Abstract
Objective
Determine the impact of cholesterol ester transfer protein (CETP) on the route of cholesterol elimination in mice.
Approach and Results
We adapted our protocol for biliary cholesterol secretion with published methods for measuring transintestinal cholesterol elimination (TICE). Bile was diverted and biliary lipid secretion maintained by infusion of bile acid. The proximal small bowel was perfused with bile acid micelles. In high fat, high cholesterol fed mice, the presence of a CETP transgene increased biliary cholesterol secretion at the expense of TICE. The increase in biliary cholesterol secretion was not associated with increases in hepatic scavenger receptor BI or ABCG5 ABCG8. The decline in intestinal cholesterol secretion was associated with an increase in intestinal Niemann-Pick disease, type C1, gene-like 1 mRNA. Finally, we followed the delivery of HDL or LDL cholesteryl esters from plasma to bile and intestinal perfusates. HDL-CE favored the biliary pathway. Following high fat feeding, the presence of CETP directed HDL-CE away from the bile and towards the intestine. The presence of CETP increased LDL-CE delivery to bile while the appearance of LDL-CE in intestinal perfusate was near the lower limit of detection.
Conclusions
Biliary and intestinal cholesterol secretion can be simultaneously measured in mice and used as a model to examine factors that alter cholesterol elimination. Plasma factors, such as CETP, alter the route of cholesterol elimination from the body. Intestinal and biliary cholesterol secretion rates are independent of transhepatic or transintestinal delivery of HDL-CE while LDL-CE was eliminated almost exclusively in the hepatobiliary pathway.
Keywords: cholesterol, CETP, liver, intestine, transporters, HDL, Lipids and Cholesterol, Physiology
Introduction
The reverse cholesterol transport (RCT) pathway delivers excess cholesterol from peripheral tissues through the plasma compartment for elimination in the feces. Although roles for both the liver and intestine for sterol excretion have been described, the process has been best characterized in the liver where cholesterol is delivered from the plasma compartment to hepatocytes by lipoprotein receptors and actively secreted into bile as unesterified cholesterol or primary bile acids by ATP-binding cassette (ABC) transporters. More recently, interest in understanding the mechanisms that mediate and regulate transintestinal cholesterol elimination (TICE) has emerged as investigators have searched for alternate, non-biliary strategies to accelerate RCT. Early evidence for TICE was inferred from observations in animal models in which biliary cholesterol secretion was compromised while fecal neutral sterols remained constant or were elevated.1 Groen and colleagues made the first attempt to measure rates of TICE by perfusing bile acid micelles through intestinal segments and measuring the delivery of radiolabeled cholesterol from the plasma compartment to the intestinal lumen.2 This, and subsequent reports, established that TICE is an active pathway in mice, is dependent on the presence of cholesterol acceptors in the intestinal lumen, and is regulated by diet, genetic, microbial, and pharmacological agents.2–13 Data consistent with a role for TICE have also been reported in rats, hamsters and humans.3, 14, 15
The delivery of sterols from the plasma compartment to the intestinal epithelium for excretion is presumed to be mediated by plasma lipoproteins. However, the relative contributions of HDL and LDL cholesterol to TICE remain unclear. Mice lacking HDL (ABCA1 deficiency) showed no difference in rates of TICE; HDL-associated radiolabeled cholesterol did not appear in intestinal perfusates when delivered to the plasma compartment.16 In addition, germline deletion of SR-BI, the primary mediator of HDL-cholesterol uptake into cells, resulted in an increase in TICE.8 Similarly, an intestinal-specific SR-BI transgene had no effect on sterol balance.17 However, a direct assessment of radiolabeled HDL cholesterol trafficking in both intestinal explants and in perfused intestine demonstrated the transport of HDL free cholesterol from the basolateral to apical compartment and from plasma to intestinal lumen, respectively.6 In the same study, the delivery of LDL free cholesterol to apical compartments in vivo and ex vivo were similar to HDL. Data on the importance of the LDL receptor (LDLR) are also conflicting. TICE was increased by germline deletion of PCSK9 and statin therapy, indicating a role for LDL and LDLR. However, TICE was largely unaffected by the absence of LDL receptor and the delivery of radiolabeled LDL cholesterol to the proximal small intestine was paradoxically elevated.6 Yet to be considered is the potential role for cholesteryl ester transfer protein (CETP), which provides an avenue for delivery of cholesterol from HDL to LDL in the RCT pathway in humans, but is absent from the mouse genome.
Studies to date in the direct measurement of TICE have been conducted with bile diversion, but in the absence of biliary cholesterol secretion once the endogenous bile acid pool has been exhausted (~30 min). In an effort to simultaneously measure the relative contribution of each pathway to cholesterol elimination, we modified the procedure of Groen and colleagues to maintain biliary cholesterol secretion during intestinal perfusion (Fig SI).2 Bile acid was infused via tail vein such that the rate of biliary bile acid output approximated the rate of bile acid perfusion of the small intestine. We then measured the relative rates of cholesterol secretion from the liver and proximal small intestine in wild-type and CETP transgenic mice. Our results are consistent with previous reports of similar rates of cholesterol secretion from the intestine and liver in mice maintained on natural ingredient diets, but there was no effect of CETP on either biliary or intestinal cholesterol secretion. In wild type mice maintained on a high fat, high cholesterol diet, biliary and intestinal cholesterol secretion rates were nearly equivalent to one another, but the presence of CETP directed cholesterol away from the intestinal pathway toward the biliary pathway. However, the opposite effect of CETP was observed for radiolabeled HDL-CE.
Materials and Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Animals and diets
All animal procedures were approved by the Institutional Animal Care and Use Committee. Mice carrying the human CETP minigene (B6.CBA-Tg(CETP) 5203Tall/J) were obtained from The Jackson Laboratory (Stock number: 003904, Major Resources Table 3). Mice were housed in individually ventilated cages with free access to water and food (Teklad global 18% protein, #2918). The colony was maintained in a temperature-controlled room under negative pressure with a 14h/10h light/dark cycle. Experimental mice were generated using hemizygous male and wild type (WT) female (C57BL/J) trios. Males were removed from breeding cages following 8 days with females. Pups were fostered by female pairs, weaned at 21 days of age, and treated as littermates for experimental purposes. Where indicated, mice were fed a high fat, high cholesterol diet (HF-HC, 41% kcal fat, 1.5% w/w cholesterol, Research Diets Inc., New Brunswick, NJ. Product number: D12079B) for two weeks. For tissue and plasma analysis, an independent cohort of wild-type and CETP transgenic littermates were analyzed. These mice did not undergo the surgical procedure, but were maintained on the HF-HC diet for 2 weeks. All mice were randomized to treatment groups. Technical staff were blinded to groups for biochemical analyses.
Serum, bile and intestinal perfusate analysis
Serum, biliary and intestinal perfusate total cholesterol content were measured by commercial colorimetric-enzymatic assays (Wako Chemicals, Richmond, VA). Total bile acids were determined enzymatically to detect 3a-hydroxylated sterols as described previously.18 Serum was fractionated by FPLC, and fractions were analyzed for cholesterol content as described previously.19 Plasma CETP activity was determined by using commercial CETP activity assay kit (Roar CETP Activity Assay Kit, Cat. No. RB-CETP). Bile flow was determined gravimetrically assuming a density of 1 mg/mL.
Lipoprotein cholesterol distributions
The cholesterol distribution among lipoprotein classes was determined after separation by gel filtration chromatography based upon the method described previously.20 An aliquot of plasma was diluted to 0.5 μg total cholesterol/μL in 0.9% NaCl, 0.05% EDTA/NaN3 and centrifuged at 2000 × g for 10 minutes to remove any particulate debris. The supernatant was transferred to a glass insert contained in a GC vial. After loading the vial into an autosampler set at 4°C (Agilent Technologies, G1329A), 40 μL of sample was injected onto a Superose 6 10/300 (GE Healthcare Life Sciences) chromatography column. Under the control of an isocratic pump (Agilent Technologies, G1310A/B), the sample was separated at a flow rate of 0.4 ml/min with eluent containing 0.9% NaCl, 0.05% EDTA/NaN3. The column effluent was mixed with total cholesterol enzymatic reagent (Pointe Scientific) running at a flow rate of 0.125 mL/min and the mixture was passed through a knitted reaction coil (Aura Industries Inc., EPOCOD) in a 37°C H2O jacket. The absorbance of the reaction mixture was read at 500 nm using a variable wavelength detector (Agilent Technologies, G1314F). The signal was subsequently integrated using Agilent OpenLAB Software Suite (Agilent Technologies). VLDL-C, LDL-C, and HDL-C concentrations were determined by multiplying the TPC concentration by the cholesterol percentage within the elution region for each lipoprotein class.
Biliary and intestinal cholesterol secretion
Perfusion fluid composition
Perfusions were carried out with a modified Krebs solution (119.95 mmol/L NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4 · 7H2O, 15 mM HEPES, 1.3 mM CaCl2 · 2H2O and 10 mM L-glutamine; final pH 7.4) supplemented with 10 mM taurocholate and 2mM lecithin (10 mM taurocholate [TC]: 2mM phosphatidylcholine [PC]). Bile acid micelles were prepared as follows: taurocholate (Sigma) was dissolved in methanol and egg yolk L-α-phosphatidylcholine (Sigma) was dissolved in chloroform. Two preparations were mixed in a volume ratio of 1:1 and solvents were evaporated under a mild stream of nitrogen at 45°C. The residue was lyophilized overnight. Lyophilized samples were sealed and stored −80°C until the day of the intestine perfusions. Before the start of the intestine perfusions, the films were suspended in perfusion buffer (room temperature).
Surgical Procedure
Mice were anesthetized with 2 mg urethane/g body weight intraperitoneally and placed in an HSE Temperature Moist Chamber Type 834/8 with metal tube heat exchanger (Harvard Apparatus #732901) at 37°C to maintain body temperature. A longitudinal cut was made from the lower abdominal incision site along the midline to the sternum. Two hemostatic forceps were used to separate the skin and expose the peritoneum. A sterile cotton- tipped applicator wetted in sterile PBS was used to push aside the liver lobes and intestine to expose the common bile duct and gall bladder. The bile duct was ligated with a 3cm length silk suture and the gall bladder cannulated with a 10-cm length of PE-tubing. The tubing was secured to the gall bladder with a 3-cm length silk suture.
Bile acid infusion/micelle perfusion
The tail vein was fitted with a catheter (Braintree Scientific, INC. No. MTV-01) attached to a syringe pump containing 20mM tauracholate. Taurocholate was infused at a rate of 100 nmol/min to maintain biliary lipid secretion. The proximal small intestine accounts for over 50% of TICE. 2 Tubing (PharMed tubing, .8mm ID Bio-RAD #7318247) attached to a peristaltic pump was inserted in the small intestine just below the fundus of stomach and an outflow tube was fitted 10 cm distal to the inflow catheter for perfusate collection. Both catheters were fixed with intestine with 3-cm length silk sutures. The proximal intestine was flushed with 5 mL PBS (37°C) to remove the luminal contents and then filled with perfusion fluid (37°C). Perfusions were performed at a fixed flow rate (3 mL/h) over the 90 minute period. Bile and perfusate were collected every 10 or 15 minutes and stored on ice.
Transhepatic and transintestinal HDL-CE and LDL-CE secretion
To examine HDL- and LDL-mediated cholesterol ester delivery to bile vs. intestine, mice were injected with 0.6μCi/mouse [3H]-cholesteryl oleate labeled lipoprotein via the retro-orbital sinus immediately after initiating infusion/perfusion. These experiments were conducted in independent cohorts of mice.
Isolation and labeling of lipoproteins
Human HDL (ρ = 1.063–1.21 g/ml) and LDL (ρ = 1.019–1.063 g/ml) were isolated from fresh plasma from healthy human volunteers by density gradient ultracentrifugation as described.21 All isolated fractions were dialyzed against 150 mM NaCl and 0.01% EDTA (saline-EDTA), and stored under nitrogen gas at 4°C. Protein concentrations were determined by the method of Lowry et al. HDL and LDL was labeled with 3H-cholesteryl oleate as described previously.22 Briefly, 3H-cholesteryl oleate in acetone (30 μCi/mg HDL or 60 μCi/mg LDL) was added dropwise with swirling to 2 ml lipoprotein deficient serum (LPDS) in a glass tube at room temperature. The acetone was then evaporated under nitrogen gas for 45 min. 5 mg HDL or LDL in 2 ml saline-EDTA was added to the labelled LPDS and was incubated at 4°C for 2 h. The density of the labelled lipoprotein was adjusted by solid KBr to 1.21 g/ml for HDL or 1.063 g/ml for LDL, and then the labeled lipoprotein was recovered by ultracentrifugation for 11.5 h at 55000 rpm for HDL, or for 5.5 h at 55000 rpm for LDL, dialyzed against saline-EDTA, and stored under nitrogen gas at 4°C. All procedures were performed under sterile conditions.
Real-time PCR
Isolation of RNA, cDNA synthesis and analysis of relative transcript abundance was conducted as previously described.23 RNA was isolated from frozen tissues using RNA STAT-60 (Tel-Test, Inc.) and the RNeasy Mini Kit (Qiagen). cDNA was synthesized using the iScript cDNA Synthesis Kit (Bio-Rad). Quantitative real-time PCR was conducted using SYBR Green and the 7900HT Fast Real-Time PCR System (Applied Biosystems). For markers of lipolysis, TaqMan (Applied Biosystems) assays were used. CT values of measured transcripts were normalized to that of Gapdh and expressed relative to control mice on control diet using the ΔΔCT method. Primer information is available in Major Resources Table 1.
SDS-PAGE and immunoblot analysis
SDS-PAGE and immunodetection of proteins were conducted as previously reported 19. Primary antibody information is located in Major Resources Tables. The secondary antibodies used were a goat anti-rabbit IgG horseradish peroxidase (HRP) conjugated antibody (Pierce Biotechnology 31460) and a horse anti-mouse IgG HRP conjugated antibody (Cell Signaling Technology 7076) at a 1:1000 dilution. Antibody Information can be found in Major Resources Table 2.
Statistical analysis
All data are expressed as mean ± SEM and were analyzed in GraphPad Prism. Data were initially analyzed by two-way ANOVA using genotype and biological sex as factors. No genotype by sex interaction was detected. Therefore, data were analyzed irrespective of sex (i.e. wild type vs CETP) and include both male and female mice. Data were analyzed using unpaired, two tailed t-tests or linear regression as indicated in figure legends. Normality of the data was confirmed by Shapiro-Wilk test.
Results
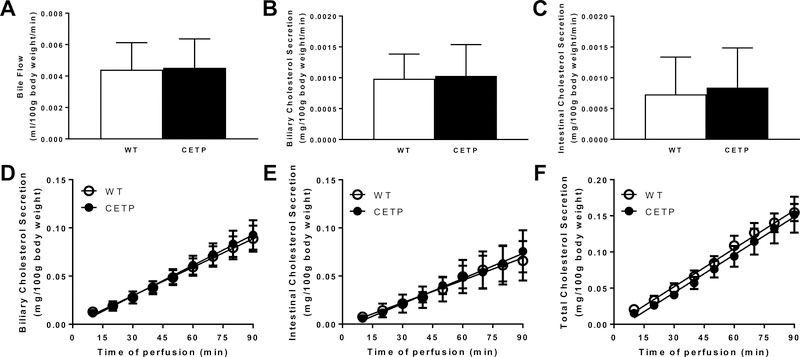
We first examined biliary and intestinal cholesterol secretion rates in male and female wild-type (WT, n=7) and CETP transgenic (CETP, n=9) littermates maintained on standard diet at 12 weeks of age. Body weight did not differ between genotypes (29.3 ± 2.0g vs. 31.3 ± 1.2g). Although plasma CETP activity was easily detected in CETP transgenic mice (41.98 ± 1.04 nmol/ml/hr), total cholesterol levels were not affected by genotype and there were only minor differences in plasma cholesterol distributions (Fig SII). Similarly, CETP did not alter bile flow or average biliary or intestinal cholesterol secretion rates (Fig 1A–C). Linear regression analysis of the cumulative cholesterol secretion over the 90-minute infusion/perfusion period revealed that biliary and intestinal cholesterol secretion were similar between genotypes, with intestinal cholesterol secretion accounting for over one third of total cholesterol secretion (Figure 1D–E). The sum of biliary and intestinal cholesterol secretion was calculated for each mouse and indicates that the presence of CETP did not alter total cholesterol secretion (Figure 1F).
Figure 1.
Biliary and intestinal cholesterol secretion in wild-type (WT, n=7) and CETP (n=9) transgenic mice. Male and female littermates were maintained on standard diet and analyzed at 12 weeks of age. Mice were anesthetized and biliary and intestinal cholesterol secretion rates were determined as described in Methods. Bile flow (A) and average cholesterol secretion rates into bile (B) and intestinal perfusates (C) over the 90 min perfusion period were calculated. The cumulative secretion of cholesterol from the liver (D) and intestine (E) as well as the sum for both organs (F) were analyzed by linear regression. Date are expressed as mean ± SEM.
The human CETP transgene (minigene) is under the control of its own promoter that increases expression in response to high fat, high cholesterol diet.24 This also allows for the accumulation of a modicum of ApoB containing lipoproteins in plasma that are generally absent in mice. Therefore, we challenged WT and CETP transgenic mice with a high fat, high cholesterol (HF-HC) diet for two weeks prior to our analysis. The HF-HC diet increased CETP activity two-fold (74.6 ± 15.41 nmol/ml/hr) relative to mice maintained on standard diet. Body weight and plasma cholesterol were slightly higher in CETP mice compared to WT controls and the expected decline in HDL cholesterol and increase in LDL cholesterol were observed (Fig SIII).
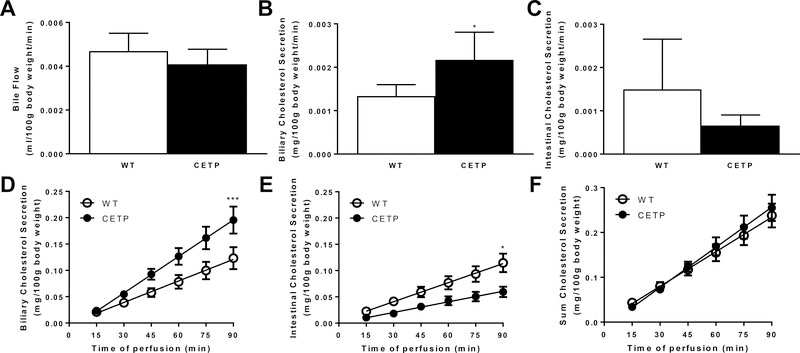
Bile flow and bile acid secretion, under basal conditions or during the surgical procedure (taurocholate-dependent), were unaffected by genotype (Fig 2A, S4). Average biliary cholesterol secretion rate was elevated in CETP mice compared to controls (Fig 2B). Average intestinal cholesterol secretion rate tended to be lower in CETP mice, but failed to reach statistical significance (Fig 2C). Linear regression analysis of the cumulative secretion of cholesterol revealed an increase in biliary and a decrease in intestinal cholesterol secretion in CETP mice, but no difference in the sum of cholesterol secreted from the liver and intestine (Fig 2D–F).
Figure 2.
Biliary and intestinal cholesterol secretion in wild-type (WT, n=7) and CETP (n=5) transgenic mice. Male and female littermates were maintained on HF-HC diet beginning at 10 weeks and analyzed at 12 weeks of age. Mice were anesthetized and biliary and intestinal cholesterol secretion rates were determined as described in Methods. Bile flow (A) and average cholesterol secretion rates into bile (B) and intestinal perfusates (C) over the 90 min perfusion period were calculated. Data are mean ± SEM and were analyzed by two-tailed t-test. *p<0.05 The cumulative secretion of cholesterol from the liver (D) and intestine (E) as well as the sum for both organs (F) were analyzed by linear regression. Asterisks indicate slopes differ at *p<0.05, ***p<0.001.
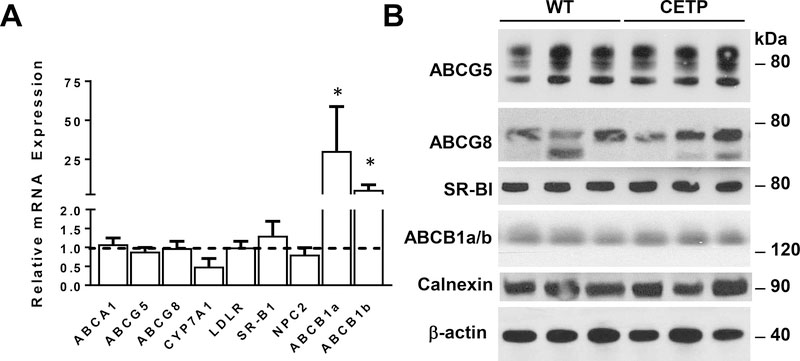
To determine if increased biliary secretion was associated with changes in hepatic gene expression, we examined mRNA levels for genes involved in cholesterol transport and metabolism in mice maintained on the HF-HC diet for two weeks. Both SR-BI and the ABCG5 ABCG8 sterol transporter regulate biliary cholesterol, but differences were not observed at the mRNA or protein level (Fig 3). At the mRNA level, we also failed to detect differences in other genes implicated in sterol uptake from the plasma compartment (LDLR) or intracellular trafficking or metabolism (NPC2, CYP7A1). The expression of ABCB1a and b were both substantially elevated at the mRNA level, but this change was not associated with an increase at the protein level. Thus, the increase in biliary cholesterol secretion in the presence of CETP was not associated with alterations in the hepatic expression of genes involved in sinusoidal uptake, intracellular trafficking, or apical secretion of cholesterol.
Figure 3.
Expression of genes implicated in hepatic cholesterol transport in WT & CETP transgenic mice maintained on HF-HC diet for two weeks. A) Total RNA was isolated from liver reverse transcribed to cDNA and analyzed for the relative abundance of mRNAs encoding genes implicated in cholesterol transport. GAPDH was used for normalization. Data are expressed as relative abundance of transcripts in CETP transgenics (n=6) compared to WT controls (n=8, dashed horizontal line). Data were analyzed by two-tailed t-test. *p<0.05. B) Representative immunoblots from liver of WT and CETP transgenic mice. Membrane proteins were prepared from liver, subjected to SDS-PAGE, and analyzed by immunoblotting. Autoradiographs were scanned and signal intensity analyzed by densitometry.
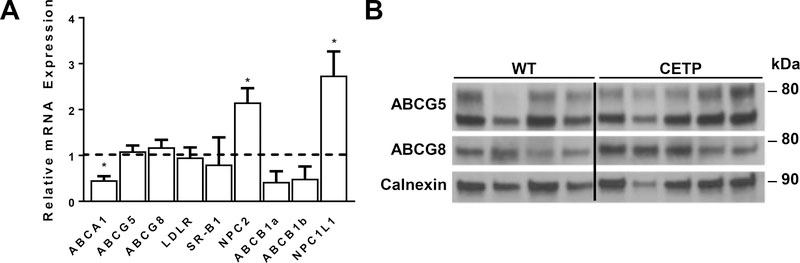
To determine if the reduction in intestinal secretion of cholesterol was associated with alterations in gene expression, we measured mRNAs for genes previously implicated in sterol transport in the gut (Fig 4A). Differences in transcript levels were not observed for genes involved in basolateral uptake (LDLR, SR-BI) or apical secretion (G5, G8), but there was a two-fold increase in the expression of NPC1L1 and a 50% reduction in ABCA1. As NPC1L1 promotes uptake of cholesterol across the apical surface, this is likely to reduce the net flux of sterol across the intestinal enterocyte into the intestinal lumen. At the protein level, differences were not appreciated for ABCG5 and ABCG8 (Fig 4B). We were unable to confirm the increase in NPC1L1 protein as commercially available antibodies failed our quality control analysis.
Figure 4.
Expression of genes implicated in intestinal cholesterol transport in WT and CETP transgenic mice maintained on HF-HC diet for two weeks. A) Total RNA was isolated from duodenum, reverse transcribed to cDNA and analyzed for the relative abundance of mRNAs encoding genes implicated in cholesterol transport. GAPDH was used for normalization. Data are expressed as relative abundance of transcripts in CETP transgenics (n=6) compared to WT controls (n=8, dashed horizontal line). Data were analyzed by two-tailed t-test. *p<0.05 B) Representative immunoblots from intestine of WT and CETP transgenic mice. Membrane proteins were prepared from the proximal small intestine, subjected to SDS-PAGE, and analyzed by immunoblotting. Three samples (1 WT and 2 CETP) were cropped from the center of the image due to apparent differences in loading or protein degradation. This is indicated by the vertical black line.
HDL is thought to be the primary plasma carrier of cholesterol in the RCT pathway. CETP serves as a shunt for esterified cholesterol from HDL (HDL-CE) to ApoB containing lipoproteins. Therefore, we determined the effect of CETP on the delivery of HDL-CE and LDL-CE to the hepatobiliary and transintestinal elimination pathways in independent cohorts of mice. To assess transhepatic and transintestinal elimination of HDL-CE and LDL-CE, we incorporated [3H]-cholesteryl oleate into human HDL and LDL. The radiolabel is positioned on the sterol moiety and traces the fate of the sterol following hydrolysis by neutral or acidic lipases in tissues. Shortly after establishing bile flow and intestinal perfusion, radiolabeled HDL or LDL were injected into the retro orbital sinus (time 0) and the appearance of radioactivity in the bile and intestinal perfusate was measured for 90 min.
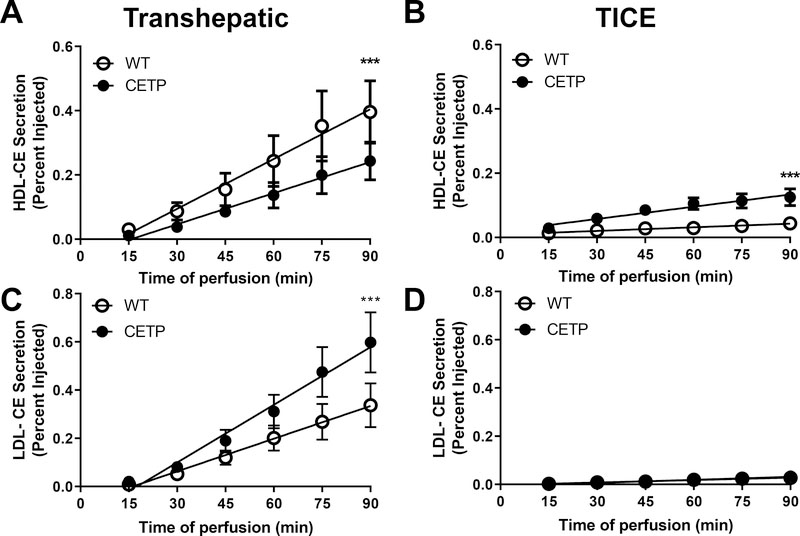
In mice maintained on standard diets, HDL-CE was preferentially eliminated through the biliary pathway, but not affected by the presence of CETP (Fig SIV). In HF-HC fed mice, the presence of CETP reduced the delivery of HDL-CE to the hepatobiliary pathway and favored the delivery of HDL-CE to the TICE pathway (Fig 5A, B). The presence of CETP promoted increased delivery of LDL-CE to bile, but had no effect on LDL-mediated TICE (Fig 5C, D). However, it should be noted that the appearance of LDL-CE in intestinal perfusates was near the lower limit of detection. At the termination of the surgical procedure, plasma and liver were collected to quantify radioactivity. Levels of radiolabeled sterol did not differ in either plasma or liver between genotypes (Fig SV). These compartments accounted for a combined 35% of the total injected dose at the termination of the procedure.
Figure 5.
Transhepatic (A,C) and transintestinal (B,D) secretion of HDL-CE (A,B) and LDL-CE (C,D) in wild-type (WT, n=6) and CETP transgenic (n=7) mice. Male and female littermates were maintained on HF-HC diet for a period of two weeks and analyzed at 12 weeks of age. Mice were anesthetized and surgically prepared for measures of biliary and intestinal cholesterol secretion. Radiolabeled lipoproteins were injected into the retroorbital sinus at time 0 and the appearance of radiolabeled sterol in the bile and intestinal perfusate monitored for 90 min. Data are mean ± SEM and are expressed as the percentage of the injected dose of radioactivity. Data were analyzed by linear regression. Asterisks indicate slopes differ at ***p<0.001.
Discussion
The present study establishes a method to simultaneously estimate biliary and intestinal cholesterol secretion rates. Using this model, we demonstrate that the presence of CETP shifts the route of cholesterol elimination towards the hepatobiliary pathway following HF-HC feeding and the accumulation of ApoB containing lipoproteins. Interestingly, the esterified cholesterol associated with HDL is almost exclusively eliminated via the hepatobiliary pathway in the absence of CETP, but is directed away from the hepatobiliary pathway in the presence of the transgene. LDL-CE is eliminated almost exclusively by the hepatobiliary pathway and was increased by CETP following HF-HC feeding. These are the first studies of the impact of CETP on TICE and indicate that alterations in plasma lipoprotein pools and sterol flux impact the route by which cholesterol is eliminated from the body.
Hepatobiliary cholesterol secretion
In the absence of the HF-HC diet, CETP had no impact on biliary cholesterol secretion or the delivery of HDL-CE to bile. This is in-line with previous studies in this mouse model indicating no effect of CETP on biliary lipid secretion.25 As expected, HF-HC feeding increased biliary cholesterol secretion (Fig 2 vs. 1) and uncovers an effect of CETP not only on biliary cholesterol secretion, but also the delivery of LDL-CE and HDL-CE to bile. However, the increase in biliary cholesterol secretion in HF-HC fed CETP mice was not associated with increased rates of bile acid secretion, indicating greater coupling of biliary cholesterol secretion to that of bile acids and the production of more saturated bile (Fig SVI).
The absence of an effect of CETP in mice maintained on the natural ingredient diet may reflect the paucity of ApoB-containing lipoproteins and the minimal number of acceptors for cholesterol ester transfer in the steady state (Fig SII). HF-HC increased total plasma cholesterol and reveals the HDL-lowering and LDL-raising effects of CETP. However, these effects are relatively modest (Fig SIII) and HDL remains the principal cholesterol carrier in plasma. The impact of CETP may be even greater in species in which ApoB-containing lipoproteins are the major carriers of plasma cholesterol.
LDL-CE was almost exclusively eliminated by the hepatobiliary route, irrespective of the presence or absence of CETP. This most likely reflects the rapid and efficient clearance of plasma LDL by the liver. The delivery of LDL-CE to bile increased in parallel with biliary cholesterol secretion in HF-HC fed CETP mice. Conversely, the effect on HDL-CE delivery to bile is in opposition to both biliary cholesterol secretion rates and the delivery of LDL-CE to bile. The opposing effects of CETP on bulk vs. radiolabeled cholesterol HDL-CE elimination in bile may be due to the miscibility of the nonesterified cholesterol with the endogenous pool in either plasma or tissue. HDL has long been thought to preferentially deliver cholesterol to the bile26, 27, but it is important to note that these studies were performed with free cholesterol associated with the surface of the particle, not the cholesterol esters within the hydrophobic core. Furthermore, the available evidence suggests that the delivery of plasma cholesterol to bile via SR-BI is independent of ABCG5 and ABCG8.28, 29
The differential effect on LDL vs HDL most likely reflects the well-established distinction for cholesterol ester delivery between LDL and HDL via receptor-mediated endocytosis and selective cholesteryl ester uptake, respectively. The concept of distinct cholesterol pools within the liver is not new but remains poorly understood. Selective uptake of cholesteryl esters is thought to take place at the plasma membrane, but membrane solubility of cholesteryl ester is extremely low. Hydrolysis is presumed to be mediated by neutral cholesteryl ester hydrolase, but the identity of the enzyme(s) has not been established. Thus, the identity and nature of the pool in which HDL-CE is delivered is unclear and the mechanism for reduced deliver of HDL-CE to bile in the presence of CETP awaits further study.
Intestinal cholesterol secretion
Using the present model, we confirm the findings of several groups suggesting that TICE accounts for a significant fraction of the overall elimination of cholesterol in the feces in mice. CETP significantly reduced intestinal secretion of cholesterol in HF-HC fed mice. This reduction was associated with an increase in mRNA for NPC1L1 and a decrease in ABCA1. NPC1L1 is known to oppose the activity of ABCG5 and ABCG8 in liver and presumably has a similar effect in the intestine.30 This increase may explain the reduction in intestinal cholesterol secretion in the presence of CETP. However, we were unable to confirm the increase in NPC1L1 protein. Conversely, ABCA1 levels are also reduced, an effect that is predicted to promote TICE. However, it should be noted that it has been previously reported that TICE is not affected by ABCA1 deficiency, suggesting that sterol transport by intestinal ABCA1 is insufficient to have a major impact on the pathway.
Cholesterol Elimination Index
In an effort to capture the relative roles of the liver and intestine on cholesterol secretion, we calculated the ratio of cholesterol secreted into the bile and intestinal perfusate over the 90 minute period for each mouse (Table 1). We have termed this the Cholesterol Elimination Index (CEI). Some fraction of cholesterol secreted in bile, and perhaps some secreted via TICE, will be reabsorbed. Consequently, a cholesterol secretion index would be a more precise term. However, CSI is commonly used to characterize biliary lipid composition and would create confusion. The CEI is slightly above 1 in wild-type mice, consistent with previous estimates that TICE accounts for up to 50% of cholesterol elimination. In HF-HC fed CETP mice, the increased biliary cholesterol secretion comes at the expense of TICE such that the CEI exceeds 3.5 and reduces the impact of TICE on cholesterol elimination to approximately 20–25%.
Table 1.
Cholesterol Elimination Index
| Diet-Genotype | Wild-type | CETP |
|---|---|---|
| Natural Ingredient Diet | 1.35 ± 0.46 | 1.63 ± 0.48 |
| HF-HC Diet | 1.33 ± 0.35 | 3.68 ± 0.81 |
Counter regulation
With respect to cholesterol secretion or the delivery of HDL-CE to the hepatobiliary or TICE pathway, changes in biliary cholesterol secretion were countered by the intestine such that total cholesterol elimination was largely unaffected. This is consistent with previous studies indicating the maintenance of sterol homeostasis despite disruptions in biliary cholesterol secretion. It remains unclear, however, how the liver and intestine communicate such changes in order to remain in neutral sterol balance. Roles for neuronal, hormonal, and microbial mechanisms have been suggested, but the molecular underpinnings of this counter regulation remain elusive.
Limitations of model
There are a number of limitations to the present study and the approach that must be acknowledged. Firstly, the mice are anesthetized. Prior studies using conscious, fistulated models have been reported, but suffer from biliary diversion without replacement. A recent report using an anastomosis approach supports a significant role for TICE in rats.3 We selected the rate of intestinal perfusion based on the initial reports of TICE such that our data would be comparable.2 However, this rate is substantially greater than the volume of fluid entering the small intestine based on drinking water intake. The small intestine is distended; the effect of which on TICE is unknown. We have subsequently reduced this rate to more physiological levels. Under these conditions, the small intestine is not distended and peristaltic contractions of the organ are observed. The data from these studies indicate similar rates of intestinal cholesterol secretion in wild-type mice (Fig SVII). Similarly, our studies employed taurocholate to maintain biliary lipid secretion and serve as the acceptor for intestinal cholesterol secretion as previously published, whereas muricholates are the predominant bile acids in mice. Our studies of THCE and TICE from radiolabeled lipoproteins employ HDL and LDL isolated from human subjects that are known to differ from endogenous pools. Finally, we only perfuse the proximal 10 cm of the small intestine. This was also based on previous reports indicating more than half of measurable TICE occurred in this portion of the small intestine.2 Consequently, our estimates of TICE may be lower than what occurs in vivo.
Future Directions
In the absence of ABCG5 and ABCG8, cholesterol synthesis is dramatically reduced in human subjects leading some investigators in the field to question the relevance of TICE in man.31–33 The impact of CETP in the present study suggests that species with CETP may be more dependent on the hepatobiliary pathway with TICE playing a less prominent role for cholesterol elimination. The results also suggest that CETP inhibitors might promote TICE at the expense of biliary cholesterol secretion in species that express the enzyme. Consistent with this idea, the CETP inhibitor, Anacetrapib, simultaneously reduced hepatic and increased fecal radiolabeled cholesterol in macrophage-to-feces RCT experiments conducted in hamsters.34
A second substantial difference in the human versus rodent lipoprotein metabolism is the prominent role of hepatic derived ApoB48 and the near absence of LDL, even in the presence of CETP in HF-HC fed mice. The relative contribution of TICE may be further diminished under conditions in which ApoB containing lipoproteins are the predominant plasma sterol carrier in the presence of CETP. Mouse models such as LDL receptor haploinsufficiency or human ApoB100 transgenic mice may be useful in further elucidating the impact of CETP on the relative rates of biliary and intestinal cholesterol secretion. Beyond this rank speculation, the relative contribution of TICE to cholesterol elimination in man is perhaps less important until the pathway is called upon under conditions of disrupted biliary secretion or potentially targeted for the development of compounds that promote extrahepatic RCT.
Supplementary Material
Highlights.
Biliary and intestinal cholesterol secretion measured simultaneously
CETP promotes hepatobiliary cholesterol secretion at the expense of intestinal
CETP diverts HDL-cholesterol ester away from the hepatobiliary pathway
Biliary cholesterol secretion is not coupled to hepatic clearance of HDL-cholesterol esters
Acknowledgments
Sources of Funding
This project was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (1R01DK113625, R01DK100892), National Center for Research Resources (P20RR021954-05), the National Institute of General Medical Sciences (8P20GM103527), and the National Center for Advancing Translational Sciences (UL1TR000117) from the National Institutes of Health.
Abbreviations
- RCT
reverse cholesterol transport
- ABC
ATP-binding cassette transporters
- TICE
transintestinal cholesterol elimination
- CETP
cholesteryl ester transfer protein
Footnotes
Disclosures
The authors have nothing to disclose.
References
- 1.Temel RE, Brown JM. A new framework for reverse cholesterol transport: Non-biliary contributions to reverse cholesterol transport. World journal of gastroenterology : WJG. 2010;16:5946–5952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Velde AE, Vrins CLJ, van den Oever K, Kunne C, Oude Elferink RPJ, Kuipers F, Groen AK. Direct intestinal cholesterol secretion contributes significantly to total fecal neutral sterol excretion in mice. Gastroenterology. 2007;133:967–975 [DOI] [PubMed] [Google Scholar]
- 3.de Boer JF, Schonewille M, Dikkers A, Koehorst M, Havinga R, Kuipers F, Tietge UJ, Groen AK. Transintestinal and biliary cholesterol secretion both contribute to macrophage reverse cholesterol transport in rats-brief report. Arterioscler Thromb Vasc Biol. 2017;37:643–646 [DOI] [PubMed] [Google Scholar]
- 4.de Boer JF, Schonewille M, Boesjes M, Wolters H, Bloks VW, Bos T, van Dijk TH, Jurdzinski A, Boverhof R, Wolters JC, Kuivenhoven JA, van Deursen JM, Oude Elferink RP, Moschetta A, Kremoser C, Verkade HJ, Kuipers F, Groen AK. Intestinal farnesoid x receptor controls transintestinal cholesterol excretion in mice. Gastroenterology. 2017;152:1126–1138 e1126 [DOI] [PubMed] [Google Scholar]
- 5.Marshall SM, Gromovsky AD, Kelley KL, Davis MA, Wilson MD, Lee RG, Crooke RM, Graham MJ, Rudel LL, Brown JM, Temel RE. Acute sterol o-acyltransferase 2 (soat2) knockdown rapidly mobilizes hepatic cholesterol for fecal excretion. PloS one. 2014;9:e98953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le May C, Berger JM, Lespine A, Pillot B, Prieur X, Letessier E, Hussain MM, Collet X, Cariou B, Costet P. Transintestinal cholesterol excretion is an active metabolic process modulated by pcsk9 and statin involving abcb1. Arterioscler Thromb Vasc Biol. 2013;33:1484–1493 [DOI] [PubMed] [Google Scholar]
- 7.Vrins CL, van der Velde AE, van den Oever K, Levels JH, Huet S, Oude Elferink RP, Kuipers F, Groen AK. Peroxisome proliferator-activated receptor delta activation leads to increased transintestinal cholesterol efflux. J Lipid Res. 2009;50:2046–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Velde AE, Vrins CL, van den Oever K, Seemann I, Oude Elferink RP, van Eck M, Kuipers F, Groen AK. Regulation of direct transintestinal cholesterol excretion in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G203–G208 [DOI] [PubMed] [Google Scholar]
- 9.van der Veen JN, van Dijk TH, Vrins CL, van Meer H, Havinga R, Bijsterveld K, Tietge UJ, Groen AK, Kuipers F. Activation of the liver x receptor stimulates trans-intestinal excretion of plasma cholesterol. J Biol Chem. 2009;284:19211–19219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brufau G, Kuipers F, Lin Y, Trautwein EA, Groen AK. A reappraisal of the mechanism by which plant sterols promote neutral sterol loss in mice. PloS one. 2011;6:e21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warrier M, Shih DM, Burrows AC, Ferguson D, Gromovsky AD, Brown AL, Marshall S, McDaniel A, Schugar RC, Wang Z, Sacks J, Rong X, Vallim TA, Chou J, Ivanova PT, Myers DS, Brown HA, Lee RG, Crooke RM, Graham MJ, Liu X, Parini P, Tontonoz P, Lusis AJ, Hazen SL, Temel RE, Brown JM. The tmao-generating enzyme flavin monooxygenase 3 is a central regulator of cholesterol balance. Cell Rep. 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meriwether D, Sulaiman D, Wagner A, Grijalva V, Kaji I, Williams KJ, Yu L, Fogelman S, Volpe C, Bensinger SJ, Anantharamaiah GM, Shechter I, Fogelman AM, Reddy ST. Transintestinal transport of the anti-inflammatory drug 4f and the modulation of transintestinal cholesterol efflux. J Lipid Res. 2016;57:1175–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall SM, Kelley KL, Davis MA, Wilson MD, McDaniel AL, Lee RG, Crooke RM, Graham MJ, Rudel LL, Brown JM, Temel RE. Reduction of vldl secretion decreases cholesterol excretion in niemann-pick c1-like 1 hepatic transgenic mice. PloS one. 2014;9:e84418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jakulj L, van Dijk TH, de Boer JF, Kootte RS, Schonewille M, Paalvast Y, Boer T, Bloks VW, Boverhof R, Nieuwdorp M, Beuers UH, Stroes ES, Groen AK. Transintestinal cholesterol transport is active in mice and humans and controls ezetimibe-induced fecal neutral sterol excretion. Cell Metab. 2016;24:783–794 [DOI] [PubMed] [Google Scholar]
- 15.Briand F, Thieblemont Q, Muzotte E, Sulpice T. High-fat and fructose intake induces insulin resistance, dyslipidemia, and liver steatosis and alters in vivo macrophage-to-feces reverse cholesterol transport in hamsters. J Nutr. 2012;142:704–709 [DOI] [PubMed] [Google Scholar]
- 16.Vrins CL, Ottenhoff R, van den Oever K, de Waart DR, Kruyt JK, Zhao Y, van Berkel TJ, Havekes LM, Aerts JM, van Eck M, Rensen PC, Groen AK. Trans-intestinal cholesterol efflux is not mediated through high density lipoprotein. J Lipid Res. 2012;53:2017–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bura KS, Lord C, Marshall S, McDaniel A, Thomas G, Warrier M, Zhang J, Davis MA, Sawyer JK, Shah R, Wilson MD, Dikkers A, Tietge UJ, Collet X, Rudel LL, Temel RE, Brown JM. Intestinal sr-bi does not impact cholesterol absorption or transintestinal cholesterol efflux in mice. J Lipid Res. 2013;54:1567–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talalay P Enzymic analysis of steroid hormones. Methods of biochemical analysis. 1960;8:119–143 [DOI] [PubMed] [Google Scholar]
- 19.Sabeva NS, Rouse EJ, Graf GA. Defects in the leptin axis reduce abundance of the abcg5-abcg8 sterol transporter in liver. J Biol Chem. 2007;282:22397–22405 [DOI] [PubMed] [Google Scholar]
- 20.Kieft KA, Bocan TM, Krause BR. Rapid on-line determination of cholesterol distribution among plasma lipoproteins after high-performance gel filtration chromatography. J Lipid Res. 1991;32:859–866 [PubMed] [Google Scholar]
- 21.Coetzee GA, Strachan AF, van der Westhuyzen DR, Hoppe HC, Jeenah MS, de Beer FC. Serum amyloid a-containing human high density lipoprotein 3. Density, size, and apolipoprotein composition. J Biol Chem. 1986;261:9644–9651 [PubMed] [Google Scholar]
- 22.Roberts DC, Miller NE, Price SG, Crook D, Cortese C, La Ville A, Masana L, Lewis B. An alternative procedure for incorporating radiolabelled cholesteryl ester into human plasma lipoproteins in vitro. Biochem J. 1985;226:319–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pijut SS, Corbett DE, Wang Y, Li J, Charnigo RJ, Graf GA. Effect of peripheral circadian dysfunction on metabolic disease in response to a diabetogenic diet. Am J Physiol Endocrinol Metab. 2016:ajpendo.00328.02015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang XC, Agellon LB, Walsh A, Breslow JL, Tall A. Dietary cholesterol increases transcription of the human cholesteryl ester transfer protein gene in transgenic mice. Dependence on natural flanking sequences. J Clin Invest. 1992;90:1290–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harada LM, Amigo L, Cazita PM, Salerno AG, Rigotti AA, Quintao EC, Oliveira HC. Cetp expression enhances liver hdl-cholesteryl ester uptake but does not alter vldl and biliary lipid secretion. Atherosclerosis. 2007;191:313–318 [DOI] [PubMed] [Google Scholar]
- 26.Robins SJ, Fasulo JM. High density lipoproteins, but not other lipoproteins, provide a vehicle for sterol transport to bile. J Clin Invest. 1997;99:380–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji Y, Wang N, Ramakrishnan R, Sehayek E, Huszar D, Breslow JL, Tall AR. Hepatic scavenger receptor bi promotes rapid clearance of high density lipoprotein free cholesterol and its transport into bile. J Biol Chem. 1999;274:33398–33402 [DOI] [PubMed] [Google Scholar]
- 28.Wiersma H, Gatti A, Nijstad N, Oude Elferink RP, Kuipers F, Tietge UJ. Scavenger receptor class b type i mediates biliary cholesterol secretion independent of atp-binding cassette transporter g5/g8 in mice. Hepatology. 2009;50:1263–1272 [DOI] [PubMed] [Google Scholar]
- 29.Dikkers A, Freak de Boer J, Annema W, Groen AK, Tietge UJ. Scavenger receptor bi and abcg5/g8 differentially impact biliary sterol secretion and reverse cholesterol transport in mice. Hepatology. 2013;58:293–303 [DOI] [PubMed] [Google Scholar]
- 30.Temel RE, Tang W, Ma Y, Rudel LL, Willingham MC, Ioannou YA, Davies JP, Nilsson LM, Yu L. Hepatic niemann-pick c1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J Clin Invest. 2007;117:1968–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salen G, Shefer S, Nguyen L, Ness GC, Tint GS, Shore V. Sitosterolemia. J Lipid Res. 1992;33:945–955 [PubMed] [Google Scholar]
- 32.Honda A, Salen G, Nguyen LB, Tint GS, Batta AK, Shefer S. Down-regulation of cholesterol biosynthesis in sitosterolemia: Diminished activities of acetoacetyl-coa thiolase, 3-hydroxy-3- methylglutaryl-coa synthase, reductase, squalene synthase, and 7- dehydrocholesterol delta7-reductase in liver and mononuclear leukocytes. J Lipid Res. 1998;39:44–50 [PubMed] [Google Scholar]
- 33.Wang J, Mitsche MA, Lutjohann D, Cohen JC, Xie XS, Hobbs HH. Relative roles of abcg5/abcg8 in liver and intestine. J Lipid Res. 2015;56:319–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castro-Perez J, Briand F, Gagen K, Wang SP, Chen Y, McLaren DG, Shah V, Vreeken RJ, Hankemeier T, Sulpice T, Roddy TP, Hubbard BK, Johns DG. Anacetrapib promotes reverse cholesterol transport and bulk cholesterol excretion in syrian golden hamsters. J Lipid Res. 2011;52:1965–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.