Abstract
Elucidating the immune mechanism by which seasonal influenza vaccines induce a protective immune response is of great importance to gain insights into the design of next-generation vaccines conferring more effective and long-lasting immune protection. Recent studies have established that T follicular helper (Tfh) cells play a major role for the generation of antibody response following influenza vaccination. Yet, the evidence is gained largely through the analysis of blood samples, and our knowledge on the role of Tfh cells in influenza vaccination is still largely limited to the generation of antigen-specific plasmablasts. Recently, influenza vaccination was shown to induce the expansion of two types of memory B cells in addition to plasmablasts. It is plausible that activated Tfh cells that remain in the lymph nodes after vaccination, a cell population missed in the analysis of blood samples, might also contribute to the diversification of memory B cell repertoire. However, current evidence shows no increase of somatic hypermutation of the expanded memory B cell clones, suggesting that this mechanism is not efficiently active in current influenza vaccines.
Introduction
Influenza continues to be a major global health problem with 3–5 million severe cases and up to 500,000 deaths globally every year [1,2]. Vaccination is considered to provide protection by generating or boosting influenza-specific antibodies (Abs). However, effectiveness of influenza vaccines has been poor, for example as low as 10% in 2013–2014 and 7% for 2014–2015 for the H3N2 [3]. Furthermore, the Ab response induced by current seasonal vaccines containing inactivated viral components is generally short-lived and does not provide long-lasting immunity. Therefore, it is of great importance to elucidate the immune mechanism by which current seasonal vaccines induce immune protection and to define the strategies to achieve more effective and long-lasting immune protection by next-generation vaccines. Recent evidence convincingly shows that T follicular helper (Tfh) cells play a fundamental role in Ab response following seasonal influenza vaccinations. Importantly, these studies have started revealing the cause of limitations in the effectiveness of current influenza vaccines. Yet, our knowledge regarding the role of Tfh cells in influenza vaccination is mainly gained from the analysis of blood samples at baseline and post-vaccination and limited to their contribution to Ab-producing plasmablasts. Recent studies revealed that Influenza vaccines expand at least two types of memory B cells in addition to plasmablasts. It is possible that activated Tfh cells that remain in the lymph nodes after vaccination might also contribute to the expansion of these memory B cell subsets. In this review, I will first summarize the recent findings on the analysis of circulating Tfh1 cell (cTfh1) cells activated by influenza vaccines and on their role for the generation of plasmablasts. Then I will describe the recently characterized two memory B cell subsets expanded by influenza vaccination and discuss how Tfh cells might contribute to the diversification of memory B cell repertoire.
Tfh cells and extrafollicular helper cells
Tfh cells are essential for the selection of high-affinity B cell clones undergoing somatic hypermutation (SHM) in GCs (reviewed in [4–6]). Within the light zone of germinal centers (GCs), B cells recognize and retrieve antigen displayed on follicular dendritic cells [7]. GC B cells processed the antigen and present the peptide-MHC class II complex on the cell surface, the density of which correlates with the affinity of the B cell receptor. Tfh cells in GCs contribute to the selection of high-affinity B cells by providing a preferential help to B cells displaying a high density of peptide-MHC class II complex. The selected high-affinity B cell clones eventually differentiate into either long-lived plasma cells that produce high-affinity Abs for many years. The contribution of Tfh cells to the differentiation of the selected GC B cells into plasma cells at post-GC period remains unclear. Extrafollicular helper cells, another Tfh-lineage CD4+ T cell subset, induce the differentiation of extrafollicular plasma cells outside B cell follicles [4–6]. Extrafollicular helper cells share the phenotype, gene profiles, and the functions with GC Tfh cells, and the extrafollicular mechanism mainly contributes to the early generation of specific antibodies after primary antigen challenge.
Tfh cell response after influenza vaccination
Part 1: What is visible: cTfh1 cells for plasmablast generation
Part 1–1: Activation of cTfh1 cells
Tfh cell precursors as well as mature Tfh cells in GCs can exit lymphoid organs into blood circulation. These emigrant Tfh cells are present in human blood as CXCR5+ CD4+ CD45RO+ memory T cells (termed circulating Tfh: cTfh cells) [8,9]. Although a fraction eventually becomes memory Tfh cells, some of which are remarkably long-lived including those induced by smallpox vaccination [10], cTfh cells do not express Bcl-6 unlike Tfh cells in lymphoid organs. Thus, Bcl-6 is not required for the maintenance of memory cTfh cells.
Human cTfh cells are composed of distinct subsets with unique phenotype and functions [8,9]. Among those, a subset expressing the chemokine receptor CXCR3, called cTfh1 cells [9], express the transcription factor T-bet and produce IFN-γ. Multiple lines of recent evidence show that cTfh1 cells play a central role for the generation of Ab responses after influenza vaccinations, likely before they exit from lymphoid organs. Vaccination with trivalent inactivated influenza vaccines, including ones adjuvanted with MF59, induces a transient increase of the activated cTfh1 cells expressing Ki-67, PD-1, CD38, and ICOS in blood [11–17]. The emergence of the activated cTfh1 cells peaks at day 7 post vaccination, and importantly, an increase of this population at day 7 positively correlated with an increase of Ab titers at day 28 [12,16–18]. Antigen-specificity of the activated cTfh1 cells at day 7 has been extensively examined by using multiple methods including the CD154 assay, the activation-induced marker assay, specific MHC class II tetramers, and TCR β chain sequencing [11,12,15]. These studies concluded that activated cTfh1 cells were highly enriched with cells specific for influenza antigens.
The induction of the activated cTfh1 cells post-vaccination was found hampered in an immunocompromised host such as the elderly [18] and HIV+ subjects [19], but partially rescued by increased vaccine dose [17]. Furthermore, the cTfh1 cells activated by vaccination in the elderly displayed impairment in the helper capacity to induce B cells to produce Igs, and there was no correlation between the increase of the activated cTfh1 cells at day 7 and the increase of Ab titers at day 28 [18]. These observations show that intact cTfh1 cell activation and function is required to induce an optimal Ab response.
Part 1–2: The role of cTfh1 cells for Ab production
The activated cTfh1 cells at day 7 post-vaccination express multiple cytokines including IL-2, IL-10, IL-21, and IFN-γ [12]. While Bcl-6 expression remains minimal, the activated cTfh1 cells express T-bet [12,15]. Importantly, the activated cTfh1 cells efficiently induce memory B cells loaded with influenza antigens to produce influenza-specific IgG in in vitro by producing IL-21 and IL-10 [11,12]. However, the activated cTfh1 cells lack the capacity to provide help to naïve B cells likely due to insufficient IL-21 production [12]. Previous studies show that resting cTfh1 cells in healthy subjects are poor to provide help to memory B cells and null to naïve B cells in vitro [9,13,20]. Thus, the capacity of cTfh1 cells to promote Ab response is very limited as compared to other cTfh subsets, and cTfh1 cells can provide help to memory B cells but not naïve B cells only when activated. This likely explains why the current influenza vaccines poorly induce Ab response in infants who have not developed influenza-specific memory B cells.
How and where do the activated cTfh1 cells provide help to memory B cells? Due to frequent vaccinations and occasional natural infections, healthy adults display a broad repertoire of memory B cells that cross-recognize HA of various influenza strains at different affinities [21]. The success of influenza vaccination is likely dependent on the expansion of memory B cells with high affinity BCRs to HA and on their differentiation into plasmablasts. Analysis of polyclonal serum Ab avidity with a real-time kinetics assay by surface plasmon resonance showed that the avidity of HA-specific Abs increases within 7 d post-vaccination but no further afterward [11]. Rapid kinetics in the increase of Ab avidity suggests that the major Ab response likely occurs at the T:B border but not within GCs after influenza vaccination. Importantly, the increase of the activated cTfh1 cells strongly correlated with the increase in the avidity of Abs at day 7 [11]. Thus, perhaps similar for the selection of high-affinity clones in GCs [7], high-affinity B cell clones have an advantage over low-affinity clones in retrieving and presenting antigens to T cells at the T:B border, and the activated cTfh1 cells preferentially interact with high-affinity clones and induce their expansion and differentiation (Fig. 1). The same kinetics in the emergence of activated cTfh1 cells and plasmablasts in blood [12,13] further suggest their interactions at the same sites in lymphoid organs.
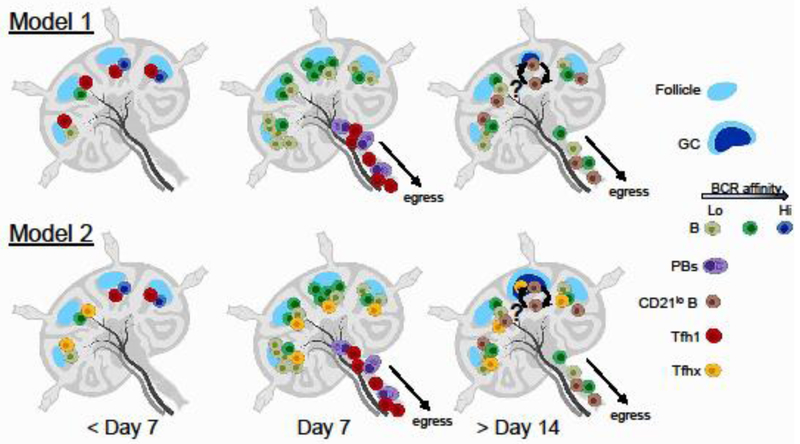
Fig. 1: Roles of Tfh cells in humoral response after influenza vaccination.
Healthy subjects display a broad range of pre-existing memory B cells recognizing influenza antigens with different affinities (indicated by different colors). Influenza-specific Tfh1 cells become activated and expanded within 7 days post-vaccination. Current evidence suggests that cTfh1 cells are mainly involved in the selection of high-affinity B cells and their differentiation towards plasmablasts. High-affinity B cells likely have an advantage over low-affinity cells to retrieve and present antigens to Tfh1 cells, and a prolonged interaction between high-affinity memory B cells and Tfh1 cells might promote B cell differentiation towards plasmablasts. These interactions likely occur at the T:B border, but not within GCs, and both the activated Tfh1 cells and plasmablasts exit the lymph nodes at day 7 post-vaccination. By contrast, where and how influenza vaccination induces the expansion and/or generation of influenza-specific CD27+CD21lo B cells and CD27+CD21hi classical memory cells, and how Tfh cells contribute to their generation remain unclear. Regarding the contribution of Tfh cells, two models can be considered. In model 1, memory B cells with lower affinities may briefly interact with the activated Tfh1 cells before Tfh1 cells exit from the lymph nodes. This short interaction might be sufficient for expansion of CD27+CD21hi classical memory B cells and differentiation towards CD27+CD21lo B cells. In models 2, memory B cells with lower affinities may primarily interact with a Tfh subset that remains in the lymph nodes for an extended period (indicated as Tfhx). Tfhx cells might be a part of the activated Tfh1 cells or different Tfh subsets. The Tfhx cells might be involved in the formation of GCs and contribute to the generation of CD27+CD21lo B cells. In either model, the current evidence suggests that the diversification of memory B cell repertoire through this mechanism by the current influenza vaccine is suboptimal. While this illustration shows the development of GCs at day > 14 days, the magnitude of GC response seems low. PB: plasmablast.
Part 1–3: Generation of memory cTfh1 cells
The frequency of the activated cTfh1 cells rapidly declines after day 7 in blood [12,16]. Nonetheless, when assessed by the CD154 assay, influenza-specific cTfh cells were still detectable even at day 60 post-vaccination, suggesting that a fraction of the activated cTfh1 cells remained as resting memory cells [11]. Consistently, a recent study further demonstrated by performing TCR β chain sequencing that the same clones became a part of the activated cTfh1 cells at day 7 post-vaccination after every vaccination in healthy adults who received successive annual vaccinations. Furthermore, these clones were found to be present mainly within the resting ICOS−CD38− cTfh cell population at 1-year post-vaccination [15]. Thus, influenza-specific memory Tfh cells remain in the blood for at least 1 year at a resting state and can be recalled after successive vaccinations. Whether the resting influenza-specific memory Tfh cells are induced originally by influenza infection or by the vaccinations remains unknown. Given the central role of cTfh1 cells for the generation of Ab response after influenza vaccination, it will be of great significance to define the immune mechanisms associated with the generation and the maintenance of influenza-specific memory cTfh1 cells.
Part 2: What is invisible: Tfh cells in the generation of humoral memory
Part 2–1: Two influenza-specific memory B cells emerging after vaccination
Recent studies demonstrated that influenza vaccination induces not only influenza-specific plasmablasts but also expands at least two types of influenza-specific memory B cells [16,22,23]. Whereas CD20loCD38hiCD27+plasmablasts emerge in blood at day 7 and disappear from blood afterward [24], two HA-specific CD20+CD38lo memory B cell subsets, CD27+CD21hi classical memory cells and CD27+CD21lo cells, emerge at day 7, peak at day 14–28, and remain high in blood circulation for at least 60–90 days [16,22,23]. Unlike plasmablasts, both HA-specific memory B cell subsets expanded after vaccination do not produce Abs unless re-activated in vitro for example by TLR9 ligand CpG, soluble CD40, and IL-21 [16,22,23].
HA-specific CD27+CD21lo cells B cells induced by influenza vaccination were found to display distinct features from HA-specific CD27+CD21hi classical memory cells [22]. CD27+CD21lo B cells diminish the expression of receptors including CXCR4, CXCR5, CCR7, and L-selectin, which are associated with trafficking into GCs and lymphoid organs. HA-specific CD27+CD21lo B cells express more T-bet than HA-specific CD27+CD21hi classical memory B cells. Given that T-bet functionally antagonizes Bcl-6 [25], a transcription factor required for GC B cell differentiation, high T-bet expression seems to provide another layer of mechanisms to sequester CD27+CD21lo B cells from GCs. By contrast, CD27+CD21lo B cells express higher levels of molecules associated with plasma cell differentiation, including PRDM1 (encoding Blimp-1), XBP1, BCMA, and IL6R. Based on these observations, Lau et al proposed that CD27+CD21lo B cells are precursors of long-lived plasma cells [22].
The frequency of HA-specific CD27+CD21lo B cells and CD27+CD21hi classical memory cells in blood gradually decrease with time and goes back to the baseline post 1-year vaccination [16]. Indeed, HA-specific CD27+CD21lo B cells express higher FAS than CD27+CD21hi classical memory cells and thus are likely more prone to apoptosis [22]. Alternatively, Koutsakos et al proposed that these memory B cells might distribute to other organs such as spleen and lung because HA-specific memory B cells were found in these organs in deceased organ donors [16]. Although HA-specific memory B cells present in organs were mainly CD27+CD21hi classical memory B cells, these cells may contain cells derived from CD27+CD21lo B cells.
Part 2–2: Clonal relationship between plasmablasts and the two memory B cell subsets
To gain insights into the origin and the clonal overlap of the B cell subsets expanded by influenza vaccination, two studies analyzed IGH gene rearrangements of the B cells. To this end, Lau et al sorted CD20loCD38hiCD27+ plasmablasts at day 7 and CD27+CD21lo B cells and CD27+CD21hi classical memory cells at day 14 [22]. Ellebedy et al sorted HA-specific plasmablasts at day 7 and HA-specific CD20+ B cells at day 14, a population likely a mixture of CD27+CD21lo B cells and CD27+CD21hi classical memory cells [23]. Both studies showed some clonal overlap (~20–30%) between plasmablasts and the two memory B cell subsets [22,23], indicating that a B cell clone can generate both plasmablasts and other B cell subsets. However, plasmablasts and memory B cells were found to be composed of largely distinct clones [22,23]. The clones found in day 7 HA-specific plasmablasts and day 14 HA-specific CD20+ B cells were present at baseline within IgD-memory B cell population [23], indicating that both populations were derived from memory B cells. Strikingly, no substantial differences were found in the frequency of IGHV SMH among day 7 plasmablasts and day 14 CD27+CD21hi memory and CD27+CD21lo memory B cell subsets [22]. Furthermore, no increase of SMH was observed within HA-specific CD20+ B cells after influenza vaccination [23]. This does not appear simply due to an extensive SMH of HA-specific memory B cells before vaccination, as even clones with less SMHs did not increase the mutations.
Part 2–3: Roles of Tfh cells in the expansion of memory B cell repertoire?
How do CD27+CD21lo B cells and CD27+CD21hi classical memory cells get expanded after influenza vaccination? Although it is largely considered that memory B cell activation requires T cell help [26], the nature of T cells associated with the expansion of antigen-specific memory B cells is unknown. Interestingly, Koutsakos et al reported that there was a positive correlation between the increase of the activated cTfh1 cells day 7 and the increase of HA-IgG+ CD27+CD21lo B cells and CD27+CD21hi classical memory cells at day 14 [16]. Thus, it is possible that cTfh1 cells also contribute to the expansion of these B cell clones (Model 1 in Fig. 1). The difference in the kinetics between plasmablast and the two memory B cells could be explained by the difference in the requirement of CD4+ T cell help. A recent study in mice demonstrated that a prolonged interaction with Tfh cells inhibit the differentiation of B cells towards GC B cells but rather promote their differentiation into plasmablasts [27]. There is also evidence that memory B cells with high affinity for the antigen show a propensity to differentiate into plasmablasts, whereas low-affinity B cell clones become either memory B cells or GC B cells [26]. Thus, in influenza vaccination, it is possible that difference in the quality and the duration of helper signals provided by cTfh1 cells determines the fate of memory B cell clones. Memory B cells with high-affinity BCR may undergo prolonged interactions with influenza-specific cTfh1 cells, and differentiate into plasmablasts. By contrast, memory B cells with low-affinity BCR may receive only suboptimal amounts of help from cTfh1 cells, expand after cTfh1 cells exit from lymph nodes, and emigrate into blood circulation.
On the other hand, it is possible that a fraction of influenza-specific Tfh cells remain in the lymph nodes for an extended period and contribute to the expansion of memory B cell clones (Model 2 in Fig. 1). This might be a fraction of the activated cTfh1 cells. Alternatively, this might be mediated by a different Tfh subset (for example lymph-resident memory Tfh cells [28–30]) whose activity correlates with the activity of cTfh1 cells after influenza vaccination. An interesting observation is that CD27+CD21lo B cells expanded post-vaccination displayed a unique pattern of SHM distinct from plasmablasts and CD27+CD21hi classical memory B cells [22]. Thus, current influenza vaccination seems capable of increasing clonal diversity at some extent of influenza-specific memory B cell repertoire by inducing CD27+CD21lo B cells. Accordingly, Lau et al proposed that CD27+CD21lo B cells are derived from ongoing GC response. Nonetheless, current evidence shows that the influenza vaccine does not induce massive GC response. A paired analysis of the biopsied draining LN samples between baseline and 14 days post-vaccination showed no difference in healthy subjects and a decrease in HIV+ subjects of the frequency of GC Tfh cells [31], although the sample number was low (4 healthy and 6 HIV+ subjects). Furthermore, as described earlier, the vaccination did not increase of SMH rate in HA-specific memory B cells after vaccination [23], and there was no difference in the frequency of SHM between plasmablasts and CD27+CD21lo B cells [22]. Thus, the precise location and the molecular requirement for the generation of CD27+CD21lo B cells remain unclear. As HA-specific memory B cells in blood seem to contain heterogenous subsets differently expressing CD21 and CD27 [16], it is possible that a unique SHM pattern of CD27+CD21lo B cells at day 14 post-vaccination might be derived from the difference in the progenitors. Collectively, the mechanism to diversify memory B cell pool seems suboptimal in the current influenza vaccines.
Conclusions
Current evidence shows the significance of cTfh1 cells for Ab response in seasonal influenza vaccines. What is well established is that cTfh1 cells interact with influenza-specific memory B cells and promote their differentiation into plasmablasts. However, this mechanism requires the presence of influenza-specific memory T cells and B cells and explains at least partly why the effectiveness of current influenza vaccines is limited. This mechanism does not operate in subjects naïve to influenza viruses for example in infants. Impaired function and activity in memory T and B cells in an immunocompromised host and elderly also causes suboptimal Ab response. Furthermore, current evidence suggests that Tfh cell response induced by influenza vaccination seems far from optimal to increase the diversity of memory B cells. It will be important to define how Tfh cells contribute to the expansion of CD27+CD21lo B cells and CD27+CD21hi classical memory cells, and how we can optimize the Tfh cell response to induce a potent Ab response and to diversify memory B cell repertoires.
Highlights.
cTfh1 cells drive Ab response after influenza vaccination
Influenza vaccination expands two types of memory B cells besides plasmablasts
Tfh cells may contribute to expand the two memory B cell subsets
Yet, Tfh cell response induced by current influenza vaccination seems very suboptimal
Acknowledgements
I would like to thank my previous and current lab members. This work is supported by the National Institutes of Health U19-AI082715.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Erbelding EJ, Post DJ, Stemmy EJ, Roberts PC, Augustine AD, Ferguson S, Paules CI, Graham BS, Fauci AS: A Universal Influenza Vaccine: The Strategic Plan for the National Institute of Allergy and Infectious Diseases. J Infect Dis 2018, 218:347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krammer F, Smith GJD, Fouchier RAM, Peiris M, Kedzierska K, Doherty PC, Palese P, Shaw ML, Treanor J, Webster RG, et al. : Influenza. Nature Reviews Disease Primers 2018, 4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belongia EA, Simpson MD, King JP, Sundaram ME, Kelley NS, Osterholm MT, McLean HQ: Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. The Lancet Infectious Diseases 2016, 16:942–951. [DOI] [PubMed] [Google Scholar]
- 4.Vinuesa CG, Linterman MA, Yu D, MacLennan IC: Follicular Helper T Cells. Annu Rev Immunol 2016, 34:335–368. [DOI] [PubMed] [Google Scholar]
- 5.Ueno H, Banchereau J, Vinuesa CG: Pathophysiology of T follicular helper cells in humans and mice. Nat Immunol 2015, 16:142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crotty S: T follicular helper cell differentiation, function, and roles in disease. Immunity 2014, 41:529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mesin L, Ersching J, Victora GD: Germinal Center B Cell Dynamics. Immunity 2016, 45:471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmitt N, Bentebibel SE, Ueno H: Phenotype and functions of memory Tfh cells in human blood. Trends Immunol 2014, 35:436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S, Sabzghabaei N, et al. : Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 2011, 34:108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rivino L, Messi M, Jarrossay D, Lanzavecchia A, Sallusto F, Geginat J: Chemokine receptor expression identifies Pre-T helper (Th)1, Pre-Th2, and nonpolarized cells among human CD4+ central memory T cells. J Exp Med 2004, 200:725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bentebibel SE, Khurana S, Schmitt N, Kurup P, Mueller C, Obermoser G, Palucka AK, Albrecht RA, Garcia-Sastre A, Golding H, et al. : ICOS(+)PD-1(+)CXCR3(+) T follicular helper cells contribute to the generation of high-avidity antibodies following influenza vaccination. Sci Rep 2016, 6:26494.**By using a real-time kinetics assay by surface plasmon resonance, this study showed that the avidity of HA-specific Abs increases within 7 d post-vaccination but no further afterward. The increase of the activated cTfh1 cells strongly correlated with the increase in the avidity of Abs at day 7.
- 12.Bentebibel SE, Lopez S, Obermoser G, Schmitt N, Mueller C, Harrod C, Flano E, Mejias A, Albrecht RA, Blankenship D, et al. : Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med 2013, 5:176ra132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He J, Tsai LM, Leong YA, Hu X, Ma CS, Chevalier N, Sun X, Vandenberg K, Rockman S, Ding Y, et al. : Circulating Precursor CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T Cells Indicate Tfh Cell Activity and Promote Antibody Responses upon Antigen Reexposure. Immunity 2013, 39:770–781. [DOI] [PubMed] [Google Scholar]
- 14.Spensieri F, Siena E, Borgogni E, Zedda L, Cantisani R, Chiappini N, Schiavetti F, Rosa D, Castellino F, Montomoli E, et al. : Early Rise of Blood T Follicular Helper Cell Subsets and Baseline Immunity as Predictors of Persisting Late Functional Antibody Responses to Vaccination in Humans. PLOS ONE 2016, 11:e0157066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herati RS, Muselman A, Vella L, Bengsch B, Parkhouse K, Del Alcazar D, Kotzin J, Doyle SA, Tebas P, Hensley SE, et al. : Successive annual influenza vaccination induces a recurrent oligoclonotypic memory response in circulating T follicular helper cells. Sci Immunol 2017, 2.**By performing TCR β chain sequencing, this study shows that that the same clones are recalled within the activated cTfh1 cells after every vaccination. This study also shows that these clones were found to be present mainly within the resting ICOS−CD38− cTfh cell population at 1-year post-vaccination.
- 16.Koutsakos M, Wheatley AK, Loh L, Clemens EB, Sant S, Nüssing S, Fox A, Chung AW, Laurie KL, Hurt AC, et al. : Circulating TFH cells, serological memory, and tissue compartmentalization shape human influenza-specific B cell immunity. Science Translational Medicine 2018, 10:eaan8405.**This comprehensive study demonstrated that the frequency of the activated cTfh1 cells at day 7 correlates with the genartion of the increase of HA-IgG+ CD27+CD21lo B cells and CD27+CD21hi classical memory cells at day 14. By using recombinant HA-binding assay, this study also characterized the phenotype of HA-specific B cells in blood post-vaccination as well as in various human organs.
- 17.Pilkinton MA, Nicholas KJ, Warren CM, Smith RM, Yoder SM, Talbot HK, Kalams SA: Greater activation of peripheral T follicular helper cells following high dose influenza vaccine in older adults forecasts seroconversion. Vaccine 2017, 35:329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herati RS, Reuter MA, Dolfi DV, Mansfield KD, Aung H, Badwan OZ, Kurupati RK, Kannan S, Ertl H, Schmader KE, et al. : Circulating CXCR5+PD-1+ Response Predicts Influenza Vaccine Antibody Responses in Young Adults but not Elderly Adults. The Journal of Immunology 2014, 193:3528–3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.George VK, Pallikkuth S, Parmigiani A, Alcaide M, Fischl M, Arheart KL, Pahwa S: HIV infection Worsens Age-Associated Defects in Antibody Responses to Influenza Vaccine. The Journal of Infectious Diseases 2015, 211:1959–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, Su LF, Cubas R, Davis MM, Sette A, et al. : Human Circulating PD-1(+)CXCR3(−)CXCR5(+) Memory Tfh Cells Are Highly Functional and Correlate with Broadly Neutralizing HIV Antibody Responses. Immunity 2013, 39:758–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller MS, Gardner TJ, Krammer F, Aguado LC, Tortorella D, Basler CF, Palese P: Neutralizing antibodies against previously encountered influenza virus strains increase over time: a longitudinal analysis. Sci Transl Med 2013, 5:198ra107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau D, Lan LY-L, Andrews SF, Henry C, Rojas KT, Neu KE, Huang M, Huang Y, DeKosky B, Palm A-KE, et al. : Low CD21 expression defines a population of recent germinal center graduates primed for plasma cell differentiation. Science Immunology 2017, 2:eaai8153.**This study was the fist to characterize extensively the influenza-specific CD27+CD21lo B cells generated post influenza-vaccination.
- 23.Ellebedy AH, Jackson KJL, Kissick HT, Nakaya HI, Davis CW, Roskin KM, McElroy AK, Oshansky CM, Elbein R, Thomas S, et al. : Defining antigen-specific plasmablast and memory B cell subsets in human blood after viral infection or vaccination. Nature Immunology 2016, 17:1226.**This study demonstrated the emergence of an HA-specific activated B cell population after influenza vaccination. By analyzing IGH gene rearrangements, this study showed that there was no increase of SMH within HA-specific CD20+ B cells after influenza vaccination.
- 24.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, et al. : Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 2008, 453:667–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oestreich KJ, Mohn SE, Weinmann AS: Molecular mechanisms that control the expression and activity of Bcl-6 in TH1 cells to regulate flexibility with a TFH-like gene profile. Nat Immunol 2012, 13:405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inoue T, Moran I, Shinnakasu R, Phan TG, Kurosaki T: Generation of memory B cells and their reactivation. Immunological Reviews 2018, 283:138–149. [DOI] [PubMed] [Google Scholar]
- 27.Zhang T-t, Gonzalez DG, Cote CM, Kerfoot SM, Deng S, Cheng Y, Magari M, Haberman AM: Germinal center B cell development has distinctly regulated stages completed by disengagement from T cell help. eLife 2017, 6:e19552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crotty S: Do Memory CD4 T Cells Keep Their Cell-Type Programming: Plasticity versus Fate Commitment? Complexities of Interpretation due to the Heterogeneity of Memory CD4 T Cells, Including T Follicular Helper Cells. Cold Spring Harb Perspect Biol 2018, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai LM, Yu D: Follicular helper T-cell memory: establishing new frontiers during antibody response. Immunol Cell Biol 2014, 92:57–63. [DOI] [PubMed] [Google Scholar]
- 30.Hale JS, Ahmed R: Memory T follicular helper CD4 T cells. Front Immunol 2015, 6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moysi E, Pallikkuth S, Armas LRD, Gonzalez LE, Ambrozak D, George V, Huddleston D, Pahwa R, Koup RA, Petrovas C, et al. : Altered immune cell follicular dynamics in HIV infection following influenza vaccination. Journal of Clinical Investigation 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]