Abstract
The pituitary adenylate cyclase-activating polypeptide (PACAP)-selective PAC1 receptor (PAC1R, ADCYAP1R1) is a member of the vasoactive intestinal peptide (VIP)/secretin/glucagon family of G protein-coupled receptors (GPCRs). PAC1R has been shown to play crucial roles in the central and peripheral nervous systems. The activation of PAC1R initiates diverse downstream signal transduction pathways, including adenylyl cyclase, phospholipase C, MEK/ERK, and Akt pathways that regulate a number of physiological systems to maintain functional homeostasis. Accordingly, at times of tissue injury or insult, PACAP/PAC1R activation of these pathways can be trophic to blunt or delay apoptotic events and enhance cell survival. Enhancing PAC1R signaling under these conditions has the potential to mitigate cellular damages associated with cerebrovascular trauma (including stroke), neurodegeneration (such as Parkinson’s and Alzheimer's disease), or peripheral organ insults. Conversely, maladaptive PACAP/PAC1R signaling has been implicated in a number of disorders, including stress-related psychopathologies (i.e., depression, posttraumatic stress disorder, and related abnormalities), chronic pain and migraine, and metabolic diseases; abrogating PAC1R signaling under these pathological conditions represent opportunities for therapeutic intervention. Given the diverse PAC1R-mediated biological activities, the receptor has emerged as a relevant pharmaceutical target. In this review, we first describe the current knowledge regarding the molecular structure, dynamics, and function of PAC1R. Then, we discuss the roles of PACAP and PAC1R in the activation of a variety of signaling cascades related to the physiology and diseases of the nervous system. Lastly, we examine current drug design and development of peptides and small molecules targeting PAC1R based on a number of structure-activity relationship studies and key pharmacophore elements. At present, the rational design of PAC1R-selective peptide or small-molecule therapeutics is largely hindered by the lack of structural information regarding PAC1R activation mechanisms, the PACAP-PAC1R interface, and the core segments involved in receptor activation. Understanding the molecular basis governing the PACAP interactions with its different cognate receptors will undoubtedly provide a basis for the development and/or refinement of receptor-selective therapeutics.
Keywords: Class B GPCR, Behavioral disorders, Neurodegenerative diseases, Molecular modeling, Structure-based drug discovery, PAC1 Receptor
1. INTRODUCTION
G Protein-coupled Receptors (GPCRs) represent the largest and one of the most important receptor families for drug discovery. The approximate 800 heptahelical receptor members can be divided into five major classes based on sequence and structural similarities: rhodopsin (class A), secretin (class B), glutamate (class C), adhesion, and frizzled/taste. Although consisting of only 15 known members, the class B GPCRs are activated by a number of critical regulatory peptides, such as corticotropin-releasing hormone (CRH), calcitonin gene-related peptide (CGRP), parathyroid hormone (PTH), glucagon, vasoactive intestinal peptide (VIP), and pituitary adenylate cyclase-activating polypeptide (PACAP, ADCYAP1). These peptides are important with respect to neural development, calcium homeostasis, glucose metabolism, circadian rhythm, thermoregulation, inflammation, feeding behavior, pain modulation, as well as stress and related endocrine responses [1-3]. Accordingly, their corresponding receptors are potential pharmacological targets for a variety of disorders including osteoporosis, hypercalcemia, type 2 diabetes, obesity, migraine, and related chronic pain disorders, as well as stress-associated psychopathologies, including depression [4-7], posttraumatic stress disorder (PTSD), and related anxiety-related abnormalities [8-10].
In this review, we focus on one specific class B GPCR, the PACAP receptor (PAC1R, ADCYAP1R1, Fig. 1), which is highly expressed in the central and peripheral nervous systems. PAC1R is activated by the alternatively posttranslationally processed PACAP neuropeptides (PACAP27 or PACAP38). PAC1R is dually coupled to Gs or Gq to stimulate adenylyl cyclase (AC)/cAMP or phospholipase C (PLC)/DAG/IP3 pathways, respectively, and can engage other signaling programs in endosomal compartments following receptor internalization. We first review the current knowledge regarding the molecular structure, motion, and function of PAC1R and its peptide-binding modes. Then, we discuss the roles of PACAP/PAC1R in homeostatic and maladaptive signaling. Lastly, we examine current approaches to the design and development of peptide and small-molecule antagonists of PAC1R based on structure-activity relationship (SAR) studies and key pharmacophore elements, which could have broad impacts on many physiological systems.
Fig. (1).
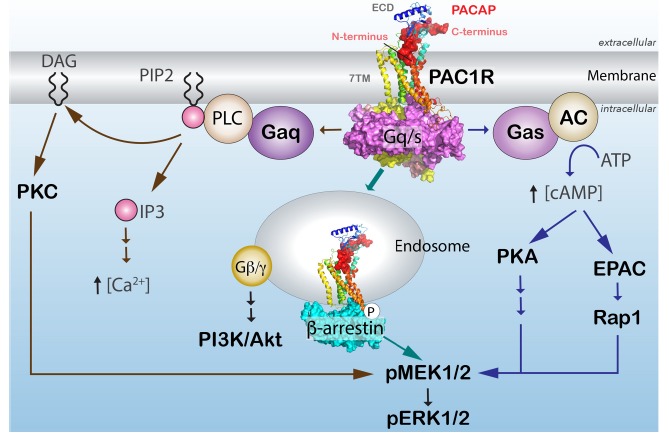
A schematic diagram of PAC1R signaling cascades. Activation of the PAC1R is dually coupled to Gs to stimulate adenylyl cyclase (AC) and Gq to stimulate phospholipase C (PLC) activity. The increase in cellular cAMP can stimulate protein kinase A (PKA)/EPAC pathways that can intersect and enhance MEK/ERK signaling. PAC1R activation and recruitment of β-arrestin also result in receptor internalization and formation of endosomal signaling platforms for long-term MEK/ERK signaling.
2. PAC1R STRUCTURE, FUNCTION, AND DYNAMICS
2.1. Structures of Class B GPCRs and PAC1R
The common basic structure of GPCRs consists of a heptahelical-transmembrane domain (7TM) tethered by three intracellular and three extracellular loops (Fig. 2). Singularly, class B GPCRs have an N-terminal extracellular domain (ECD) composed of 120 to 160 residues which appear critical for the recognition of the C-terminal regions of peptide ligands [4]. Atomic structures of eleven of the fifteen ECDs have been determined by X-ray crystallography or nuclear magnetic resonance (NMR), either with or without their peptide ligands in the complex. Notably, these structures reveal a similar arrangement of a three-layered α-β-β/α fold [5] as well as a conserved peptide binding interface [6] (Fig. 2). The 7TM structures for three class B receptors, the glucagon receptor (GCGR) [7-8], the glucagon-like peptide 1 receptor (GLP-1R) [9], and the corticotrophin-releasing factor receptor 1 (CRF1R) [10] have been determined. These structures provide templates to model peptide and allosteric modulator interactions within the 7TM and have enabled initial, structure-based interpretations of SARs. The recently published full-length crystal structures of peptide bound GLP-1R [11] and antibody or peptide bound GCGR [12, 13] reveal how the peptides can bind to the orthosteric pockets within the 7TM to trigger downstream signaling cascades. Further, the cryo-electron microscopy (cryo-EM) structures of GLP-1R [14, 15] and Calcitonin Receptor (CTR) [16] and CGRP receptor [17] bound to their downstream Gs trimeric complex reveal how the class B GPCR 7TMs undergo conformational changes at their intracellular regions (from TM5 to helix 8) to allow G protein binding and signaling. The binding poses of Gαs with TM5-TM6 of class A and class B GPCRs are similar, but the class B GPCRs have helix 8 in the cytoplasmic tail segment, tilted (~25o) to enable additional interactions with the Gβ subunit. Overall, these structures have extended understandings of ligand-receptor interactions and orthosteric versus allosteric modulation mechanisms of class B GPCRs and have provided the underlying basis for structure-based drug discovery, targeting the VIP/secretin/glucagon family of GPCRs.
Fig. (2).
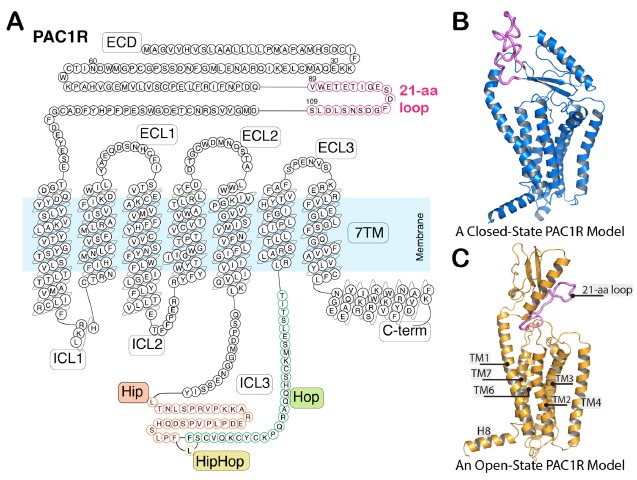
(A) The PAC1R sequence and topography with potential splice variants. PAC1R can have Hip and/or Hop 28-aa cassette inserts into the ICL3 region. Accordingly, the PAC1R can be Null (neither Hip nor Hop), Hip, Hop, or HipHop variants, as described in the text. Unlike most class B receptors, PAC1R also has a 21-aa loop segment in the ECD. (B) A representative conformation of PAC1R in the ECD-closed state [21]. (C) A representative conformation of PAC1R in the ECD-open state [21]. Helix 8 (H8) is located within the intracellular C-terminal tail region.
There are several variants of the PAC1R (Fig. 2) based on the alternative splicing of two 84-base pair exons that encode Hip and Hop 28-amino acid cassettes within the third intracellular loop (ICL3) [18, 19]. Hence, the PAC1R may be Null (neither Hip nor Hop inserts), Hip, Hop (Hop1, or additional short Hop variant Hop2), or HipHop (an aggregate of the two cassettes). The PAC1Null and PAC1Hop receptor variants are preferentially expressed in the central and peripheral nervous systems. In addition to the ICL3 Hip/Hop inserts, the deletion of a 21-amino acid (21-aa) loop segment within the β3 and β4 strands of the ECD has also been described [20], although this ECD deletion has not been observed in most tissues. The functional roles of these receptor variants are unclear but have been proposed to increase receptor selectivity for different neuropeptides and/or G proteins.
2.2. Structure-Dynamics-Function Relationship of PAC1R
Molecular dynamics (MD) studies of class A GPCRs have revealed valuable information about the transition between receptor active and inactive states as well as intervening conformations [22-29]. In recent years, an understanding of the structural basis and dynamic details of class B receptors has been greatly advanced. Unlike class A receptors, class B GPCRs seem to operate through large conformational shifts of the ECD and the 7TM, which are connected by a flexible linker [9, 12-13]. This shapeshifter feature [30] of PAC1R has been assessed by a recent MD simulation study on the microsecond timescale [21]. During the first few hundred nanoseconds, sweeping dynamics of the PAC1R ECD are observed. These ECD motions diminish once interactions with the 7TM developed, which generate several populated conformational communities distinct in the ECD position relative to the 7TM. The two major states of PAC1R, as identified according to the ECD-7TM relative position, are the ECD-open and ECD-closed states (Fig. 2). Using the Markov state model [31], the transition pathways from one microstate (small conformational community) to another have been mapped along a series of intermediate conformations; accordingly, the transitions between the ECD-open and closed states are estimated on a timescale of hundreds of microseconds to milliseconds [21].
More specifically, large-scale conformational changes generate diverse microstates between the ECD and the 7TM and reveal diverse rearrangements and displacements within the 7TM helices (Fig. 3). Along the extracellular face of the TMs, major displacements are observed at the stalk/linker region affecting TM1, TM6, and TM7. While the movement of TM1 is closely impacted by the ECD motion, the movements of TM6 and TM7 correlate with the dynamics of extracellular loop 3 (ECL3). The interactions between the ECL3 and the ECD are altered during ECD motion from open to closed conformations, which causes the movement of TM6 and TM7. At the intracellular face of the receptor, the TM5-ICL3-TM6 region undergoes a large displacement [30, 31], similar to class A receptor dynamics. Furthermore, in the transition between different states, TM6 displays larger displacements than other TM helices. The unique flexibility of TM6 and its association with ICL3 likely plays a key role in the function of PAC1R.
Fig. (3).
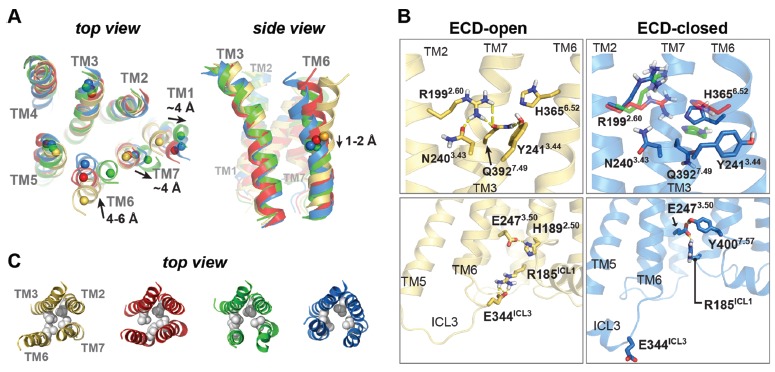
(A) The top and side views of four PAC1R models to illustrate the helical rearrangements during the open-to-closed transition. (B) Key hydrogen-bonds and salt bridges within the 7TM domain. Key residues are labelled with the Wootten numbering scheme [32]. (C) Helical rearrangements involving TM2, TM3, TM6, and TM7 with the hydrophobic region of L1922.53, L2443.47, L3586.45, and V3967.53 (spheres) in four PAC1R models. Reprinted with permission from Liao et al. [21].
As a result of rearrangements in the TM helices, a reshuffling of interaction networks is observed for residues within TM2, TM3, TM6, and TM7 (Fig. 3). For instance, the transitions between PAC1R states involve the reshuffling of hydrogen bonds and salt bridges around the orthosteric site (N2403.43-R1992.60-Q3927.49-Y2413.44, Wootten numbering [32]) and near the intracellular face of the receptor (altered interactions between E344ICL3-R185ICL1 and R185ICL1- E2473.50-Y4007.57). In addition, there is a change in the hydrophobic packing of L1922.53, L2443.47, L3586.45, and V3967.53 [21]. In aggregate, the ECD dynamics, rearrangements within the 7TM, and reshuffling of interaction networks describe a sequence by which changes in communication among the domains can contribute to PAC1R function. Hence, the three-dimensional (3D) structures obtained from these simulations not only further our understandings of the mechanism governing PAC1R activation but also allow insights for structure-based drug design targeting this receptor class.
2.3. Peptide-Binding Sites of Class B GPCRs and PAC1R
As described earlier, unlike the class A GPCRs most of which have a relatively small extracellular N-terminus domain, the class B receptors have evolved large ECDs to capture peptide ligands for presentation to the orthosteric receptor activation site in the 7TM. Among the 15 crystal and NMR structures of the ECDs, 11 class B GPCR ECD structures have solved in complex with their peptide ligands [33-37], including those with full-length crystal and cryo-EM structures [13-14]. In a majority of these structures, the C-terminus of the peptide ligand binds to a hydrophobic patch principally formed by the ECD α-β-β/α fold (α1-β1-β2-α2), while the N-terminus of the peptide ligand points in the same direction as the N-terminus α1 of the ECD (Fig. 4A). Based on the conserved three-layered α-β-β/α fold [5] and the orientation of the bound peptide, a “two-site” activation model of class B receptors is supported. In this model, the α-helical C-terminal region of the peptide binds to the ECD domain of the class B GPCR, which allows presentation of the peptide N-terminus to the orthosteric site within the 7TM domain eliciting receptor activation [11-13, 38] (Fig. 4B).
Fig. (4).
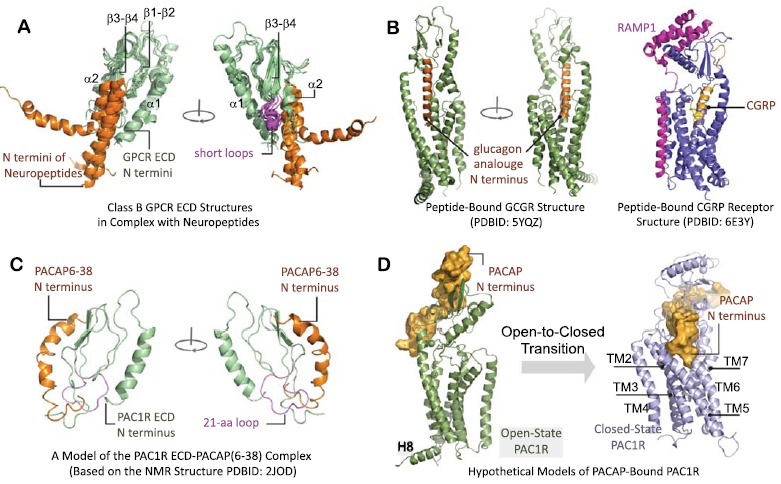
(A) Overlapped ECD structures of five class B GPCRs in complex with their peptide ligands (PDBIDs: 2QKH (GIP receptor), 3IOL (GLP-1 receptor), 3C4M (PTHR), 2L27 (CRF receptor with α-helical CRF), 3N96 (CRF receptor with urocortin) [34-37]. A three-layered α1-(β1-β2)-(β3-β4)/α2 fold is presented. (B) The full-length crystal structure of GCGR in complex with a glucagon analogue [13], in comparison with the full-length cryo-EM structure of the CGRP receptor. The CGRP receptor is shown in association with its receptor associated modifying protein 1 (RAMP1). (C) The PACAP-bound PAC1R ECD model. (D) Hypothetical models of full-length PAC1R bound to PACAP in open- and closed-states.
However, NMR studies [37] indicate that the truncated PACAP(6-38) PAC1R antagonist binds PAC1R at a distinct site and in the opposite orientation [33] as illustrated in Fig. (4C). This binding orientation was also suggested in the ligand-free PAC1R ECD crystal structure and mutation study [39]. Although clarifications may be needed, the distinct binding geometry of PACAP(6-38) to PAC1R ECD by NMR [37] raises obvious issues on how the N-terminus of the endogenous peptide can interact with the 7TM orthosteric site. Despite the apparent difference in ECD peptide orientation, the two-site activation model, which operates through a series of ECD motion transitions [21], still appears applicable for PAC1R activation. The large domain motion of the ECD leads to several distinct conformational states of PAC1R [21], which may provide structural details relevant to peptide binding and signaling. Compared to the ECD-closed conformations, the ECD-open conformations render the PACAP binding site [37] more exposed to the extracellular environment, and therefore more accessible for PACAP binding. Through the open-to-closed conformational transition process of PAC1R, the N-terminus of PACAP is likely inserted into the orthosteric ligand-binding site (Fig. 4D).
Another structural element that may regulate ligand binding is the loop between the β3-β4 strands (referred to as the 21-aa loop), which has been found to contribute to the hydrophobic interactions between the ligand and ECD in all available peptide-bound structures [13, 34-37]. Even though PACAP adopts a different binding geometry in the NMR models [37], dominant hydrophobic interactions have been identified at the ligand-ECD interfaces. The prevalent PAC1R isoforms, containing the 21-aa ECD segment, exhibits a long loop between the β3-β4 strands (Fig. 2), which is rare in other class B receptors. The 21-aa loop can interact with ECL3 to facilitate the ECD-open conformation (Fig. 4); also, in simulations of ligand-free PAC1R, the 21-aa insert was found to transform between a random coil and a partially helical conformation at its C-terminal region, which may lead to different ligand-binding sites for PACAP [21]. Altogether, the ECD motion suggests that the receptor may adopt multiple ECD conformations to increase the probability of capturing a peptide ligand by displaying a variety of potential binding sites and then deliver the bound ligand to the orthosteric pocket through a series of ECD motions.
3. PAC1R AND SIGNALING PATHWAYS
3.1. Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP)
PACAP is a peptide widely expressed in central and peripheral neurons and behaves as a neurotransmitter and neurotrophic regulator with critical roles in signaling, physiological homeostasis, development, survival, proliferation, differentiation, and regeneration [40]. PACAP belongs to the VIP/secretin/glucagon family of related peptides. The mature bioactive peptides are endoproteolytically cleaved from a 176-amino acid precursor molecule into two alternatively processed α-amidated bioactive PACAP38 or PACAP27 peptides (Fig. 5), but PACAP38 is typically more than 100-fold more abundant than PACAP27 in nervous tissues [41-43]. PACAP is well conserved in evolution; PACAP can be found in tunicates and the PACAP amino acid sequence is identical in all mammals examined to date [44].
Fig. (5).
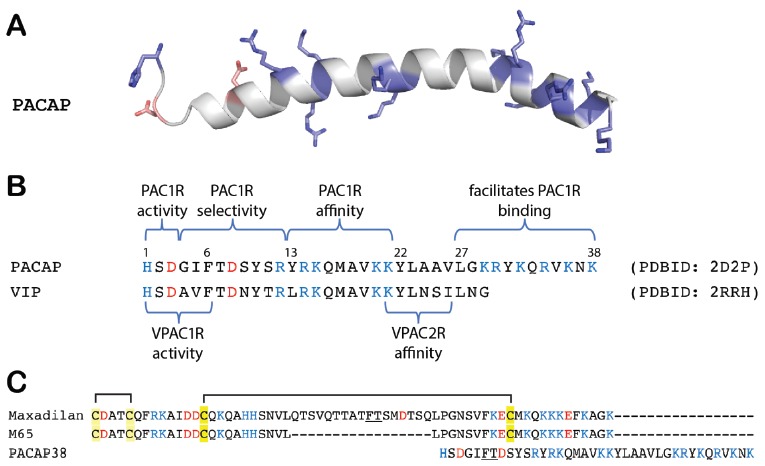
(A) Structure of PACAP38 (PDBID: 2D2P). (B) Sequences alignments of PACAP38 and VIP indicating domains responsible for PAC1R recognition, activation, selectivity, and binding affinity determined by SAR studies [40]. (C) Sequences alignments for maxadilan (a PAC1R agonist) [49], M65 (a PAC1R antagonist) [49], and PACAP38 by UniProt. Basic residues are colored in blue and acidic residues in red.
3.2. PACAP Receptor Subtypes, Selectivity, and Signaling
Because PACAP shares nearly 70% amino acid homology with VIP (a 29-residue family-related peptide), the PACAP/VIP peptides share three receptor subtypes: PAC1R, VPAC1R (VIPR1), and VPAC2R (VIPR2) receptors [1, 20]. Structurally, PAC1R shares nearly 60% sequence identity with VPAC1R and VPAC2R in the transmembrane domains. PACAP binds with high affinity and specificity to PAC1R variants; both PACAP and VIP bind to VPAC1R and VPAC2R with near equal high affinity [40]. The N-terminally truncated PACAP(6-38) acts as a PAC1R and VPAC2R antagonist [45]. The sand fly saliva peptide maxadilan, which does not share significant sequence similarity to PACAP, (Fig. 5) and its shortened deletion variant (maxD4 and M65) behave as a selective PAC1R agonist and antagonist, respectively [46-48].
As described above, there are several alternatively spliced forms of the PAC1R, e.g., Null, Hip, Hop, Hop2, or HipHop. Depending on the cellular expression of the PAC1R variant, the receptor may be differentially coupled to adenylyl cyclase, phospholipase C, MAPK, or Akt pathways, among others. Most of the neural and peripheral tissues examined to date contain the 21-aa splice insert in the PAC1R ECD. The 21-aa loop insert may have primary roles in stabilizing the ECD-open receptor state although early work has suggested that it may have some roles in peptide ligand specificity and function. However, splice variants have not been described for the VPAC1R and VPAC2R, which appear to be preferentially coupled to adenylyl cyclase. Recent work has shown that β-arrestin-mediated PAC1R internalization and endosomal signaling is the primary driver of long-term cellular ERK activation [50].
4. PAC1R AND BEHAVIORAL DISORDERS
4.1. PACAP/PAC1R Signaling Regulates Stress-related Behaviors
Over the last several years, the PACAP/PAC1R system has been associated with stress-related anxiety-like behavior [51], including PTSD [52, 53]. Chronic stress increased PACAP and PAC1R transcripts in the rat bed nucleus of the stria terminalis (BNST) and hypothalamus [51], two limbic brain regions that participate in stress responses and behavior. The changes in BNST PACAP expression were dependent on chronic rather than acute stress and did not appear secondary to corticosterone, suggesting the direct effects of stress in limbic circuit phenotypic plasticity [54]. Infusions of PACAP or maxadilan (a specific PAC1R agonist) into the BNST were anxiogenic and anorexic (analogous to stress-mediated decreases in feeding behavior) [55]. Unlike other neuroregulators, the BNST PACAP effects were long-lasting; a single injection of PACAP sustained anxiety-like responses for several days [51]. Similar long-term anxiogenic responses were elicited following PACAP infusions directly into the central amygdala (CeA) [56-57]. Accordingly, these results suggest that chronic traumatic stress and the accompanying increases in limbic PACAP expression/signaling could drive the maladaptive neuroplasticity leading to behavioral abnormalities. In testing this hypothesis, rats were chronically infused with the PAC1R antagonist during stressor exposures; as proof of principle, antagonism of PAC1R signaling with PACAP(6-38) during chronic stress blocked both anxiety-related responses and decreased feeding behavior [55]. The observations were in good agreement with many related studies. The PACAP and PAC1R knockout mice appeared to be less anxious [58-61] and demonstrated impaired corticosterone responses to emotional and chronic stressors [62-65].
These observations have direct translational relevancy. Notably, elevated blood PACAP levels and polymorphism in the estrogen responsive element (ERE) of the PAC1R gene have been associated with PTSD symptoms in the female population [52, 66]. The changes in PACAP levels and receptor polymorphism were correlated with higher acoustic startle indices, which have been associated previously with PTSD risk. Correspondingly, PAC1R transcripts were increased in the rat extended amygdala after classical fear conditioning and as a function of estrogen exposure. Although these PACAP/PAC1R results were associated primarily with female PTSD symptoms, epigenetic signatures for the PAC1R may be markers for PTSD-related abnormalities in both males and females [52]. These observations have been corroborated independently [53, 67-71], supporting the PACAPergic system as a novel and relevant target for therapeutics. More recent work has suggested that the maladaptive PACAP signaling may be sustained in stress-related behavioral disorders and that the benefits of PAC1R antagonists may be meaningful even during ongoing disorder progression, and may not have to stem solely from prophylactic treatments [52]. These results are particularly notable from the vantage of potential therapeutics.
4.2. PACAP Pain Pathways and Intersections with Behavior Circuits
PACAP was initially characterized as a sensory peptide. PACAP is expressed in a population of sensory dorsal root and trigeminal ganglion neurons, and from neuroplasticity, can be induced upon injury or inflammation paradigms. Axotomy or inflammatory challenges dramatically increased PACAP expression in sensory neurons, resulting in augmented PACAP projections and signaling onto second-order dorsal horn neurons. In delineating PACAP sensory circuits, it was recently shown that PACAP in the nociceptive (pain) spino-parabrachioamygdaloid pathway is important in mediating the emotional components of pain [56-57]. Briefly, PACAP in the peripheral pain circuits relayed signals that ultimately terminated in the lateral capsular division of the amygdala (CeLC), another limbic nucleus well studied for its roles in stress, anxiety, and fear [72-73]. Stress and chronic pain (including migraine) are comorbid. Nearly 50% of patients with psychopathologies have chronic pain; conversely, 30 to 40% of patients with chronic pain present anxiety disorders including PTSD [74-80] Hence this particular PACAP neurocircuit may represent a means by which chronic pain can be integrated with limbic functions leading to heightened pain responses and psychopathologies. PACAP or maxadilan infusions into the amygdala increased anxiety-like responses and nociceptive thermal sensitivity; further, the PACAP responses were long-term [56]. As before, to establish the physiological relevance of this pathway, a partial sciatic nerve ligation protocol was used to induce chronic pain without impairing locomotion [57]. The paradigm produced stress- and anxiety-related behavior in parallel with increased thermal pain sensitivity. Of note, acute amygdala infusion with the PAC1R antagonist PACAP(6-38), during the progression of heightened anxiety and pain, was able to block these maladaptive responses [57]. This implicated augmented and sustained PACAP signaling in mediating these responses and that therapeutic interventions, even during ongoing disorder processes, may have beneficial effects.
Commensurate with PACAP roles in pain, several recent studies have now shown that PACAP and PAC1R signaling is associated with migraine [81-83]. Migraine is a complex and often debilitating neurological disorder characterized by intense unilateral pulsating headache, frequently accompanied by aura, photophobia, phonophobia, nausea, and related symptoms. The causes of migraine are not understood and are likely to be multifactorial. One prototypic neuroregulator in migraine is the sensory and potent vasodilatory peptide, calcitonin gene-related peptide (CGRP) [84]. Among many models, sensory (trigeminal) and/or autonomic (sphenopalatine) ganglion neuronal peptide signaling along central pathways or in the perivasculature may contribute to the experience of migraine pain. CGRP is localized to these pathways, exogenous CGRP administration has been shown to induce migraine-like headaches indistinguishable from spontaneous migraine attacks [85] and notably, CGRP receptor antagonists are effective in treating migraine [86]. The CGRP receptor antagonists (Olcegepant and Telcagepant), however, demonstrated adverse reactions, and consequently, antibody-based functional blockage of CGRP receptors (Aimovig; erenumab) represents the most current therapeutic means for migraine. PACAP and CGRP share a number of similarities, including parallel tissue expression and distribution patterns (colocalization in nearly 70% of sensory and central neurons), and potent vasodilatory effects through comparable signaling mechanisms. Importantly, like CGRP, PACAP infusions into humans can also induce migraine-like attacks in normal subjects and migraineurs [87-88]. However, whereas CGRP signaling and responses in ex vivo pressurized vessels preparations appear short-term or transient, PACAP acts more potent with long-term sustained responses [81, 89]. These observations are novel and suggest that reagents that target PAC1R function may be more efficacious than those for CGRP in migraine and chronic pain management.
4.3. PACAP/PAC1R Responses are Mediated by Internalized Receptor Endosomal Signaling
Importantly, behavioral and chronic pain responses are dependent on cellular ERK activation. Stress and chronic pain paradigms increase phosphorylated ERK signaling in the brain; conversely, stress and chronic pain responses can be attenuated by inhibitors of the MEK/ERK signaling cascade [90]. PACAP infusions into limbic areas increase ERK activation and c-fos induction (a marker for increased neuronal activity) in parallel with behavioral and pain responses; these PACAP responses can be attenuated with MEK/ERK inhibitors [57]. Notably, current work has shown that cellular PACAP/PAC1R-ERK signaling is mediated largely following PAC1R internalization and endosomal signaling [50, 91]. In coherence, inhibitors of endocytic mechanisms can block PACAP-stimulated pain sensitivity [57].
4.4. PACAP Roles in Metabolism
Some of the most consistent and robust responses of PACAP are related to feeding behavior, which can ultimately impact obesity and diabetes. PACAP and PAC1R are highly expressed in several hypothalamic nuclei important to appetite, satiety, and homeostatic metabolic regulation, including the paraventricular nucleus (PVN), ventromedial hypothalamic nucleus (VMN), arcuate nucleus (ARC), and the supraoptic nucleus (SON) [92]. Both peripheral and central administration of PACAP leads to decreased feeding or anorexic responses, resulting in decreased weight gain. Intracerebroventricular injections of PACAP reduced food intake [93, 94] and similarly, intraperitoneal PACAP injections suppressed appetite by decreasing ghrelin (resulting in diminished hunger) and increasing GLP-1 and leptin (resulting in increased satiety) in plasma [95, 96]. The central hypothalamic mechanisms appear multifaceted as direct PACAP injections into the different sites appeared to yield slightly different attributes. Direct PACAP infusions into PVN decreased meal size, duration, and total eating time, whereas PACAP activation of the VMN resulted in increased body temperature and locomotion (increased energy expenditure) [97, 98]. These responses were blocked with PAC1R selective antagonist and did not appear to be mediated by VPAC receptor signaling. The PACAP/PAC1R-mediated signals in feeding are well integrated into established feeding and appetite circuits. PACAP stimulated ARC neuron POMC transcript expression in parallel with augmented αMSH production, and as the PACAP-mediated hypophagic effects could be attenuated with MC4R receptor antagonists, the anorexic responses of PACAP/PAC1R signaling appeared upstream of POMC/melanocortin signaling [93]. The expression of hypothalamic orexigenic peptides neuropeptide Y (NPY) and agouti-related peptide (AgRP) were not regulated by PACAP although more recent work has suggested that during fasting, PVN PACAP signaling can stimulate ARC AgRP to enhance appetite [99]. Hence, PACAP/PAC1R signaling appears to participate in anorexic and orexigenic responses depending on metabolic status.
Whether coordinate or separate, central PACAP regulation of autonomic pathways can also have significant effects on metabolic states. Intracerebroventricular (ICV) PACAP infusions, for example, were shown to elevate plasma glucose levels and increase hepatic glucose production that appeared dependent on sympathetic innervation of the liver [100]. However, PACAP expression and function in vagal parasympathetic inputs to the pancreas appear to function synergistically with cholinergic signaling to increase islet β-cell proliferation via FoxM1 (forkhead box cell cycle transcriptional factor) for potential diabetes management [101]. These observations echo the well-studied PACAP trophic (protective and prosurvival) effects against a variety of challenges in many tissue systems [40] (see below). Hence unlike for chronic pain and stress-related disorders in which PAC1R antagonism at central pathways may be beneficial, the targeted application PAC1R agonists may be novel approaches for cellular protection, proliferation and regeneration, and maintenance of energy homeostasis.
5. PACAP AND PAC1R NEUROTROPHIC AND NEUROPROTECTIVE ACTIVITIES
5.1. PACAP/PAC1R Neurotrophic Signaling
PACAP/PAC1R signaling can engage multiple signaling cascades including adenylyl cyclase/cAMP, phospholipase C/DAG/PKC, MEK/ERK, and PI3K/Akt pathways to maintain physiological homeostasis. These signaling events intersect and likely engage a wider network of trophic signaling pathways that promote cellular survival, proliferation, and differentiation in neurodevelopment and block or attenuate pro-apoptotic processes following injury or degenerative states. Hence, the same PACAP/PAC1R signaling events that can contribute to the maladaptive neuroplasticity observed following repeated or chronic physiological insults (i.e., stress-related psychopathologies and chronic pain as described above) have important beneficial attributes in development and in mitigating damages from tissue injury, disease-related degeneration, and inflammation.
PACAP/PAC1R signaling has been shown to facilitate trophic signaling in a variety of central and peripheral neurons. PACAP promotes the proliferation astrocytes, sympathetic neuroblasts, and neural stem cells [102, 103]; PAC1R is expressed in neurogenic regions of the mouse brain and PACAP can promote the in vitro proliferation of cells isolated from the lateral ventricle wall of adult mice [104]. Similar observations were also made in vivo following the intracerebroventricular injection of PACAP [104]. Altogether, these results, which have been reproduced using the PAC1R selective agonist maxadilan [46], implicate the involvement of PAC1R in neurogenesis. Consistent with these trophic effects, PACAP can also promote the differentiation and neurite outgrowth of neuroprogenitor, neuroepithelial, neuroblastoma, cerebellar granule, and pheochromocytoma cells.
5.2. PACAP/PAC1R Signaling in Neuroprotection
Trophic PACAP/PAC1R signaling is also neuroprotective. PACAP mitigates naturally occurring programmed cell death during cerebellar development [105, 106]; PACAP can abrogate, mitigate, or delay neural damages against a variety of insults including stroke, glutamate excitotoxicity, hydrogen peroxide-mediated oxidative stress, ethanol, and ceramide [107-111]. PACAP administration before or after ischemia in a middle cerebral artery occlusion (MCAO) model of stroke, decreased infarct size, mitigated cell death, and improved functional recovery [111-117]. Similarly, PACAP and the PAC1R agonist maxadilan protected retinal neuroblastic cells from anisomycin-induced apoptosis [118]. These neuroprotective effects of PACAP have also been observed following insults in a variety of peripheral tissues, including kidney, cardiomyocytes, and intestinal cell layers [119-122], suggesting that PACAP/PAC1R signaling has broad tissue protection abilities under many proapoptotic challenges.
Hence from these observations and attributes, PACAP/PAC1R signaling can be protective in neurodegenerative states. In a variety of in vitro culture models, cell death induced by reagents, such as rotenone, paraquat, salsolinol, MPP+, or 6-hydroxydopamine (6-OHDA) to simulate Parkinson’s disease mechanisms, can be ameliorated by PACAP treatments [108, 123, 124]. In accord, PACAP protected dopaminergic cells from apoptosis in the 6-OHDA lesion model of Parkinson’s disease and in parallel, ameliorated the disease-associated neurological and behavioral defects [125, 126]. PACAP attenuated β-amyloid-induced toxicity in PC12 pheochromocytoma cells [127]; further, long-term PACAP treatments of the APP[V717] transgenic mouse model of Alzheimer’s disease improved cognitive functions [128]. Similarly, repeated intranasal PACAP administration to the R6/1 transgenic mouse model of Huntington's disease improved memory and cognitive performance, and diminished mutant huntingtin aggregates [129]. From these examples, reagents that enhance PACAP/PAC1R trophic signaling may be novel in their potential to mitigate the cellular damage and functional deficits associated with a number of neurodegenerative diseases.
6. DEVELOPMENT OF PEPTIDES TARGETING PAC1R
With a few exceptions, class B GPCRs have proven difficult targets for the development of either peptide or small-molecule therapeutics [130]. For instance, the large PACAP-PAC1R interface, which makes critical residue interactions dispersed across the entire receptor structure, and the lack of insights related to the interactions involved in core sequence receptor activation, have so far prevented the rational design of small-molecule modulators or PAC1R-selective analogs [130]. However, some metabolically stable PACAP derivatives specifically targeting PAC1R can be poised to make a significant breakthrough in the treatment of neurodegenerative diseases [130]. Understanding the molecular basis governing PACAP interactions with its different PAC1R, VPAC1R, and VPAC2R subtypes will undoubtedly provide a rational basis for the development and/or refinement of receptor-active peptidergic compounds.
From the consensus two-site model for ligand binding and signaling of class B GPCRs, understanding the spatial positioning of specific residues and/or structural features within PACAP becomes critical in the design of pharmacological specific peptidergic reagents to the different PAC1/VPAC receptors. Similar to other members of the VIP/ secretin/glucagon family, PACAP exhibits a disorder N-terminal segment (residues 1-5), which might adopt a specific pose upon interaction with its receptor, and a C-terminal α-helix for ECD binding [130, 131]. Over the years, SARs have highlighted the existence and the essential role played by the Asx-turn present at the N-terminus, the N-capping motif encompassing amino acid residues 6 to 10, the C-terminal (residues 28 to 38) region, as well as the Tyr residue at position 22, for the binding and biological activity of PACAP38 [130].
6.1. Structure-Activity Relationships (SARs) of PACAP N-Terminal Segment
The putative Asx-turn-like structure in PACAP is characterized by two hydrogen bonds involving i) nitrogen at position 3 but not 1 of the imidazole ring and the amide proton of the Asp residue at position 3, and ii) the carbonyl of the His residue and the amide proton of the glycine at position 4 [132]. Stabilization of this secondary structure, through the replacement of the native Ser2 with Ala, Pro, or Hyp residues, did not alter the ability of PACAP27 to bind PAC1R and VPAC1R but significantly affected its binding affinity for VPAC2R [132, 133]. Accordingly, [Pro2]PACAP27 was observed to adopt a well-defined structure, similar to a turn, that could restrict PACAP conformations for receptor activation [133]. By contrast, the introduction of an Aib moiety or a D-Ser did not particularly affect the binding affinity of PACAP27 for PAC1R and VPAC2R but significantly reduced its propensity to bind VPAC1R [132]. This exploratory work led to the identification of a PACAP/VIP analog, i.e., [Hyp2]PACAP27, with potent neuroprotective action in vitro [132]. In a recent study, the substitution of His at position 1 with 4-imidazole acetic acid ([Iaa1]PACAP38) or 4-imidazole acrylic acid ([Iac1]PACAP38) increased PACAP affinity to PAC1R and VPAC2R but reduced its binding to VPAC1R [134]. Finally, modifications introduced at position 3 of this PACAP variant, including the introduction of a proline, a piperidine-2-carboxylic acid, or a N-methyl-glycine, generated several analogs with reduced affinities for all receptors, corroborating again the importance of the amide proton of the residue at position 3 [134]. While no particular physio-chemical parameters favoring PAC1R binding over VPAC receptors have been identified, these studies demonstrate that the secondary structure at the N-terminus of the peptide is an important determinant of receptor specificity.
Apart from this specific turn, other determinants, also within the N-terminal domain of PACAP contribute to the selectivity of PACAP towards its different receptors. First, residues at positions 4 and 5 have been suggested to form an encrypted receptor selective dipeptide. Indeed, when the Gly4-Ile5 dipeptide in PACAP27 replaced Ala4-Val5 in the VIP sequence, the new [Gly4, Ile5]VIP analog was able to bind and activate PAC1R [135]. Similarly, the substitution of the Ala4-Val5 dipeptide for Gly4-Ile5 in PACAP abolished its propensity to bind PAC1R but improved its binding to VPAC receptors [135]. The intrinsic characteristics of these amino acids and their differential impact on the secondary structure of PACAP27 and its binding characteristics might reflect the presence of specific structures (β-turn versus helix) involved in the discrimination by PACAP for PAC1R and VPAC receptors. Hence, the stabilization of a β-turn structure around residues 4 and 5 in PACAP could achieve specificity toward PAC1R while the extension of the C-terminal α-helix to residue 4 may shift peptide specificity to VPAC1R [136].
6.2. SAR of PACAP N-Capping Motif
The N-capping motif of PACAP, VIP, and other Class B GPCR ligands constitutes another potential determinant of receptor activation [137]. Accordingly, the folded backbone conformation imposed by the N-capping structure has been proposed to serve as an element for the rational design of PAC1R selective ligands. The structure, encompassing amino acid residues 6 to 10, is mostly characterized by hydrophobic interactions between aromatic residue (Phe6 and Tyr10) and specific hydrogen bonding (between Thr7 and Tyr10 or Ser11). Over the years, the replacement of Phe6 by Tyr, L-cyclohexylalanine (Cha), L-biphenylalanine (Bip), or L-naphtylalanine (Nal) has been shown not to significantly alter the binding properties of PACAP analogs but modulated their specificity for the different receptors [130-132]. The incorporation at position 6 of bulky aromatic residues like Bip or Nal, for example, produced analogs with apparent potent activity toward PAC1R but reduced activity and affinity toward VPACs receptors [130-132]. Overall, SAR studies suggested that the bulky and hydrophobic features of PACAP Phe6 are more essential than its aromaticity for proper receptor recognition. From these observations, the construction of the Ac-[Phe(pI)6,Nle17]PACAP27 analog was shown to be selective for PAC1R/VPAC1R with potent neuroprotective actions in vitro and in vivo [138]. The Thr7 residue of PACAP may facilitate N-capping motif stability and accordingly, the [Ala7]PACAP27 analog can efficiently bind and activate PAC1R and VPAC1R, with almost no activation of VPAC2R. Accordingly, this residue, which is also present in VIP, appears to represent a specific key pharmacophore for VPAC2 selectivity [132, 139]. In sum, these results implicate the N-capping motif as a structural feature in receptor recognition.
6.3. SAR of PACAP C-Terminal Segment
Over the years, various modifications have been introduced within the C-terminal region of PACAP with mixed results [130-131]. However, Ramos-Alvarez et al. suggested recently that L-Ala substitution of PACAP Tyr22 could be also a good strategy to uncover PAC1R specific ligands [134]. The Tyr22 residue, found in PACAP and VIP, has been previously associated with their propensity to bind VPAC2 receptors [140]. Hence, [Ala22]PACAP27/38 has decreased affinity towards the three receptors but the decrement was more acute for VPAC receptors [134, 141].
In sum, the development of PAC1R-specific peptide agonists and antagonists remains elusive, although a few PACAP analogs have exhibited higher affinities for PAC1R and VPAC1R than VPAC2R. While single residue substitutions have failed to generate the specific ligands, multiple residue substitutions informed by PAC1R computational modeling may offer new opportunities. In addition to the design of peptide analogs specific to each of the three PACAP/VIP GPCR subtypes, there are other significant challenges with respect to drug development, including the abilities of the compounds to cross the BBB and the preferential engagement of second messengers for biased receptor signaling. GPCR activation results in canonical plasma membrane-delimited activities of G proteins, but rather than desensitization following receptor endocytosis, the cellular internalization and trafficking of GPCRs are now understood to represent a reprogramming of signaling events in endosomal compartments. GPCR endosomal signaling is best understood with respect to β-arrestin recruitment and scaffolding of proteins for MEK/ERK activation, although sustained cellular cAMP signaling has also been attributed to endosomal receptor mechanisms. Endosomal signaling can direct second messengers to specific intracellular sites with a high spatial and temporal resolution for distinct cellular responses and many of the maladaptive PACAP/PAC1R-mediated effects have been attributed to internalized receptor-endosome mechanisms. Hence a more comprehensive understanding of PACAP/PAC1R signaling messengers for the many homeostatic and maladaptive responses is needed and the design of compounds that can bias the activation or inhibition of specific second messenger pathways becomes attractive to better target therapeutic outcomes. A recent study has started to assess the impact of chemical and structural modifications introduced into specific regions of the PACAP variants on the signaling signatures of PACAP analogs [141].
7. DEVELOPMENT OF SMALL MOLECULES TARGETING PAC1R
Concurrent with efforts to develop receptor specific PACAP analogs, the identification and development of small-molecule ligands for class B GPCRs have also remained a challenge in drug discovery [143-144]. In recent years, a diverse array of orthosteric and allosteric nonpeptide ligands that include antagonists, agonists, and positive allosteric modulators with intrinsic efficacy has been reported for GLP-1R [145], GCGR [8], CRF1R [10], PAC1R [142, 146, 147], VPAC1/2R [148]. Specific small-molecule PAC1R agonists and antagonists have utility in neurotrophic signaling and blocking the maladaptive effects of PACAP, respectively, but progress has been difficult. Below describe recent efforts to develop small-molecule ligands targeting PAC1R.
7.1. Small-Molecule PAC1R Antagonists
From earlier sections, PAC1R antagonism may have therapeutic potential to attenuate or abrogate the maladaptive effects of PACAP/PAC1R signaling in stress-related disorders, including chronic pain and migraine. The studies with PAC1R antagonist PACAP(6-38) have provided proof-of-principle that PAC1R antagonism, even during disorder progression, may be beneficial. As these studies have suggested that PACAP/PAC1R-initiated endosomal ERK signaling is the primary mediator of these effects, small molecules that are biased to preferentially block PAC1R endocytosis and β-arrestin-mediated ERK signaling may be particularly useful.
Beebe and colleagues [142] first reported potent small-molecule hydrazide compounds as antagonists for the PAC1R. SAR studies around two lead hydrazides (Fig. 6) were carried out by competitive 125I-PACAP27 radioligand binding and calcium influx assays. SARs were undertaken on four regions: the acyl phenol portion, the hydrazide linker, the middle aromatic ring, and the distal aromatic ring (Fig. 6). The acyl phenol tolerated no changes and the hydrazide linker tolerated small changes. Both middle aromatic ring and the distal aromatic ring accepted changes for potency. A lipophilic group attached to the distal aromatic ring was preferred, but not crucial for potency. As these lead compounds blocked PACAP/PAC1R-stimulated calcium flux but had little effect on PAC1R endocytosis or ERK signaling, the acyl hydrazides may be biased to block predominantly membrane-delimited G-protein signaling. Accordingly, these compounds may require additional studies for full therapeutic potential.
Fig. (6).
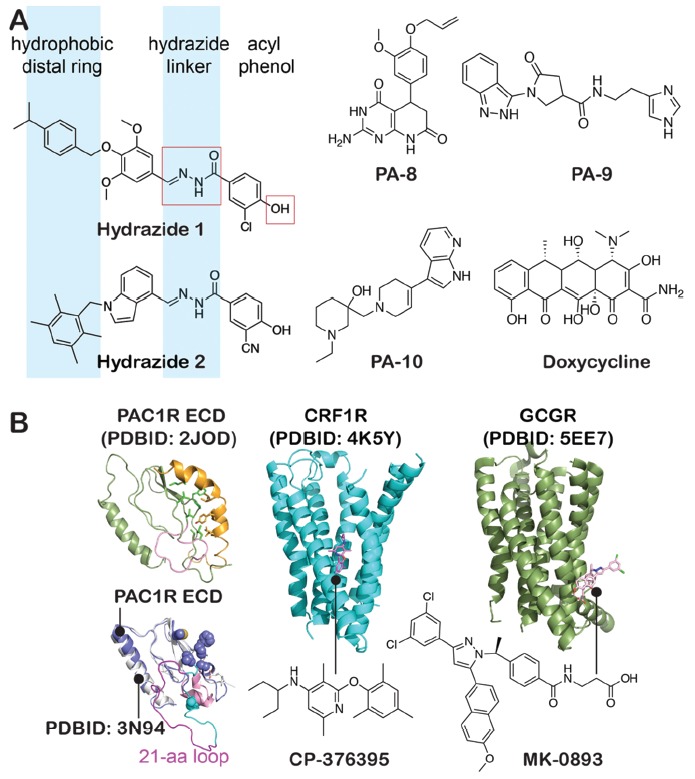
(A) Small molecule compounds previously reported as PAC1R antagonists from calcium and cAMP assays [142]. (B) Potential small-molecule binding sites of PAC1R ECD. The hydrophobic residues that interact with PACAP(6-38) in the NMR structure [37], displayed by green sticks and spheres in the PAC1R model, correspond to residues M72, L74, L80, V113, F127, P128, and A133. The ECD of PAC1R (in purple-blue) was built on the crystal structure (PDBID: 3N94) [39]. The β3-β4 region is in pink and the 21-aa loop in magenta. The deep allosteric pocket site of CRF1R, and the extra-helical site of GCGR targeted by small-molecule compounds CP-376395 and MK-0893, respectively.
More recently, Takasaki and colleagues [146] used virtual screening and in vitro/in vivo pharmacological assays to identify new small-molecule antagonists for PAC1R. The NMR structure [37] of ECD of PAC1R in complex with its peptide antagonist PACAP(6-38) was used, and the conformational positions of residues Tyr22, Val26, and Arg30 were structured to create 3D pharmacophore models. After multifilter virtual screening protocol, ten candidate compounds (PA-1 to PA-10) were selected to evaluate the pharmacological activity. PA-8, PA-9, and PA-10 (1 nM, Fig. 6) significantly attenuated PACAP (1 nM)-induced cAMP Response Element Binding Protein (CREB) phosphorylation; PA-8 and PA-9 showed comparable inhibitory effect of PACAP(6-38). Overall, PA-8, PA-9, and PA-10 showed specific and potent antagonistic activities toward the PAC1R but no detectable agonistic activity toward PAC1R, VPAC1R, and VPAC2R. As these compounds also demonstrated abilities to block PACAP-mediated physiological responses, these compounds may have the potential for further development.
7.2. Small Molecules Targeting PAC1R as Positive Allosteric Modulators
Doxycycline (Fig. 6) and minocycline from the tetracycline family are mostly known as antibiotics to treat various bacterial infections [149]. The neuroprotective effects of doxycycline have been associated with its nonantibiotic properties including inhibition of matrix metalloproteinases [150]. Recently, doxycycline and minocycline were suggested to enhance PAC1R activity in mediating the neuroprotective effects [147]. Doxycycline might affect the binding of PACAP C-terminus to the ECD domain of PAC1R, based on evidence from molecular modeling and a single-site mutation (D116A) in the linker region [147]. Given the observation of doxycycline to promote cellular proliferation, these tetracycline compounds may enhance PACAP binding to PAC1R as well as the resulted PAC1R activation, which leads to a further hypothesis of their roles as positive allosteric modulators [147]. However, how these compounds impact PAC1R signaling remains to be evaluated in detail.
7.3. Small-Molecule Binding Sites of PAC1R
Apart from the ECD, targeting the druggable interface of PAC1R and PACAP to mimic the orthosteric receptor activation site provides additional means to design small-molecule antagonists. The hydrazides [147], PA compounds [146], and doxycycline [147] have been studied in targeting the ECD of PAC1R based on the PACAP(6-38) – PAC1R interface [37]. From many studies, other orthosteric and allosteric GPCR sites may provide opportunities as drug targets. In addition to ECD binding to the C-terminus of PACAP, the orthosteric peptide-binding/activation pocket within the PAC1R 7TM is also promising for drug interventions. Unexpectedly, the crystal structure of CRF1R [10] has revealed that the small-molecule antagonist CP-376395 binds at a deep allosteric site distinct from the orthosteric peptide-binding pocket (Fig. 6B). Further, the GCGR antagonist MK-0893 was found to bind to the receptor at an extra-helical site between TM6 and TM7 in direct contact with the lipid bilayer (Fig. 6B) [8]. As the PAC1R may contain comparable allosteric sites, these regions may represent unique targets that can define PAC1R versus VPAC receptors specificity. Given the dynamics of the ECD and 7TM of PAC1R, there may be other sites and approaches to modulate the function of the PACAP receptor.
7.4. Molecular Docking to Develop Small-Molecule PAC1R Ligands
The knowledge of small-molecule binding sites enables computational molecular docking studies to develop new PAC1R ligands. Despite the lack of an experimentally determined 7TM structure of PAC1R, the receptor models from homology modeling have been employed [21, 147, 151]. A few amino acid residues may be key for the definition of the orthosteric binding pocket, including R1992.60 in TM2, N2403.43 and Y2413.44 in TM3, S3546.41 and H3656.52 in TM6, and Y4007.57 in TM7. In addition, the crystal 7TM structures of CRF1R and GCGR in complex with small molecules (PDBIDs: 4K5Y and 5EE7 respectively, Fig. (6), as well as the PACAP-bound PAC1R ECD structure (PDBID: 2JOD, Fig. (6), may provide useful details of alternative sites for docking. Notably, docking small molecules to the extra-helical site of PAC1R should be performed with caution, as ligand contacts to the lipid bilayer have to be included during sampling and scoring. Furthermore, ensemble docking (use of multiple representative receptor models) [152] and various post-processing steps [153] have been suggested to improve the accuracy of GPCR docking. With a growing understanding of the SARs, molecular docking can be expected to aid future development of effective small molecules to modulate PAC1R.
CONCLUSION AND FUTURE DIRECTIONS
In this review, we summarize the current understanding of PAC1R structure and dynamics, PACAP/PAC1R signaling mechanisms in physiological homeostasis and maladaptive states, and recent progress in peptide and small-molecule development to target PAC1R. PAC1Rs are involved in a variety of signaling cascades, including adenylyl cyclase, phospholipase C, MEK/ERK, and Akt pathways to maintain mature CNS and PNS function. These pathways may participate in neurogenesis, neuronal protection, migration, neuronal differentiation, and regeneration; the same mechanisms may be the underlying basis for their neuroprotection activities after CNS insults or neurodegeneration. Conversely, after chronic or repeated insults, the same PAC1R signaling pathways may result in maladaptive responses that can precipitate stress-related and chronic pain disorders. Thus, studies of the PACAP/PAC1R system and the signaling pathways prove new avenues and medical strategies to treat pathophysiological conditions.
Given its diverse roles, PAC1R has emerged as a potential target for therapeutics. PAC1R agonism may enhance its trophic attributes to mitigate neurodegeneration disorders and maintain energy balance functions in the metabolic regulation against obesity and diabetes. Conversely, PAC1R antagonism bears potential in treating stress-related disorders, chronic pain/migraine, and other behavioral abnormalities. SAR studies for a number of PACAP/VIP analogs have identified some of the molecular determinants responsible for the recognition and activation of PAC1R as well as VPAC1/2R. A few important motifs have been determined, including a putative Asx-turn at the N-terminal segment (residues 1 to 5) and a key pharmacophore element Phe6 for PAC1R recognition and activation, Gly4 - Ile5 for apparent PACAP selectivity for PAC1R, and the C-terminal helix essential for PAC1R binding affinity. Among issues, peptide agonists are subject to problems in PAC1R/VPAC receptor selectivity, transport across the BBB, and rapid degradation. The development of small-molecule compounds provides a means to overcome some of these problems, but challenges still remain. To facilitate the future development of peptide and small-molecule reagents for PAC1R, better insights into the key pharmacophores involved in the specific activation of PAC1R signaling are still needed. In this light, PAC1R 3D structures and dynamics from computational modeling can provide novel opportunities and insights to accelerate structure-based drug design for PAC1R and other class B GPCRs in the treatment of a number of disease conditions.
ACKNOWLEDGEMENTS
The authors thank Dr. Jacob Remington for help discussions.
LIST OF ABBREVIATIONS
- aa
Amino Acid
- AC
Adenylyl Cyclase
- Akt
Protein Kinase B or PKB
- ARC
Arcuate Nucleus
- AgRP
Agouti-Related Peptide
- BNST
Bed nucleus of the Stria Terminalis
- BBB
Blood Brain Barrier
- cAMP
Cyclic Adenosine Monophosphate
- CeA
Central Amygdala
- CeLC
Capsular Division of the Amygdala
- CGRP
Calcitonin Gene-Related Peptide
- CREB
cAMP Response Element Binding Protein
- CRF1R
Corticotropin-Releasing Factor Receptor 1
- cryo-EM
Cryo-Electron Microscopy
- CTR
Calcitonin Receptor
- DAG
Diacylglycerol
- ECD
Extracellular Domain
- ECL
GPCR Extracellular Loop (1, 2 or 3)
- EPAC
Exchange Factor Activated by cAMP
- ERE
Estrogen Responsive Element
- ERK
Extracellular Signal-Regulated Kinases
- Gαq
G Protein, Gq Alpha Subunit
- Gαs
G Protein, Gs Alpha Subunit
- Gβ/γ
G Proteins, beta/gamma subunits
- GPCR
G Protein-Coupled Receptor
- GCGR
Glucagon Receptor
- GIP
Gastric Inhibitory Peptide
- GLP-1R
Glucagon-Like Peptide 1 Receptor
- GRK G
Protein-Coupled Receptor Kinases
- ICL
GPCR Intercellular Loop (1, 2 or 3)
- ICV
Intracerebroventricular
- IP3
Inositol Triphosphate
- MAPK
Mitogen-Activated Protein Kinase
- MC4Rreceptor
Melanocortin 4 Receptor
- MEK
Mitogen-Activated Protein Kinase Kinase
- αMSH
α-Melanocyte Stimulating Hormone
- MPP+
1-Methyl-4-Phenylpyridinium
- NPY
Neuropeptide Y
- NMR
Nuclear Magnetic Resonance
- PACAP
Pituitary Adenylate Cyclase-Activating Polypeptide
- PACAP38
PACAP, 38 Residues
- PACAP27
PACAP, 27 Residues
- PACAP(6-38)
PACAP Residues 6-38
- PAC1R
Pituitary Adenylate Cyclase Activating Polypeptide Type 1 Receptor
- PDBID
Protein Data Bank Identifier
- PI3K
Phosphatidylinositol-3-Kinase
- PIP2
Phosphatidylinositiol 4,5-Bisphosphate
- PKA
Protein Kinase A
- PKC
Protein Kinase C
- PLC
Phospholipase C
- POMC
Pro-Opiomelanocortin
- PTHR
Parathyroid Hormone Receptor
- PTSD
Posttraumatic Stress Disorder
- PVN
Paraventricular Nucleus
- RAP1
Ras-Related Protein 1
- SAR
Structure–Activity Relationship
- SON
Supraoptic Nucleus
- 7TM
Heptahelical Transmembrane Domain
- TM
Transmembrane Domain, (same as 7TM)
- VIP
Vasoactive Intestinal Polypeptide
- VPAC1R or VIPR
Vasoactive Intestinal Polypeptide Receptor 1
- VPAC2R or VIPR2
Vasoactive Intestinal Polypeptide Receptor 2
- VMN
Ventromedial Hypothalamic Nucleus
- 3D
Three-Dimensional
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
Research reported in this publication was supported by the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health (NIH) under Award Number R01GM129431 to J. L., V. M., and M. B. The con- tent is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Partial support was also provided by the U.S. Army Research Office (Grant 71015-CH-YIP) to S.T.S.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Harmar A.J., Fahrenkrug J., Gozes I., Laburthe M., May V., Pisegna J.R., Vaudry D., Vaudry H., Waschek J.A., Said S.I. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. Br. J. Pharmacol. 2012;166(1):4–17. doi: 10.1111/j.1476-5381.2012.01871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bortolato A., Doré A.S., Hollenstein K., Tehan B.G., Mason J.S., Marshall F.H. Structure of Class B GPCRs: new horizons for drug discovery. Br. J. Pharmacol. 2014;171(13):3132–3145. doi: 10.1111/bph.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Culhane K.J., Liu Y., Cai Y., Yan E.C.Y. Transmembrane signal transduction by peptide hormones via family B G protein-coupled receptors. Front. Pharmacol. 2015;6:264. doi: 10.3389/fphar.2015.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graaf Cd., Donnelly D., Wootten D., Lau J., Sexton P.M., Miller L.J., Ahn J.M., Liao J., Fletcher M.M., Yang D., Brown A.J., Zhou C., Deng J., Wang M.W. Glucagon-like peptide-1 and its class B G protein-coupled receptors: A long march to therapeutic successes. Pharmacol. Rev. 2016;68(4):954–1013. doi: 10.1124/pr.115.011395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pal K., Melcher K., Xu H.E. Structure and mechanism for recognition of peptide hormones by Class B G-protein-coupled receptors. Acta Pharmacol. Sin. 2012;33(3):300–311. doi: 10.1038/aps.2011.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Graaf C., Song G., Cao C., Zhao Q., Wang M.W., Wu B., Stevens R.C. Extending the structural view of class B GPCRs. Trends Biochem. Sci. 2017;42(12):946–960. doi: 10.1016/j.tibs.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Siu F.Y., He M., de Graaf C., Han G.W., Yang D., Zhang Z., Zhou C., Xu Q., Wacker D., Joseph J.S., Liu W., Lau J., Cherezov V., Katritch V., Wang M.W., Stevens R.C. Structure of the human glucagon class B G-protein-coupled receptor. Nature. 2013;499(7459):444–449. doi: 10.1038/nature12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jazayeri A., Doré A.S., Lamb D., Krishnamurthy H., Southall S.M., Baig A.H., Bortolato A., Koglin M., Robertson N.J., Errey J.C., Andrews S.P., Teobald I., Brown A.J.H., Cooke R.M., Weir M., Marshall F.H. Extra-helical binding site of a glucagon receptor antagonist. Nature. 2016;533(7602):274–277. doi: 10.1038/nature17414. [DOI] [PubMed] [Google Scholar]
- 9.Song G., Yang D., Wang Y., de Graaf C., Zhou Q., Jiang S., Liu K., Cai X., Dai A., Lin G., Liu D., Wu F., Wu Y., Zhao S., Ye L., Han G.W., Lau J., Wu B., Hanson M.A., Liu Z-J., Wang M-W., Stevens R.C. Human GLP-1 receptor transmembrane domain structure in complex with allosteric modulators. Nature. 2017;546(7657):312–315. doi: 10.1038/nature22378. [DOI] [PubMed] [Google Scholar]
- 10.Hollenstein K., Kean J., Bortolato A., Cheng R.K.Y., Doré A.S., Jazayeri A., Cooke R.M., Weir M., Marshall F.H. Structure of class B GPCR corticotropin-releasing factor receptor 1. Nature. 2013;499(7459):438–443. doi: 10.1038/nature12357. [DOI] [PubMed] [Google Scholar]
- 11.Jazayeri A., Rappas M., Brown A.J.H., Kean J., Errey J.C., Robertson N.J., Fiez-Vandal C., Andrews S.P., Congreve M., Bortolato A., Mason J.S., Baig A.H., Teobald I., Doré A.S., Weir M., Cooke R.M., Marshall F.H. Crystal structure of the GLP-1 receptor bound to a peptide agonist. Nature. 2017;546(7657):254–258. doi: 10.1038/nature22800. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H., Qiao A., Yang D., Yang L., Dai A., de Graaf C., Reedtz-Runge S., Dharmarajan V., Zhang H., Han G.W., Grant T.D., Sierra R.G., Weierstall U., Nelson G., Liu W., Wu Y., Ma L., Cai X., Lin G., Wu X., Geng Z., Dong Y., Song G., Griffin P.R., Lau J., Cherezov V., Yang H., Hanson M.A., Stevens R.C., Zhao Q., Jiang H., Wang M.W., Wu B. Structure of the full-length glucagon class B G-protein-coupled receptor. Nature. 2017;546(7657):259–264. doi: 10.1038/nature22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H., Qiao A., Yang L., Van Eps N., Frederiksen K.S., Yang D., Dai A., Cai X., Zhang H., Yi C., Cao C., He L., Yang H., Lau J., Ernst O.P., Hanson M.A., Stevens R.C., Wang M.W., Reedtz-Runge S., Jiang H., Zhao Q., Wu B. Structure of the glucagon receptor in complex with a glucagon analogue. Nature. 2018;553(7686):106–110. doi: 10.1038/nature25153. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y., Sun B., Feng D., Hu H., Chu M., Qu Q., Tarrasch J.T., Li S., Sun Kobilka T., Kobilka B.K., Skiniotis G. Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein. Nature. 2017;546(7657):248–253. doi: 10.1038/nature22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang Y.L., Khoshouei M., Glukhova A., Furness S.G.B., Zhao P., Clydesdale L., Koole C., Truong T.T., Thal D.M., Lei S., Radjainia M., Danev R., Baumeister W., Wang M.W., Miller L.J., Christopoulos A., Sexton P.M., Wootten D. Phase-plate cryo-EM structure of a biased agonist-bound human GLP-1 receptor-Gs complex. Nature. 2018;555(7694):121–125. doi: 10.1038/nature25773. [DOI] [PubMed] [Google Scholar]
- 16.Liang Y.L., Khoshouei M., Radjainia M., Zhang Y., Glukhova A., Tarrasch J., Thal D.M., Furness S.G.B., Christopoulos G., Coudrat T., Danev R., Baumeister W., Miller L.J., Christopoulos A., Kobilka B.K., Wootten D., Skiniotis G., Sexton P.M. Phase-plate cryo-EM structure of a class B GPCR-G-protein complex. Nature. 2017;546(7656):118–123. doi: 10.1038/nature22327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang Y-L., Khoshouei M., Deganutti G., Glukhova A., Koole C., Peat T.S., Radjainia M., Plitzko J.M., Baumeister W., Miller L.J., Hay D.L., Christopoulos A., Reynolds C.A., Wootten D., Sexton P.M. Cryo-EM structure of the active, Gs-protein complexed, human CGRP receptor. Nature. 2018;561(7724):492–497. doi: 10.1038/s41586-018-0535-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spengler D., Waeber C., Pantaloni C., Holsboer F., Bockaert J., Seeburg P.H., Journot L. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365(6442):170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- 19.Braas K.M., May V. Pituitary adenylate cyclase-activating polypeptides directly stimulate sympathetic neuron neuropeptide Y release through PAC(1) receptor isoform activation of specific intracellular signaling pathways. J. Biol. Chem. 1999;274(39):27702–27710. doi: 10.1074/jbc.274.39.27702. [DOI] [PubMed] [Google Scholar]
- 20.Blechman J., Levkowitz G. Alternative splicing of the pituitary adenylate cyclase-activating polypeptide receptor PAC1: Mechanisms of fine tuning of brain activity. Front. Endocrinol. (Lausanne) 2013;4:55. doi: 10.3389/fendo.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao C., Zhao X., Brewer M., May V., Li J. Conformational transitions of the pituitary adenylate cyclase-activating polypeptide receptor, a human class B GPCR. Sci. Rep. 2017;7(1):5427. doi: 10.1038/s41598-017-05815-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Millar R.P., Newton C.L. The year in G protein-coupled receptor research. Mol. Endocrinol. 2010;24(1):261–274. doi: 10.1210/me.2009-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lebon G., Warne T., Edwards P.C., Bennett K., Langmead C.J., Leslie A.G.W., Tate C.G. Agonist-bound adenosine A2A receptor structures reveal common features of GPCR activation. Nature. 2011;474(7352):521–525. doi: 10.1038/nature10136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J., Jonsson A.L., Beuming T., Shelley J.C., Voth G.A. Ligand-dependent activation and deactivation of the human adenosine A(2A) receptor. J. Am. Chem. Soc. 2013;135(23):8749–8759. doi: 10.1021/ja404391q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao C., Zhao X., Liu J., Schneebeli S.T., Shelley J.C., Li J. Capturing the multiscale dynamics of membrane protein complexes with all-atom, mixed-resolution, and coarse-grained models. Phys. Chem. Chem. Phys. 2017;19(13):9181–9188. doi: 10.1039/C7CP00200A. [DOI] [PubMed] [Google Scholar]
- 26.Dror R.O., Arlow D.H., Maragakis P., Mildorf T.J., Pan A.C., Xu H., Borhani D.W., Shaw D.E. Activation mechanism of the β2-adrenergic receptor. Proc. Natl. Acad. Sci. USA. 2011;108(46):18684–18689. doi: 10.1073/pnas.1110499108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan S., Hu Z., Filipek S., Vogel H. W246(6.48) opens a gate for a continuous intrinsic water pathway during activation of the adenosine A2A receptor. Angew. Chem. Int. Ed. Engl. 2015;54(2):556–559. doi: 10.1002/anie.201409679. [DOI] [PubMed] [Google Scholar]
- 28.Latorraca N.R., Venkatakrishnan A.J., Dror R.O. GPCR dynamics: Structures in motion. Chem. Rev. 2017;117(1):139–155. doi: 10.1021/acs.chemrev.6b00177. [DOI] [PubMed] [Google Scholar]
- 29.Kohlhoff K.J., Shukla D., Lawrenz M., Bowman G.R., Konerding D.E., Belov D., Altman R.B., Pande V.S. Cloud-based simulations on Google Exacycle reveal ligand modulation of GPCR activation pathways. Nat. Chem. 2014;6(1):15–21. doi: 10.1038/nchem.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao C., May V., Li J. PAC1 receptors: Shapeshifters in motion. J. Mol. Neurosci. 2019;68(3):331–339. doi: 10.1007/s12031-018-1132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beauchamp K.A., Bowman G.R., Lane T.J., Maibaum L., Haque I.S., Pande V.S. MSMBuilder2: Modeling conformational dynamics on the picosecond to millisecond scale. J. Chem. Theory Comput. 2011;7(10):3412–3419. doi: 10.1021/ct200463m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wootten D., Simms J., Miller L.J., Christopoulos A., Sexton P.M. Polar transmembrane interactions drive formation of ligand-specific and signal pathway-biased family B G protein-coupled receptor conformations. Proc. Natl. Acad. Sci. USA. 2013;110(13):5211–5216. doi: 10.1073/pnas.1221585110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hollenstein K., de Graaf C., Bortolato A., Wang M.W., Marshall F.H., Stevens R.C. Insights into the structure of class B GPCRs. Trends Pharmacol. Sci. 2014;35(1):12–22. doi: 10.1016/j.tips.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grace C.R.R., Perrin M.H., Gulyas J., Rivier J.E., Vale W.W., Riek R. NMR structure of the first extracellular domain of corticotropin-releasing factor receptor 1 (ECD1-CRF-R1) complexed with a high affinity agonist. J. Biol. Chem. 2010;285(49):38580–38589. doi: 10.1074/jbc.M110.121897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parthier C., Kleinschmidt M., Neumann P., Rudolph R., Manhart S., Schlenzig D., Fanghänel J., Rahfeld J-U., Demuth H-U., Stubbs M.T. Crystal structure of the incretin-bound extracellular domain of a G protein-coupled receptor. Proc. Natl. Acad. Sci. USA. 2007;104(35):13942–13947. doi: 10.1073/pnas.0706404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pioszak A.A., Xu H.E. Molecular recognition of parathyroid hormone by its G protein-coupled receptor. Proc. Natl. Acad. Sci. USA. 2008;105(13):5034–5039. doi: 10.1073/pnas.0801027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun C., Song D., Davis-Taber R.A., Barrett L.W., Scott V.E., Richardson P.L., Pereda-Lopez A., Uchic M.E., Solomon L.R., Lake M.R., Walter K.A., Hajduk P.J., Olejniczak E.T. Solution structure and mutational analysis of pituitary adenylate cyclase-activating polypeptide binding to the extracellular domain of PAC1-RS. Proc. Natl. Acad. Sci. USA. 2007;104(19):7875–7880. doi: 10.1073/pnas.0611397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang L., Yang D., de Graaf C., Moeller A., West G.M., Dharmarajan V., Wang C., Siu F.Y., Song G., Reedtz-Runge S., Pascal B.D., Wu B., Potter C.S., Zhou H., Griffin P.R., Carragher B., Yang H., Wang M.W., Stevens R.C., Jiang H. Conformational states of the full-length glucagon receptor. Nat. Commun. 2015;6:7859. doi: 10.1038/ncomms8859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar S., Pioszak A., Zhang C., Swaminathan K., Xu H.E. Crystal structure of the PAC1R extracellular domain unifies a consensus fold for hormone recognition by class B G-protein coupled receptors. PLoS One. 2011;6(5):e19682. doi: 10.1371/journal.pone.0019682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaudry D., Falluel-Morel A., Bourgault S., Basille M., Burel D., Wurtz O., Fournier A., Chow B.K.C., Hashimoto H., Galas L., Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev. 2009;61(3):283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- 41.Miyata A., Jiang L., Dahl R.D., Kitada C., Kubo K., Fujino M., Minamino N., Arimura A. Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38). Biochem. Biophys. Res. Commun. 1990;170(2):643–648. doi: 10.1016/0006-291X(90)92140-U. [DOI] [PubMed] [Google Scholar]
- 42.Miyata A., Arimura A., Dahl R.R., Minamino N., Uehara A., Jiang L., Culler M.D., Coy D.H. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem. Biophys. Res. Commun. 1989;164(1):567–574. doi: 10.1016/0006-291X(89)91757-9. [DOI] [PubMed] [Google Scholar]
- 43.Arimura A., Somogyvári-Vigh A., Miyata A., Mizuno K., Coy D.H., Kitada C. Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology. 1991;129(5):2787–2789. doi: 10.1210/endo-129-5-2787. [DOI] [PubMed] [Google Scholar]
- 44.Sherwood N.M., Krueckl S.L., McRory J.E. The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocr. Rev. 2000;21(6):619–670. doi: 10.1210/edrv.21.6.0414. [DOI] [PubMed] [Google Scholar]
- 45.Robberecht P., Gourlet P., De Neef P., Woussen-Colle M.C., Vandermeers-Piret M.C., Vandermeers A., Christophe J. Structural requirements for the occupancy of pituitary adenylate-cyclase-activating-peptide (PACAP) receptors and adenylate cyclase activation in human neuroblastoma NB-OK-1 cell membranes. Discovery of PACAP(6-38) as a potent antagonist. Eur. J. Biochem. 1992;207(1):239–246. doi: 10.1111/j.1432-1033.1992.tb17043.x. [DOI] [PubMed] [Google Scholar]
- 46.Lerner E.A., Iuga A.O., Reddy V.B. Maxadilan, a PAC1 receptor agonist from sand flies. Peptides. 2007;28(9):1651–1654. doi: 10.1016/j.peptides.2007.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soares M.B., Titus R.G., Shoemaker C.B., David J.R., Bozza M. The vasoactive peptide maxadilan from sand fly saliva inhibits TNF-alpha and induces IL-6 by mouse macrophages through interaction with the pituitary adenylate cyclase-activating polypeptide (PACAP) receptor. J. Immunol. 1998;160(4):1811–1816. [PubMed] [Google Scholar]
- 48.Tatsuno I., Uchida D., Tanaka T., Saeki N., Hirai A., Saito Y., Moro O., Tajima M. Maxadilan specifically interacts with PAC1 receptor, which is a dominant form of PACAP/VIP family receptors in cultured rat cortical neurons. Brain Res. 2001;889(1-2):138–148. doi: 10.1016/S0006-8993(00)03126-7. [DOI] [PubMed] [Google Scholar]
- 49.Uchida D., Tatsuno I., Tanaka T., Hirai A., Saito Y., Moro O., Tajima M. Maxadilan is a specific agonist and its deleted peptide (M65) is a specific antagonist for PACAP type 1 receptor. Ann. N. Y. Acad. Sci. 1998;865:253–258. doi: 10.1111/j.1749-6632.1998.tb11185.x. [DOI] [PubMed] [Google Scholar]
- 50.May V., Parsons R.L. G protein-coupled receptor endosomal signaling and regulation of neuronal excitability and stress responses: Signaling options and lessons from the PAC1 receptor. J. Cell. Physiol. 2017;232(4):698–706. doi: 10.1002/jcp.25615. [DOI] [PubMed] [Google Scholar]
- 51.Hammack S.E., Cheung J., Rhodes K.M., Schutz K.C., Falls W.A., Braas K.M., May V. Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology. 2009;34(6):833–843. doi: 10.1016/j.psyneuen.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ressler K.J., Mercer K.B., Bradley B., Jovanovic T., Mahan A., Kerley K., Norrholm S.D., Kilaru V., Smith A.K., Myers A.J., Ramirez M., Engel A., Hammack S.E., Toufexis D., Braas K.M., Binder E.B., May V. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470(7335):492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hammack S.E., May V. Pituitary adenylate cyclase activating polypeptide in stress-related disorders: data convergence from animal and human studies. Biol. Psychiatry. 2015;78(3):167–177. doi: 10.1016/j.biopsych.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lezak K.R., Roman C.W., Braas K.M., Schutz K.C., Falls W.A., Schulkin J., May V., Hammack S.E. Regulation of bed nucleus of the stria terminalis PACAP expression by stress and corticosterone. J. Mol. Neurosci. 2014;54(3):477–484. doi: 10.1007/s12031-014-0269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roman C.W., Lezak K.R., Hartsock M.J., Falls W.A., Braas K.M., Howard A.B., Hammack S.E., May V. PAC1 receptor antagonism in the bed nucleus of the stria terminalis (BNST) attenuates the endocrine and behavioral consequences of chronic stress. Psychoneuroendocrinology. 2014;47:151–165. doi: 10.1016/j.psyneuen.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Missig G., Roman C.W., Vizzard M.A., Braas K.M., Hammack S.E., May V. Parabrachial nucleus (PBn) pituitary adenylate cyclase activating polypeptide (PACAP) signaling in the amygdala: implication for the sensory and behavioral effects of pain. Neuropharmacology. 2014;86:38–48. doi: 10.1016/j.neuropharm.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Missig G., Mei L., Vizzard M.A., Braas K.M., Waschek J.A., Ressler K.J., Hammack S.E., May V. Parabrachial pituitary adenylate cyclase-activating polypeptide activation of amygdala endosomal extracellular signal-regulated kinase signaling regulates the emotional component of pain. Biol. Psychiatry. 2017;81(8):671–682. doi: 10.1016/j.biopsych.2016.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hashimoto H., Shintani N., Tanaka K., Mori W., Hirose M., Matsuda T., Sakaue M., Miyazaki J., Niwa H., Tashiro F., Yamamoto K., Koga K., Tomimoto S., Kunugi A., Suetake S., Baba A. Altered psychomotor behaviors in mice lacking pituitary adenylate cyclase-activating polypeptide (PACAP). Proc. Natl. Acad. Sci. USA. 2001;98(23):13355–13360. doi: 10.1073/pnas.231094498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Girard B.A., Lelievre V., Braas K.M., Razinia T., Vizzard M.A., Ioffe Y., El Meskini R., Ronnett G.V., Waschek J.A., May V. Noncompensation in peptide/receptor gene expression and distinct behavioral phenotypes in VIP- and PACAP-deficient mice. J. Neurochem. 2006;99(2):499–513. doi: 10.1111/j.1471-4159.2006.04112.x. [DOI] [PubMed] [Google Scholar]
- 60.Hattori S., Takao K., Tanda K., Toyama K., Shintani N., Baba A., Hashimoto H., Miyakawa T. Comprehensive behavioral analysis of pituitary adenylate cyclase-activating polypeptide (PACAP) knockout mice. Front. Behav. Neurosci. 2012;6:58. doi: 10.3389/fnbeh.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Otto C., Kovalchuk Y., Wolfer D.P., Gass P., Martin M., Zuschratter W., Gröne H.J., Kellendonk C., Tronche F., Maldonado R., Lipp H.P., Konnerth A., Schütz G. Impairment of mossy fiber long-term potentiation and associative learning in pituitary adenylate cyclase activating polypeptide type I receptor-deficient mice. J. Neurosci. 2001;21(15):5520–5527. doi: 10.1523/JNEUROSCI.21-15-05520.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stroth N., Eiden L.E. Stress hormone synthesis in mouse hypothalamus and adrenal gland triggered by restraint is dependent on pituitary adenylate cyclase-activating polypeptide signaling. Neuroscience. 2010;165(4):1025–1030. doi: 10.1016/j.neuroscience.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hatanaka M., Tanida M., Shintani N., Isojima Y., Kawaguchi C., Hashimoto H., Kakuda M., Haba R., Nagai K., Baba A. Lack of light-induced elevation of renal sympathetic nerve activity and plasma corticosterone levels in PACAP-deficient mice. Neurosci. Lett. 2008;444(2):153–156. doi: 10.1016/j.neulet.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 64.Tsukiyama N., Saida Y., Kakuda M., Shintani N., Hayata A., Morita Y., Tanida M., Tajiri M., Hazama K., Ogata K., Hashimoto H., Baba A. PACAP centrally mediates emotional stress-induced corticosterone responses in mice. Stress. 2011;14(4):368–375. doi: 10.3109/10253890.2010.544345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Otto C., Martin M., Wolfer D.P., Lipp H.P., Maldonado R., Schütz G. Altered emotional behavior in PACAP-type-I-receptor-deficient mice. Brain Res. Mol. Brain Res. 2001;92(1-2):78–84. doi: 10.1016/S0169-328X(01)00153-X. [DOI] [PubMed] [Google Scholar]
- 66.Dias B.G., Ressler K.J. PACAP and the PAC1 receptor in post-traumatic stress disorder. Neuropsychopharmacology. 2013;38(1):245–246. doi: 10.1038/npp.2012.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Almli L.M., Mercer K.B., Kerley K., Feng H., Bradley B., Conneely K.N., Ressler K.J. ADCYAP1R1 genotype associates with post-traumatic stress symptoms in highly traumatized African-American females. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2013;162B(3):262–272. doi: 10.1002/ajmg.b.32145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jovanovic T., Norrholm S.D., Davis J., Mercer K.B., Almli L., Nelson A., Cross D., Smith A., Ressler K.J., Bradley B. PAC1 receptor (ADCYAP1R1) genotype is associated with dark-enhanced startle in children. Mol. Psychiatry. 2013;18(7):742–743. doi: 10.1038/mp.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Uddin M., Chang S.C., Zhang C., Ressler K., Mercer K.B., Galea S., Keyes K.M., McLaughlin K.A., Wildman D.E., Aiello A.E., Koenen K.C. Adcyap1r1 genotype, posttraumatic stress disorder, and depression among women exposed to childhood maltreatment. Depress. Anxiety. 2013;30(3):251–258. doi: 10.1002/da.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang L., Cao C., Wang R., Qing Y., Zhang J., Zhang X.Y. PAC1 receptor (ADCYAP1R1) genotype is associated with PTSD’s emotional numbing symptoms in Chinese earthquake survivors. J. Affect. Disord. 2013;150(1):156–159. doi: 10.1016/j.jad.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 71.Pohlack S.T., Nees F., Ruttorf M., Cacciaglia R., Winkelmann T., Schad L.R., Witt S.H., Rietschel M., Flor H. Neural mechanism of a sex-specific risk variant for posttraumatic stress disorder in the type I receptor of the pituitary adenylate cyclase activating polypeptide. Biol. Psychiatry. 2015;78(12):840–847. doi: 10.1016/j.biopsych.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 72.Rouwette T., Vanelderen P., Roubos E.W., Kozicz T., Vissers K. The amygdala, a relay station for switching on and off pain. Eur. J. Pain. 2012;16(6):782–792. doi: 10.1002/j.1532-2149.2011.00071.x. [DOI] [PubMed] [Google Scholar]
- 73.Gauriau C., Bernard J-F. Pain pathways and parabrachial circuits in the rat. Exp. Physiol. 2002;87(2):251–258. doi: 10.1113/eph8702357. [DOI] [PubMed] [Google Scholar]
- 74.Otis J.D., Keane T.M., Kerns R.D. An examination of the relationship between chronic pain and post-traumatic stress disorder. J. Rehabil. Res. Dev. 2003;40(5):397–405. doi: 10.1682/JRRD.2003.09.0397. [DOI] [PubMed] [Google Scholar]
- 75.McFarlane A.C., Atchison M., Rafalowicz E., Papay P. Physical symptoms in post-traumatic stress disorder. J. Psychosom. Res. 1994;38(7):715–726. doi: 10.1016/0022-3999(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 76.McWilliams L.A., Cox B.J., Enns M.W. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. 2003;106(1-2):127–133. doi: 10.1016/S0304-3959(03)00301-4. [DOI] [PubMed] [Google Scholar]
- 77.Asmundson G.J., Katz J. Understanding the co-occurrence of anxiety disorders and chronic pain: state-of-the-art. Depress. Anxiety. 2009;26(10):888–901. doi: 10.1002/da.20600. [DOI] [PubMed] [Google Scholar]
- 78.Moeller-Bertram T., Keltner J., Strigo I.A. Pain and post traumatic stress disorder - review of clinical and experimental evidence. Neuropharmacology. 2012;62(2):586–597. doi: 10.1016/j.neuropharm.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 79.Norman S.B., Stein M.B., Dimsdale J.E., Hoyt D.B. Pain in the aftermath of trauma is a risk factor for post-traumatic stress disorder. Psychol. Med. 2008;38(4):533–542. doi: 10.1017/S0033291707001389. [DOI] [PubMed] [Google Scholar]
- 80.Scioli-Salter E.R., Forman D.E., Otis J.D., Gregor K., Valovski I., Rasmusson A.M. The shared neuroanatomy and neurobiology of comorbid chronic pain and PTSD: therapeutic implications. Clin. J. Pain. 2015;31(4):363–374. doi: 10.1097/AJP.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 81.Syed A.U., Koide M., Braas K.M., May V., Wellman G.C. Pituitary adenylate cyclase-activating polypeptide (PACAP) potently dilates middle meningeal arteries: implications for migraine. J. Mol. Neurosci. 2012;48(3):574–583. doi: 10.1007/s12031-012-9851-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Edvinsson L., Tajti J., Szalárdy L., Vécsei L. PACAP and its role in primary headaches. J. Headache Pain. 2018;19(1):21. doi: 10.1186/s10194-018-0852-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Akerman S., Goadsby P.J. Neuronal PAC1 receptors mediate delayed activation and sensitization of trigeminocervical neurons: Relevance to migraine. Sci. Transl. Med. 2015;7(308):308ra157. doi: 10.1126/scitranslmed.aaa7557. [DOI] [PubMed] [Google Scholar]
- 84.Brain S.D., Williams T.J., Tippins J.R., Morris H.R., MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313(5997):54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- 85.Lassen L.H., Haderslev P.A., Jacobsen V.B., Iversen H.K., Sperling B., Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22(1):54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- 86.Tepper S.J. History and review of anti-calcitonin gene-related peptide (CGRP) therapies: From translational research to treatment. Headache. 2018;58(Suppl. 3):238–275. doi: 10.1111/head.13379. [DOI] [PubMed] [Google Scholar]
- 87.Schytz H.W., Birk S., Wienecke T., Kruuse C., Olesen J., Ashina M. PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain. 2009;132(Pt 1):16–25. doi: 10.1093/brain/awn307. [DOI] [PubMed] [Google Scholar]
- 88.Amin F.M., Asghar M.S., Guo S., Hougaard A., Hansen A.E., Schytz H.W., van der Geest R.J., de Koning P.J., Larsson H.B., Olesen J., Ashina M. Headache and prolonged dilatation of the middle meningeal artery by PACAP38 in healthy volunteers. Cephalalgia. 2012;32(2):140–149. doi: 10.1177/0333102411431333. [DOI] [PubMed] [Google Scholar]
- 89.Syed A.U., Koide M., May V., Wellman G.C. In: PACAP regulation of vascular tone: differential mechanism among vascular beds.Pituitary Adenylate Cyclase Activating Polypeptide — PACAP; Reglodi, D. Tamas A., editor. Cham: Springer International Publishing; 2016. pp. 617–630. [DOI] [Google Scholar]
- 90.Carrasquillo Y., Gereau R.W., IV Activation of the extracellular signal-regulated kinase in the amygdala modulates pain perception. J. Neurosci. 2007;27(7):1543–1551. doi: 10.1523/JNEUROSCI.3536-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.May V., Buttolph T.R., Girard B.M., Clason T.A., Parsons R.L. PACAP-induced ERK activation in HEK cells expressing PAC1 receptors involves both receptor internalization and PKC signaling. Am. J. Physiol. Cell Physiol. 2014;306(11):C1068–C1079. doi: 10.1152/ajpcell.00001.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hannibal J. Pituitary adenylate cyclase-activating peptide in the rat central nervous system: an immunohistochemical and in situ hybridization study. J. Comp. Neurol. 2002;453(4):389–417. doi: 10.1002/cne.10418. [DOI] [PubMed] [Google Scholar]
- 93.Mounien L., Do Rego J.C., Bizet P., Boutelet I., Gourcerol G., Fournier A., Brabet P., Costentin J., Vaudry H., Jégou S. Pituitary adenylate cyclase-activating polypeptide inhibits food intake in mice through activation of the hypothalamic melanocortin system. Neuropsychopharmacology. 2009;34(2):424–435. doi: 10.1038/npp.2008.73. [DOI] [PubMed] [Google Scholar]
- 94.Mizuno Y., Kondo K., Terashima Y., Arima H., Murase T., Oiso Y. Anorectic effect of pituitary adenylate cyclase activating polypeptide (PACAP) in rats: lack of evidence for involvement of hypothalamic neuropeptide gene expression. J. Neuroendocrinol. 1998;10(8):611–616. doi: 10.1046/j.1365-2826.1998.00244.x. [DOI] [PubMed] [Google Scholar]
- 95.Vu J.P., Larauche M., Flores M., Luong L., Norris J., Oh S., Liang L.J., Waschek J., Pisegna J.R., Germano P.M. Regulation of appetite, body composition, and metabolic hormones by vasoactive intestinal polypeptide (VIP). J. Mol. Neurosci. 2015;56(2):377–387. doi: 10.1007/s12031-015-0556-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vu J.P., Goyal D., Luong L., Oh S., Sandhu R., Norris J., Parsons W., Pisegna J.R., Germano P.M. PACAP intraperitoneal treatment suppresses appetite and food intake via PAC1 receptor in mice by inhibiting ghrelin and increasing GLP-1 and leptin. Am. J. Physiol. Gastrointest. Liver Physiol. 2015;309(10):G816–G825. doi: 10.1152/ajpgi.00190.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Resch J.M., Boisvert J.P., Hourigan A.E., Mueller C.R., Yi S.S., Choi S. Stimulation of the hypothalamic ventromedial nuclei by pituitary adenylate cyclase-activating polypeptide induces hypophagia and thermogenesis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;301(6):R1625–R1634. doi: 10.1152/ajpregu.00334.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Resch J.M., Maunze B., Gerhardt A.K., Magnuson S.K., Phillips K.A., Choi S. Intrahypothalamic pituitary adenylate cyclase-activating polypeptide regulates energy balance via site-specific actions on feeding and metabolism. Am. J. Physiol. Endocrinol. Metab. 2013;305(12):E1452–E1463. doi: 10.1152/ajpendo.00293.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Krashes M.J., Shah B.P., Madara J.C., Olson D.P., Strochlic D.E., Garfield A.S., Vong L., Pei H., Watabe-Uchida M., Uchida N., Liberles S.D., Lowell B.B. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014;507(7491):238–242. doi: 10.1038/nature12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yi C-X., Sun N., Ackermans M.T., Alkemade A., Foppen E., Shi J., Serlie M.J., Buijs R.M., Fliers E., Kalsbeek A. Pituitary adenylate cyclase-activating polypeptide stimulates glucose production via the hepatic sympathetic innervation in rats. Diabetes. 2010;59(7):1591–1600. doi: 10.2337/db09-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yamamoto J., Imai J., Izumi T., Takahashi H., Kawana Y., Takahashi K., Kodama S., Kaneko K., Gao J., Uno K., Sawada S., Asano T., Kalinichenko V.V., Susaki E.A., Kanzaki M., Ueda H.R., Ishigaki Y., Yamada T., Katagiri H. Neuronal signals regulate obesity induced β-cell proliferation by FoxM1 dependent mechanism. Nat. Commun. 2017;8(1):1930. doi: 10.1038/s41467-017-01869-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tatsuno I., Morio H., Tanaka T., Uchida D., Hirai A., Tamura Y., Saito Y. Pituitary adenylate cyclase-activating polypeptide (PACAP) is a regulator of astrocytes: PACAP stimulates proliferation and production of interleukin 6 (IL-6), but not nerve growth factor (NGF), in cultured rat astrocyte. Ann. N. Y. Acad. Sci. 1996;805:482–488. doi: 10.1111/j.1749-6632.1996.tb17508.x. [DOI] [PubMed] [Google Scholar]
- 103.Lu N., Zhou R., DiCicco-Bloom E. Opposing mitogenic regulation by PACAP in sympathetic and cerebral cortical precursors correlates with differential expression of PACAP receptor (PAC1-R) isoforms. J. Neurosci. Res. 1998;53(6):651–662. doi: 10.1002/(SICI)1097-4547(19980915)53:6<651:AID-JNR3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 104.Mercer A., Rönnholm H., Holmberg J., Lundh H., Heidrich J., Zachrisson O., Ossoinak A., Frisén J., Patrone C. PACAP promotes neural stem cell proliferation in adult mouse brain. J. Neurosci. Res. 2004;76(2):205–215. doi: 10.1002/jnr.20038. [DOI] [PubMed] [Google Scholar]
- 105.Vaudry D., Gonzalez B.J., Basille M., Fournier A., Vaudry H. Neurotrophic activity of pituitary adenylate cyclase-activating polypeptide on rat cerebellar cortex during development. Proc. Natl. Acad. Sci. USA. 1999;96(16):9415–9420. doi: 10.1073/pnas.96.16.9415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Allais A., Burel D., Isaac E.R., Gray S.L., Basille M., Ravni A., Sherwood N.M., Vaudry H., Gonzalez B.J. Altered cerebellar development in mice lacking pituitary adenylate cyclase-activating polypeptide. Eur. J. Neurosci. 2007;25(9):2604–2618. doi: 10.1111/j.1460-9568.2007.05535.x. [DOI] [PubMed] [Google Scholar]
- 107.Manecka D-L., Boukhzar L., Falluel-Morel A., Lihrmann I., Anouar Y. PACAP signaling in neuroprotection. In: Reglodi D., Tamas A., editors. Pituitary Adenylate Cyclase Activating Polypeptide — PACAP. Cham: Springer International Publishing; 2016. pp. 549–561. [DOI] [Google Scholar]
- 108.Reglodi D., Tamas A., Jungling A., Vaczy A., Rivnyak A., Fulop B.D., Szabo E., Lubics A., Atlasz T. Protective effects of pituitary adenylate cyclase activating polypeptide against neurotoxic agents. Neurotoxicology. 2018;66:185–194. doi: 10.1016/j.neuro.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 109.Vaudry D., Falluel-Morel A., Basille M., Pamantung T.F., Fontaine M., Fournier A., Vaudry H., Gonzalez B.J. Pituitary adenylate cyclase-activating polypeptide prevents C2-ceramide-induced apoptosis of cerebellar granule cells. J. Neurosci. Res. 2003;72(3):303–316. doi: 10.1002/jnr.10530. [DOI] [PubMed] [Google Scholar]
- 110.Falluel-Morel A., Aubert N., Vaudry D., Basille M., Fontaine M., Fournier A., Vaudry H., Gonzalez B.J. Opposite regulation of the mitochondrial apoptotic pathway by C2-ceramide and PACAP through a MAP-kinase-dependent mechanism in cerebellar granule cells. J. Neurochem. 2004;91(5):1231–1243. doi: 10.1111/j.1471-4159.2004.02810.x. [DOI] [PubMed] [Google Scholar]
- 111.Reglodi D., Vaczy A., Rubio-Beltran E. MaassenVanDenBrink, A. Protective effects of PACAP in ischemia. J. Headache Pain. 2018;19(1):19. doi: 10.1186/s10194-018-0845-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ohtaki H., Nakamachi T., Dohi K., Aizawa Y., Takaki A., Hodoyama K., Yofu S., Hashimoto H., Shintani N., Baba A., Kopf M., Iwakura Y., Matsuda K., Arimura A., Shioda S. Pituitary adenylate cyclase-activating polypeptide (PACAP) decreases ischemic neuronal cell death in association with IL-6. Proc. Natl. Acad. Sci. USA. 2006;103(19):7488–7493. doi: 10.1073/pnas.0600375103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang Y., Malmberg A.B., Yaksh T.L., Sjölund B., Sundler F., Håkanson R. Capsaicin-evoked release of pituitary adenylate cyclase activating peptide (PACAP) and calcitonin gene-related peptide (CGRP) from rat spinal cord in vivo. Regul. Pept. 1997;69(2):83–87. doi: 10.1016/S0167-0115(97)02133-2. [DOI] [PubMed] [Google Scholar]
- 114.Nakamachi T., Ohtaki H., Yofu S., Dohi K., Watanabe J., Hayashi D., Matsuno R., Nonaka N., Itabashi K., Shioda S. Pituitary adenylate cyclase-activating polypeptide (PACAP) type 1 receptor (PAC1R) co-localizes with activity-dependent neuroprotective protein (ADNP) in the mouse brains. Regul. Pept. 2008;145(1-3):88–95. doi: 10.1016/j.regpep.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 115.Gillardon F., Hata R., Hossmann K-A. Delayed up-regulation of Zac1 and PACAP type I receptor after transient focal cerebral ischemia in mice. Brain Res. Mol. Brain Res. 1998;61(1-2):207–210. doi: 10.1016/S0169-328X(98)00202-2. [DOI] [PubMed] [Google Scholar]
- 116.Brifault C., Gras M., Liot D., May V., Vaudry D., Wurtz O. Delayed pituitary adenylate cyclase-activating polypeptide delivery after brain stroke improves functional recovery by inducing m2 microglia/macrophage polarization. Stroke. 2015;46(2):520–528. doi: 10.1161/STROKEAHA.114.006864. [DOI] [PubMed] [Google Scholar]
- 117.Dejda A., Sokołowska P., Nowak J.Z. Neuroprotective potential of three neuropeptides PACAP, VIP and PHI. Pharmacol. Rep. 2005;57(3):307–320. [PubMed] [Google Scholar]
- 118.Silveira M.S., Costa M.R., Bozza M., Linden R. Pituitary adenylyl cyclase-activating polypeptide prevents induced cell death in retinal tissue through activation of cyclic AMP-dependent protein kinase. J. Biol. Chem. 2002;277(18):16075–16080. doi: 10.1074/jbc.M110106200. [DOI] [PubMed] [Google Scholar]
- 119.Rőth E., Wéber G., Kiss P., Horváth G., Tóth G., Gasz B., Ferencz A., Gallyas F., Jr, Reglődi D., Rácz B. Effects of PACAP and preconditioning against ischemia/reperfusion-induced cardiomyocyte apoptosis in vitro. Ann. N. Y. Acad. Sci. 2009;1163:512–516. doi: 10.1111/j.1749-6632.2008.03635.x. [DOI] [PubMed] [Google Scholar]
- 120.Laszlo E., Juhasz T., Varga A., Czibere B., Kovacs K., Degrell P., Horvath G., Jancso G., Szakaly P., Tamas A., Reglodi D. Protective effect of PACAP on ischemia/reperfusion-induced kidney injury of male and female rats: gender differences. J. Mol. Neurosci. 2018;68(3):408–419. doi: 10.1007/s12031-018-1207-y. [DOI] [PubMed] [Google Scholar]
- 121.Ferencz A., Kiss P., Weber G., Helyes Z., Shintani N., Baba A., Reglodi D. Comparison of intestinal warm ischemic injury in PACAP knockout and wild-type mice. J. Mol. Neurosci. 2010;42(3):435–442. doi: 10.1007/s12031-010-9357-6. [DOI] [PubMed] [Google Scholar]
- 122.Ferencz A., Weber G., Helyes Z., Hashimoto H., Baba A., Reglodi D. Presence of endogenous PACAP-38 ameliorated intestinal cold preservation tissue injury. J. Mol. Neurosci. 2010;42(3):428–434. doi: 10.1007/s12031-010-9352-y. [DOI] [PubMed] [Google Scholar]
- 123.Wang G., Qi C., Fan G-H., Zhou H-Y., Chen S-D. PACAP protects neuronal differentiated PC12 cells against the neurotoxicity induced by a mitochondrial complex I inhibitor, rotenone. FEBS Lett. 2005;579(18):4005–4011. doi: 10.1016/j.febslet.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 124.Deguil J., Jailloux D., Page G., Fauconneau B., Houeto J-L., Philippe M., Muller J-M., Pain S. Neuroprotective effects of pituitary adenylate cyclase-activating polypeptide (PACAP) in MPP+-induced alteration of translational control in Neuro-2a neuroblastoma cells. J. Neurosci. Res. 2007;85(9):2017–2025. doi: 10.1002/jnr.21318. [DOI] [PubMed] [Google Scholar]
- 125.Reglődi D., Lubics A., Tamás A., Szalontay L., Lengvári I. Pituitary adenylate cyclase activating polypeptide protects dopaminergic neurons and improves behavioral deficits in a rat model of Parkinson’s disease. Behav. Brain Res. 2004;151(1-2):303–312. doi: 10.1016/j.bbr.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 126.Reglődi D., Tamás A., Lubics A., Szalontay L., Lengvári I. Morphological and functional effects of PACAP in 6-hydroxydopamine-induced lesion of the substantia nigra in rats. Regul. Pept. 2004;123(1-3):85–94. doi: 10.1016/j.regpep.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 127.Onoue S., Endo K., Ohshima K., Yajima T., Kashimoto K. The neuropeptide PACAP attenuates β-amyloid (1-42)-induced toxicity in PC12 cells. Peptides. 2002;23(8):1471–1478. doi: 10.1016/S0196-9781(02)00085-2. [DOI] [PubMed] [Google Scholar]
- 128.Rat D., Schmitt U., Tippmann F., Dewachter I., Theunis C., Wieczerzak E., Postina R., van Leuven F., Fahrenholz F., Kojro E. Neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) slows down Alzheimer’s disease-like pathology in amyloid precursor protein-transgenic mice. FASEB J. 2011;25(9):3208–3218. doi: 10.1096/fj.10-180133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cabezas-Llobet N., Vidal-Sancho L., Masana M., Fournier A., Alberch J., Vaudry D., Xifró X. Pituitary adenylate cyclase-activating polypeptide (PACAP) enhances hippocampal synaptic plasticity and improves memory performance in Huntington’s disease. Mol. Neurobiol. 2018;55(11):8263–8277. doi: 10.1007/s12035-018-0972-5. [DOI] [PubMed] [Google Scholar]
- 130.Bourgault S., Chatenet D., Wurtz O., Doan N.D., Leprince J., Vaudry H., Fournier A., Vaudry D. Strategies to convert PACAP from a hypophysiotropic neurohormone into a neuroprotective drug. Curr. Pharm. Des. 2011;17(10):1002–1024. doi: 10.2174/138161211795589337. [DOI] [PubMed] [Google Scholar]
- 131.Bourgault S., Vaudry D., Dejda A., Doan N.D., Vaudry H., Fournier A. Pituitary adenylate cyclase-activating polypeptide: Focus on structure-activity relationships of a neuroprotective Peptide. Curr. Med. Chem. 2009;16(33):4462–4480. doi: 10.2174/092986709789712899. [DOI] [PubMed] [Google Scholar]
- 132.Doan N.D., Bourgault S., Dejda A., Létourneau M., Detheux M., Vaudry D., Vaudry H., Chatenet D., Fournier A. Design and in vitro characterization of PAC1/VPAC1-selective agonists with potent neuroprotective effects. Biochem. Pharmacol. 2011;81(4):552–561. doi: 10.1016/j.bcp.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 133.Bourgault S., Vaudry D., Ségalas-Milazzo I., Guilhaudis L., Couvineau A., Laburthe M., Vaudry H., Fournier A. Molecular and conformational determinants of pituitary adenylate cyclase-activating polypeptide (PACAP) for activation of the PAC1 receptor. J. Med. Chem. 2009;52(10):3308–3316. doi: 10.1021/jm900291j. [DOI] [PubMed] [Google Scholar]
- 134.Ramos-Álvarez I., Mantey S.A., Nakamura T., Nuche-Berenguer B., Moreno P., Moody T.W., Maderdrut J.L., Coy D.H., Jensen R.T. A structure-function study of PACAP using conformationally restricted analogs: Identification of PAC1 receptor-selective PACAP agonists. Peptides. 2015;66:26–42. doi: 10.1016/j.peptides.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Onoue S., Waki Y., Nagano Y., Satoh S., Kashimoto K. The neuromodulatory effects of VIP/PACAP on PC-12 cells are associated with their N-terminal structures. Peptides. 2001;22(6):867–872. doi: 10.1016/S0196-9781(01)00411-9. [DOI] [PubMed] [Google Scholar]
- 136.Inooka H., Ohtaki T., Kitahara O., Ikegami T., Endo S., Kitada C., Ogi K., Onda H., Fujino M., Shirakawa M. Conformation of a peptide ligand bound to its G-protein coupled receptor. Nat. Struct. Biol. 2001;8(2):161–165. doi: 10.1038/84159. [DOI] [PubMed] [Google Scholar]
- 137.Neumann J.M., Couvineau A., Murail S., Lacapère J.J., Jamin N., Laburthe M., Class B. Class-B GPCR activation: is ligand helix-capping the key? Trends Biochem. Sci. 2008;33(7):314–319. doi: 10.1016/j.tibs.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 138.Lamine A., Létourneau M., Doan N.D., Maucotel J., Couvineau A., Vaudry H., Chatenet D., Vaudry D., Fournier A. Characterizations of a synthetic pituitary adenylate cyclase-activating polypeptide analog displaying potent neuroprotective activity and reduced in vivo cardiovascular side effects in a Parkinson’s disease model. Neuropharmacology. 2016;108:440–450. doi: 10.1016/j.neuropharm.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 139.Igarashi H., Ito T., Pradhan T.K., Mantey S.A., Hou W., Coy D.H., Jensen R.T. Elucidation of the vasoactive intestinal peptide pharmacophore for VPAC(2) receptors in human and rat and comparison to the pharmacophore for VPAC(1) receptors. J. Pharmacol. Exp. Ther. 2002;303(2):445–460. doi: 10.1124/jpet.102.038075. [DOI] [PubMed] [Google Scholar]
- 140.Gourlet P., Vandermeers-Piret M.C., Rathé J., De Neef P., Cnudde J., Robberecht P., Waelbroeck M. Vasoactive intestinal peptide modification at position 22 allows discrimination between receptor subtypes. Eur. J. Pharmacol. 1998;348(1):95–99. doi: 10.1016/S0014-2999(98)00133-2. [DOI] [PubMed] [Google Scholar]
- 141.Poujol de Molliens M., Létourneau M., Devost D., Hébert T.E., Fournier A., Chatenet D. New insights about the peculiar role of the 28-38 C-terminal segment and some selected residues in PACAP for signaling and neuroprotection. Biochem. Pharmacol. 2018;154:193–202. doi: 10.1016/j.bcp.2018.04.024. [DOI] [PubMed] [Google Scholar]
- 142.Beebe X., Darczak D., Davis-Taber R.A., Uchic M.E., Scott V.E., Jarvis M.F., Stewart A.O. Discovery and SAR of hydrazide antagonists of the pituitary adenylate cyclase-activating polypeptide (PACAP) receptor type 1 (PAC1-R). Bioorg. Med. Chem. Lett. 2008;18(6):2162–2166. doi: 10.1016/j.bmcl.2008.01.052. [DOI] [PubMed] [Google Scholar]
- 143.Wacker D., Stevens R.C., Roth B.L. How ligands illuminate GPCR molecular pharmacology. Cell. 2017;170(3):414–427. doi: 10.1016/j.cell.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kufareva I., Katritch V. Stevens, Raymond C.; Abagyan, R. Advances in GPCR modeling dvaluated by the GPCR Dock 2013 assessment: Meeting new challenges. Structure. 2014;22:1120–1139. doi: 10.1016/j.str.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Willard F.S., Bueno A.B., Sloop K.W. Small molecule drug discovery at the glucagon-like peptide-1 receptor. Exp. Diabetes Res. 2012;2012:709–893. doi: 10.1155/2012/709893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Takasaki I., Watanabe A., Yokai M., Watanabe Y., Hayakawa D., Nagashima R., Fukuchi M., Okada T., Toyooka N., Miyata A., Gouda H., Kurihara T. In silico screening identified novel small-molecule antagonists of PAC1 receptor. J. Pharmacol. Exp. Ther. 2018;365(1):1–8. doi: 10.1124/jpet.117.245415. [DOI] [PubMed] [Google Scholar]
- 147.Yu R., Zheng L., Cui Y., Zhang H., Ye H. Doxycycline exerted neuroprotective activity by enhancing the activation of neuropeptide GPCR PAC1. Neuropharmacology. 2016;103:1–15. doi: 10.1016/j.neuropharm.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 148.Chu A., Caldwell J.S., Chen Y.A. Identification and characterization of a small molecule antagonist of human VPAC(2) receptor. Mol. Pharmacol. 2010;77(1):95–101. doi: 10.1124/mol.109.060137. [DOI] [PubMed] [Google Scholar]
- 149.Chopra I., Roberts M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001;65(2):232–260. doi: 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bortolanza M., Nascimento G.C., Socias S.B., Ploper D., Chehín R.N., Raisman-Vozari R., Del-Bel E. Tetracycline repurposing in neurodegeneration: focus on Parkinson’s disease. J. Neural Transm. (Vienna) 2018;125(10):1403–1415. doi: 10.1007/s00702-018-1913-1. [DOI] [PubMed] [Google Scholar]
- 151.Yu R., Zheng L., Cui Y., Zhang H., Ye H. Doxycycline exerted neuroprotective activity by enhancing the activation of neuropeptide GPCR PAC1. Neuropharmacology. 2016;103:1–15. doi: 10.1016/j.neuropharm.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 152.Amaro R.E., Baudry J., Chodera J., Demir Ö., McCammon J.A., Miao Y., Smith J.C. Ensemble docking in drug discovery. Biophys. J. 2018;114(10):2271–2278. doi: 10.1016/j.bpj.2018.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Beuming T., Lenselink B., Pala D., McRobb F., Repasky M., Sherman W. Docking and virtual screening strategies for GPCR drug discovery. In: Filizola M., editor. G Protein-Coupled Receptors in Drug Discovery: Methods and Protocols. New York, NY: Springer; 2015. pp. 251–276. [DOI] [PubMed] [Google Scholar]


