Abstract
Objectives/Hypothesis:
The larynx is a highly responsive organ exposed to mechanical, thermal, and chemical stimuli. Chemicals elicit responses both in intraepithelial nerve fibers and in specialized chemosensory cells, including scattered solitary cells as well as taste cells organized into taste buds. Activation of both chemosensory cells and taste buds in the larynx elicit cough, swallow, or apnea with exposure to sour or bitter substances, and even by water or sweet-tasting chemicals. In an effort to begin understanding their function, we sought to compare the distribution, density, and types of chemosensory cells and chemoresponsive nerve fibers in laryngeal epithelium of humans and mice.
Study Design:
Animal and human laboratory analysis.
Methods:
Using immunohistochemistry, we identified taste cells and polymodal nociceptive nerve fibers in the arytenoid area of the laryngeal epithelium of the following: 1) infants undergoing supraglottoplasty for laryngomalacia, and 2) a cadaveric specimen procured from a 34-year-old donor. We then compared these findings to both preweanling and mature mouse tissue.
Results:
Arytenoid tissue from both human and mouse contained many taste buds containing type II taste cells—bitter, sweet, or umami sensing—which were innervated by nerve fibers expressing P2X3 type adenosine triphosphate receptors. Type III cells (acid responsive) were also present, but they were fewer in human tissue than in equivalent tissue from mice. In both species, the epithelium was densely innervated by free nerve endings.
Conclusions:
Our findings suggest that from a standpoint of chemosensation, human and mouse larynges are biologically similar. This suggests that a murine model can be used effectively in laryngeal chemosensory research.
Keywords: Laryngomalacia, arytenoid, epithelium, chemoreceptors, irritation, taste buds
INTRODUCTION
In humans, the larynx functions as both a valve to protect the airway and as a biomechanical vibrator to produce voice. It is also a highly responsive sensory organ triggering airway protective responses such as cough, swallow, and apnea when stimulated by mechanical, thermal, or chemical substances. In human infants, the larynx lies high and anterior at the level of the C1 to C4 vertebrae, with the epiglottis opposing the soft palate, allowing coordination of breathing and sucking in the positions commonly employed for feeding. Neonatal infants demonstrate both swallow and apnea responses when small amounts of water are injected into the pharynx,1,2 thereby protecting the lower airways from potentially damaging aspiration. Coughing is rare and appears to develop in infants with exposure to upper airway infections.3
The larynges of quadrupedal mammals demonstrate similar protective responses,4–6 but differ in anatomy and configuration. What is anterior in a human larynx is ventral in a quadruped. Compared to humans, rodents have a longer oral cavity and shorter pharynx along with a more rostral laryngeal complex,7 reducing the probability of aspiration.8
In humans, prolonged irritation of the laryngeal mucosa leads to inflammation ranging from subtle edema to severe mucosal changes. Diffuse inflammation in the larynx is commonly attributed to direct effects of extraesophageal reflux9; however, double-blind controlled trials of antireflux therapy have shown no reduction in laryngeal signs and symptoms in treated participants.10 The most commonly prescribed class of antireflux therapy are proton pump inhibitors, which act to reduce the acidity of refluxate rather than to eliminate reflux events. Thus, even if acidity is neutralized, potentially irritating bitter refluxate components, such as bile, pepsin, and trypsin, can still contact the laryngeal mucosa.
Bitter substances activate the chemosensitive cells of laryngeal taste, which are assumed to play a role in airway protection. The elongate cells within taste buds, taste cells, are classified into types based on morphologic, molecular, and functional features. Type II cells express G-protein coupled receptors for umami, sweet, or bitter taste transduction, whereas type III cells are responsible for sour taste transduction.11 The oropharynx and airways also contain scattered chemoresponsive cells (i.e., taste-like cells) termed solitary chemosensory cells (SCCs), that express taste receptors. In rats, SCCs are reported to be densely packed in the vicinity of the epiglottis and arytenoids.12,13 Tizzano et al.14 report that SCCs in mice occur primarily in the epiglottis and portions of the arytenoids, in epithelium innervated by the superior laryngeal nerve.
Recent research findings in the mouse indicate that detection of irritants by SCCs as well as by chemosensitive nerve fibers can evoke local inflammation.15 Although SCCs are morphologically distinct from taste buds, both SCCs and type II taste cells (responsive to sweet, bitter, or umami stimuli) utilize G-protein-coupled taste (T1R or T2R) receptors to generate responses and are identifiable by their expression of common downstream transduction molecules such as the G-protein α subunit, gustducin (GNAT3) or the β2 isoform of phospholipase C (PLCβ2). When activated, SCCs release acetylcholine to excite the peptidergic polymodal nociceptive nerve fibers to release inflammatory peptides.16
We hypothesize that laryngeal inflammation may result similarly from activation of local chemosensory elements within the laryngeal epithelium. As a first step toward developing a mechanistic laryngeal model of neurogenic inflammation, we sought to map the distribution, density, and types of chemosensory cells and nociceptive polymodal nerve fibers in the arytenoid area of the laryngeal epithelium of infants who underwent supraglottoplasty for airway obstruction and/or laryngomalacia. We then compared these findings to a single postmortem adult specimen and both preweanling pups and adult mice to elucidate the translational utility of a murine model of chemoreceptor-induced neurogenic laryngeal inflammation.
MATERIALS AND METHODS
Human Participants
The parents of pediatric patients between the ages of 1 day and 21 years undergoing possible supraglottoplasty at Children’s Hospital Colorado (Aurora, Colorado) were approached for inclusion in this study under a protocol approved by the Colorado Multiple Institution Review Board (COMIRB), and informed consent was obtained from all participants. Study data including gestational age, sex, race, medical history, and medications were collected and managed using Research Electronic Data Capture (https://projectredcap.org/ ) hosted at the University of Colorado.
Thirty-nine patients ranging from 1 month to 20 years of age were consented for the study. Twenty-three participants (59%) were male, and the median age was 4.31 months. Seven participants were born premature (i.e., prior to 37 weeks gestation; 18%), and the median gestational age was 39 weeks (range, 34–41 weeks). Indications for supraglottoplasty included laryngomalacia (n = 15) and obstructive sleep apnea (n = 8). Other diagnoses were stridor and failure to thrive. A common comorbidity was reflux. Four patients had Down syndrome, one had a Chiari malformation, one had congenital hydrocephalus, and one had a dysplastic pulmonary valve. Common medications included proton pump inhibitors (n = 21), histamine 2 (H2) blockers (n = 7), bronchodilators (n = 5), and inhaled corticosteroids (n = 3). Four participants were using supplemental oxygen.
Tissue was collected from the superior most mucosa of the arytenoids using cold microsurgical resection during suspension microlaryngoscopy (Fig. 1). Resected tissue was put on ice and submerged in 4% paraformaldehyde (PFA) (cat no. 158127; Sigma-Aldrich, St. Louis, MO) in 0.1 M phosphate buffer (PB) (pH 7.2) within 20 minutes following the procedure. Fixation time in 4% PFA ranged from 3 hours at room temperature to 20 hours at 4○C. After fixation, tissues were cryoprotected in 20% sucrose in 0.1 M PB and then frozen in tissue blocks of Tissue-Plus O.C.T. Compound (Fisher Scientific, Waltham, MA). Serial sections were cut on a cryostat at 14 to 16 μm, collected onto Superfrost Plus slides (Fisher Scientific)m and stored at −20°C until processing.
Fig. 1.
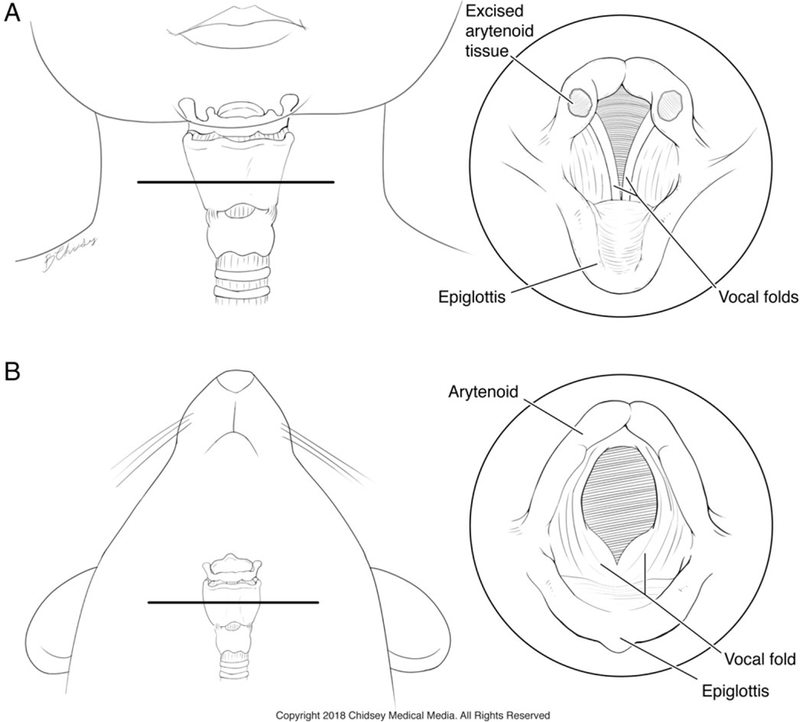
Comparison of laryngeal anatomy between human infants and mice. (A) Frontal view showing the relative position and anatomy of the larynx in an infant. The horizontal line indicates the level of the panel at right depicting the location of tissue procurement in human infants undergoing supraglottoplasty. (B) Ventral view of a mouse showing the position of the larynx. Inset at right shows an equivalent view of the mouse larynx indicating the relative position of the arytenoids and other key laryngeal structures. Printed with permission. © 2018 Chidsey Medical Media. All Rights Reserved.
Although there was heterogeneity in the samples and not all samples were systematically analyzed section by section, the human infant data presented herein are representative of 143 slides analyzed from 19 participants enrolled in this study.
Cadaveric Specimen
Following COMIRB approval, a single whole excised larynx was procured from a 34-year-old male donor within 2 hours postmortem and immediately immersion fixed in 4% PFA in 0.1 M PB (pH 7.2) for 24 hours. The specimen was then washed in 0.1 M phosphate-buffered saline (PBS), and one arytenoid was dissected and cryoprotected in 20% sucrose in 0.1 M PB. Tissues were cut into serial sections for immunohistochemical analysis as above.
Mice
All mice were housed at the University of Colorado Anschutz Medical Campus, and all procedures were approved by the Animal Care and Use Committee at the University of Colorado School of Medicine. Four mice were used for final analysis. Two were aged 2 months and two were 15 days old at the time of sacrifice. Younger mice were preweanlings and were selected due to their younger developmental stage similar to the infants enrolled in the study.
Mice were either anesthetized with sodium pentobarbital, intraperitoneal injection at 50 mg/kg, and transcardially perfused with 4% PFA or euthanized by CO2 inhalation. Larynges were extracted before immersion in 4% PFA for 2 hours. After fixation and post-fix PFA treatment, tissues were transferred to a 20% sucrose solution overnight at 4°C before being cut into 12- to 16-μm sections on a cryostat.
Immunohistochemistry
Slides were washed 3 times in 0.1 M PBS (pH 7.2), and sections were incubated in blocking buffer (2% normal donkey serum, 1% bovine serum albumin, 0.3% Triton in 0.1 M PBS) for 1 hour at room temperature. Sections were then exposed to primary antibodies in blocking buffer (see Tables I and II for dilutions) at 4°C for 12 to 36 hours. Control slides were incubated with blocking buffer without primary antibody. After three washes in PBS, sections were exposed to secondary antibodies diluted 1:400 in blocking buffer (Tables I and II) for 2 hours at room temperature. Slides were washed in PB and coverslipped with 4′,6-diamidino-2-phenylindole (DAPI) Fluoromount-G (SouthernBiotech, Birmingham, AL). All images were collected with an Olympus epifluorescence microscope (Olympus, Tokyo, Japan) equipped with a Q-imaging monochrome camera and/or with an Olympus Fluoview or Leica S5 (Leica, Wetzlar, Germany) confocal laser scanning microscope. Brightness and contrast were adjusted by Adobe Photoshop (Adobe Systems, Inc., San Jose, CA).
TABLE I.
Antibodies for Human Tissue
| Antisera Against | Marks | Company, Catalog No., RRID No. | Host, Dilution | Secondary Antibody | Antisera Against |
|---|---|---|---|---|---|
| Acetylated tubulin | Nerve fibers, ciliated cells | Sigma, T7451, AB_609894 | Mouse, 1:5000 | Alexa 647 | Donkey anti-mouse |
| α-gustducin (GNAT3) | G-protein subunit in type II taste cells and solitary chemosensory cells | Aviva System Biology Corp., OAEB00418, AB_10882823 | Goat, 1:500 | Alexa 488; Alexa 647 | Donkey anti-goat |
| Carbonic anhydrase 4 (CA4) | Type III cells | R&D Systems, AF2414, AB_2070332 | Goat, 1:500 | Alexa 488 | Donkey anti-goat |
| PGP9.5 (UCHL-1) | Neurons and nerve fibers; subpopulation of type III cells | Novus Biologicals, NB110–58872SS AB_877619 | Chicken, 1:2000 | Alexa 488 | Donkey anti-chicken |
| P2X3 | ATP receptor on taste nerves and pain fibers | Alomone Labs, APR-016, AB_2341047 | Rabbit, 1:1000 | Alexa 568 | Donkey anti-rabbit |
| P2X3 | ATP receptor on taste nerves and pain fibers | Neuromics, GP10108, AB_2283325 | Guinea pig, 1:500 | Alexa 488 | Donkey anti-guinea pig |
| PGP9.5 | Neurons and nerve fibers; subpopulation of type III cells | ABD Serotec, 7863–0504, AB_2210505 | Rabbit, 1:500 | Alexa 568 | Donkey anti-rabbit |
| PLCβ2 | Transduction component in type II taste cells | Santa Cruz Biotechnology, SC-206, AB_632197 | Rabbit, 1:500 | Alexa 568 | Donkey anti-rabbit |
| SNAP25 | Neurons and nerve fibers; subpopulation of type III cells | Sigma, S9684, AB_261576 | Rabbit, 1:500 | Alexa 568 | Donkey anti-rabbit |
ATP = adenosine triphosphate; RRID = research resource identifier.
TABLE II.
Antibodies for Mouse Tissue
| Antisera Against | Marks | Company, Catalog No., RRID No. | Host, Dilution | Secondary Antibody | Antisera Against |
|---|---|---|---|---|---|
| Acetylated tubulin* | Nerve fibers, ciliated cells | Sigma, T7451, AB_609894 | Mouse, 1:5000 | Alexa 647 | Donkey anti-mouse |
| α-gustducin (GNAT3) | G-protein subunit in type II taste cells and solitary chemosensory cells | Aviva System Biology Corp., OAEB00418, AB_10882823 | Goat, 1:500 | Alexa 488; Alexa 647 | Donkey anti-goat |
| Carbonic anhydrase 4 (CA4) | Type III cells | R&D System, AF2414, AB_2070332 | Goat, 1:1000 | Alexa 488 | Donkey anti-goat |
| P2X3 | ATP receptor on taste nerves and pain fibers | Neuromics, GP10108, AB_2283325 | Guinea pig, 1:500 | Alexa 488 | Donkey anti-guinea pig |
| PGP9.5 (UCHL-1) | Neurons and nerve fibers, subpopulation of type III cells | Novus Biologicals, NB110–58872SS, AB_877619 | Chicken, 1:2000 | Alexa 488 | Donkey anti-chicken |
| PLCβ2 | Transduction component in type II taste cells | Santa Cruz Biotechnology, SC-206, AB_632197 | Rabbit, 1:500 | Alexa 568 | Donkey anti-rabbit |
| SNAP25 | Neurons and nerve fibers, subpopulation of type III cells | GeneTex, GTX89577, AB_10724125 | Goat, 1:1000 | Alexa 488 | Donkey anti-goat |
| Substance P | Nerve fibers | Accurate Chemicals, YMC1021, AB_2333091 | Rat, 1:1000 | Alexa 488 | Donkey anti-rat |
AffiniPure Fab Fragment Donkey anti-mouse immunoglobulin G (H + L), Jackson ImmunoResearch, 715–007-003, used for blocking of endogenous immunoglobulins on mouse tissue
ATP = adenosine triphosphate; RRID = research resource identifier.
RESULTS
Taste Buds
Taste buds occur within the arytenoid epithelium in both human and mouse, regardless of age, and were similar across species. Immunohistochemical analysis with various antibodies revealed type II and type III taste cells within buds and dense innervation of the arytenoid epithelium. Of the 19 human infants analyzed, 16 showed morphological evidence of taste buds. The single adult specimen also showed taste buds in the arytenoid epithelium. Similarly, all four specimens procured from mice demonstrated evidence of taste buds on the arytenoids.
In both human and mouse arytenoid samples, taste buds were spaced irregularly across the epithelium, with some areas showing a high density, whereas other regions had no taste buds at all. Based on spacing between taste buds, the maximum observed density of taste buds in arytenoid tissue from both mice and human infants was roughly estimated (by applying an Abercrombie correction) to be five taste buds per 100 μm2 (Fig. 2 and Fig. 4C). In mice, clusters of taste buds were most prevalent on the dorsal surface of the arytenoids at the esophageal face of the arytenoid complex (Fig. 4C,F).
Fig. 2.
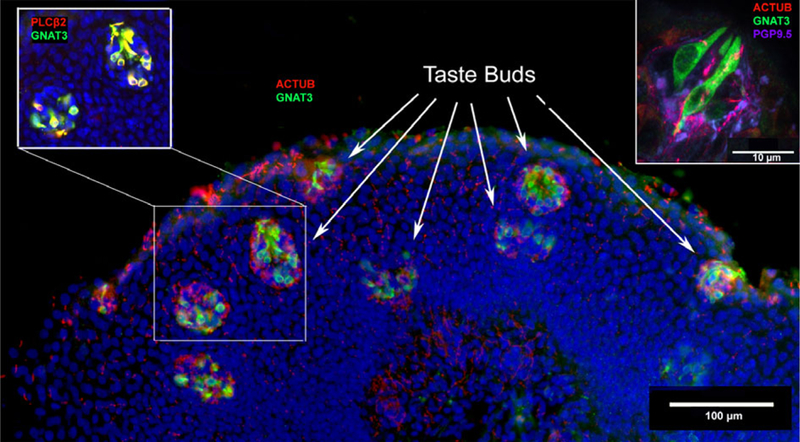
Taste buds in human arytenoid tissue. The main figure is a section from a full-term, 5-month-old infant. Staining for markers of type II taste cells, GNAT3 (green) and PLCβ2 (red, upper left inset), show numerous taste buds within a small area of the epithelium. An antibody directed against acetylated tubulin (red, main figure) demonstrates dense innervation of immunoreactive taste cells. In some regions of the epithelium the taste buds are densely packed, making up a density of approximately five taste buds per 100 μm2. Upper right: confocal image taken from the arytenoid tissue of a 34-year-old male donor. Type II taste cells (GNAT3-positive = green) are innervated by nerve fibers shown by acetylated tubulin (red) staining and occur within epithelium that is densely innervated with PGP9.5-positive (magenta) nerve fibers.
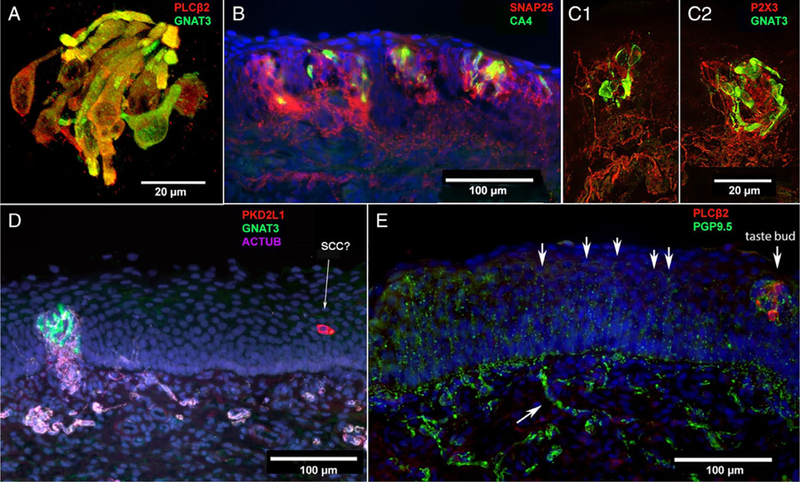
Fig. 4.
Mouse arytenoid taste buds, taste cells, and polymodal nociceptive nerve fibers. (A) Confocal image from a 15-day-old mouse pup depicting a taste bud with near complete colocalization of GNAT3 (green) and PLCβ2 (red), along with innervation by acetylated tubulinpositive (blue) nerve fibers. (B) Confocal image of a taste bud from 15-day-old mouse pup depicting GNAT3-positive (green) type II taste cells innervated by P2X3-positive (red) nerve fibers. (C) Confocal image depicting innervation of a taste bud by acetylated tubulin (red) in a 2-month-old (adult) mouse. Although there are more type II taste cells in the bud (green), the innervation pattern is similar to the 15-day-old mouse shown in (A). (D) Confocal image of three adjacent taste buds situated just proximal to the esophageal face of the arytenoid complex in a 2-month-old (adult) mouse. These taste buds include multiple GNAT3-positive (red) type II taste cells, some SNAP25-positive (green) type III taste cells, and dense innervation by PGP9.5-positive (blue) nerve fibers. PGP9.5-positive nerve fibers also innervate the epithelium surrounding the taste buds reaching near the surface of the tissue. (E) Confocal image from a 15-day-old mouse pup showing CA4-positive (type III) cells adjacent to PLCβ2-positive (type II) cells within a taste bud. (F) Two adjacent taste buds on the esophageal-adjacent surface of the arytenoid in a 2-month-old (adult) mouse. As with the 15-day-old mouse, taste buds shown in (A) and the human infant taste buds in Figure 3A, GNAT3 and PLCβ2 demonstrate near-complete colocalization in type II taste cells. These taste buds are also innervated by small substance P-positive (magenta) neural fibers (arrows).
Type II Taste Cells
Taste buds in humans and in mice exhibited numerous type II (bitter, sweet, umami detecting) cells (Fig. 2, Fig. 4C) evidenced by staining for GNAT3 or PLCβ2. In both species, these substances mostly colocalized (Figs. 3A and 4A). In neither humans nor mice did we observe isolated GNAT3- positive SCCs within the arytenoid epithelium as would be expected for SCCs. All GNAT3-positive and PLCβ2-positive cells were contained within multicellular aggregates that we identified as taste buds. Infant mice were similar to adult mice in terms of colocalization of type II taste cell markers, GNAT3 and PLCβ2 (Fig. 4A,E), and presence of multiple type II taste cells within buds (Fig. 4B,D).
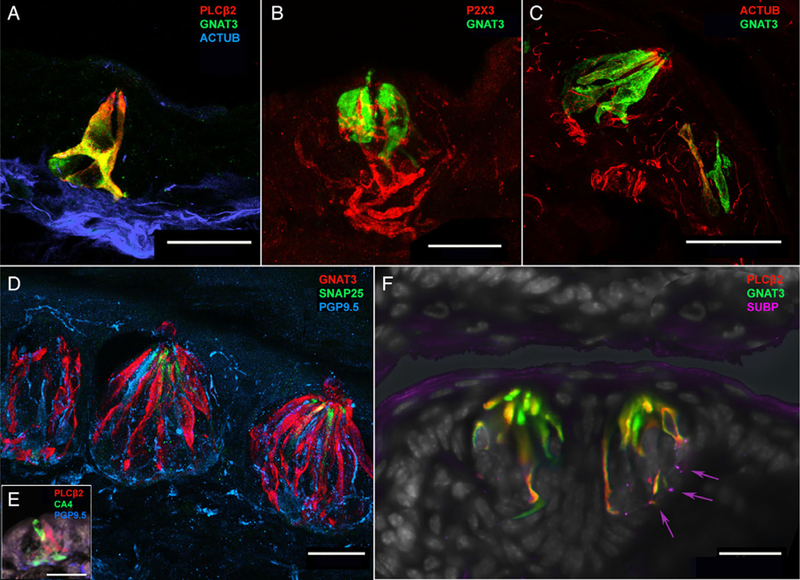
Fig. 3.
Human infant arytenoid taste buds, taste cells, and polymodal nociceptive nerve fibers. (A) Confocal image from a 5-month-old, fullterm infant showing near complete colocalization of GNAT3 (green) and PLCβ2 (red) staining. This suggests that gustducin is the predominant taste-associated G-protein in these tissues. (B) Taste buds from a full-term, 3-month-old infant. Few taste cells express CA4 (green), a marker for sour-responsive type III taste cells. SNAP25 (red) stains nerve fibers showing dense innervation of taste buds. (C1, C2) Two adjacent taste buds from a full-term, 3-month-old infant. Confocal imaging shows dense innervation of GNAT3-positive (green) type II taste cells within taste buds by P2X3-positive (red) nerve fibers. (D) Image from an 8-month-old, premature infant showing a densely innervated (nerve fibers from acetylated tubulin staining = pink) taste bud with GNAT3-positive (green) type II taste cells. Nearby in the epithelium, a PKD2L1-positive (red) solitary cell that is not innervated by acetylated tubulin-positive (magenta) neural fibers. (E) At right is a taste bud with PLCβ2-positive (red) taste cells, closely associated with PGP9.5-positive (green) nerve fibers. At left, numerous free nerve endings, also stained by PGP9.5, extend upward ending near the luminal surface of the epithelium (arrows). The lower arrow indicates a subepithelial axon bundle.
Type III Taste Cells
In human infants we detected rare type III cells (acid responsive) identified as being immunoreactive for PKD2L1 or CA4 (Fig. 3B,D). In contrast, in mice, numerous type III cells occur within each taste bud (Fig. 4C). We used multiple probes to identify type III cells including CA4, PKD2L1, SNAP25, and PGP9.5. In one infant human specimen, CA4 stained type III taste cells within buds (Fig. 3B). In another infant human specimen, we found a single PKD2L1-positive cell in the epithelium, possibly representing an SCC (Fig. 3D). In mice, we found SNAP25 and PGP9.5 to be robust markers for type III cells (Fig. 4C), whereas PKD2L1 and CA4 demonstrated weaker staining within buds (Fig. 4E).
Innervation
Acetylated tubulin and PGP9.5 are ubiquitous markers of nerve fibers in diverse species. Nerve fibers stained for acetylated tubulin were present in both human and mouse laryngeal tissue (Figs. 2, Fig. 3A,D, Fig. 4B–D). Similarly, PGP9.5 antisera labeled nerve fibers including numerous free nerve endings in human (Fig. 3E) and in mouse (Fig. 4C) specimens. The purinergic ionotropic receptor, P2X3, is expressed by gustatory nerve fibers as well as by many polymodal nociceptors.17,18 In both humans and mice, nerve fibers positive for P2X3 occur within the arytenoid epithelium and taste buds (Figs. 3B and Fig. 4C1,C2). The density of P2X3-positive nerve fibers within the taste buds in human samples appeared higher than in mice. In human tissues, SNAP25 antibodies labelled intraepithelial nerve fibers (Fig. 3B), whereas in mice, SNAP25 also stained some type III taste cells.
DISCUSSION
This study compares the expression of taste cell and neuronal markers in human arytenoid tissue to that in mice to identify similarities in chemoresponsive architecture between the two species and in immature and mature specimens for each species. The major similarities included the presence of numerous type II taste cells within taste buds and colocalization of type II taste cell markers PLCβ2 and GNAT3. Differences were characterized by variability in taste bud innervation, where human taste buds had a greater degree of P2X3 and acetylated tubulin fiber innervation relative to mouse. The density of the taste buds was variable across infants, with certain areas and certain specimens demonstrating a high density of taste buds. In mice, specific locations on the arytenoids (e.g., esophageal face of the arytenoid complex), contained a higher density of taste buds than other regions of the complex. The taste-like SCCs, which are prevalent in the respiratory mucosa, were not present on the arytenoid process of either mouse or humans.
Previous investigators have reported the presence of taste buds in the infant larynx. Lalonde and Eglitis19 published a single case study of a newborn infant, noting taste buds in the epiglottic valleculae, epiglottis, aryepiglottic folds, posterior and lateral walls of the pharynx, posterior commissure down to the cricoid cartilage, anterior commissure, and ventricular folds.19 A more recent immunohistochemical investigation of vocal fold innervation examined cadaveric laryngeal tissue procured from three preterm infants and revealed increased nerve fiber density in the posterior glottis.20
Although the presence of laryngeal taste buds has been established both in this study and historically, the function of these taste receptor end organs has not fully been elucidated in humans. Two major hypotheses were initially developed concerning the function of extralingual taste buds. One hypothesis posited that laryngeal taste buds are phylogenetic residue with no functional role, whereas the other suggested they are taste organs that augment laryngeal closure reflexes during deglutition.21,22 Wilson22 reported that he dropped tastants onto his larynx and could taste each, though the sensation was reduced compared to tongue.
In human infants, the larynx lies high in the neck to allow nasal breathing simultaneously with suckling. Coordinated movement of the arytenoids to prevent liquid from entering the airway during the transfer of a bolus of liquid is a major facet of the suck–swallow reflex. The presence of taste buds in arytenoid tissue may represent a protective mechanism, whereby stimulation of taste receptor cells results in closure of the glottis to prevent liquid from entering the airway. Supporting the hypothesis that extraoral taste buds serve a protective function, Perkett and Vaughan23 first documented a chemoreflexive response to water in 10 preterm infants. Dickman and Smith24 demonstrated an excitatory response in the superior laryngeal nerves of hamsters upon application of distilled water to the larynx. Following up on this work, Davies et al.25 identified a laryngeal chemoreflex (LCR) in preterm infants characterized most frequently by swallowing, central apnea, and airway obstruction in response to pharyngeal stimulation with water and saline. The LCR, which is initiated by chemoreceptors, protects the airway from aspiration; however, it has also been implicated in hypoxia associated with sudden infant death syndrome.26 Infants undergoing supraglottoplasty for laryngomalacia are subjected to removal of multiple taste buds. Although these procedures are necessary for airway maintenance, the removal of potentially protective tissue from the junction between the airway and esophagus should not be overlooked.
Inhaled, ingested, and refluxed irritants come into contact with the laryngeal mucosa and have the potential to be detected by chemical receptors. Acids in refluxate would likely activate both taste buds (sour-responsive type III cells, bittersensing type II cells) and polymodal nociceptive free nerve endings expressing acid-sensitive Trp channels or acid-sensing ion channels. Many of the patients enrolled in the current study were taking proton pump inhibitors or H2 blockers, which would stop or reduce acid secretion in the stomach, resulting in refluxate that is less acidic. Until 4 to 6 months of age, an infant’s diet consists primarily of human milk, infant formula, or a combination of both. When casein, a protein in milk, or soybean protein in infant formula is hydrolyzed with pepsin and/or trypsin, hydrolysates are perceived as bitter.27 Another component of refluxate is bile acids, which themselves are bitter. These findings suggest that the refluxate of infants treated with acid reflux medications could still stimulate bitter receptors in the larynx including those in type II taste cells or SCCs.
Interestingly, we did not identify any morphologically distinct SCCs in the arytenoid mucosa of humans or mice using GNAT3 or PLCβ2. This finding is similar to cow, wherein SCCs were detected in the trachea and bronchi but not in the arytenoids.28 Conversely, in the rat, GNAT3-immunoreactive SCCs were present throughout arytenoid tissue, although few were specifically located in the region investigated in the current work (i.e., at the tip of the corniculate process of the arytenoid cartilage).13 Tizzano et al.14 reported SCCs within the laryngeal epithelium, and specifically in the area of the arytenoids referred to as the hypoglossal region, which likely refers to the lingual face of the arytenoids. Finger et al.18 also described gustducin-expressing SCCs in the laryngeal epithelium of transgenic mice. Having sectioned whole specimens, we observed GNAT- and PLCβ2-reactive SCCs in the mouse airway, particularly in the trachea and subglottis; thus, it is possible that the differences observed between these studies relate to sampling location.
Similarities in arytenoid taste bud density and structure exist between human infants and the single human adult specimen and between mouse pups and adult mice. This finding is important because it supports the notion of conducting future laryngeal chemosensory research on mature adult mice. In sheep, the numbers of epiglottal taste buds increase across the lifespan starting during the prenatal period.29 Conversely, density decreases as a function of age, likely due to growth of the epiglottis over time.29 A study of human epiglottal taste buds procured from 237 cadaveric specimens that ranged in age from 10 to 98 years also demonstrated decreased density in older adults (mean age of 80 years) compared to younger adults (mean age of 26.4 years).30 The results of the present study indicate that sensory receptors associated with apnea in animal models are present in arytenoid tissue in humans, and it is possible that, when stimulated by mechanical or chemical (e.g., refluxate) irritants, evoke a neurogenic inflammatory response in local tissue.
CONCLUSION
Taste buds are abundant in the arytenoid mucosa of humans and contain numerous type II and fewer type III taste cells. These structures are densely innervated and likely contribute to airway protection and coordination of vital laryngeal functions. Their role in neurogenic inflammation remains to be determined; however, a mouse model for examining this mechanism is warranted given the similarities between the two species.
Acknowledgments
The authors thank Mei Li for histological preparations, Amanda Ruiz, Emily Jensen, and Christopher Greenlee for assisting with tissue retrieval, Drs. Todd Wine and Melissa Scholes for procuring infant tissue, and Drs. Sue Kinnamon and Vijay Ramakrishnan for valuable insights and critical reflection.
This work was supported by the Seymour Cohen Award of the American Laryngological Association granted to M.E.J. and M.S.C. This work was also supported by the National Institute on Deafness and other Communication Disorders, at the National Institutes of Health (grant numbers T32DC012280, R01DC014728 to T.E.F., and K23DC014747).
Footnotes
Presented at the Combined Otolaryngology Spring Meetings, National Harbor, Maryland, U.S.A., April 18–22, 2018.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Contributor Information
Marie E. Jetté, Department of Otolaryngology, School of Medicine at the University of Colorado, Rocky Mountain Taste and Smell Center, Aurora, Colorado, U.S.A..
Matthew S. Clary, Department of Otolaryngology, School of Medicine at the University of Colorado.
Jeremy D. Prager, Department of Otolaryngology, School of Medicine at the University of Colorado, School of Medicine at the University of Colorado, Children’s Hospital Colorado, Aurora, Colorado, U.S.A..
Thomas E. Finger, Department of Cell and Developmental Biology, Aurora, Colorado, U.S.A., Rocky Mountain Taste and Smell Center, Aurora, Colorado, U.S.A..
BIBLIOGRAPHY
- 1.Jadcherla SR, Gupta A, Wang M, Coley BD, Fernandez S, Shaker R. Definition and implications of novel pharyngo-glottal reflex in human infants using concurrent manometry ultrasonography. Am J Gastroenterol 2009; 104:2572–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jadcherla SR, Gupta A, Coley BD, Fernandez S, Shaker R. Esophago-glottal closure reflex in human infants: a novel reflex elicited with con-current manometry and ultrasonography. Am J Gastroenterol 2007;102: 2286–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thach BT. Maturation of cough and other reflexes that protect the fetal and neonatal airway. Pulm Pharmacol Ther 2007;20:365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucier GE, Storey AT, Sessle BJ. Effects of upper respiratory tract stimuli on neonatal respiration: reflex and single neuron analyses in the kitten. Biol Neonate 1979;35:82–89. [DOI] [PubMed] [Google Scholar]
- 5.Boggs DF, Bartlett D Jr. Chemical specificity of a laryngeal apneic reflex in puppies. J Appl Physiol Respir Environ Exerc Physiol 1982;53:455–462. [DOI] [PubMed] [Google Scholar]
- 6.Storey AT, Johnson P. Laryngeal water receptors initiating apnea in the lamb. Exp Neurol 1975;47:42–55. [DOI] [PubMed] [Google Scholar]
- 7.Russell JA, Ciucci MR, Hammer MJ, Connor NP. Videofluorographic assessment of deglutitive behaviors in a rat model of aging and Parkinson disease. Dysphagia 2013;28:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inagi K, Schultz E, Ford CN. An anatomic study of the rat larynx: establishing the rat model for neuromuscular function. Otolaryngol Head Neck Surg 1998;118:74–81. [DOI] [PubMed] [Google Scholar]
- 9.Gaynor EB. Otolaryngologic manifestations of gastroesophageal reflux. Am J Gastroenterol 1991;86:801–808. [PubMed] [Google Scholar]
- 10.Qadeer MA, Phillips CO, Lopez AR, et al. Proton pump inhibitor therapy for suspected GERD-related chronic laryngitis: a meta-analysis of randomized controlled trials. Am J Gastroenterol 2006;101:2646–2654. [DOI] [PubMed] [Google Scholar]
- 11.Roper SD, Chaudhari N. Taste buds: cells, signals and synapses. Nat Rev Neurosci 2017;18:485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sbarbati A, Merigo F, Benati D, et al. Identification and characterization of a specific sensory epithelium in the rat larynx. J Comp Neurol 2004;475:188–201. [DOI] [PubMed] [Google Scholar]
- 13.Masuda H, Nakamuta N, Yamamoto Y. Morphology of GNAT3-immunoreactive chemosensory cells in the rat larynx. J Anat 2019;234:149–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tizzano M, Cristofoletti M, Sbarbati A, Finger TE. Expression of taste receptors in solitary chemosensory cells of rodent airways. BMC Pulm Med 2011;11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tizzano M, Finger TE. Chemosensors in the nose: guardians of the airways. Physiology 2013;28:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tizzano M, Gulbransen BD, Vandenbeuch A, et al. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci U S A 2010;107:3210–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vandenbeuch A, Larson ED, Anderson CB, et al. Postsynaptic P2X3-containing receptors in gustatory nerve fibres mediate responses to all taste qualities in mice. J Physiol 2015;593:1113–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finger TE, Danilova V, Barrows J, et al. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 2005;310: 1495–1499. [DOI] [PubMed] [Google Scholar]
- 19.Lalonde ER, Eglitis JA. Number and distribution of taste buds on the epiglottis, pharynx, larynx, soft palate and uvula in a human newborn. Anat Rec 1961;140:91–95. [DOI] [PubMed] [Google Scholar]
- 20.Gonçalves da Silva Leite J, Costa Cavalcante ML, Fechine-Jamacaru FV, et al. Morphology of nerve endings in vocal fold of human newborn. Int J Pediatr Otorhinolaryngol 2016;89:55–59. [DOI] [PubMed] [Google Scholar]
- 21.Kiesow F Sur la presence de boutons gustatifs a la surface linguale de l’epiglotte humain, avec quelques reflexions sur les memes organes qui se trouvent dans la muqueuse du larynx. Arch Ital Anat Biol 1902;38: 334–336. [Google Scholar]
- 22.Wilson JG. The structure and function of the taste-buds of the larynx. Brain 1905;28:339–351. [Google Scholar]
- 23.Perkett EA, Vaughan RL. Evidence for a laryngeal chemoreflex in some human preterm infants. Acta Paediatr Scand 1982;71:969–972. [DOI] [PubMed] [Google Scholar]
- 24.Dickman JD, Smith DV. Response properties of fibers in the hamster superior laryngeal nerve. Brain Res 1988;450:25–38. [DOI] [PubMed] [Google Scholar]
- 25.Davies AM, Koenig JS, Thach BT. Upper airway chemoreflex responses to saline and water in preterm infants. J Appl Physiol 1988;64:1412–1420. [DOI] [PubMed] [Google Scholar]
- 26.Lanier B, Richardson MA, Cummings C. Effect of hypoxia on laryngeal reflex apnea—implications for sudden infant death. Otolaryngol Head Neck Surg 1983;91:597–604. [DOI] [PubMed] [Google Scholar]
- 27.Maehashi K, Matsuzaki M, Yamamoto Y, Udaka S. Isolation of peptides from an enzymatic hydrolysate of food proteins and characterization of their taste properties. Biosci Biotechnol Biochem 1999;63:555–559. [DOI] [PubMed] [Google Scholar]
- 28.Tizzano M, Merigo F, Sbarbati A. Evidence of solitary chemosensory cells in a large mammal: the diffuse chemosensory system in Bos taurus airways. J Anat 2006;209:333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradley RM, Cheal ML, Kim YH. Quantitative analysis of developing epiglottal taste buds in sheep. J Anat 1980;130(pt 1):25–32. [PMC free article] [PubMed] [Google Scholar]
- 30.Kano M, Shimizu Y, Okayama K, Kikuchi M. Quantitative study of ageing epiglottal taste buds in humans. Gerodontology 2007;24:169–172. [DOI] [PubMed] [Google Scholar]


