Abstract
For decades the field of radiation oncology has sought to improve the therapeutic ratio through innovations in physics, chemistry, and biology. To date, technological advancements in image guided beam delivery techniques have provided clinicians with their best options for improving this critical tool in cancer care. Medical physics has focused on the preferential targeting of tumors while minimizing the collateral dose to the surrounding normal tissues, yielding only incremental progress. However, recent developments involving ultra-high dose rate irradiation termed FLASH radiotherapy (FLASH-RT), that were initiated nearly 50 years ago, have stimulated a renaissance in the field of radiotherapy, long awaiting a breakthrough modality able to enhance therapeutic responses and limit normal tissue injury. Compared to conventional dose rates used clinically (0.1 – 0.2 Gy/s), FLASH can implement dose rates of electrons or x- rays in excess of 100 Gy/s. The implications of this ultra-fast delivery of dose are significant and need to be re-evaluated to appreciate the fundamental aspects underlying this seemingly unique radiobiology. The capability of FLASH to significantly spare normal tissue complications in multiple animal models, when compared to conventional rates of dose-delivery, while maintaining persistent growth inhibition of select tumor models has generated considerable excitement, as well as skepticism. Based on fundamental principles of radiation physics, radio- chemistry, and tumor vs. normal cell redox metabolism, this article presents a series of testable, biologically relevant hypotheses, which may help rationalize the differential effects of FLASH irradiation observed between normal tissue and tumors.
Keywords: Free Radical Chemistry, Organic Hydroperoxides, Oxygen, Redox Active Iron, FLASH Radiation, Tumor versus Normal Tissue Responses
Introduction
Changes in the methodologies used to deliver radiotherapy may soon be approaching, challenging many of the classical approaches that have been a cornerstone of current clinical practice. At the center of this paradigm shift are the long-standing radiation therapy treatment approaches for multiple cancer types that utilize low doses and low dose rates in a variety of fractionation schedules, all designed to exploit potential differences between normal tissue and tumor radiosensitivity. The underlying premise is that dose fractionation preferentially favors the recovery of normal tissue over tumors from the harmful effects of radiation, thereby avoiding unacceptable, or even lethal normal tissue complications during the course of tumor sterilization. In fact, normal tissue injury dictates the maximum tolerated dose that can be safely delivered to a tumor bed, and typically involves modulating low doses per fraction (i.e. 2 Gy) through intensity-modulated, volumetric-modulated, or particle-based radiotherapeutic approaches.
Recent advances in image guided stereotactic ablative or body radiotherapy have revealed certain benefits of hypofractionation, using doses in excess of 2 Gy per fraction. Despite these recent trends, change has been slow and relatively incremental, as iso-dose effect curves have long pointed to the benefits of minimizing the dose per fraction so as not to induce severe late effects. Until recently, radiotherapy has not fully considered changes in dose rate as an adjustable parameter important for therapeutic gain, and the vast majority of all treatment protocols implementing various radiation modalities operate at relatively constant, low dose rates around 0.1 − 0.2 Gy s−1. Recently, increasing evidence suggests that increasing the dose rate to extremely high levels (≥ 40 Gy s−1) provides some rather remarkable benefits in terms of sparing of normal tissue [1, 2]. Here we present a novel theoretical construct and hypothesis, based on radiochemical principles and differences in oxidative metabolism of radiation-induced damage products that might help provide a useful paradigm to help understand the benefits of ultra-high dose rate FLASH radiotherapy (FLASH-RT) in sparing normal tissue while retaining tumor therapeutic responses.
Re-analysis of Radiobiological Concepts in the Context of FLASH-RT
Given traditional radiobiological concepts governing the free radical chemistry initiated at the time of irradiation by conventional radiation dose delivery, it is difficult to reconcile the mounting in vivo evidence demonstrating the sparing of normal tissue in multiple organ sites exposed to FLASH-RT while maintaining persistent inhibition of tumor growth. However in the past two decades it has also become well appreciated that free radical and oxidative metabolism involving the formation of reactive oxygen species (ROS), such as superoxide (O •−2), hydrogen peroxide (H2O2), organic hydroperoxides (ROOH), as well as redox active metal ions, such as labile iron, can significantly contribute to the effects of ionizing radiation in normal tissues for significant periods of time following exposure [3–6]. It is also becoming clearly evident that cancer (compared to normal) cell metabolism demonstrates profoundly altered steady-state levels of ROS and redox active metals that can significantly impact therapy responses to agents that can disrupt redox metabolism [6–9]. This differential response between normal and tumor tissue to redox active agents has been harnessed recently in preclinical and clinical trials for the purpose of improving responses to therapy by exacerbating oxidative stress and DNA damage in cancer tissues while sparing normal tissue injury [6–9]. Most provocative, in the context of differences in redox metabolism between cancer vs. normal tissue, is the evidence that ultra- high dose rate FLASH-RT energy deposition led to less normal tissue damage, despite retaining tumor response. This finding is in contrast to decades of experimental findings demonstrating that reduced dose rates (cGy h−1) accomplish similar outcomes, due to the temporal superposition of dose delivery and the repair of DNA damage.
It is intriguing to speculate that the clearly emerging differences in the redox biology of oxygen metabolism may contribute to the differential effects of FLASH-RT in cancer vs. normal tissues. In this emerging field of redox cancer biology, the formation and processing of FLASH-RT- induced oxidative damage to organic molecules in actively respiring cancer vs. normal tissues can be re-evaluated in the context of what is known about radiation chemistry and oxidative metabolism. Our re-evaluation is an attempt to develop a framework for deriving testable hypotheses for determining mechanisms by which ultra-high rate of dose-delivery might elicit such striking and clinically promising results.
Ionizing radiation is known to generate aqueous electrons (eaq−) and oxidizing free radicals that elicit covalent changes in the macromolecular constituents of cells [10–12]. Under typical irradiation scenarios using lower LET radiation therapy modalities, ionizing radiation-induced DNA damage has long been attributed to the combination of both indirect and direct effects that contribute to 67% and 33% of the total damage, respectively [10–12]. Direct effects of ionizing radiation lead to the formation of organic radicals (R•) on biomolecules; while indirect effects, through the ionization of water, generate the highly reactive hydroxyl radical (HO•) that reacts rapidly (t1/2 ≈ 1 nanosecond) through hydrogen atom abstraction and addition reactions to also generate R•. This results in the accumulation of oxidative damage to critical organic biomolecules (such as DNA) in living cells. Importantly, the resulting radiation chemistry is stochastic and linear with dose, leading to a plethora of non-linear biological responses involving specific types of stress responsive signaling pathways in cells that mediate cell cycle arrest, DNA damage repair, mutations, genomic instability, metabolic disruption, and multiple modes of cell kill. Over the years a variety of approaches have been designed aimed at modulating the contribution of the indirect and direct effects of ionizing radiation through changes in bound water (phage, dehydrated spores), oxygen tension (multiple test systems from bacteria, to yeast, to fish and mammalian cells) and in exo/endogenous free radical scavengers. Whether implied or not, the practical focus of these studies was ultimately aimed at determining fundamental principles of radiobiology that could be useful in sensitizing tumor tissues while protecting normal tissues exposed to ionizing radiation at conventional dose rates (≈0.03 Gy s−1).
Oxygen (O2) is known to be a potent radiosensitizer. A wealth of past work has defined both tumor and normal tissue oxygen levels, and the doses required to deplete oxygen in those respective target sites [13–17]. In the current reanalysis of the tissue micro-environmental factors governing the generation and reactions of free radicals and eaq− generated by pulses of low LET ultra-high dose rate FLASH-RT vs. conventional pulses of fractionated low LET radiation will be considered. Further, the radiochemical yields of free radicals, organic peroxyl radicals (ROO•), organic hydroperoxides (ROOH), and their participation in subsequent reaction cascades in tissues involving labile metal ions that can intensify the depletion of oxygen under ultra-high dose rate delivery will also be considered. The hypothesis is that differences between the decay rates of ROO• and ROOH produced in normal tissue vs. tumors, along with the differences in the labile iron pool may provide a plausible framework for explaining the beneficial therapeutic ratio of FLASH-RT compared to conventional dose rate irradiations.
FLASH vs. Conventional Dose Rate Radiation Chemistry
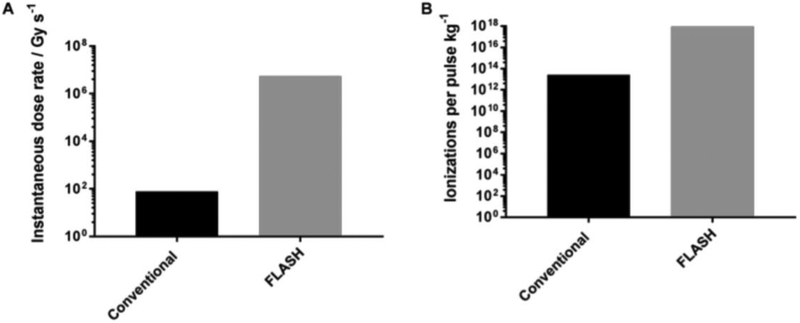
To initiate our analysis, we begin with a comparison of the pulse-to-pulse dose rate amplification from FLASH, Figure 1A. If we consider the dose rate from a conventional linear accelerator as described by Karzmark and Morton [18], the photon beam mean dose rate at 6 MV is 4 Gy min−1; a 6 MeV electron beam corresponds to a dose rate is 5 Gy min−1. Therefore a 6 MV photon beam has a flattened mean dose rate of 0.07 Gy s−1 (0.19 Gy s−1 for flattening filter free (FFF) deliveries), and an conventional 6 MeV electron beam mean dose rate of 0.08 Gy s−1. The resulting dose per pulse, assuming 300 pulses per second, is 2.2 × 10−4 Gy per pulse for a conventional flattened 6 MV beam (6.3 × 10−4 Gy per pulse, FFF) and 2.8 × 10−4 Gy per pulse for a conventional 6 MeV electron beam. Assuming a conventional pulse width of approximately 3.5 μs, this corresponds to an instantaneous 6 MV photon dose rate of 64 Gy s−1 (181 Gy s−1, FFF), and a 6 MeV electron beam dose rate of 79 Gy s−1. A recent FLASH study published by Montay-Gruel et al. [19] produced 10 Gy in a single 1.8 μs electron beam pulse at 4.5 – 6 MeV, resulting in an instantaneous dose rate of approximately 5.6 × 106 Gy s−1. Based on the given assumptions, FLASH deliveries represent a greater than four order of magnitude increase in instantaneous dose rate over conventional photon and electron beam deliveries. Although modern accelerators produce dose rates slightly above the values stated in Karzmark [18], the general conclusions presented here remain the same.
Figure 1.
Graphical representation of theoretical results comparing an instantaneous pulse from a conventional and FLASH electron beam. (A) instantaneous dose rate; and (B) number of ionizations produced following an instantaneous pulse (ionizations kg−1).
If we consider the radiolysis of water to be the initiating process for the formation of free radicals and subsequent formation of ROS following the incidence of Low-LET radiation (i.e. photons and electrons), then we can calculate the number of ions generated per instantaneous pulse based on the energy being imparted from each pulse, Figure 1B. The initial, physio-chemical step in the radiolysis of water is the separation of an electron from the water molecule (Eqn 1):
| (1) |
If we only choose to focus on the release of a single electron from water in soft tissue as the key initiating step in the generation of free radicals and ROS, we can compute the number of ions released from a single pulse of a conventional 6 MV flattened photon beam and 6 MeV electron beam as compared to a single FLASH pulse of 10 Gy delivered in 1.8 μs.
Here we assume that all ionizations occurring in a 1 kg mass of soft tissue following an instantaneous pulse of radiation are the result of an electron being released from soft tissue with an effective ionization energy (Ieff) of 66.2 eV per liberated electron [20]. Comparing conventional and FLASH electron beam deliveries, 2.8 × 10−4 Gy is delivered in a single conventional beam pulse and 10 Gy is delivered in a single FLASH pulse. Thus, 1.75 × 1015 eV and 6.2 × 1019 eV of energy is deposited per kg of tissue for conventional and FLASH deliveries, respectively.
Using an effective ionization energy, Ieff, of 66.2 eV per liberated electron from soft tissue (Ieff ≈ 66.0 eV for water), this analysis indicates that 2.6 × 1013 electrons kg−1 are liberated in a single conventional pulse, whereas 9.4 × 1017 electrons kg−1 are mobilized after a pulse of FLASH. If we further assume that all electron interactions are elastic collisions (i.e. all of the energy creates ionizations), then a single pulse of FLASH leads to ≈ 36,000 more ionization events than a corresponding pulse of conventional electron beam irradiation. If the same calculations are done comparing a FLASH pulse and a conventional 6 MV photon beam, the difference would be on the same order of magnitude, resulting in ≈ 45,000 more ionization events from a FLASH pulse.
After the initial separation of an electron from water by ionizing radiation, Eqn 1 (ps time-scale), in oxygenated aqueous solution the two entities will be converted to the species of Eqn 2 (ns time-scale):
| (2) |
From a radiation chemistry perspective, the expected stoichiometry and yields of the species of Eqn 2 can then be estimated based on G-values used to convert dose (Gy) to radical concentration [21]. As an example, an estimation of the chemical yield of species following a 10 Gy pulse at dose rates from 20 – 200 Gy s−1 in brain tissue is shown in Table 1.
Table 1.
G-values for radiolysis of water.
| Primary Product | G-value | G-value (μM/Gy)a | μM after 10 Gy pulse |
|---|---|---|---|
| HO• | 2.8 | 0.28 | 2.8 |
| 2.7 | 0.27 | 2.7 | |
| H• | 0.57 | 0.057 | 0.6 |
| H2O2 | 0.71 | 0.071 | 0.7 |
| H2 | 0.47 | 0.047 | 0.5 |
Chemical yield in water; the nominal concentration at the end of a very short pulse of ionizing radiation.
In an oxygenated aqueous solution, both eaq− and H• will react with dioxygen to produce 0.33 μM Gy−1 superoxide (O •−2 and its protonated form HO •2). With the aid of superoxide dismutase (SOD), superoxide will dismute producing O2 and H2O2 thereby removing O2, G-value ≈ −0.2 uM Gy−1.
If there are organic species present, reactions with HO• will principally yield carbon-centered radicals that will rapidly react with oxygen [22] removing additional oxygen. Indeed, radiolysis of a minimum essential medium (MEM) yields a larger G-value for the loss of oxygen, −0.44 μM Gy−1 [23, 24] If cells and medium are both present, the apparent G-value is −0.68 μM Gy−1 [25]. Media, such as MEM, are only about 10 mM in small-molecule organic substances. In the organic dense environment of tissue (≈ 1000 mM in organics), the G-value for O2 uptake would be expected to be much larger, especially if there are many polyunsaturated lipids (PUFA) present. Nerve tissue is second only to adipose tissue in the amount of lipid: ≈ 73% water, 13% lipids, and 12% protein [26]. In brain about 30% of fatty acids are PUFA [27] which are highly prone to oxidation via chain reactions [28].
The ensuing redox reactions of the reactive species following an “instantaneous” FLASH pulse would propagate in the biological tissues and eventually decay in a series of biochemical and biophysical reactions that would be expected to take a kinetically different path than similar reactions following a conventional pulse of radiation. After a 10 Gy pulse, HO• will immediately generate ≈ 2.8 μM of organic radicals (R•), most of which will react rapidly with O (k ≈ 108 M−1 s−1 to nearly 1010 M−1 s−1, depending on the nature of R•) resulting in the immediate removal of up to ≈ 2.8 μM O2 [22]. The ROO• formed in lipids (highly prevalent in brain tissue), and other organic molecules, will lead to abstraction of bis-allylic hydrogens (H•) from other PUFA yielding ROOH + R’•. This new R’• will continue to consume O2 (R’• + O2 → R’OO•) in lipid peroxidation chain reactions until an ROO• encounters a chain-terminating antioxidant, such as vitamin E (ROO• + Vit-E-OH → ROOH + Vit-E-O•) [29, 30]. Since the ratio of PUFA to vitamin E molecules is ≈ 1/3000 to 1/1000 in lipid bilayers [31], these lipid peroxidation chain reactions could consume ≈ 5 O2 for each initiating reaction before encountering a termination reaction; the total consumption of O2 could be as much as ≈ 15 μM.
Aqueous electrons (eaq−) and H• formed during the FLASH pulse will react rapidly with O2 to generate superoxide (O •−2). After a 10 Gy pulse, there will be about 3.3 μM O •−2. Some of this O •−2 can then react with iron-containing proteins (i.e., aconitase, ferritin, Fe-S proteins) to release labile, redox active iron (Fe2+) that will magnify the oxidative damage via Fe2+-O2 complexes [32], and especially by reacting through Fenton reactions with H2O2 and ROOH to form additional HO• and RO•. Superoxide will also dismute via superoxide dismutase (SOD) (k = 2 × 109 M−1 s−1) to produce ≈ 1.6 μM H2O2. Thus, the immediate loss of O2 will be at least ≈ 1.6 μM. However, when the H2O2 and ROOH encounter a redox active metal ion such as Fe2+ another HO• and RO• will be formed initiating more peroxidation chain reactions; as much as another 8 – 10 μM O2 could be consumed.
If we assume brain tissue has an oxygen tension of 20 mm Hg, and the partial pressure of oxygen in the atmosphere is about 160 mm Hg, then oxygen availability can be calculated to be [O2] = (20/160) × 195 μM = 25 μM O2, where 195 μM is the solubility of O2 in water at 37 °C and ionic strength of about 150 mM [33]. The oxygen tension of 20 mm Hg may be generous as oxygen electrode measurements had many readings of 6 mm Hg and many much less [34].
The 20 mm Hg of O2 does not consider oxygen present in oxyhemoglobin in the blood stream. However, the unloading of this oxygen occurs on the timescale of ≈ 50 ms, and would be released long after the immediate free radical chemistry of the FLASH has occurred [35]. Importantly, and based on the above theoretical construct, the total O2 that could be consumed by FLASH can be estimated to be as much as 15 + 1.6 + 10 ≈ 25 μM, suggesting that essentially all the tissue O2 would be consumed at the instant of the FLASH in both normal and cancerous brain tissue. At lower oxygen tensions, the oxygen would also be depleted.
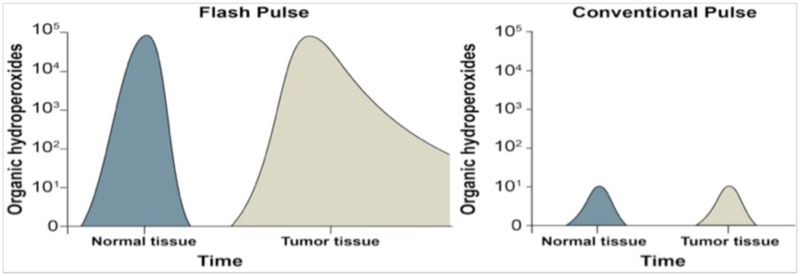
Moreover, based on initial recombination rates, it would be expected that recombination of HO• and eaq− /H• will be negligible; but, reactions of O2•− with Fe-containing proteins would be expected to release labile iron (Fe2+), and even though these reactions may represent only ≈ 1% of the reactions of O2•−, essentially all reactive iron will encounter a O2•−. This has potentially important implications, as reactions of O2•−with iron could double or even triple the size of the intracellular redox active labile iron pools at the time of the flash. This labile Fe2+ would be available to participate in damaging Fenton-type reactions [32, 36, 37]. Tumor cells, relative to normal cells, have 2- to 4-fold higher levels of labile iron accompanied with increased levels of transferrin receptor to bring Fe into the cell [9, 38–41]. Fenton-type chain reactions of Fe2+ with ROOH and H2O2 could greatly magnify free radical chain reactions in tumor tissues exposed to FLASH-RT (relative to normal tissues), leading to significantly higher levels of organic hydroperoxide and oxidative damage in cancer cells compared to normal cells/tissue. Conversely, normal tissues have less labile iron, lower levels of transferrin receptor, and can more easily regulate and sequester labile iron pools. This differential ability of normal tissues to regulate labile iron could allow for the more rapid removal of the FLASH-induced hydroperoxides prior to undergoing Fenton chemistry as well as limiting the peroxidation chain reactions, which could account for the relative normal tissue protection seen with ultra-high dose rate radiation (Figure 2).
Figure 2.
Proposed mechanism for differential dissipation of organic hydroperoxide levels in FLASH vs. conventional radiation damage to normal versus tumor tissue.
The foregoing arguments have several built in assumptions and caveats and we are cognizant that this will undoubtedly raise certain concerns and questions. We have chosen to focus our present thesis on a single dose of 10 Gy to the brain, since this has been shown to elicit neurocognitive sparing with FLASH delivery. The FLASH effect will of course depend on many physiological factors and will exhibit dose dependency. This will also depend in large part, on the starting levels of O2 in specific tissues and regions of the tumor, and will result in different threshold doses for sufficient oxygen depletion. Despite such limitations, we have sought to provide a framework useful for designing additional experiments to test the underlying basis of the FLASH effect, and hope that these radiochemical calculations stimulate additional investigators to generate the necessary data sets to critically test these hypotheses.
Summary
Based on fundamental radiation chemistry and the theoretical construct, vida supra, some interesting hypotheses can be derived to explain the differential biological effects of FLASH irradiation (vs. conventional dose rates) on normal tissue vs. cancer tissue.
FLASH radiation doses that consume all the local tissue O2 to form reactive organic hydroperoxide products will show the maximal effective differences between normal and tumor tissues;
Since normal tissues can more effectively regulate endogenous levels of labile Fe, as well as having a greater capacity to sequester available labile Fe, Fenton type reactions will be limited in normal vs. cancer tissues;
Normal cells also have lower prooxidant burdens during normal steady-state metabolism and a greater reserve capacity for the enzymatic reduction of hydroperoxides, relative to cancer cells, so normal tissues would be expected to be able to remove organic hydroperoxides more effectively, relative to tumor tissues; and
If the dose rate of the FLASH is high enough to convert all of the locally available O2 in tissue to organic hydroperoxides (ROOH) in both cancer and normal tissues, and if these hydroperoxides are removed more readily by antioxidant pathways in normal cells prior to inducing Fenton chemistry or peroxidation chain reactions, then this theoretical construct could be used to provide a biochemical rationale for explaining the differential biological effects of FLASH irradiation in tumor and normal tissues, Figure 2.
The model in Figure 2 proposes that the instantaneous production of organic hydroperoxides starts at equal levels immediately after FLASH or conventional pulse in cancer vs. normal tissue. However, it is the more rapid removal and decay of the organic hydroperoxides and free radicals derived from peroxidation chain reactions in normal tissue compared to tumor tissue that defines the beneficial therapeutic index of the FLASH effect. Importantly, inundating the system that removes the copious organic hydroperoxides generated by FLASH irradiation is the means by which the differences in redox metabolism between cancerous vs. normal tissues can be maximized. The much, much lower total yield of free radicals and organic hydroperoxides (4 orders of magnitude) produced by the low dose rates of conventional radiation does not appear to be sufficient to uncover these tumor vs. normal tissue differences in oxidative metabolism. This coupled with higher levels of redox-active iron, i.e. labile iron, in tumor tissue, compared to normal tissue, underpin the differential redox biology leveraged by FLASH-RT. With this theoretical construct in mind, the field of radiobiology now has a rationale on which to generate testable hypotheses that could advance the ability to manipulate radiation physics, chemistry, and biochemistry for the purpose of developing novel and more effective FLASH-RT treatment paradigms. It is now necessary to test these hypotheses experimentally in order to validate the underlying mechanisms of the remarkable biological effects of FLASH-RT.
FLASH radiation dose-rates consume all the local tissue O2 to form reactive organic hydroperoxides.
Fenton type reactions will be limited in normal vs. cancer tissues due to lower levels of labile Fe.
Normal tissues are expected to remove organic hydroperoxides more effectively relative to tumor tissues.
Since tumor tissue cannot remove hydroperoxides as effectively, FLASH and conventional dose rate irradiation are more isoefficient at killing tumor cells compared to normal cells.
Acknowledgements
The authors would like to that Gareth Smith for helpful assistance with graphics. This work was supported by NIH grants R01CA182804 (DRS), R01CA169046 (GRB), P01CA217797 (DRS, GRB) P30CA086862A1 (DRS), T32CA078586 (MP), R01 NS089575 (CLL) and the Department of Radiation Oncology at the University of Iowa.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The Authors of this manuscript declare no conflict of interest
References
- [1].Favaudon V, Caplier L, Monceau V, Pouzoulet F, Sayarath M, Fouillade C, et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med. 2014;6:245ra93. [DOI] [PubMed] [Google Scholar]
- [2].Vozenin MC, De Fornel P, Petersson K, Favaudon V, Jaccard M, Germond JF, et al. The Advantage of FLASH Radiotherapy Confirmed in Mini-pig and Cat-cancer Patients. Clin Cancer Res. 2019;25:35–42. [DOI] [PubMed] [Google Scholar]
- [3].Dayal D, Martin SM, Limoli CL, Spitz DR. Hydrogen peroxide mediates the radiation-induced mutator phenotype in mammalian cells. Biochem J. 2008;413:185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dayal D, Martin SM, Owens KM, Aykin-Burns N, Zhu Y, Boominathan A, et al. Mitochondrial complex II dysfunction can contribute significantly to genomic instability after exposure to ionizing radiation. Radiat Res. 2009;172:737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Spitz DR, Azzam EI, Li JJ, Gius D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: a unifying concept in stress response biology. Cancer Metastasis Rev. 2004;23:311–22. [DOI] [PubMed] [Google Scholar]
- [6].Zhu Y, Dean AE, Horikoshi N, Heer C, Spitz DR, Gius D. Emerging evidence for targeting mitochondrial metabolic dysfunction in cancer therapy. J Clin Invest. 2018;128:3682–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Alexander MS, Wilkes JG, Schroeder SR, Buettner GR, Wagner BA, Du J, et al. Pharmacologic Ascorbate Reduces Radiation-Induced Normal Tissue Toxicity and Enhances Tumor Radiosensitization in Pancreatic Cancer. Cancer Res. 2018;78:6838–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Aykin-Burns N, Ahmad IM, Zhu Y, Oberley LW, Spitz DR. Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem J. 2009;418:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schoenfeld JD, Sibenaller ZA, Mapuskar KA, Wagner BA, Cramer-Morales KL, Furqan M, et al. O2(−) and H2O2-Mediated Disruption of Fe Metabolism Causes the Differential Susceptibility of NSCLC and GBM Cancer Cells to Pharmacological Ascorbate. Cancer Cell. 2017;32:268. [DOI] [PubMed] [Google Scholar]
- [10].Altman KI, Gerber GB, Okada S. Radiation biochemistry, by Altman Kurt I., Gerber George B. [and] Okada Shigefumi. New York,: Academic Press; 1970. [Google Scholar]
- [11].Arena V Ionizing radiation and life; an introduction to radiation biology and biological radiotracer methods. St. Louis,: Mosby; 1971. [Google Scholar]
- [12].Hall EJ, Giaccia AJ. Radiobiology for the radiologist. Eighth edition. ed. Philadelphia: Wolters Kluwer; 2019. [Google Scholar]
- [13].Epp ER, Weiss H, Ling CC. Irradiation of cells by single and double pulses of high intensity radiation: oxygen sensitization and diffusion kinetics. Curr Top Radiat Res Q. 1976;11:201–50. [PubMed] [Google Scholar]
- [14].Hornsey S, Bewley DK. Hypoxia in mouse intestine induced by electron irradiation at high dose-rates. Int J Radiat Biol Relat Stud Phys Chem Med. 1971;19:479–83. [DOI] [PubMed] [Google Scholar]
- [15].Ling CC, Michaels HB, Gerweck LE, Epp ER, Peterson EC. Oxygen sensitization of mammalian cells under different irradiation conditions. Radiat Res. 1981;86:325–40. [PubMed] [Google Scholar]
- [16].Michaels HB, Epp ER, Ling CC, Peterson EC. Oxygen sensitization of CHO cells at ultrahigh dose rates: prelude to oxygen diffusion studies. Radiat Res. 1978;76:510–21. [PubMed] [Google Scholar]
- [17].Hendry JH, Moore JV, Hodgson BW, Keene JP. The constant low oxygen concentration in all the target cells for mouse tail radionecrosis. Radiat Res. 1982;92:172–81. [PubMed] [Google Scholar]
- [18].Karzmark CJ, Nunan CS, Tanabe E. Medical electron accelerators. New York: McGraw-Hill, Health Professions Division; 1993. [Google Scholar]
- [19].Montay-Gruel P, Petersson K, Jaccard M, Boivin G, Germond JF, Petit B, et al. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100Gy/s. Radiother Oncol. 2017;124:365–9. [DOI] [PubMed] [Google Scholar]
- [20].Hines G, Brownell G. Radiation Dosimetry. New York: 1956. [Google Scholar]
- [21].Bensasson RV, Land EJ, Truscott TG. Flash photolysis and pulse radiolysis: contributions to the chemistry of biology and medicine. 1st ed. Oxford ; New York: Pergamon; 1983. [Google Scholar]
- [22].Neta P, Huie R, AB R. Rate constants for reactions of peroxyl radicals in fluid solutions. J Phys Chem Ref Data. 1990;19:413–513. [Google Scholar]
- [23].Michaels HB. Oxygen depletion in irradiated aqueous solutions containing electron affinic hypoxic cell radiosensitizers. Int J Radiat Oncol Biol Phys. 1986;12:1055–8. [DOI] [PubMed] [Google Scholar]
- [24].Palcic B, Skarsgard LD. Reduced oxygen enhancement ratio at low doses of ionizing radiation. Radiat Res. 1984;100:328–39. [PubMed] [Google Scholar]
- [25].Epp ER, Weiss H, Djordjevic B, Santomasso A. The radiosensitivity of cultured mammalian cells exposed to single high intensity pulses of electrons in various concentrations of oxygen. Radiat Res. 1972;52:324–32. [PubMed] [Google Scholar]
- [26].Mitchell H, Hamilton T, Steggerda F, Bean H. The composition of the adult human body and its bearing on the biochemistry of growth. J Biol Chem. 1945;158:625–37. [Google Scholar]
- [27].Carrie I, Clement M, de Javel D, Frances H, Bourre JM. Specific phospholipid fatty acid composition of brain regions in mice. Effects of n-3 polyunsaturated fatty acid deficiency and phospholipid supplementation. J Lipid Res. 2000;41:465–72. [PubMed] [Google Scholar]
- [28].Wagner BA, Buettner GR, Burns CP. Free radical-mediated lipid peroxidation in cells: oxidizability is a function of cell lipid bis-allylic hydrogen content. Biochemistry. 1994;33:4449–53. [DOI] [PubMed] [Google Scholar]
- [29].Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha- tocopherol, and ascorbate. Arch Biochem Biophys. 1993;300:535–43. [DOI] [PubMed] [Google Scholar]
- [30].Schafer FQ, Qian SY, Buettner GR. Iron and free radical oxidations in cell membranes. Cell Mol Biol (Noisy-le-grand). 2000;46:657–62. [PMC free article] [PubMed] [Google Scholar]
- [31].Kelley EE, Buettner GR, Burns CP. Relative alpha-tocopherol deficiency in cultured cells: free radical-mediated lipid peroxidation, lipid oxidizability, and cellular polyunsaturated fatty acid content. Arch Biochem Biophys. 1995;319:102–9. [DOI] [PubMed] [Google Scholar]
- [32].Qian SY, Buettner GR. Iron and dioxygen chemistry is an important route to initiation of biological free radical oxidations: an electron paramagnetic resonance spin trapping study. Free Radic Biol Med. 1999;26:1447–56. [DOI] [PubMed] [Google Scholar]
- [33].Wagner BA, Venkataraman S, Buettner GR. The rate of oxygen utilization by cells. Free Radic Biol Med. 2011;51:700–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Collingridge DR, Piepmeier JM, Rockwell S, Knisely JP. Polarographic measurements of oxygen tension in human glioma and surrounding peritumoural brain tissue. Radiother Oncol. 1999;53:127–31. [DOI] [PubMed] [Google Scholar]
- [35].Clark A Jr., Federspiel WJ, Clark PA, Cokelet GR. Oxygen delivery from red cells. Biophys J. 1985;47:171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Miller DM, Buettner GR, Aust SD. Transition metals as catalysts of “autoxidation” reactions. Free Radic Biol Med. 1990;8:95–108. [DOI] [PubMed] [Google Scholar]
- [37].Wardman P, Candeias LP. Fenton chemistry: an introduction. Radiat Res. 1996;145:523–31. [PubMed] [Google Scholar]
- [38].Du J, Wagner BA, Buettner GR, Cullen JJ. Role of labile iron in the toxicity of pharmacological ascorbate. Free Radic Biol Med. 2015;84:289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Torti SV, Manz DH, Paul BT, Blanchette-Farra N, Torti FM. Iron and Cancer. Annu Rev Nutr. 2018;38:97–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Torti SV, Torti FM. Ironing out cancer. Cancer Res. 2011;71:1511–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Torti SV, Torti FM. Iron and cancer: more ore to be mined. Nat Rev Cancer. 2013;13:342–55. [DOI] [PMC free article] [PubMed] [Google Scholar]