Abstract
Mammalian cells metabolize glucose primarily for energy production, biomass synthesis and post-translational glycosylation; and maintaining glucose metabolic homeostasis is essential for normal physiology of cells. Impaired glucose homeostasis leads to hyperglycemia, a hallmark of diabetes. Chronically increased glucose in diabetes promotes pathological changes accompanied by impaired cellular function and tissue damage, which facilitates the development of cardiovascular complications, the major cause of morbidity and mortality of diabetic patients. Emerging roles of glucose metabolism via the hexosamine biosynthesis pathway (HBP) and increased protein modification via O-linked β-N-acetylglucosamine (O-GlcNAcylation) have been demonstrated in diabetes mellitus, and implicated in the development of diabetic cardiovascular complications. This review will discuss the biological outcomes of the glucose metabolism via the hexosamine biogenesis pathway and protein O-GlcNAcylation in regulating cellular homeostasis, and highlight the regulations and contributions of elevated O-GlcNAcylation to the pathogenesis of diabetic cardiovascular disease.
Keywords: Metabolic stress, diabetes, cardiovascular disease, HBP, O-GlcNAcylation
INTRODUCTION
Cardiovascular complications are the most prevalent causes of morbidity and mortality in patients with diabetes1, 2. According to the American Heart Association, diabetes mellitus is rising to be one of the major risk factors for cardiovascular diseases and cardiovascular death (https://www.heart.org/en/health-topics/diabetes/why-diabetes-matters/cardiovascular-disease--diabetes) The pathogenesis of the cardiovascular diseases in diabetes is complex, regulated by numerous factors via integrated molecular signaling pathways and cellular processes3. Insulin deficiency and hyperglycemia are the characteristic features of diabetes that trigger diabetic complications, as well as other metabolic disorders, such as hyperlipidemia and obesity that contribute to pathogenesis of cardiovascular disease. Glucose is the key energy-producing molecule that is metabolized via multiple pathways, which provides carbon source for cellular biosynthesis. Maintaining glucose metabolic homeostasis is essential for normal physiological functions of cells. In diabetes, chronic increase in glucose uptake and metabolism promotes pathological transformation that results in impaired cellular function and tissue damage. The emerging roles of glucose metabolism via the hexosamine biosynthesis pathways (HBP) and increased protein modification via O-linked β-N-acetylglucosamine (O-GlcNAcylation) have been linked to the pathogenesis of diabetic cardiomyopathy and vasculopathy, including diabetic microvascular complications, such as neuropathy, nephropathy, retinopathy, as well as diabetic macrovascular complications, including hypertension, atherosclerosis, vascular calcification and stroke. The precise mechanisms responsible for the abnormal glucose metabolism-induced diabetic vasculopathy and the roles of HBP and protein O-GlcNAcylation have not been fully elucidated. This review will summarize the biological function of the HBP and protein O-GlcNAcylation in maintaining cellular homeostasis, and highlight the findings that emphasize the regulations and contributions of increased O-GlcNAcylation to the pathogenesis of diabetic cardiovascular complications.
Hyperglycemia and Cardiovascular Disease in Diabetes Mellitus
Diabetes mellitus represents a complex metabolic disorder featuring high levels of blood sugar (hyperglycemia), insulin insufficiency and excessive oxidative stress. Cardiovascular disease remains a leading cause of morbidity and mortality in diabetes mellitus4. The major diabetic complications in the cardiovascular system are the diabetic cardiomyopathy and diabetic vasculopathy, including microvascular and macrovascular complications. It has been well established that hyperglycemia is a major cause of cardiovascular complications and diseases in diabetes. Insulin insufficiency results in acute increase in the blood glucose and ketone concentrations that cause diabetic ketoacidosis, a metabolic disorder associated with mortality in diabetic patients. On the other hand, chronically increased glucose uptake and metabolisms lead to impaired cellular function and damages of multiple tissues, including skeletal muscle, liver, kidney, adipose tissues and blood vessels. For instance, hyperglycemia has been shown to induce the expression of cytokines, growth factors and extracellular matrix proteins in renal cells, which in turn activates the renin-angiotensin system and induces hypertension in diabetic patients5. Increased glucose oxidation generates acetyl-CoA that promotes fatty acid synthesis, which leads to increased production of very-low-density lipoproteins and other lipoproteins in diabetic patients, thus contributing to increased cardiovascular disease in diabetic patients. In addition, hyperglycemia activates the polyol pathway, in which glucose is converted, by the enzyme aldose reductase, to sorbitol that is further oxidized to fructose6. Increased activation of polyol pathway induces oxidative stress via consumption of the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) and generation of the advanced glycation end products (AGEs), a heterogeneous group of nonenzymatic glycation products of proteins, lipids, and nucleic acids. Hyperglycemia-induced AGEs and enhanced expression and activation of the receptor for AGEs (RAGE) have been linked to increased synthesis of diacylglycerol and activation of the protein kinase C (PKC) signaling pathway that consequently increases activation of inflammatory and oxidative signals4, 6–9. Moreover, increased glucose metabolism via the hexosamine biosynthesis pathway and oxidative stress promote posttranslational protein modification via O-linked β-N-acetylglucosamine modification (O-GlcNAcylation) in diabetes10, 11, which contributes to diabetes cardiovascular disease12–14. Altogether, hyperglycemia-induced glycolytic flux, glucose oxidation, activation of polyol pathway, HBP and AGEs/RAGE-activated PKC signaling and metabolic signals may converge to increase oxidative stress in the cardiovascular systems, which promotes diabetic cardiovascular complications, including diabetes-specific microvascular disease that causes blindness, renal failure and nerve damage, as well as diabetes-accelerated atherosclerosis and vascular calcification, increased risk of myocardial infarction, stroke and limb amputation6, 7, 15–19.
Controlling glycemic levels remains the main treatment strategy for diabetes mellitus and diabetic cardiovascular disease. The long-term Diabetes Control and Complications Trial (DCCT) and the Epidemiology of Diabetes Interventions and Complications (EDIC) follow-up study have demonstrated that intensive control of glucose delays the onset and slows the progression of diabetic retinopathy, nephropathy and neuropathy in type 1 diabetes mellitus2, 5. Other studies did not seem to exclusively support the effects of aggressive blood glucose-lowering regimens on cardiovascular outcome in diabetes mellitus, particularly in patients with type 2 diabetes20–24. The United Kingdom Prospective Diabetes Study (UKPDS) has shown that glycemic control reduces diabetes-related complications in newly diagnosed patients with type 2 diabetes20. However, in the large randomized control trials, ADVANCE and ACCORD trials, aggressive glucose control on type 2 diabetes patients with a history of major macrovascular or microvascular disease only resulted in a small reduction in microvascular events, but did not reduce the risk of myocardial infarct and cardiovascular mortality and increased incidence of hypoglycemia2, 21, 22. Similarly, the Veterans Affairs Diabetes Trial (VADT) reported that intensive glucose lowering, compared with standard therapy, in patients with poorly controlled type 2 diabetes had no significant effect on the rates of major cardiovascular events, death, or microvascular complications among 1791 military veterans (median follow-up of 5.6 years)23. A recent report further demonstrated that intensive glucose control in patients with type 2 diabetes did not reduce the risks of major cardiovascular events or death, compared with those in the standard-therapy group, over a period of 15 years of follow-up of the VADT24. Accordingly, chronic hyperglycemia and metabolic disorder in diabetic conditions, particularly in type 2 diabetes, may cause pathologic changes in micro- and macro-vascular that cannot be reversed by further glucose lowering therapies.
It is encouraging that the 21-years follow-up studies of the randomized controlled multifactorial Steno-2 (a small study with a big heart) trial of type 2 diabetes mellitus have demonstrated increased lifespan, reduced cardiovascular events, mortality and microvascular complications25, 26. The beneficial effects in the Steno-2 trial were achieved with multifactorial and target-driven treatment including glucose control combining with individually targeting diet, exercise, blood pressure and lipid lowering25, 26, suggesting that it is critical to understand the regulation of the glucose metabolism and its interactions with other metabolic abnormalities in the development of cardiovascular diseases in diabetes.
Glucose Metabolism and Protein O-GlcNAcylation
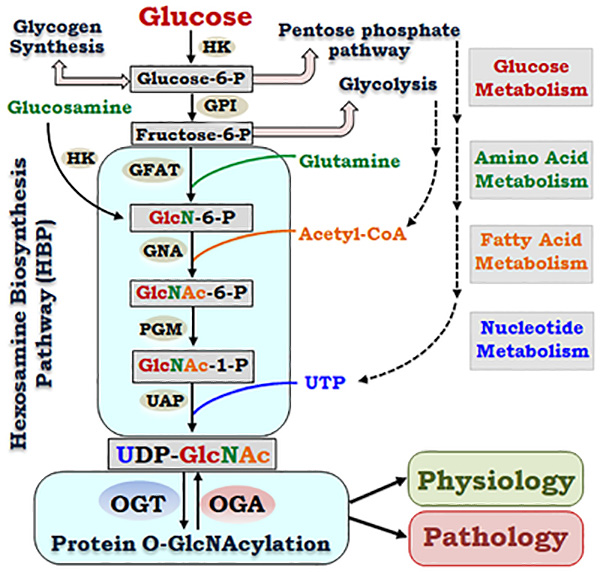
Cellular metabolisms encompass chemical reactions that generate energy and produce essential metabolic molecules, such as lipids, amino acids, nucleic acids and carbohydrates, required for cellular function, growth and reproduction. Under normal conditions, intracellular metabolic pathways cross talk and coordinate to maintain cellular homeostasis in response to environmental cues27. Glucose is the major intracellular energy-producing molecule and provides a carbon source for cellular biosynthesis. Therefore, maintaining glucose metabolic homeostasis is essential for normal physiological functions and survival of cells. Upon entering cells via the glucose transporter, the key first step of intracellular glucose metabolism is the conversion of glucose into glucose 6-phosphate (glucose-6-P, Figure 1), which is catalyzed by the hexokinase (HK) that uses adenosine triphosphate (ATP) as the phosphoryl donor28. Glucose-6-P is the substrate for the synthesis of glycogen, a storage form of glucose polymers, which can be broken down to generate glucose when needed. Glucose-6-P can also be utilized via the pentose phosphate pathway (PPP) that generates ribose-5-phosphate for DNA and RNA synthesis and produces NADPH, which is involved in regulating the cellular redox status and the synthesis of lipids and fatty acids29. As the oxidative reactions of the PPP are inhibited by NADPH, increased use of NADPH by aldose reductase-driven polyol pathway may promote the glucose flux via the PPP.
Figure 1. Glucose metabolism via hexosamine biosynthesis pathway links amino acid, fatty acid and nucleotide metabolisms to regulate cell physiology and pathology via protein O-GlcNAcylation.
In addition to glycolysis, glycogen synthesis and pentose phosphate pathway, glucose metabolism generates UDP-GlcNAc via the hexosamine biosynthesis pathway (HBP). The HBP utilizes a series of enzymes and substrates, which integrates glucose metabolism to amino acid metabolism, fatty acid metabolism and nucleotide metabolism. Dynamically regulated by the two enzymes, OGT and OGA, that catalyze the addition and removal of GlcNAc moiety on proteins respectively, O-GlcNAcylation affects a variety of cellular functions under physiological and pathological conditions. (HK: Hexokinase. GPI: Glucose-6-phosphate isomerase. GFAT: Glucosamine-fructose-6-phosphate aminotransferase. GNA: Glucosamine 6-phosphate N-acetyltransferase. PGM: Phospho-acetylglucosamine mutase, UAP: UDP-N-acetylglucosamine pyrophosphorylase. OGT: O-GlcNAc transferase. OGA: O-GlcNAcase)
Glycolysis is the major glucose metabolism pathway, which converts fructose-6-P, derived from glucose-6-P by the glucose-6-phosphate isomerase (GPI), into pyruvate in the cytoplasm and produces ATP. Oxidation of pyruvate produces acetyl-CoA, which enters the tricarboxylic acid (TCA) cycle to generate more ATPs in the mitochondria. Pyruvate-derived acetyl-CoA can also be used to produce fatty acids, and acetyl-glucosamine in the hexosamine biosynthesis pathway. Glucose metabolism via the HBP is catalyzed by a series of enzymes, which metabolize glucose to generate uridine-diphosphate-β-D-N-acetylglucosamine (UDP-GlcNAc), an active sugar donor for glycosylation, including the modification by O-linked β-N-acetylglucosamine (O-GlcNAcylation)30. The HBP pathways utilize a variety of metabolites that integrate glucose metabolism with metabolisms of amino acids, fatty acids and nucleotides (Figure 1). Glucose metabolism through the HBP is required to sustain sufficient growth factor signaling and glutamine uptake to support cell growth and survival31.
O-GlcNAcylation is a posttranslational protein modification that is different from the classic N-glycosylation and O-glycosylation, as it is a dynamic modification on serine or threonine residues of proteins by a single sugar moiety GlcNAc. O-GlcNAcylation is functionally analogous to phosphorylation, a common posttranslational modification that regulates many cellular signals, as O-GlcNAcylation and phosphorylation may occur on the same or adjacent serine or threonine residues on many proteins32. While phosphorylation is regulated by a large number of kinases and phosphatases, O-GlcNAcylation is tightly controlled by only two specific enzymes. The O-GlcNAc transferase (OGT) catalyzes the addition of the GlcNAc moiety to the hydroxyl group of the serine or threonine residues on the target proteins; and the O-GlcNAcase (OGA) removes the O-GlcNAc from the modified targets.
Three forms of OGT proteins generated from alternative RNA splicing activity are identified: the nucleocytoplasmic OGT (ncOGT), mitochondrial OGT (mOGT), and a shorter form of OGT (sOGT)33. While the mitochondrial-targeting domain determines the specific subcellular localization of the mOGT, the different numbers of tetratricopeptide-repeat (TPR) domains on OGT mediate interactions of OGT with other proteins and their complex formation for specificity and temporal activation of the enzyme34–36. Two OGA alternative splicing variants, a larger cytoplasmic isoform and a smaller nuclear isoform37, have been implicated in spatial distribution and function of OGA variants. The interplays of the OGT and OGA, in different cells and subcellular compartments, dynamically modulate O-GlcNAcylation homeostasis in response to changes in nutrients and environmental cues, thus regulating multiple cellular signals and functions in different tissues13, 14, 38. Protein O-GlcNAcylation plays crucial roles in maintaining normal cellular functions; whereas disrupted O-GlcNAcylation homeostasis contribute to the pathogenesis of diseases, especially chronic diseases such as diabetes and cardiovascular disease39–41.
O-GlcNAcylation and Diabetic Cardiovascular Disease
Emerging evidence has linked the glucose metabolism via HBP and protein modification by O-GlcNAcylation to the pathogenesis of diabetic cardiovascular disease. Protein O-GlcNAcylation, serving as an environmental nutrient and stress sensor, modulates stability, cellular localization and function of many nuclear and cytoplasmic proteins in response to glucose levels38 and other environmental signals, such as ischemia, hypoxia, oxidative stress, osmotic pressure, and ultraviolet light42. Thus, hyperglycemia and other abnormal metabolisms in diabetes may synergistically induce elevation of O-GlcNAcylation via increased HBP flux, thus promoting pathogenesis of cardiovascular disease.
• O-GlcNAcylation and diabetic cardiomyopathy
Hyperglycemia and impaired insulin signaling pathways in the heart promote diabetic cardiomyopathy, the diastolic dysfunction of the heart due to structural and functional abnormality in myocardium in the absence of vascular dysfunction, such as hypertension, coronary arterial disease and hyperlipidemia43, 44. Clinical and experimental studies have established the function of HBP and protein O-GlcNAcylation in regulating diabetes cardiomyopathy12–14. Increased O-GlcNAcylation via inhibition of OGA reduces hypoxic-induced cell death of cardiomyocytes in vitro; whereas reduced O-GlcNAcylation by overexpression of OGA significantly increased hypoxic-induced cell death12. In ischemia-reperfusion model of heart failure, acute increase in O-GlcNAcylation protects cardiomyocytes from oxidative stress-induced calcium overload and structural damage45–47. In contrast to the protective effects of the acute increase in O-GlcNAcylation on cardiac injury, chronic elevation of O-GlcNAcylation in diabetes is associated with impaired calcium handling and contractile properties, as well as mitochondria dysfunction and cardiac hypertrophy. For example, increased OGT and decreased mitochondrial-specific OGA observed in isolated heart mitochondria from diabetic rats, are accompanied with increased protein O-GlcNAcylation48. Inhibition of OGT or OGA activity in rat cardiomyocytes affects energy production, mitochondrial membrane potential, and mitochondrial oxygen consumption, suggesting that dysregulation of O-GlcNAcylation promotes mitochondrial dysfunction thus contributing to cardiomyopathy in diabetes48. Additionally, increase in nuclear O-GlcNAcylation impairs cardiomyocyte calcium cycling and cardiomyocyte function, which can be rescued by overexpression of OGA that reduces O-GlcNAcylation49. These data support that the O-GlcNAcylation-regulated calcium flux is critical for cardiomyocyte function and the development of diabetic cardiomyopathy. Consistently, studies with diabetic mouse models have shown that inhibition of O-GlcNAcylation with adenovirus-mediated OGA overexpression improves calcium handling and contractile function of the diabetic heart50. As HBP integrates the glucose metabolism into metabolism of fatty acids, amino acids and nucleotides, chronic elevation of O-GlcNAcylation not only disrupts the glucose homeostasis but also induces other metabolic disorders, which further promotes oxidative stress and generation of advanced glycation end products, leading to diabetic cardiomyopathy.
Collectively, these studies support the concept that acute increase in protein O-GlcNAcylation may serve as a stress sensor that triggers a protective mechanism for stress-induced cardiac injury, whereas chronically elevated O-GlcNAcylation in diabetes impairs normal function of cardiomyocytes, which leads to diabetic cardiomyopthy51.
• O-GlcNAcylation and diabetic vasculopathy
Hyperglycemia is the major cause of the pathogenesis and severity of diabetic vascularopathy, the complications in the blood vessels that affect multiple organ systems, including microvascular diseases and macrovascular diseases7. Microvascular complications are the damaging of small blood vessels in the eye (retinopathy), the kidney (nephropathy) and the peripheral neural system (neuropathy)3, 52. Macrovascular complications include coronary artery disease, peripheral arterial disease, and stroke. Diabetic vasculopathy leads to blindness, limb amputation, kidney failure, atherosclerosis, stroke and heart failure, which increases the incidence of morbidity and mortality of diabetic patients4, 53, 54.
Emerging studies have linked increased O-GlcNAcylation to diabetic vasculopathy, including microvascular and macrovascular diseases. In chronic hyperglycemia conditions, elevation of O-GlcNAcylation promotes diabetic retinopathy, via blocking the neuroprotective effect of insulin and inducing apoptosis in retinal neurons and retinal pericytes55, 56. High glucose-induced O-GlcNAcylation stimulates mesangial cell lipogenesis and fibrosis, which contributes to diabetic nephropathy57. The detrimental effects of elevation of O-GlcNAcylation have also been documented in the macrovasculature, although acute increase in O-GlcNAcylation appears to be protective for injury-induced vascular inflammatory and neointimal formation58. In the hypertension rat models, increased vascular O-GlcNAcylation was associated with impaired vasodilator activity and hypertension59. In addition, increased HBP flux, OGT and O-GlcNAcylation are identified in patients of idiopathic pulmonary arterial hypertension60. Inhibition of O-GlcNAcylation by OGT knockdown in the culture pulmonary smooth muscle cells (PASMC) blocks cell proliferation, supporting the contribution of the increased OGT and O-GlcNAcylation-induced PASMC proliferation in the development of PAH60. Nonetheless, a causative role of O-GlcNAcylation in regulating the major diabetic macrovascular diseases, such as atherosclerosis, has yet to be determined.
• O-GlcNAcylation and diabetic vascular calcification
Increased incidence of atherosclerosis is observed in human subjects with diabetes, which is associated with higher levels of arterial calcification61, 62. In addition to the calcification identified in the atherosclerotic intimal lesions, medial calcification is also prevalent in the peripheral arteries in diabetic patients without coronary arterial disease63–65. Importantly, vascular calcification promotes coronary arterial disease and peripheral arterial disease, which greatly increases the risk of cardiovascular disease and cardiovascular death in diabetes63, 66.
Hyperglycemia induces oxidative stress, AGEs/RAGE and activation of inflammation signaling in the vascularture4, 8, 9, which all have been shown to promote vascular calcification in vitro and in vivo9, 67, 68. We and others have reported that increased oxidative stress is a major cause of vascular calcification67–71. The mechanisms underlying redox signaling-regulated normal vascular function and oxidative stress-induced vascular cell dysfunction that promotes vascular calcification have been discussed in our recent review72. As both high glucose and oxidative stress increase O-GlcNAcylaton10, 11, it is likely that increased O-GlcNAcylation may mediate the effects of hyperglycemia and oxidative stress-induced vascular calcification. Consistently, elevation of O-GlcNAcylation and increased vascular calcification are evident in human and mouse diabetic vascular lesions40, 63, 73–75, suggesting a close correlation of increased O-GlcNAcylation with vascular calcification in diabetes.
Our recent studies have uncovered a novel function of O-GlcNAcylation in promoting diabetic vascular calcification75. With the low-dose streptozocin (STZ)-induced mouse model of diabetes, we have demonstrated a causative link of increased O-GlcNAcylation with vascular calcification in the diabetic vasculature75. Diabetic vascular calcification promoted by elevated O-GlcNAcylation is associated with increased vascular stiffness in mice75. By inhibition of OGA, we have determined a direct effect of increased O-GlcNAcylation on inducing calcification of vascular smooth muscle cells (VSMC) in vitro. Furthermore, knockdown of OGT inhibits high glucose and oxidative stress-induced O-GlcNAcylation and concurrently attenuates VSMC calcification. VSMC are the key vascular cells that undergo calcification in the atherosclerosis-related intimal calcification or atherosclerosis-independent medial calcification, both commonly observed in the diabetic arteries63–65. Accordingly, targeting OGT and O-GlcNAcylation in VSMC can be an effective approach to inhibit vascular calcification in diabetes. Further studies with smooth muscle cell-specific OGT ablation animal models are warranted to determine a definitive role of OGT-mediated protein O-GlcNAcylation in the development of vascular calcification and other cardiovascular complications in diabetes in vivo.
Regulation of O-GlcNAcylation in Diabetes
Multifaceted regulatory mechanisms increase O-GlcNAcylation in diabetes. Upregulation of OGT and downregulation of OGA are observed in response to chronic hyperglycemia in diabetes, suggesting that the dysregulation of OGT and OGA expression contributes to the increased O-GlcNAcylation in diabetes34, 76. Insulin signaling has been implicated in upregulating OGT via activation of the PI3K signaling pathway77. It has been reported that the regulation of OGT and OGA occurs at the transcriptional level. Jones and colleagues have demonstrated that E2F1 negatively regulates Ogt and Mgea5 (OGA) gene expression in HEK293 and mouse embryonic fibroblasts78. In addition, E2F1 has recently been shown to promote hepatic gluconeogenesis and contributes to hyperglycemia during diabetes79. It is likely that E2F1 may regulate OGA/OGT protein expression via directly modulating the OGA/OGT transcription, as well as indirectly via hyperglycemia-activated signals in diabetes. As O-GlcNAc levels change rapidly in cells in response to extracellular environmental fluctuations, the dynamic and coordinated regulation O- GlcNAcylation by OGT and OGA may integrate multiple pathways via different mechanisms in addition to the transcriptional regulation34. Studies that support this later concept include the observations that insulin signaling also promotes OGT translocation from the nucleus to the cytoplasm, and its localization and activation in the lipid rafts in the plasma membrane77, 80, 81. In addition, phosphorylation of OGT by calcium and calmodulin-dependent protein kinase IV (CaMK IV) is critical for activation of OGT82. Furthermore, a regulatory feedback loop of OGT and OGA has been suggested in maintaining O-GlcNAcylation homeostasis, thus the overall O-GlcNAcylation levels can be determined by mutual regulation and direct interactions between OGA and OGT in response to glucose and other stimuli34. Taken together, these studies suggest that multiple mechanisms may regulate the transcription, expression, posttranslational modification, spatial distribution of OGT and OGA as well as their close interaction in response to metabolic stress in diabetes, thus increasing the intracellular O-GlcNAcylation levels.
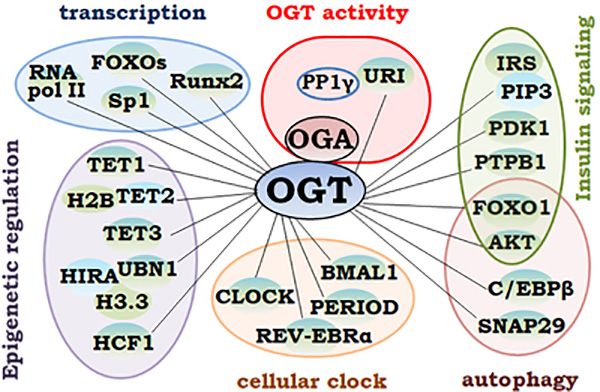
The UDP-GlcNAc concentrations modulate the OGT activities36, suggesting that increased O-GlcNAcylation in diabetes may be attributed, at least in part, to the increased HBP influx. Glucosamine has been shown to increase glucose production and expression of gluconeogenic enzymes, glucose 6-phosphatase and phosphoenolpyruvate carboxykinase. Glucosamine increases the O-GlcNAc transferase (OGT)-dependent protein O-GlcNAc level. Moreover, inhibition of OGT (by ST045849) decreases glucose production83. Of note, reduced UDP-GlcNAc levels due to glucose deprivation have also been associated with increased O-GlcNAcylation; and acute response to glucose starvation-induced O-GlcNAcylation may be linked to increased binding of OGT to its modified proteins84, 85. Given that the enzymatic activity of OGT can be regulated by its interacting proteins, OGT binding partners can modulate O-GlcNAcylation via regulating OGT activity. For example, OGT binds to the unconventiaonl prefolding RPB5 interactor (URI) and protein phosphatase 1γ, which regulates OGT activity86. Phosphorylation of URI inhibits OGT activity; while protein phosphatase1γ dephosphorylates URI and thus maintaining OGT activity (Figure 2). The spatiotemporal distribution of the adaptor proteins in different cellular compartments and the amount of the target proteins may determine biological outcomes of O-GlcNAc modification in a context-dependent manner. Consequently, cells with abnormal O-GlcNAc modification may discriminatively promote the signaling pathways that cause impaired cardiovascular functions and the development of cardiovascular diseases in diabetes.
Figure 2. OGT interaction with various targeting proteins.
1) regulates its enzymatic activity (top center); and 2) forms complexes that lead to O-GlcNAcylation of a variety of proteins that modulate molecular and cellular functions. The OGT-interacted and O-GlcNAc-regulated molecules involved in epigenetic gene regulation, transcription, circadian clock and autophagy, as well as intracellular signaling pathways, including the insulin signaling pathways, are shown and described in the text.
O-GlcNAcylation-regulated Molecular and Cellular Signals
The distinct effects of acute and chronic elevation of O-GlcNAcylation on cardiovascular injury and the development of cardiovascular disease support the spatiotemporal control of O-GlcNAcylation on important molecules that mediate cellular physiology and pathology. As a key ubiquitous nutrient and stress sensor, the protein O-GlcNAcylation via HBP regulates multiple cellular pathways in all cell types, in a cell autonomous manner as well as through cell-cell cross talks. The impact of O-GlcNAcylation on insulin resistance serves as a good model to illustrate how the elevation of O-GlcNAcylation in pancreatic β cells, hepatic cells and adipose cells not only interferes with normal cellular function but also contributes to systemic metabolic dysregulation76, 87, which further enhances diabetic cardiovascular complications88, 89.
• O-GlcNAc regulation of insulin resistance
Hyperglycemia is positively associated with elevated HBP and O-GlcNAcylation in diabetic animals, which have also been found associated with insulin resistance, another hallmark of diabetes75, 88–90. The impacts of spatiotemporal regulation of protein O-GlcNAcylation on insulin resistance in diabetes have been documented at multiple levels. Increased HBP flux by overexpression of GFAT (glutamine: fructose-6-phosphate amidotransferase, Figure 1) causes insulin resistance in mice91 and decreases insulin sensitivity in rat fibroblasts92. In contrast, inhibition of the HBP flux by GFAT inactivation blocks hyperglycemia-induced insulin resistance93. Consistent with the observations that increased O-GlcNAcylation promotes insulin resistance88, 89, inhibition of O-GlcNAcylation by overexpression of OGA improves glucose tolerance and insulin sensitivity in diabetic mice87. These studies support the important role of the HBP and O-GlcNAcylation in regulating glucose intolerance and insulin resistance in diabetes.
Increased O-GlcNAcylation is associated with hyperglycemia-induced pancreatic β cell death76. Pancreatic β cells are the primary regulator of glucose flux by sensing the plasma glucose concentration and secreting appropriate amounts of insulin to direct glucose uptake and storage. Inhibition of the HBP flux and O-GlcNAcylation in pancreatic β cells attenuates hyperglycemia-induced apoptosis of the pancreatic β cells in vivo76, supporting the role of O-GlcNAcylation in mediating hyperglycemia-induced β cell death and insulin resistance.
O-GlcNAcylation can trigger hepatic gluconeogenesis, via direct O-GlcNAc modification on the central transcriptional regulator of gluconeogenic genes, the transducer of regulated cyclic adenosine monophosphate response element–binding protein 2, which inhibits its phosphorylation and promotes its cytoplasm to nuclear translocation87. Furthermore, increased HBP flux alters the activity and translocation of protein kinase C isoforms. In hepatocytes, O-GlcNAc modifications are identified on serine or threonine residuals at similar positions in several PKC isozyme; and increased O-GlcNAcylation of PKC alpha correlates negatively with PKCalpha activity94. Considering that activation of PKC family of enzymes is an important regulator of insulin signaling pathway and oxidative status, O-GlcNAcylation of PKC isoforms should modulate their activity in a cell-dependent manner.
In addition, direct O-GlcNAc modification has been identified on several key cellular mediators in the insulin signaling pathways, including the insulin receptor substrate (IRS) and the insulin-regulated signals, such as protein kinase B/Akt, protein-tyrosine phosphatase 1B (PTP 1B), forkhead box protein O1 (FOXO1) and phosphoinositide-dependent kinase-1 (PDK-1)89, 95–97. Insulin also induces the recruitment of OGT from the nucleus to the plasma membrane through its interaction with the phosphatidylinositol 3,4,5-trisphosphate (PIP3), thus catalyzing O-GlcNAc modification of the insulin receptor and insulin signaling molecules80. O-GlcNAc modification on these key insulin signals inhibits insulin-activated phosphorylation, translocation and activity of these molecules, leading to insulin resistance.
• O-GlcNAc regulation of cellular functions
Cellular microenvironment plays a key role in regulating the O-GlcNAc levels in a specific cell type, thus determining the unique cellular responses. Peripheral circadian clocks modulate cell homeostasis by coupling the autonomous intracellular rhythms with changes in extracellular environment98, 99. Direct modification by O-GlcNAc has been identified on several key transcriptional regulators of the circadian rhythm, including BMAL1, CLOCK and PERIOD, which prevents ubiquitination and hence prolongs the stability of these transcriptional regulators100–102. Increased O-GlcNAcylation of these key cellular circadian regulators disrupts peripheral circadian clocks98, 99. In addition, a recent study uncovers an important role of the clock regulator REV-ERBα as an OGT binding partner that protects cytoplasmic OGT from degradation and facilitates O-GlcNAcylation of cytosolic and nuclear proteins103. Accordingly, increased O-GlcNAcylation coupled with cellular circadian clock may upregulate signaling pathways that promote cardiovascular disease in diabetes.
Similarly, O-GlcNAcylation regulates autophagy104, 105, an important cellular process that contribute to maintain cellular homeostasis in response to changes of nutrients, via recycling intracellular energy or removing cytotoxic proteins and damaged organelles. Inhibition of O-GlcNAcylation on an autophagy vesicle protein SNAP-29 promotes autophagosomes and endosomes/lysosomes fusion and autophagic flux, and facilitates autophagic degradation of protein aggregates104. In addition, OGT/O-GlcNAcylation regulates expression of autophagy genes and shapes autophagy structures via Akt/FOXO signaling106. It remains to be determined whether direct O-GlcNAcylation of AKT and FOXO transcription factors affects autophagy activation, flux, and lysosomal fusion, thereby contributing to diabetic cardiomyopathies107–109. Of note, the expression of the major autophagy transcription factors, C/EBPβ and FOXO1, are highly rhythmically regulated in a cell-autonomous manner110, 111. Coincidently, these transcriptional factors are also modified by O-GlcNAcylation96, 112. Therefore, O-GlcNAcylation may represent a common regulation of the nutrient sensing cellular process, such as autonomous circadian rhythm and autophagy, which contributes to the temporal compartmentalization of cellular signaling in response to metabolic abnormality in diabetes.
• GlcNAc and epigenetic regulation of gene expression
O-GlcNAc modification on targeting proteins is regulated by OGT binding to its adaptors selectively, via its tetratricopeptide repeat domains. The binding of OGT to a variety of partners enables the formation of distinct functional complexes. The binding of OGT with ubinuclein1 (UBN1), for example, mediates O-GlcNAcylation of histone proteins that directly modulates chromatin structure and hence cell survival113. UBN1 binds with the calcineurin binding protein 1 and the histone cell cycle regulator (HIRA) to form the histone chaperone HIRA complex that deposits histone variant H3.3 on chromatin in a replication-independent manner114. Active genes often have histone H3.3 deposited115. Ablation of HIRA-mediated chromatin assembly and histone H3.3 nucleosome causes hypertrophy and increased susceptibility to oxidative stress in the HIRA deficiency skeletal muscle cells and cardiomyocytes116, 117. The binding of OGT to UBN1 recruits OGT to the HIRA complex and thus modifies HIRA on serine 231, which promotes the assembly of the HIRA-H3.3 complex and H3.3 nucleosome. OGT inhibition or expression of the HIRA serine 231 mutant delays premature cellular senescence in primary human fibroblasts, whereas overexpression of OGT accelerates senescence, supporting an important role of OGT-mediated O-GlcNAcylation in modulating chromatin structures that regulate cellular function and survival 113.
In addition, OGT has been found in the histone H3K4 methyltransferase complexes via binding to one of the key components, the host cell factor-1 (HCF-1), a transcriptional co-regulator of human cell-cycle progression118. HCF-1 undergoes proteolytic maturation in which any of six repeated sequences is cleaved. The tetratricopeptide-repeat domain of OGT binds to a proteolytic repeat at the carboxyl-terminal of HCF-1, which results in cleavage and proteolytic maturation of HCF-1 that directly promotes histone H3K4 trimethylaton118, an indicator for active transcription.
Although the precise mechanisms and the biological significance of the crosstalk between O-GlcNAcylation and DNA demethylation remains elusive, OGT has been reported to bind to the three known members in the ten-eleven translocation methylcytosine dioxygenase family (TET), which converts 5-methylcytosine to 5-hydroxymethylcytosine and regulates gene transcription. OGT binds to the C-terminal region of TET1 that stimulates TET1 demethylation activity and thus controlling the developmental fate of embryonic stem cells119. Recent studies have shown that TET1 binds a significant proportion of polycomb group target genes, supporting a role of TET1 in transcriptional repression120. Accordingly, OGT may repress gene transcription via TET1. In contrast, TET2 recruits OGT to the transcriptional start site, and thus modifying histone H2B by O-GlcNAcylation, the dual epigenetic modifications on both DNA and histones by TET2 and OGT coordinate the regulation of gene transcription121. On the other hand, OGT binding and O-GlcNAcylation of TET3 promotes its nuclear export, and thus inhibiting the formation of 5-hydroxymethylcytosine catalyzed by TET3122. Taken together, OGT binding and differential regulation of TET family proteins link glucose metabolism to DNA epigenetic modification. Considering the important role of TET family, particularly TET2 in cardiovascular disease123, it is likely that O-GlcNAcylation of these epigenetic regulators contributes to diabetic cardiovascular disease.
• O-GlcNAc regulation of signaling molecules in cardiovascular cells
As reviewed by Hart and colleagues14, 124, the function of O-GlcNAcylation in regulating gene transcription is documented in every step during transcription process, via direct O-GlcNAc modification on RNA polymerase II, transcription factors such as Sp1, FOXOs and RUNX2, and proteins involved in multiple essential transcriptional processes, including ubiquitination and methylation of histones, DNA demethylation by the TET proteins, and the binding timing of TATA-binding protein on promoters. Thus, increased O-GlcNAcylation may affect the overall transcriptional process as well as regulate specific transcription factors to modulate the transcription of disease-related signals. In addition, the finding of O-GlcNAcylation of ribosome proteins suggest a role of O-GlcNAcylation in regulating protein translation125. Nonetheless, posttranslational modification by O-GlcNAcylation represents an important molecular mechanism that affects stability, cellular localization, protein-protein interaction, and function of proteins. For instance, O-GlcNAcylation of transcription factor Sp1 affects Sp1 interaction with other transcription factors, thus regulating the transcriptional activity of Sp1126, including the intercellular adhesion molecule-1127 and the key angiogenic growth factor vascular endothelial growth factor A128. Therefore, dysregulation of multiple signaling pathways by increased O-GlcNAcylation at transcription, translation and posttranslational levels promotes insulin resistance and diabetic cardiovascular disease.
O-GlcNAc modification on serine and threonine residues on proteins may compete with phosphorylation on the same or adjacent sites that cause conformational changes of these proteins, thus affecting the activity, stability or cellular locations. O-GlcNAc modification on Akt, for example, affects Akt phosphorylation, and Akt signaling-activated translocation of the glucose transporter and glucose uptake in cardiomyocytes, thus increasing intracellular Ca2+ 129. Activation of Akt signaling promotes coronary endothelial nitric oxide synthase130, and the increased nitric oxide mediates coronary vasodilation, myocardial substrate flexibility and energy homeostasis131. Thus, impaired Akt activation by O-GlcNAcylation may not only affect glucose handling in the cardiac cells, but also inhibit insulin-stimulated coronary endothelial nitric oxide synthase activity and nitric oxide production, leading to impaired endothelial/cardiomyocyte coupling, cardiac stiffness and diastolic dysfunction129. Consistently, inhibition of O-GlcNAcylation by OGA overexpression in endothelial cells increases capillary density and improves endothelium-dependent relaxation in diabetes132.
In VSMC, O-GlcNAc modification on Akt promotes Akt phosphorylation and vascular calcification in diabetes75. Different from the previously identified O-GlcNAc modification sites at Akt threonine (Thr) 305/312 that inhibit the phosphorylation of Akt at Thr 308 in breast cancer cells133, O-GlcNAc modification on Akt at Thr 430/479 in VSMC does not affect AKT phosphorylation at Thr 308 but enhances Akt phosphorylation/activation at serine 47375. Therefore, the regulation of O-GlcNAcylation on Akt activity appears to be cell type-specific and depend on the sites of modification. This may explain the observations that Akt activation is decreased in diabetic cardiomyocytes and myotubes134, 135, while VSMC in diabetic models exhibits sustained activation of Akt after chronic hyperglycemia75. O-GlcNAcylation-induced Akt activation in VSMC upregulates the osteogenic transcription factor Runx2, the master regulator for VSMC calcification in vitro and promotes vascular calcification in vivo67, 68, 136. Chronic hyperglycemia-induced Akt and Runx2 expression in the diabetic vasculature is associated with increased O-GlcNAcylation and vascular calcification75. O-GlcNAc modification of Runx2 has also been reported in bone marrow mesenchymal stem cells137, but the functional consequence of such modification remains unclear. Further investigations to determine whether Runx2 is modified by O-GlcNAc in VSMC and how O-GlcNAcylation of Runx2 contributes to its osteogenic function will provide new molecular insights into the pathogenesis of diabetic vascular disease and strategies for prevention and therapy.
Taken together, elevation of O-GlcNAcylation impairs the cellular nutrient sensing process, such as circadian clock and/or autophagy, which spatiotemporally modulates the expression, stability, cellular localization and function of diverse signaling molecules that promote the development of insulin resistance and diabetic cardiovascular disease. As chronic hyperglycemia is also linked to abnormal metabolisms of lipids/fatty acids and amino acids in diabetes, the elevated protein O-GlcNAcylation likely interacts with other posttranslational modification systems, such as phosphorylation, ubiquitination, acetylation, and methylation34. The disrupted interplays in a cell specific manner may impair protein stability and function, thus leading to impaired cellular function and tissue damage that promotes diabetic cardiovascular disease.
Conclusions and Prospective
Serum glucose level is the primary biomarker for diabetes, and glucose-lowering regimen remains the major treatment strategy for diabetes and diabetic cardiovascular disease. However, chronic hyperglycemia and metabolic stress in diabetic conditions, particularly in type 2 diabetes, may cause pathologic changes that cannot be reversed by simple control of glucose. Pantalone et al found that the current diagnostic marker, hemoglobin A1c (HbA1c), measured at the time of diabetes diagnosis had no apparent effects on longitudinal changes in cardiovascular complication138. Emerging evidence suggest that impaired glucose intake and metabolism may integrate with abnormal metabolisms of lipids/fatty acids, amino acids and nucleotides in diabetes, leading to elevation of O-GlcNAcylation. Recent studies have detected increased O-GlcNAc in erythrocytes of pre-diabetic and diabetic patients139, 140. In a young adult population, Myslicki JP et al identified a positive association between O-GlcNAc and HOMA-IR (insulin resistance), whereas no correlation was found between HbA1c and HOMA-IR, suggesting that O-GlcNAc may serve as a potential sensitive marker of early metabolic dysfunction, compared to HbA1c, in the young population free of clinically relevant pathologies141.
Glucose metabolism via HBP and elevation of O-GlcNAcylation have emerged to be an important regulating axis in promoting insulin resistance and the development of cardiovascular disease. Increased HBP flux and O-GlcNAcylation promotes glucose tolerance and insulin resistance, possibly via regulating pancreatic β cell death, hepatic gluconeogenesis, and direct O-GlcNAc modification on several key cellular mediators in the insulin signaling pathways. Acute elevation of O-GlcNAcylation appears to protect stress-induced cardiovascular inflammation and injury. In contrast, chronic elevation of O-GlcNAcylation in diabetes causes impaired calcium handling and contractile properties, as well as mitochondria dysfunction and cardiac hypertrophy. Increased O-GlcNAcylation has been associated with hyperglycemia and oxidative stress that promotes diabetic vascular calcification and vascular stiffness in mice. As a causative regulation of O-GlcNAcylation on the development of major diabetic macrovascular disease, such as atherosclerosis and vascular calcification, has yet to be determined, future investigation in this area with the use of vascular cell-specific OGT ablation animal models will provide important molecular and mechanistic insights into OGT-mediated protein O-GlcNAcylation in the development of cardiovascular complications in diabetes.
The elevation of O-GlcNAcylation in diabetes is mediated by multifaceted regulatory mechanisms. Both the HBP flux and the concentration of UDP-GlcNAc determine the levels of O-GlcNAcylation (Figure 1). As OGT/OGA is the only pair of enzymes that dynamically regulate O-GlcNAcylation, dysregulation of the expression of OGT and OGA appears to modulate the increased O-GlcNAcylation in diabetes. However, the rapid changes of O-GlcNAcylation levels in response to environmental fluctuations also support the involvement of dynamic posttranslational regulatory mechanisms, including OGT phosphorylation, translocation, OGA/OGT interaction, and most importantly OGT interaction with its targeting proteins (Figure 2).
O-GlcNAcylation-regulated molecular and cellular functions have been demonstrated in multiple levels. At the cellular level, increased O-GlcNAcylation is linked to nutrient sensing cellular process, such as peripheral cellular circadian clock, autophagy and cell death. It is reasonable to assume that O-GlcNAcylation acts as a common sensor for these cellular processes to maintain cellular homeostasis in response to extracellular changes. However, chronic elevation of O-GlcNAcylation and other metabolic abnormalities may cause epigenetic changes that are not “reversible”, thus leading to tissue damage. In fact, dysregulation of O-GlcNAc modification on many cytoplasmic and nuclear proteins has been linked to altered expression, stability, translocation, protein-protein interaction and function of key regulators that promote insulin resistance and the development of cardiovascular disease. In addition, O-GlcNAcylation may interplay with other protein posttranslational modifications, including phosphorylation, ubiquitination, acetylation and methylation, to modulate protein stability and function. Therefore, understanding the spatiotemporal regulation of O-GlcNAcylation in specific cardiovascular cells and subcellular compartments under physiological and pathological conditions is critical to uncovering the mechanisms underlying O-GlcNAcylation-regulated pathogenesis of cardiovascular diseases in diabetes.
O-GlcNAcylation appears to be the “integrated sensor” for metabolic stress in diabetes, including oxidative stress, insulin resistance and other metabolic abnormality, and a potential sensitive marker for early detection of diabetes and metabolic dysfunction. Large scale clinical studies are warranted to validate whether O-GlcNAc may represent a promising biomarker for diabetes and for prediction of the development of diabetic cardiovascular complications. Further development of sensitive, reliable and cost-effective tools and approaches for studying O-GlcNAcylation as well as their clinical applications should facilitate the translation of basic science discovery to clinic. Since chronically elevated-O-GlcNAcylation promotes the development of diabetic cardiovascular disease, it is plausible to explore O-GlcNAcylation as a target for prevention and therapy. In this regard, specifically cellular/tissue deliver of low toxic and potent antagonists for OGT/O-GlcNAcylation is critical. As we are uncovering the cell-specific O-GlcNAcylation-regulated signaling network and disease-specific O-GlcNAc modification proteins and mechanisms, new strategies and targets will emerge for precision therapies that prevent and treat cardiovascular disease in diabetes.
Supplementary Material
HIGHLIGHTS.
This review discusses glucose metabolism via the hexosamine biogenesis pathway and protein O-GlcNAcylation in regulating cellular homeostasis and highlights:
The emerging role of chronic elevation of O-GlcNAcylation in promoting diabetic cardiovascular disease.
The multifaceted regulatory mechanisms underlying upregulation of O-GlcNAcylation in diabetes.
The spatiotemporal regulation of increased O-GlcNAcylation on molecular and cellular function, including transcription, translation, nutrient sensing cellular processes, such as peripheral cellular circadian clock and autophagy, and key molecular signals that promote insulin resistance and pathogenesis of cardiovascular diseases in diabetes.
The potential of O-GlcNAcylation as an “integrated sensor” for detecting diabetes and predicting the development of diabetic cardiovascular disease.
ACKNOWLEDGEMENTS
Due to the scope and limitation, we apologize for not being able to include all the important work in the field.
SOURCES OF FUNDING
The original research of the authors has been supported by grants from the National Institutes of Health HL092215, HL136165, HL146103 and DK100847 as well as United States Department of Veterans Affairs BX0003617 and BX004426 to YC.
ABBREVIATIONS
- AGEs
advanced glycation end products
- ATP
adenosine triphosphate
- DCCT
Diabetes Control and Complications Trial
- EDIC
Epidemiology of Diabetes Interventions and Complications
- FOXO1
forkhead box protein O1
- GFAT
glutamine: fructose-6-phosphate amidotransferase
- glucose-6-P
glucose 6-phosphate
- GNA
Glucosamine 6-phosphate N-acetyltransferase
- GPI
Glucose-6-phosphate isomerase
- HBP
hexosamine biosynthesis pathways
- HIRA
histone cell cycle regulator
- HK
Hexokinase
- IRS
insulin receptor substrate
- mOGT
mitochondral OGT
- NADPH
nicotinamide adenine dinucleotide phosphate
- ncOGT
nucleocytoplasmic OGT
- OGA
O-GlcNAcase
- O-GlcNAcylation
O-linked β-N-acetylglucosamine modification
- OGT
O-GlcNAc transferase
- PASMC
pulmonary arterial smooth muscle cells
- PDK-1
phosphoinositide-dependent kinase-1
- PGM
Phospho-acetylglucosamine mutase
- PIP3
phosphatidylinositol 3,4,5-trisphosphate
- PKC
Protein Kinase C
- PPP
pentose phosphate pathway
- PTP 1B
protein-tyrosine phosphatase 1B
- RAGE
Receptor for advanced glycation end products
- ROS
reactive oxygen species
- sOGT
shorter form of OGT
- STZ
streptozocin
- TCA
tricarboxylic acid
- TET
ten-of-eleven translocation
- TPR
tetratricopeptide-repeat
- UAP
UDP-N-acetylglucosamine pyrophosphorylase
- UBN1
ubinuclein1
- UDP-GlcNAc
uridine-diphosphate-β-D-N-acetylglucosamine
- UKPDS
United Kingdom Prospective Diabetes Study
- URI
unconventional prefolding RPB5 interactor
- VADT
Veterans Affairs Diabetes Trial
- VSMC
vascular smooth muscle cells
Footnotes
DISCLOSURES
The authors have no potential conflicts of interests to disclose.
REFERENCES
- 1.Matheus AS, Tannus LR, Cobas RA, Palma CC, Negrato CA, Gomes MB. Impact of diabetes on cardiovascular disease: An update. Int J Hypertens. 2013;2013:653789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leon BM, Maddox TM. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World journal of diabetes. 2015;6:1246–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiological reviews. 2013;93:137–188 [DOI] [PubMed] [Google Scholar]
- 4.Schmidt AM. Diabetes mellitus and cardiovascular disease. Arteriosclerosis, thrombosis, and vascular biology. 2019;39:558–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The New England journal of medicine. 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 6.Ramasamy R, Yan SF, Schmidt AM. Polyol pathway and rage: A central metabolic and signaling axis in diabetic complications. Expert Rev Endocrinol Metab. 2010;5:65–75 [DOI] [PubMed] [Google Scholar]
- 7.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813+ [DOI] [PubMed] [Google Scholar]
- 8.Nishikawa T, Edelstein D, Du XL, Yamagishi S-i, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes H-P, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790 [DOI] [PubMed] [Google Scholar]
- 9.Wei Q, Ren X, Jiang Y, Jin H, Liu N, Li J. Advanced glycation end products accelerate rat vascular calcification through rage/oxidative stress. BMC cardiovascular disorders. 2013;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing sp1 glycosylation. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:12222–12226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the akt site. The Journal of clinical investigation. 2001;108:1341–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ngoh GA, Facundo HT, Hamid T, Dillmann W, Zachara NE, Jones SP. Unique hexosaminidase reduces metabolic survival signal and sensitizes cardiac myocytes to hypoxia/reoxygenation injury. Circulation research. 2009;104:41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ong Q, Han W, Yang X. O-glcnac as an integrator of signaling pathways. Frontiers in endocrinology. 2018;9:599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hart GW. Nutrient regulation of signaling and transcription. The Journal of biological chemistry. 2019;294:2211–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brodeur MR, Bouvet C, Bouchard S, Moreau S, Leblond J, Deblois D, Moreau P. Reduction of advanced-glycation end products levels and inhibition of rage signaling decreases rat vascular calcification induced by diabetes. PloS one. 2014;9:e85922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cannizzo B, Lujan A, Estrella N, Lembo C, Cruzado M, Castro C. Insulin resistance promotes early atherosclerosis via increased proinflammatory proteins and oxidative stress in fructose-fed apoe-ko mice. Exp Diabetes Res. 2012;2012:941304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konior A, Schramm A, Czesnikiewicz-Guzik M, Guzik TJ. Nadph oxidases in vascular pathology. Antioxid Redox Signal. 2014;20:2794–2814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nowotny K, Jung T, Hohn A, Weber D, Grune T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules. 2015;5:194–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tada Y, Yano S, Yamaguchi T, Okazaki K, Ogawa N, Morita M, Sugimoto T. Advanced glycation end products-induced vascular calcification is mediated by oxidative stress: Functional roles of nad(p)h-oxidase. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2013;45:267–272 [DOI] [PubMed] [Google Scholar]
- 20.King P, Peacock I, Donnelly R. The uk prospective diabetes study (ukpds): Clinical and therapeutic implications for type 2 diabetes. British journal of clinical pharmacology. 1999;48:643–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. The New England journal of medicine. 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 22.Riddle MC. Effects of intensive glucose lowering in the management of patients with type 2 diabetes mellitus in the action to control cardiovascular risk in diabetes (accord) trial. Circulation. 2010;122:844–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD, Investigators V. Glucose control and vascular complications in veterans with type 2 diabetes. The New England journal of medicine. 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 24.Reaven PD, Emanuele NV, Wiitala WL, Bahn GD, Reda DJ, McCarren M, Duckworth WC, Hayward RA, Investigators V. Intensive glucose control in patients with type 2 diabetes - 15-year follow-up. The New England journal of medicine. 2019;380:2215–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaede P, Oellgaard J, Carstensen B, Rossing P, Lund-Andersen H, Parving HH, Pedersen O. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow-up on the steno-2 randomised trial. Diabetologia. 2016;59:2298–2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bajaj H, Zinman B. Steno-2 — a small study with a big heart. Nature Reviews Endocrinology. 2016;12:692. [DOI] [PubMed] [Google Scholar]
- 27.Brand MD. Regulation analysis of energy metabolism. The Journal of experimental biology. 1997;200:193–202 [DOI] [PubMed] [Google Scholar]
- 28.Butterworth PJ. Lehninger: Principles of biochemistry (4th edn) Nelson d. L. and Cox m. C., w. H. Freeman & co., new york, 1119 pp (plus 17 pp glossary), isbn 0–7167-4339–6 (2004). Cell Biochemistry and Function. 2005;23:293–294 [Google Scholar]
- 29.Bouche C, Serdy S, Kahn CR, Goldfine AB. The cellular fate of glucose and its relevance in type 2 diabetes. Endocr Rev. 2004;25:807–830 [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Liu R, Hawkins M, Barzilai N, Rossetti L. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature. 1998;393:684–688 [DOI] [PubMed] [Google Scholar]
- 31.Wellen KE, Lu C, Mancuso A, Lemons JM, Ryczko M, Dennis JW, Rabinowitz JD, Coller HA, Thompson CB. The hexosamine biosynthetic pathway couples growth factor-induced glutamine uptake to glucose metabolism. Genes & development. 2010;24:2784–2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeidan Q, Hart GW. The intersections between o-glcnacylation and phosphorylation: Implications for multiple signaling pathways. Journal of cell science. 2010;123:13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanover JA, Yu S, Lubas WB, Shin SH, Ragano-Caracciola M, Kochran J, Love DC. Mitochondrial and nucleocytoplasmic isoforms of o-linked glcnac transferase encoded by a single mammalian gene. Archives of biochemistry and biophysics. 2003;409:287–297 [DOI] [PubMed] [Google Scholar]
- 34.Yang X, Qian K. Protein o-glcnacylation: Emerging mechanisms and functions. Nature reviews. Molecular cell biology. 2017;18:452–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lazarus MB, Nam Y, Jiang J, Sliz P, Walker S. Structure of human o-glcnac transferase and its complex with a peptide substrate. Nature. 2011;469:564–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kreppel LK, Hart GW. Regulation of a cytosolic and nuclear o-glcnac transferase. Role of the tetratricopeptide repeats. The Journal of biological chemistry. 1999;274:32015–32022 [DOI] [PubMed] [Google Scholar]
- 37.Comtesse N, Maldener E, Meese E. Identification of a nuclear variant of mgea5, a cytoplasmic hyaluronidase and a beta-n-acetylglucosaminidase. Biochemical and biophysical research communications. 2001;283:634–640 [DOI] [PubMed] [Google Scholar]
- 38.Wells L, Vosseller K, Hart GW. Glycosylation of nucleocytoplasmic proteins: Signal transduction and o-glcnac. Science (New York, N.Y.). 2001;291:2376–2378 [DOI] [PubMed] [Google Scholar]
- 39.Lunde IG, Aronsen JM, Kvaloy H, Qvigstad E, Sjaastad I, Tonnessen T, Christensen G, Gronning-Wang LM, Carlson CR. Cardiac o-glcnac signaling is increased in hypertrophy and heart failure. Physiological genomics. 2012;44:162–172 [DOI] [PubMed] [Google Scholar]
- 40.Federici M, Menghini R, Mauriello A, Hribal ML, Ferrelli F, Lauro D, Sbraccia P, Spagnoli LG, Sesti G, Lauro R. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by o-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation. 2002;106:466–472 [DOI] [PubMed] [Google Scholar]
- 41.Dassanayaka S, Jones SP. O-glcnac and the cardiovascular system. Pharmacology & therapeutics. 2014;142:62–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chatham JC, Not LG, Fulop N, Marchase RB. Hexosamine biosynthesis and protein o-glycosylation: The first line of defense against stress, ischemia, and trauma. Shock (Augusta, Ga.). 2008;29:431–440 [DOI] [PubMed] [Google Scholar]
- 43.Boudina S, Abel ED. Diabetic cardiomyopathy, causes and effects. Reviews in endocrine & metabolic disorders. 2010;11:31–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. 2014;57:660–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bennett CE, Johnsen VL, Shearer J, Belke DD. Exercise training mitigates aberrant cardiac protein o-glcnacylation in streptozotocin-induced diabetic mice. Life Sciences. 2013;92:657–663 [DOI] [PubMed] [Google Scholar]
- 46.Watson LJ, Facundo HT, Ngoh GA, Ameen M, Brainard RE, Lemma KM, Long BW, Prabhu SD, Xuan YT, Jones SP. O-linked beta-n-acetylglucosamine transferase is indispensable in the failing heart. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17797–17802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ngoh GA, Watson LJ, Facundo HT, Jones SP. Augmented o-glcnac signaling attenuates oxidative stress and calcium overload in cardiomyocytes. Amino acids. 2011;40:895–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Banerjee PS, Ma J, Hart GW. Diabetes-associated dysregulation of o-glcnacylation in rat cardiac mitochondria. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:6050–6055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clark RJ, McDonough PM, Swanson E, Trost SU, Suzuki M, Fukuda M, Dillmann WH. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear o-glcnacylation. The Journal of biological chemistry. 2003;278:44230–44237 [DOI] [PubMed] [Google Scholar]
- 50.Hu Y, Belke D, Suarez J, Swanson E, Clark R, Hoshijima M, Dillmann WH. Adenovirus-mediated overexpression of o-glcnacase improves contractile function in the diabetic heart. Circulation research. 2005;96:1006–1013 [DOI] [PubMed] [Google Scholar]
- 51.Medford HM, Chatham JC, Marsh SA. Chronic ingestion of a western diet increases o-linked-beta-n-acetylglucosamine (o-glcnac) protein modification in the rat heart. Life sciences. 2012;90:883–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barrett EJ, Liu Z, Khamaisi M, King GL, Klein R, Klein BEK, Hughes TM, Craft S, Freedman BI, Bowden DW, Vinik AI, Casellini CM. Diabetic microvascular disease: An endocrine society scientific statement. The Journal of clinical endocrinology and metabolism. 2017;102:4343–4410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun YM, Su Y, Li J, Wang LF. Recent advances in understanding the biochemical and molecular mechanism of diabetic nephropathy. Biochemical and biophysical research communications. 2013;433:359–361 [DOI] [PubMed] [Google Scholar]
- 54.Ola MS, Nawaz MI, Siddiquei MM, Al-Amro S, Abu El-Asrar AM. Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. Journal of diabetes and its complications. 2012;26:56–64 [DOI] [PubMed] [Google Scholar]
- 55.Nakamura M, Barber AJ, Antonetti DA, LaNoue KF, Robinson KA, Buse MG, Gardner TW. Excessive hexosamines block the neuroprotective effect of insulin and induce apoptosis in retinal neurons. The Journal of biological chemistry. 2001;276:43748–43755 [DOI] [PubMed] [Google Scholar]
- 56.Gurel Z, Sheibani N. O-linked β-<em>n</em>-acetylglucosamine (o-glcnac) modification: A new pathway to decode pathogenesis of diabetic retinopathy. Clinical Science. 2018;132:185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park MJ, Kim DI, Lim SK, Choi JH, Han HJ, Yoon KC, Park SH. High glucose-induced o-glcnacylated carbohydrate response element-binding protein (chrebp) mediates mesangial cell lipogenesis and fibrosis: The possible role in the development of diabetic nephropathy. The Journal of biological chemistry. 2014;289:13519–13530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xing D, Feng W, Not LG, Miller AP, Zhang Y, Chen YF, Majid-Hassan E, Chatham JC, Oparil S. Increased protein o-glcnac modification inhibits inflammatory and neointimal responses to acute endoluminal arterial injury. American journal of physiology. Heart and circulatory physiology. 2008;295:H335–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lima VV, Giachini FR, Choi H, Carneiro FS, Carneiro ZN, Fortes ZB, Carvalho MH, Webb RC, Tostes RC. Impaired vasodilator activity in deoxycorticosterone acetate-salt hypertension is associated with increased protein o-glcnacylation. Hypertension. 2009;53:166–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barnes JW, Tian L, Heresi GA, Farver CF, Asosingh K, Comhair SAA, Aulak KS, Dweik RA. O-linked β-acetylglucosamine transferase directs cell proliferation in idiopathic pulmonary arterial hypertension. Circulation. 2015;131:1260–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yahagi K, Kolodgie FD, Lutter C, Mori H, Romero ME, Finn AV, Virmani R. Pathology of human coronary and carotid artery atherosclerosis and vascular calcification in diabetes mellitus. Arteriosclerosis, Thrombosis, and Vascular Biology. 2017;37:191–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Katz R, Wong ND, Kronmal R, Takasu J, Shavelle DM, Probstfield JL, Bertoni AG, Budoff MJ, O’Brien KD. Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the multi-ethnic study of atherosclerosis. Circulation. 2006;113:2113–2119 [DOI] [PubMed] [Google Scholar]
- 63.Carr JJ, Register TC, Hsu FC, Lohman K, Lenchik L, Bowden DW, Langefeld CD, Xu J, Rich SS, Wagenknecht LE, Freedman BI. Calcified atherosclerotic plaque and bone mineral density in type 2 diabetes: The diabetes heart study. Bone. 2008;42:43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. Journal of the American College of Cardiology. 2004;43:1663–1669 [DOI] [PubMed] [Google Scholar]
- 65.Hoff JA, Quinn L, Sevrukov A, Lipton RB, Daviglus M, Garside DB, Ajmere NK, Gandhi S, Kondos GT. The prevalence of coronary artery calcium among diabetic individuals without known coronary artery disease. Journal of the American College of Cardiology. 2003;41:1008–1012 [DOI] [PubMed] [Google Scholar]
- 66.Chiha M, Njeim M, Chedrawy EG. Diabetes and coronary heart disease: A risk factor for the global epidemic. International Journal of Hypertension. 2012;2012:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, McDonald JM, Chen Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor runx2 by akt signaling. The Journal of biological chemistry. 2008;283:15319–15327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Byon CH, Sun Y, Chen J, Yuan K, Mao X, Heath JM, Anderson PG, Tintut Y, Demer LL, Wang D, Chen Y. Runx2-upregulated receptor activator of nuclear factor kappab ligand in calcifying smooth muscle cells promotes migration and osteoclastic differentiation of macrophages. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:1387–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mody N, Parhami F, Sarafian TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free radical biology & medicine. 2001;31:509–519 [DOI] [PubMed] [Google Scholar]
- 70.Liberman M, Bassi E, Martinatti MK, Lario FC, Wosniak J Jr., Pomerantzeff PM, Laurindo FR. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:463–470 [DOI] [PubMed] [Google Scholar]
- 71.Miller JD, Chu Y, Brooks RM, Richenbacher WE, Pena-Silva R, Heistad DD. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. Journal of the American College of Cardiology. 2008;52:843–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Byon CH, Heath JM, Chen Y. Redox signaling in cardiovascular pathophysiology: A focus on hydrogen peroxide and vascular smooth muscle cells. Redox Biol. 2016;9:244–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bostrom KI, Jumabay M, Matveyenko A, Nicholas SB, Yao Y. Activation of vascular bone morphogenetic protein signaling in diabetes mellitus. Circulation research. 2011;108:446–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stabley JN, Towler DA. Arterial calcification in diabetes mellitus: Preclinical models and translational implications. Arteriosclerosis, thrombosis, and vascular biology. 2017;37:205–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heath JM, Sun Y, Yuan K, Bradley WE, Litovsky S, Dell’Italia LJ, Chatham JC, Wu H, Chen Y. Activation of akt by o-linked n-acetylglucosamine induces vascular calcification in diabetes mellitus. Circulation research. 2014;114:1094–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu K, Paterson AJ, Chin E, Kudlow JE. Glucose stimulates protein modification by o-linked glcnac in pancreatic beta cells: Linkage of o-linked glcnac to beta cell death. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:2820–2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perez-Cervera Y, Dehennaut V, Aquino Gil M, Guedri K, Solorzano Mata CJ, Olivier-Van Stichelen S, Michalski JC, Foulquier F, Lefebvre T. Insulin signaling controls the expression of o-glcnac transferase and its interaction with lipid microdomains. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27:3478–3486 [DOI] [PubMed] [Google Scholar]
- 78.Muthusamy S, Hong KU, Dassanayaka S, Hamid T, Jones SP. E2f1 transcription factor regulates o-linked n-acetylglucosamine (o-glcnac) transferase and o-glcnacase expression. The Journal of biological chemistry. 2015;290:31013–31024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giralt A, Denechaud PD, Lopez-Mejia IC, Delacuisine B, Blanchet E, Bonner C, Pattou F, Annicotte JS, Fajas L. E2f1 promotes hepatic gluconeogenesis and contributes to hyperglycemia during diabetes. Mol Metab. 2018;11:104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang X, Ongusaha PP, Miles PD, Havstad JC, Zhang F, So WV, Kudlow JE, Michell RH, Olefsky JM, Field SJ, Evans RM. Phosphoinositide signalling links o-glcnac transferase to insulin resistance. Nature. 2008;451:964–969 [DOI] [PubMed] [Google Scholar]
- 81.Whelan SA, Lane MD, Hart GW. Regulation of the o-linked beta-n-acetylglucosamine transferase by insulin signaling. The Journal of biological chemistry. 2008;283:21411–21417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Song M, Kim H-S, Park J-M, Kim S-H, Kim I-H, Ryu SH, Suh P-G. O-glcnac transferase is activated by camkiv-dependent phosphorylation under potassium chloride-induced depolarization in ng-108–15 cells. Cellular Signalling. 2008;20:94–104 [DOI] [PubMed] [Google Scholar]
- 83.Jeon JH, Suh HN, Kim MO, Ryu JM, Han HJ. Glucosamine-induced ogt activation mediates glucose production through cleaved notch1 and foxo1, which coordinately contributed to the regulation of maintenance of self-renewal in mouse embryonic stem cells. Stem Cells Dev. 2014;23:2067–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheung WD, Hart GW. Amp-activated protein kinase and p38 mapk activate o-glcnacylation of neuronal proteins during glucose deprivation. The Journal of biological chemistry. 2008;283:13009–13020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taylor RP, Geisler TS, Chambers JH, McClain DA. Up-regulation of o-glcnac transferase with glucose deprivation in hepg2 cells is mediated by decreased hexosamine pathway flux. The Journal of biological chemistry. 2009;284:3425–3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Buren S, Gomes AL, Teijeiro A, Fawal MA, Yilmaz M, Tummala KS, Perez M, Rodriguez-Justo M, Campos-Olivas R, Megias D, Djouder N. Regulation of ogt by uri in response to glucose confers c-myc-dependent survival mechanisms. Cancer Cell. 2016;30:290–307 [DOI] [PubMed] [Google Scholar]
- 87.Dentin R, Hedrick S, Xie J, Yates J 3rd, Montminy M. Hepatic glucose sensing via the creb coactivator crtc2. Science (New York, N.Y.). 2008;319:1402–1405 [DOI] [PubMed] [Google Scholar]
- 88.Arias EB, Kim J, Cartee GD. Prolonged incubation in pugnac results in increased protein o-linked glycosylation and insulin resistance in rat skeletal muscle. Diabetes. 2004;53:921–930 [DOI] [PubMed] [Google Scholar]
- 89.Vosseller K, Wells L, Lane MD, Hart GW. Elevated nucleocytoplasmic glycosylation by o-glcnac results in insulin resistance associated with defects in akt activation in 3t3-l1 adipocytes. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:5313–5318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Akimoto Y, Kreppel LK, Hirano H, Hart GW. Increased o-glcnac transferase in pancreas of rats with streptozotocin-induced diabetes. Diabetologia. 2000;43:1239–1247 [DOI] [PubMed] [Google Scholar]
- 91.Hebert LF Jr., Daniels MC, Zhou J, Crook ED, Turner RL, Simmons ST, Neidigh JL, Zhu JS, Baron AD, McClain DA. Overexpression of glutamine:Fructose-6-phosphate amidotransferase in transgenic mice leads to insulin resistance. The Journal of clinical investigation. 1996;98:930–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Crook ED, Daniels MC, Smith TM, McClain DA. Regulation of insulin-stimulated glycogen synthase activity by overexpression of glutamine: Fructose-6-phosphate amidotransferase in rat-1 fibroblasts. Diabetes. 1993;42:1289–1296 [DOI] [PubMed] [Google Scholar]
- 93.Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. The Journal of biological chemistry. 1991;266:4706–4712 [PubMed] [Google Scholar]
- 94.Robles-Flores M, Melendez L, Garcia W, Mendoza-Hernandez G, Lam TT, Castaneda-Patlan C, Gonzalez-Aguilar H. Posttranslational modifications on protein kinase c isozymes. Effects of epinephrine and phorbol esters. Biochimica et biophysica acta. 2008;1783:695–712 [DOI] [PubMed] [Google Scholar]
- 95.Zhao Y, Tang Z, Shen A, Tao T, Wan C, Zhu X, Huang J, Zhang W, Xia N, Wang S, Cui S, Zhang D. The role of ptp1b o-glcnacylation in hepatic insulin resistance. International journal of molecular sciences. 2015;16:22856–22869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kuo M, Zilberfarb V, Gangneux N, Christeff N, Issad T. O-glcnac modification of foxo1 increases its transcriptional activity: A role in the glucotoxicity phenomenon? Biochimie. 2008;90:679–685 [DOI] [PubMed] [Google Scholar]
- 97.Park SY, Ryu J, Lee W. O-glcnac modification on irs-1 and akt2 by pugnac inhibits their phosphorylation and induces insulin resistance in rat primary adipocytes. Experimental & molecular medicine. 2005;37:220–229 [DOI] [PubMed] [Google Scholar]
- 98.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science (New York, N.Y.). 2010;330:1349–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Boden G, Chen X, Polansky M. Disruption of circadian insulin secretion is associated with reduced glucose uptake in first-degree relatives of patients with type 2 diabetes. Diabetes. 1999;48:2182–2188 [DOI] [PubMed] [Google Scholar]
- 100.Li M-D, Ruan H-B, Hughes Michael E, Lee J-S, Singh Jay P, Jones Steven P, Nitabach Michael N, Yang X. O-glcnac signaling entrains the circadian clock by inhibiting bmal1/clock ubiquitination. Cell Metabolism. 2013;17:303–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim EY, Jeong EH, Park S, Jeong HJ, Edery I, Cho JW. A role for o-glcnacylation in setting circadian clock speed. Genes & development. 2012;26:490–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kaasik K, Kivimae S, Allen JJ, Chalkley RJ, Huang Y, Baer K, Kissel H, Burlingame AL, Shokat KM, Ptacek LJ, Fu YH. Glucose sensor o-glcnacylation coordinates with phosphorylation to regulate circadian clock. Cell metabolism. 2013;17:291–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Berthier A, Vinod M, Porez G, Steenackers A, Alexandre J, Yamakawa N, Gheeraert C, Ploton M, Marechal X, Dubois-Chevalier J, Hovasse A, Schaeffer-Reiss C, Cianferani S, Rolando C, Bray F, Duez H, Eeckhoute J, Lefebvre T, Staels B, Lefebvre P. Combinatorial regulation of hepatic cytoplasmic signaling and nuclear transcriptional events by the ogt/rev-erbalpha complex. Proceedings of the National Academy of Sciences of the United States of America. 2018;115:E11033–e11042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Guo B, Liang Q, Li L, Hu Z, Wu F, Zhang P, Ma Y, Zhao B, Kovacs AL, Zhang Z, Feng D, Chen S, Zhang H. O-glcnac-modification of snap-29 regulates autophagosome maturation. Nature cell biology. 2014;16:1215–1226 [DOI] [PubMed] [Google Scholar]
- 105.Parker ZM, Murphy AA, Leib DA. Role of the DNA sensor sting in protection from lethal infection following corneal and intracerebral challenge with herpes simplex virus 1. J Virol. 2015;89:11080–11091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Park S, Lee Y, Pak JW, Kim H, Choi H, Kim JW, Roth J, Cho JW. O-glcnac modification is essential for the regulation of autophagy in drosophila melanogaster. Cellular and molecular life sciences : CMLS. 2015;72:3173–3183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lampert MA, Gustafsson AB. Balancing autophagy for a healthy heart. Current opinion in physiology. 2018;1:21–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bhattacharya D, Mukhopadhyay M, Bhattacharyya M, Karmakar P. Is autophagy associated with diabetes mellitus and its complications? A review. EXCLI J. 2018;17:709–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rabinowitz JD, White E. Autophagy and metabolism. Science (New York, N.Y.). 2010;330:1344–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ma D, Panda S, Lin JD. Temporal orchestration of circadian autophagy rhythm by c/ebpbeta. The EMBO journal. 2011;30:4642–4651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sengupta A, Molkentin JD, Yutzey KE. Foxo transcription factors promote autophagy in cardiomyocytes. The Journal of biological chemistry. 2009;284:28319–28331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li X, Molina H, Huang H, Zhang YY, Liu M, Qian SW, Slawson C, Dias WB, Pandey A, Hart GW, Lane MD, Tang QQ. O-linked n-acetylglucosamine modification on ccaat enhancer-binding protein beta: Role during adipocyte differentiation. The Journal of biological chemistry. 2009;284:19248–19254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee JS, Zhang Z. O-linked n-acetylglucosamine transferase (ogt) interacts with the histone chaperone hira complex and regulates nucleosome assembly and cellular senescence. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E3213–3220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tagami H, Ray-Gallet D, Almouzni G, Nakatani Y. Histone h3.1 and h3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell. 2004;116:51–61 [DOI] [PubMed] [Google Scholar]
- 115.Jin C, Felsenfeld G. Distribution of histone h3.3 in hematopoietic cell lineages. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:574–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Valenzuela N, Fan Q, Fa’ak F, Soibam B, Nagandla H, Liu Y, Schwartz RJ, McConnell BK, Stewart MD. Cardiomyocyte-specific conditional knockout of the histone chaperone hira in mice results in hypertrophy, sarcolemmal damage and focal replacement fibrosis. Dis Model Mech. 2016;9:335–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Valenzuela N, Soibam B, Li L, Wang J, Byers LA, Liu Y, Schwartz RJ, Stewart MD. Hira deficiency in muscle fibers causes hypertrophy and susceptibility to oxidative stress. Journal of cell science. 2017;130:2551–2563 [DOI] [PubMed] [Google Scholar]
- 118.Lazarus MB, Jiang J, Kapuria V, Bhuiyan T, Janetzko J, Zandberg WF, Vocadlo DJ, Herr W, Walker S. Hcf-1 is cleaved in the active site of o-glcnac transferase. Science (New York, N.Y.). 2013;342:1235–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hrit J, Goodrich L, Li C, Wang BA, Nie J, Cui X, Martin EA, Simental E, Fernandez J, Liu MY, Nery JR, Castanon R, Kohli RM, Tretyakova N, He C, Ecker JR, Goll M, Panning B. Ogt binds a conserved c-terminal domain of tet1 to regulate tet1 activity and function in development. Elife. 2018;7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Williams K, Christensen J, Pedersen MT, Johansen JV, Cloos PA, Rappsilber J, Helin K. Tet1 and hydroxymethylcytosine in transcription and DNA methylation fidelity. Nature. 2011;473:343–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen Q, Chen Y, Bian C, Fujiki R, Yu X. Tet2 promotes histone o-glcnacylation during gene transcription. Nature. 2013;493:561–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang Q, Liu X, Gao W, Li P, Hou J, Li J, Wong J. Differential regulation of the ten-eleven translocation (tet) family of dioxygenases by o-linked beta-n-acetylglucosamine transferase (ogt). The Journal of biological chemistry. 2014;289:5986–5996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu CL, Sano S, Muralidharan S, Rius C, Vuong J, Jacob S, Muralidhar V, Robertson AA, Cooper MA, Andres V, Hirschi KK, Martin KA, Walsh K. Clonal hematopoiesis associated with tet2 deficiency accelerates atherosclerosis development in mice. Science (New York, N.Y.). 2017;355:842–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hardiville S, Hart GW. Nutrient regulation of signaling, transcription, and cell physiology by o-glcnacylation. Cell metabolism. 2014;20:208–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zeidan Q, Wang Z, De Maio A, Hart GW. O-glcnac cycling enzymes associate with the translational machinery and modify core ribosomal proteins. Molecular biology of the cell. 2010;21:1922–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yang X, Su K, Roos MD, Chang Q, Paterson AJ, Kudlow JE. O-linkage of n-acetylglucosamine to sp1 activation domain inhibits its transcriptional capability. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6611–6616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang Y, Qu Y, Niu T, Wang H, Liu K. O-glcnac modification of sp1 mediates hyperglycaemia-induced icam-1 up-regulation in endothelial cells. Biochemical and biophysical research communications. 2017;484:79–84 [DOI] [PubMed] [Google Scholar]
- 128.Donovan K, Alekseev O, Qi X, Cho W, Azizkhan-Clifford J. O-glcnac modification of transcription factor sp1 mediates hyperglycemia-induced vegf-a upregulation in retinal cells. Investigative ophthalmology & visual science. 2014;55:7862–7873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nature reviews. Endocrinology. 2016;12:144–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dhalla NS, Pierce GN, Innes IR, Beamish RE. Pathogenesis of cardiac dysfunction in diabetes mellitus. The Canadian journal of cardiology. 1985;1:263–281 [PubMed] [Google Scholar]
- 131.Kim JA, Jang HJ, Martinez-Lemus LA, Sowers JR. Activation of mtor/p70s6 kinase by ang ii inhibits insulin-stimulated endothelial nitric oxide synthase and vasodilation. American journal of physiology. Endocrinology and metabolism. 2012;302:E201–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Makino A, Dai A, Han Y, Youssef KD, Wang W, Donthamsetty R, Scott BT, Wang H, Dillmann WH. O-glcnacase overexpression reverses coronary endothelial cell dysfunction in type 1 diabetic mice. Am J Physiol Cell Physiol. 2015;309:C593–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang S, Huang X, Sun D, Xin X, Pan Q, Peng S, Liang Z, Luo C, Yang Y, Jiang H, Huang M, Chai W, Ding J, Geng M. Extensive crosstalk between o-glcnacylation and phosphorylation regulates akt signaling. PloS one. 2012;7:e37427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Greulich S, Maxhera B, Vandenplas G, de Wiza DH, Smiris K, Mueller H, Heinrichs J, Blumensatt M, Cuvelier C, Akhyari P, Ruige JB, Ouwens DM, Eckel J. Secretory products from epicardial adipose tissue of patients with type 2 diabetes mellitus induce cardiomyocyte dysfunction. Circulation. 2012;126:2324–2334 [DOI] [PubMed] [Google Scholar]
- 135.Boon J, Hoy AJ, Stark R, Brown RD, Meex RC, Henstridge DC, Schenk S, Meikle PJ, Horowitz JF, Kingwell BA, Bruce CR, Watt MJ. Ceramides contained in ldl are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes. 2013;62:401–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sun Y, Byon CH, Yuan K, Chen J, Mao X, Heath JM, Javed A, Zhang K, Anderson PG, Chen Y. Smooth muscle cell-specific runx2 deficiency inhibits vascular calcification. Circulation research. 2012;111:543–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nagel AK, Ball LE. O-glcnac modification of the runt-related transcription factor 2 (runx2) links osteogenesis and nutrient metabolism in bone marrow mesenchymal stem cells. Molecular & cellular proteomics : MCP. 2014;13:3381–3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pantalone KM, Misra-Hebert AD, Hobbs TM, Wells BJ, Kong SX, Chagin K, Dey T, Milinovich A, Weng W, Bauman JM, Burguera B, Zimmerman RS, Kattan MW. Effect of glycemic control on the diabetes complications severity index score and development of complications in people with newly diagnosed type 2 diabetes. J Diabetes. 2018;10:192–199 [DOI] [PubMed] [Google Scholar]
- 139.Park K, Saudek CD, Hart GW. Increased expression of beta-n-acetylglucosaminidase in erythrocytes from individuals with pre-diabetes and diabetes. Diabetes. 2010;59:1845–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang Z, Park K, Comer F, Hsieh-Wilson LC, Saudek CD, Hart GW. Site-specific glcnacylation of human erythrocyte proteins: Potential biomarker(s) for diabetes. Diabetes. 2009;58:309–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Myslicki JP, Shearer J, Hittel DS, Hughey CC, Belke DD. O-glcnac modification is associated with insulin sensitivity in the whole blood of healthy young adult males. Diabetology & metabolic syndrome. 2014;6:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.