Abstract
Background:
Syncope is a common chief complaint among older adults in the Emergency Department (ED), and orthostatic vital signs are often a part of their evaluation. We assessed whether abnormal orthostatic vital signs in the ED are associated with composite 30-day serious outcomes in older adults presenting with syncope.
Methods:
We performed a secondary analysis of a prospective, observational study at 11 EDs in adults ≥ 60 years who presented with syncope or near syncope. We excluded patients lost to follow up. We used the standard definition of abnormal orthostatic vital signs or subjective symptoms of lightheadedness upon standing to define orthostasis. We determined the rate of composite 30-day serious outcomes, including those during the index ED visit, such as cardiac arrhythmias, myocardial infarction, cardiac intervention, new diagnosis of structural heart disease, stroke, pulmonary embolism, aortic dissection, subarachnoid hemorrhage, cardiopulmonary resuscitation, hemorrhage/anemia requiring transfusion, with major traumatic injury from fall, recurrent syncope, and death) between the groups with normal and abnormal orthostatic vital signs.
Results:
The study cohort included 1974 patients, of whom 51.2% were male and 725 patients (37.7%) had abnormal orthostatic vital signs. Comparing those with abnormal to those with normal orthostatic vital signs, we did not find a difference in composite 30-serious outcomes (111/725 (15.3%) vs 184/1249 (14.7%); unadjusted odds ratio, 1.05 [95%CI, 0.81–1.35], p=0.73). After adjustment for gender, coronary artery disease, congestive heart failure (CHF), history of arrhythmia, dyspnea, hypotension, any abnormal ECG, physician risk assessment, medication classes and disposition, there was no association with composite 30-serious outcomes (adjusted odds ratio, 0.82 [95%CI, 0.62–1.09], p=0.18).
Conclusions:
In a cohort of older adult patients presenting with syncope who were able to have orthostatic vital signs evaluated, abnormal orthostatic vital signs did not independently predict composite 30-day serious outcomes.
Introduction
Syncope is a common chief complaint among patients presenting to the emergency department (ED), accounting for 740,000 ED visits annually.1 Differentiating between the serious and benign causes of syncope can be challenging, particularly in the older adult. Orthostatic hypotension affects up to 50 percent of all older adults.2 Orthostatic hypotension causing syncope can be the manifestation of simple volume depletion in an otherwise healthy patient or herald a more serious etiology, such as acute blood loss or cardiac dysfunction.
The 2017 AHA/ACC/HRS guidelines on the evaluation of syncope in the ED recommend orthostatic vital signs as part of the standard evaluation.3 Prior studies have conflicting data regarding the utility of orthostatic vital signs in the diagnostic work up of syncope in the ED.4,5,6 Older patients are more likely to have baseline abnormal orthostatic vital signs due to medications and autonomic dysfunction, and the finding of orthostasis in the ED may be unrelated to the cause of syncope.7,8,9,10,11 On the other hand, abnormal orthostatic vital signs in older patients with syncope could herald potentially modifiable causes such as gastrointestinal hemorrhage, medication side-effects or dehydration.
The purpose of this study was to evaluate whether abnormal orthostatic vital signs in the setting of syncope were independently associated with 30-day composite serious events in older adults.
Methods
We conducted a secondary analysis of a large, multicenter, prospective cohort study (ClinicalTrials.gov ) to determine whether abnormal orthostatic vital signs are predictive of composite 30-day serious adverse outcomes in older adults presenting to the ED with syncope or near-syncope. The institutional review boards at all sites (Appendix A) approved the study and we obtained written informed consent from all participating subjects. We report data per STROBE guidelines (Appendix B).12
Setting and Patient Population
Eligible patients were ≥60 years of age with a complaint of syncope or near-syncope at 11 academic EDs across the United States. Exclusion criteria were as follows: intoxication, medical or electrical intervention to restore consciousness and inability or unwillingness to provide informed consent or follow-up information. Patients with a presumptive cause of loss of consciousness due to seizure, stroke or transient ischemic attack, or hypoglycemia were also excluded. For this analysis, we also excluded patients that did not have orthostatic vital signs obtained or documented, or were lost to follow up. The full study protocol has been published elsewhere.13
Study Protocol
All patients included in this analysis underwent standardized history, physical examination including orthostatic vital signs, laboratory testing, and 12-lead electrocardiogram (ECG) testing. Patient disposition was directed by the treating clinical providers. We conducted 30-day patient follow-up through a process that included review of the electronic medical records by local research personnel to evaluate for serious outcomes within 30 days from the index ED visit. Additionally, all patients were called at 30 days by a research assistant blinded to clinical course to identify out-of-hospital deaths and subsequent ED visits and hospitalizations that occurred outside of the study sites. If a patient or their authorized representative reported an ED or hospital visit that occurred outside of the study site, their medical charts associated with those visits were reviewed. All potential serious outcomes identified by research staff were reviewed and adjudicated by a study physician blinded to clinical course.
Measurements
Data variables collected were consistent with reporting guidelines for ED based syncope research.12 Data on current medications were organized by class of drug and included beta-blockers, calcium channel blockers, and other antiarrhythmic agents (e.g., amiodarone). We based ECG interpretations on the first ECG obtained in the ED, which were abstracted by one of five research study physicians who were blinded to all clinical data. Clinical staff obtained orthostatic vital signs during the ED evaluation. When not collected, the reason for them not be obtained was recorded as a free text field. Abnormal orthostatic vital signs were defined as a systolic blood pressure drop of 20 mmHg after two minutes of standing OR 10 mmHg upon standing OR symptoms of dizziness or lightheadedness upon standing.3,5
Outcome
Our primary study outcome was a composite endpoint of 30-day serious events. We defined serious outcomes as any of the following: a significant arrhythmia (ventricular fibrillation, symptomatic ventricular tachycardia >30 seconds, sick sinus syndrome, sinus pause >30 seconds, Mobitz II heart block, complete heart block, symptomatic supraventricular tachycardia, or symptomatic bradycardia <40 beats per minute), myocardial infarction, a cardiac intervention, new diagnosis of structural heart disease, stroke, pulmonary embolism, aortic dissection, subarachnoid hemorrhage, cardiopulmonary resuscitation, internal hemorrhage/anemia requiring transfusion, recurrent syncope/fall resulting in major traumatic injury, or death. Although not part of our pre-specified analysis, we also reported short-term serious events, those that occurred during the ED or hospital course (prior to discharge).
Analysis
Continuous variables are presented as means and standard deviations and categorical variables and percent frequency of occurrence. We tested independence between categorical variables with a chi-square test or with Fisher’s exact test, as appropriate. In the study cohort, we compared patients with and without orthostatic findings on demographic and medical characteristics. After assessing the univariate effect of orthostatic findings on composite 30-day serious events, we ran a multivariable logistic regression of composite 30-day serious events on orthostatic findings with pre-specified adjustments for gender, coronary artery disease, congestive heart failure (CHF), history of arrhythmia, dyspnea, hypotension, any abnormal ECG, physician risk assessment, medication classes and disposition. We selected these variables based on prior literature that suggests these are important predictors of serious outcomes in patients with syncope.2 We used similar analytical techniques for the short-term outcomes. We recorded the reasons for not obtaining orthostatic vital signs and compared patients who did and did not receive orthostatic vital signs to assess bias (Appendix C). All statistical analyses were performed in the R package.15 All p-values are two-sided and considered significant at the 5% level.
Results
Characteristics of the Subjects
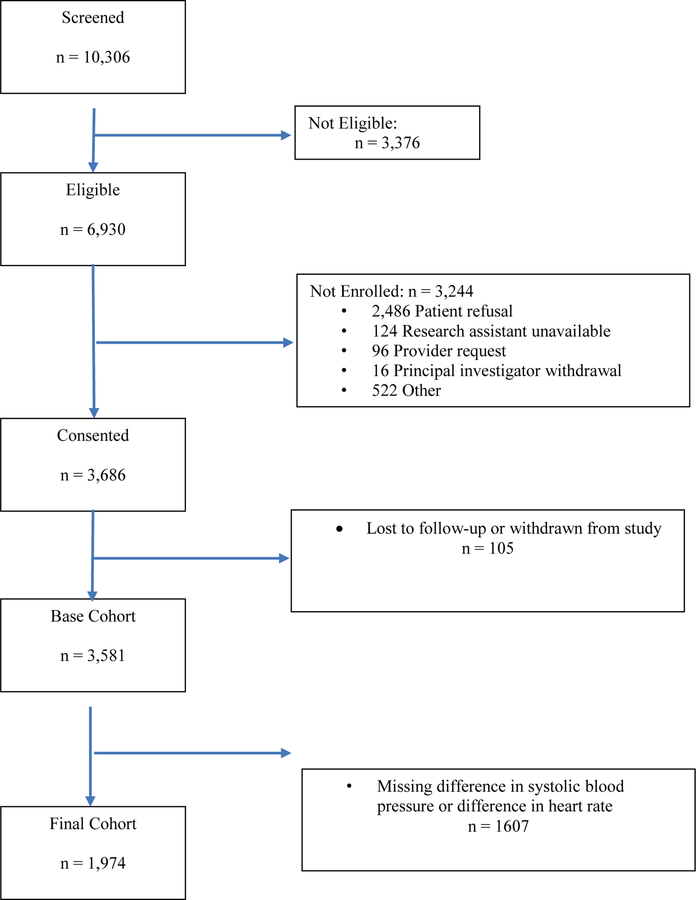
There were 6930 subjects that met eligibility criteria for the primary study, of which 3686 (53.2%) consented and were enrolled (Figure 1). Of the 3686 enrolled subjects, there were 1974 patients (53.6%) who had orthostatic vital signs performed in the ED, representing the cohort for this study. Compared to the study cohort, subjects not receiving assessment of orthostatic vital signs were more likely to be older, have CAD, HF, dyspnea, or an abnormal ECG, and more likely to have a higher physician risk estimate or be hospitalized (see Appendix C). Reasons recorded, in free text, for not obtaining orthostatic vital signs (n=1125) most commonly included too symptomatic at baseline {395 (35%)], unable to stand [305 (27%)] most often related to injuries from fall or baseline condition, provider determined it was not indicated [170 (15%)], and the patient refused [129 (11%)].
Figure 1.
Flow diagram of study cohort
Study subjects had a mean age of 72.1 years and 1010 (51.2%) were male (Table 1). Compared to patients who had normal orthostatic findings, those that had abnormal orthostatic findings were more likely to have heart failure, an abnormal ECG, hypotension on initial triage vital signs, and be hospitalized (Table 2).
Table 1.
Baseline characteristics of study cohort
| Patient characteristics | Overall Cohort (n=1974) | Normal Orthostatic Vital Signs (n=1249) | Abnormal Orthostatic Vital Signs (n=725) | p-value |
|---|---|---|---|---|
| Age, mean6 | 72.1 (8.6) | 72.1 (8.8) | 72.1 (8.4) | 0.969 |
| Age | 0.017 | |||
| 60 to <70 | 898 (45.5) | 586 (46.9) | 312 (43.0) | |
| 70 to <80 | 647 (32.8) | 388 (31.1) | 259 (35.7) | |
| 80 to <90 | 367 (18.6) | 227 (18.2) | 140 (19.3) | |
| 90+ | 62 (3.1) | 48 (3.8) | 14 (1.9) | |
| Gender | 0.510 | |||
| Male | 1010 (51.2) | 632 (50.6) | 378 (52.1) | |
| Female | 964 (48.8) | 617 (49.4) | 347 (47.9) | |
| Race | 0.348 | |||
| White or Caucasian | 1646 (83.7) | 1055 (84.8) | 591 (81.7) | |
| Black or African American | 255 (13.0) | 149 (12.0) | 106 (14.7) | |
| Asian | 29 (1.5) | 18 (1.4) | 11 (1.5) | |
| Other | 37 (1.9) | 22 (1.8) | 15 (2.1) | |
| History of | ||||
| Congestive Heart Failure | 210 (10.6) | 112 (9.0) | 98 (13.5) | 0.002 |
| Coronary Artery Disease | 503 (25.5) | 300 (24.0) | 203 (28.0) | 0.052 |
| Arrhythmia | 432 (21.9) | 259 (20.8) | 173 (23.9) | 0.107 |
| Prescribed Medication | ||||
| Beta Blockers | 763 (38.7) | 481 (38.5) | 282 (38.9) | 0.876 |
| Calcium Channel Blockers | 346 (17.5) | 216 (17.3) | 130 (17.9) | 0.726 |
| Diuretics | 543 (27.5) | 328 (26.3) | 215 (29.7) | 0.106 |
| Dyspnea | 387 (20.0) | 228 (18.7) | 159 (22.2) | 0.060 |
| <0.00 | ||||
| Hypotension | 212 (10.7) | 82 (6.6) | 130 (17.9) | 1 |
| Abnormal ECG | 1030 (52.8) | 625 (50.6) | 405 (56.6) | 0.012 |
| Physician Risk Assessment, mean6 | 8.0 (11.3) | 8.2 (12.0) | 7.7 (10.0) | 0.370 |
| Disposition | 0.004 | |||
| Hospitalized | 1538 (79.0) | 945 (77.0) | 593 (82.5) | |
| Discharged | 409 (21.0) | 283 (23.0) | 126 (17.5) |
Unless otherwise noted, data are presented as number (%)
Table 2.
Individual and composite 30-day serious outcomes stratified by normal and abnormal orthostatic vital signs
| Predictor Variables | Odds for 30-day Serious Outcome (95% CI) | 95% CI | p-value | |
|---|---|---|---|---|
| | ||||
| Outcome | Overall Cohort (n=1974) | Normal Orthostatic Vital Signs (n=1249) | Abnormal Orthostatic Vital Signs (n=725) | p-value |
| Any 30-day serious outcome | 295 (14.9) | 184 (14.7) | 111 (15.3) | 0.728 |
| Pulmonary embolism OR internal hemorrhage/anemia | 73 (3.7) | 36 (2.9) | 37 (5.1) | 0.012 |
| 30 Day Death | 7 (0.4) | 3 (0.2) | 4 (0.6) | 0.262 |
| Serious Cardiac Arrhythmia | ||||
| Ventricular Fibrillation | 6 (0.3) | 4 (0.3) | 2 (0.3) | 0.863 |
| Ventricular tachycardia (>30 secs) | 5 (0.3) | 2 (0.2) | 3 (0.4) | 0.280 |
| Symptomatic ventricular tachycardia (<30 secs) | 8 (0.4) | 7 (0.6) | 1 (0.1) | 0.154 |
| Sick sinus disease | 11 (0.6) | 8 (0.6) | 3 (0.4) | 0.514 |
| Sinus Pause > 3 seconds | 6 (0.3) | 5 (0.4) | 1 (0.1) | 0.307 |
| Mobitz II atrioventricular heart block | 8 (0.4) | 6 (0.5) | 2 (0.3) | 0.491 |
| Complete heart block | 10 (0.5) | 8 (0.6) | 2 (0.3) | 0.271 |
| Symptomatic supraventricular tachycardia | 70 (3.5) | 45 (3.6) | 25 (3.4) | 0.858 |
| Symptomatic bradycardia | 24 (1.2) | 18 (1.4) | 6 (0.8) | 0.230 |
| Pacemaker/ICD | 2 (0.1) | 1 (0.1) | 1 (0.1) | 0.697 |
| Other Serious Outcomes | ||||
| Myocardial Infarction | 30 (1.5) | 21 (1.7) | 9 (1.2) | 0.441 |
| Cardiac Intervention | 85 (4.3) | 62 (5.0) | 23 (3.2) | 0.059 |
| New diagnosis of structural heart disease | 24 (1.2) | 19 (1.5) | 5 (0.7) | 0.104 |
| Stroke | 14 (0.7) | 5 (0.4) | 9 (1.2) | 0.032 |
| Pulmonary Embolism | 14 (0.7) | 9 (0.7) | 5 (0.7) | 0.937 |
| Aortic Dissection | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 |
| Subarachnoid Hemorrhage | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 |
| Cardiopulmonary resuscitation | 2 (0.1) | 1 (0.1) | 1 (0.1) | 0.697 |
| GI hemorrhage/anemia | 60 (3.0) | 28 (2.2) | 32 (4.4) | 0.007 |
| Recurrent syncope/fall with major injury | 4 (0.2) | 1 (0.1) | 3 (0.4) | 0.112 |
| Abnormal Orthostatic | 1.05 | (0.81, 1.35) | 0.728 | |
| Male | 1.40 | (1.09, 1.81) | 0.008 | |
| History of Congestive Heart Failure | 2.12 | (1.50, 2.96) | <0.001 | |
| History of Coronary Artery Disease | 1.59 | (1.22, 2.07) | 0.001 | |
| History of Arrhythmia | 2.55 | (1.95, 3.32) | <0.001 | |
| Abnormal ECG | 2.34 | (1.79, 3.07) | <0.001 | |
| Dyspnea | 2.17 | (1.64, 2.86) | <0.001 | |
| Physician Risk Assessment | 1.03 | (1.02, 1.04) | <0.001 | |
| Hypotension | 2.03 | (1.43, 2.84) | <0.001 | |
| Discharged | 0.25 | (0.15, 0.39) | <0.001 | |
| Beta Blocker | 1.41 | (1.10, 1.81) | 0.007 | |
| Diuretics | 1.21 | (0.92, 1.58) | 0.166 | |
| Calcium Channel Blocker | 0.93 | (0.66, 1.28) | 0.650 | |
Unless otherwise noted, data are presented as number (%)
Main Results
Overall, 295 (14.9%) study subjects had a composite 30-day serious outcome (Table 3). One hundred eighty-four of 1249 (14.7%) of patients with normal orthostatic vital signs and 111/725 (15.3%) of patients with abnormal orthostatic vital signs had a composite 30-day serious outcome (odds ratio [OR] 1.05; 95% CI 0.81–1.35). After adjustment for pre-specified co-variates (Table 4), the adjusted OR was 0.83 (95% CI 0.62–1.10). Similarly, orthostatic vital signs were not associated with short-term serious outcomes. (Appendix D) Of the 20 different items within the composite 30-day serious event outcome, events rates for 18 conditions were similar between the two groups (Table 2). Patients with abnormal orthostatic vital signs were more likely to have GI hemorrhage/anemia 4.4% vs 2.2% (p=0.007), and stroke 1.2% v 0.4% (p=0.032). Both of these conditions were also associated with serious short term (Appendix E).
Table 3.
Unadjusted odd’s ratios for composite 30-day serious outcomes
| Hosmer-Lemeshow Goodness of Fit Test | |
|---|---|
| X-squared | 6.06 |
| df | 8 |
| p-value | 0.640 |
There is no evidence of poor fit.
Table 4.
Multivariate Logistic Regression Model Predicting Composite 30-Day Serious Outcomes
| Predictor Variables | Odds for 30-day Serious Outcome (95% CI) | 95% CI | p-value |
|---|---|---|---|
| Abnormal Orthostatic | 0.83 | (0.62, 1.10) | 0.192 |
| Male | 1.22 | (0.93, 1.61) | 0.158 |
| History of Congestive Heart Failure | 1.44 | (0.95, 2.16) | 0.083 |
| History of Coronary Artery Disease | 0.9 | (0.65, 1.24) | 0.524 |
| History of Arrhythmia | 2.14 | (1.59, 2.88) | <0.001 |
| Abnormal ECG | 1.78 | (1.32, 2.40) | <0.001 |
| Dyspnea | 2.05 | (1.51, 2.76) | <0.001 |
| Physician Risk Assessment | 1.02 | (1.01, 1.03) | <0.001 |
| Hypotension | 1.84 | (1.24, 2.69) | 0.002 |
| Discharged | 0.29 | (0.17, 0.46) | <0.001 |
| Beta Blocker | 1.07 | (0.80, 1.45) | 0.639 |
| Diuretics | 0.78 | (0.56, 1.07) | 0.128 |
| Calcium Channel Blocker | 0.93 | (0.64, 1.33) | 0.683 |
Discussion
In a large cohort of older adults with syncope with orthostatic vital signs measured, abnormal orthostatic vital signs in the ED did not have increased composite 30-day serious outcomes compared to patients with normal orthostatic vital signs. Current AHA/ACC/HRS guidelines recommend incorporation of a set of orthostatic vital signs into the standard ED work up for syncope despite conflicting evidence.3 Our study specifically focused on patients where the clinicians felt orthostatic vital signs may have some role, as patients that clinicians felt were too sick did not receive orthostatic vital signs. It also adjusted for multiple co-morbidities including physician risk assessment. We found that orthostatic vital sign abnormalities did not predict 30-day serious outcomes in unadjusted or adjusted models. This does not mean that they are useless, but does suggest that they should not be a required standard of care for all patients.
The reliability of abnormal orthostatic vital signs in patients with syncope has not been well established. In fact, the Canadian Syncope rule did not evaluate orthostatic vital signs.4 Abnormal orthostatic vital signs may be associated with both serious and non-serious conditions such as pulmonary embolism, GI hemorrhage, cardiac tamponade dehydration, autonomic dysfunction and sleep deprivation.2, 8,15,16,19 Older adults have altered physiologic response compared to younger patients making the interpretation of abnormal orthostatic vital signs in older adults more difficult.18 In theory, abnormalities may suggest intra-vascular depletion or an occult process that has not yet been recognized. However, this assumes a normal physiologic state; one without peripheral arterial disease, baseline hypertension, or medications that may distort heart rate or blood pressure responsiveness to normal shifts in position.
Older adults with baseline abnormal orthostatic vital signs and atherosclerosis, hypertension, stroke, and neurologic conditions have worse long term outcomes in prior studies. 9,19, 20, 21,22, 23 When symptomatic (with syncope), as in our study, abnormal orthostatic vital signs are not an independent predictor of adverse events. This is analogous to cardiac risk factors predicting long-term cardiovascular risk in asymptomatic patients but not being predictive of short-term events in symptomatic ED patients. 6
In our univariate analysis assessing the relationship between orthostatic vitals signs and 20 outcomes, there were two 30-day serious outcomes that were more common in patients with abnormal orthostatic vital signs: GI hemorrhage/anemia and stroke. Biological plausibility for the findings in GI bleeding is obvious, but it is less clear in stroke patients. It might be an artifact of multiple testing (e.g., Type 1 error). As such, this finding should be considered exploratory. It is worth noting that patients who presented with stroke as the cause of syncope were excluded so these patients represent only those without clinically obvious stroke prior to enrollment.
One criticism of our study could be that not all patients had measurement of orthostatic vital signs. Bias may result from not obtaining orthostatic vital signs on patients in the two extremes – those judged too well to need them, and those judged too sick to obtain them. In general, orthostatic vital signs are not measured on critically ill patients that cannot stand up or those that have a clear etiology of their syncope, excluding an important group from most large studies. This was also seen in our study, where approximately half of all study patients did not have orthostatic vital signs measured in the ED. Although this may be considered a flaw in our ability to determine the value of orthostatic vital signs, it does reflect what is seen in the “real world’ and thus should not limit the generalizability of our findings. This is consistent with our analysis of the characteristics of these patients which found that the cohort who did not get a set of orthostatic vital signs in the ED were statistically older, had more cardiovascular co-morbidities, and were more likely to be admitted to the hospital. Patients judged to be too sick and require inpatient admission or ICU level care were not able to stand up and did not get orthostatic vital signs.
In addition, although our protocol did standardize the ascertainment of orthostatic vital signs - the method of measurement was determined locally. As a result, we cannot be sure that all providers waited the appropriate amount of time when altering positioning. Although obtaining blood pressure too rapidly or too slowly can change the likelihood of the test being abnormal, it does represent the real-world experience and allows our study to be generalizable. Although we used only academic sites, patients presenting with syncope are usually those from the local community, rather than high complex referrals. Finally, our results cannot be applied to younger patients or others with characteristics who were ineligible for our study.
Conclusions
In a cohort of older adult patients presenting with syncope who were able to have orthostatic vital signs evaluated, abnormal orthostatic vital signs did not independently predict composite 30-day serious outcomes.
Acknowledgments
Funding Sources and Support:
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number R01HL111033. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix A–
Characteristics of Enrolling Sites
| Name and Location | Annual ED Volume | Hospital Beds |
|---|---|---|
| Oregon Heath & Science University, Portland, OR | 46,782 | 576 |
| UC Davis School of Medicine, Sacramento, CA | 69,293 | 625 |
| University of Rochester, NY | 99,519 | 739 |
| William Beaumont Hospital-Troy, Troy, MI | 80,000 | 418 |
| William Beaumont Hospital-Royal Oak, Royal Oak, MI | 119,950 | 1,070 |
| Brigham & Women’s Hospital, Boston, MA | 59,851 | 769 |
| Ohio State University Wexner Medical Center, Columbus, OH | 72,000 | 971 |
| Thomas Jefferson University Hospital, Philadelphia, PA | 60,270 | 717 |
| Wake Forest School of Medicine, Winston Salem, NC | 109,687 | 885 |
| Summa Health System, Akron, OH | 90,656 | 544 |
| Vanderbilt University, Nashville, TN | 65,000 | 864 |
Appendix B-
Checklist STROBE Statement
| Item No | Recommendation | |
|---|---|---|
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract Done. “Orthostatic Vital Signs Do Not Predict 30 Day Serious Outcomes in Older Emergency Department Patients with Syncope: A multicenter observational study” |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found Done. | ||
| Introduction | ||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported. Done. Para 1 & 2. |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses Done. Para 3 |
| Methods | ||
| Study design | 4 | Present key elements of study design early in the paper Done. Methods, paragraph 1. |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection Done. Methods, paragraph 2, 3, 4, 5 |
| Participants | 6 | (a) Give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow-up Methods, paragraph 3 |
| (b) For matched studies, give matching criteria and number of exposed and unexposed. N/A | ||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable Methods, paragraph 5 & 6 |
| Data sources/measurement | 8* | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group Methods, paragraph 5 & 6 |
| Bias | 9 | Describe any efforts to address potential sources of bias. See limitations section |
| Study size | 10 | Explain how the study size was arrived at noted, secondary analysis of clinical trial |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why Page 7, Analysis section |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding Page 7, Analysis section |
| (b) Describe any methods used to examine subgroups and interactions Page 7, Analysis section | ||
| (c) Explain how missing data were addressed Not imputed | ||
| (d) If applicable, explain how loss to follow-up was addressed noted | ||
| (e) Describe any sensitivity analyses N/A | ||
| Results | ||
| Participants | 13* | (a) Report numbers of individuals at each stage of study—eg numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analyzed Page 8 and flow diagram |
| (b) Give reasons for non-participation at each stage Page 8 and flow diagram | ||
| (c) Consider use of a flow diagram Figure 1 | ||
| Descriptive data | 14* | (a) Give characteristics of study participants (eg demographic, clinical, social) and information on exposures and potential confounders Table 1 |
| (b) Indicate number of participants with missing data for each variable of interest See tables | ||
| (c) Summarize follow-up time (eg, average and total amount) See sfigure 1 | ||
| Outcome data | 15* | Report numbers of outcome events or summary measures over time Results and tables 2, 3, 4 |
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (eg, 95% confidence interval). Make clear which confounders were adjusted for and why they were included Results and tables 2, 3, 4 |
| (b) Report category boundaries when continuous variables were categorized Results and tables 2, 3, 4 | ||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period Results and tables 2, 3, 4 | ||
| Other analyses | 17 | Report other analyses done—eg analyses of subgroups and interactions, and sensitivity analyses see appendecies |
| Discussion | ||
| Key results | 18 | Summarize key results with reference to study objectives Discussion, para 1 |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias Discussion para 4 & 5 |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence in Discussion |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results in discussion |
| Other information | ||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based see title page |
Appendix C.–
Comparison of patients who did not have any orthostatic vital signs obtained compared to the group that did get orthostatic vital signs assessed (normal and abnormal)
| Variable | Overall Cohort (n=3581) | Did Not Obtain Orthostatic Vital Signs (n=1607) | Obtained OVS (n=1974) | p-value |
|---|---|---|---|---|
| Age, mean6 | 72.8 (9.0) | 73.6 (9.3) | 72.1 (8.6) | <0.001 |
| Age | <0.001 | |||
| 60 to <70 | 1539 (43.0) | 641 (39.9) | 898 (45.5) | |
| 70 to <80 | 1156 (32.3) | 509 (31.7) | 647 (32.8) | |
| 80 to <90 | 729 (20.4) | 362 (22.5) | 367 (18.6) | |
| 90+ | 157 (4.4) | 95 (5.9) | 62 (3.1) | |
| Gender | 0.559 | |||
| Male | 1848 (51.6) | 838 (52.1) | 1010 (51.2) | |
| Female | 1733 (48.4) | 769 (47.9) | 964 (48.8) | |
| Race | 0.196 | |||
| White or Caucasian | 2974 (83.5) | 1328 (83.4) | 1646(83.7) | |
| Black or African American | 478 (13.4) | 223 (14.0) | 255 (13.0) | |
| Asian | 41 (1.2) | 12 (0.8) | 29 (1.5) | |
| Other | 67 (1.9) | 30 (1.9) | 37 (1.9) | |
| History of | ||||
| Congestive Heart Failure | 449 (12.5) | 239 (14.9) | 210 (10.6) | <0.001 |
| Coronary Artery Disease | 979 (27.4) | 476 (29.7) | 503 (25.5) | 0.005 |
| Arrhythmia | 803 (22.4) | 371 (23.1) | 432 (21.9) | 0.384 |
| Prescribed Medication | ||||
| Beta Blockers | 1422 (39.7) | 659 (41.1) | 763 (38.7) | 0.147 |
| Calcium Channel Blockers | 657 (18.4) | 311 (19.4) | 346 (17.5) | 0.157 |
| Diuretics | 1048 (29.3) | 505 (31.5) | 543 (27.5) | 0.010 |
| Dyspnea | 747 (21.4) | 360 (23.1) | 387 (20.0) | 0.026 |
| Hypotension | 382 (10.7) | 170 (10.6) | 212 (10.7) | 0.877 |
| Abnormal ECG | 1948 (55.4) | 918 (58.5) | 1030 (52.8) | 0.001 |
| Physician Risk Assessment, mean6 | 9.2 (13.2) | 10.7 (15.1) | 8.0 (11.3) | <0.001 |
| Disposition | 0.001 | |||
| Hospitalized | 2860 (80.9) | 1322 (83.3) | 1538 (79.0) | |
| Discharged | 674 (19.1) | 265 (16.7) | 409 (21.0) |
Unless otherwise noted, data are presented as number (%)
Appendix D.
Multivariate Logistic Regression Model Predicting Short Term Serious Outcomes
| Variables | OR | 95% CI | p-value |
|---|---|---|---|
| Abnormal Orthostatic | 0.81 | (0.60, 1.09) | 0.165 |
| Male | 1.20 | (0.89, 1.61) | 0.225 |
| History of Heart Failure | 1.21 | (0.78, 1.89) | 0.393 |
| History of Coronary Artery Disease | 0.84 | (0.59, 1.19) | 0.331 |
| History of Arrhythmia | 2.45 | (1.8,0 3.34) | 0.000 |
| Abnormal ECG | 1.95 | (1.41, 2.69) | <0.001 |
| Dyspnea | 2.12 | (1.55, 2.92) | <0.001 |
| Physician Risk Assessment | 1.02 | (1.01, 1.03) | <0.001 |
| Hypotension | 1.67 | (1.11, 2.53) | 0.014 |
| Discharged | 0.13 | (0.06, 0.27) | <0.001 |
| Beta Blocker | 1.11 | (0.81, 1.52) | 0.523 |
| Diuretics | 0.71 | (0.50, 0.99) | 0.045 |
| Calcium Channel Blocker | 0.93 | (0.63, 1.37) | 0.717 |
| Hosmer-Lemeshow Goodness of Fit Test | |
|---|---|
| X-squared | 4.91 |
| df | 8 |
| p-value | 0.767 |
There is no evidence of poor fit.
Appendix E.
Supplementary analysis of short term serious outcomes
| Outcome | Overall Cohort (n=1974) | Normal Orthostatic Vital Signs (n=1249) | Abnormal Orthostatic Vital Signs (n=725) | p-value |
|---|---|---|---|---|
| Any serious outcome | 257 (13.0) | 162 (13.0) | 95 (13.1) | 0.933 |
| Pulmonary embolism OR Internal hemorrhage/anemia | 66 (3.3) | 31 (2.5) | 35 (4.8) | 0.005 |
| Death | 3 (0.2) | 1 (0.1) | 2 (0.3) | 0.559 |
| Serious Cardiac Arrhythmia | ||||
| Ventricular fibrillation | 5 (0.3) | 3 (0.2) | 2 (0.3) | 1.000 |
| Ventricular tachycardia (>30 secs) | 4 (0.2) | 1 (0.1) | 3 (0.4) | 0.143 |
| Symptomatic ventricular tachycardia (<30 secs) | 7 (0.4) | 6 (0.5) | 1 (0.1) | 0.434 |
| Sick sinus disease with alternating sinus bradycardia and tachycardia | 8 (0.4) | 6 (0.5) | 2 (0.3) | 0.718 |
| Sinus Pause > 3 seconds | 6 (0.3) | 5 (0.4) | 1 (0.1) | 0.424 |
| Mobitz II atrioventricular heart block | 6 (0.3) | 4 (0.3) | 2 (0.3) | 1.000 |
| Complete heart block | 8 (0.4) | 7 (0.6) | 1 (0.1) | 0.271 |
| Symptomatic supraventricular tachycardia | 64 (3.2) | 43 (3.4) | 21 (2.9) | 0.509 |
| Symptomatic bradycardia | 21 (1.1) | 16 (1.3) | 5 (0.7) | 0.217 |
| Pacemaker or implantable cardioverter-defibrillator malfunction with cardiac pauses | 2 (0.1) | 1 (0.1) | 1 (0.1) | 1.000 |
| Other Serious Outcomes | ||||
| Myocardial infarction | 24 (1.2) | 17 (1.4) | 7 (1.0) | 0.439 |
| Cardiac intervention | 69 (3.5) | 52 (4.2) | 17 (2.3) | 0.034 |
| New diagnosis of structural heart disease | 21 (1.1) | 18 (1.4) | 3 (0.4) | 0.039 |
| Stroke | 10 (0.5) | 3 (0.2) | 7 (1.0) | 0.044 |
| Pulmonary embolism | 13 (0.7) | 8 (0.6) | 5 (0.7) | 0.896 |
| Aortic dissection | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 |
| Subarachnoid hemorrhage | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 |
| Cardiopulmonary resuscitation | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 |
| GI hemorrhage/anemia | 54 (2.7) | 24 (1.9) | 30 (4.1) | 0.004 |
| Recurrent syncope/fall resulting in major injury | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
JLW has no conflicts to report
JEH has received research funding from Alere, Siemens, Roche, Portola and Trinity
AMC has received research funding from Abbott, Akers, Alere, Nanomix, Siemens, Roche, Ortho Diagnostics, Portola and Trinity
ALL has no conflicts to report
ES has no conflicts to report
REW has no conflicts to report
ANY has no conflicts to report
SEM has no conflicts to report
DHA has received research funding from Roche
AB has received research funding from Radiometer and Portola and has been a consultant for Portola
CWB has received advisory board and speaker’s fees from Roche, research funding from Janssen and Boehringer Ingelheim and consulting and advisory board fees from Janssen
JMC has received research funding from Aztra Zeneca
CLC has received institutional research funding from Radiometer, Ortho Clinical Trials, Janssen, Pfizer, Portola, Glaxo Smith Klein, and Hospital Quality Foundation and is a consultant for Portola, Janssen, and Hospital Quality Foundation
DBD is a consultant for Janssen and Roche, has received institutional research support from Novartis, ortho Scientific, and Roche and is on the editorial board for AEM and Circulation
DKN has received honorarium for Pfizer
BAN has no conflicts to report
MNS has received research funding from Roche Molecular Systems
KAS has no conflicts to report
ABS is a consultant for Quidel, Siemens, and MCM Education
STW has no conflicts to report
BCS is a consultant for Medtronic.
References
- 1.Probst MA, Kanzaria HK, Gbedemah M, Richardson LD, Sun BC. National trends in resource utilization associated with ED visits for syncope. Am J Emerg Med 2015;33:998–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold AC, Raj SR. Orthostatic Hypotension: A Practical Approach to Investigation and Management. Can J Cardiol 2017;33:1725–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen WK, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS Guideline for the Evaluation and Management of Patients with Syncope: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, and the Heart Rhythm Society. Heart Rhythm 2017. [DOI] [PubMed]
- 4.Thiruganasambandamoorthy V, Kwong K, Wells GA, et al. Development of the Canadian Syncope Risk Score to predict serious adverse events after emergency department assessment of syncope. CMAJ 2016;188:E289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibbons CH, Schmidt P, Biaggioni I, et al. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J Neurol 2017;264:1567–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han JH, Lindsell CJ, Storrow AB, Luber S, Hoekstra JW, Hollander JE, Peacock WF IV, Pollack CV, Gibler WB. Cardiac risk factor burden and its association with acute coronary syndrome. Ann Emerg Med, 2007;49:145–152. [DOI] [PubMed] [Google Scholar]
- 7.D’Ascenzo F, Biondi-Zoccai G, Reed MJ, et al. Incidence, etiology and predictors of adverse outcomes in 43,315 patients presenting to the Emergency Department with syncope: an international meta-analysis. Int J Cardiol 2013;167:57–62. [DOI] [PubMed] [Google Scholar]
- 8.Raiha I, Luutonen S, Piha J, Seppanen A, Toikka T, Sourander L. Prevalence, predisposing factors, and prognostic importance of postural hypotension. Arch Intern Med 1995;155:930–5. [DOI] [PubMed] [Google Scholar]
- 9.Ooi WL, Hossain M, Lipsitz LA. The association between orthostatic hypotension and recurrent falls in nursing home residents. Am J Med 2000;108:106–11. [DOI] [PubMed] [Google Scholar]
- 10.Ooi WL, Barrett S, Hossain M, Kelley-Gagnon M, Lipsitz LA. Patterns of orthostatic blood pressure change and their clinical correlates in a frail, elderly population. JAMA 1997;277:1299–304. [PubMed] [Google Scholar]
- 11.Shibao C, Lipsitz LA, Biaggioni I, American Society of Hypertension Writing G. Evaluation and treatment of orthostatic hypotension. J Am Soc Hypertens 2013;7:317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharp MK, Utrobicic A, Gomez G, Cobo E, Wager E, Hren D. The STROBE extensions: protocol for a qualitative assessment of content and a survey of endorsement. BMJ Open 2017;7:e019043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishijima DK, Lin AL, Weiss RE, et al. ECG Predictors of Cardiac Arrhythmias in Older Adults With Syncope. Ann Emerg Med, 2018;71:452–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson TA, Weiss RE, Sun BC. Predictors of Short-Term Outcomes after Syncope: A Systematic Review and Meta-Analysis. West J Emerg Med 2018;19:517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Team RC. R: A language and environment for statistical computing. R. Foundation for Statistical Computing, Vienna Austria: 2016. [Google Scholar]
- 16.Writing Committee M, Shen WK, Sheldon RS, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm 2017;14:e155–e217. [DOI] [PubMed] [Google Scholar]
- 17.Muenter NK, Watenpaugh DE, Wasmund WL, Wasmund SL, Maxwell SA, Smith ML. Effect of sleep restriction on orthostatic cardiovascular control in humans. J Appl Physiol (1985) 2000;88:966–72. [DOI] [PubMed] [Google Scholar]
- 18.Mussi C, Ungar A, Salvioli G, et al. Orthostatic hypotension as cause of syncope in patients older than 65 years admitted to emergency departments for transient loss of consciousness. J Gerontol A Biol Sci Med Sci 2009;64:801–6. [DOI] [PubMed] [Google Scholar]
- 19.Diedrich A, Biaggioni I. Segmental orthostatic fluid shifts. Clin Auton Res 2004;14:146–7. [DOI] [PubMed] [Google Scholar]
- 20.Aronow WS, Lee NH, Sales FF, Etienne F. Prevalence of postural hypotension in elderly patients in a long-term health care facility. Am J Cardiol 1988;62:336. [DOI] [PubMed] [Google Scholar]
- 21.Mar PL, Raj SR. Orthostatic hypotension for the cardiologist. Curr Opin Cardiol 2018;33:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Probst MA, McConnell JK, Weiss RE, et al. Estimating the Cost of Care for Emergency Department Syncope Patients: Comparison of Three Models. West J Emerg Med 2017;18:253–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali NJ, Grossman SA. Geriatric Syncope and Cardiovascular Risk in the Emergency Department. J Emerg Med 2017;52:438–48 e3. [DOI] [PubMed] [Google Scholar]