitoneal injection of GW4869, a neutral sphingomyelinase 2 (nSMase2) inhibitor to prevent exosome excretion in 5XFAD mice, levels of exosomes were decreased along with total brain Aβ levels [82].
Investigations have also been conducted to determine the plausibility of tau being secreted and spread through exosomes. It was found that tau is secreted through exosomes [83]. It was also determined that exosomal tau is similar to the tau isoforms secreted into cerebrospinal fluid (CSF) of early AD patients [83]. In another study, the extracted neuron-derived exosomes from blood samples of mild cognitive impairment patients had overall significantly higher levels of total tau, p-tau T181, and p-tau S396 than the ones from cognitively normal control subjects [84]. Moreover, one study demonstrated that microglia facilitated tau dissemination via phagocytosis of tau aggregates followed by exosome secretion, and that inhibition of exosome biosynthesis by knocking down sphingomyelinase 2 or inhibiting its enzymatic activities prevented tau propagation in vitro and in vivo in an adeno-associated virus-based mouse model inducing rapid tau dissemination from entorhinal cortex to dentate gyrus [85]. Together, these studies suggest that exosomes may propagate the release of tau between cells, critical for disease progression.
Finally, another promising characteristic of exosomes is the potential as an AD biomarker. Exosomes contain a large selection of molecules, including, but not limited to, DNA, mRNAs, and microRNAs [86]. This is of particular interest because of the role of microRNAs (miRNAs) in regulation of gene expression. Some investigations have been conducted to better understand how exosomal miRNAs can play a role in AD. One study compared the miRNA expression profiles between AD patients and age-matched controls and identified sixty miRNAs that were differentially expressed between the two groups [87]. In another study assessing exosomal miRNAs in AD, it was found that levels of twenty miRNAs were significantly different between AD samples and controls [88]. Further studies are needed to determine the validity of applying exosomes as AD biomarkers, more specifically to investigate the sensitivity and specificity of changes in exosomal contents like miRNA changes in disease development and progression in longitudinal studies using large sample cohorts.
2.4. Autophagy
Autophagy is a lysosome-dependent degradation process to eliminate the accumulation of cellular waste by degrading and recycling defective organelles and misfolded proteins [89]. It is categorized into microautophagy, chaperone-mediated autophagy and macroautophagy. Autophagy dysfunction has been associated with neurodegenerative processes with an increasing number of autophagy genes associated with neurodegenerative diseases such as PICALM, autophagy-related 7 (ATG7), beclin 1 (BECN1/ATG6), clusterin, cathepsin D and PS1 [89,90]. There are proposed mechanisms of action for identified risk genes on various steps of autophagic process including impaired autophagosome formation [90], disruption of cargo recognition [90], inhibition of the fusion of autophagy with lysosome [91], accumulation of misfolded proteins within autophagosomes and autophagolysosomes [92], and inability to degrade cellular wastes [93]. However, the autophagy pathway is rather complex and how dysregulated autophagy machinery is involved in AD pathogenesis remains controversial. For example, Aβ can be cleared through the autophagy pathway and hampering autophagosome formation or its fusion with endo-lysosomal compartments increased βCTF and Aβ levels significantly [94]. Upregulation of autophagy functions reduced Aβ levels seen in neurons and AD animal models [95]. On the other hand, Aβ can be generated within autophagosomes during turnover of APP-containing organelles [91,96–98]. Autophagy pathway can also regulate Aβ secretion, e.g. ATG7 knockout in APP transgenic mice reduced extracellular Aβ levels and plaque formation but led to increased intracellular Aβ accumulation and cognitive deficits [94].
Recently, studies suggest the link between autophagy and neuroinflammation in AD pathogenesis. A study showed that IL1β-induced inflammation elevated expression of certain autophagy proteins in cultured microglia [99]. In addition, in APP/PS1 mutant AD transgenic mice, accumulation of autophagic vacuoles within dystrophic neurons and expression of autophagy regulators BECN1 and mTOR correlated with increased levels of inflammatory cytokines [100]. Moreover, recent studies have shown that knockdown of autophagy protein beclin 1 impaired microglial phagocytosis and recycling of phagocytic receptors by retromers as described in the earlier section [66]. In contrary, others have shown that inhibition of autophagy may enhance microglial activities such as the release of inflammatory cytokines in vitro [101]. Nevertheless, the cross-talk between autophagy and neuroinflammation likely play a role in AD.
2.5. Mitochondria and mitophagy
Mitochondria is an organelle that produces adenosine triphosphate (ATP), which supplies energy and maintains cellular integrity and function [102–104]. It plays a major role in the formation of synapses and neuroplasticity [105–107]. Previous studies indicated that mitochondrial dysfunction as one of early AD pathological features, including accumulation of dysfunctional mitochondria seen in cellular and animal models of AD and in brain tissue of AD patients [108–110], as well as defects in mitochondrial motility and dynamics [106,111,112]. For example, in a knockout mouse model of mitofusion 2 (CAMKII-Mfn2−/−), a key regulator protein involved in mitochondrial fusion, mitochondrial membrane abnormalities and cristae damage were seen with progressive transition into neurodegeneration, neuronal death and cortical volume loss over time mirroring AD pathological changes [113].
Evidence further suggests that accumulation of APP and Aβ play important roles in mitochondrial toxicities. For example, Aβ was found in purified mitochondria from brain tissue of AD patients and mouse models. The interaction between Aβ and mitochondrial matrix proteins induced cytotoxic effects [110,114], affected mitochondrial fusion and motility [115,116]. Mitochondrial dysfunction and dysfunctional mitochondria accumulation generate the excessive reactive oxygen species and lower ATP levels, which exacerbate mitochondrial damage and cause abnormal processing of APP and p-tau in turn, leading to increased formation of Aβ plaques and neurofibrillary tangles [106,117]. Mitochondrial dysfunction also results in dysregulation of Ca2+ homeostasis, rendering stress resistance of cells, and accumulation of dysfunctional mitochondria leads to neuronal apoptosis through activating caspase-9 dependent pathway [106,107,117].
Mitophagy is the major pathway to sequester damaged mitochondria into autophagosomes for lysosomal degradation and maintain mitochondria in high quality of function [118], through Parkin-dependent and Parkin-independent manners [119–122]. Mitophagy has been found to be compromised in AD [116]. For example, undigested mitochondria have been found in lysosomes of AD neurons, as well as in soma due to impaired lysosomal function and mitochondrial transport [118]. In addition, up-regulation of mitophagy associated gene expression was seen in early stages of AD, suggesting compromised lysosomal function leading to autophagosomes accumulation [123,124]. On the other hand, reduced expression mitophagy proteins such as SIRT1, SIRT3, PGC1 and NRF2 associated with defects in mitochondrial biogenesis and responses to oxidative stress [125–127]. It has also been reported that the levels of cellular metabolite NAD+ are decreased in AD leading to compromised mitophagy function [128–130]. Genetic manipulations to knock down NAD+ consuming enzymes like CD38 or PARP1 and elevate NAD+ levels reduced Aβ plaques and improved learning and memory in AD mouse models [131–133]. Similarly, conditions like excessive dietary intake and diabetes can impair mitophagy function leading to neurodegeneration [134,135]. Therapeutic interventions targeting at mitochondrial health may protect neurons against aging and neurodegenerative processes.
2.6. Lysosomes
Lysosome is the last step to degrade cargo-like macromolecules, organelles or protein aggregates by the endocytic and autophagic pathways [136]. As the degradation center of cells, lysosomes house over 60 unique hydrolytic enzymes with the purpose of degrading both intracellular and extracellular proteins, lipids, and a myriad of macromolecules into basic molecular building blocks [137]. From a physiological standpoint, lysosomes must maintain an optimal pH of 4–5 for hydrolytic enzymes to function properly [137]. Additionally, lysosomes must fuse properly with other cellular compartments (trafficking), as exemplified by endosomal fusion, in order to function properly [23]. A defect in any of these mechanisms could culminate in various neurological pathology states [23].
Evidence suggests the roles of lysosomal dysfunction in cellular aging, longevity, and neurodegenerative diseases such as Parkinson’s disease and AD. Previous studies showed a broad range of genes and proteins associated with lysosomal network dysregulated in AD patients including several well-known risk genes of AD such as Apolipoprotein E4 (ApoE4) [138,139]. It has been shown that the presence of ApoE4 increases Aβ aggregation and decreases Aβ clearance [140–142]. In addition, the presence of ApoE4 increases the pathogenicity of tau and α-synclein [143]. Recent RNA-sequencing studies of the entorhinal cortex of AD patients have identified several endosomal-lysosomal genes deeply affected by ApoE4 expression supporting the role of ApoE4 in the lysosomal degradation pathway [144]. These results were further strengthened by immunohistochemistry studies of brain sections from ApoE4 versus ApoE3 mice showing significant morphological impairments in endosomal-lysosomal compartments of ApoE4 mouse brains. Besides ApoE4, cohorts with genetic defects in v-ATPase or v-ATPase related proteins also show increased susceptibilities for neurodegenerative processes [145].
Lysosomal proteolysis defects result in neuropathological changes such as Aβ accumulation in AD [146,147]. Inhibition of lysosomal function increased amyloid accumulation and memory deficits in mouse models of AD [148]. Aβ aggregates together with oxidized lipids and lipoproteins prevent lysosomal proper proteolysis, exacerbating toxic cargo accumulation and neuronal degeneration Aβ aggregates together with oxidized lipids and lipoproteins prevent lysosomal proper proteolysis, exacerbating toxic cargo accumulation and neuronal degeneration [148]. For example, studies demonstrated that a chloride transporter, ClC-7 plays a critical role in lysosomal acidification and fibrillary Aβ degradation in cultured primary microglia. Activation of microglia with colony-stimulating factor promotes CIC-7 delivery to lysosomes, leading to optimal lysosomal acidification and increased Aβ degradation [149]. Another protein of interest that influences AD via lysosomal trafficking is synaptojanin 1 (synj1) and phosphoinositol (4,5)-biphosphate (PIP2) [138]. Synj1 is a phosphatase that dephosphorylate PIP2, which acts as a signaling molecule that stimulates clathrin-mediated endocytosis, a vital process in the endo-lysosomal pathway [150,151]. Experimental data demonstrated synj1 knockdown reduced Aβ levels through accelerated cellular Aβ uptake, internalization and lysosomal degradation in conjunction with increased PIP2 levels [138], ultimately supporting an important role for synj1 in modulating endo-lysosomal degradation of Aβ through PIP2 signaling.
Defects in lysosomal trafficking and vesicular fusion can also result in Aβ-independent pathological changes [23]. In this context, disruption in calcium homeostasis within lysosomal compartments play an extremely integral role, particularly in endo-lysosome and autophagy pathway [152]. Experimental efforts involving cell-free vesicle fusion assays showed that lysosomes and late endosomes require Ca++ for fusion, and that lysosomes are the source of Ca++ in late endosomal and lysosome fusion [153]. Familial AD mutations of PS1 can compromise lysosomal Ca++ efflux, as well as v-ATPase assembly and its proton pumping activity [154]. PS1 knockout cells had elevated lysosomal Ca++ efflux. Interestingly, lysosomal proteolysis, autophagy, and Ca++ homeostasis of PS1 knockout cells were restored when acidic nanoparticles were introduced to resume optimal lysosomal pH levels but not when correcting for Ca++ deficits alone [154].
Because of v-ATPase’s important role in maintaining the integrity of optimal intra-lysosomal pH, its functional disturbance could alter and diminish the effectiveness of many hydrolases of lysosomes [145]. Experimental models of yeast, Drosophila, and mice have demonstrated the detrimental effects of increased intra-lysosomal pH values. In these models, early age onset of pH increases in intra-lysosomal compartment yielded mitochondrial dysfunction and shorted lifespan, all of which were accompanied by further intra-lysosomal pH increase along with aging [155–158]. Conversely, overexpression of v-ATPase and v-ATPase related proteins extended lifespan in yeast model [155–158].
Another class of important lysosomal proteins of interest is leucine rich repeat and immunoglobulin-like domain-containing protein (LINGO)-1 and its analogs LINGO-2 and LINGO-3. LINGO-1 is predominately expressed in the central nervous system [202]. Experimental reports demonstrated that the physical interaction between LINGO-1 and APP promotes lysosomal degradation of APP, and thereby reduces available APP for amyloidogenic process [159]. It is also shown that expression of LINGO-1 was down-regulated in AD cortical gray matter [160]. Over-expression of LINGO-1 in cortical neurons significantly reduced APP levels, which supports the hypothesis that LINGO-1 promotes APP degradation and inhibits Aβ generation [159].
The clearance of aberrant tau proteins through the autophagy-lysosomal pathway [161] can be regulated via transcription factor EB (TFEB) [162]. Normally, TFEB is contained in the cytoplasm in a phosphorylated state due to the influence of mechanistic target of rapamycin complex 1 (mTORC1) [163]. Inhibition of mTORC1 causes TFEB dephosphorylation and nuclear translocation, which subsequently increases lysosomal expression [164]. Experiments showed that TFEB overexpression reduced neurofibrillary tangle burden and ameliorated behavioral deficits in the Tg4510 tau mouse model, while no adverse side effects seen in wildtype mice with TFEB overexpression [165]. Additionally, the brain mass of treatment group (AAV-TFEB delivery) was increased brain mass compared to that of the control group [165]. These promising results do suggest the possibility of upregulating TFEB expression to modulate lysosomal activity as a potential therapeutic approach.
Therapeutic approaches targeting at lysosomal function are rather limited, possibly due to the challenges derived from the diversity and redundancy of lysosomal hydrolases to be targeted on. On the other hand, some lysosomal regulators have been assessed as AD biomarkers such as lysosome-associated membrane glycoprotein (LAMP) family proteins [166]. Studies demonstrated about 1.4-fold and 2.8-fold increases in CSF LAMP-1 and LAMP-2 levels of AD individuals when compared to those of non-AD subjects, respectively [166]. Specifically, the levels of LAMP-2 correlated well with CSF pTau levels [166].
3. Ubiquitin and proteasome system dysfunction during AD pathogenesis
While the endo-lysosome autophagy system clears long-lived proteins and intracellular organelles, the UPS provides the fundamental molecular machinery for short-lived protein degradation [11], and helps maintain overall proteostasis in eukaryotic cells. In addition, UPS is an essential player in the modulation of other crucial cellular functions, such as differentiation, inflammation, cell cycle, cell signaling during stress, apoptosis and antigen processing [167]. In the brain, UPS elements are involved in synaptic function [168]. As one of the primary intracellular proteolytic pathways, UPS holds a critical role in maintaining a dynamic state of equilibrium [169]. Several lines of evidence suggest that certain modifications can alter UPS activities and consequentially induce pathological conditions with defective brain function such as neurodegenerative diseases with a commonly shared feature by which accumulation of toxic protein aggregates deeply interferes with normal proteostasis [167,170].
UPS mediates the removal of damaged soluble proteins and degradation of short-lived regulatory proteins by two fundamental steps: Ubiquitination, followed by proteolytic degradation of ubiquitinated proteins [167]. During ubiquitination, a polyubiquitin chain is covalently attached to a lysine residue of a substrate protein. This ATP-dependent process serves as a recognition signal for later degradation processing by the proteasome. Three classes of enzymes are involved: ubiquitin-activating enzymes (E1), ubiquitin-conjugating enzymes (E2), and ubiquitin ligases (E3) [171]. The ubiquitination process has a high degree of substrate specificity, which is accomplished by a large number of distinct E2 and E3 enzymes [172]. When a minimum of four ubiquitin groups are assembled on a substrate protein, this polyubiquitinated substrate is then recognized, unfolded and degraded by the proteasome to subsequently generate short peptides and amino acids that are later recycled for new protein synthesis. The proteasome is a multi-subunit complex with a central catalytic core, with a cylindrical structure, and one or two regulatory particles [173].
3.1. The UPS machinery and AD
The disturbance of the UPS machinery plays a critical role in AD development. A significant decrease in proteasome activities has been evidenced in the hippocampus, middle temporal gyri, and inferior temporal lobe of AD patients [16,174]. Defective UPS proteolysis induces synaptic dysfunction [175]. Furthermore, disruption of UPS correlates with several AD pathological features, such as Aβ accumulation, tau hyper-phosphorylation (HP-tau) and autophagy impairment [176,177]. Studies have demonstrated that the accumulation of HP-tau oligomers at pre- and postsynaptic terminals are directly associated with increased ubiquitinated substrates and proteasome elements in brains of AD patients, suggesting that HP-tau oligomers influence and mediate UPS proteotoxicity, thus impairing synaptic function [176].
3.2. Aβ modulates UPS function
The relationship between Aβ and the proteasome has been well-studied since the discovery of ubiquitin in senile plaques [178]. Since then, many reports indicate the effects of UPS on Aβ toxicities. A study demonstrated that when the proteolytic activity of the proteasome was inhibited in primary culture of cortical neurons and astrocytes, there was a significant decrease in Aβ degradation suggesting that Aβ is a substrate for the UPS machinery [179]. Impairment of UPS affects Aβ degradation leading to its accumulation and amyloid plaque formation [179].
On the other hand, Aβ inhibits the proteolytic activity of the proteasome [180]. Evidence suggests that Aβ induced a significant increase of ubiquitin-protein conjugates and expression of E1 in neurons [179], considered as AD pathological traits. With growing concentrations of toxic oligomeric Aβ, Aβ competes against natural proteasomal substrates leading to the proteasomal malfunction [181]. In addition, a study indicates that the inhibition of UPS by intraneuronal Aβ was originated from impaired MVB sorting pathway [180], where different materials or substances are carried from neuronal terminals to cell bodies through retrograde transport for signaling cascades or lysosomal degradation. Further research on the link between MVB impairment and UPS defects in AD might shed a light into molecular mechanisms of Aβ-induced neuro-toxicities.
3.3. The interplay between UPS and tau through UPS
Tau can be cleaved by a number of proteases (e.g. caspases, cathepsins, calpains and thrombin). Increasing evidence suggests the involvement of UPS in tau turnover [8]. Studies have shown that the proteasome was co-immunoprecipitated with tau aggregates and the amounts of tau aggregates pulled-down were significantly higher in samples with low proteasome activities [8], suggesting that the interaction between tau aggregates and proteasome inhibits the proteasomal activities. Moreover, in vitro experiments using tau aggregates isolated from human AD brains confirmed that tau inhibits the proteasome, whereas non-aggregated tau does not have this effect [8]. Additional data supports the involvement of the UPS in tau turnover in vitro [7,182]. Inhibiting the proteasome resulted in reduced tau degradation [7], and conversely, tau degradation was accelerated after incubation with the proteasome [182].
Studies further suggest that the proteasome holds the molecular link between Aβ and tau pathogenesis. Promoting Aβ clearance can reduce early tau pathology [183]. On the other hand, increased Aβ damages the proteasomal function thus exacerbating tau accumulation [183]. Notably, both Aβ and tau accumulation was reported on transgenic mice after directly inhibiting the proteasome activity [184]. Further research is needed to elucidate how proteasome inhibition induced by Aβ, leads to tau accumulation, and which molecular regulators within UPS play key roles in the interplay between Aβ and tau pathology of AD.
UPS also plays a role within signaling pathways that modulate neurotransmitter release, synaptic plasticity, and recycling of membrane receptors [185,186]. Particularly in neurons, the signaling pathway of cAMP-dependent protein kinase A (PKA)-cAMP response element binding protein (CREB) is directly involved in cognitive function, and the PKA activation and CREB phosphorylation are key mechanisms for memory formation [187]. This pathway is finely modulated by UPS through controlling the degradation of the PKA regulatory subunit [188]. Moreover, studies have evidenced that CREB signaling is compromised by Aβ both in vitro, using neuronal culture treated with oligomeric Aβ, and in vivo by overproduction of Aβ in transgenic mice [188,189]. Therefore, reduction of PKA-pCREB levels is suggested to contribute to impaired synaptic plasticity and diminished learning function during AD progression [190]. A number of compounds such as cAMP phosphodiesterases inhibitors have been found to enhance pCREB levels in the brain, providing a beneficial effect on cognitive function [186]. Another UPS regulator is the ubiquitin C-terminal hydrolase (Uch-L1), an abundant deubiquitinase mainly expressed in neurons [191]. The reduction of cytosolic Uch-L1 is linked to AD progression, specifically by inducing the formation of Aβ plaques and abnormal tau metabolism [192]. Moreover, studies showed that Uch-L1 levels were significantly reduced in brains of AD patients and AD transgenic mouse models, corresponding with the accumulation of ubiquitinated proteins in Aβ plaques and NFT [193].
4. Implications for developing therapeutic strategies for AD
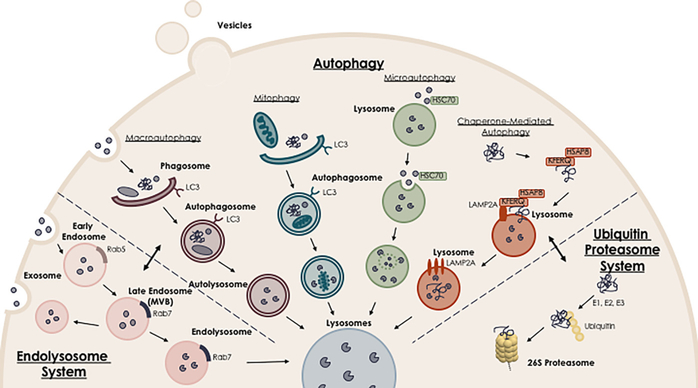
Dysregulation of proteostasis contributes to the accumulation of misfolded proteins and cellular waste in AD. While we review the critical roles of two major protein homeostasis governing pathways in AD pathogenesis (Fig. 1; endo-lysosomal autophagy pathway and ubiquitin proteasome system), targeted therapy at key components of these pathways may have great potential in developing novel therapeutic interventions for AD.
Fig. 1.
Protein degradation pathways affected in Alzheimer’s Disease. The endo-lysosomal pathway, autophagy, and ubiquitin-proteasome system are the three main regulatory routes of protein degradation. During AD progression, aberrant expression and/or impaired function of key components of these pathways, as well as defects in the interactions between upstream and downstream components involved in protein homeostasis induce dysregulation of proteostasis contributing to AD pathogenesis. Targeted therapy to key players of these pathways becomes a promising strategy for developing novel therapeutic interventions for AD.
Different strategies targeted at various steps of endo-lysosomal pathway have been tested for AD therapies. For example, it was hypothesized that stabilizing the retromer complex may enhance its function and correct cargo protein trafficking defects. Using pharmacological chaperones to stabilize and increase retromer levels in neurons, APP trafficking toward Golgi was increased and thereby it was moved away from endosomes for amyloidogenic processes [194]. Evidence suggests a role for PIP2 in regulating Aβ clearance through endolysosomal pathway. Reduction of a PIP2 degrading enzyme in the brain, synj1 accelerated Aβ degradation through lysosome and improved cognitive function in an AD transgenic mouse model [138]. These studies indicate a potential therapeutic strategy for AD targeted at synj1 to increase PIP2 levels and accelerate Aβ clearance (Cao et al., manuscript in preparation).
Several interventions to stimulate autophagy or mitophagy pathways through fasting, caloric restriction, exercise or rapamycin treatment have also been shown to provide neuroprotective effects against degeneration in AD experimental models [195–197]. It is reported that accumulation of Aβ and p-Tau activated the serine/threonine kinase mTOR [198], and that inhibitors of mTOR activity like rapamycin and its analogs as autophagy inducers have shown beneficial effects against AD pathologies in AD animal models [90,195].
While UPS dysfunction is intimately associated with AD pathogenesis [186], UPS regulators such as PDEs and Uch-L1 that can modulate brain PKA-pCREB levels become attractive drug targets For AD. A number of studies have supported this idea by demonstrating that overexpression of Uch-L1, or pharmacological enhancement of Uch-L1 activity greatly improved cognition in AD transgenic mouse models. Conversely, knocking out Uch-L1 or pharmacological inhibition of UchL1 activity will cause a notorious cognition decline [199,200]. Therefore, enhancing the expression of Uch-L1 in the brain is suggested to have beneficial effects in both accelerating protein degradation and improving cognitive and synaptic functions.
It should be noted that while targeting specific steps of protein degradation pathways, defects of downstream machinery need to be taken into consideration. For example, up-regulating expression of autophagy components may be futile or even detrimental if the clearance or degradation of autophagosome is impaired. Approaches to stimulate the activities of the whole pathway may be more efficacious. Alternatively, directly targeting at the downstream machinery like lysosomal function could be more effective but limited by the diversity and redundancy of various lysosomal hydrolases to be targeted on as we discussed in the previous section.
5. Conclusions
In summary, several lines of evidence support a direct link between the efficiency of degradation systems and cellular dysfunction in AD. The functional diversity of protein degradation systems has important implications of our understanding of AD pathogenesis. While we have gathered some critical information regarding the role of dysregulated proteostasis in AD pathogenesis, a detailed understanding of the aberrant circuits triggering the disease is yet to be accomplished. It is critical to elucidate the interactions and interplay between upstream and downstream key components of the protein homeostasis governing pathways which will improve our abilities to manipulate transport pathways with higher efficacy and less side effects. More importantly, future investigations are needed to determine how this complex regulatory system becomes malfunctional during AD development and progression, which will help tailor future development of novel therapeutic interventions targeted at specific stages of disease [201].
Acknowledgments
DC is supported by NIH R01 (1R01AG048923) and RF1 (1RF1AG054014), by Department of Veteran Affairs BLR&D (1I01BX003380) and RR&D (1I01RX002290), as well as by New York State SCI Foundation. JC is supported by NNSFC (81771162) and RF1 (1RF1AG054014 for DC). CAT is supported by New York State SCI Foundation. LZ is supported by NIH R01 (1R01AG048923 for DC) and RF1 (1RF1AG054014 for DC). We thank Dr. Jianwei Hou for his help in preparation of the figure.
Abbreviations
- Aβ
amyloid beta
- AD
Alzheimer’s disease
- APP
amyloid precursor protein
- ApoE4
Apolipoprotein E4
- ATG7
autophagy-related 7
- ATP
adenosine triphosphate
- AVs
autophagic vacuoles
- BACE1
beta-secretase 1
- BBB
blood-brain barrier
- βCTFs
β C-terminal fragments
- CTFs
C-terminal fragments
- CREB
cAMP response element binding protein
- CSF
cerebrospinal fluid
- E1
ubiquitin-activating enzymes
- E2
ubiquitin-conjugating enzymes
- E3
ubiquitin ligases
- HP-tau
tau hyperphosphorylation
- ILVs
intraluminal vesicles
- LAMP
lysosome-associated membrane glycoprotein
- LE
late endosomes
- LINGO
leucine rich repeat and immunoglobin-like domain-containing protein
- MVB
multivesicular body
- mTORC1
mechanistic target of rapamycin complex 1
- PICALM
phosphatidylinositol binding clathrin assembly protein
- PIP2
phosphoinositol (4,5)-biphosphate
- PKA
protein kinase A
- PS1
Presenilin 1
- TFEB
transcription factor EB
- TGN
trans-Golgi network
- TREM2
triggering receptor expressed on myeloid cells 2
- SNX
sorting nexin family
- SYNJ1
synaptojanin 1
- Uch-L1
ubiquitin C-terminal hydrolase
- UPS
ubiquitin-proteasome system
- VPS
vacuolar protein sorting-associated proteins
References
- [1].Blennow K, de Leon MJ, Zetterberg H, Alzheimer’s disease, Lancet 368 (2006) 387–403. [DOI] [PubMed] [Google Scholar]
- [2].Armstrong RA, What causes Alzheimer’s disease? Folia Neuropathol. 51 (2013) 169–188. [DOI] [PubMed] [Google Scholar]
- [3].Selkoe DJ, The cell biology of beta-amyloid precursor protein and presenilin in Alzheimer’s disease, Trends Cell Biol. 8 (1998) 447–453. [DOI] [PubMed] [Google Scholar]
- [4].Greenfield JP, Gross RS, Gouras GK, Xu H, Cellular and molecular basis of beta-amyloid precursor protein metabolism, Front. Biosci 5 (2000) D72–D83. [DOI] [PubMed] [Google Scholar]
- [5].Small SA, Gandy S, Sorting through the cell biology of Alzheimer’s disease: intracellular pathways to pathogenesis, Neuron 52 (2006) 15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Marcus JN, Schachter J, Targeting post-translational modifications on tau as a therapeutic strategy for Alzheimer’s disease, J. Neurogenet 25 (2011) 127–133. [DOI] [PubMed] [Google Scholar]
- [7].David DC, Layfield R, Serpell L, Narain Y, Goedert M, Spillantini MG, Proteasomal degradation of tau protein, J. Neurochem 83 (2002) 176–185. [DOI] [PubMed] [Google Scholar]
- [8].Keck S, Nitsch R, Grune T, Ullrich O, Proteasome inhibition by paired helical filament-tau in brains of patients with Alzheimer’s disease, J. Neurochem 85 (2003) 115–122. [DOI] [PubMed] [Google Scholar]
- [9].Petrucelli L, Dickson D, Kehoe K, Taylor J, Snyder H, Grover A, De Lucia M, McGowan E, Lewis J, Prihar G, Kim J, Dillmann WH, Browne SE, Hall A, Voellmy R, Tsuboi Y, Dawson TM, Wolozin B, Hardy J, Hutton M, CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation, Hum. Mol. Genet 13 (2004) 703–714. [DOI] [PubMed] [Google Scholar]
- [10].Dolan PJ, Johnson GV, A caspase cleaved form of tau is preferentially degraded through the autophagy pathway, J. Biol. Chem 285 (2010) 21978–21987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lee MJ, Lee JH, Rubinsztein DC, Tau degradation: the ubiquitin-proteasome system versus the autophagy-lysosome system, Prog. Neurobiol 105 (2013) 49–59. [DOI] [PubMed] [Google Scholar]
- [12].Gonzalez AE, Munoz VC, Cavieres VA, Bustamante HA, Cornejo VH, Januario YC, Gonzalez I, Hetz C, daSilva LL, Rojas-Fernandez A, Hay RT, Mardones GA, Burgos PV, Autophagosomes cooperate in the degradation of intracellular C-terminal fragments of the amyloid precursor protein via the MVB/lysosomal pathway, FASEB J. 31 (2017) 2446–2459. [DOI] [PubMed] [Google Scholar]
- [13].Xu W, Fang F, Ding J, Wu C, Dysregulation of Rab5-mediated endocytic pathways in Alzheimer’s disease, Traffic 19 (2018) 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Xue S, Jia J, Genetic association between Ubiquitin Carboxy-terminal Hydrolase-L1 gene S18Y polymorphism and sporadic Alzheimer’s disease in a Chinese Han population, Brain Res. 1087 (2006) 28–32. [DOI] [PubMed] [Google Scholar]
- [15].Orr ME, Oddo S, Autophagic/lysosomal dysfunction in Alzheimer’s disease, Arthritis Res. Ther 5 (2013) 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhang Y, Chen X, Zhao Y, Ponnusamy M, Liu Y, The role of ubiquitin proteasomal system and autophagy-lysosome pathway in Alzheimer’s disease, Rev. Neurosci 28 (2017) 861–868. [DOI] [PubMed] [Google Scholar]
- [17].Cecarini V, Bonfili L, Cuccioloni M, Mozzicafreddo M, Rossi G, Buizza L, Uberti D, Angeletti M, Eleuteri AM, Crosstalk between the ubiquitin-proteasome system and autophagy in a human cellular model of Alzheimer’s disease, Biochim. Biophys. Acta 1822 (2012) 1741–1751. [DOI] [PubMed] [Google Scholar]
- [18].Perez SE, He B, Nadeem M, Wuu J, Ginsberg SD, Ikonomovic MD, Mufson EJ, Hippocampal endosomal, lysosomal, and autophagic dysregulation in mild cognitive impairment: correlation with abeta and tau pathology, J. Neuropathol. Exp. Neurol 74 (2015) 345–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gruenberg J, Griffiths G, Howell KE, Characterization of the early endosome and putative endocytic carrier vesicles in vivo and with an assay of vesicle fusion in vitro, J. Cell Biol 108 (1989) 1301–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mayor S, Presley JF, Maxfield FR, Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process, J. Cell Biol 121 (1993) 1257–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mellman I, Endocytosis and molecular sorting, Annu. Rev. Cell Dev. Biol 12 (1996) 575–625. [DOI] [PubMed] [Google Scholar]
- [22].Jovic M, Sharma M, Rahajeng J, Caplan S, The early endosome: a busy sorting station for proteins at the crossroads, Histol. Histopathol 25 (2010) 99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Xu H, Ren D, Lysosomal physiology, Annu. Rev. Physiol 77 (2015) 57–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].McCray BA, Taylor JP, The role of autophagy in age-related neurodegeneration, Neurosignals 16 (2008) 75–84. [DOI] [PubMed] [Google Scholar]
- [25].Karch CM, Goate AM, Alzheimer’s disease risk genes and mechanisms of disease pathogenesis, Biol. Psychiatry 77 (2015) 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cataldo AM, Barnett JL, Pieroni C, Nixon RA, Increased neuronal endocytosis and protease delivery to early endosomes in sporadic Alzheimer’s disease: neuropathologic evidence for a mechanism of increased beta-amyloidogenesis, J. Neurosci 17 (1997) 6142–6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cataldo AM, Petanceska S, Terio NB, Peterhoff CM, Durham R, Mercken M, Mehta PD, Buxbaum J, Haroutunian V, Nixon RA, Abeta localization in abnormal endosomes: association with earliest Abeta elevations in AD and Down syndrome, Neurobiol. Aging 25 (2004) 1263–1272. [DOI] [PubMed] [Google Scholar]
- [28].Nixon RA, Endosome function and dysfunction in Alzheimer’s disease and other neurodegenerative diseases, Neurobiol. Aging 26 (2005) 373–382. [DOI] [PubMed] [Google Scholar]
- [29].Cataldo AM, Mathews PM, Boiteau AB, Hassinger LC, Peterhoff CM, Jiang Y, Mullaney K, Neve RL, Gruenberg J, Nixon RA, Down syndrome fibroblast model of Alzheimer-related endosome pathology: accelerated endocytosis promotes late endocytic defects, Am. J. Pathol 173 (2008) 370–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jiang Y, Mullaney KA, Peterhoff CM, Che S, Schmidt SD, Boyer-Boiteau A, Ginsberg SD, Cataldo AM, Mathews PM, Nixon RA, Alzheimer’s-related endosome dysfunction in Down syndrome is Abeta-independent but requires APP and is reversed by BACE-1 inhibition, Proc. Natl. Acad. Sci. U. S. A 107 (2010) 1630–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cossec JC, Lavaur J, Berman DE, Rivals I, Hoischen A, Stora S, Ripoll C, Mircher C, Grattau Y, Olivomarin JC, de Chaumont F, Lecourtois M, Antonarakis SE, Veltman JA, Delabar JM, Duyckaerts C, Di Paolo G, Potier MC, Trisomy for synaptojanin1 in Down syndrome is functionally linked to the enlargement of early endosomes, Hum. Mol. Genet 21 (2012) 3156–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kim S, Sato Y, Mohan PS, Peterhoff C, Pensalfini A, Rigoglioso A, Jiang Y, Nixon RA, Evidence that the rab5 effector APPL1 mediates APP-betaCTF-induced dysfunction of endosomes in Down syndrome and Alzheimer’s disease, Mol. Psychiatry 21 (2016) 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lauritzen I, Pardossi-Piquard R, Bourgeois A, Pagnotta S, Biferi MG, Barkats M, Lacor P, Klein W, Bauer C, Checler F, Intraneuronal aggregation of the beta-CTF fragment of APP (C99) induces Abeta-independent lysosomal-autophagic pathology, Acta Neuropathol. 132 (2016) 257–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chiti F, Dobson CM, Protein misfolding, amyloid formation, and human disease: a summary of progress over the last decade, Annu. Rev. Biochem 86 (2017) 27–68. [DOI] [PubMed] [Google Scholar]
- [35].Schreij AM, Fon EA, McPherson PS, Endocytic membrane trafficking and neurodegenerative disease, Cell. Mol. Life Sci 73 (2016) 1529–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cataldo AM, Peterhoff CM, Troncoso JC, Gomez-Isla T, Hyman BT, Nixon RA, Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer’s disease and Down syndrome: differential effects of APOE genotype and presenilin mutations, Am. J. Pathol 157 (2000) 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Urwin H, Ghazi-Noori S, Collinge J, Isaacs A, The role of CHMP2B in frontotemporal dementia, Biochem. Soc. Trans 37 (2009) 208–212. [DOI] [PubMed] [Google Scholar]
- [38].Nixon RA, Amyloid precursor protein and endosomal-lysosomal dysfunction in Alzheimer’s disease: inseparable partners in a multifactorial disease, FASEB J. 31 (2017) 2729–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Grbovic OM, Mathews PM, Jiang Y, Schmidt SD, Dinakar R, SummersTerio NB, Ceresa BP, Nixon RA, Cataldo AM, Rab5-stimulated up-regulation of the endocytic pathway increases intracellular beta-cleaved amyloid precursor protein carboxyl-terminal fragment levels and Abeta production, J. Biol. Chem 278 (2003) 31261–31268. [DOI] [PubMed] [Google Scholar]
- [40].Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD, Recycling endosomes supply AMPA receptors for LTP, Science 305 (2004) 1972–1975. [DOI] [PubMed] [Google Scholar]
- [41].Brown TC, Tran IC, Backos DS, Esteban JA, NMDA receptor-dependent activation of the small GTPase Rab5 drives the removal of synaptic AMPA receptors during hippocampal LTD, Neuron 45 (2005) 81–94. [DOI] [PubMed] [Google Scholar]
- [42].Kessels HW, Malinow R, Synaptic AMPA receptor plasticity and behavior, Neuron 61 (2009) 340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Granholm AC, Sanders LA, Crnic LS, Loss of cholinergic phenotype in basal forebrain coincides with cognitive decline in a mouse model of Down’s syndrome, Exp. Neurol 161 (2000) 647–663. [DOI] [PubMed] [Google Scholar]
- [44].Salehi A, Delcroix JD, Belichenko PV, Zhan K, Wu C, Valletta JS, Takimoto-Kimura R, Kleschevnikov AM, Sambamurti K, Chung PP, Xia W, Villar A, Campbell WA, Kulnane LS, Nixon RA, Lamb BT, Epstein CJ, Stokin GB, Goldstein LS, Mobley WC, Increased App expression in a mouse model of Down’s syndrome disrupts NGF transport and causes cholinergic neuron degeneration, Neuron 51 (2006) 29–42. [DOI] [PubMed] [Google Scholar]
- [45].Xu W, Weissmiller AM, White JA 2nd, Fang F, Wang X, Wu Y, Pearn ML, Zhao X, Sawa M, Chen S, Gunawardena S, Ding J, Mobley WC, Wu C, Amyloid precursor protein-mediated endocytic pathway disruption induces axonal dysfunction and neurodegeneration, J. Clin. Invest 126 (2016) 1815–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kajiho H, Saito K, Tsujita K, Kontani K, Araki Y, Kurosu H, Katada T, RIN3: a novel Rab5 GEF interacting with amphiphysin II involved in the early endocytic pathway, J. Cell Sci 116 (2003) 4159–4168. [DOI] [PubMed] [Google Scholar]
- [47].Xiao Q, Gil SC, Yan P, Wang Y, Han S, Gonzales E, Perez R, Cirrito JR, Lee JM, Role of phosphatidylinositol clathrin assembly lymphoid-myeloid leukemia (PICALM) in intracellular amyloid precursor protein (APP) processing and amyloid plaque pathogenesis, J. Biol. Chem 287 (2012) 21279–21289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kanatsu K, Morohashi Y, Suzuki M, Kuroda H, Watanabe T, Tomita T, Iwatsubo T, Decreased CALM expression reduces Abeta42 to total Abeta ratio through clathrin-mediated endocytosis of gamma-secretase, Nat. Commun 5 (2014) 3386. [DOI] [PubMed] [Google Scholar]
- [49].Treusch S, Hamamichi S, Goodman JL, Matlack KE, Chung CY, Baru V, Shulman JM, Parrado A, Bevis BJ, Valastyan JS, Han H, Lindhagen-Persson M, Reiman EM, Evans DA, Bennett DA, Olofsson A, DeJager PL, Tanzi RE, Caldwell KA, Caldwell GA, Lindquist S, Functional links between Abeta toxicity, endocytic trafficking, and Alzheimer’s disease risk factors in yeast, Science 334 (2011) 1241–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tian Y, Chang JC, Fan EY, Flajolet M, Greengard P, Adaptor complex AP2/PICALM, through interaction with LC3, targets Alzheimer’s APP-CTF for terminal degradation via autophagy, Proc. Natl. Acad. Sci. U. S. A 110 (2013) 17071–17076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhao Z, Sagare AP, Ma Q, Halliday MR, Kong P, Kisler K, Winkler EA, Ramanathan A, Kanekiyo T, Bu G, Owens NC, Rege SV, Si G, Ahuja A, Zhu D, Miller CA, Schneider JA, Maeda M, Maeda T, Sugawara T, Ichida JK, Zlokovic BV, Central role for PICALM in amyloid-beta blood-brain barrier transcytosis and clearance, Nat. Neurosci 18 (2015) 978–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Small SA, Petsko GA, Retromer in Alzheimer disease, Parkinson disease and other neurological disorders, Nat. Rev. Neurosci 16 (2015) 126–132. [DOI] [PubMed] [Google Scholar]
- [53].Zhang D, Isack NR, Glodowski DR, Liu J, Chen CC, Xu XZ, Grant BD, Rongo C, RAB-6.2 and the retromer regulate glutamate receptor recycling through a retrograde pathway, J. Cell Biol 196 (2012) 85–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Choy RW, Park M, Temkin P, Herring BE, Marley A, Nicoll RA, von Zastrow M, Retromer mediates a discrete route of local membrane delivery to dendrites, Neuron 82 (2014) 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hussain NK, Diering GH, Sole J, Anggono V, Huganir RL, Sorting Nexin 27 regulates basal and activity-dependent trafficking of AMPARs, Proc. Natl. Acad. Sci. U. S. A 111 (2014) 11840–11845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Loo LS, Tang N, Al-Haddawi M, Dawe GS, Hong W, A role for sorting nexin 27 in AMPA receptor trafficking, Nat. Commun 5 (2014) 3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Vardarajan BN, Bruesegem SY, Harbour ME, Inzelberg R, Friedland R, St George-Hyslop P, Seaman MN, Farrer LA, Identification of Alzheimer disease-associated variants in genes that regulate retromer function, Neurobiol. Aging 33 (2012) 2231.e15–2231.e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Muhammad A, Flores I, Zhang H, Yu R, Staniszewski A, Planel E, Herman M, Ho L, Kreber R, Honig LS, Ganetzky B, Duff K, Arancio O, Small SA, Retromer deficiency observed in Alzheimer’s disease causes hippocampal dysfunction, neurodegeneration, and Abeta accumulation, Proc. Natl. Acad. Sci. U. S. A 105 (2008) 7327–7332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Vieira SI, Rebelo S, Esselmann H, Wiltfang J, Lah J, Lane R, Small SA, Gandy S, da Cruz ESEF, da Cruz ESOA, Retrieval of the Alzheimer’s amyloid precursor protein from the endosome to the TGN is S655 phosphorylation state-dependent and retromer-mediated, Mol. Neurodegener 5 (2010) 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Bhalla A, Vetanovetz CP, Morel E, Chamoun Z, Di Paolo G, Small SA, The location and trafficking routes of the neuronal retromer and its role in amyloid precursor protein transport, Neurobiol. Dis 47 (2012) 126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Huang TY, Zhao Y, Li X, Wang X, Tseng IC, Thompson R, Tu S, Willnow TE, Zhang YW, Xu H, SNX27 and SORLA interact to reduce amyloidogenic subcellular distribution and processing of amyloid precursor protein, J. Neurosci 36 (2016) 7996–8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Wen L, Tang FL, Hong Y, Luo SW, Wang CL, He W, Shen C, Jung JU, Xiong F, Lee DH, Zhang QG, Brann D, Kim TW, Yan R, Mei L, Xiong WC, VPS35 haploinsufficiency increases Alzheimer’s disease neuropathology, J. Cell Biol 195 (2011) 765–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wang CL, Tang FL, Peng Y, Shen CY, Mei L, Xiong WC, VPS35 regulates developing mouse hippocampal neuronal morphogenesis by promoting retrograde trafficking of BACE1, Biol. Open 1 (2012) 1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Lane RF, Raines SM, Steele JW, Ehrlich ME, Lah JA, Small SA, Tanzi RE, Attie AD, Gandy S, Diabetes-associated SorCS1 regulates Alzheimer’s amyloidbeta metabolism: evidence for involvement of SorL1 and the retromer complex, J. Neurosci 30 (2010) 13110–13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Huang TY, Zhao Y, Jiang LL, Li X, Liu Y, Sun Y, Pina-Crespo JC, Zhu B, Masliah E, Willnow TE, Pasquale EB, Xu H, SORLA attenuates EphA4 signaling and amyloid beta-induced neurodegeneration, J. Exp. Med 214 (2017) 3669–3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lucin KM, O’Brien CE, Bieri G, Czirr E, Mosher KI, Abbey RJ, Mastroeni DF, Rogers J, Spencer B, Masliah E, Wyss-Coray T, Microglial beclin 1 regulates retromer trafficking and phagocytosis and is impaired in Alzheimer’s disease, Neuron 79 (2013) 873–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert JC, Amouyel P, Goate A, Rademakers R, Morgan K, Powell J, St George-Hyslop P, Singleton A, Hardy J, Alzheimer Genetic Analysis Group, TREM2 variants in Alzheimer’s disease, N. Engl. J. Med 368 (2013) 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Yin J, Liu X, He Q, Zhou L, Yuan Z, Zhao S, Vps35-dependent recycling of Trem2 regulates microglial function, Traffic 17 (2016) 1286–1296. [DOI] [PubMed] [Google Scholar]
- [69].Khurana V, Elson-Schwab I, Fulga TA, Sharp KA, Loewen CA, Mulkearns E, Tyynela J, Scherzer CR, Feany MB, Lysosomal dysfunction promotes cleavage and neurotoxicity of tau in vivo, PLoS Genet. 6 (2010) e1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Desdin-Mico G, Mittelbrunn M, Role of exosomes in the protection of cellular homeostasis, Cell Adh. Migr 11 (2017) 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Edgar JR, Q&A: What are exosomes, exactly? BMC Biol. 14 (2016) 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].McDonald B, Martin-Serrano J, No strings attached: the ESCRT machinery in viral budding and cytokinesis, J. Cell Sci 122 (2009) 2167–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Fevrier B, Raposo G, Exosomes: endosomal-derived vesicles shipping extracellular messages, Curr. Opin. Cell Biol 16 (2004) 415–421. [DOI] [PubMed] [Google Scholar]
- [74].Xiao T, Zhang W, Jiao B, Pan CZ, Liu X, Shen L, The role of exosomes in the pathogenesis of Alzheimer’ disease, Transl. Neurodegener 6 (2017) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ, B lymphocytes secrete antigen-presenting vesicles, J. Exp. Med 183 (1996) 1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S, Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes, Nat. Med 4 (1998) 594–600. [DOI] [PubMed] [Google Scholar]
- [77].Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, Inskoe E, Piroyan A, Sokolsky M, Okolie O, Hingtgen SD, Kabanov AV, Batrakova EV, Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells, Nanomedicine 12 (2016) 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Vingtdeux V, Sergeant N, Buee L, Potential contribution of exosomes to the prion-like propagation of lesions in Alzheimer’s disease, Front. Physiol 3 (2012) 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K, Alzheimer’s disease beta-amyloid peptides are released in association with exosomes, Proc. Natl. Acad. Sci. U. S. A 103 (2006) 11172–11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Takahashi RH, Milner TA, Li F, Nam EE, Edgar MA, Yamaguchi H, Beal MF, Xu H, Greengard P, Gouras GK, Intraneuronal Alzheimer abeta42 accumulates in multivesicular bodies and is associated with synaptic pathology, Am. J. Pathol 161 (2002) 1869–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Langui D, Girardot N, El Hachimi KH, Allinquant B, Blanchard V, Pradier L, Duyckaerts C, Subcellular topography of neuronal Abeta peptide in APPxPS1 transgenic mice, Am. J. Pathol 165 (2004) 1465–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Dinkins MB, Dasgupta S, Wang G, Zhu G, Bieberich E, Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer’s disease, Neurobiol. Aging 35 (2014) 1792–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Saman S, Kim W, Raya M, Visnick Y, Miro S, Saman S, Jackson B, McKee AC, Alvarez VE, Lee NC, Hall GF, Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease, J. Biol. Chem 287 (2012) 3842–3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB, Abner EL, Petersen RC, Federoff HJ, Miller BL, Goetzl EJ, Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case-control study, Alzheimers Dement. 11 (2015) 600–607 e601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T, Wolozin B, Butovsky O, Kugler S, Ikezu T, Depletion of microglia and inhibition of exosome synthesis halt tau propagation, Nat. Neurosci 18 (2015) 1584–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Simpson RJ, Kalra H, Mathivanan S, ExoCarta as a resource for exosomal research, J. Extracell. Vesicles (2012) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Cogswell JP, Ward J, Taylor IA, Waters M, Shi Y, Cannon B, Kelnar K, Kemppainen J, Brown D, Chen C, Prinjha RK, Richardson JC, Saunders AM, Roses AD, Richards CA, Identification of miRNA changes in Alzheimer’s disease brain and CSF yields putative biomarkers and insights into disease pathways, J. Alzheimers Dis 14 (2008) 27–41. [DOI] [PubMed] [Google Scholar]
- [88].Lugli G, Cohen AM, Bennett DA, Shah RC, Fields CJ, Hernandez AG, Smalheiser NR, Plasma exosomal miRNAs in persons with and without Alzheimer disease: altered expression and prospects for biomarkers, PLOS One 10 (2015) e0139233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Uddin MS, Stachowiak A, Mamun AA, Tzvetkov NT, Takeda S, Atanasov AG, Bergantin LB, Abdel-Daim MM, Stankiewicz AM, Autophagy and Alzheimer’s disease: from molecular mechanisms to therapeutic implications, Front. Aging Neurosci 10 (2018) 04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Menzies FM, Fleming A, Caricasole A, Bento CF, Andrews SP, Ashkenazi A, Fullgrabe J, Jackson A, Jimenez Sanchez M, Karabiyik C, Licitra F, Lopez Ramirez A, Pavel M, Puri C, Renna M, Ricketts T, Schlotawa L, Vicinanza M, Won H, Zhu Y, Skidmore J, Rubinsztein DC, Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities, Neuron 93 (2017) 1015–1034. [DOI] [PubMed] [Google Scholar]
- [91].Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH, Nixon RA, Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer’s disease, J. Neurosci 28 (2008) 6926–6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Garcia-Arencibia M, Hochfeld WE, Toh PP, Rubinsztein DC, Autophagy, a guardian against neurodegeneration, Semin. Cell Dev. Biol 21 (2010) 691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Shen HM, Mizushima N, At the end of the autophagic road: an emerging understanding of lysosomal functions in autophagy, Trends Biochem. Sci 39 (2014) 61–71. [DOI] [PubMed] [Google Scholar]
- [94].Nilsson P, Loganathan K, Sekiguchi M, Matsuba Y, Hui K, Tsubuki S, Tanaka M, Iwata N, Saito T, Saido TC, Abeta secretion and plaque formation depend on autophagy, Cell Rep. 5 (2013) 61–69. [DOI] [PubMed] [Google Scholar]
- [95].Vingtdeux V, Chandakkar P, Zhao H, d’Abramo C, Davies P, Marambaud P, Novel synthetic small-molecule activators of AMPK as enhancers of autophagy and amyloid-beta peptide degradation, FASEB J. 25 (2011) 219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Yu WH, Cuervo AM, Kumar A, Peterhoff CM, Schmidt SD, Lee JH, Mohan PS, Mercken M, Farmery MR, Tjernberg LO, Jiang Y, Duff K, Uchiyama Y, Naslund J, Mathews PM, Cataldo AM, Nixon RA, Macroautophagy—a novel Beta-amyloid peptide-generating pathway activated in Alzheimer’s disease, J. Cell Biol 171 (2005) 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Nixon RA, Autophagy, amyloidogenesis and Alzheimer disease, J. Cell Sci 120 (2007) 4081–4091. [DOI] [PubMed] [Google Scholar]
- [98].Steele JW, Ju S, Lachenmayer ML, Liken J, Stock A, Kim SH, Delgado LM, Alfaro IE, Bernales S, Verdile G, Bharadwaj P, Gupta V, Barr R, Friss A, Dolios G, Wang R, Ringe D, Protter AA, Martins RN, Ehrlich ME, Yue Z, Petsko GA, Gandy S, Latrepirdine stimulates autophagy and reduces accumulation of alpha-synuclein in cells and in mouse brain, Mol. Psychiatry 18 (2013) 882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Francois A, Terro F, Janet T, Rioux Bilan A, Paccalin M, Page G, Involvement of interleukin-1beta in the autophagic process of microglia: relevance to Alzheimer’s disease, J. Neuroinflammation 10 (2013) 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Francois A, Rioux Bilan A, Quellard N, Fernandez B, Janet T, Chassaing D, Paccalin M, Terro F, Page G, Longitudinal follow-up of autophagy and inflammation in brain of APPswePS1dE9 transgenic mice, J. Neuroinflammation 11 (2014) 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Ye J, Jiang Z, Chen X, Liu M, Li J, Liu N, The role of autophagy in pro-inflammatory responses of microglia activation via mitochondrial reactive oxygen species in vitro, J. Neurochem 142 (2017) 215–230. [DOI] [PubMed] [Google Scholar]
- [102].Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ, Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions, Neuron 47 (2005) 365–378. [DOI] [PubMed] [Google Scholar]
- [103].Sun T, Qiao H, Pan PY, Chen Y, Sheng ZH, Motile axonal mitochondria contribute to the variability of presynaptic strength, Cell Rep. 4 (2013) 413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Rangaraju V, Calloway N, Ryan TA, Activity-driven local ATP synthesis is required for synaptic function, Cell 156 (2014) 825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Nicholls DG, Budd SL, Mitochondria and neuronal survival, Physiol. Rev 80 (2000) 315–360. [DOI] [PubMed] [Google Scholar]
- [106].Sheng ZH, Cai Q, Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration, Nat. Rev. Neurosci 13 (2012) 77–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Mishra P, Chan DC, Mitochondrial dynamics and inheritance during cell division, development and disease, Nat. Rev. Mol. Cell Biol 15 (2014) 634–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Swerdlow RH, Burns JM, Khan SM, The Alzheimer’s disease mitochondrial cascade hypothesis, J. Alzheimers Dis 20 (Suppl. 2) (2010) S265–S279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Reddy PH, Reddy TP, Manczak M, Calkins MJ, Shirendeb U, Mao P, Dynamin-related protein 1 and mitochondrial fragmentation in neurodegenerative diseases, Brain Res. Rev 67 (2011) 103–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Du H, Guo L, Yan SS, Synaptic mitochondrial pathology in Alzheimer’s disease, Antioxid. Redox Signal 16 (2012) 1467–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Baloyannis SJ, Mitochondrial alterations in Alzheimer’s disease, J. Alzheimers Dis 9 (2006) 119–126. [DOI] [PubMed] [Google Scholar]
- [112].Chen H, Chan DC, Mitochondrial dynamics—fusion, fission, movement, and mitophagy—in neurodegenerative diseases, Hum. Mol. Genet 18 (2009) R169–R176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Jiang S, Nandy P, Wang W, Ma X, Hsia J, Wang C, Wang Z, Niu M, Siedlak SL, Torres S, Fujioka H, Xu Y, Lee HG, Perry G, Liu J, Zhu X, Mfn2 ablation causes an oxidative stress response and eventual neuronal death in the hippocampus and cortex, Mol. Neurodegener 13 (2018) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, Wu H, ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease, Science 304 (2004) 448–452. [DOI] [PubMed] [Google Scholar]
- [115].Cai Q, Tammineni P, Alterations in mitochondrial quality control in Alzheimer’s disease, Front. Cell Neurosci 10 (2016) 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Kerr JS, Adriaanse BA, Greig NH, Mattson MP, Cader MZ, Bohr VA, Fang EF, Mitophagy and Alzheimer’s disease: cellular and molecular mechanisms, Trends Neurosci. 40 (2017) 151–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Court FA, Coleman MP, Mitochondria as a central sensor for axonal degenerative stimuli, Trends Neurosci. 35 (2012) 364–372. [DOI] [PubMed] [Google Scholar]
- [118].Ashrafi G, Schwarz TL, PINK1- and PARK2-mediated local mitophagy in distal neuronal axons, Autophagy 11 (2015) 187–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W, PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1, Nat. Cell Biol 12 (2010) 119–131. [DOI] [PubMed] [Google Scholar]
- [120].Rakovic A, Shurkewitsch K, Seibler P, Grunewald A, Zanon A, Hagenah J, Krainc D, Klein C, Phosphatase and tensin homolog (PTEN)-induced putative kinase 1 (PINK1)-dependent ubiquitination of endogenous Parkin attenuates mitophagy: study in human primary fibroblasts and induced pluripotent stem cell-derived neurons, J. Biol. Chem 288 (2013) 2223–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, Sideris DP, Fogel AI, Youle RJ, The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy, Nature 524 (2015) 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Strappazzon F, Nazio F, Corrado M, Cianfanelli V, Romagnoli A, Fimia GM, Campello S, Nardacci R, Piacentini M, Campanella M, Cecconi F, AMBRA1 is able to induce mitophagy via LC3 binding, regardless of PARKIN and p62/SQSTM1, Cell Death Differ. 22 (2015) 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Goetzl EJ, Boxer A, Schwartz JB, Abner EL, Petersen RC, Miller BL, Kapogiannis D, Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease, Neurology 85 (2015) 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Bordi M, Berg MJ, Mohan PS, Peterhoff CM, Alldred MJ, Che S, Ginsberg SD, Nixon RA, Autophagy flux in CA1 neurons of Alzheimer hippocampus: increased induction overburdens failing lysosomes to propel neuritic dystrophy, Autophagy 12 (2016) 2467–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Julien C, Tremblay C, Emond V, Lebbadi M, Salem N Jr., Bennett DA, Calon F, Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease, J. Neuropathol. Exp. Neurol 68 (2009) 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Rice AC, Keeney PM, Algarzae NK, Ladd AC, Thomas RR, Bennett JP Jr., Mitochondrial DNA copy numbers in pyramidal neurons are decreased and mitochondrial biogenesis transcriptome signaling is disrupted in Alzheimer’s disease hippocampi, J. Alzheimers Dis 40 (2014) 319–330. [DOI] [PubMed] [Google Scholar]
- [127].Yang W, Zou Y, Zhang M, Zhao N, Tian Q, Gu M, Liu W, Shi R, Lu Y, Yu W, Mitochondrial Sirt3 expression is decreased in APP/PS1 double transgenic mouse model of Alzheimer’s disease, Neurochem. Res 40 (2015) 1576–1582. [DOI] [PubMed] [Google Scholar]
- [128].Verdin E, NAD(+) in aging, metabolism, and neurodegeneration, Science 350 (2015) 1208–1213. [DOI] [PubMed] [Google Scholar]
- [129].Zhou M, Ottenberg G, Sferrazza GF, Hubbs C, Fallahi M, Rumbaugh G, Brantley AF, Lasmezas CI, Neuronal death induced by misfolded prion protein is due to NAD+ depletion and can be relieved in vitro and in vivo by NAD+ replenishment, Brain 138 (2015) 992–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Fang EF, Scheibye-Knudsen M, Chua KF, Mattson MP, Croteau DL, Bohr VA, Nuclear DNA damage signalling to mitochondria in ageing, Nat. Rev. Mol. Cell Biol 17 (2016) 308–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Strosznajder JB, Czapski GA, Adamczyk A, Strosznajder RP, Poly(ADP-ribose) polymerase-1 in amyloid beta toxicity and Alzheimer’s disease, Mol. Neurobiol 46 (2012) 78–84. [DOI] [PubMed] [Google Scholar]
- [132].Blacher E, Dadali T, Bespalko A, Haupenthal VJ, Grimm MO, Hartmann T, Lund FE, Stein R, Levy A, Alzheimer’s disease pathology is attenuated in a CD38-deficient mouse model, Ann. Neurol 78 (2015) 88–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Martire S, Fuso A, Mosca L, Forte E, Correani V, Fontana M, Scarpa S, Maras B, d’Erme M, Bioenergetic impairment in animal and cellular models of Alzheimer’s disease: PARP-1 inhibition rescues metabolic dysfunctions, J. Alzheimers Dis 54 (2016) 307–324. [DOI] [PubMed] [Google Scholar]
- [134].Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP, Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats, Hippocampus 18 (2008) 1085–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Jung HS, Lee MS, Role of autophagy in diabetes and mitochondria, Ann. N. Y. Acad. Sci 1201 (2010) 79–83. [DOI] [PubMed] [Google Scholar]
- [136].Huotari J, Helenius A, Endosome maturation, EMBO J. 30 (2011) 3481–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Luzio JP, Pryor PR, Bright NA, Lysosomes: fusion and function, Nat. Rev. Mol. Cell Biol 8 (2007) 622–632. [DOI] [PubMed] [Google Scholar]
- [138].Zhu L, Zhong M, Zhao J, Rhee H, Caesar I, Knight E, Volpicelli-Daley L, Bustos V, Netzer W, Liu L, Lucast L, Ehrlich M, Robakis NK, Gandy SE, Cai D,Reduction of synaptojanin 1 accelerates Abeta clearance and attenuates cognitive deterioration in an Alzheimer mouse model, J. Biol. Chem 288 (44) (2013) 32050–32063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Zhu L, Zhong M, Elder GA, Sano M, Holtzman DM, Gandy S, Cardozo C, Haroutunian V, Robakis NK, Cai D, Phospholipid dysregulation contributes toApoE4-associated cognitive deficits in Alzheimer’s disease pathogenesis, Proc. Natl. Acad. Sci. U. S. A 112 (2015) 11965–11970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Rebeck GW, Reiter JS, Strickland DK, Hyman BT, Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions, Neuron 11 (1993) 575–580. [DOI] [PubMed] [Google Scholar]
- [141].Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D, Paul SM, Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease, Proc. Natl. Acad. Sci. U. S. A 97 (2000) 2892–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, Fagan AM, Morris JC, Mawuenyega KG, Cruchaga C, Goate AM, Bales KR, Paul SM, Bateman RJ, Holtzman DM, Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance, Sci. Transl. Med 3 (2011) 89ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Huynh TV, Davis AA, Ulrich JD, Holtzman DM, Apolipoprotein E and Alzheimer’s disease: the influence of apolipoprotein E on amyloid-beta and other amyloidogenic proteins, J. Lipid Res 58 (2017) 824–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Nuriel T, Peng KY, Ashok A, Dillman AA, Figueroa HY, Apuzzo J, Ambat J, Levy E, Cookson MR, Mathews PM, Duff KE, The endosomal-lysosomal pathway is dysregulated by APOE4 expression in vivo, Front Neurosci. 11 (2017) 702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Colacurcio DJ, Nixon RA, Disorders of lysosomal acidification—the emerging role of v-ATPase in aging and neurodegenerative disease, Ageing Res. Rev 32 (2016) 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Haass C, Kaether C, Thinakaran G, Sisodia S, Trafficking and proteolytic processing of APP, Cold Spring Harb. Perspect. Med 2 (2012) a006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Selkoe DJ, Hardy J, The amyloid hypothesis of Alzheimer’s disease at 25 years, EMBO Mol. Med 8 (2016) 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Gowrishankar S, Yuan P, Wu Y, Schrag M, Paradise S, Grutzendler J, De Camilli P, Ferguson SM, Massive accumulation of luminal protease-deficient axonal lysosomes at Alzheimer’s disease amyloid plaques, Proc. Natl. Acad. Sci. U. S. A 112 (2015) E3699–E3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Majumdar A, Capetillo-Zarate E, Cruz D, Gouras GK, Maxfield FR, Degradation of Alzheimer’s amyloid fibrils by microglia requires delivery of ClC-7 to lysosomes, Mol. Biol. Cell 22 (2011) 1664–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].McPherson PS, Garcia EP, Slepnev VI, David C, Zhang X, Grabs D, Sossin WS, Bauerfeind R, Nemoto Y, De Camilli P, A presynaptic inositol-5-phosphatase, Nature 379 (1996) 353–357. [DOI] [PubMed] [Google Scholar]
- [151].Slepnev VI, De Camilli P, Accessory factors in clathrin-dependent synaptic vesicle endocytosis, Nat. Rev. Neurosci 1 (2000) 161–172. [DOI] [PubMed] [Google Scholar]
- [152].Morgan AJ, Platt FM, Lloyd-Evans E, Galione A, Molecular mechanisms of endolysosomal Ca2+ signalling in health and disease, Biochem. J 439 (2011) 349–374. [DOI] [PubMed] [Google Scholar]
- [153].Pryor PR, Mullock BM, Bright NA, Gray SR, Luzio JP, The role of intraorganellar Ca(2+) in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles, J. Cell Biol 149 (2000) 1053–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Lee JH, McBrayer MK, Wolfe DM, Haslett LJ, Kumar A, Sato Y, Lie PP, Mohan P, Coffey EE, Kompella U, Mitchell CH, Lloyd-Evans E, Nixon RA, Presenilin 1 maintains lysosomal Ca(2+) homeostasis via TRPML1 by regulating vATPase-mediated lysosome acidification, Cell Rep. 12 (2015) 1430–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Hughes AL, Gottschling DE, An early age increase in vacuolar pH limits mitochondrial function and lifespan in yeast, Nature 492 (2012) 261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Ruckenstuhl C, Netzberger C, Entfellner I, Carmona-Gutierrez D, Kickenweiz T, Stekovic S, Gleixner C, Schmid C, Klug L, Hajnal I, Sorgo AG, Eisenberg T, Buttner S, Marin OG, Koziel R, Magnes C, Sinner F, Pieber TR, Jansen-Durr P, Frohlich KU, Kroemer G, Madeo F, Autophagy extends lifespan via vacuolar acidification, Microb. Cell 1 (2014) 160–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Ruckenstuhl C, Netzberger C, Entfellner I, Carmona-Gutierrez D, Kickenweiz T, Stekovic S, Gleixner C, Schmid C, Klug L, Sorgo AG, Eisenberg T, Buttner S, Marino G, Koziel R, Jansen-Durr P, Frohlich KU, Kroemer G, Madeo F, Lifespan extension by methionine restriction requires autophagy-dependent vacuolar acidification, PLoS Genet. 10 (2014) e1004347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Stacchini A, Aliberti S, Pacchioni D, Demurtas A, Isolato G, Gazzera C, Veltri A, Maletta F, Molinaro L, Novero D, Flow cytometry significantly improves the diagnostic value of fine needle aspiration cytology of lymphoproliferative lesions of salivary glands, Cytopathology 25 (2014) 231–240. [DOI] [PubMed] [Google Scholar]
- [159].de Laat R, Meabon JS, Wiley JC, Hudson MP, Montine TJ, Bothwell M, LINGO-1 promotes lysosomal degradation of amyloid-beta protein precursor, Pathobiol. Aging Age Relat. Dis 5 (2015) 25796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Ehehalt R, Keller P, Haass C, Thiele C, Simons K, Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts, J. Cell Biol 160 (2003) 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Wang Y, Martinez-Vicente M, Kruger U, Kaushik S, Wong E, Mandelkow EM, Cuervo AM, Mandelkow E, Tau fragmentation, aggregation and clearance: the dual role of lysosomal processing, Hum. Mol. Genet 18 (2009) 4153–4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, Sardiello M, Rubinsztein DC, Ballabio A, TFEB links autophagy to lysosomal biogenesis, Science 332 (2011) 1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Roczniak-Ferguson A, Petit CS, Froehlich F, Qian S, Ky J, Angarola B, Walther TC, Ferguson SM, The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis, Sci. Signal 5 (2012) ra42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, Di Malta C, Donaudy F, Embrione V, Polishchuk RS, Banfi S, Parenti G, Cattaneo E, Ballabio A, A gene network regulating lysosomal biogenesis and function, Science 325 (2009) 473–477. [DOI] [PubMed] [Google Scholar]
- [165].Polito VA, Li H, Martini-Stoica H, Wang B, Yang L, Xu Y, Swartzlander DB, Palmieri M, di Ronza A, Lee VM, Sardiello M, Ballabio A, Zheng H, Selective clearance of aberrant tau proteins and rescue of neurotoxicity by transcription factor EB, EMBO Mol. Med 6 (2014) 1142–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Armstrong A, Mattsson N, Appelqvist H, Janefjord C, Sandin L, Agholme L, Olsson B, Svensson S, Blennow K, Zetterberg H, Kagedal K, Lysosomal network proteins as potential novel CSF biomarkers for Alzheimer’s disease, Neuromolecular Med. 16 (2014) 150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Schmidt M, Finley D, Regulation of proteasome activity in health and disease, Biochim. Biophys. Acta 1843 (2014) 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Caldeira MV, Salazar IL, Curcio M, Canzoniero LM, Duarte CB, Role of the ubiquitin-proteasome system in brain ischemia: friend or foe? Prog. Neurobiol 112 (2014) 50–69. [DOI] [PubMed] [Google Scholar]
- [169].McKinnon C, Tabrizi SJ, The ubiquitin-proteasome system in neurodegeneration, Antioxid. Redox Signal 21 (2014) 2302–2321. [DOI] [PubMed] [Google Scholar]
- [170].Ciechanover A, Kwon YT, Degradation of misfolded proteins in neurodegenerative diseases: therapeutic targets and strategies, Exp. Mol. Med 47 (2015) e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Finley D, Recognition and processing of ubiquitin-protein conjugates by the proteasome, Annu. Rev. Biochem 78 (2009) 477–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Weissman AM, Themes and variations on ubiquitylation, Nat. Rev. Mol. Cell Biol 2 (2001) 169–178. [DOI] [PubMed] [Google Scholar]
- [173].Voutsadakis IA, The ubiquitin-proteasome system and signal transduction pathways regulating Epithelial Mesenchymal transition of cancer, J. Biomed. Sci 19 (2012) 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Riederer BM, Leuba G, Vernay A, Riederer IM, The role of the ubiquitin proteasome system in Alzheimer’s disease, Exp. Biol. Med. (Maywood) 236 (2011) 268–276. [DOI] [PubMed] [Google Scholar]
- [175].Hong L, Huang HC, Jiang ZF, Relationship between amyloid-beta and the ubiquitin-proteasome system in Alzheimer’s disease, Neurol. Res 36 (2014) 276–282. [DOI] [PubMed] [Google Scholar]
- [176].Tai HC, Serrano-Pozo A, Hashimoto T, Frosch MP, Spires-Jones TL, Hyman BT, The synaptic accumulation of hyperphosphorylated tau oligomers in Alzheimer disease is associated with dysfunction of the ubiquitin-proteasome system, Am. J. Pathol 181 (2012) 1426–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Gadhave K, Bolshette N, Ahire A, Pardeshi R, Thakur K, Trandafir C, Istrate A, Ahmed S, Lahkar M, Muresanu DF, Balea M, The ubiquitin proteasomal system: a potential target for the management of Alzheimer’s disease, J. Cell. Mol. Med 20 (2016) 1392–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Perry G, Friedman R, Shaw G, Chau V, Ubiquitin is detected in neurofibrillary tangles and senile plaque neurites of Alzheimer disease brains, Proc. Natl. Acad. Sci. U. S. A 84 (1987) 3033–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Lopez Salon M, Pasquini L, Besio Moreno M, Pasquini JM, Soto E, Relationship between beta-amyloid degradation and the 26S proteasome in neural cells, Exp. Neurol 180 (2003) 131–143. [DOI] [PubMed] [Google Scholar]
- [180].Almeida CG, Takahashi RH, Gouras GK, Beta-amyloid accumulation impairs multivesicular body sorting by inhibiting the ubiquitin-proteasome system, J. Neurosci 26 (2006) 4277–4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Zhao X, Yang J, Amyloid-beta peptide is a substrate of the human 20S proteasome, ACS Chem. Neurosci 1 (2010) 655–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Cardozo C, Michaud C, Proteasome-mediated degradation of tau proteins occurs independently of the chymotrypsin-like activity by a nonprocessive pathway, Arch. Biochem. Biophys 408 (2002) 103–110. [DOI] [PubMed] [Google Scholar]
- [183].Oddo S, The ubiquitin-proteasome system in Alzheimer’s disease, J. Cell. Mol. Med 12 (2008) 363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Tseng BP, Green KN, Chan JL, Blurton-Jones M, LaFerla FM, Abeta inhibits the proteasome and enhances amyloid and tau accumulation, Neurobiol. Aging 29 (2008) 1607–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Patrick GN, Synapse formation and plasticity: recent insights from the perspective of the ubiquitin proteasome system, Curr. Opin. Neurobiol 16 (2006) 90–94. [DOI] [PubMed] [Google Scholar]
- [186].Gong B, Radulovic M, Figueiredo-Pereira ME, Cardozo C, The ubiquitin-proteasome system: potential therapeutic targets for Alzheimer’s disease and spinal cord injury, Front. Mol. Neurosci 9 (2016) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Fioravante D, Byrne JH, Protein degradation and memory formation, Brain Res. Bull 85 (2011) 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Vitolo OV, Sant’Angelo A, Costanzo V, Battaglia F, Arancio O, Shelanski M, Amyloid beta -peptide inhibition of the PKA/CREB pathway and long-term potentiation: reversibility by drugs that enhance cAMP signaling, Proc. Natl. Acad. Sci. U. S. A 99 (2002) 13217–13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Smith DL, Pozueta J, Gong B, Arancio O, Shelanski M, Reversal of long-term dendritic spine alterations in Alzheimer disease models, Proc. Natl. Acad. Sci. U. S. A 106 (2009) 16877–16882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Atkin G, Paulson H, Ubiquitin pathways in neurodegenerative disease, Front. Mol. Neurosci 7 (2014) 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Wilkinson KD, Lee KM, Deshpande S, Duerksen-Hughes P, Boss JM, Pohl J, The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl-terminal hydrolase, Science 246 (1989) 670–673. [DOI] [PubMed] [Google Scholar]
- [192].Chen J, Huang RY, Turko IV, Mass spectrometry assessment of ubiquitin carboxyl-terminal hydrolase L1 partitioning between soluble and particulate brain homogenate fractions, Anal. Chem 85 (2013) 6011–6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Zetterberg M, Sjolander A, von Otter M, Palmer MS, Landgren S, Minthon L, Wallin A, Andreasen N, Blennow K, Zetterberg H, Ubiquitin carboxy-terminal hydrolase L1 (UCHL1) S18Y polymorphism in Alzheimer’s disease, Mol. Neurodegener 5 (2010) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Mecozzi VJ, Berman DE, Simoes S, Vetanovetz C, Awal MR, Patel VM, Schneider RT, Petsko GA, Ringe D, Small SA, Small, Pharmacological chaperones stabilize retromer to limit APP processing, Nat. Chem. Biol 10 (2014) 443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V, Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease, PLoS One 5 (2010) e9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Mattson MP, Energy intake and exercise as determinants of brain health and vulnerability to injury and disease, Cell Metab. 16 (2012) 706–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Geisler JG, Marosi K, Halpern J, Mattson MP, DNP, mitochondrial uncoupling, and neuroprotection: a little dab’ll do ya, Alzheimers Dement. 13 (2017) 582–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Cheng J, North BJ, Zhang T, Dai X, Tao K, Guo J, Wei W, The emerging roles of protein homeostasis-governing pathways in Alzheimer’s disease, Aging Cell 17 (2018) e12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Gong B, Cao Z, Zheng P, Vitolo OV, Liu S, Staniszewski A, Moolman D, Zhang H, Shelanski M, Arancio O, Ubiquitin hydrolase Uch-L1 rescues beta-amyloid-induced decreases in synaptic function and contextual memory, Cell 126 (2006) 775–788. [DOI] [PubMed] [Google Scholar]
- [200].Chen F, Sugiura Y, Myers KG, Liu Y, Lin W, Ubiquitin carboxyl-terminal hydrolase L1 is required for maintaining the structure and function of the neuromuscular junction, Proc. Natl. Acad. Sci. U. S. A 107 (2010) 1636–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Chamberland JP, Ritter B, Retromer revisited: evolving roles for retromer in endosomal sorting, J. Cell Biol 216 (2017) 3433–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Mosyak L, Wood A, Dwyer B, Buddha M, Johnson M, Aulabaugh A, Zhong X, Presman E, Benard S, Kelleher K, Wilhelm J, Stahl ML, Kriz R, Gao Y, Cao Z, Ling HP, Pangalos MN, Walsh FS, Somers WS, The structure of the Lingo-1 ectodomain, a module implicated in central nervous system repair inhibition, J. Biol. Chem 281 (2006) 36378–36390. [DOI] [PubMed] [Google Scholar]