Abstract
Objective:
Refrigeration-induced binding of von Willebrand factor (VWF) to platelets contributes to the rapid clearance of refrigerated platelets. In this study, we investigate whether inhibiting VWF binding by a DNA-based aptamer ameliorates the clearance of refrigerated platelets without significantly impeding hemostatic functions.
Approach and Results:
Platelets were refrigerated with or without aptamer ARC1779 for 48 hours. VWF binding, the effective lifetime of ARC1779, platelet post-transfusion recovery and survival, and the hemostatic function were measured. ARC1779 treatment during refrigeration inhibited the platelet-VWF interaction. ARC1779-treated refrigerated murine platelets exhibited increased post-transfusion recovery and survival than untreated ones (recovery of ARC1779-treated platelets: 76.7±5.5%; Untreated: 63.7±0.8%, p<0.01. Half-life: 31.4±2.36 hr vs. 28.1±0.86 hr, p<0.05). A similar increase was observed for refrigerated human platelets (Recovery: 49.4±4.4% vs. 36.8±2.1%, p<0.01; Half-life: 9.2±1.5 hr vs. 8.7±0.9 hr, n.s.). The effective lifetime of ARC1779 in mice was 2 hours. Additionally, ARC1779 improved the long-term (2 hours after transfusion) hemostatic function of refrigerated platelets (tail bleeding time of mice transfused with ARC1779-treated refrigerated platelets: 160±65s; Untreated: 373±96s, p<0.01). The addition of an ARC1779 antidote before transfusion improved the immediate (15 minutes after transfusion) hemostatic function (bleeding time of treated platelets: 149±21s; Untreated: 320±36s, p<0.01).
Conclusion:
ARC1779 improves the post-transfusion recovery of refrigerated platelets, and preserves the long-term hemostatic function of refrigerated platelets. These results suggest that a short-acting inhibitor of the platelet-VWF interaction may be a potential therapeutic option to improve refrigeration of platelets for transfusion treatment.
Keywords: aptamer, VWF, platelet refrigeration, transfusion, Basic Sciences, Platelets
Graphical Abstract
Introduction
Compared to erythrocytes, which can be refrigerated for 42 days, platelets can only be stored for up to 5 days at room temperature (RT) under constant agitation1, 2. One main reason for the short shelf life of platelets is the risk of bacterial contamination at RT1, 3. Storing platelets at cold temperatures (1–6°C) is a potential option for reducing bacterial contamination and increasing storage time. Refrigerated platelets have recently been approved by U.S. Food and Drug Administration4, 5. One major purpose for transfusing refrigerated platelets is for the treatment of massive hemorrhage, because refrigerated platelets are preactivated and can stop bleeding effectively4. Another purpose for transfusing refrigerated platelets is for the prophylactic treatment of thrombocytopenia. However, refrigerated platelets are rapidly cleared upon transfusion6–8. The mechanism of fast clearance of refrigerated platelets is not fully understood.
A significant increase of von Willebrand factor (VWF) binding to platelets after platelet refrigeration was previously reported9, 10. Glycoprotein (GP)Ibα is the receptor of VWF on the platelet surface, and the interaction between GPIbα and VWF often leads to platelet clearance. Patients with type 2B von Willebrand disease, in which VWF binds GPIbα spontaneously11–13, often present with thrombocytopenia14. Consistently, in a mouse model expressing type 2B VWF, platelet-VWF complexes are cleared by macrophages in the liver and spleen15. We previously demonstrated that the interaction between the ligand-binding domain (LBD) of GPIbα and the A1 domain of VWF leads to unfolding of the mechanosensory domain (MSD) in GPIbα when a mechanical pulling force is applied16. Unfolding of the MSD thereafter results in intracellular signaling events mediated by glycoprotein Ib-IX complex (GPIb-IX), including platelet desialylation, phosphatidylserine (PS) exposure, P-selectin expression, and intracellular Ca2+ elevation, and eventually platelet clearance17–19.
During refrigeration VWF binding to human and WT mouse platelets is increased9, 10. Interestingly, refrigerated VWF−/− platelets exhibited much better post-transfusion recovery and survival compared to refrigerated WT platelets, indicating that VWF binding contributes to rapid clearance of refrigerated platelets. Further, the addition of OS1 peptide (CTERMALHNLC)20, 21, which binds the LBD and blocks the GPIbα-VWF interaction, during platelet refrigeration improved the recovery and survival of refrigerated platelets10. However, OS1 has a robust binding affinity to the GPIbα LBD (KD=0.74 nM)21, and the GPIbα-OS1 complex is highly stable20. The GPIbα LBD on OS1-treated refrigerated platelets may be completely occupied by OS1 after transfusion, resulting in the impaired hemostatic function of the refrigerated platelets. Therefore, a short-acting antagonist for either the LBD of GPIbα or the A1 domain of VWF, which can be quickly removed from circulation after transfusion, should be a more applicable alternative.
The DNA aptamer ARC1779 was previously developed to bind the VWF A1 domain22. The binding site of ARC1779 is located within Phe507-Thr705 on VWF A1, which clashes with the GPIbα-binding site23. ARC1779 has a high binding affinity to VWF A1 domain (KD ~ 2 nM)24, and binding assays suggested that 2 μg/ml of ARC1779 could inhibit nearly 90% of VWF activity and 10 μg/ml could completely inhibit VWF activity22. ARC1779 was developed to treat VWF-related platelet disorders and diseases. In several Phase II clinical trials, ARC1779 was found to successfully ameliorate thrombotic thrombocytopenia purpura (), type 2B VWD (), cerebral microembolism () and thrombotic microangiopathy ()25–28. However, because ARC1779 is an inhibitor of VWF, ARC1779 treatment may decrease the hemostatic function of refrigerated platelets. Importantly, the half-life of ARC1779 in human is about 2 hours22. Due to the short in vivo half-life of ARC1779, the hemostatic function of ARC1779-treated refrigerated platelets may not be severely impaired after transfusion. In this study, we investigated the effect of ARC1779 in improving the recovery and survival of refrigerated platelets, and in preserving their hemostatic function.
Materials and methods
The authors declare that all supporting data are available within the article and its online supplementary files.
Materials and animals
DNA aptamer ARC1779 (PEG20K-NH2-mGmCmGmUdGdCdAmGmUmGmCmCmU-mUmCmGmGmCdCmG-s-TmGdCdGdGTmGmCdCmUdCdCmGmUdCmAmCmGmC-3T) and the reverse complementary DNA strand (GCGTGACGGAGGCA) were synthesized by IDT (Coralville, Iowa). Allophycocyanin (APC)-labeled anti-mouse CD41 antibody (Cat: 133913), and carboxyfluorescein succinimidyl ester (CFSE, Cat: 423801) were purchased from Biolegend (San Diego, CA). APC anti-human CD41 antibody (clone HIP8, Cat: 559777) was from BD Pharmingen (San Diego, CA). Fluorescein isothiocyanate (FITC)-labeled anti-mouse CD41 antibody (Cat: 553848) was obtained from BD Biosciences (San Jose, CA). FITC-labeled anti-VWF (Cat: P150–1) was from Emfret Analytics (Eibelstadt, Germany). Horseradish peroxidase (HRP)-conjugated anti-VWF antibody (Cat: P0226) was from DAKO (Denmark). 1-Step Ultra TMB (Cat: 34–28) was purchased from Thermo Scientific (Rockford, IL). Apyrase (Cat: A7646–500UN), prostaglandin I2 (PGI2, Cat: P6188–1MG), PGE1 (Cat: P5515), protease inhibitor cocktail (Cat: P8340), and DNAse I (Cat: 10104159001) were from Sigma-Aldrich (Saint Louis, MO). Human Type IV Collagen was from Southern Biotech (Cat: 1250–01S). Gas-permeable bag (Cat: PL07) was from OriGen (Austin, TX). Snake venom from Bothrops jararaca (Cat: V5625–100MG) was obtained from Sigma-Aldrich (Saint Louis, MO).
C57BL/6J (wild-type, WT; Stock No. 000664) and NOD.CB17-Prkdcscid/J (NOD-SCID; Stock No. 001303) mice were purchased from The Jackson Laboratory. Transgenic mice expressing chimeric human IL4Rα/ human GPIbα (IL4R-IbαTg) have been described2, 29. Six- to 8-week-old mice were used in the study as approved by the IACUC of Emory University. Both sexes of age-matched littermates were generally used for the study. All animals were randomized before the experiment, and investigators were blinded to group allocation during data collection. Human leukoreduced apheresis-derived platelets (LR-ADP) were obtained from Emory Hospital. Human whole blood was drawn from healthy volunteers in 0.38% sodium chloride. The informed consent and related protocols were approved by Emory University institutional review boards.
Refrigeration of platelets
Whole blood from WT mice was collected into sodium citrate and platelet-rich plasma (PRP) was isolated as described30, 31. Apyrase (0.02 U/ml) and PGI2 (0.1 μg/ml) were added to inhibit platelet activation during PRP preparation. Human whole blood was drawn from healthy volunteers in 0.38% sodium citrate and PRP was separated by centrifugation of 140g, 12 min. PRPs were refrigerated with or without 10 μg/ml ARC1779 in gas-permeable bags (OriGen, Austin, TX) at 4°C for 48 hours. Unless otherwise noted, after refrigeration PRP was rewarmed to 37˚C for 10 min before further characterization.
Measurement of VWF binding to platelets
Fresh and refrigerated PRPs were incubated with FITC anti-VWF antibody at room temperature (RT) for 10 min. After incubation, platelets were fixed with 2% PFA and analyzed on a CytoFLEX flow cytometer. VWF binding was quantified by mean fluorescence intensity (MFI) of the measured cell population, subtracted by that of a negative control, and normalized with the relative MFI of fresh platelets (without storage) being 1.
Post-transfusion recovery and survival of platelets in vivo
Fresh or refrigerated human platelets were concentrated by centrifugation in the presence of 3 μM PGE1. After that, human platelets were injected into the retro-orbital plexus of recipient NOD-SCID mice at 108 platelets per 10g of body weight. Fresh or refrigerated WT platelets were labeled with 2 μg/ml CFSE, and concentrated in the presence of apyrase and PGI22. CFSE-labeled WT platelets were infused into WT mice at 108 platelets per 10g of body weight. At indicated time points afterwards, ~30 μl of whole blood was collected via the facial vein of infused NOD-SCID and WT mice into heparinized capillary tubes (Fisher Scientific, West Chester, PA) and incubated with appropriate antibodies for 30 min. All samples were then treated with 200 μl RBC Fix/Lyse solution (eBioscience, San Diego, CA) for 10 min, centrifuged at 400 g for 5 min, and platelet pellets were washed once, resuspended in 100 μl PBS, then fixed in 100 μl 4% PFA for flow cytometry analysis2. Human platelets recovery was calculated as , and normalized to that of fresh human platelets at 20-min post transfusion as 100%. WT mouse platelets recovery was calculated as , and normalized to that of fresh WT platelets at 1-hour post transfusion as 100%. Platelet survival (half-life time, T1/2) was calculated using one phase exponential decay32, 33.
Tail bleeding time
PBS, fresh platelets, untreated refrigerated platelets, ARC1779-treated refrigerated platelets, and ARC1779/antidote-treated refrigerated platelets (adding 0.9 μM ARC1779 antidote, three times of the molar mass as 10 μg/ml ARC1779, before transfusion) were transfused into IL4R-IbαTg at 108/10g of body weight. Two hours or 15 minutes after infusion, mice were placed on a heat plate and anesthetized by mask inhalation of isoflurane vaporized at concentrations of up to 4%. A 2-mm segment of the tail tip was cut off with a scalpel, and the tail was immediately placed in warmed (37°C) saline. Bleeding time from starting bleeding to occlusion was recorded and blood loss was measured. Occlusion was defined when the tail bleeding ceased for 30 seconds34. The blood loss was calculated as .
In vivo lifetime of ARC1779
A 96-well plate was coated with 6 μg/ml human GPIb-IX (purified as described35, 36) at 4°C overnight, and was blocked by 1% BSA at RT for 1 hour. Refrigerated WT platelets with 10 μg/ml ARC1779 were transfused into WT mice. At various time points (before transfusion, 20 min, 1 hour, 2 hours, 4 hours, 8 hours and 24 hours after transfusion), 60 μl blood was collected via facial vein and plasma was isolated by centrifugation. The isolated plasma then was added into the ELISA plate in the presence of 1 μg/ml botrocetin (Botrocetin is purified from Bothrops jararaca venom as described17), and incubated at RT for 1 hour. An HRP-conjugated anti-VWF antibody was applied and incubated at RT for 1 hour to detect VWF-GPIbα association. The bound anti-VWF antibody was quantified by adding TMB substrates and recording optical density (OD) at 450 nm. The OD450 reading from the plasma isolated before transfusion was normalized as 100%, indicating the endogenous VWF activity before transfusion was 100%.
The interaction of collagen IV and VWF
A 96-well ELISA plate was coated with 5 μg/ml human Collagen IV at 4°C overnight, and was blocked by 1% BSA. Human plasma was incubated with 10 μg/ml ARC1779 or saline for 10 minutes. The plasma was then added into the plate, and incubated at RT for 1 hour. An HRP-conjugated anti-VWF antibody was applied to detect the bound VWF. The OD450 reading from plasma incubated with saline was normalized as 100%.
Platelet aggregometry
Human whole blood was obtained from healthy donors, and PRP was isolated from the whole blood by centrifugation. The PRP then was adjusted to the concentration of 5 × 105 platelets/μl2. After that, the PRP was incubated with or without 10 μg/ml ARC1779 for 10 minutes, and ARC1779-incubated PRP was further incubated with 0.9 μM ARC1779 antidote or PBS for 10 minutes. Then, the PRP was stimulated by 0.9 mg/ml ristocetin, and the aggregation/agglutination was monitored in a dual-channel Chrono-Log aggregometer (Havertown, PA).
Statistics
The Kolmogorov-Smirnov test was used to confirm that all data had a normal distribution. Bartlett’s test was used to check variance between groups, and in all cases the results showed that equal variance was accepted. Figures 1 and 4 were analyzed by a two-way ANOVA with Tukey’s posthoc test. Figure 2 was analyzed by a one-way ANOVA with Tukey’s posthoc test. Figure 3 was analyzed by repeated measures ANOVA with Tukey’s posthoc test. Supplementary Figure 1 was analyzed by Student t-test. P<0.05 was considered as statistically significant.
Figure 1.
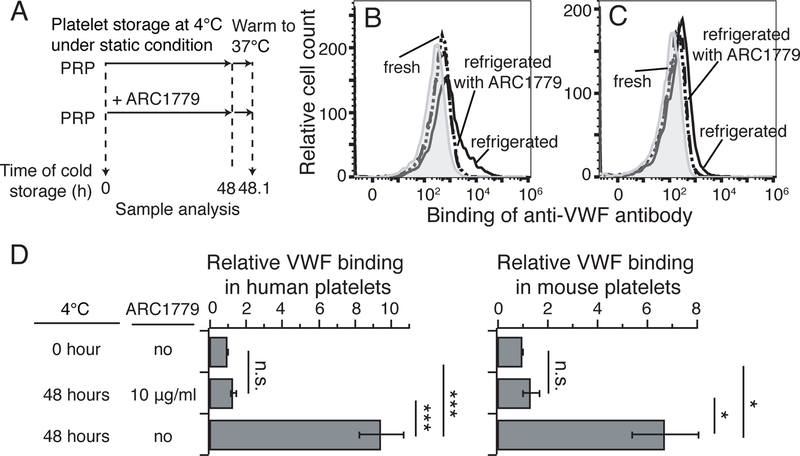
ARC1779 inhibits refrigeration-mediated VWF binding. A, Platelet refrigeration strategy. Human or murine WT PRPs were refrigerated with or without ARC1779 for 48 hours. Refrigerated platelets and fresh platelets were then warmed to 37°C, and VWF binding was analyzed by flow cytometry. B and C, Flow cytometry histogram of VWF binding to human platelets (B) and mouse platelets (C). The histogram of the negative control is filled by gray area. The dotted black line is fresh platelets. The dashed black line is refrigerated platelets treated with ARC1779, and the solid black line is untreated refrigerated platelets. D, Quantification of VWF binding to human platelets (n=3, left) and mouse platelets (n=3, right). Each VWF binding was quantified by mean fluorescence intensity of the histogram, subtracted by that of negative control, and normalized with that of fresh platelets as 1. Statistical analysis was performed by 2-way ANOVA. All data are shown as mean±SD. *, P < 0.05; and ***, P < 0.001.
Figure 4.
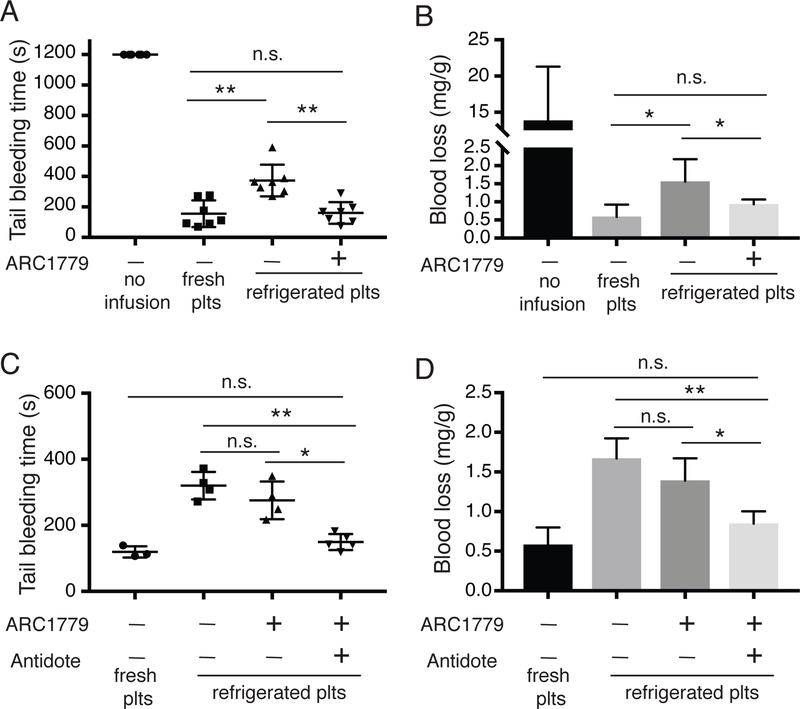
Antidote treatment increases the hemostatic function of ARC1779-treated refrigerated platelets. A-B, Tail bleeding assay two hours after transfusion. IL4R-IbαTg mice were transfused with PBS, fresh, refrigerated, and refrigerated with ARC1779 WT platelets. Two hours after transfusion, 2 mm segment of the mouse tail was cut off, and the tail vein was put into warmed saline. Bleeding time (A) and the blood loss (B) then was recorded. C-D, Tail bleeding assay fifteen minutes after transfusion. IL4R-IbαTg mice were transfused with fresh, refrigerated, ARC1779-treated refrigerated platelets, and ARC1779/antidote-treated refrigerated platelets. Tail bleeding time assays were performed at 15 minutes after transfusion, and bleeding time (C) and blood loss (D) were measured. Statistical analysis was performed by 2-way ANOVA. All data are presented as mean±SD (n=3–7). n.s., not significant; *, P < 0.05; and **, P < 0.01.
Figure 2.
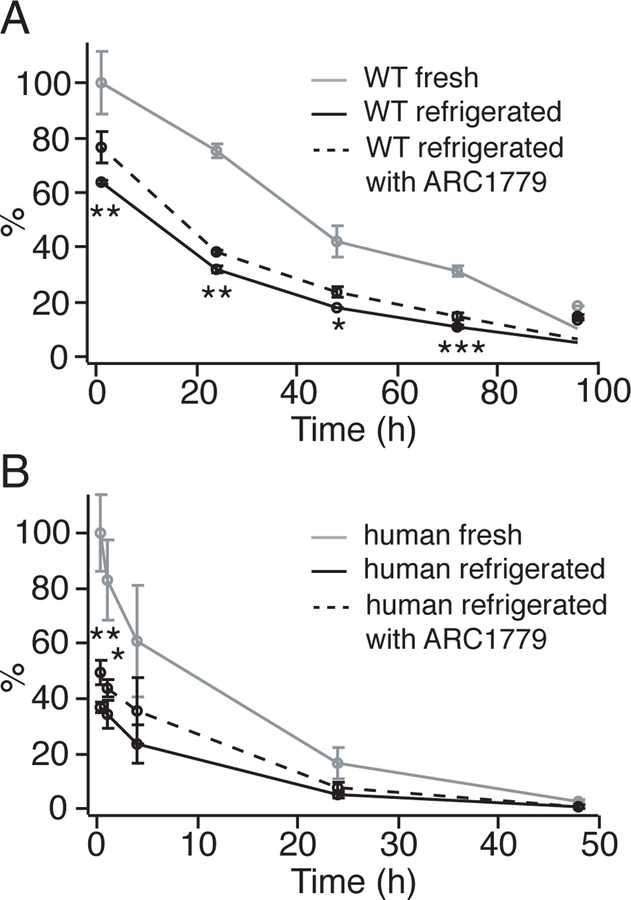
Improved post-transfusion recovery and survival of ARC1779 treated platelets. A, Fresh (gray), refrigerated (black) and refrigerated with ARC1779 (black dotted) WT platelets were labeled with CFSE, and transfused into WT mice at 108/10g. At 1, 24, 48, 72, and 96 hours after transfusion, about 30 μl of whole blood was drawn from recipient mice. The survival of the infused platelets was measured by flow cytometry. The recovery of WT fresh platelets at 1-hour post transfusion was normalized as 100% (n=6). B, Fresh (gray), refrigerated (black) and refrigerated with ARC1779 (black dotted) human platelets were transfused into NOD-SCID mice at 108/10g. Blood was drawn from recipient mice at 20 min, 1, 4, 24, and 48 hours after transfusion. The recovery of fresh human platelet at 20 min was normalized as 100% (n=6–8). Statistical analysis was performed by 1-way ANOVA. All data are shown as mean±SD. *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
Figure 3.
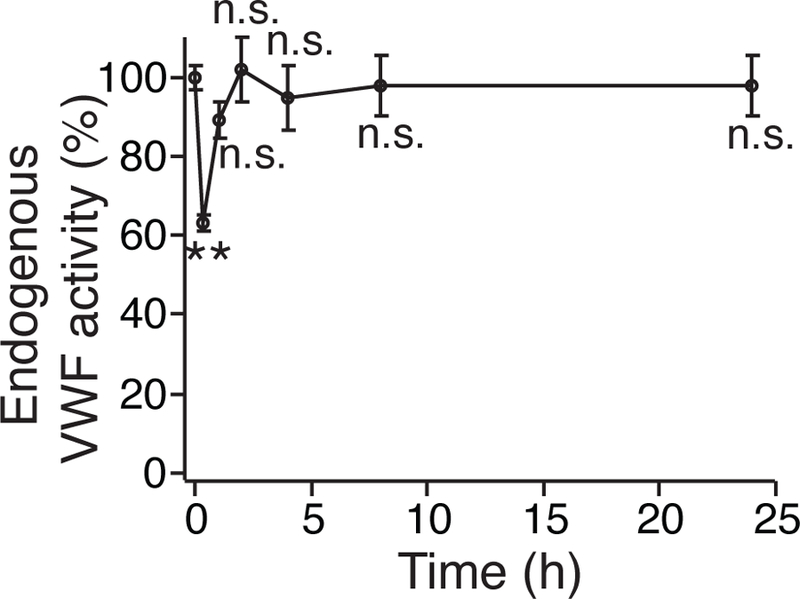
Short in vivo life time of ARC1779. WT PRP was refrigerated with 10 μg/ml ARC1779 for 48 hours, and then transfused into WT recipient mice. At 0 min (considered as before transfusion), 20 min, 1, 2, 4, 8 and 24 hours after transfusion, 50 μl of whole blood was collected and plasma was isolated. An ELISA assay was performed to detect 1 μg/ml botrocetin-induced VWF-GPIb association, which can indicate the inhibiting activity of ARC1779. The OD at 450 nm of plasma collected at 0 min was normalized as 100% of endogenous VWF activity, meaning the inhibiting effect of ARC1779 at this time point was 0%. The values at 20 min, 1, 2, 4, 8 and 24 hours were compared to that at 0 min. Statistical analysis was performed by repeated measures ANOVA. Data are presented as mean±SD (n=3). n.s., not significant; **, P < 0.01.
Results
ARC1779 inhibits refrigeration-mediated VWF binding
To investigate the effect of ARC1779 in inhibiting refrigeration-mediated VWF binding, PRPs obtained from human and WT mouse whole blood were refrigerated with or without 10 μg/ml ARC1779 in gas-permeable bags at 4˚C for 48 hours under static conditions, then re-warmed at 37°C for 10 min10. VWF binding was detected by flow cytometry using a FITC-labelled anti-VWF antibody (Fig. 1A). PRPs freshly isolated from humans and WT mice were also measured for comparison. Illustrated in Fig. 1B and 1C, both human and mouse platelets exhibited significantly increased VWF binding after refrigeration. Compared to fresh human platelets, VWF binding to refrigerated human platelets was increased approximately 10-fold. Similarly, refrigerated mouse platelets exhibited a more than 6-fold increase of VWF binding compared to fresh mouse platelets (Fig. 1D). The extent of the increased VWF binding is similar to our previous report10. Importantly, this increased VWF binding was abolished by the addition of ARC1779. In conjunction with our previously published data showing that anti-GPIbα LBD peptide OS1 inhibited refrigeration-mediated VWF binding, these results further confirm that refrigeration leads to the increased interaction between GPIbα and VWF, and antagonists against either the VWF A1 domain or the GPIbα LBD can inhibit this interaction.
In addition to platelets, the A1 domain of VWF binds collagen IV37. As ARC1779 interacts with the A1 domain of VWF, it may have an inhibitory effect on the interaction of VWF and collagen IV. To investigate this, an ELISA assay was performed to demonstrate that ARC1779 can reduce the VWF-collagen IV interaction by 34% (Supplementary Figure I).
Improved post-transfusion recovery and survival in ARC1779-treated refrigerated platelets
We previously reported that refrigerated VWF−/− platelets, as well as refrigerated human platelets incubated with peptide OS1, had higher post-transfusion recovery and longer survival time10. Here we investigated whether inhibiting VWF binding by ARC1779 could achieve a similar effect. Both human and WT mouse platelets were refrigerated for 48 hours in the presence or absence of 10 μg/ml ARC1779. Refrigerated human platelets, as well as fresh human platelets, were transfused into NOD-SCID mice at 108 platelets per 10 g of body weight, and the recovery and survival of the transfused human platelets were monitored. Similarly, fresh or refrigerated WT mouse platelets were transfused into WT mice after fluorescence labeling. The post-transfusion recovery and survival were measured by flow cytometry. ARC1779-treated refrigerated mouse platelets exhibited significantly better post-transfusion recovery (76.71 ± 5.51% vs. 63.78 ± 0.81%) and prolonged survival time (31.45 ± 2.37 hours vs. 28.16 ± 0.86 hours) compared to untreated refrigerated platelets (Fig. 2A, Table 1). Consistent with these data, ARC1779 treatment also improved the post-transfusion recovery (49.46 ± 4.44% vs. 36.89 ± 2.13%) of refrigerated human platelets (Fig. 2B, Table 2). These results demonstrate that inhibition of VWF binding by ARC1779 ameliorates quick clearance of refrigerated platelets.
Table 1.
Blocking VWF binding with ARC1779 enhanced the post-transfusion recovery and survival of refrigerated WT platelets.
| Platelets (108/10g) | Recovery (%) | Survival (T1/2; hour) |
|---|---|---|
| Fresh WT platelets | 100 ± 11.71 | 43.89 ± 5.23 |
| Refrigerated WT platelets | 63.78 ± 0.81 (**) | 28.16 ± 0.86 (*) |
| Refrigerated WT platelets with ARC1779 | 76.71 ± 5.51 | 31.45 ± 2.37 |
The indicated platelets were transfused into WT mice (108/10g), and one hour later the recovery and half-life time (T1/2) of the transfused platelets were measured. The recovery of fresh WT platelets was considered as 100%. As indicated, the recovery of ARC1779 treated refrigerated WT platelets was higher than those without ARC1779 (n=6). Statistical analysis was performed by 1-way ANOVA.
, P < 0.05
, P < 0.01.
Table 2.
ARC1779 treatment improved the post-transfusion recovery of refrigerated human platelets.
| Platelets (108/10g) | Recovery (%) | Survival (T1/2; hour) |
|---|---|---|
| Fresh human platelets | 100 ± 13.72 | 9.98 ± 1.2 |
| Refrigerated human platelets | 36.89 ± 2.13 (**) | 9.21 ± 2.75 |
| Refrigerated human platelets with ARC1779 | 49.46 ± 4.44 | 8.74 ± 0.91 |
Fresh or refrigerated human platelets were transfused into NOD-SCID recipient mice (108/10g). Twenty minutes after transfusion, the recovery and half-life time (T1/2) of the platelets was measured. The recovery of fresh human platelets was normalized as 100%. Significantly lower recovery was found in refrigerated platelets without ARC1779 compared to those with ARC1779. Statistical analysis was performed by 1-way ANOVA.
, P < 0.01.
Short lifetime of ARC1779 in mouse
ARC1779 has a relatively short half-life of about 2 hours in healthy human volunteers22. In the present study, we investigated the lifetime of ARC1779 in mice after transfusing ARC1779-treated platelets. Mouse platelets were refrigerated for 48 hours with 10 μg/ml ARC1779, and were transfused into WT recipient mice. At indicated time points before (0 min) and after transfusion, plasma was isolated from 60 μl of recipient mouse whole blood. An ELISA assay was performed to measure the plasma VWF activity by detecting botrocetin-mediated GPIbα-VWF binding. OD450 reading at 0 min was normalized as 100%. As shown in Fig. 3, the VWF activity was dropped at 20 min after transfusion by approximately 40%, but was fully recovered at 2 hours. These data suggest that the in vivo lifespan of ARC1779, which was contained in refrigerated PRPs, was 2 hours after transfusion.
The hemostatic function of ARC1779-treated refrigerated platelets
ARC1779 binds the VWF A1 domain and inhibits the GPIbα-VWF interaction. Since the GPIbα-VWF interaction is critical for primary hemostasis, one limitation of ARC1779 is that it may inhibit the hemostatic function of refrigerated platelets. Therefore, measuring the hemostatic function of ARC1779-treated refrigerated platelets is critical. Figure 3 suggests that the hemostatic function of ARC1779-treated refrigerated platelets may return to normal when the VWF activity recovered at 2 hours after transfusion. We performed tail bleeding time assays to determine the hemostatic function of ARC1779-treated and untreated refrigerated platelets in vivo. Refrigerated platelets (with or without ARC1779 treatment), fresh platelets, or PBS, were transfused into IL4R-IbαTg mice at 108 platelets per 10 g of body weight. Two hours after transfusion, a 2-mm tail tip of IL4R-IbαTg mice was cut off with a scalpel, and the tail was put into pre-warmed saline immediately. Bleeding time (Fig. 4A) and blood loss (Fig. 4B) were measured. Mice transfused with ARC1779-treated refrigerated platelets exhibited shorter bleeding time (160 ± 65 s) compared to those transfused untreated refrigerated platelets (373 ± 96 s). When the bleeding time of mice transfused with fresh platelets (155 s) is normalized as 100%, the bleeding time of mice transfused with ARC1779-treated refrigerated platelets is 103 ± 42% and untreated refrigerated platelets is 240 ± 62%. Similarly, blood loss in mice transfused with ARC1779-treated platelets (0.93 ± 0.12 mg/g, 158 ± 20% compared to fresh platelets) was less than those transfused with untreated platelets (1.56 ± 0.57 mg/g, 263 ± 97% compared to fresh platelets). Importantly, no significant difference could be observed between mice transfused with ARC1779-treated refrigerated platelets and mice transfused with fresh platelets (bleeding time: 155 ± 80 s; blood loss: 0.59 ± 0.31 mg/g). These results demonstrate that 2 hours after transfusion, ARC1779-treated refrigerated platelets had better hemostatic function compared to untreated refrigerated platelets.
Clinically, one major purpose of transfusing refrigerated platelets is to treat trauma patients with severe bleeding4, 5. The immediate hemostatic function of transfused platelets is required to stop severe bleeding. Here, we assessed the immediate hemostatic function of ARC1779-treated refrigerated platelets by measuring the tail bleeding time and blood loss at only 15 minutes after transfusion of refrigerated platelets. As shown in Fig. 4C and 4D, mice transfused with ARC1779-treated refrigerated platelets displayed similar tail bleeding time (275 ± 49 s, or 229 ± 41% of fresh platelets) and blood loss (1.39 ± 0.27 mg/g, 240 ± 42%) when compared to untreated ones (Bleeding time: 320 ± 36 s, 266 ± 30%; Blood loss: 1.67 ± 0.25 mg/g, 288 ± 38%). These results suggest that although ARC1779-treated refrigerated platelets have better post-transfusion recovery, their immediate hemostatic function is not better than untreated refrigerated platelets. The main reason is that the VWF activity in the mice transfused with ARC1779-treated platelets was transiently reduced by 40% (Fig. 3). To overcome this limitation, an antidote, which is the oligonucleotide complementary to ARC1779 and inhibitory to ARC1779 (Supplementary Figure II), was added into ARC1779-treated refrigerated platelets before transfusion. The immediate hemostatic function of these platelets was measured by the tail bleeding time assay. As shown in Fig. 4C and 4D, the bleeding time of the mice transfused with these antidote-treated platelets (149 ± 21 s, 124 ± 18% of fresh platelets) was shorter than those without antidote, and blood loss (0.84 ± 0. mg/g, 145 ± 24 %) was less. Taken together, these results indicate that ARC1779-treated refrigerated platelets may be effective in the prophylactic treatment of thrombocytopenia, but they may not be very effective in the treatment of acute hemorrhage, and that the addition of the ARC1779 antidote before transfusion can be applied to refrigerated platelets to treat acute hemorrhage.
Discussion
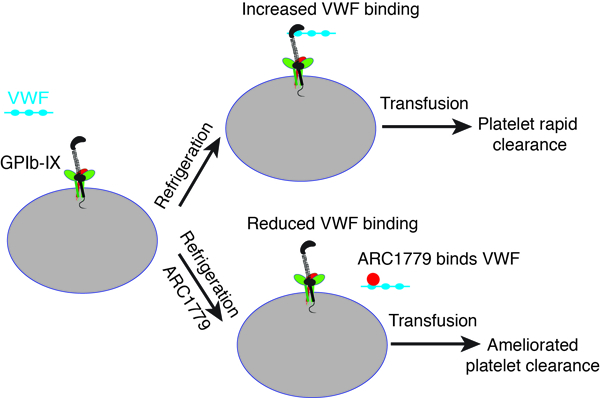
Although refrigerated platelets are less likely to be contaminated by bacteria and have higher hemostatic function compared to platelets stored at RT38, they are rapidly removed from circulation upon transfusion6–8. Previous studies showed that VWF binding to platelets was strongly enhanced after refrigeration9, 10. While it remains unclear how refrigeration induces VWF binding, there are some potential explanations. First, during refrigeration, VWF and GPIbα may undergo conformational changes to promote their interaction39, 40. Second, platelet-derived VWF, which is more active41, may be secreted by platelets during refrigeration, leading to the increased binding. However, additional studies are required to understand the mechanism of refrigeration-induced VWF binding. Based on refrigeration-mediated VWF binding, we demonstrated that this VWF binding resulted in MSD unfolding after physiological shear treatment, and subsequent GPIb-IX signaling events, including intracellular Ca2+ elevation, PS exposure and β-galactose exposure, which consequently induced platelet clearance10. In the present study, we used the DNA aptamer ARC1779 to inhibit refrigeration-mediated VWF binding to platelets. We found that the addition of ARC1779 during refrigeration significantly improved the post-transfusion recovery and survival of both refrigerated human and mouse platelets. In addition, due to the short in vivo lifetime of ARC1779, aptamer-treated refrigerated platelets exhibited preserved hemostatic function compared to untreated ones. These results indicate the efficacy of ARC1779 as a potential therapeutic option for improving the quality of refrigerated platelets.
ARC1779 was developed to treat VWF-related disorders, including Type 2B VWD, thrombotic thrombocytopenia, thrombotic microangiopathy, and stroke25–28. In addition, ARC1779 can inhibit botrocetin-mediated VWF binding to platelets and subsequent platelet agglutination24. However, the effect of ARC1779 on platelet refrigeration and transfusion has not been investigated so far. In our previous research, we demonstrated that inhibiting GPIbα-VWF interaction by OS1 enhanced the recovery and survival of refrigerated platelets upon transfusion10. In the present study, we demonstrate that ARC1779 can completely inhibit refrigeration-mediated VWF binding in both human and murine platelets (Fig. 1). Subsequently, inhibiting GPIbα-VWF binding by ARC1779 improved the recovery of refrigerated human platelets by approx. 13%, and refrigerated mouse platelets by approx. 13% (Fig. 2; Table 1 and 2). This effect is very similar to that of OS1, which improved the recovery of refrigerated platelets by approx. 14%10. Additionally, ARC1779 improved the post-transfusion survival of refrigerated mouse platelets (from T1/2 of 28.16 ± 0.86 hours to 31.45 ± 2.37 hours). These results demonstrate for the first time that ARC1779 plays a positive role in facilitating platelet refrigeration and transfusion.
One major limitation of nucleic acid as therapeutics is that it may be quickly degraded or cleared in blood stream. Although ARC1779 contains many modifications, including 26 modified 2’-O-methyl-substituted nucleotides and 1 inverted deoxythymidine nucleotide as a 3’ terminus ‘cap’, to minimized endonuclease and exonuclease digestions22, 23, the in vivo half-life in human body is relatively short, approximately 2 hours22. However, the activities of these enzymes are highly temperature-dependent and at refrigeration temperature (1–6°C), the activities of most of the endonucleases and exonucleases are extremely low. Transfusing 10 μg/ml ARC1779-treated refrigerated PRP into mice still decreased the endogenous VWF activity of recipient mice by approximately 40%, demonstrating that 48-hour refrigeration did not result in excessive degradation of ARC1779 (Fig. 3). Further, the endogenous VWF activity was fully recovered 2 hours after transfusion, suggesting that ARC1779 in refrigerated PRPs could survive for only 2 hours in mice, where the temperature is 37°C and a large number of endonucleases and exonucleases present (Fig. 3).
Although the ARC1779 inhibits VWF-mediated platelet activation and thrombus formation, the extent of bleeding complication caused by ARC1779 treatment is much less than the GPIIb/IIIa inhibitor abciximab, especially at low concentrations22, 24. In this study, we further investigated the hemostatic function of ARC1779-treated refrigerated platelets by a tail bleeding time assay. IL4R-IbαTg mice, which have severe bleeding phenotype due to the lack of normal GPIbα, were used as recipient mice. Two hours after transfusing platelets, a 2-mm tail tip of the recipient mice was cut off, and the bleeding time and blood loss were quantified. Refrigerated platelets with ARC1779 exhibited significantly higher hemostatic function compared to those without ARC1779 (Fig. 4A and 4B). In addition to detecting the hemostatic function two hours after transfusion, we also determined the immediate hemostatic activity (15 minutes after transfusion) of ARC1779-treated refrigerated platelets. The immediate hemostatic function of ARC-1779 treated refrigerated platelet was not different compared to untreated refrigerated platelets. However, the addition of ARC1779 antidote before transfusion can improve the immediate hemostatic function of ARC1779-treated refrigerated platelets. Several factors may contribute to these results. First, refrigerated platelets with ARC1779 have better post-transfusion recovery and survival than those without ARC1779. Second, transfusing ARC1779-treated platelets leads to a 40% reduction of endogenous VWF activity and this effect is completely eliminated at 2 hours after transfusion due to its short in vivo survival term. Third, the amount of ARC1779 in the refrigerated PRPs is not sufficient to binding all endogenous VWF in recipient mice after transfusion. Upon transfusion, more than 60% of endogenous VWF in recipient were active (Fig. 3) and capable of binding the transfused platelets to initiate hemostasis. Taken together, these results suggest that ARC1779 can be a novel option for adding into refrigerated platelets and treating thrombocytopenia, but ARC1779-treated refrigerated platelets are not effective for treating acute hemorrhage. The antidote of ARC1779 may be required to add into ARC1779-treated refrigerated platelets before transfusion to treat acute hemorrhage.
Supplementary Material
Highlights.
An anti-VWF aptamer improves the post-transfusion recovery and survival of refrigerated platelets.
Adding an antidote into aptamer-treated refrigerated platelets before transfusion can improve their immediate hemostatic function.
Acknowledgements
WC, CDJ, and RL designed research; WC and KMV performed research; WC analyzed the results; WC and RL wrote the paper; CDJ edited manuscript and provided critical comments. We thank the Emory Children’s Pediatric Research Center Flow Cytometry Core for technical support.
Sources of Funding
This work was supported in part by National Institutes of Health grants HL082808 and HL123984.
RL is a co-founder of Hemoletix LLC, and consults for Neoletix Labs, Inc.
Nonstandard Abbreviations and Acronyms
- GPIbα
Glycoprotein Ibα
- GPIb-IX
Glycoprotein Ib-IX complex
- VWF
von Willebrand factor
- RT
Room temperature
- WT
wild-type
- CFSE
carboxyfluorescein succinimidyl ester
- MSD
mechanosensory domain
- LBD
ligand-binding domain
- PRP
platelet-rich plasma
- LR-ADP
Human leukoreduced apheresis-derived platelets
Footnotes
Disclosures
Other authors declared no conflicts of interest.
References
- 1.Devine DV, Serrano K. The platelet storage lesion. Clinics in laboratory medicine 2010;30:475–487 [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Liang X, Syed AK, Jessup P, Church WR, Ware J, Josephson CD, Li R. Inhibiting gpibα shedding preserves post-transfusion recovery and hemostatic function of platelets after prolonged storage. Arterioscler Thromb Vasc Biol 2016;36:1821–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandler SG, Yu H, Rassai N. Risks of blood transfusion and their prevention. Clin Adv Hematol Oncol 2003;1:307–313 [PubMed] [Google Scholar]
- 4.Stubbs JR, Tran SA, Emery RL, Hammel SA, Haugen AL, Zielinski MD, Zietlow SP, Jenkins D. Cold platelets for trauma-associated bleeding: Regulatory approval, accreditation approval, and practice implementation-just the “tip of the iceberg”. Transfusion 2017;57:2836–2844 [DOI] [PubMed] [Google Scholar]
- 5.Cap AP, Spinella PC. Just chill-it’s worth it! Transfusion 2017;57:2817–2820 [DOI] [PubMed] [Google Scholar]
- 6.Becker GA, Tuccelli M, Kunicki T, Chalos MK, Aster RH. Studies of platelet concentrates stored at 22 c nad 4 c. Transfusion 1973;13:61–68 [DOI] [PubMed] [Google Scholar]
- 7.Berger G, Hartwell DW, Wagner DD. P-selectin and platelet clearance. Blood 1998;92:4446–4452 [PubMed] [Google Scholar]
- 8.Murphy S, Gardner FH. Effect of storage temperature on maintenance of platelet viability--deleterious effect of refrigerated storage. N Engl J Med 1969;280:1094–1098 [DOI] [PubMed] [Google Scholar]
- 9.Rumjantseva V, Grewal PK, Wandall HH, Josefsson EC, Sorensen AL, Larson G, Marth JD, Hartwig JH, Hoffmeister KM. Dual roles for hepatic lectin receptors in the clearance of chilled platelets. Nat Med 2009;15:1273–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen W, Druzak SA, Wang Y, Josephson CD, Hoffmeister KM, Ware J, Li R. Refrigeration-induced binding of von willebrand factor facilitates fast clearance of refrigerated platelets. Arterioscler Thromb Vasc Biol 2017;37:2271–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruggeri ZM, Pareti FI, Mannucci PM, Ciavarella N, Zimmerman TS. Heightened interaction between platelets and factor viii/von willebrand factor in a new subtype of von willebrand’s disease. N Engl J Med 1980;302:1047–1051 [DOI] [PubMed] [Google Scholar]
- 12.Randi AM, Jorieux S, Tuley EA, Mazurier C, Sadler JE. Recombinant von willebrand factor arg578-->gln. A type iib von willebrand disease mutation affects binding to glycoprotein ib but not to collagen or heparin. J Biol Chem 1992;267:21187–21192 [PubMed] [Google Scholar]
- 13.Miyata S, Goto S, Federici AB, Ware J, Ruggeri ZM. Conformational changes in the a1 domain of von willebrand factor modulating the interaction with platelet glycoprotein ibalpha. J Biol Chem 1996;271:9046–9053 [DOI] [PubMed] [Google Scholar]
- 14.Lillicrap D Von willebrand disease: Advances in pathogenetic understanding, diagnosis, and therapy. Blood 2013;122:3735–3740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casari C, Du V, Wu YP, Kauskot A, de Groot PG, Christophe OD, Denis CV, de Laat B, Lenting PJ. Accelerated uptake of vwf/platelet complexes in macrophages contributes to vwd type 2b-associated thrombocytopenia. Blood 2013;122:2893–2902 [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Deng W, Zhou L, Xu Y, Yang W, Liang X, Wang Y, Kulman JD, Zhang XF, Li R. Identification of a juxtamembrane mechanosensitive domain in the platelet mechanosensor glycoprotein ib-ix complex. Blood 2015;125:562–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng W, Xu Y, Chen W, Paul DS, Syed AK, Dragovich MA, Liang X, Zakas P, Berndt MC, Di Paola J, Ware J, Lanza F, Doering CB, Bergmeier W, Zhang XF, Li R. Platelet clearance via shear-induced unfolding of a membrane mechanoreceptor. Nat Commun 2016;7:12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quach ME, Dragovich MA, Chen W, Syed AK, Cao W, Liang X, Deng W, De Meyer SF, Zhu G, Peng J, Ni H, Bennett CM, Hou M, Ware J, Deckmyn H, Zhang XF, Li R. Fc-independent immune thrombocytopenia via mechanomolecular signaling in platelets. Blood 2018;131:787–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quach ME, Chen W, Li R. Mechanisms of platelet clearance and translation to improve platelet storage. Blood 2018;131:1512–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McEwan PA, Andrews RK, Emsley J. Glycoprotein ibα inhibitor complex structure reveals a combined steric and allosteric mechanism of von willebrand factor antagonism. Blood 2009;114:4883–4885 [DOI] [PubMed] [Google Scholar]
- 21.Benard SA, Smith TM, Cunningham K, Jacob J, DeSilva T, Lin L, Shaw GD, Kriz R, Kelleher KS. Identification of peptide antagonists to glycoprotein ibalpha that selectively inhibit von willebrand factor dependent platelet aggregation. Biochemistry 2008;47:4674–4682 [DOI] [PubMed] [Google Scholar]
- 22.Gilbert JC, DeFeo-Fraulini T, Hutabarat RM, Horvath CJ, Merlino PG, Marsh HN, Healy JM, Boufakhreddine S, Holohan TV, Schaub RG. First-in-human evaluation of anti von willebrand factor therapeutic aptamer arc1779 in healthy volunteers. Circulation 2007;116:2678–2686 [DOI] [PubMed] [Google Scholar]
- 23.Huang RH, Fremont DH, Diener JL, Schaub RG, Sadler JE. A structural explanation for the antithrombotic activity of arc1172, a DNA aptamer that binds von willebrand factor domain a1. Structure 2009;17:1476–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diener JL, Daniel Lagasse HA, Duerschmied D, Merhi Y, Tanguay JF, Hutabarat R, Gilbert J, Wagner DD, Schaub R. Inhibition of von willebrand factor-mediated platelet activation and thrombosis by the anti-von willebrand factor a1-domain aptamer arc1779. J Thromb Haemost 2009;7:1155–1162 [DOI] [PubMed] [Google Scholar]
- 25.Mayr FB, Knobl P, Jilma B, Siller-Matula JM, Wagner PG, Schaub RG, Gilbert JC, Jilma-Stohlawetz P. The aptamer arc1779 blocks von willebrand factor-dependent platelet function in patients with thrombotic thrombocytopenic purpura ex vivo. Transfusion 2010;50:1079–1087 [DOI] [PubMed] [Google Scholar]
- 26.Jilma-Stohlawetz P, Gilbert JC, Gorczyca ME, Knobl P, Jilma B. A dose ranging phase i/ii trial of the von willebrand factor inhibiting aptamer arc1779 in patients with congenital thrombotic thrombocytopenic purpura. Thromb Haemost 2011;106:539–547 [DOI] [PubMed] [Google Scholar]
- 27.Jilma-Stohlawetz P, Knobl P, Gilbert JC, Jilma B. The anti-von willebrand factor aptamer arc1779 increases von willebrand factor levels and platelet counts in patients with type 2b von willebrand disease. Thromb Haemost 2012;108:284–290 [DOI] [PubMed] [Google Scholar]
- 28.Markus HS, McCollum C, Imray C, Goulder MA, Gilbert J, King A. The von willebrand inhibitor arc1779 reduces cerebral embolization after carotid endarterectomy: A randomized trial. Stroke 2011;42:2149–2153 [DOI] [PubMed] [Google Scholar]
- 29.Kanaji T, Russell S, Ware J. Amelioration of the macrothrombocytopenia associated with the murine bernard-soulier syndrome. Blood 2002;100:2102–2107 [DOI] [PubMed] [Google Scholar]
- 30.Chen W, Thielmann I, Gupta S, Subramanian H, Stegner D, van Kruchten R, Dietrich A, Gambaryan S, Heemskerk JW, Hermanns HM, Nieswandt B, Braun A. Orai1-induced store-operated ca(2+) entry enhances phospholipase activity and modulates canonical transient receptor potential channel 6 function in murine platelets. J Thromb Haemost 2014;12:528–539 [DOI] [PubMed] [Google Scholar]
- 31.Gotru SK, Chen W, Kraft P, Becker IC, Wolf K, Stritt S, Zierler S, Hermanns HM, Rao D, Perraud AL, Schmitz C, Zahedi RP, Noy PJ, Tomlinson MG, Dandekar T, Matsushita M, Chubanov V, Gudermann T, Stoll G, Nieswandt B, Braun A. Trpm7 kinase controls calcium responses in arterial thrombosis and stroke in mice. Arterioscler Thromb Vasc Biol 2018;38:344–352 [DOI] [PubMed] [Google Scholar]
- 32.Tsukada T, Tango T. On the methods calculating mean survival time in 51cr-platelet survival study. Am. J. Hematol 1980;8:281–290 [DOI] [PubMed] [Google Scholar]
- 33.Baker GR, Sullam PM, Levin J. A simple, fluorescent method to internally label platelets suitable for physiological measurements. Am J Hematol 1997;56:17–25 [DOI] [PubMed] [Google Scholar]
- 34.Bender M, May F, Lorenz V, Thielmann I, Hagedorn I, Finney BA, Vogtle T, Remer K, Braun A, Bosl M, Watson SP, Nieswandt B. Combined in vivo depletion of glycoprotein vi and c-type lectin-like receptor 2 severely compromises hemostasis and abrogates arterial thrombosis in mice. Arterioscler Thromb Vasc Biol 2013;33:926–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan R, Mo X, Paredes AM, Dai K, Lanza F, Cruz MA, Li R. Reconstitution of the platelet glycoprotein ib-ix complex in phospholipid bilayer nanodiscs. Biochemistry 2011;50:10598–10606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang X, Russell SR, Estelle S, Jones LH, Cho S, Kahn ML, Berndt MC, Bunting ST, Ware J, Li R. Specific inhibition of ectodomain shedding of glycoprotein ibalpha by targeting its juxtamembrane shedding cleavage site. J Thromb Haemost 2013;11:2155–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flood VH, Schlauderaff AC, Haberichter SL, Slobodianuk TL, Jacobi PM, Bellissimo DB, Christopherson PA, Friedman KD, Gill JC, Hoffmann RG, Montgomery RR, Zimmerman Program I. Crucial role for the vwf a1 domain in binding to type iv collagen. Blood 2015;125:2297–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reddoch KM, Pidcoke HF, Montgomery RK, Fedyk CG, Aden JK, Ramasubramanian AK, Cap AP. Hemostatic function of apheresis platelets stored at 4 degrees c and 22 degrees c. Shock 2014;41 Suppl 1:54–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deng W, Wang Y, Druzak SA, Healey JF, Syed AK, Lollar P, Li R. A discontinuous autoinhibitory module masks the a1 domain of von willebrand factor. submitted 2017 [DOI] [PMC free article] [PubMed]
- 40.Tischer A, Madde P, Moon-Tasson L, Auton M. Misfolding of vwf to pathologically disordered conformations impacts the severity of von willebrand disease. Biophys J 2014;107:1185–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verhenne S, Denorme F, Libbrecht S, Vandenbulcke A, Pareyn I, Deckmyn H, Lambrecht A, Nieswandt B, Kleinschnitz C, Vanhoorelbeke K, De Meyer SF. Platelet-derived vwf is not essential for normal thrombosis and hemostasis but fosters ischemic stroke injury in mice. Blood 2015;126:1715–1722 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.