Abstract
Objective:
Regulation of tissue factor (TF):coagulation factor VIIa (FVIIa) complex procoagulant activity is especially critical in tissues where plasma can contact TF-expressing cells. One example is the liver, where hepatocytes are routinely exposed to plasma because of the fenestrated sinusoidal endothelium. Although liver-associated TF contributes to coagulation, the mechanisms controlling the TF:FVIIa complex activity in this tissue are not known.
Approach and Results:
Common bile duct ligation in mice triggered rapid hepatocyte TF-dependent intrahepatic coagulation coincident with increased plasma bile acids, which occurred at a time before observable liver damage. Similarly, plasma thrombin-antithrombin levels increased in cholestatic patients without concurrent hepatocellular injury. Pathologically-relevant concentrations of the bile acid glycochenodeoxycholic acid (GCDCA) rapidly increased hepatocyte TF-dependent procoagulant activity in vitro, independent of de novo TF synthesis and necrotic or apoptotic cell death. GCDCA increased hepatocyte TF activity even in the presence of the phosphatidylserine-blocking protein lactadherin. Interestingly, GCDCA and taurochenodeoxycholic acid (TCDCA) increased the procoagulant activity of the TF:FVIIa complex relipidated in unilamellar phosphatidylcholine vesicles, which was linked to an apparent decrease in the Km for coagulation factor X. Notably, the zwitterionic detergent CHAPS, a bile acid structural analog, did not increase relipidated TF:FVIIa activity. Bile acids directly enhanced factor X activation by recombinant soluble TF:FVIIa complex, but had no effect on FVIIa alone.
Conclusions:
The results indicate that bile acids directly accelerate TF:FVIIa-driven coagulation reactions, suggesting a novel mechanism whereby elevation in a physiological mediator can directly increase TF:FVIIa procoagulant activity.
Keywords: tissue factor, coagulation, thrombosis
Subject terms: Basic Science Research, Mechanisms, Thrombosis
Introduction
Tissue factor (TF) is the primary activator of blood coagulation and plays a critical role in both primary hemostasis and thrombosis. Cellular expression of TF is regulated through transcriptional and translational mechanisms.1 A cellular barrier separating extravascular TF from its plasma ligand, coagulation factor VIIa (FVIIa), prevents inappropriate intravascular coagulation.2, 3 Procoagulant activity of the TF:FVIIa complex is also regulated by post-translational mechanisms, wherein the TF:FVIIa complex can exist in an encrypted state defined by little-to-no procoagulant function.4, 5 Proposed mechanisms underlying the decryption (i.e., activation) of TF:FVIIa procoagulant activity focus on interactions with anionic phospholipids (e.g., phosphatidylserine) in the cell membrane and oxidation of extracellular disulfides on the TF molecule.5 Discovery of these mechanisms has been enabled by chemical tools including calcium ionophores and oxidizing agents (e.g., HgCl2).6–8 However, examples of TF decryption triggered by (patho)physiologically-relevant small molecules are lacking.
Liver parenchymal cells (i.e., hepatocytes) have been shown to express low levels of TF.3,9 However, unlike other tissues, the fenestrated endothelium of the liver microvasculature allows plasma unrestricted access to hepatocytes. In addition, hepatocytes are the primary site of synthesis of several coagulation factors, including FVII(a). Consequently, TF expression by hepatocytes occurs in a microenvironment lacking the traditional “hemostatic envelope”. Indeed, isolated hepatocytes seem to express TF complexed with FVIIa,9 highlighting the theoretical importance of TF:FVIIa encryption as a means to prevent inappropriate coagulation. However, the molecular mechanisms and mediators controlling procoagulant function of the TF:FVIIa complex in the liver are largely unknown. Notably, hepatocyte TF-dependent coagulation is evident in experimental settings where the concentration of plasma bile acids is increased.10–12 Although bile acids have well-described proinflammatory activity,13, 14 the effect of these molecules on the TF:FVIIa procoagulant activity has not been examined.
We tested the hypothesis that bile acids stimulate TF:FVIIa procoagulant activity and used a combination of in vivo and in vitro approaches to determine whether this could form a novel mechanism of TF:FVIIa decryption by a (patho)physiologically-relevant molecule elevated in conditions of liver disease.
Materials and Methods
Data disclosure statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Mice and bile duct ligation (BDL)
Wild-type C57Bl/6J male mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Littermate TFflox/flox mice (control mice) and TFflox/flox/AlbCre mice (HPCΔTF mice) backcrossed 8 generations onto a C57Bl/6J background have been described previously.9, 11 Approximately 50 male mice between the ages of 10–18 weeks were used in this study. The hypothesis to be tested for mouse experiments relied on baseline knowledge obtained from the literature on the time course of BDL-induced hepatic injury, TF-dependent coagulation, and biliary pressure after BDL in males.11, 25–27 Thus, male mice were used for BDL experiments. Mice were housed at an ambient temperature of approximately 22°C with alternating 12-hour light/12-hour dark cycles and provided purified water and normal rodent diet (Teklad Irradiated 22/5 Rodent Diet 8940, Envigo, Indianapolis, Indiana) ad libitum. Obstructive cholestasis was induced in mice by surgical ligation of the common bile duct, as described previously.14 Under deep surgical isoflurane (3–5%) anesthesia, an abdominal incision was made to reveal the common bile duct. Cotton-tipped applicators were used to visualize the bile duct, which was then separated from other tissue using forceps. The bile duct was then ligated with sutures between the gallbladder and the intestine, the incision site covered with gauze soaked in warm sterile saline, and the mouse was kept on a warming pad under deep surgical anesthesia for 30 minutes. For sham surgeries, the same steps were performed except ligation of the bile duct. After 30 minutes, sodium citrate (3.2%) was injected at 8 mL/kg into the vena cava. Thirty seconds after injection of sodium citrate, anticoagulated whole blood was collected into the same syringe. The liver was then removed, the left lateral lobe fixed in 10% neutral-buffered formalin and remaining lobes were snap-frozen in liquid nitrogen. Mice were maintained in Association for Assessment and Accreditation of Laboratory Animal Care International–accredited facilities at Michigan State University. All animal procedures were approved by the Michigan State University Institutional Animal Care and Use Committee.
Human plasma samples
Patients admitted to the University of Kansas Hospital were enrolled into an Institutional Review Board (IRB)-approved protocol by the Kansas University Liver Center Staff or University of Kansas Medical Center Staff for the isolation of plasma from patients with suspected cholestasis. The study fully adhered to the Helsinki Declaration and informed consent was acquired from all patients before the onset of the study. The primary inclusion criterion was patients with suspected cholestasis undergoing a planned endoscopic retrograde cholangiopancreatography (ERCP) for diagnosis and treatment. Blood was drawn before the initial ERCP and stored at –80°C until use. For the purpose of this study, the patients were subdivided into three categories based on the clinical values obtained by the University of Kansas Hospital labs. Uninjured patients had alanine aminotransferase (ALT) levels <50 U/L and alkaline phosphatase (ALP) levels <110 U/L (4 males and 5 females). Patients presenting with cholestasis, but without liver injury had no elevation in ALT (ALT <50 U/L), but an ALP <110 U/L (7 males and 7 females). Patients with cholestatic liver injury had ALT values ≥50 U/L and defined cholestasis as evidenced by ERCP and ALP ≥110 U/L (10 males and 8 females).
Hepatocyte isolation and treatment
Rat tail collagen (Corning) was diluted in acidified sterile water (0.05N HCl), and coated on 6-well culture plates (Greiner Bio-One, Monroe, NC) at 100 μg/mL overnight at 4°C. The plates were then washed three times with sterile phosphate-buffered saline and air-dried for 10 minutes. Hepatocytes from male mice were isolated by perfusion and collagenase digestion, as previously described.9 Cell viability was determined by trypan blue exclusion, and hepatocytes with at least 80% viability were used. Hepatocytes were plated at a density of 500,000 cells/well in Williams E medium (Sigma-Aldrich) containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Sigma-Aldrich). After 2 hours, non-adherent cells were removed and replaced with fresh media containing 10% FBS. The cells were then incubated overnight at 37°C and 5% CO2. The next day, the cells were treated with various concentrations of sodium glycochenodeoxycholate (GCDCA, CHEM-IMPEX International, Wood Dale, IL) or the vehicle HEPES [N-2-hydroxyethylpiperazine-N’−2-ethanesulfonic acid] buffered saline (HBS; 137 mM NaCl, 5.38 mM KCl, 5.55 mM glucose, 10 mM HEPES) for 15 minutes in HBS containing albumin (HBSA) prior to assessment of the TF procoagulant activity (see below). In select experiments, the cells were pretreated with actinomycin D (ActD, 0.2 μg/mL, Sigma-Aldrich), GW4064 (10 μM, Tocris, Minneapolis, MN), or caspase 3/7 inhibitor (Ac-DEVD-CHO, 50 μM, Biolegend, San Diego, CA) 30 minutes before the bile acid treatment. The vehicle for each compound was DMSO, and the final concentration of DMSO in culture was 0.1%. To assess the effect of bile acid treatment on the activation of apoptosis, levels of cleaved caspase-3 were assessed by western blot with Jo2/ActD co-treatment (0.5 μg/mL Jo2, BD, Franklin Lakes, NJ and 0.2 μg/mL ActD) used as a positive control.15
Western blot for cleaved caspase-3
Cleaved caspase-3 levels were determined by western blotting as described previously.15 Hepatocyte pellets were homogenized in 1X RIPA buffer containing protease and phosphatase inhibitors (G-Biosciences, St. Louis, MO) and the homogenate was rotated end-over-end for 30 minutes at 4°C, spun at 10,000 × g for 10 minutes and the supernatants were saved. Equal amounts of protein were resolved using SDS-PAGE. Following semi-dry transfer, the PVDF membrane was blocked in 5% BSA in Tris-buffered saline + Tween-20 (50 mM Tris, 150 mM NaCl, 0.1% Tween-20, pH 7.4) and then incubated overnight with a rabbit monoclonal anti-cleaved caspase-3 antibody (Asp175, clone 5A1E, 1:1000 dilution, Cell Signaling Technology, Danvers, MA) at 4°C. Next, the membrane was washed in TBST and then incubated with a goat anti-rabbit horseradish peroxidase (HRP)-conjugated secondary antibody (1:2000, Jackson ImmunoResearch Laboratories, West Grove, PA) for 2 hours at room temperature. The membrane was washed and then incubated with Clarity Western enhanced chemiluminescence substrate solution (BioRad, Hercules, CA) and exposed to blue autoradiography film (ISC BioExpress, Kaysville, UT). The membrane was stripped in Restore Stripping Buffer (Thermo Fisher Scientific, Waltham, MA) and probed for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using a mouse monoclonal antibody (Proteintech, Rosemont, IL, 1:10000 dilution) overnight at 4°C and developed as described earlier with a goat anti-mouse HRP-conjugated secondary antibody.
Measurement of plasma thrombin-antithrombin levels, hepatic fibrin(ogen) deposition, and liver procoagulant activity
Plasma thrombin-antithrombin (TAT) levels were determined using a commercial ELISA (Siemens Healthcare Diagnostics, Malvern, PA). Paraffin-embedded formalin-fixed livers were sectioned and stained for fibrin(ogen) using a polyclonal rabbit anti-human fibrin(ogen) antibody (Agilent, Dako, Santa Clara, CA), as described previously.16 Labeling was performed by the Michigan State University Investigative Histopathology Laboratory, a Division of Human Pathology. Images were captured from the scanned sections obtained using an Olympus VS110 system. Liver procoagulant activity was determined using a single-stage clotting assay, as described previously.9
Determination of plasma bile acid concentration and hepatocyte injury in vitro and in vivo
Bile acids were measured using a commercial kit (Diazyme, Poway, CA) with the following modification in sample and reagent volume: 10 μL of plasma, 135 μL of R1, and 45 μL of R2. Plasma alanine aminotransferase (ALT) activity was determined using a commercial reagent (Thermo Fisher Scientific, Watham, MA). In vitro cytotoxicity was assessed by the ALT release into the culture medium. Absorbance changes were evaluated using an Infinite M200 plate reader (Tecan Durham, NC).
Relipidation of full-length TF into unilamellar vesicles
Full-length recombinant TF (Enzyme Research Laboratories, South Bend, IN) was relipidated in unilamellar vesicles composed of L-α-phosphatidylcholine (PC, isolated from chicken egg) and cholesterol at a 1:5 molar ratio, containing various amounts of L-α-phosphatidylserine (PS, isolated from porcine brain) (Avanti Polar Lipids, Alabaster, AL), as described previously.17 For some studies, TF-deficient vesicles composed of a molar ratio of 50:50 PC:PS were synthesized as described18.
Determination of TF:FVIIa procoagulant activity
TF:FVIIa activity was determined as described previously9 by measuring conversion of human factor X (FX) to FXa. For studies involving hepatocytes, the culture medium was removed from the wells, and the cells were treated with HBSA containing vehicle (HBS) or various concentrations (0–500 μM) of GCDCA (CHEM-IMPEX International) or sodium taurochenodeoxycholate (TCDCA, Matrix Science, Columbia, SC). After 15 minutes, FX (100 nM final, Enzyme Research Laboratories) was added to the wells. For studies using relipidated TF, vesicles containing TF were diluted (0.1 nM TF final), incubated with human FVIIa (5 pM final, Enzyme Research Laboratories) in HBSA containing calcium (5 mM final) for 5 minutes at 37°C.Various concentrations (0–125 μM) of GCDCA, TCDCA, or CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, Pierce, Thermo Fisher Scientific) were added before addition of FX (100 nM final). In some studies, relipidated TF (0.1 nM final) (in PC or PC (99%):PS (1%) was treated with GCDCA (125 μM final) and FVIIa (5 pM final) as above, with the addition of lactadherin (1–40 nM final, Haematologic Technologies Inc., Essex Junction, VT) or vehicle (HBS). For kinetic studies using relipidated TF, the FX concentration was increased up to 5 μM and the reaction was stopped after 60 minutes. For studies using soluble TF (sTF) (100 nM final; kindly provided by Dr. James Morrissey, University of Michigan), a higher concentration of FVIIa was used (5 nM), as reported previously.19 In some experiments involving sTF:FVIIa, reactions were performed in the presence of PC:PS (50:50). FXa generation was allowed to proceed at 37°C for 15 minutes for experiments with hepatocytes, and 30 minutes for experiments using relipidated TF and sTF. FXa generation was stopped with EDTA (pH 7.4, 5 mM final). FXa activity in each sample was assessed using a chromogenic substrate (RGR-Xachrom; 0.667 mM final; Enzyme Research Laboratories) and the data were collected using an Infinite M200 microplate reader (Tecan).
Thrombin generation
Thrombin generation was measured using a Fluoroskan Microplate Fluorometer (ThermoFisher Scientific,Watham, MA) by calibrated automated thrombography, as described previously.20–22 Briefly, 10 μL of trigger (0.5 or 2 nM relipidated TF [in 100% PC vesicles] final) was added to 40 μL of platelet-poor plasma mixed with various concentrations of GCDCA (0–125 μM). Reactions were initiated by adding 10 μL of FluCa solution (0.416 mM ZGGR-AMC substrate, 16.6 mM CaCl2, 60 mg/mL bovine serum albumin in 20 mM HEPES, 0.02% NaN3, pH 7.3) to the wells. Data were analyzed according to Kremers and Hemker21 to yield parameters: lagtime (time at 6 nM of thrombin), time to peak, velocity index (Peak/[time to peak-lagtime]), peak, and endogenous thrombin potential (ETP, expressed as nM thrombin × minute).
Statistics
Comparisons of two groups were made using Student’s t-test. Comparisons of three or more groups were made by one- or two-way analysis of variance, with or without paired analysis for studies involving vesicles and sTF. Student-Newman-Keuls test was used for multiple comparisons. Datasets without a normal distribution or lacking homogeneity of variance were transformed (generally Log10) prior to analysis. Michaelis-Menton parameters were determined using GraphPad Prism 8. All data are reported as mean +SEM and the criterion for statistical significance was P<0.05.
Results
BDL triggers rapid coagulation cascade activation
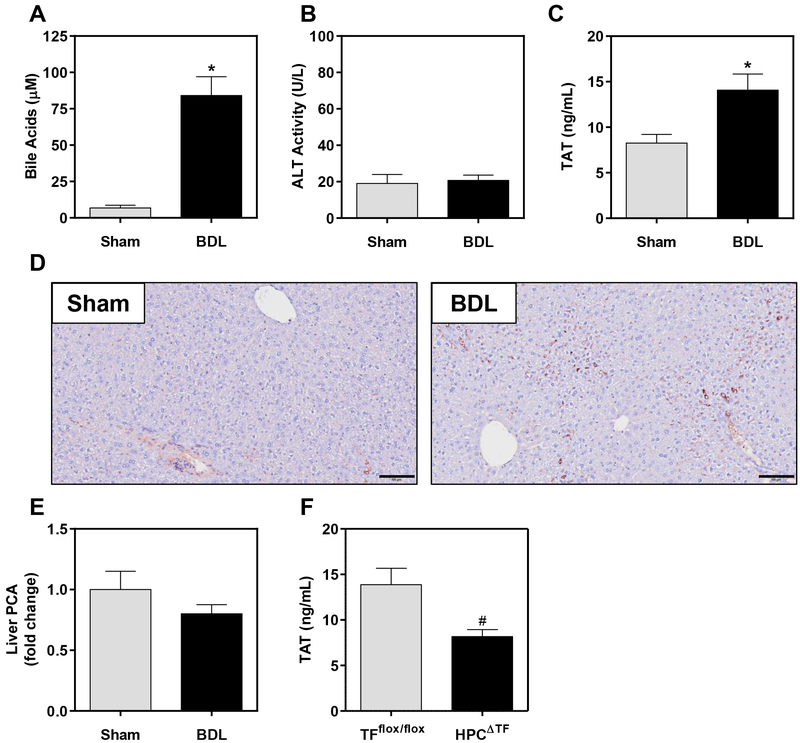
Cholestasis is associated with the activation of coagulation in patients and in experimental mice.11, 23, 24 Hepatocyte TF has been shown to activate coagulation after BDL in mice, but well after liver pathology (i.e., necrosis and fibrosis) has developed.11 Biliary pressure increases within minutes after BDL,25, 26 suggesting rapid exposure of liver parenchyma to abnormally high levels of bile. We found that BDL increased plasma bile acid concentrations within 30 minutes (Fig. 1A). Although BDL ultimately causes liver damage,27, 28 hepatocellular necrosis was not evident 30 minutes after BDL, as plasma ALT levels were not increased (Fig. 1B). Despite no evidence of hepatocellular injury, plasma TAT levels were significantly increased 30 minutes after BDL, indicating activation of coagulation (Fig. 1C). Similar results were observed in a small preliminary study (n=4–5 mice per group) in female mice (not shown). Hepatic fibrin(ogen) deposits also increased in BDL mice and resembled microthrombi primarily localized within the sinusoids (Fig. 1D). BDL did not increase total liver procoagulant activity (Fig. 1E), but the procoagulant response after BDL was significantly reduced in mice with hepatocyte TF deficiency (HPCΔTF), as indicated by reduced plasma TAT levels (Fig. 1F). Collectively, these results indicate that BDL triggers rapid TF-dependent coagulation prior to the development of liver injury.
Figure 1. Obstructive cholestasis in mice triggers rapid intrahepatic coagulation.
Male wild-type mice were subjected to bile duct ligation (BDL) or sham surgery for 30 minutes, and plasma (A) total bile acids, (B) alanine aminotransferase (ALT) activity, and (C) thrombin-antithrombin (TAT) were assessed. (D) Immunohistochemical labeling of hepatic fibrin(ogen) deposition (brown, original magnification 100X) was assessed. Scale bar = 100 μm. (E) Levels of liver procoagulant activity (PCA) were determined using a single-stage clotting assay. N=6 mice for sham surgery and N=8 mice for BDL surgery. (F) TFflox/flox mice (N=10) and TFflox/flox/AlbCre (HPCΔTF, N=9) were subjected to BDL for 30 minutes and plasma TAT levels were assessed. Data are expressed as mean + SEM. *p<0.05 vs. sham mice. #p<0.05 vs. TFflox/flox mice.
Association of coagulation with cholestasis in humans
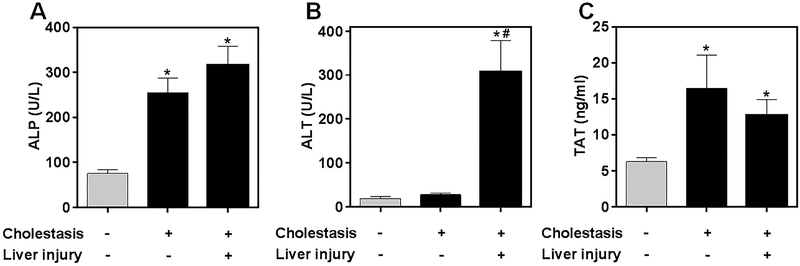
Patients with cholestasis often present with coincident hepatocellular injury, making it challenging to distinguish changes in coagulation biomarkers from hepatocellular injury itself. We examined plasma TAT levels in a group of patients referred for ERCP for confirmation of a diagnosis of cholestasis. These patients were separated into cohorts with biochemical evidence of cholestasis (i.e., elevation in serum ALP) (Fig. 2A) with or without concurrent hepatocellular injury (i.e., elevation in serum ALT activity) (Fig. 2B). Interestingly, plasma TAT levels were increased in cholestatic patients irrespective of biochemical indication of liver damage (Fig. 2C). This suggests that, similar to experimental cholestasis in mice, clinical conditions associated with increased bile acids are associated with increased coagulation without concurrent hepatocellular injury.
Figure 2. Coagulation activation is independent of hepatocellular injury in patients with cholestasis.
Plasma samples were obtained from patients hospitalized for suspected cholestasis. Patients were defined as either uninjured (N=9; 4 males and 5 females), cholestatic without liver injury (N=14; 7 males and 7 females), or cholestatic with liver injury (N=18; 10 males and 8 females), as described in Materials and Methods. (A) Serum alkaline phosphatase (ALP), (B) serum alanine aminotransferase (ALT), and (C) plasma thrombin-antithrombin (TAT) levels were determined. Data are expressed as mean + SEM. *p<0.05 vs. uninjured patients. #p<0.05 vs. patients with cholestasis and without liver injury.
GCDCA increases hepatocyte TF:FVIIa procoagulant activity
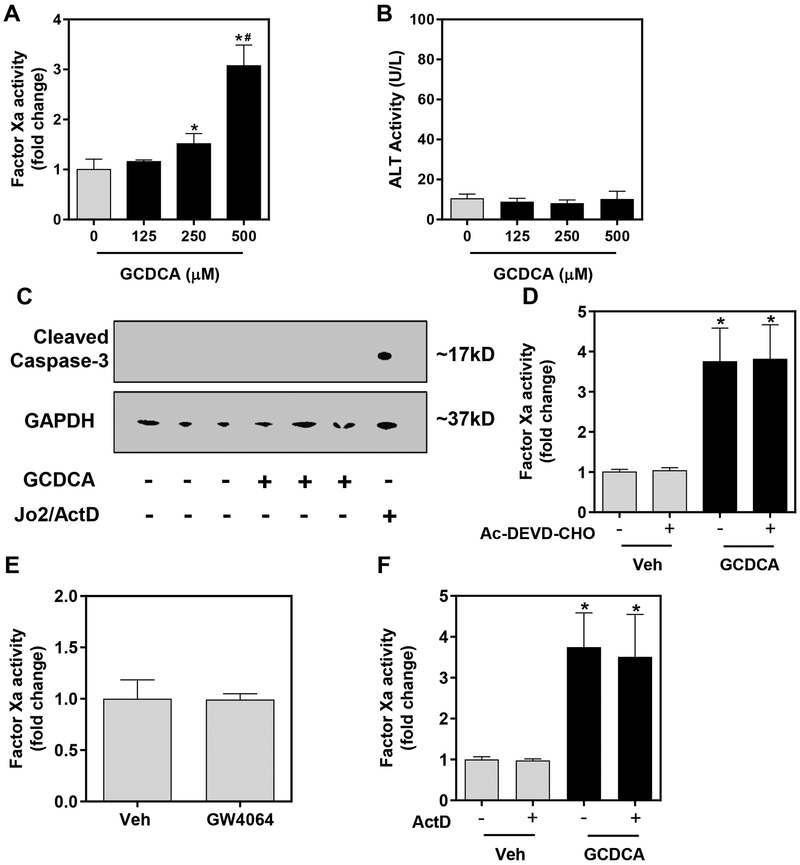
Bile acids are highly concentrated (i.e., >2 mM) in bile, and obstruction of bile flow causes exposure of liver parenchymal cells (i.e., hepatocytes) to high levels of bile acids. Total plasma bile acid concentration increases in patients with various liver diseases,29–31 with glycine-conjugated bile acids comprising the majority of bile acids in humans.32 Because hepatocytes are likely exposed to high bile acid concentrations, we next determined whether bile acids affect hepatocyte TF:FVIIa procoagulant activity. Treatment of primary mouse hepatocytes with GCDCA for 15 minutes significantly increased TF:FVIIa procoagulant activity in a concentration-dependent manner (Fig. 3A). Importantly, GCDCA treatment did not cause necrotic cell death at this time point, indicated by no significant changes in ALT release (Fig. 3B). In agreement with prior studies,32 GCDCA did not cause apoptosis, denoted by the lack of caspase-3 cleavage (Fig. 3C). Moreover, the rapid GCDCA-mediated increase in TF:FVIIa activity was not affected by pretreatment with an inhibitor of caspase 3/7 (Ac-DEVD-CHO), suggesting that TF activity was not increased as a consequence of caspase-directed apoptosis (Fig. 3D). Direct activation of the bile acid nuclear receptor FXR with GW4064 had no effect on TF:FVIIa procoagulant activity (Fig. 3E) and the GCDCA-mediated increase in the TF:FVIIa activity was unaffected by pretreatment with ActD, an inhibitor of transcription (Fig. 3F). Collectively, the results indicate that pathologically-relevant concentrations of GCDCA rapidly increase TF:FVIIa procoagulant activity on primary hepatocytes.
Figure 3. Sodium glycochenodeoxycholate (GCDCA) increases hepatocyte TF:FVIIa activity independent of known mechanisms.
Primary hepatocytes were isolated from male wild-type mice and treated with GCDCA or vehicle (HBS) for 30 minutes. (A) TF:FVIIa procoagulant activity was measured by FXa generation and (B) ALT activity was assessed in the culture medium. (C) Cleaved caspase-3 (marker of apoptosis) and GAPDH were determined by western blot. Jo2/ActD treatment was used as a positive control for caspase-3 cleavage. (D) Hepatocytes were pretreated with Ac-DEVD-CHO (caspase-3/7 inhibitor, 50 μM) or DMSO vehicle (0.1% final) 30 minutes prior to GCDCA treatment (500 μM, 15 minutes) and TF:FVIIa procoagulant activity was determined by FXa generation. (E) Wild-type hepatocytes were treated with the FXR agonist GW4064 (10 μM) or (F) actinomycin D (ActD, 0.2 μg/mL) or DMSO (Veh, 0.1% final) in the presence of GCDCA (500 μM) for 30 minutes prior to determination of FXa activity. Data are expressed as mean + SEM, representing experiments from at least three separate hepatocyte isolations. *p<0.05 vs. respective vehicle treatments. #p<0.05 vs. 250 μM GCDCA treatment.
Direct effects of bile acids on relipidated TF:FVIIa procoagulant activity
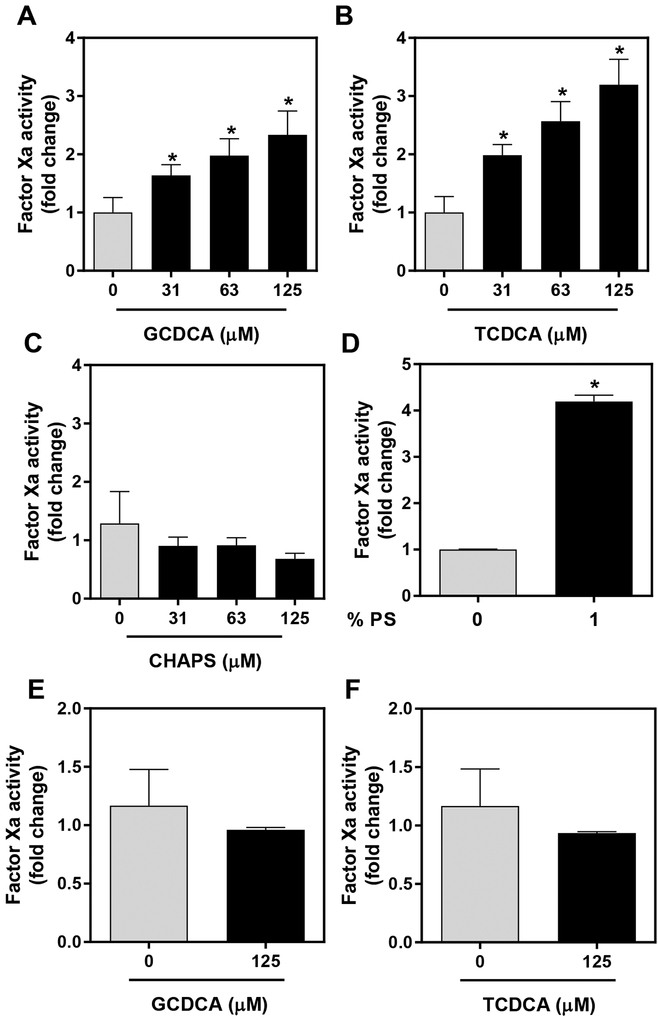
The rapid activation of TF:FVIIa by GCDCA in the absence of cell death and without de novo TF gene expression (i.e. activation occurred in the presence of an inhibitor of transcription) exhibited the hallmarks of TF decryption. We wondered if bile acids could directly enhance TF:FVIIa procoagulant activity. To explore this possibility, we relipidated recombinant, full-length human TF into PC/cholesterol unilamellar vesicles with limited procoagulant activity. In this baseline condition, GCDCA significantly increased TF:FVIIa-dependent FXa generation (Fig. 4A). Glycine-conjugated bile acids (e.g. GCDCA) are the predominant species in humans;32 mice carry comparable bile acids, but conjugated largely to taurine (e.g. TCDCA).33 Interestingly TCDCA also evoked a concentration-dependent increase in TF:FVIIa-dependent FXa generation (Fig. 4B). TCDCA and GCDCA are, in principle, physiologically-relevant anionic detergents. Thus, we also performed similar studies with the non-physiologic detergent CHAPS, a zwitterionic bile acid homolog. Notably, at equimolar concentrations, CHAPS did not increase TF:FVIIa-dependent FXa generation (Fig. 4C).
Figure 4. Direct effect of bile acids on relipidated TF:FVIIa procoagulant activity.
TF relipidated in PC/cholesterol (see Methods) was incubated with FVIIa (5 pM final) for 5 minutes and then incubated with FX (100 nM), vehicle (HBS) or various concentrations of (A) GCDCA, (B) TCDCA, (C) CHAPS prior to assessment of FXa generation 30 minutes later. (D) FXa generation by TF relipidated in vesicles containing PC and cholesterol or vesicles containing 1% phosphatidylserine (PS). (E-F) Vesicles containing 1% PS were treated with FVIIa (5 pM final) for 5 minutes and then treated with vehicle (HBS), GCDCA or TCDCA for 30 minutes before FXa generation was assessed. Data are expressed as mean + SEM, representing data from at least 3 separate experiments. *p<0.05 vs. vehicle.
In studies performed on phosphatidylcholine vesicles containing TF, GCDCA (150 μM) decreased the apparent Km for TF:FVIIa-dependent FXa generation (2.0 ± 0.3 μM to 1.0 ± 0.1 μM, p<0.05) without affecting the apparent Vmax (2.6 ± 0.2 pM/sec (HBS) and 2.4 ± 0.1 pM/sec (GCDCA). Prior studies have shown that PS (6%) reduces the Km for this reaction by nearly 36-fold.34 Thus, we next determined whether bile acid enhancement of TF:FVIIa procoagulant activity would still be evident in the presence of PS, a potent activator of TF:FVIIa activity. In agreement with previous studies35, the addition of low levels of PS (1%) to the vesicles dramatically increased TF:FVIIa-dependent Xa generation (Fig. 4D). Interestingly, bile acid enhancement of TF:FVIIa activity was absent in the presence of PS (Fig. 4E and 4F). Collectively, the results suggest that bile acids directly increase TF:FVIIa-dependent FXa generation, but this effect is mitigated by the presence of a strong activator such as PS.
Next, we evaluated the effect of GCDCA on thrombin generation initiated by TF relipidated in PC. Importantly, relipidation of TF in vesicles without PS required a final concentration of TF much higher than traditionally applied in this assay (i.e., low nM) to initiate thrombin generation. Relipidated TF (in PC only) was used to trigger coagulation in normal human plasma containing various concentrations of GCDCA. Interestingly, the addition of GCDCA to plasma subtly enhanced thrombin generation (Supplemental Figure I). Although effects were modest, analysis by ANOVA suggested a significant concentration-dependent effect of GCDCA on time to peak, peak, and velocity of thrombin generation. These results are consistent with the effect of bile acids on TF:FVIIa activity and the concept that GCDCA may amplify the procoagulant response in vivo, suggested by our observations in both humans and mice with cholestasis.
Direct effects of bile acids on soluble TF:FVIIa procoagulant activity
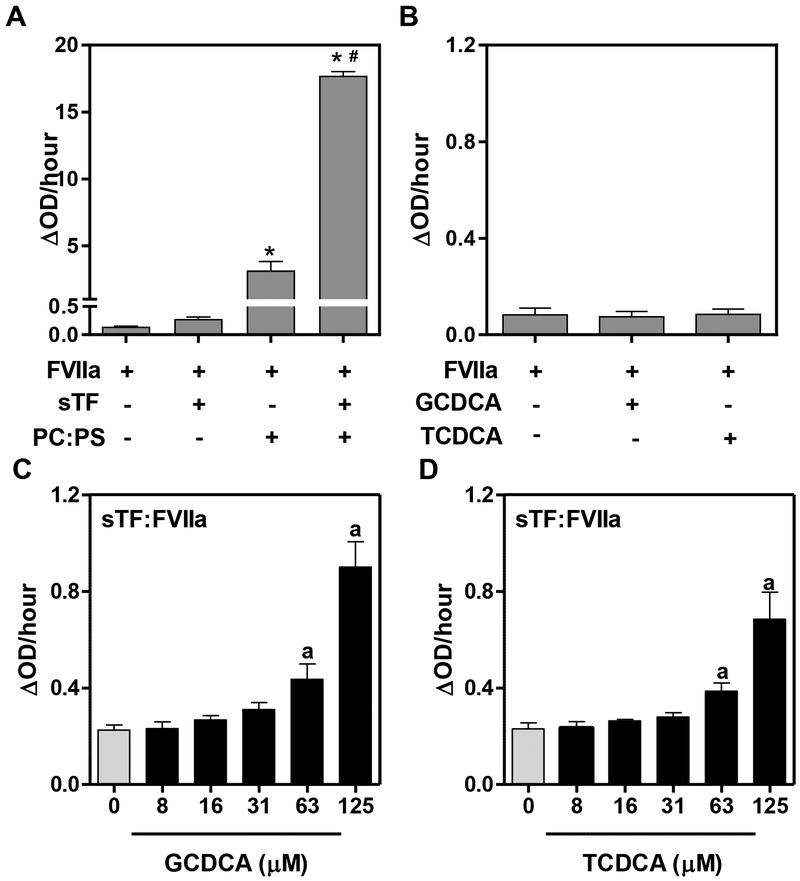
Next, we determined whether bile acids had direct effects on FVIIa catalytic activity. In the absence of TF, high levels of FVIIa have been shown to convert FX to FXa in the presence of phospholipids.17, 18 Indeed, as a positive control, we found that vesicles composed of equal amounts of PC:PS increased TF-independent activation of FX by FVIIa (Fig. 5A). The addition of sTF to the reaction further increased FX activation in the presence of phospholipids (Fig. 5A). In contrast to phospholipids, neither TCDCA nor GCDCA had any effect on TF-independent activation of FX by FVIIa (Fig. 5B). Remarkably, both TCDCA and GCDCA caused a significant and concentration-dependent increase in FX activation by the sTF:FVIIa complex (Fig. 5C and 5D). The results suggest that the amplification of FXa generation by bile acids requires the TF:FVIIa complex.
Figure 5. Direct effect of bile acids on soluble TF:FVIIa procoagulant activity.
(A) FX (100 nM) was incubated with FVIIa (5 nM) in the presence or absence of soluble TF (sTF, 100 nM) with or without unilamellar vesicles (PC:PS 50:50, 100 μM) or respective vehicle (HBS), and FXa generated for 30 minutes. (B) The effect of bile acids (125 μM) on TF-independent FVIIa (5nM)-mediated FX activation. (C-D) sTF:FVIIa complex (100 nM sTF, 5nM FVIIa) was incubated with various concentrations of GCDCA or TCDCA. FXa generation was assessed in all experiments and is represented as a change in absorbance over time (ΔOD/hour). Data are expressed as mean + SEM from at least 3 separate experiments.*p<0.05 vs. FVIIa group. #p<0.05 vs. respective treatment without sTF. ap<0.05 vs. vehicle.
Direct effects of GCDCA independent of phosphatidylserine.
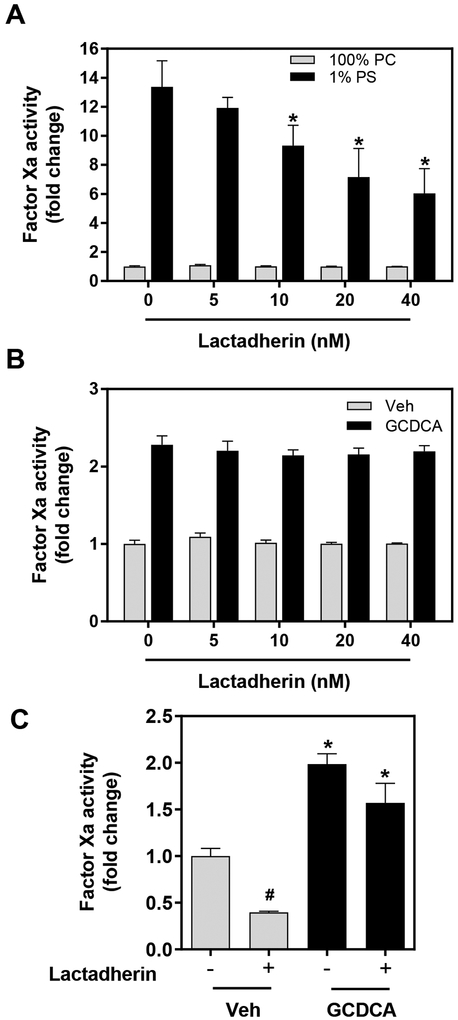
The effect of GCDCA on relipidated and sTF:FVIIa activity suggests a direct effect on procoagulant activity. To distinguish this potential direct mechanism from the effects of PS exposure, we used the PS-binding protein lactadherin. Lactadherin binds to PS and inhibits the PS-dependent enhancement of TF:FVIIa activity.36 In a concentration-dependent manner, lactadherin reduced the activity of TF:VIIa relipidated in PS:PC (1:99%) vesicles, but had no effect on TF relipidated solely in PC (Fig. 6A). There was also no effect of lactadherin on GCDCA-stimulated TF:FVIIa activity (Fig. 6B), allowing us to apply lactadherin to selectively inhibit PS in cultured hepatocytes. We selected the lowest concentration of lactadherin that inhibited PS-dependent TF:FVIIa activity by approximately 50%. Lactadherin (20 nM) significantly reduced hepatocyte TF procoagulant activity, even in the absence of GCDCA, implying baseline PS exposure in cultured hepatocytes (Fig. 6C). Remarkably, GCDCA treatment increased hepatocyte TF procoagulant activity even in the presence of lactadherin (Fig. 6C). Together, the results suggest that PS exposure is not a vital component of the mechanism whereby GDCCA increases hepatocyte TF procoagulant activity.
Figure 6. Effect of blocking phosphatidylserine on bile acid driven relipidated and hepatocyte TF procoagulant activity.
(A) TF relipidated (0.1 nM final) in vesicles of various phospholipid composition were incubated with FVIIa (5 pM final) for 5 minutes. For (B), TF relipidated in PC vesicles was treated with GCDCA (125 μM final) or vehicle (HBS). For both A and B, 100 nM FX was added in the presence or absence of various concentrations of lactadherin and FXa generation was measured 60 minutes later. (C) Primary mouse hepatocytes were isolated from wild-type male mice and treated with GCDCA (500 μM) or vehicle (HBS; Veh) for 15 minutes in the presence or absence of lactadherin (20nM). FXa generation was assessed 30 minutes later. Data are expressed as mean + SEM, representing experiments from at least three separate hepatocyte isolations and four separate vesicle experiments. For panel A, *p<0.05 vs. 1% PS vesicles without lactadherin. For panel C, *p<0.05 compared to respective Veh-treated cells. #p<0.05 compared to respective group without lactadherin.
Discussion
Mechanisms of TF decryption have been discovered using non-physiological chemical triggers (e.g. calcium ionophores, HgCl2, etc.) of TF:FVIIa procoagulant activity.6–8 To identify new pathologically-relevant mediators of TF:FVIIa decryption, we considered TF expressed by liver parenchymal cells. Because the liver lacks a traditional hemostatic envelope, regulation of TF:FVIIa by encryption seems essential to restrict intrahepatic coagulation. We demonstrate here that bile acids can increase hepatocyte TF:FVIIa procoagulant activity. Notably, bile acid activation of hepatocyte TF:FVIIa procoagulant activity occurred rapidly, in the absence of apoptotic or necrotic cell death, and did not require de novo transcription, which are hallmarks of direct TF:FVIIa decryption.5 Paired with the finding that bile acids amplify TF driven thrombin generation in plasma, rapid intrahepatic coagulation in experimental BDL and evidence of injury-independent coagulation activation in small group of cholestatic patients, these results strongly suggest bile acids are a pathologically-relevant amplifier of the TF-driven coagulation. Collectively, the results reveal a novel connection between a physiological mediator elevated in disease and direct amplification of TF:FVIIa procoagulant activity.
Mechanisms underlying the decryption of TF:FVIIa activity have focused on changes in cellular membrane composition, because PS externalization causes robust increases in TF:FVIIa activity.37–40 Additionally, prior studies have shown that apoptotic hepatocytes are highly procoagulant.15 However, apoptosis does not contribute to liver injury after BDL,41 making it unlikely to contribute to rapid coagulation in this model, nor is apoptosis a primary lesion in clinical obstructive cholestasis.32 Although GCDCA causes hepatocyte necrosis over time,32 we observed increased TF:FVIIa activity prior to cell injury. In agreement with prior reports,32 we did not observe apoptosis in the GCDCA-treated hepatocytes, and a caspase inhibitor had no effect on GCDCA-driven TF:FVIIa activity. These results correspond with our observation that coagulation is activated in the absence of detectable liver injury after BDL in mice and in cholestatic patients. Overall, it seems unlikely that the rapid bile acid-mediated increase in the TF:FVIIa activity we observed is simply driven by cell death.
Multiple mechanisms have been reported to influence TF:FVIIa activity.42 The most studied are isomerization of TF extracellular disulfides by protein disulfide isomerase and interactions with exposed anionic phospholipids such as PS. Although it is difficult to exclude a contribution of these processes to the effect of bile acids on TF:FVIIa activity, our in vitro studies suggest neither mechanism is essential. Specifically, we found that addition of the inhibitory anti-PDI antibody (RL-90)43 had no effect on GCDCA-induced hepatocyte TF procoagulant activity (data not shown). Moreover, while hepatocyte apoptosis drives PS exposure,15 in preliminary studies we were not able to detect an increase in cell-surface PS in GCDCA-treated cells using annexin V labeling and flow cytometry. However, this assay could overlook small changes in PS that could affect coagulation reactions. Notably, GCDCA treatment increased hepatocyte TF activity even in the presence of the PS-binding protein lactadherin. Viewed in the context of direct effects of GCDCA on relipidated and sTF:FVIIa activity suggests a direct mechanism not requiring reorganization of membrane phospholipids such as PS.
The catalytic activity of FVIIa alone was not affected by GCDCA nor TCDCA, implying formation of the TF:FVIIa complex is required for the bile acid effect. Interestingly, we found that GCDCA reduced the apparent Km for TF:FVIIa-driven FX activation. One possibility is that GCDCA increases TF:FVIIa activity by a mechanism analogous to that of PS, allowing bile acids to substitute for membrane phospholipids as drivers of TF:FVIIa activity, albeit less efficiently based on the more robust reduction in Km by PS.34 This hypothesis is consistent with bile acids directly increasing sTF:FVIIa activity and our related observation that modest amounts of PS limit the effect of bile acids on relipidated TF. Even in light of these direct effects, our studies do not exclude a mechanism whereby bile acids increase TF activity through changes in components of the plasma membrane. Indeed, at physiological pH, GCDCA and TCDCA are essentially anionic detergents, a property critical for their role in digestion.44 However, the concentrations used in this study are well below their critical micelle concentration in physiological solutions.45, 46 Moreover, the zwitterionic detergent CHAPS, a bile acid structural homolog, did not increase TF:FVIIa activity. This result, combined with the lack of effect on markers of membrane permeability (i.e., ALT release), is inconsistent with a non-specific detergent-like effect being a primary mechanism. Overall, our studies suggest a direct, perhaps “PS-like” mechanism whereby GCDCA and other bile acids alter TF:FVIIa activity.
In summary, we discovered that bile acids directly increase TF:FVIIa procoagulant activity, displaying hallmarks of TF:FVIIa decryption. This may be particularly relevant to the liver, as subtle changes in TF:FVIIa activity in the leaky sinusoidal vasculature may be sufficient to drive pathologic coagulation. Indeed, we report evidence of rapid intrahepatic coagulation without concurrent liver injury in experimental cholestasis in mice and increased coagulation in human patients with cholestasis. Importantly, plasma and hepatic bile acid levels are increased in patients with the most common forms of liver disease,31, 47, 48 and our results suggest that bile acids may be coupled to intrahepatic activation of coagulation or hypercoagulability in these patients. Overall, these studies advance the field by pinpointing mechanisms of the TF decryption driven by pathologic elevation of a physiologically-relevant mediator. Identification of bile acids as a trigger of coagulation suggests a novel stimulus capable of disrupting the delicate hemostatic balance in liver disease, leading to pathologic intrahepatic coagulation.
Supplementary Material
Highlights:
Elevated plasma bile acids are associated with increased coagulation in mice and humans.
Bile acids decrypt hepatocyte tissue factor:factor VIIa activity independent of cell death.
Bile acids directly increase tissue factor:factor VIIa complex activity.
Acknowledgements:
The authors wish to thank Dr. Nigel Mackman for kindly providing the TFflox/flox mice and Dr. James Morrissey for providing the soluble TF. K.S.B, A.K.K, A.S.W and J.P.L. developed the hypothesis; K.S.B, A.K.K., A.P., L.G.P., H.C.F., M.O., B.W., and A.M. performed experiments and/or collected data; K.S.B, A.K.K, and J.P.L analyzed data; K.S.B., A.K.K., A.P., L.G.P., D.I. B.W., H.J., A.S.W, and J.P.L interpreted data; J.P.L performed statistical analysis; K.S.B and J.P.L wrote the manuscript, which was revised by and approved by all authors.
Sources of funding: This project was supported by grants from the National Institutes of Health National Institute of Environmental Health Sciences [R01 ES017537], National Institute of General Medical Sciences [T32 GM092715], and National Heart, Lung, and Blood Institute [HL126974] and by the USDA National Institute of Food and Agriculture. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or USDA. K.S.B. and J.P.L wish to thank the Michigan State University College of Natural Sciences and the Family of John A. Penner for supporting this research, in part through receipt of the John A. Penner Endowed Research Assistantship.
Nonstandard Abbreviations and Acronyms:
- TF
tissue factor
- FVIIa
coagulation factor VIIa
- BDL
bile duct ligation
- GCDCA
glycochenodeoxycholic acid
- TCDCA
taurochenodeoxycholic acid
- FX
coagulation factor X
- ERCP
endoscopic retrograde cholangiopancreatography
- TAT
thrombin-antithrombin
- PC
phosphatidylcholine
- PS
phosphatidylserine
Footnotes
Disclosures: There are no relevant conflicts of interest to disclose.
References
- 1.Grover SP, Mackman N. Tissue factor: An essential mediator of hemostasis and trigger of thrombosis. Arteriosclerosis, thrombosis, and vascular biology. 2018;38:709–725 [DOI] [PubMed] [Google Scholar]
- 2.Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:1687–1693 [DOI] [PubMed] [Google Scholar]
- 3.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. The American journal of pathology. 1989;134:1087–1097 [PMC free article] [PubMed] [Google Scholar]
- 4.Rao LV, Pendurthi UR. Regulation of tissue factor coagulant activity on cell surfaces. Journal of thrombosis and haemostasis : JTH. 2012;10:2242–2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao LV, Kothari H, Pendurthi UR. Tissue factor encryption and decryption: Facts and controversies. Thrombosis research. 2012;129 Suppl 2:S13–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolberg AS, Monroe DM, Roberts HR, Hoffman MR. Tissue factor de-encryption: Ionophore treatment induces changes in tissue factor activity by phosphatidylserine-dependent and -independent mechanisms. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 1999;10:201–210 [PubMed] [Google Scholar]
- 7.Chen VM, Ahamed J, Versteeg HH, Berndt MC, Ruf W, Hogg PJ. Evidence for activation of tissue factor by an allosteric disulfide bond. Biochemistry. 2006;45:12020–12028 [DOI] [PubMed] [Google Scholar]
- 8.Kothari H, Nayak RC, Rao LV, Pendurthi UR. Cystine 186-cystine 209 disulfide bond is not essential for the procoagulant activity of tissue factor or for its de-encryption. Blood. 2010;115:4273–4283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan BP, Kopec AK, Joshi N, Cline H, Brown JA, Bishop SC, Kassel KM, Rockwell C, Mackman N, Luyendyk JP. Hepatocyte tissue factor activates the coagulation cascade in mice. Blood. 2013;121:1868–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woolbright BL, McGill MR, Staggs VS, Winefield RD, Gholami P, Olyaee M, Sharpe MR, Curry SC, Lee WM, Jaeschke H. Glycodeoxycholic acid levels as prognostic biomarker in acetaminophen-induced acute liver failure patients. Toxicological Sciences. 2014;142:436–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rautou PE, Tatsumi K, Antoniak S, Owens AP, 3rd, Sparkenbaugh E, Holle LA, Wolberg AS, Kopec AK, Pawlinski R, Luyendyk JP, Mackman N. Hepatocyte tissue factor contributes to the hypercoagulable state in a mouse model of chronic liver injury. Journal of hepatology. 2016;64:53–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Hong JY, Rockwell CE, Copple BL, Jaeschke H, Klaassen CD. Effect of bile duct ligation on bile acid composition in mouse serum and liver. Liver International. 2012;32:58–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Copple BL, Li T. Pharmacology of bile acid receptors: Evolution of bile acids from simple detergents to complex signaling molecules. Pharmacological research. 2016;104:9–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen K, Jaeschke H, Copple BL. Bile acids induce inflammatory genes in hepatocytes: A novel mechanism of inflammation during obstructive cholestasis. Am J Pathol. 2011;178:175–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez M, Kopec AK, Joshi N, Geddings JE, Cline H, Towery KL, Rockwell CE, Mackman N, Luyendyk JP. Fas-induced apoptosis increases hepatocyte tissue factor procoagulant activity in vitro and in vivo. Toxicological Sciences. 2014;141:453–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joshi N, Kopec AK, Ray JL, Cline-Fedewa H, Nawabi A, Schmitt T, Nault R, Zacharewski TR, Rockwell CE, Flick MJ, Luyendyk JP. Fibrin deposition following bile duct injury limits fibrosis through an alphambeta2-dependent mechanism. Blood. 2016;127:2751–2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neuenschwander PF, Fiore MM, Morrissey JH. Factor vii autoactivation proceeds via interaction of distinct protease-cofactor and zymogen-cofactor complexes. Implications of a two-dimensional enzyme kinetic mechanism. Journal of Biological Chemistry. 1993;268:21489–21492 [PubMed] [Google Scholar]
- 18.Bom VJ, Bertina RM. The contributions of ca2+, phospholipids and tissue-factor apoprotein to the activation of human blood-coagulation factor x by activated factor vii. Biochemical Journal. 1990;265:327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neuenschwander PF, Branam DE, Morrissey JH. Importance of substrate composition, ph and other variables on tissue factor enhancement of factor vlla activity. Thrombosis and haemostasis. 1993;70:0970–0977 [PubMed] [Google Scholar]
- 20.Dargaud Y, Spronk HMH, Leenders P, Hemker HC, Ten Cate H. Monitoring platelet dependent thrombin generation in mice. Thrombosis Research. 2010;126:436–441 [DOI] [PubMed] [Google Scholar]
- 21.Hemker HC, Kremers R. Data management in thrombin generation. Thrombosis Research. 2013;131:3–11 [DOI] [PubMed] [Google Scholar]
- 22.Kessels H, Willems G, Hemker HC. Analysis of thrombin generation in plasma. Computers in Biology and Medicine. 1994;24:277–288 [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Vohra BP, Zhang Y, Heuckeroth RO. Transcriptional profiling after bile duct ligation identifies pai-1 as a contributor to cholestatic injury in mice. Hepatology. 2005;42:1099–1108 [DOI] [PubMed] [Google Scholar]
- 24.Kloek JJ, Heger M, van der Gaag NA, Beuers U, van Gulik TM, Gouma DJ, Levi M. Effect of preoperative biliary drainage on coagulation and fibrinolysis in severe obstructive cholestasis. Journal of clinical gastroenterology. 2010;44:646–652 [DOI] [PubMed] [Google Scholar]
- 25.Wiener SM, Hoyt RF Jr., Deleonardis JR, RR Clevenger, KR Jeffries, Nagashima K, Mandel M, Owens J, Eckhaus M, Lutz RJ, Safer B. Manometric changes during retrograde biliary infusion in mice. American journal of physiology. Gastrointestinal and liver physiology. 2000;279:G49–66 [DOI] [PubMed] [Google Scholar]
- 26.Wagner M, Fickert P, Zollner G, Fuchsbichler A, Silbert D, Tsybrovskyy O, Zatloukal K, Guo GL, Schuetz JD, Gonzalez FJ, Marschall HU, Denk H, Trauner M. Role of farnesoid x receptor in determining hepatic abc transporter expression and liver injury in bile duct-ligated mice. Gastroenterology. 2003;125:825–838 [DOI] [PubMed] [Google Scholar]
- 27.Yang M, Ramachandran A, Yan H-M, Woolbright BL, Copple BL, Fickert P, Trauner M, Jaeschke H. Osteopontin is an initial mediator of inflammation and liver injury during obstructive cholestasis after bile duct ligation in mice. Toxicology letters. 2014;224:186–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Georgiev P, Jochum W, Heinrich S, Jang J, Nocito A, Dahm F, Clavien PA. Characterization of time‐related changes after experimental bile duct ligation. British Journal of Surgery. 2008;95:646–656 [DOI] [PubMed] [Google Scholar]
- 29.Stiehl A, Rudolph G, Raedsch R, Moller B, Hopf U, Lotterer E, Bircher J, Fölsch U, Klaus J, Endele R. Ursodeoxycholic acid–induced changes of plasma and urinary bile acids in patients with primary biliary cirrhosis. Hepatology. 1990;12:492–497 [DOI] [PubMed] [Google Scholar]
- 30.Makino I, Hashimoto H, Shinozaki K, Yoshino K, Nakagawa S. Sulfated and nonsulfated bile acids in urine, serum, and bile of patients with hepatobiliary diseases. Gastroenterology. 1975;68:545–553 [PubMed] [Google Scholar]
- 31.Dasarathy S, Yang Y, McCullough AJ, Marczewski S, Bennet C, Kalhan SC. Elevated hepatic fatty acid oxidation, high plasma fibroblast growth factor 21, and fasting bile acids in nonalcoholic steatohepatitis. European journal of gastroenterology & hepatology. 2011;23:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woolbright BL, Dorko K, Antoine DJ, Clarke JI, Gholami P, Li F, Kumer SC, Schmitt TM, Forster J, Fan F, Jenkins RE, Park BK, Hagenbuch B, Olyaee M, Jaeschke H. Bile acid-induced necrosis in primary human hepatocytes and in patients with obstructive cholestasis. Toxicol Appl Pharmacol. 2015;283:168–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alnouti Y, Csanaky IL, Klaassen CD. Quantitative-profiling of bile acids and their conjugates in mouse liver, bile, plasma, and urine using lc–ms/ms. Journal of Chromatography B. 2008;873:209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neuenschwander PF, Bianco-Fisher E, Rezaie AR, Morrissey JH. Phosphatidylethanolamine augments factor viia-tissue factor activity: Enhancement of sensitivity to phosphatidylserine. Biochemistry. 1995;34:13988–13993 [DOI] [PubMed] [Google Scholar]
- 35.Bach R, Gentry R, Nemerson Y. Factor vii binding to tissue factor in reconstituted phospholipid vesicles: Induction of cooperativity by phosphatidylserine. Biochemistry. 1986;25:4007–4020 [DOI] [PubMed] [Google Scholar]
- 36.Shi J, Gilbert GE. Lactadherin inhibits enzyme complexes of blood coagulation by competing for phospholipid-binding sites. Blood. 2003;101:2628–2636 [DOI] [PubMed] [Google Scholar]
- 37.Tavoosi N, Davis-Harrison RL, Pogorelov TV, Ohkubo YZ, Arcario MJ, Clay MC, Rienstra CM, Tajkhorshid E, Morrissey JH. Molecular determinants of phospholipid synergy in blood clotting. The Journal of biological chemistry. 2011;286:23247–23253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao LV, Kothari H, Pendurthi UR. Tissue factor: Mechanisms of decryption. Frontiers in bioscience. 2012;4:1513–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ke K, Yuan J, Morrissey JH. Tissue factor residues that putatively interact with membrane phospholipids. PloS one. 2014;9:e88675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ansari SA, Pendurthi UR, Sen P, Rao LV. The role of putative phosphatidylserine-interactive residues of tissue factor on its coagulant activity at the cell surface. PloS one. 2016;11:e0158377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gujral JS, Liu J, Farhood A, Jaeschke H. Reduced oncotic necrosis in fas receptor-deficient c57bl/6j-lpr mice after bile duct ligation. Hepatology. 2004;40:998–1007 [DOI] [PubMed] [Google Scholar]
- 42.Bach RR. Tissue factor encryption. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:456–461 [DOI] [PubMed] [Google Scholar]
- 43.Ahamed J, Versteeg HH, Kerver M, Chen VM, Mueller BM, Hogg PJ, Ruf W. Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proceedings of the National Academy of Sciences. 2006;103:13932–13937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.St-Pierre MV, Kullak-Ublick GA, Hagenbuch B, Meier PJ. Transport of bile acids in hepatic and non-hepatic tissues. Journal of Experimental Biology. 2001;204:1673–1686 [DOI] [PubMed] [Google Scholar]
- 45.Carey MC, Small DM. Micelle formation by bile salts: Physical-chemical and thermodynamic considerations. Archives of Internal Medicine. 1972;130:506–527 [PubMed] [Google Scholar]
- 46.Stevens RD, Lack L, Collins RH, Meyers WC, Killenberg PG. Effects of monosulfate esters of taurochenodeoxycholate on bile flow and biliary lipids in hamsters. Journal of Lipid Research. 1989;30:673–679 [PubMed] [Google Scholar]
- 47.Pennington C, Ross P, Bouchier I. Serum bile acids in the diagnosis of hepatobiliary disease. Gut. 1977;18:903–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aranha MM, Cortez-Pinto H, Costa A, da Silva IBM, Camilo ME, de Moura MC, Rodrigues CM. Bile acid levels are increased in the liver of patients with steatohepatitis. European journal of gastroenterology & hepatology. 2008;20:519–525 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.