Abstract
Effective ventilation of the lungs is essential in mediating pulmonary vasodilation at birth to allow effective gas exchange and an increase in systemic oxygenation. Unsuccessful transition prevents the increase in pulmonary blood flow after birth resulting in hypoxemia and persistent pulmonary hypertension of the newborn (PPHN). Management of neonates with PPHN includes ventilation of the lungs with supplemental oxygen to correct hypoxemia. Optimal oxygenation should meet oxygen demand to the tissues and avoid hypoxic pulmonary vasoconstriction (HPV) while preventing oxidative stress. The optimal target for oxygenation in PPHN is not known. Animal models have demonstrated that PaO2<45mmHg exacerbates HPV. However, there are no practical methods of assessing oxygen levels associated with oxidant stress. Oxidant stress can be due to free radical generation from underlying lung disease or from free radicals generated by supplemental oxygen. Free radicals act on the nitric oxide pathway reducing cGMP and promoting pulmonary vasoconstriction. Antioxidant therapy improves systemic oxygenation in an animal model of PPHN but there are no clinical trials to support such therapy. Targeting preductal SpO2 between 90-97% and PaO2 at 50-80 mmHg appears prudent in PPHN but clinical trials to support this practice are lacking. Preterm infants with PPHN present unique challenges due to lack of antioxidant defenses and functional and structural immaturity of the lungs. This review highlights the need for additional studies to mitigate the impact of oxidative stress in the lung and pulmonary vasculature in PPHN.
Preface: appropriate clinical application of oxygen for the infant with PPHN
Introduction
A 39-week gestation infant with meconium aspiration syndrome (MAS), hypoxic-ischemic encephalopathy (HIE) and cyanosis is transferred to the regional perinatal center. During transport, the baby’s oxygen saturations (SpO2) were labile and fluctuating between 78 to 94%. Hence, inspired oxygen was increased to 100%. An umbilical arterial blood gas obtained soon after arrival to the neonatal intensive care unit (NICU) demonstrated a pH of 7.12, PaCO2 of 64 mmHg, PO2 of 42 mmHg with a base deficit of 8 mEq/L. The infant was placed on high frequency oscillatory ventilation (HFOV) and treated with surfactant and inhaled nitric oxide (iNO). Umbilical arterial PaO2 increased to 180 mmHg with SpO2 of 99% with these measures.
The clinical conundrum described in this vignette is commonly encountered in the NICU. Perinatal distress and HIE increase oxidative stress. [1] Hyperoxic ventilation increases oxygen toxicity to the lung. Despite hyperoxic ventilation, systemic hypoxemia results in inadequate oxygen delivery to systemic tissues. Difficulties in titrating inspired oxygen result in intermittent systemic hyperoxia and hypoxemia exacerbating oxidative stress. The clinician faces dilemma in an attempt to achieve the goals of supplemental oxygen therapy: (a) reduce pulmonary vascular resistance (PVR), (b) increases oxygen delivery to the tissues and (c) minimize formation of free radicals.
This review discusses the use of supplemental oxygen to overcome the abnormal fetal to newborn transition at birth evident in PPHN, methods to determine oxygen saturation and optimal oxygenation, and sources and targets of reactive oxygen species (ROS) generation in experimental PPHN and due to oxygen exposure. We then discuss oxidant stress in pulmonary hypertension (PH) associated with prematurity, and identify potential therapeutic targets that may improve the efficacy of antioxidants to alleviate the consequences of increased oxidant stress during supplemental oxygen ventilation for term and preterm infants with PPHN.
The fetal to newborn transition.
At birth, the lung adapts to replace the placenta as the organ of gas exchange. This is facilitated by a dramatic decrease in pulmonary vascular resistance, regulated by complex physiological and biochemical processes with a central role for NO, resulting in an 8-10 fold increase in pulmonary blood flow [2]. eNOS converts L-arginine and molecular oxygen to L-citrulline and the vasodilator NO using O2 as well as electrons from NADPH. NO stimulates vasorelaxation by activating soluble guanylate cyclase (sGC) to generate cGMP, while phosphodiesterase type 5 (PDE5) impairs vasorelaxation by degrading cGMP (figure 1). The activity of eNOS is regulated by protein phosphorylation as well as by the availability of substrate and several cofactors including calcium-calmodulin, HSP90, and tetrahydrobiopterin (BH4). Mechanisms that inhibit eNOS activity or attenuate downstream NO signaling can induce vasoconstriction.
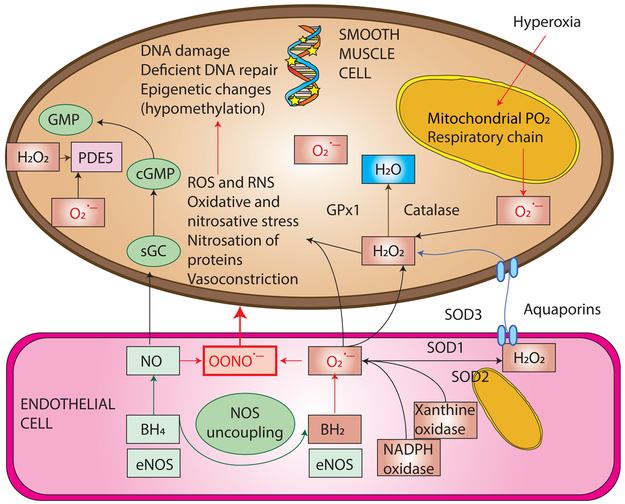
Figure 1. Free radicals - reactive oxygen (ROS) and nitrogen species (RNS) in persistent pulmonary hypertension of the newborn.
Reactive oxygen species such as superoxide anions (O2.−) can be produced by the electron transport chain (ETC) in the mitochondria, due to exposure to hyperoxia or from enzymes such as uncoupled nitric oxide synthase (NOS), NADPH oxidase, and xanthine oxidase or the Fenton reaction. Nitric oxide (NO) is a free radical and avidly binds to superoxide anion to form peroxynitrite (OONO.−) at the rate of 6.7/M/s. This rate is considerably faster than the rate of dismutation of superoxide anions by superoxide dismutase (SOD) to form hydrogen peroxide (H2O2). Hydrogen peroxide can diffuse across membranes through aquaporins. Hydrogen peroxide is broken down by catalase and glutathione peroxidase (GPx1) to water. NO and oxygen are vasodilators but peroxynitrite is a potent vasoconstrictor. ROS (superoxide anions and hydrogen peroxide) stimulate phosphodiesterase 5 (PDE5) enzyme and breakdown cGMP limiting vasodilator effect of NO. Copyright Satyan Lakshminrusimha.
BH4, tetrahydrobiopterin; SOD1 – superoxide dismutase; SOD3, extracellular superoxide dismutase; eNOS, endothelial nitric oxide synthase; SOD2 or MnSOD, manganese superoxide dismutase; sGC, soluble guanylate cyclase; cGMP – cyclic guanosine monophosphate;
Abnormal vascular transition at birth:
In conditions such as birth asphyxia, [1] MAS, [3] [4] intrauterine stress, [5] maldevelopment leading to alveolar and vascular hypoplasia (e.g., congenital diaphragmatic hernia – CDH) [6] [7] and maternal ingestion of non-steroidal anti-inflammatory drugs (NSAIDs) [8] during late pregnancy (by constriction of ductus arteriosus), the increase in pulmonary blood flow soon after birth may not occur. [9, 10] In these conditions, the high pulmonary vascular resistance (PVR) characteristic of the fetal lung persists into the neonatal period leading to PPHN. PPHN is characterized by extrapulmonary right-to-left shunting resulting in labile hypoxemia. [9] Management of neonates with PPHN includes ventilation of the lungs with supplemental oxygen to correct hypoxemia and use of pulmonary vasodilators such as inhaled nitric oxide (iNO).
Delivery room management of term infants at risk for PPHN:
The diagnosis of PPHN is often made in the postnatal period. However, infants with an antenatal diagnosis of CDH, presence of meconium stained amniotic fluid and perinatal asphyxia can be identified at birth to be at risk for PPHN. [11] Current guidelines recommend initiation of resuscitation for all term newborn with 21% oxygen and titrating inspired oxygen based on preductal SpO2. [12] The Canadian guidelines for management of CDH recommend supplemental oxygen to achieve SpO2 ≥ 85%. [13] The European guidelines recommend initiation of ventilation with FiO2 < 1.0 and titrated to achieve preductal SpO2 between 80 and 95%. [14] Investigators at Children’s Hospital of Philadelphia have demonstrated that reducing initial FiO2 from 1.0 to 0.5 did not result in an untoward events. [15]
Stuides in lambs without PPHN have shown that use of 100% oxygen for initial ventilation results in a greater decrease in PVR compared to 21% oxygen but impairs subsequent vasodilator response to iNO and systemic acetylcholine. [16] Lambs with PPHN induced by antenatal ductal ligation showed similar degree of decrease in PVR with ventilation with 21%, 50% and 100% oxygen. [17] After the first 30 minutes, all the three groups of lambs were ventilated with 50% oxygen and treated with iNO. Prior exposure to 100% oxygen impaired vasodilator response to iNO. [17] An increase in pulmonary arterial superoxide anions is observed in asphyxiated lambs after 30 min of ventilation with 100% oxygen. [18] Resuscitation with 100% oxygen was associated with increased pulmonary arterial contractility to norepinephrine, a phenomenon that was reversed with prior treatment with superoxide dismutase. [18] We speculate that even brief exposure to 100% oxygen at birth increases free radical generation that can impair effectiveness of iNO. Similar increase in oxidative stress has been observed in human term neonates after resuscitation with 100% oxygen. [19] In an ovine model of MAS with PPHN, initiation of resuscitation with 21% oxygen followed by titration to achieve target SpO2 recommended by the Neonatal Resuscitation Program [12] resulted in higher pulmonary blood flow compared to 21% oxygen ventilation only. [20] These results suggest that initial resuscitation of term infants at risk for PPHN with 21-50% oxygen with titration to achieve target SpO2 recommended by Neonatal Resuscitation Program is probably a safe practice and may potentially enhance subsequent response to pulmonary vasodilators such as iNO.
Assessment of oxygenation in PPHN:
Site of measurement of oxygenation status:
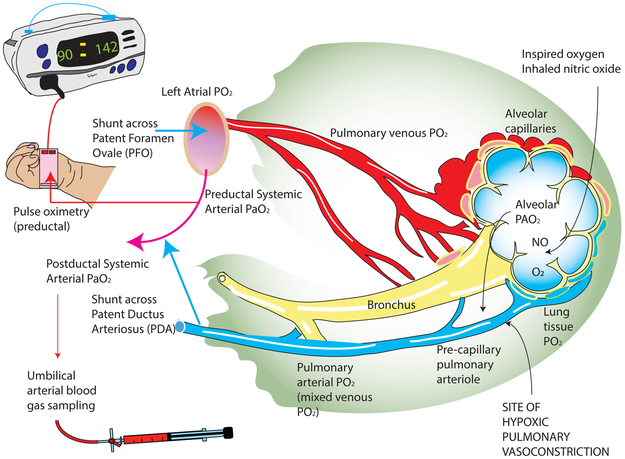
Traditional management of PPHN assessed oxygenation by measurement of postductal PaO2 using umbilical arterial catheters and calculation of oxygenation index (OI) using the formula – OI = Mean airway pressure (cm H2O) × FiO2 × 100 + Postductal PaO2. [21, 22] The primary determinant of PVR is the oxygen level in the precapillary pulmonary arteriole [23] which is influenced by alveolar oxygen (PAO2). [24] Clinically systemic arterial oxygenation is used as a surrogate of alveolar oxygenation and in parenchymal lung disease with ventilation perfusion mismatch and high alveolar-arterial oxygen gradients (A-a DO2), systemic PaO2 levels will not accurately reflect alveolar or pre-pulmonary capillary arteriolar oxygen levels (figure 2). In patients with right-to-left ductal shunting, assessment of preductal oxygenation using a right radial arterial line or right upper limb pulse oximetry might be a more accurate strategy in the management of PPHN. [25]
Figure 2. Oxygen tension in different sites of pulmonary circulation and pulmonary vascular resistance (PVR).
The precise site of hypoxic pulmonary vasoconstriction and the sensing mechanisms are not clear but is thought by most to be the precapillary pulmonary arteriole in the lung. The pulmonary arterial smooth muscle cells (PASMC) are exposed to lung tissue PO2, alveolar PAO2, and pulmonary arterial (mixed venous) PO2. It is thought that the rapid diffusion from alveolar PAO2 is the predominant determinant of oxygen tension in PASMC. In infants with persistent pulmonary hypertension of the newborn (PPHN), PAO2 can be approximately calculated using preductal PaO2 values. The presence of a right-to-left shunt at the atrial level or ductal level can reduce PaO2 levels in PPHN. Heterogeneous lung disease can also interfere with the relationship between alveolar PAO2 and PVR. Copyright Satyan Lakshminrusimha. Modified from Hemodynamics and Cardiology: Neonatology Questions and Controversies 3rd Edition.
Targets for preductal PaO2 in the management of PPHN in term infants:
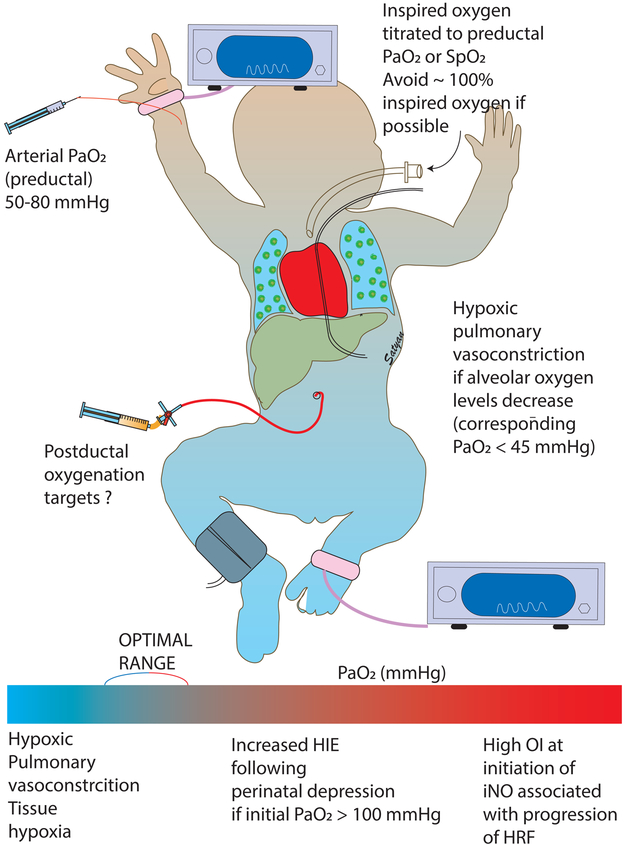
The optimal lower limit for PaO2 in the management of PPHN should meet oxygen demand to the tissues and avoid hypoxic pulmonary vasoconstriction (figure 3). In healthy newborn calves, the limit of PaO2 below which hypoxic pulmonary vasoconstriction occurs appears to be 45 mmHg. [26] Similar values were observed in our laboratory newborn lambs with PPHN induced by meconium aspiration and control lambs without lung disease. [27] However, the upper limit of optimal oxygenation is not clear and is probably related to toxicity and oxidative stress. While measures of oxidative stress are measured in experimental settings, we do not have a practical method of assessing oxygen toxicity in the neonatal intensive care unit. Increasing PaO2 above 100 mmHg does not result in enhanced pulmonary vasodilation. [17] Hypoxemia (<45 mmHg) causes pulmonary vasoconstriction and normoxia (50-80 mmHg) results in pulmonary vasodilation but, hyperoxia (PaO2 > 100 mmHg) does not result in additional pulmonary vasodilation but can lead to oxidative stress. [28] Kapadia et al have demonstrated that the incidence of HIE increases if neonates with perinatal asphyxia had PaO2 > 100 mmHg in the first hour of postnatal period. [29] Exposure to prolonged hyperoxic ventilation and high oxygenation indices at the time of randomization to iNO in clinical trials is associated with a higher incidence of ECMO/death. [30] [31] In lambs with PPHN induced by antenatal ligation of ductus arteriosus, exposure to 100% oxygen at birth impairs vasodilator response to iNO. [17] These associations with hyperoxic ventilation, high OI and impaired response to iNO can partly be explained by generation of ROS in the pulmonary vasculature (figure 1). Based on these results, we recommend targeting preductal PaO2 between 50 and 80 mmHg during the management of PPHN in term infants.
Figure 3. Optimal targets for oxygenation in the management of acute PPHN in term infants.
Based on preclinical data, we recommend a preductal PaO2 of 50 to 80 mmHg in infants with PPHN. The corresponding SpO2 targets are approximately 90 to 97%. Oxygen targets below this range are associated with hypoxic pulmonary vasoconstriction. Targets above this range are associated with poor response to inhaled nitric oxide and higher incidence of HIE following perinatal depression.
Targets for oxygen saturation by pulse oximetry (SpO2) in PPHN:
Non-invasive and continuous assessment of oxygenation is typically done using pulse oximeters. There are very few published guidelines for optimal SpO2 targets in term infants with PPHN. The Canadian [13] and European [14] guidelines for the management of PPHN associated with congenital diaphragmatic hernia (CDH) recommend a preductal SpO2 targets between 85 to 95% and 80 to 95% respectively. Postductal SpO2 targets > 70% are considered adequate during management of CDH patients in the absence of lactic acidosis. The American Thoracic Society (ATS) guidelines for Pediatric Pulmonary Hypertension recommend SpO2 targets between 92-95% for the management of pulmonary hypertension associated with bronchopulmonary dysplasia (BPD). [32] In lambs with PPHN induced by antenatal ductal ligation, preductal SpO2 targets of 90-97% are associated with low PVR. [17] Based on these published guidelines and preclinical data, we recommend preductal SpO2 target of 90 to 97% and postductal target of >70% (in the absence of lactic acidosis) during the management of acute phase of PPHN in term infants (figure 3).
Oxygen-hemoglobin dissociation curve – relationship between SpO2 and PaO2:
Several factors such as type of hemoglobin, pH and body temperature can shift the oxygen-hemoglobin dissociation curve. [33] It is not uncommon to observe a preterm neonate admitted from the delivery room have a preductal SpO2 of 90% with an umbilical arterial PaO2 in the high 30s and low 40s (mmHg). Preductal SpO2 is an important determinant of oxygen content of arterial blood (CaO2) and oxygen delivery to essential organs such as brain and heart. [25, 27] However, it may be prudent to periodically check an arterial blood gas to assess PaO2 (see paragraph below).
Asphyxia, therapeutic hypothermia and PPHN:
Birth asphyxia is a common cause of oxidative stress [34] and is often associated with PPHN. [1] Therapeutic hypothermia either by selective head cooling or whole body cooling is standard of care in the management of moderate to severe hypoxic-ischemic encephalopathy (HIE) in term infants. PPHN is associated with approximately a fourth of patients undergoing whole body hypothermia for moderate to severe HIE. [3] Due to a shift in oxygen-hemoglobin dissociation curve to the left during hypothermia, higher preductal SpO2 targets of 95-98% may be necessary to achieve PaO2 of 50 to 80 mmHg during whole body hypothermia. [35] Providing supplemental oxygen right from birth is unlikely to benefit these patients at risk for HIE and PPHN. Data from asphyxiated lambs demonstrates that hyperoxic ventilation at birth increases PaO2 but the decrease in PVR by 30 minutes is similar to that achieved with normoxic ventilation. [28] In addition, following perinatal asphyxia, infants with hyperoxemia on their first blood gas (PaO2>100 mmHg) were more likely to develop moderate to severe HIE compared to infants with normoxemia. [29] The same study demonstrated that among infants with moderate to severe HIE, hyperoxemia on admission was more likely to be associated with abnormal brain MRI findings.
Free radicals in PPHN:
Free radicals are important vascular signaling molecules that regulate pulmonary vascular tone and function. Multiple enzymatic oxidase systems contribute to the production of free radicals in the vessel wall, and each system has specific roles in vascular physiology (figure 1). In the endothelium, mitochondria, xanthine oxidase, and NADPH oxidases (Nox) generate free radicals, while an uncoupling of endothelial nitric oxide synthase (eNOS) can also contribute [36, 37]. In vascular smooth muscle, Nox isoforms and mitochondria are significant sources of free radicals [38-42]. Antioxidants regulate vascular signaling pathways by scavenging free radicals. Enzyme systems donate an electron to molecular oxygen to generate superoxide anion (O2.−), and superoxide dismutases (SODs) generate the non-radical reactive oxygen species (ROS) H2O2 through dismutation of superoxide. H2O2 is diffusible across membranes via aquaporins and is thus an important intracellular and intercellular signaling molecule. H2O2 is scavenged by enzymes including catalase and glutathione peroxidase. Superoxide can also react with other radicals such as nitric oxide (.NO) to form peroxynitrite (ONOO.−), thus depleting bioavailable NO and impairing NO-mediated vasorelaxation. ONOO is an extremely reactive oxidant that can promote further vascular dysfunction by non-specific nitration of proteins resulting in a significant alteration in their functions. Impaired regulation of free radicals potentially induces vascular injury due to their interaction with proteins, DNA, RNA and lipids [43, 44].
Experimental PPHN and ROS.
In fetal lambs, ligation or compression of the ductus arteriosus rapidly induces fetal and neonatal pulmonary hypertension. Similar to newborns that die of PPHN, these lambs have medial hypertrophy within the small pulmonary arteries, complete muscularization of normally partially muscularized pulmonary arteries, and extension of muscle to non-muscularized arteries. The endothelium and vascular smooth muscle of PPHN pulmonary arteries display elevated levels of superoxide [45, 46] and H2O2 [47, 48]. NADPH oxidase (Nox) enzymes are membrane proteins that transfer electrons from NADPH to molecular oxygen, producing ROS intracellularly or extracellularly depending on the isoform and subcellular location of the enzyme. The Nox2 isoform is expressed in cells comprising the vascular wall and increased Nox2 subunit expression correlates with increased superoxide levels and impaired pulmonary vasorelaxation in both lamb and piglet models of neonatal pulmonary hypertension [45, 49]. Furthermore, extracellular SOD (ecSOD or SOD3) activity is decreased in PPHN pulmonary arteries [50] exacerbating the effects of Nox2-derived superoxide. The Nox4 isoform has been shown to generate both superoxide and H2O2 depending upon the stimulus and cell type [51], and increased Nox4 expression correlates with increased H2O2 in PPHN pulmonary arteries [48].
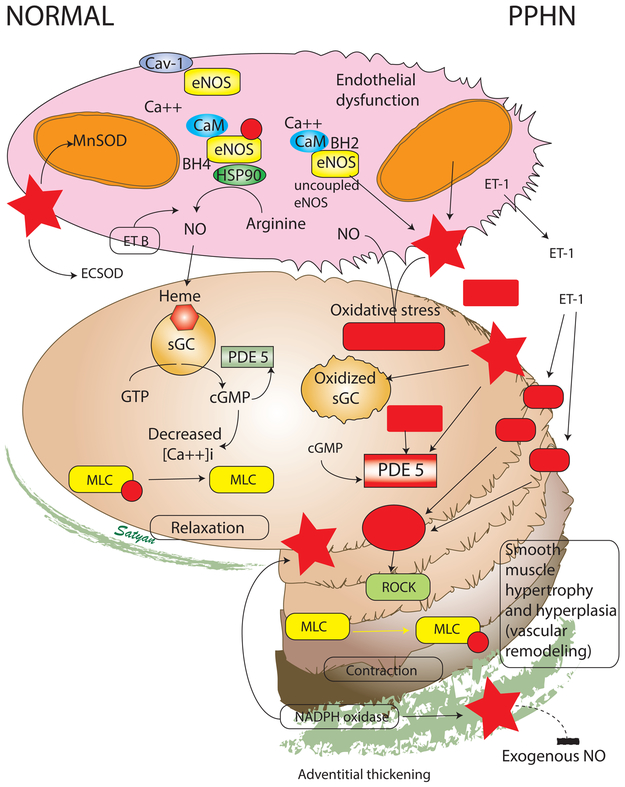
As shown in figure 4, PPHN lambs exhibit alterations in major components of NO-mediated pulmonary vasodilation including decreased NOS expression and activity [52], decreased expression of soluble guanylate cyclase [53], increased PDE5 expression and activity [54], and increased ET-1 levels [55]. These alterations may be due, in part, to increased pulmonary artery H2O2, which potentially contributes to decreased eNOS expression [56], impaired cGMP production [57], elevated PDE5 activity [57] and decreased ecSOD activity [50]. Peroxynitrite formation is elevated in PPHN lambs [58], and inhibits NOS activity via mechanisms that include decreased association with HSP90 [59, 60]. eNOS becomes a source of ROS when the enzyme becomes uncoupled, resulting in incomplete reduction of molecular oxygen with the formation of superoxide. Uncoupled eNOS is evident in pulmonary arteries isolated from PPHN lambs [61] and may be a consequence of increased Nox activity [62, 63]. The role of superoxide in impaired NO-mediated pulmonary vasodilation in PPHN is demonstrated by studies showing improved NO-mediated relaxation in isolated pulmonary arteries treated with SOD or superoxide scavengers [64]. The negative impact of elevated H2O2 in PPHN is confirmed by similar studies showing improved relaxation to exogenous NO in PPHN pulmonary arteries treated with catalase [47]. Overall these data highlight the positive feedback mechanisms leading to increased ROS and impaired NO-mediated vasodilation in PPHN.
Figure 4. Cellular and biochemical changes in PPHN secondary to oxidative and nitrosative stress.
Pulmonary arteries from human neonates and animal models of PPHN demonstrate thickening of the muscular layer and adventitia. The normal pulmonary arterial endothelium produces nitric oxide (NO) from phosphorylated endothelial nitric oxide synthase (eNOS) coupled to heat shock protein 90 (HSP90) with tetrahydrobiopterin (BH4) as a cofactor with adequate supply of arginine as substrate. The eNOS protein is bound to caveolin-1 (Cav-1) prior to its release by a calcium-calmodulin (CaM) dependent process. Endothelin acting through endothelin-B receptor (ETB) on the endothelium stimulates NO production. Manganese superoxide dismutase (MnSOD or SOD-2) is present in the mitochondria and scavenges superoxide anions. Extracellular superoxide dismutase (ecSOD) limits the interaction (and inactivation) of NO with superoxide anions in the endothelial-smooth muscle interface. NO reaches the smooth muscle cell and binds to reduced soluble guanylate cyclase (sGC) which in turn catalyzes the conversion of GTP to cGMP. Cyclic GMP is an important second messenger that reduces the cytosolic concentration of ionic calcium [Ca++]i. Reduced concentration of ionic calcium leads to dephosphorylation of myosin light chains (MLC) resulting in smooth muscle relaxation. In PPHN, endothelial dysfunction leads to uncoupling of eNOS. Low levels of MnSOD and possibly ecSOD increase oxidative stress and formation of superoxide anions. Superoxide anions inactive nitric oxide resulting in the formation of toxic peroxynitrite. Oxidized sGC cannot be activated by NO to produce cGMP. Superoxide anions stimulate phosphodiesterase 5 (PDE5) activity and enhance breakdown of cGMP. Pulmonary arterial endothelial cells from PPHN pulmonary arteries produce increased levels of endothelin-1 (ET-1), a powerful pulmonary vasoconstrictor. ET-1 acts through ETA receptor and stimulates Rho-A, Rho-kinase (ROCK) pathway leading to phosphorylation of MLC and smooth muscle contraction. The pulmonary arterial endothelial cells have low levels of ETB receptors. The net effect is reduced cGMP (vasodilator second messenger) and sensitization of the smooth muscle to ionic calcium. (Copyright-Lakshminrusimha and Steinhorn). Modified from Polin and Fox – Fetal and Neonatal Physiology 5th Edition
In vitro studies.
Pulmonary artery smooth muscle cells (PASMC) isolated from PPHN lambs exhibit elevated cytosolic ROS, increased Nox4 expression and decreased ecSOD activity relative to control PASMC [48]. Nox4 siRNA knockdown attenuates cytosolic ROS levels and elevates ecSOD activity in these cells [48]. PPHN PASMC also display elevated mitochondrial matrix ROS, associated with increased activity of the mitochondrial SOD MnSOD, increased PDE5 activity and decreased cGMP-responsiveness to NO [65]. These data raise the possibility that PPHN-induced mitochondrial superoxide is converted to H2O2 by MnSOD and crosses mitochondrial membranes thereby contributing to elevated cytosolic ROS. Increasing evidence indicates cross talk between the mitochondria and Nox isoforms [66]. ROS stimulate vascular SMC growth suggesting that altered ROS generation and scavenging also contribute to pulmonary vascular remodeling in PPHN [67, 68].
Pulmonary artery endothelial cells (PAEC) isolated from PPHN lambs also exhibit elevated ROS associated with increased expression of Nox2 and Nox4 and impaired angiogenesis [69]. Further studies indicate that uncoupled eNOS and decreased MnSOD (or SOD2) activity also contribute to elevated ROS and impaired angiogenesis in PPHN PAEC [59, 70].
Oxygen exposure and ROS.
Chronic exposure to hyperoxia (60% O2 and greater) induces oxidant stress, pulmonary hypertension and vascular remodeling in newborn rats [71] and mice [72]. Increased lung Nox1 expression was reported in neonatal mice on postnatal day 7 after 24h exposure to 75% O2 from birth [73]. Furthermore, ROS generation and lung injury is attenuated in Nox1- but not in Nox2-deficient adult mice following exposure to 100% O2 for 72h [74]. Conversely, lung Nox2 and Nox4 expression is increased in neonatal mice following exposure to 75% O2 for 7d [75]. In PASMC isolated from control lambs, exposure to 95% O2 for 24h increases cytosolic ROS and increases PDE5 activity [57]. Furthermore, exposure to 30 minutes of 95% O2 increases mitochondrial but not cytosolic ROS while overexpression of mitochondrial catalase attenuates PDE5 activity after 24h [76]. Together these data suggest that acute hyperoxia elevates mitochondrial ROS, which results in elevated cytosolic ROS upon prolonged exposure. Chronic hyperoxia exposure activates PDE5 in PASMC and may have additional consequences for NO signaling as highlighted above.
Antioxidant therapy.
As described above, PPHN and hyperoxia elevate pulmonary ROS via common and distinct mechanisms and suggest that ROS scavengers may be effective in limiting the oxidant stress in PPHN infants ventilated with oxygen. In neonatal lamb models of PPHN, intratracheal administration of antioxidants decreases ROS, increases eNOS expression and normalizes tetrahydrobiopterin levels after ventilation with 100% O2 for 24h [77, 78]. Intratracheal recombinant human SOD (rhSOD) also reduces ONOO-mediated protein nitration (figure 5) [58], decreases PDE5 activity and increases cGMP in the pulmonary arteries of ventilated PPHN lambs [79]. Similarly, Intratracheal catalase improves oxygenation, increases lung ecSOD activity, decreases PA superoxide levels, decreases PA PDE5 activity and increases PA cGMP in ventilated PPHN lambs [50, 65]. These data suggest that antioxidant therapy may increase oxygenation in PPHN lambs by improving NO-mediated vasodilation at multiple points in the signaling pathway.
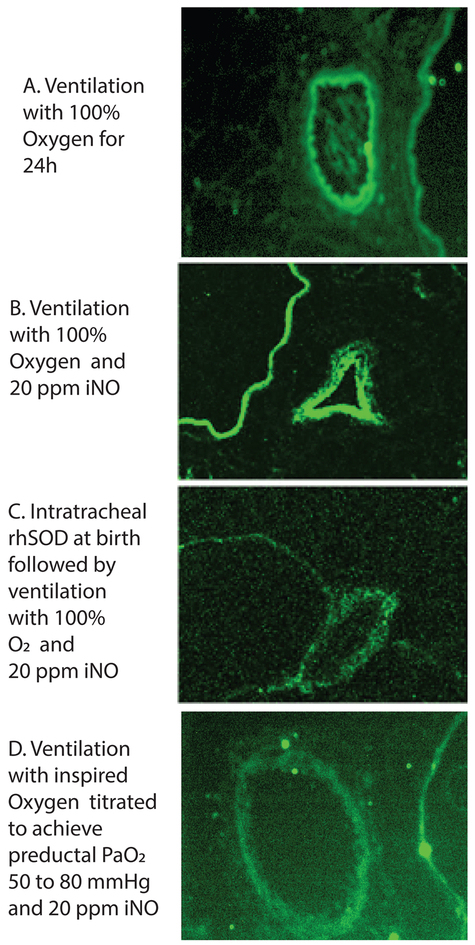
Figure 5. Nitrosative stress and the role of inhaled nitric oxide and inspired oxygen.
- Lambs were ventilated with 100% oxygen for 24 hours irrespective of PaO2 levels.
- Lambs were ventilated with 100% oxygen and 20 ppm iNO for 24 hours irrespective of PaO2 levels.
- Lambs were ventilated with 100% oxygen and 20 ppm iNO for 24 hours irrespective of PaO2 levels. These lambs received a dose of intratracheal recombinant human superoxide dismutase (rhSOD) mixed with surfactant at birth.
- Lambs were ventilated with titrated inspired oxygen to maintain preductal PaO2 between 50 and 80 mmHg and 20 ppm iNO for 24 hours.
Modified from reference # 44
Using the same PPHN lamb model, both antenatal betamethasone [80] and postnatal hydrocortisone significantly improved oxygenation, in part by increasing superoxide dismutase activity and reducing oxidant stress [81, 82] However, it is important to note that clinical trials of antioxidant therapy for neonatal pulmonary diseases have had only limited success [83]. This may be due to the timing of treatment relative to disease progression, the subcellular location of ROS in different diseases, and the involvement of ROS in normal cell signaling. It is likely that antioxidant therapies will need to be precisely targeted at a cellular and subcellular level to be most effective in the treatment of ROS-induced neonatal pulmonary hypertension.
The preterm lung:
The preterm lung is meant to develop under hypoxic conditions that favor rapid parenchymal and pulmonary vascular growth, which means that exposure to even ambient levels of oxygen after birth produces relative hyperoxia. [84] Various factors that promote oxidative stress in preterm infants can be associated with development of pulmonary hypertension (figure 6). The controversies surrounding oxygen use and pulmonary hypertension in preterm infants can be discussed under three sub-headings – delivery room and immediate postnatal management, early acute preterm PPHN and bronchopulmonary dysplasia (BPD) with pulmonary hypertension.
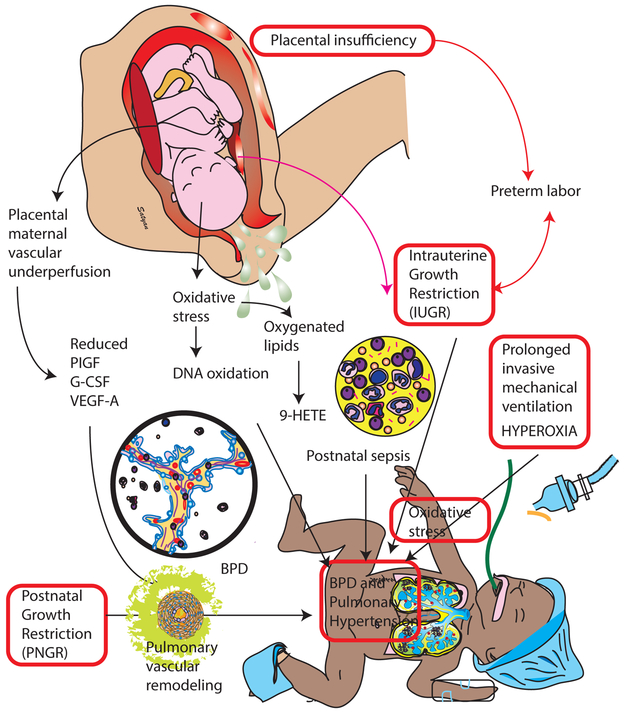
Figure 6.
Role of prenatal and postnatal oxidative stress in the pathogenesis of bronchopulmonary dysplasia (BPD) and pulmonary hypertension (PH). Growth restriction, both prenatal (IUGR) and postnatal (PNGR) contribute to development of PH. Cord blood placental growth factor (PIGF), granulocyte-colony stimulating factor (G-CSF), and vascular endothelial growth factor-A (VEGF-A) Copyright Satyan Lakshminrusimha
Delivery room and postnatal management:
Oxygen supplementation during delivery room resuscitation of extremely preterm infants has been a subject of controversy and is reviewed in a recent manuscript. [85] Extremely preterm infants with poor respiratory effort at birth are unable to establish functional residual capacity (FRC) or achieve physiologically appropriate SpO2 and/or heart rate by 5 minutes after birth and are at high risk of negative outcomes. [86] [87] Several multi-center randomized trials attempting to minimize oxidative stress in this extremely premature population by (a) promoting placental transfusion through delayed cord clamping [88] or umbilical cord milking [89], (b) limiting inspired oxygen [90] or targeting lower SpO2 [91] and establishing FRC through sustained inflation [92] have failed to demonstrate benefit. Some of these trials have in fact shown harm with these interventions in very immature infants (figure 7).
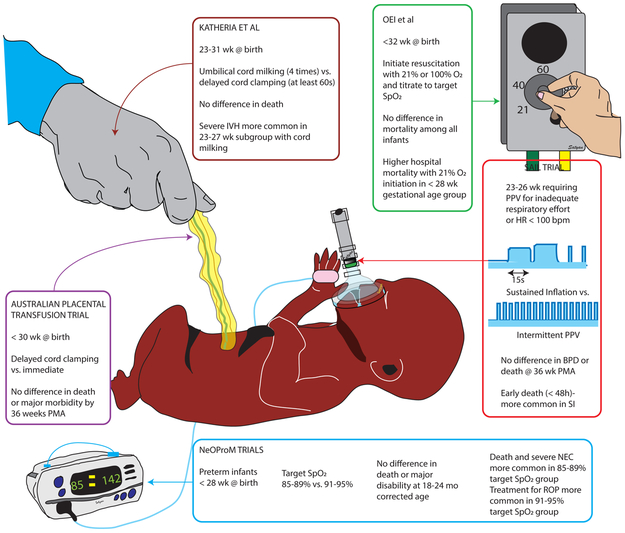
Figure 7.
Recent randomized controlled trials in extremely preterm infants with interventions that could potentially reduce oxidative stress and their outcomes. Some of these trials were stopped early due to negative outcomes in the intervention group. The Australian placental transfusion trial [88], Oei et al [90], Katheria et al [89], NeOProM trials [91] and the SAIL randomized control trial [92] are highlighted in this figure. The patient population studied, intervention, primary outcome and negative findings in subgroups (if any) are shown in boxes. Copyright Satyan Lakshminrusimha
After birth, supplemental oxygen and hyperoxia are well-known sources of oxidant stress and risk factors for chronic lung disease. However, recent clinical trials have revealed the complexities of oxygen supplementation and oxygen targets for premature infants.
Five large trials from Australia, New Zealand, the United States, Canada, and the United Kingdom randomized extremely preterm infants to management with either 85-89% or 91-95% saturation. [91] The Neonatal Oxygenation Prospective Metaanalysis (NeOProM) Collaboration performed a metaanalysis of the individual patient data for 4965 patients in these trials and found that rates of the primary outcome of death or major disability at 18 to 24 months corrected age were not different between the two groups (53.5% for the lower target vs. 51.6% for the higher target). Rates of bronchopulmonary dysplasia were significantly reduced in the lower vs. higher target saturation group (25% vs. 30%, P<.001). However, this pulmonary benefit was offset by an increased risk of death at 18 to 24 months in the lower vs. higher saturation group (19.9% vs. 17.1%; P = .02) and severe necrotizing enterocolitis was more frequent in the lower vs. higher saturation group (9.2% vs. 6.9%; P = .003). Fetal growth restriction or other risk factors did not alter the risk of morbidity and mortality for either saturation range. This higher target range of 91-95% has now been widely adopted in most neonatal units in the US and Europe. Finding ways to selectively reduce the pulmonary toxicity of oxygen in this population, while retaining the systemic benefits, is an important research and clinical goal for the next decade.
Early PPHN in preterm infants:
Early respiratory failure after preterm birth commonly occurs due to surfactant deficiency and lung immaturity, and supplemental oxygen is required to support life. These infants often demonstrate clinical, echocardiographic and histological evidence of pulmonary hypertension. [93] This problem is compounded by antenatal stressors such as placental insufficiency that appear to increase oxidant stress prior to birth. Mothers with pregnancies subsequently affected by intrauterine growth restriction have been reported to have elevated urinary 8-oxodG in the first and second trimester, indicating that oxidative stress (as evidenced by DNA oxidation) may precede the placental and clinical changes of placental insufficiency. [94] In other prospective cohort studies, infants that developed pulmonary hypertension as a complication of prematurity were more likely to have experienced placental insufficiency and fetal growth restriction prior to birth. [95, 96] Therapies targeted towards addressing hypoxemic respiratory failure and PPHN such as supplemental oxygen and iNO can exacerbate free radical mediated damage. [97]
Oxidative stress, bronchopulmonary dysplasia (BPD) and pulmonary hypertension:
Preterm infants with severe BPD are at high risk of pulmonary hypertension presenting later in their NICU course. [98] There is evidence to suggest that oxidative stress early in the course of these preterm infants is associated with later onset of BPD and pulmonary hypertension. Examination of cord blood metabolomic profiles revealed that markers of oxidative stress were strongly associated with the later onset of PH and chronic lung disease in preterm infants. [99] Oxygenated lipids derived from linoleic acid or α-linolenic acid were uniformly elevated by a variety of mechanisms including soluble epoxide hydrolase activity and levels of 9-HETE, a non-enzymatic oxidation product of arachidonic acid, were elevated more than 2-fold. A recent large meta-analysis of 12 non-overlapping studies reinforces these associations, and found that PH was associated with fetal growth restriction. [100] Collectively, these findings suggest that smaller and more premature infants may experience abnormal pulmonary vascular development long before they are challenged by the pulmonary insults such as mechanical ventilation and sepsis associated with postnatal care (figure 6)
Rodent models have been widely used to identify the mechanisms that give rise to PH and BPD in preterm infants. Neonatal rats and mice are born with developmentally immature lungs at the saccular stage in development, similar to extremely premature infants at <28 weeks gestation. Exposure of rodent pups to hyperoxia (>60% oxygen) leads to pulmonary hypertension, right ventricular hypertrophy, pulmonary vascular remodeling, and alveolar simplification, similar to human infants with PH and BPD. Recent studies indicate that just 24 hours of hyperoxic exposure shortly after birth is sufficient to cause PH and right ventricular hypertrophy that persists to day 14, along with abnormal vascular function due to diminished soluble guanylate cyclase and increased cGMP phosphodiesterase activity. [101]
Other stresses common to the sick preterm infant, such as nutritional deficiency, likely amplify the effect of hyperoxia on the pulmonary vasculature. [102] A novel rodent model causes postnatal growth restriction (PNGR) by increasing the number of pups per dam, which limits milk and fat intake. Combining PNGR with exposure to 75% oxygen for the first 14 days of life produced a more severe phenotype of PH with decreased pulmonary vessel density, thickened pulmonary arteriolar walls and right ventricular hypertrophy (figure 6). PNGR and hyperoxia, both individually and in combination, also caused decreased expression of key modulators of angiogenesis and vascular tone including VEGF, VEGF receptor 2, HIF1α, HIF2α, eNOS and NOS metabolites. In addition, PNGR was associated with increased activity of soluble epoxide hydrolase (sEH) as evidenced by an increase in the ratio of 14,15-DiHETE (pro-inflammatory oxylipins) to 14,15-EET (anti-inflammatory oxylipins) in the plasma of exposed rat pups. [103] Soluble epoxide hydrolase rapidly converts the more biologically active and anti-inflammatory EpFAs to less active and more pro-inflammatory di-hydroxy fatty acids.
There has been limited progress in the prevention of PH associated with prematurity. As noted above, antioxidant therapy with rhSOD had no effect on prevention of chronic lung disease, although it did reduce some aspects of chronic respiratory morbidity at a year of life. Inhibitors of sEH have been promising developments for treatment of adult diseases of chronic inflammation such as diabetes, metabolic syndrome, atherosclerosis, asthma, COPD, and systemic hypertension. In adult rodents, sEH inhibition attenuated PH and deletion of sEH reduced acute hyperoxic lung injury [104, 105] but this novel approach still needs to be tested in neonatal models of PH. It is also possible that nutritional modifications or supplements could reduce the PH and lung injury associated with postnatal growth restriction. For instance, the offspring of pregnant rats fed ω-3 polyunsaturated fatty acids (PUFA) and exposed to hyperoxia had decreased lung injury typical of bronchopulmonary dysplasia while ω-6 PUFA supplementation had no benefit. [106] Whether these nutritional approaches selectively reduce the pulmonary impact of oxidant stress is an important research question.
Conclusion: Clinical implications of free radicals in PPHN
Supplemental oxygen is a necessary and life-saving therapy for infants who experience a delayed or failed pulmonary vascular transition in the delivery room, or who have neonatal respiratory failure due to PPHN or prematurity. However, supplemental oxygen also increases oxidant stress through production of free radicals by the mitochondrial matrix and enzymatic sources in the cytosol. Longer exposures to hyperoxia also activate PDE5 in the pulmonary vasculature which reduces cGMP generation in response to pulmonary vasodilators such as inhaled NO. Therapies to mitigate the impact of oxidant stress in the lung and pulmonary vasculature are not yet selective enough to the lung and/or to subcellular oxidant signaling. The effects of free radical damage may extend well beyond infancy [97], as suggested by the increased risk of childhood malignancy after neonatal exposure to hyperoxia [107] and iNO. [108] There is an urgent need for basic science, translational, clinical, and epidemiologic studies to solve these important problems.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Lapointe A, Barrington KJ, Pulmonary hypertension and the asphyxiated newborn, The Journal of pediatrics 158(2 Suppl) (2011) e19–24. [DOI] [PubMed] [Google Scholar]
- [2].Dawes GS, Mott JC, Widdicombe JG, Wyatt DG, Changes in the lungs of the newborn lamb., J. Physiol 121 (1953) 141–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lakshminrusimha S, Shankaran S, Laptook A, McDonald S, Keszler M, Van Meurs K, Guillet R, Chawla S, Sood BG, Bonifacio S, Das A, Higgins RD, Pulmonary Hypertension Associated with Hypoxic-Ischemic Encephalopathy-Antecedent Characteristics and Comorbidities, The Journal of pediatrics 196 (2018) 45–51 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Murphy JD, Vawter GF, Reid LM, Pulmonary vascular disease in fatal meconium aspiration, The Journal of pediatrics 104(5) (1984) 758–62. [DOI] [PubMed] [Google Scholar]
- [5].Murphy JD, Rabinovitch M, Goldstein JD, Reid LM, The structural basis of persistent pulmonary hypertension of the newborn infant, The Journal of pediatrics 98(6) (1981) 962–7. [DOI] [PubMed] [Google Scholar]
- [6].N. The Neonatal Inhaled Nitric Oxide Study Group, Inhaled nitric oxide and hypoxic respiratory failure in infants with congenital diaphragmatic hernia. The Neonatal Inhaled Nitric Oxide Study Group (NINOS), Pediatrics 99(6) (1997) 838–45. [DOI] [PubMed] [Google Scholar]
- [7].Lakshminrusimha S, Keszler M, Kirpalani H, Van Meurs K, Chess P, Ambalavanan N, Yoder B, Fraga MV, Hedrick H, Lally KP, Nelin L, Cotten M, Klein J, Guilford S, Williams A, Chaudhary A, Gantz M, Gabrio J, Chowdhury D, Zaterka-Baxter K, Das A, Higgins RD, Milrinone in congenital diaphragmatic hernia - a randomized pilot trial: study protocol, review of literature and survey of current practices, Maternal health, neonatology and perinatology 3 (2017) 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Alano MA, Ngougmna E, Ostrea EM Jr., Konduri GG, Analysis of nonsteroidal antiinflammatory drugs in meconium and its relation to persistent pulmonary hypertension of the newborn, Pediatrics 107(3) (2001) 519–23. [DOI] [PubMed] [Google Scholar]
- [9].Lakshminrusimha S, Keszler M, Persistent Pulmonary Hypertension of the Newborn, Neoreviews 16(12) (2015) e680–e692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lakshminrusimha S, Steinhorn RH, Pulmonary vascular biology during neonatal transition, Clinics in perinatology 26(3) (1999) 601–19. [PubMed] [Google Scholar]
- [11].Sharma V, Berkelhamer S, Lakshminrusimha S, Persistent pulmonary hypertension of the newborn, Maternal health, neonatology and perinatology 1 (2015) 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].A.A.o. Pediatrics Weiner GM, A.H. Association, Zaichkin J, Textbook of Neonatal Resuscitation, American Academy of Pediatrics; 2016. [Google Scholar]
- [13].C. Canadian Congenital Diaphragmatic Hernia Puligandla PS, Skarsgard ED, Offringa M, Adatia I, Baird R, Bailey M, Brindle M, Chiu P, Cogswell A, Dakshinamurti S, Flageole H, Keijzer R, McMillan D, Oluyomi-Obi T, Pennaforte T, Perreault T, Piedboeuf B, Riley SP, Ryan G, Synnes A, Traynor M, Diagnosis and management of congenital diaphragmatic hernia: a clinical practice guideline, CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne 190(4) (2018) E103–E112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Snoek KG, Reiss IK, Greenough A, Capolupo I, Urlesberger B, Wessel L, Storme L, Deprest J, Schaible T, van Heijst A, Tibboel D, Consortium CE, Standardized Postnatal Management of Infants with Congenital Diaphragmatic Hernia in Europe: The CDH EURO Consortium Consensus - 2015 Update, Neonatology 110(1) (2016) 66–74. [DOI] [PubMed] [Google Scholar]
- [15].Riley JS, Antiel RM, Rintoul NE, Ades AM, Waqar LN, Lin N, Herkert LM, D'Agostino JA, Hoffman C, Peranteau WH, Flake AW, Adzick NS, Hedrick HL, Reduced oxygen concentration for the resuscitation of infants with congenital diaphragmatic hernia, Journal of perinatology : official journal of the California Perinatal Association 38(7) (2018) 834–843. [DOI] [PubMed] [Google Scholar]
- [16].Lakshminrusimha S, Russell JA, Steinhorn RH, Swartz DD, Ryan RM, Gugino SF, Wynn KA, Kumar VH, Mathew B, Kirmani K, Morin FC 3rd, Pulmonary hemodynamics in neonatal lambs resuscitated with 21%, 50%, and 100% oxygen, Pediatric research 62(3) (2007) 313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lakshminrusimha S, Swartz DD, Gugino SF, Ma CX, Wynn KA, Ryan RM, Russell JA, Steinhorn RH, Oxygen concentration and pulmonary hemodynamics in newborn lambs with pulmonary hypertension, Pediatric research 66(5) (2009) 539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lakshminrusimha S, Steinhorn RH, Wedgwood S, Savorgnan F, Nair J, Mathew B, Gugino SF, Russell JA, Swartz DD, Pulmonary hemodynamics and vascular reactivity in asphyxiated term lambs resuscitated with 21 and 100% oxygen, J Appl Physiol (1985) 111(5) (2011) 1441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vento M, Asensi M, Sastre J, Lloret A, Garcia-Sala F, Vina J, Oxidative stress in asphyxiated term infants resuscitated with 100% oxygen, The Journal of pediatrics 142(3) (2003) 240–6. [DOI] [PubMed] [Google Scholar]
- [20].Rawat M, Chandrasekharan PK, Swartz DD, Mathew B, Nair J, Gugino SF, Koenigsknecht C, Vali P, Lakshminrusimha S, Neonatal resuscitation adhering to oxygen saturation guidelines in asphyxiated lambs with meconium aspiration, Pediatric research 79(4) (2016) 583–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fox WW, Duara S, Persistent pulmonary hypertension in the neonate: diagnosis and management, The Journal of pediatrics 103(4) (1983) 505–14. [DOI] [PubMed] [Google Scholar]
- [22].Duara S, Gewitz MH, Fox WW, Use of mechanical ventilation for clinical management of persistent pulmonary hypertension of the newborn, Clinics in perinatology 11(3) (1984) 641–52. [PubMed] [Google Scholar]
- [23].Moudgil R, Michelakis ED, Archer SL, Hypoxic pulmonary vasoconstriction, Journal of applied physiology 98(1) (2005) 390–403. [DOI] [PubMed] [Google Scholar]
- [24].Lumb AB, Nunn's Applied Respiratory Physiology E-Book, Elsevier Health Sciences 2012. [Google Scholar]
- [25].Gien J, Kinsella JP, Differences in preductal and postductal arterial blood gas measurements in infants with severe congenital diaphragmatic hernia, Archives of disease in childhood. Fetal and neonatal edition 101(4) (2016) F314–8. [DOI] [PubMed] [Google Scholar]
- [26].Rudolph AM, Yuan S, Response of the pulmonary vasculature to hypoxia and H+ ion concentration changes, The Journal of clinical investigation 45(3) (1966) 399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lakshminrusimha S, Konduri GG, Steinhorn RH, Considerations in the management of hypoxemic respiratory failure and persistent pulmonary hypertension in term and late preterm neonates, Journal of perinatology : official journal of the California Perinatal Association 36 Suppl 2 (2016) S12–9. [DOI] [PubMed] [Google Scholar]
- [28].Lakshminrusimha S, Steinhorn RH, Wedgwood S, Savorgnan F, Nair J, Mathew B, Gugino SF, Russell JA, Swartz DD, Pulmonary Hemodynamics and Vascular Reactivity in Asphyxiated Term Lambs Resuscitated with 21% and 100% Oxygen, Journal of applied physiology (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kapadia VS, Chalak LF, DuPont TL, Rollins NK, Brion LP, Wyckoff MH, Perinatal asphyxia with hyperoxemia within the first hour of life is associated with moderate to severe hypoxic-ischemic encephalopathy, The Journal of pediatrics 163(4) (2013) 949–54. [DOI] [PubMed] [Google Scholar]
- [30].Konduri GG, New approaches for persistent pulmonary hypertension of newborn, Clinics in perinatology 31(3) (2004) 591–611. [DOI] [PubMed] [Google Scholar]
- [31].Lakshminrusimha S, The pulmonary circulation in neonatal respiratory failure, Clinics in perinatology 39(3) (2012) 655–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, Hanna BD, Rosenzweig EB, Raj JU, Cornfield D, Stenmark KR, Steinhorn R, Thebaud B, Fineman JR, Kuehne T, Feinstein JA, Friedberg MK, Earing M, Barst RJ, Keller RL, Kinsella JP, Mullen M, Deterding R, Kulik T, Mallory G, Humpl T, Wessel DL, Pediatric Pulmonary Hypertension: Guidelines From the American Heart Association and American Thoracic Society, Circulation (2015). [DOI] [PubMed] [Google Scholar]
- [33].Lakshminrusimha S, Manja V, Mathew B, Suresh GK, Oxygen targeting in preterm infants: a physiological interpretation, Journal of perinatology : official journal of the California Perinatal Association 35(1) (2015) 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nunez A, Benavente I, Blanco D, Boix H, Cabanas F, Chaffanel M, Fernandez-Colomer B, Fernandez-Lorenzo JR, Loureiro B, Moral MT, Pavon A, Tofe I, Valverde E, Vento M, c. co-investigadores del ensayo, [Oxidative stress in perinatal asphyxia and hypoxic-ischaemic encephalopathy], Anales de pediatria 88(4) (2018) 228 e1–228 e9. [DOI] [PubMed] [Google Scholar]
- [35].Afzal B, Chandrasekharan P, Tancredi DJ, Russell J, Steinhorn RH, Lakshminrusimha S, Monitoring Gas Exchange During Hypothermia for Hypoxic-Ischemic Encephalopathy, Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 20(2) (2019) 166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kukreja RC, Kontos HA, Hess ML, Ellis EF, PGH synthase and lipoxygenase generate superoxide in the presence of NADH or NADPH, Circ Res 59(6) (1986) 612–9. [DOI] [PubMed] [Google Scholar]
- [37].Mueller CF, Laude K, McNally JS, Harrison DG, ATVB in focus: redox mechanisms in blood vessels, Arterioscler Thromb Vasc Biol 25(2) (2005) 274–8. [DOI] [PubMed] [Google Scholar]
- [38].Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK, Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer, Am J Physiol Heart Circ Physiol 294(2) (2008) H570–8. [DOI] [PubMed] [Google Scholar]
- [39].Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK, Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells, Arterioscler Thromb Vasc Biol 24(4) (2004) 677–83. [DOI] [PubMed] [Google Scholar]
- [40].Lyle AN, Griendling KK, Modulation of vascular smooth muscle signaling by reactive oxygen species, Physiology (Bethesda) 21 (2006) 269–80. [DOI] [PubMed] [Google Scholar]
- [41].Waypa GB, Schumacker PT, Oxygen sensing in hypoxic pulmonary vasoconstriction: using new tools to answer an age-old question, Exp Physiol 93(1) (2008) 133–8. [DOI] [PubMed] [Google Scholar]
- [42].Wolin MS, Ahmad M, Gupte SA, Oxidant and redox signaling in vascular oxygen sensing mechanisms: basic concepts, current controversies, and potential importance of cytosolic NADPH, Am J Physiol Lung Cell Mol Physiol 289(2) (2005) L159–73. [DOI] [PubMed] [Google Scholar]
- [43].Rhoades R, Packer C, Roepke D, Jin N, Meiss R, Reactive oxygen species alter contractile properties of pulmonary arterial smooth muscle., Can J Physiol Pharmacol 68 (1990) 1581–1589. [DOI] [PubMed] [Google Scholar]
- [44].Demiryürek A, Wadsworth R, Superoxide in the pulmonary circulation., Pharmacol Ther. 84 (1999) 355–365. [DOI] [PubMed] [Google Scholar]
- [45].Brennan LA, Steinhorn RH, Wedgwood S, Mata-Greenwood E, Roark EA, Russell JA, Black SM, Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: a role for NADPH oxidase, Circulation research 92(6) (2003) 683–91. [DOI] [PubMed] [Google Scholar]
- [46].Chandrasekar I, Eis A, Konduri GG, Betamethasone attenuates oxidant stress in endothelial cells from fetal lambs with persistent pulmonary hypertension, Pediatric research 63(1) (2008) 67–72. [DOI] [PubMed] [Google Scholar]
- [47].Wedgwood S, Steinhorn RH, Bunderson M, Wilham J, Lakshminrusimha S, Brennan LA, Black SM, Increased hydrogen peroxide downregulates soluble guanylate cyclase in the lungs of lambs with persistent pulmonary hypertension of the newborn, American journal of physiology. Lung cellular and molecular physiology 289(4) (2005) L660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wedgwood S, Lakshminrusimha S, Czech L, Schumacker PT, Steinhorn RH, Increased p22phox/Nox4 Expression Triggers Remodeling through Hydrogen Peroxide Signaling in Persistent Pulmonary Hypertension of the Newborn Antioxid Redox Signal 18 (2013) 1765–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Fike CD, Slaughter JC, Kaplowitz MR, Zhang Y, Aschner JL, Reactive oxygen species from NADPH oxidase contribute to altered pulmonary vascular responses in piglets with chronic hypoxia-induced pulmonary hypertension, Am J Physiol Lung Cell Mol Physiol 295(5) (2008) L881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wedgwood S, Lakshminrusimha S, Fukai T, Russell J, Schumacker P, Steinhorn R, Hydrogen peroxide regulates extracellular superoxide dismutase activity and expression in neonatal pulmonary hypertension., Antioxid Redox Signal 15 (2011) 1497––1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Dikalov S, Dikalova A, Bikineyeva A, Schmidt H, Harrison D, Griendling K, Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production., Free Radic Biol Med 45 (2008) 1340–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Shaul PW, Yuhanna IS, German Z, Chen Z, Steinhorn RH, Morin FC 3rd, Pulmonary endothelial NO synthase gene expression is decreased in fetal lambs with pulmonary hypertension, The American journal of physiology 272(5 Pt 1) (1997) L1005–12. [DOI] [PubMed] [Google Scholar]
- [53].Steinhorn RH, Russell JA, Morin FC 3rd, Disruption of cGMP production in pulmonary arteries isolated from fetal lambs with pulmonary hypertension, The American journal of physiology 268(4 Pt 2) (1995) H1483–9. [DOI] [PubMed] [Google Scholar]
- [54].Black SM, Johengen MJ, Soifer SJ, Coordinated regulation of genes of the nitric oxide and endothelin pathways during the development of pulmonary hypertension in fetal lambs., Pediatr Res 44 (1998) 821–830. [DOI] [PubMed] [Google Scholar]
- [55].Ivy DD, Ziegler JW, Dubus MF, Fox JJ, Kinsella JP, Abman SH, Chronic intrauterine pulmonary hypertension alters endothelin receptor activity in the ovine fetal lung, Pediatr Res 39 (1996) 435–442. [DOI] [PubMed] [Google Scholar]
- [56].Wedgwood S, Black SM, Endothelin-1 decreases endothelial NOS expression and activity through ETA receptor-mediated generation of hydrogen peroxide., Am J Physiol Lung Cell Mol Physiol 288 (2005) L480–L487. [DOI] [PubMed] [Google Scholar]
- [57].Farrow KN, Groh BS, Schumacker PT, Lakshminrusimha S, Czech L, Gugino SF, Russell JA, Steinhorn RH, Hyperoxia increases phosphodiesterase 5 expression and activity in ovine fetal pulmonary artery smooth muscle cells, Circulation research 102(2) (2008) 226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lakshminrusimha S, Russell J, Wedgwood S, Gugino S, Kazzaz J, Davis J, Steinhorn RH, Superoxide dismutase improves oxygenation and reduces oxidation in neonatal pulmonary hypertension., Am J Respir Crit Care Med 174 (2006) 1370–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Teng RJ, Wu TJ, Bisig CG, Eis A, Pritchard KA, Konduri GG, Nitrotyrosine impairs angiogenesis and uncouples eNOS activity of pulmonary artery endothelial cells isolated from developing sheep lungs, Pediatr Res 69(2) (2011) 112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Wedgwood S, McMullan DM, Bekker JM, Fineman JR, Black SM, Role for endothelin-1-induced superoxide and peroxynitrite production in rebound pulmonary hypertension associated with inhaled nitric oxide therapy., Circ Res 89(4) (2001) 357–64. [DOI] [PubMed] [Google Scholar]
- [61].Konduri G, Bakhutashvili I, Eis A, Pritchard KJ, Oxidant stress from uncoupled nitric oxide synthase impairs vasodilation in fetal lambs with persistent pulmonary hypertension., Am J Physiol Heart Circ Physiol 292 (2007) H1812–H1820. [DOI] [PubMed] [Google Scholar]
- [62].Dikalova AE, Gongora MC, Harrison DG, Lambeth JD, Dikalov S, Griendling KK, Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling, Am J Physiol Heart Circ Physiol 299(3) (2010) H673–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG, Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension, J Clin Invest 111(8) (2003) 1201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Brennan LA, Steinhorn RH, Wedgwood S, Mata-Greenwood E, Roark EA, Russell JA, Black SM, Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: a role for NADPH oxidase., Circ Res 92 (2003) 683–691. [DOI] [PubMed] [Google Scholar]
- [65].Farrow K, Wedgwood S, Lee K, Czech L, Gugino S, Lakshminrusimha S, Schumacker P, Steinhorn R, Mitochondrial oxidant stress increases PDE5 activity in persistent pulmonary hypertension of the newborn., Respir Physiol Neurobiol 174 (2010) 272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Dikalov S, Cross talk between mitochondria and NADPH oxidases, Free Radic Biol Med 51(7) (2011) 1289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Rao G, Berk B, Active oxygen species stimulate vascular smooth muscle cell growth and proto-oncogene expression, Circ Res 18 (1992) 775–794. [DOI] [PubMed] [Google Scholar]
- [68].Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T, Requirement for generation of H2O2 for platelet-derived growth factor signal transduction, Science 270(5234) (1995) 296–299. [DOI] [PubMed] [Google Scholar]
- [69].Teng RJ, Eis A, Bakhutashvili I, Arul N, Konduri GG, Increased superoxide production contributes to the impaired angiogenesis of fetal pulmonary arteries with in utero pulmonary hypertension, Am J Physiol Lung Cell Mol Physiol 297(1) (2009) L184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Afolayan AJ, Eis A, Teng RJ, Bakhutashvili I, Kaul S, Davis JM, Konduri GG, Decreases in manganese superoxide dismutase expression and activity contribute to oxidative stress in persistent pulmonary hypertension of the newborn, American journal of physiology. Lung cellular and molecular physiology 303(10) (2012) L870–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Koppel R, Han RN, Cox D, Tanswell AK, Rabinovitch M, Alpha 1-antitrypsin protects neonatal rats from pulmonary vascular and parenchymal effects of oxygen toxicity, Pediatr Res 36(6) (1994) 763–70. [DOI] [PubMed] [Google Scholar]
- [72].Warner BB, Stuart LA, Papes RA, Wispe JR, Functional and pathological effects of prolonged hyperoxia in neonatal mice, Am J Physiol 275(1 Pt 1) (1998) L110–7. [DOI] [PubMed] [Google Scholar]
- [73].Berkelhamer SK, Kim GA, Radder JE, Wedgwood S, Czech L, Steinhorn RH, Schumacker PT, Developmental differences in hyperoxia-induced oxidative stress and cellular responses in the murine lung, Free radical biology & medicine 61C (2013) 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Carnesecchi S, Deffert C, Pagano A, Garrido-Urbani S, Metrailler-Ruchonnet I, Schappi M, Donati Y, Matthay MA, Krause KH, Barazzone Argiroffo C, NADPH oxidase-1 plays a crucial role in hyperoxia-induced acute lung injury in mice, Am J Respir Crit Care Med 180(10) (2009) 972–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Harijith A, Pendyala S, Reddy NM, Bai T, Usatyuk PV, Berdyshev E, Gorshkova I, Huang LS, Mohan V, Garzon S, Kanteti P, Reddy SP, Raj JU, Natarajan V, Sphingosine kinase 1 deficiency confers protection against hyperoxia-induced bronchopulmonary dysplasia in a murine model: role of S1P signaling and Nox proteins, Am J Pathol 183(4) (2013) 1169–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Farrow KN, Lee KJ, Perez M, Schriewer JM, Wedgwood S, Lakshminrusimha S, Smith CL, Steinhorn RH, Schumacker PT, Brief Hyperoxia Increases Mitochondrial Oxidation and Increases Pde5 Activity in Fetal Pulmonary Artery Smooth Muscle Cells, Antioxid Redox Signal 17 (2012) 460–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Wedgwood S, Lakshminrusimha S, Farrow KN, Czech L, Gugino SF, Soares F, Russell JA, Steinhorn RH, Apocynin Improves Oxygenation and Increases eNOS in Persistent Pulmonary Hypertension of the Newborn, Am J Physiol Lung Cell Mol Physiol 302 (2012) L616–L626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Farrow KN, Lakshminrusimha S, Reda WJ, Wedgwood S, Czech L, Gugino SF, Davis JM, Russell JA, Steinhorn RH, Superoxide dismutase restores eNOS expression and function in resistance pulmonary arteries from neonatal lambs with persistent pulmonary hypertension, American journal of physiology. Lung cellular and molecular physiology 295(6) (2008) L979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Farrow KN, Lakshminrusimha S, Czech L, Groh BS, Gugino S, Davis JM, Russell JA, Steinhorn RH, SOD and inhaled nitric oxide normalize phosphodiesterase 5 expression and activity in neonatal lambs with persistent pulmonary hypertension., Am J Physiol Lung Cell Mol Physiol 299 (2010) L109–L116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Konduri GG, Bakhutashvili I, Eis A, Afolayan A, Antenatal betamethasone improves postnatal transition in late preterm lambs with persistent pulmonary hypertension of the newborn, Pediatric research 73(5) (2013) 621–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Perez M, Wedgwood S, Lakshminrusimha S, Farrow KN, Steinhorn RH, Hydrocortisone normalizes phosphodiesterase-5 activity in pulmonary artery smooth muscle cells from lambs with persistent pulmonary hypertension of the newborn, Pulmonary Circulation 4(1) (2014) 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Perez M, Lakshminrusimha S, Wedgwood S, Czech L, Gugino SF, Russell JA, Farrow KN, Steinhorn RH, Hydrocortisone normalizes oxygenation and cGMP regulation in lambs with persistent pulmonary hypertension of the newborn, American journal of physiology. Lung cellular and molecular physiology 302(6) (2012) L595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Davis JM, Parad RB, Michele T, Allred E, Price A, Rosenfeld W, Pulmonary outcome at 1 year corrected age in premature infants treated at birth with recombinant human CuZn superoxide dismutase, Pediatrics 111(3) (2003) 469–76. [DOI] [PubMed] [Google Scholar]
- [84].Smith CV, Hansen TN, Martin NE, McMicken HW, Elliott SJ, Oxidant stress responses in premature infants during exposure to hyperoxia, Pediatric research 34(3) (1993) 360–5. [DOI] [PubMed] [Google Scholar]
- [85].Lara-Canton I, Solaz A, Parra-Llorca A, Garcia-Robles A, Vento M, Optimal Inspired Fraction of Oxygen in the Delivery Room for Preterm Infants, Children (Basel) 6(2) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Oei JL, Finer NN, Saugstad OD, Wright IM, Rabi Y, Tarnow-Mordi W, Rich W, Kapadia V, Rook D, Smyth JP, Lui K, Vento M, Outcomes of oxygen saturation targeting during delivery room stabilisation of preterm infants, Archives of disease in childhood. Fetal and neonatal edition 103(5) (2018) F446–F454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Perlman J, Delivery Room Resuscitation of Extremely Preterm Infants, JAMA : the journal of the American Medical Association 321(12) (2019) 1161–1162. [DOI] [PubMed] [Google Scholar]
- [88].Tarnow-Mordi W, Morris J, Kirby A, Robledo K, Askie L, Brown R, Evans N, Finlayson S, Fogarty M, Gebski V, Ghadge A, Hague W, Isaacs D, Jeffery M, Keech A, Kluckow M, Popat H, Sebastian L, Aagaard K, Belfort M, Pammi M, Abdel-Latif M, Reynolds G, Ariff S, Sheikh L, Chen Y, Colditz P, Liley H, Pritchard M, de Luca D, de Waal K, Forder P, Duley L, El-Naggar W, Gill A, Newnham J, Simmer K, Groom K, Weston P, Gullam J, Patel H, Koh G, Lui K, Marlow N, Morris S, Sehgal A, Wallace E, Soll R, Young L, Sweet D, Walker S, Watkins A, Wright I, Osborn D, Simes J, Australian G Placental Transfusion Study Collaborative, Delayed versus Immediate Cord Clamping in Preterm Infants, The New England journal of medicine 377(25) (2017) 2445–2455. [DOI] [PubMed] [Google Scholar]
- [89].Katheria A, Reister F, Essers J, Mendler M, Hummler H, Kraft K, Subramaniam A, Carlo W, Tita AT, Truong G, Davis-Nelson S, Schmolzer G, Chari R, Kaempf J, Tomlinson M, Yanowitz T, Beck S, Simhan H, Dempsey E, O'Donoghue K, Bhat S, Hoffman M, Faksh A, Arnell K, Rich W, Finer N, Vaucher Y, Varner M, Feese M, Allman P, Szychowski J, Cutter G, Premature infants receiving cord milking or delayed cord clamping: a randomized controlled non-inferiority trial, Pediatric Academic Societies, Baltimore, MD, USA, 2019. [Google Scholar]
- [90].Oei JL, Saugstad OD, Lui K, Wright IM, Smyth JP, Craven P, Wang YA, McMullan R, Coates E, Ward M, Mishra P, De Waal K, Travadi J, See KC, Cheah IG, Lim CT, Choo YM, Kamar AA, Cheah FC, Masoud A, Tarnow-Mordi W, Targeted Oxygen in the Resuscitation of Preterm Infants, a Randomized Clinical Trial, Pediatrics 139(1) (2017). [DOI] [PubMed] [Google Scholar]
- [91].Askie LM, Darlow BA, Finer N, Schmidt B, Stenson B, Tarnow-Mordi W, Davis PG, Carlo WA, Brocklehurst P, Davies LC, Das A, Rich W, Gantz MG, Roberts RS, Whyte RK, Costantini L, Poets C, Asztalos E, Battin M, Halliday HL, Marlow N, Tin W, King A, Juszczak E, Morley CJ, Doyle LW, Gebski V, Hunter KE, Simes RJ, Neonatal C Oxygenation Prospective Meta-analysis, Association Between Oxygen Saturation Targeting and Death or Disability in Extremely Preterm Infants in the Neonatal Oxygenation Prospective Meta-analysis Collaboration, JAMA : the journal of the American Medical Association 319(21) (2018) 2190–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Kirpalani H, Ratcliffe SJ, Keszler M, Davis PG, Foglia EE, Te Pas A, Fernando M, Chaudhary A, Localio R, van Kaam AH, Onland W, Owen LS, Schmolzer GM, Katheria A, Hummler H, Lista G, Abbasi S, Klotz D, Simma B, Nadkarni V, Poulain FR, Donn SM, Kim HS, Park WS, Cadet C, Kong JY, Smith A, Guillen U, Liley HG, Hopper AO, Tamura M, Investigators SS, Effect of Sustained Inflations vs Intermittent Positive Pressure Ventilation on Bronchopulmonary Dysplasia or Death Among Extremely Preterm Infants: The SAIL Randomized Clinical Trial, JAMA : the journal of the American Medical Association 321(12) (2019) 1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Chandrasekharan P, Kozielski R, Kumar VH, Rawat M, Manja V, Ma C, Lakshminrusimha S, Early Use of Inhaled Nitric Oxide in Preterm Infants: Is there a Rationale for Selective Approach?, American journal of perinatology 34(5) (2017) 428–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Potdar N, Singh R, Mistry V, Evans MD, Farmer PB, Konje JC, Cooke MS, First-trimester increase in oxidative stress and risk of small-for-gestational-age fetus, BJOG : an international journal of obstetrics and gynaecology 116(5) (2009) 637–42. [DOI] [PubMed] [Google Scholar]
- [95].Check J, Gotteiner N, Liu X, Su E, Porta N, Steinhorn R, Mestan KK, Fetal growth restriction and pulmonary hypertension in premature infants with bronchopulmonary dysplasia, Journal of perinatology : official journal of the California Perinatal Association 33(7) (2013) 553–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Mestan KK, Gotteiner N, Porta N, Grobman W, Su EJ, Ernst LM, Cord Blood Biomarkers of Placental Maternal Vascular Underperfusion Predict Bronchopulmonary Dysplasia-Associated Pulmonary Hypertension, The Journal of pediatrics 185 (2017) 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Vali P, Vento M, Underwood M, Lakshminrusimha S, Free radical damage can cause serious long-lasting effects, Acta paediatrica (Oslo, Norway : 1992) 107(12) (2018) 2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Berkelhamer SK, Mestan KK, Steinhorn R, An update on the diagnosis and management of bronchopulmonary dysplasia (BPD)-associated pulmonary hypertension, Seminars in perinatology 42(7) (2018) 432–443. [DOI] [PubMed] [Google Scholar]
- [99].La Frano MR, Fahrmann JF, Grapov D, Pedersen TL, Newman JW, Fiehn O, Underwood MA, Mestan K, Steinhorn RH, Wedgwood S, Umbilical cord blood metabolomics reveal distinct signatures of dyslipidemia prior to bronchopulmonary dysplasia and pulmonary hypertension, American journal of physiology. Lung cellular and molecular physiology 315(5) (2018) L870–L881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Arjaans S, Zwart EAH, Ploegstra MJ, Bos AF, Kooi EMW, Hillege HL, Berger RMF, Identification of gaps in the current knowledge on pulmonary hypertension in extremely preterm infants: A systematic review and meta-analysis, Paediatr Perinat Epidemiol 32(3) (2018) 258–267. [DOI] [PubMed] [Google Scholar]
- [101].Lee KJ, Berkelhamer SK, Kim GA, Taylor JM, O'Shea KM, Steinhorn RH, Farrow KN, Disrupted pulmonary artery cyclic guanosine monophosphate signaling in mice with hyperoxia-induced pulmonary hypertension, American journal of respiratory cell and molecular biology 50(2) (2014) 369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Wedgwood S, Warford C, Agvateesiri SC, Thai P, Berkelhamer SK, Perez M, Underwood MA, Steinhorn RH, Postnatal growth restriction augments oxygen-induced pulmonary hypertension in a neonatal rat model of bronchopulmonary dysplasia, Pediatric research 80(6) (2016) 894–902. [DOI] [PubMed] [Google Scholar]
- [103].La Frano MR, Fahrmann JF, Grapov D, Fiehn O, Pedersen TL, Newman JW, Underwood MA, Steinhorn RH, Wedgwood S, Metabolic perturbations of postnatal growth restriction and hyperoxia-induced pulmonary hypertension in a bronchopulmonary dysplasia model, Metabolomics 13(4) (2017) 32. [Google Scholar]
- [104].Revermann M, Barbosa-Sicard E, Dony E, Schermuly RT, Morisseau C, Geisslinger G, Fleming I, Hammock BD, Brandes RP, Inhibition of the soluble epoxide hydrolase attenuates monocrotaline-induced pulmonary hypertension in rats, J Hypertens 27(2) (2009) 322–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Liu LP, Li B, Shuai TK, Zhu L, Li YM, Deletion of soluble epoxide hydrolase attenuates mice Hyperoxic acute lung injury, BMC Anesthesiol 18(1) (2018) 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Sharma D, Nkembi AS, Aubry E, Houeijeh A, Butruille L, Houfflin-Debarge V, Besson R, Deruelle P, Storme L, Maternal PUFA omega-3 Supplementation Prevents Neonatal Lung Injuries Induced by Hyperoxia in Newborn Rats, International journal of molecular sciences 16(9) (2015) 22081–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Spector LG, Klebanoff MA, Feusner JH, Georgieff MK, Ross JA, Childhood cancer following neonatal oxygen supplementation, The Journal of pediatrics 147(1) (2005) 27–31. [DOI] [PubMed] [Google Scholar]
- [108].Dixon F, Ziegler DS, Bajuk B, Wright I, Hilder L, Abdel Latif ME, Somanathan A, Oei JL, Treatment with nitric oxide in the neonatal intensive care unit is associated with increased risk of childhood cancer, Acta paediatrica (Oslo, Norway : 1992) 107(12) (2018) 2092–2098. [DOI] [PubMed] [Google Scholar]