Abstract
Aims
To test the efficacy of the Tobacco Status Project (TSP) Facebook smoking cessation intervention for young adults relative to referral to an on-line program on biochemically verified 7-day abstinence from smoking.
Design
Two-group parallel randomized controlled trial, comparing TSP (n = 251) to on-line control (n = 249) with follow-up to 12 months.
Setting
On-line, throughout the United States.
Participants
Young adult cigarette smokers (mean age 21 years; 73% white, 55% female, 87% daily smokers).
Interventions and comparator
TSP provided private Facebook groups tailored to stage of change to quit smoking, daily contacts, weekly live counseling sessions, and for those ready to quit, six cognitive behavioral therapy counseling sessions. Some TSP groups were assigned randomly to receive a monetary incentive for engagement. Control provided referral to the National Cancer Institute Smokefree.gov website.
Measurements: primary outcome
Biochemically verified 7-day abstinence over 12 months.
Secondary outcomes
Post-treatment (3-month) abstinence; reported abstinence, quit attempt, reduction in smoking, readiness to quit smoking over 12 months.
Findings
Verified 7-day abstinence was not significantly different for intervention compared with control over 1 year: month 3 (8.3 versus 3.2%), 6 (6.2 versus 6.0%), and 12 (5.9 versus 10.0%); odds ratio (OR) = 1.07; 95% confidence interval (CI) = 0.23, 4.97; retention = 71%. There was an effect at 3 months (OR = 2.52; CI = 1.56, 4.04; P < 0.0001). There were no 12-month treatment effects for reported abstinence (P = 0.746), reduction in smoking by 50% or more (P = 0.533), likelihood of having made a quit attempt (P = 0.387) or stage of change over time (0.968). Participants in TSP engaged more and rated the intervention more favorably than those in the control condition.
Conclusions
Compared with referral to a smoking cessation website, a novel USA-focused Facebook smoking cessation intervention did not improve abstinence from smoking over 1 year, but increased abstinence at the end of treatment and was engaging to participants.
Keywords: Facebook, randomized trial, smoking cessation, social media, tobacco, young adults
INTRODUCTION
Tobacco kills more than 7 million people each year world-wide [1], and young adulthood is the age at which people are most likely to smoke. In the United States, one in four young adults reported past month cigarette use in 2016 [2]. Almost all smokers (98%) report starting before the age of 26 years [3], and more than 2000 US youth and young adults become daily cigarette smokers each day [3,4].
Young adults are just as motivated to quit as other adult age groups, yet are less likely to use evidence-based treatments for smoking cessation (e.g. medication, counseling, quit lines [5–7]. On-line programs offer the potential for expanding the reach of cessation services; however, large drop-offs in engagement have been observed over time [8–13].
Social media have been harnessed to disseminate information widely about a broad range of health concerns, including smoking cessation [14–17]. Facebook, the most popular social media platform in the United States, is used by 88% of US young adults aged 18–29, 79% of whom access it daily [18], making it promising to deliver public health messaging. Reports of smoking cessation support groups on Facebook have shown the platform to be useful for sharing experiences and providing encouragement and information [19], engaging young adults concerning tobacco prevention [20] and show positive short-term outcomes (e.g. 25% reported 7-day abstinence at 2 weeks in a pilot feasibility study of adults motivated to quit smoking (n = 15) [21]; 47% reported 7-day abstinence at 3 months in a trial including web and social-media components for young adults ready to quit smoking (n = 102) [22]. Research is needed to determine whether a Facebook intervention, without additional supports, is efficacious for both those ready and not ready to quit smoking; whether abstinence can be verified biochemically; and whether abstinence rates can be maintained past 3 months.
Using Facebook, our group developed the Tobacco Status Project (TSP), a motivationally tailored smoking cessation intervention. TSP is a 90-day cessation program combining Facebook posts tailored to participants’ readiness to quit smoking with weekly live group counseling sessions. Given the success of monetary incentives in recruiting participants to randomized clinical trials [23] and in yielding short-term abstinence to substance use [24] among previous studies, we additionally randomized participants to receive a monetary incentive tied to engagement in the intervention. An initial feasibility trial with 79 young adults in seven Facebook groups achieved self-reported 7-day abstinence rates of 21% at 6 months (9% of 79 biochemically verified) and 18% at 12 months (9% verified) [25]; 92% of participants remained in a Facebook group for the full 3-month intervention; and 61% (48/79) commented on at least one post, with more commenting among those randomized to receive a monetary incentive (median 16) compared to no incentive (median = 7) [26].
The purpose of this randomized controlled trial was to test the hypothesis that among young adult smokers of cigarettes, the TSP Facebook intervention would result in greater biochemically verified abstinence from smoking relative to awebsite referral control condition over 12 months. Secondary outcomes included a comparison of biochemically verified point prevalence at the end of treatment (3 months), and reported 7-day abstinence, reduction in cigarettes smoked, whether a quit attempt was made and readiness to quit smoking (proportion in preparation, action or maintenance stage of change) over 12 months. We also examined patterns and correlates of engagement in both groups, and evaluated a monetary incentive for engagement in the TSP intervention as a moderator of engagement and smoking abstinence.
METHODS
Study design
A parallel, two-group, randomized controlled trial with follow-up assessments conducted at 3, 6 and 12 months was used. Details about study design, intervention and control condition, and measures are reported elsewhere [27].
Participants
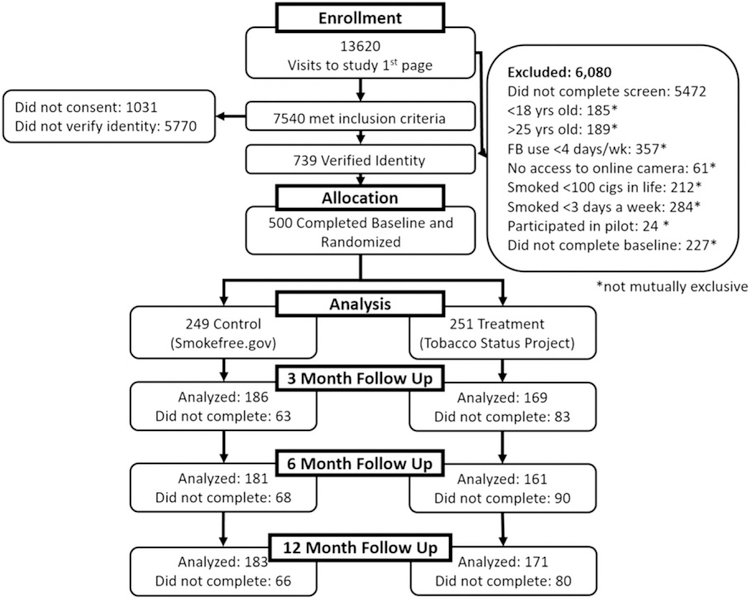
Participants were recruited over 10 months from October 2014 to July 2015, primarily from Facebook, based on an advertisement campaign developed and used previously by the team [28]. Advertisements included a link to the study’s website on Qualtrics [29] with a description of the study and an eligibility survey. Inclusion criteria were English-literate young adults, aged 18–25 years, who reported smoking ≥ 100 cigarettes in their life-times; and at the time of recruitment reported smoking at least one cigarette per day on 3 or more days of the week on average. Intention to quit smoking was not required for trial enrollment; the intervention and control conditions were tailored to stage of change for quitting smoking. Additional inclusion criteria were regular Facebook use (≥ 4 days per week) and access to a digital camera (e.g. on a phone or computer) to send a picture as part of the biochemical validation procedure (see ‘Outcome measures’ below). Individuals who had participated in the TSP feasibility study were excluded [25]. In total, 500 participants completed a baseline assessment and were randomized to a study condition (Fig. 1).
Figure 1.
Participants’ flow through a Facebook smoking cessation clinical trial
Study procedure
The UCSF Institutional Review Board approved the study procedures. Informed consent to participate was obtained on-line through the study website. Three multiple-choice questions confirmed understanding of study risks [30]; identity was verified by e-mail or social media; the on-line baseline assessment link was then e-mailed. Following baseline completion, the participants were randomized to TSP (n = 251) or control (n = 249) 1 : 1 using a blocked random assignment sequence generated by the study biostatistician (K.L.D.). The randomization table was held by D.E.R. and the research assistants obtained the group assignment once the baseline assessment was completed. Randomization was stratified by daily smoking status (yes/no) and stage of change for quitting smoking pre-contemplation, contemplation and preparation [31], variables known to be related to outcomes and addressed by the intervention [32]. Within the TSP condition, participants were placed in a Facebook group tailored to stage of change. All groups were assigned randomly 1 : 1 to a monetary incentive condition (daily, weekly, monthly or no incentive), within stage of change (pre-contemplation, contemplation, preparation), using a scheme generated by the biostatistician and held by the first author. TSP groups began on a rolling basis starting when the first participant had been waiting no longer than 2 weeks; thus, group size varied [26,27]. Twenty-nine Facebook secret groups were created (nine pre-contemplation, 11 contemplation and nine preparation; group size ranged from three to 18). Groups were open for the duration of the trial (12 months), although content was generated by the study team only for the first 3 months.
Immediately after randomization, participants in both conditions were linked by e-mail to the Smokefree.gov website and encouraged to use it actively for the duration of the trial. Assessments were conducted on-line at baseline, 3, 6 and 12 months’ follow-up. In both conditions, participants received their choice of gift cards in the amount of $20 per assessment and a $20 bonus for completing all three assessments, for a total possible incentive of $100.
Interventions
Tobacco Status Project (TSP) intervention
The TSP was implemented entirely through ‘secret’ (i.e. entirely private) Facebook groups. TSP participants were assigned to a Facebook group matched to their baseline stage of change for quitting smoking (pre-contemplation, contemplation, preparation). The group-based intervention had three main features.
First, Facebook posts containing evidence-based smoking cessation strategies were designed to be delivered each day for 90 days via Facebook. Posts were based on the US Clinical Practice Guidelines for smoking cessation [5] and the Transtheoretical Model (TTM) of behavior change [33]. Posts in all groups included a combination of images, videos and text designed to reflect the experience of young adults and elicit a response from participants (see Supporting information, Appendix S1 for a sample of posts in each group).
Secondly, the intervention incorporated weekly ‘The Dr Is In’ live sessions with a PhD-level smoking cessation counselor, during which the counselor provided some limited content for discussion and participants could ask questions and receive support using Facebook commenting features. Content for sessions was based initially on motivational interviewing, and cognitive behavioral coping skills for smoking cessation were discussed as participants were ready to make a quit attempt.
Thirdly, in the preparation groups, six manualized 45-minute cognitive–behavioral treatment (CBT) sessions over 12 weeks were delivered biweekly through Facebook events (a tool for scheduling live communications on Facebook’s newsfeed within private groups). Sessions were adapted for social media delivery from a tobacco treatment manual targeted to adolescents and young adults [34,35] that attended to peer relationships, family influences and the co-use of alcohol and illicit drugs. Group members could attend the events live, and had access to session content throughout the 90-day intervention period.
Additionally, and only in TSP groups, groups were randomized to one of four incentive conditions tied to engagement in the intervention (daily, weekly, monthly or no incentive). Participants in incentive groups could earn giftcards based on comments made to Facebook posts at the end of the assigned period [$1 (or $0) each day in the daily condition, $7 (or $0) each week in the weekly condition, and $30 (or $0) each month in the monthly condition], up to a maximum of $90 at the end of the 90-day intervention. This was in addition to the $100 incentive that all study participants could earn for completing follow-up assessments. Of the 29 groups, seven were assigned randomly to receive no incentive, six daily, eight weekly and eight monthly incentives.
Control group
Participants received a referral to the National Cancer Institute’s Smokefree.gov website. Features include a website tailored to readiness to quit smoking, a texting program, Smartphone application, on-line live chat and a Facebook page. The site includes programs for general adults, women, Spanish-speaking adults and teens. The treatments available to control participants met US Clinical Practice Guidelines for treating nicotine dependence [5].
Measures
Primary outcome measure
The primary outcome was biochemically verified 7-day point prevalence abstinence over 12 months as recommended by the Society for Research on Nicotine and Tobacco Workgroup on abstinence measures [36]. At each follow-up assessment participants reporting ‘no smoking, not even a puff’ in the past 7 days were coded as abstinent from cigarettes and mailed a NicAlert saliva cotinine test strip with previously established diagnostic accuracy [37] and asked to record two pictures: one giving a saliva sample and another of the test result. Participants with a salivary cotinine level < 11 ng/ml [38] were considered confirmed non-smokers. If participants indicated active use of nicotine replacement therapy (NRT) or an electronic nicotine delivery system (ENDS; e.g. an e-cigarette) to aid in smoking cessation, saliva cotinine confirmed abstinence and reported NRT/ENDS use were recorded and reported separately from biochemically verified abstinence. In analyses, those who reported abstinence from all other tobacco than an e-cigarette to quit smoking and returned saliva cotinine results showing a cotinine range between 11–30 ng/ml were treated as abstinent.
Secondary outcomes
Secondary outcomes included: (1) biochemically verified abstinence at treatment end (3 months); (2) reported 7-day abstinence from cigarettes (including all reports of abstinence not verified biochemically); (3) reduction of cigarette consumption by 50% or more (yes/no) between baseline and each follow-up; (4) presence of at least one 24-hour tobacco quit attempt in the assessment time period (yes/no); and (5) proportion of participants in preparation, action or maintenance stages of change at all time-points [33].
Baseline measures
We assessed participants’ age, gender, race/ethnicity, completed education, annual income, housing stability, employment, marital status and smoking history [39]. Additional measures included the Fagerström Test of Cigarette Dependence (FTCD) [40]; a three-item Social Smoking measure used previously with young adults [41]; and the Thoughts about Abstinence scale (desire, success and difficulty, rated on 10-point scales and goal-related to smoking coded as 0 = no goal, 1 = intermediate goal or 2 = quitting for good) [42].
Treatment acceptability/engagement
An eight-item measure, used in our prior work [25], was administered at intervention end (3 months) to assess whether the intervention components in each condition were accessed and general reactions (e.g. ‘The [intervention] was helpful’). The items were reported on a four-point scale from ‘strongly disagree’ to ‘strongly agree’. Proportions of those reporting ‘agree’ or ‘strongly agree’ were computed for each item. In addition, for TSP, participant comments were tallied across all intervention content (study-generated posts, user-generated posts, live sessions and CBT sessions) during the 3-month intervention period (comment volume).
Data analyses
To examine abstinence versus smoking status at the 3–12- month follow-ups by condition (the primary hypothesis), we estimated and tested a logistic regression model using a mixed-effects model (via PROC GLIMMIX in SAS version 9.4; SAS Institute, Cary, NC, USA). The model accounted for dependence of responses within individuals attributable to repeated measures and, clustering effects due to treatment group membership, for dependence of responses within Facebook groups, and allowed us to derive effect estimates from all available data. Analysis was conducted first using all available data included in the modeling and participants assigned to the treatment condition to which they were randomized. We chose this strategy because it is consistent with current statistical practice [43,44], concerns put forth by a Society for Research on Nicotine and Tobacco task force on analysis in clinical trials [45], and published trials from our group [39,46]. The independent variables were TSP versus control condition and time, plus variables that are known to be related to successful quitting (daily smoking status and stage of change at baseline). The outcome variable was verified abstinence, treating reported ENDS or NRT use for cessation as abstinent as long as no other nicotine or tobacco product was used. The study was powered to detect differences of approximately 5% in rates of use [27]. Follow-up logistic regression analyses compared abstinence at treatment end (3 months) between treatment and control groups, with the same covariates as the primary analysis. Reported abstinence, 50% reduction in cigarettes/week and making a 24-hour quit attempt were modeled similarly. To be consistent with some of the literature, the analysis of the primary outcome was repeated after imputing all missing data as positive for smoking (i.e. intent-to-treat).
Kruskal–Wallis tests were used to compare comment volume by stage of change and incentive condition. Bivariate models predicted whether demographic and smoking variables predicted comment volume. Two analyses tested the effects of comment volume (Wilcoxon’s signed rank) and incentive (Person’s χ2) on 3-month abstinence. Outcome variables were biochemically verified abstinence and self-reported abstinence.
RESULTS
Retention
Participant characteristics are in Table 1. Follow-up completion was 71.0% (355 of 500) at 3 months, 68.4% (342 of 500) at 6 months and 70.8% (354 of 500) at 12 months with no difference in number of follow-up assessments completed between treatment and control (χ2 = 3.64, P = 0.302), baseline readiness to quit smoking (χ2 = 6.673, P = 0.352), daily smoking status (χ2 = 1.231, P = 0.746) nor, among those in the TSP condition (n = 251), assignment to a group with a monetary incentive (χ2 = 5.69, P = 0.770). Forty participants (16%) left their Facebook group at some point during the 3-month intervention period, with dropout greatest among those in pre-contemplation (24%) compared to contemplation (10%) or preparation (18%; χ2 = 6.79, P = 0.033).
Table 1.
Participant characteristics (n = 500).
| Variable | Full sample (n = 500) | Treatment (n = 251) | Control (n = 249) |
|---|---|---|---|
| Age (mean/SD) | 20.9 (2.0) | 20.9 (2.0) | 20.9 (2.0) |
| Sex (%/n) | |||
| Male | 44.8 (224) | 44.2 | 45.4 |
| Female | 54.6 (273) | 55.0 | 54.2 |
| Sexual minority | 0.6 (3) | 0.8 | 0.4 |
| Race or ethnicitya (%/n) | |||
| Non-Hispanic Caucasian | 73.8 (366) | 77.0 | 70.6 |
| Native American | 1 (5) | 1.2 | 0.0 |
| African American | 2.6 (13) | 3.6 | 1.6 |
| Asian/Pacific Islander | 1.2 (6) | 1.2 | 1.2 |
| Hispanic | 6.9 (34) | 5.2 | 8.5 |
| More than one | 14.5 (72) | 11.7 | 17.3 |
| Employment status (%/n) | |||
| Employed, part-time | 19.8 (99) | 18.3 | 21.3 |
| Employed, full-time | 43.4 (108) | 46.6 | 40.2 |
| Unemployed, looking | 30.6 (153) | 29.1 | 32.1 |
| Unemployed, not looking | 6.2 (31) | 6.0 | 6.4 |
| Education (%/n) | |||
| High school degree or less | 48.0 (240) | 46.3 | 49.8 |
| Some college | 46.2 (231) | 46.6 | 45.8 |
| College degree or higher | 5.8 (29) | 7.3 | 4.4 |
| Education status (%/n) | |||
| Not in school | 69.6 (348) | 67.3 | 71.9 |
| Part-time | 8.8 (44) | 10.0 | 7.6 |
| Full-time | 21.6 (108) | 22.7 | 20.5 |
| Household income (%/n) | |||
| Less than $20 000 | 28.8 (144) | 29.5 | 28.1 |
| $21 000–60 000 | 49.0 (245) | 51.4 | 46.6 |
| $61 000–100 000 | 15.4 (77) | 16.5 | 14.3 |
| More than $100 000 | |||
| Geographic region (%/n)b | 6.8 (34) | 8.8 | 4.8 |
| South | 32.4 (161) | 36.4 | 28.3 |
| Midwest | 29.2 (149) | 28.4 | 30.4 |
| Northeast | 15.4 (77) | 11.6 | 19.4 |
| West | 22.7 (113) | 23.6 | 21.9 |
| Cigarettes per day (%/n) | |||
| 10 or fewer | 48.0 (240) | 50.2 | 45.8 |
| 11–20 | 46.6 (233) | 43.4 | 49.8 |
| 21–30 | 4.0 (20) | 5.2 | 2.8 |
| 31 or more | 1.4 (7) | 1.2 | 1.6 |
| Cigarettes per day (mean/SD) | 11.6 (6.8) | 10.8 (6.3) | 11.4 (7.2) |
| Days per week smoked (mean/SD) | 6.8 (.86) | 6.7 (0.9) | 6.7 (0.9) |
| Stage of change at baseline (%/n) | |||
| Pre-contemplation | 30.0 (150) | 29.9 | 30.1 |
| Contemplation | 48.6 (243) | 47.4 | 49.8 |
| Preparation | 21.4 (107) | 22.7 | 20.1 |
| Past year 24-hour quit attempt (% yes/SD) | 62.2 (311) | 62.5 | 61.8 |
| FTCD (mean/SD) | 3.2 (2.1) | 3.2 (2.1) | 3.1 (2.1) |
| Smoke within first 30 minutes of waking (% yes/n) | 53.2 (266) | 53.8 | 52.6 |
| Daily smoking (% yes/n) | 86.6% (433) | 87.3 | 85.9 |
| Social smoker (% yes/n) | 71.2 (356) | 73.3 | 69.1 |
| Desire to quit (range: 0–9) | 5.6 (2.9) | 5.5 (2.9) | 5.7 (2.8) |
| Perceived quit success (range: 0–9) | 4.8 (2.6) | 4.8 (2.6) | 4.8 (2.6) |
| Perceived quit difficulty (range: 0–9) | 7.5 (2.4) | 7.5 (2.5) | 7.5 (2.3) |
| Sustained abstinence goal (% yes) | 11.4% (205) | 12.0 | 10.8 |
FTCD = Fagerström Test of Cigarette Dependence; SD = standard deviation.
n = 496
n = 497.
Primary outcome: biochemically verified 7-day abstinence
Smoking status throughout 12 months is reported in Table 2. We obtained saliva cotinine test results on approximately half of participants self-reporting abstinence at 3 months (22 of 36; 61%), 6 months (24 of 50; 48%) and 12 months (34 of 69; 49%), with no difference in receipt of cotinine test results by treatment condition. In analyses using available data, and considering those who reported using only ENDS as abstinent from cigarettes, we modeled the abstinence rates over 12 months and found no significant difference by treatment condition [(month 3 [8.3% vs. 3.2%]), 6 [6.2% vs. 6.0%], and 12 [5.9% vs. 10.0%]; odds ratio (OR) = 1.07; 95% confidence interval (CI) = 0.23, 4.97; P = 0.924; Table 3]. Re-analysis with missing data coded as smoking produced similar results (OR = 1.0; CI = 0.24, 4.46; P = 0.969). Five participants reported sustained abstinence throughout 12 months (two treatment; three control).
Table 2.
Smoking cessation outcomes among young adults in the Tobacco Status Project intervention (treatment) versus referral to Smokefree.gov (control) over 12 months (n = 500).
| 3 months |
6 months |
12 months |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment |
Control |
Treatment |
Control |
Treatment |
Control |
|||||||||||||
| n | % available datab | % missing = smokingb | n | % available data | % missing = smoking | n | % available data | % missing = smoking | n | % available data | % missing = smoking | n | % available data | % missing = smoking | n | % available data | % missing = smoking | |
| Reported abstinence, verified | 12 | 7.1 | 4.8 | 5 | 2.7 | 2.0 | 9 | 5.6 | 3.6 | 9 | 4.9 | 3.6 | 11 | 5.9 | 4.4 | 14 | 8.2 | 5.6 |
| Reported used ENDS | 2 | 1.2 | 0.8 | 1 | 0.5 | 0.4 | 1 | 0.6 | 0.4 | 2 | 1.1 | 0.8 | 0 | 0 | – | 3 | 1.8 | 1.2 |
| Reported abstinence not verified | 9 | 5.3 | 3.6 | 8 | 4.3 | 3.2 | 15 | 9.3 | 6.0 | 18 | 9.9 | 7.2 | 26 | 14.1 | 10.4 | 21 | 12.4 | 8.4 |
| Reported smoking | 145 | 86.3 | 57.8 | 172 | 92.5 | 69.1 | 136 | 84.5 | 54.6 | 152 | 84.0 | 60.6 | 148 | 80.0 | 60.0 | 132 | 77.6 | 53.0 |
| No response | 83 | – | 33.1 | 63 | – | 25.3 | 90 | 35.3 | 68 | – | 27.9 | 66 | 26.3 | 79 | 31.7 | |||
| Totala | 251 | 99.9 | 100.0 | 249 | 100.0 | 100.0 | 251 | 100.0 | 100.0 | 249 | 99.9 | 100.1 | 251 | 100.0 | 100.0 | 249 | 100.0 | 100.0 |
ENDS = electronic nicotine delivery system.
Total percentage of values may not add up to 100 due to rounding
In primary outcome analyses reported abstinence, verified and reported used ENDS values were pooled.
Table 3.
Mixed effects regression models testing the effect of intervention (Tobacco Status Project versus Smokefree.gov) on biochemically verified abstinence, reported abstinence, reduction in smoking, likelihood of making a quit attempt and readiness to quit over 12 months (n = 500).
| Biochemically verified abstinence (% yes) |
Reported abstinence (% yes |
Reduction in smoking by 50% or more (% yes) |
Quit attempt (% yes) |
Ready to quit or quit (% in preparation, action or maintenance) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Treatment (versus control) | 1.07 | 0.23–4.97 | 0.925 | 1.29 | 0.26–6.36 | 0.746 | 1.43 | 0.45–4.54 | .533 | 0.94 | 0.23–3.78 | 0.929 | 0.927 | 0.089–9.68 | 0.947 |
| Daily smoking status (versus non-daily) | 2.31 | 1.21–4.41 | 0.015 | 0.001 | 0.16 | 1.06–2.43 | 0.027 | 0.53 | .348–.804 | 00.005 | 0.50 | 0.349–0.731 | 0.001 | ||
| Baseline stage of change | |||||||||||||||
| Preparation (versus pre-contemplation) | 0.21 | 0.05–0.83 | 0.036 | 0.16 | 0.05–0.45 | 0.011 | 0.29 | 0.15–0.57 | 0.010 | 5.86 | 2.87–11.96 | 0.004 | |||
| Contemplation (versus pre-contemplation) | 0.42 | 0.11–1.64 | 0.136 | 0.28 | 0.10–0.78 | 0.029 | 0.51 | 0.29–0.90 | 0.032 | 3.57 | 2.02–6.32 | 0.006 | |||
CI = confidence interval; OR = odds ratio.
Secondary outcomes
Comparisons between treatment and control at treatment end (3 months), controlling for baseline stage of change and daily smoking status, found a significant difference between treatment and control (OR = 2.52; CI = 1.56, 4.04; P < 0.0001). Findings were similar with missing = smoking (OR = 2.71 (CI = 1.02–7.22; P = 0.039). Readiness to quit and daily smoking at baseline predicted abstinence over the 12 months, with daily smokers and those in preparation more likely to be abstinent over time than those in pre-contemplation (Table 3).
Self-reported 7-day point prevalence abstinence was 13.6% (23 of 169) for treatment and 7.5% (14 of 186) for control participants at the 3-month follow-up, 18.6% (29 of 156) and 14.5% (25 of 172) at 6 months and 21.8% (37 of 170) and 20.8% (38 of 183) at 12 months, respectively, with OR = 1.29; 95% CI = 0.26, 6.36; P = 0.746 for the overall model; Table 3. Abstinence increased over 1 year in both groups, with the largest absolute difference between groups at 3 months (6.1%). There were no significant treatment effects over 12 months for reduction in cigarettes smoked, quit attempts or likeli-hood of being ready to quit or quit (Table 3).
Treatment engagement
Participants in both groups rated the extent to which study treatment materials were engaging and useful (Fig. 2). TSP participants gave significantly higher ratings on all measures compared to the control condition (all P < 0.001). Highest ratings were for ease of understanding the intervention (96%), thinking about what they read (92%) and believing the material gave sound advice (91%).
Figure 2.
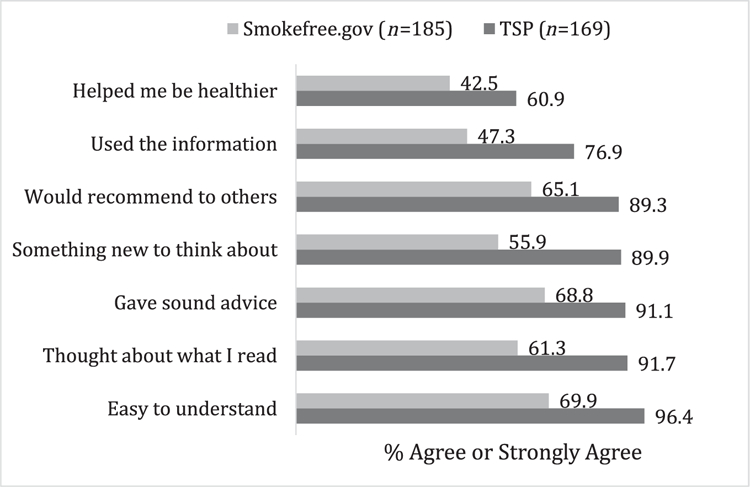
Proportion of ‘agree’ or ‘strongly agree’ reports about content in the Tobacco Status Project (TSP) intervention versus control condition (Smokefree.gov); n = 355. All comparisons were significant with P < 0.001
Among TSP participants, 77% (n = 192) commented at least once to their Facebook group. Median commenting among the full TSP sample was 13 [interquartile range (IQR) = 1–66], and among those who commented at least once was 31 (IQR = 7–84); 101 participants (40.6%) commented at least once during a live counseling session. Ten (15.9% of participants in preparation) participated in one or more CBT treatment sessions during the 90-day intervention period. Stage of change (χ2 = 6.96, P = 0.031) and incentive condition (χ2 = 17.64, P = 0.001) was related to comment volume. Comments were greater among those in pre-contemplation (median = 22; IQR = 3–82) and preparation (median = 23; IQR = 2–70) than contemplation (median = 7; IQR = 0–57). Groups with an incentive had higher comment volume than no-incentive, and monthly and weekly incentives had the highest volumes (none: median = 5; IQR = 0–25; monthly: median = 30; IQR: 2–87; weekly: median = 31; IQR: 2–94; daily: median = 11; IQR = 1–50). Incentives were related significantly to comment volume in contemplation (χ2 = 14.59, P = 0.002) and preparation (χ2 = 9.95, P = 0.019), but not pre-contemplation (χ2 = 6.80, P = 0.079).
No other individual predictors of comment volume (demographics, smoking characteristics) were significant in bivariate models. Comment volume did not relate significantly to either verified abstinence (abstinent: median = 62; non-abstinent: median = 45; Wilcoxon’s Z = 1.22, P = 0.224) or reported abstinence at 3 months (abstinent: median = 60; non-abstinent: median = 45; Wilcoxon’s Z = 1.26, P = 0.208). Incentive condition was not associated with either verified (χ2 = 3.29, P = 0.349) or reported abstinence (χ2 = 1.21, P = 0.750) at 3 months.
Among control participants, 30.8% (n = 57) reported ever use of Smokefree.gov services (29.2% Smokefree.gov, 6.5% SmokefreeWomen, 3.8% SmokefreeTeen; 0% Smokefree en Español). Use of any Smokefree.gov tools did not relate significantly to either verified (χ2 = 1.02, P = 0.312) or reported abstinence (χ2 = 2.49, P = 0.114) at 3 months.
DISCUSSION
The TSP Facebook quit smoking intervention did not reduce significantly the odds of a biochemically verified quit over 12 months when compared to an evidence-based website. However, this study, the first to report use of Facebook as a smoking cessation intervention, resulted in a high level of engagement, a good level of active participation and limited dropout. In secondary analyses, end-of-treatment differences were found between TSP and the control condition on 3-month biochemically verified abstinence. The lack of differences over 1 year may reflect as much the relatively low compliance to biochemical verification procedures as actual lack of differences between conditions; certainly, compliance needs to be increased if biochemically verification is to be used as the gold standard in internet-based studies. The loss of differences in abstinence to 12 months also suggests the potential value of extending the intervention duration. The intervention did not result in differences in reported abstinence, reduction in smoking, likelihood of a quit attempt or readiness to quit smoking over 12 months. The null findings to 1-year follow-up may be due to the relatively short duration of the 3-month intervention and the inclusion of young adult smokers not ready to quit. Interventions aimed at engaging and treating smokers not prepared to quit have typically extended intervention contacts to 12 months with an 18-month follow-up, and have demonstrated increases in abstinence over time [46,47].
TSP was engaging for young adults, with limited dropout, and generally solicited active participation comparable to or greater than other social media intervention programs [22,48,49], especially given that only one-quarter of the TSP group indicated a desire to quit at intervention start. In the control condition, our quit rates were similar to those found in other clinical trials with referral to Smokefree.gov as a condition (e.g. 6.8% [50] and 10% [51] reported abstinence among motivated adults after 3 months).
In both treatment conditions, as expected, abstinence over time was more likely among those in preparation compared to contemplation or pre-contemplation. Within the TSP condition, despite not being ready to quit, those in pre-contemplation commented as frequently as those ready in the next month (preparation). This suggests that the social media environment can be an engaging tobacco treatment tool for those not ready to quit. Those not ready to quit may have participated in the trial solely for incentives, yet the engagement in these groups suggests that a longer intervention could aid this group in moving toward abstinence. In our feasibility trial, content related to decisional balance (increasing the pros and decreasing the cons of change) was most engaging in both pre-contemplation and contemplation groups [52], and its prominence in the pre-contemplation groups was probably also engaging in the clinical trial. Those in contemplation, however, were less engaged, suggesting that the content may not have been as well designed as in the other two groups. Indeed, commenting was also less frequent in contemplation groups than other groups in the feasibility trial [25]; while changes were made, additional changes may be needed to engage those in contemplation in a future delivery of the TSP. A more detailed examination of engagement by content type and group factors (e.g. size) is warranted.
The effectiveness of a monetary incentive at increasing engagement in the TSP group shows that, for widespread dissemination, monetary incentives may be needed to maximize participation, especially for those ready to quit during the next 6 months. Cost-effectiveness analysis is warranted and planned for the future. Although engagement in TSP was unrelated to abstinence in this study, this is probably due in part to low power to detect an effect given a relatively low abstinence rate.
Three intervention strategies were used with varying success. A high proportion of engagement with daily posting and ‘The Dr Is In’ live sessions (77% commented at least once) suggest that content delivered ‘publicly’ within groups was effective at engaging users. While it is impossible to discern from a wholly digital study whether viewing a post was associated with behavior change, and we used a proxy of commenting to measure engagement, it is likely that many more users who did not comment still engaged in some way with the intervention and may have changed their thoughts and/or behaviors as a result. Additional research should evaluate whether engagement varied by content or design features of postings or individual characteristics of participants. CBT sessions implemented in ‘events’ within the Facebook groups were less engaging. CBT sessions that were implemented using Facebook’s private messaging feature in the feasibility trial of TSP [25] were moved to a more public format within the private Facebook groups for this trial to maximize reach within groups. Unfortunately, engagement in the organized events for these sessions still remained low (16% of those in preparation commented at least once), suggesting that live sessions within groups were a better use of counselor time. ‘The Dr Is In’ sessions were more effective, and may be the only strategy needed for live sessions in future intervention delivery.
Our study sample was similar to the US population of smokers, with almost half men and almost three-quarters non-Hispanic white. Our study recruited 45% males, more than other on-line smoking cessation trials, which tend to have a majority of women [53,54]. Social media, or at least Facebook, may be particularly useful for engaging young men in tobacco treatment. Our intervention was designed to appeal to the general audience of young adult smokers and it is unknown whether it would be as engaging to vulnerable smokers [e.g. mental health populations, lesbian, gay, bisexual, transgender, questioning, queer (LGBTQ)+-identified young adults]. Trials evaluating the efficacy of the TSP to tailored interventions for special populations are under way (ClinicalTrials.gov: , ). Although nicotine replacement is recommended by the clinical practice guidelines for smoking cessation [5], the large proportion of participants smoking fewer than 10 cigarettes per day suggested that nicotine replacement would not be indicated for most participants. We did not provide nicotine replacement in this study, and no participant reported using nicotine replacement in a cessation attempt during the trial, despite a majority of participants reporting at least one quit attempt at each time-point. In contrast, ENDS use, common among young adults [55], was reported as a quit strategy. The pros and cons of using ENDS for cessation should be addressed in smoking cessation interventions with young adults. Overall quit rates in the trial were fairly low; provision of nicotine replacement as an adjunct to the on-line treatment may help to improve these rates in future studies, albeit with a threat to external validity given its lack of dissemination in the real world.
Limitations include that some groups in the TSP condition (rather than control) received a monetary incentive for engagement. There is some debate as to the utility of the Transtheoretical Model as a predictor of smoking cessation [56], yet many studies have found it to lead to short- and long-term smoking cessation [57–65].
CONCLUSIONS
The social media intervention had a significant effect on abstinence while the intervention was active. Once removed, the treatment effects were not sustained in follow-up assessments to 1 year. The intervention modality and channel appears effective in reaching and engaging young adult smokers, which has been a challenging group to treat. Future work should examine feasible strategies for sustaining the effects, perhaps with more extended interventions, given the chronicity of tobacco addiction and the major health harms accumulated with continued smoking.
Supplementary Material
Acknowledgements
This study was funded by a grant from the National Institute on Drug Abuse (K23DA032578, Ramo, PI).
Footnotes
Trial registration number
.
Declaration of interests
D.R. has consulted to Carrot Inc., which makes a tobacco cessation device. S.H. has consulted to Carrot Inc. and Biorealm; J.J.P. has consulted to Pfizer, which makes smoking cessation medications; is an advisor to Carrot Inc.; and has been an expert witness for plaintiffs’ counsel in court cases against the tobacco companies. All other authors declare no completing interests.
Supporting Information
Additional Supporting Information may be found online in the supporting information tab for this article.
Appendix Sample Tobacco Status Project posts tailored to readiness to quit smoking according to Transtheoretical Model processes of change and posts for all levels of readiness based on motivational interviewing and electronic cigarette content.
References
- 1.World Health Organization. Tobacco: World Health Organization; 2017. Available at: http://www.who.int/mediacentre/factsheets/fs339/en/ (accessed 5 December 2017) (Archived at http://www.webcitation.org/6vglGPggm).
- 2.Substance Abuse and Mental Health Services Administration. Results from the 2015 National Survey on Drug Use and Health: Detailed Tables Rockville, MD: Department of Health and Human Services, Substance Abuse and Mental Health Services Administration; 2016. [Google Scholar]
- 3.US Department of Health and Human Services. Preventing tobacco use among youth and young adults: A Report of the Surgeon General Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2012. [Google Scholar]
- 4.US Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 5.Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz N, Curry SJ et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline Rockville, MD: US Department of Health and Human Services, Public Health Service; 2008. [Google Scholar]
- 6.Curry SJ, Sporer AK, Pugach O, Campbell RT, Emery S Use of tobacco cessation treatments among young adult smokers: 2005 National Health Interview Survey. Am J Public Health 2007; 97: 1464–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thrul J, Ramo DE Cessation strategies young adult smokers use after participating in a Facebook intervention. Subst Use Misuse 2017; 52: 259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swartz LH, Noell JW, Schroeder SW, Ary DV A randomised control study of a fully automated internet based smoking cessation programme. Tob Control 2006; 15: 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKay HG, Danaher BG, Seeley JR, Lichtenstein E, Gau JM Comparing two web-based smoking cessation programs: randomized controlled trial. J Med Internet Res 2008; 10: e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eysenbach G Issues in evaluating health Web sites in an Internet-based randomized controlled trial. J Med Internet Res 2002; 4: e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feil EG, Noell J, Lichtenstein E, Boles SM, McKay HG Evaluation of an internet-based smoking cessation program: lessons learned from a pilot study. Nicotine Tob Res 2003; 5: 189–94. [DOI] [PubMed] [Google Scholar]
- 12.Cantrell J, Ilakkuvan V, Graham AL, Richardson A, Xiao H, Mermelstein RJ et al. Young adult utilization of a smoking cessation website: an observational study comparing young and older adult patterns of use. JMIR Res Protoc 2016; 5: e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hung WT, Dunlop SM, Perez D, Cotter T Use and perceived helpfulness of smoking cessation methods: results from a population survey of recent quitters. BMC Public Health 2011; 11: 592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christakis NA Health care in a web. BMJ 2008; 336: 1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cobb NK, Graham AL, Abrams DB Social network structure of a large online community for smoking cessation. Am J Public Health 2010; 100: 1282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simoni JM, Nelson KM, Franks JC, Yard SS, Lehavot K Are peer interventions for HIV efficacious? A systematic review. AIDS Behav 2011; 15: 1589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gold J, Pedrana AE, Sacks-Davis R, Hellard ME, Chang S, Howard S et al. A systematic examination of the use of on-line social networking sites for sexual health promotion. BMC Public Health 2011; 11: 583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenwood S, Perrin A, Duggan M Social media update 2016: Pew Research Center: internet, science, and technology 2016. Available at: http://www.pewinternet.org/2016/11/11/social-media-update-2016/ (accessed 29 November 2016) (Archived at http://www.webcitation.org/6vglS0nRR).
- 19.Cheung YT, Chan CH, Wang MP, Li HC, Lam TH Online social support for the prevention of smoking relapse: a content analysis of the WhatsApp and Facebook social groups. Telemed J E Health 2016; 23: 6. [DOI] [PubMed] [Google Scholar]
- 20.Strekalova YA, Damiani RE Message design and audience engagement with tobacco prevention posts on social media. J Cancer Educ 2016; 10.1007/s13187-016-1135-x. [DOI] [PubMed]
- 21.Kim SJ, Marsch LA, Brunette MF, Dallery J Harnessing Facebook for smoking reduction and cessation interventions: Facebook user engagement and social support predict smoking reduction. J Med Internet Res 2017; 19: e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baskerville NB, Azagba S, Norman C, McKeown K, Brown KS Effect of a digital social media campaign on young adult smoking cessation. Nicotine Tob Res 2015; 18: 351–60. [DOI] [PubMed] [Google Scholar]
- 23.Caldwell PH, Hamilton S, Tan A, Craig JC Strategies for increasing recruitment to randomised controlled trials: systematic review. PLOS Med 2010; 7: e1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benishek LA, Dugosh KL, Kirby KC, Matejkowski J, Clements NT, Seymour BL et al. Prize-based contingency management for the treatment of substance abusers: a meta-analysis. Addiction 2014; 109: 1426–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramo DE, Thrul J, Chavez K, Delucchi KL, Prochaska JJ Feasibility and quit rates of the tobacco status project: a Facebook smoking cessation intervention for young adults. J Med Internet Res 2015; 17: e291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ping Q, Yang C, Ramo DE Engagement in a Facebook smoking cessation intervention for young adults: effects of motivation and monetary incentive Philadelphia, PA: Society for Research on Nicotine and Tobacco Annual Meeting; 2015. [Google Scholar]
- 27.Ramo DE, Thrul J, Delucchi KL, Ling PM, Hall SM, Prochaska JJ The Tobacco Status Project (TSP): study protocol for a randomized controlled trial of a Facebook smoking cessation intervention for young adults. BMC Public Health 2015; 15: 897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramo DE, Rodriguez TMS, Chavez K, Sommer M, Prochaska JJ Facebook recruitment of young adult smokers for a cessation trial: methods, metrics, and lessons learned. Internet Interv 2014; 1: 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qualtrics. Qualtrics Provo: UT; 2017. [Google Scholar]
- 30.Palmer BW, Cassidy EL, Dunn LB, Spira AP, Sheikh JI Effective use of consent forms and interactive questions in the consent process. IRB Ethics Hum Res 2008; 30: 8–12. [PubMed] [Google Scholar]
- 31.DiClemente CC, Prochaska JO, Fairhurst S, Velicer WF, Velasquez MM, Rossi JS The process of smoking cessation: an analysis of precontemplation, contemplation, and preparation stages of change. J Consult Clin Psychol 1991; 59: 295–304. [DOI] [PubMed] [Google Scholar]
- 32.Sargent JD, Mott LA, Stevens M. Predictors of smoking cessation in adolescents. Arch Pediatr Adolesc Med 1998; 152: 388–93. [DOI] [PubMed] [Google Scholar]
- 33.Prochaska JO, DiClemente CC Stages and processes of self-change for smoking: toward an integrative model of change. J Consult Clin Psychol 1983; 51: 390–5. [DOI] [PubMed] [Google Scholar]
- 34.Brown RA, Ramsey SE, Strong DR, Myers MG, Kahler CW, Lejuez CW et al. Effects of motivational interviewing on smoking cessation in adolescents with psychiatric disorders. Tob Control 2003; 12: IV3–IV10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prochaska JJ, Fromont SC, Ramo DE, Young-Wolff KC, Delucchi K, Brown RA et al. Gender differences in a randomized controlled trial treating tobacco use among adolescents and young adults with mental health concerns. Nicotine Tob Res 2015; 17: 479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res 2003; 5: 13–25. [PubMed] [Google Scholar]
- 37.Cooke F, Bullen C, Whittaker R, McRobbie H, Chen MH, Walker N Diagnostic accuracy of NicAlert cotinine test strips in saliva for verifying smoking status. Nicotine Tob Res 2008; 10: 607–12. [DOI] [PubMed] [Google Scholar]
- 38.Jarvis MJ, Tunstall-Pedoe H, Feyerabend C, Vesey C, Saloojee Y Comparison of tests used to distinguish smokers from nonsmokers. Am J Public Health 1987; 77: 1435–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall SM, Tsoh JY, Prochaska JJ, Eisendrath S, Rossi JS, Redding CA et al. Treatment for cigarette smoking among depressed mental health outpatients: a randomized clinical trial. Am J Public Health 2006; 96: 1808–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict 1991; 86: 1119–27. [DOI] [PubMed] [Google Scholar]
- 41.Schane RE, Glantz SA, Ling PM Nondaily and social smoking: an increasingly prevalent pattern. Arch Intern Med 2009; 169: 1742–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hall SM, Havassy BE, Wasserman DA Commitment to abstinence and acute stress in relapse to alcohol, opiates, and nicotine. J Consult Clin Psychol 1990; 58: 175–81. [DOI] [PubMed] [Google Scholar]
- 43.Leigh JP, Ward MM, Fries JF Reducing attrition bias with an instrumental variable in a regression model: results from a panel of rheumatoid arthritis patients. Stat Med 1993; 12: 1005–18. [DOI] [PubMed] [Google Scholar]
- 44.Heyting A, Tolboom JT, Essers JG Statistical handling of drop-outs in longitudinal clinical trials. Stat Med 1992; 11: 2043–61. [DOI] [PubMed] [Google Scholar]
- 45.Hall SM, Delucchi KL, Velicer WF, Kahler CW, Ranger-Moore J, Hedeker D et al. Statistical analysis of randomized trials in tobacco treatment: longitudinal designs with dichotomous outcome. Nicotine Tob Res 2001; 3: 193–202. [DOI] [PubMed] [Google Scholar]
- 46.Hall SM, Humfleet GL, Munoz RF, Reus VI, Prochaska JJ, Robbins JA Using extended cognitive behavioral treatment and medication to treat dependent smokers. Am J Public Health 2011; 101: 2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prochaska JJ, Hall SE, Delucchi K, Hall SM Efficacy of initiating tobacco dependence treatment in inpatient psychiatry: a randomized controlled trial. Am J Public Health 2014; 104: 1557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naslund JA, Kim SJ, Aschbrenner KA, McCulloch LJ, Brunette MF, Dallery J et al. Systematic review of social media interventions for smoking cessation. Addict Behav 2017; 73: 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheung YT, Chan CH, Lai CK, Chan WF, Wang MP, Li HC et al. Using WhatsApp and Facebook on-line social groups for smoking relapse prevention for recent quitters: a pilot pragmatic cluster randomized controlled trial. J Med Internet Res 2015; 17: e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stoddard JL, Augustson EM, Moser RP Effect of adding a virtual community (bulletin board) to smokefree. gov: randomized controlled trial. J Med Internet Res 2008; 10: e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bricker J, Wyszynski C, Comstock B, Heffner JL Pilot randomized controlled trial of web-based acceptance and commitment therapy for smoking cessation. Nicotine Tob Res 2013; 15: 1756–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thrul J, Klein AB, Ramo DE Smoking cessation intervention on Facebook: which content generates the best engagement? J Med Internet Res 2015; 17: e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Graham AL, Carpenter KM, Cha S, Cole S, Jacobs MA, Raskob M et al. Systematic review and meta-analysis of Internet interventions for smoking cessation among adults. Subst Abuse Rehabil 2016; 7: 55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Civljak M, Stead LF, Hartmann-Boyce J, Sheikh A, Car J Internet-based interventions for smoking cessation. Cochrane Database Syst Rev 2013; Issue 7 Art. No.: CD007078. 10.1002/14651858.CD007078.pub4. [DOI] [PubMed] [Google Scholar]
- 55.Delnevo CD, Giovenco DP, Steinberg MB, Villanti AC, Pearson JL, Niaura RS et al. Patterns of electronic cigarette use among adults in the United States. Nicotine Tob Res 2016; 18: 715–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.West R Time for a change: putting the transtheoretical (stages of change) model to rest. Addiction 2005; 100: 1036–9. [DOI] [PubMed] [Google Scholar]
- 57.Hollis JF, Polen MR, Whitlock EP, Lichtenstein E, Mullooly JP, Velicer WF et al. Teen reach: outcomes from a randomized, controlled trial of a tobacco reduction program for teens seen in primary medical care. Pediatrics 2005; 115: 981–9. [DOI] [PubMed] [Google Scholar]
- 58.O’Connell ML, Freeman M, Jennings G, Chan W, Greci LS, Manta ID et al. Smoking cessation for high school students. Impact evaluation of a novel program. Behav Modif 2004; 28: 133–46. [DOI] [PubMed] [Google Scholar]
- 59.Aveyard P, Sherratt E, Almond J, Lawrence T, Lancashire R, Griffin C et al. The change-in-stage and updated smoking status results from a cluster-randomized trial of smoking prevention and cessation using the transtheoretical model among British adolescents. Prev Med 2001; 33: 313–24. [DOI] [PubMed] [Google Scholar]
- 60.Stanton A, Grimshaw G Tobacco cessation interventions for young people. Cochrane Database Syst Rev 2013; Issue 8 Art. No.: CD003289. 10.1002/14651858.CD003289.pub5. [DOI] [PubMed] [Google Scholar]
- 61.Velicer WF, Prochaska JO, Fava JL, Laforge RG, Rossi JS Interactive versus noninteractive interventions and dose-response relationships for stage-matched smoking cessation programs in a managed care setting. Health Psychol 1999; 18: 21–8. [DOI] [PubMed] [Google Scholar]
- 62.Velicer WF, Friedman RH, Fava JL, Gulliver SB, Keller S, Sun X et al. Evaluating nicotine replacement therapy and stage-based therapies in a population-based effectiveness trial. J Consult Clin Psychol 2006; 74: 1162–72. [DOI] [PubMed] [Google Scholar]
- 63.Prochaska JO, Velicer WF, Fava JL, Ruggiero L, Laforge RG, Rossie JB et al. Counselor and stimulus control enhancements of a stage-matched expert system intervention for smokers in a managed care setting. Prev Med 2001; 32: 23–32. [DOI] [PubMed] [Google Scholar]
- 64.Prochaska JO, Velicer WF, Fava JL, Rossi JS, Tsoh JY Evaluating a population-based recruitment approach and a stage-based expert system intervention for smoking cessation. Addict Behav 2001; 26: 583–602. [DOI] [PubMed] [Google Scholar]
- 65.Prochaska JO, DiClemente CC, Velicer WF, Rossi JS Standardized, individualized, interactive, and personalized self-help programs for smoking cessation. Health Psychol 1993; 12: 399–405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.