Abstract
Oligodendrocytes (OL) are the only myelinating cells of the central nervous system thus interferences, either environmental or genetic, with their maturation or function have devastating consequences. Albeit so far neglected, one of the less appreciated, nevertheless possible, regulators of OL maturation and function is the circadian cycle. Yet, disruptions in these rhythms are unfortunately becoming a common “disorder” in the today’s world. The temporal patterning of behaviour and physiology is controlled by a circadian timing system based in the anterior hypothalamus. At the molecular level, circadian rhythms are generated by a transcriptional/translational feedback system that regulates transcription and has a major impact on cellular function(s). Fundamental cellular properties/functions in most cell types vary with the daily circadian cycle: OL are unlikely an exception! To be clear, the presence of circadian oscillators or the cell-specific function(s) of the circadian clock in OL has yet to be defined. Furthermore, we wish to entertain the idea of links between the “thin” evidence on OL intrinsic circadian rhythms and their interjection(s) at different stages of lineage progression as well as in supporting/regulating OL crucial function: myelination. Individuals with intellectual and developmental syndromes as well as neurodegenerative diseases present with a disrupted sleep/wake cycle; hence, we raise the possibility that these disturbances in timing can contribute to the loss of white matter observed in these disorders. Preclinical and clinical work in this area is needed for a better understanding of how circadian rhythms influence OL maturation and function(s), to aid the development of new therapeutic strategies and standards of care for these patients.
Keywords: circadian rhythms, myelination, oligodendrocytes, oligodendrocyte progenitors, sleep
In the central nervous system (CNS), mature myelinating oligodendrocytes (OL) send processes that wrap around the axons to form myelin sheaths, which insulate axons and have a critical influence on the passive electrical properties of neurons. Myelination involves a finely-tuned pathway of OPC specification, proliferation and migration followed by differentiation. This is an ongoing dynamic process in the CNS and alterations due to external environmental influences, genetic deficiencies or disease will directly affect the speed at which action potentials can travel down an axon and thus change the functional connections among circuits in the CNS, affecting cognitive functions [1–4]. Prominent myelinated tracks in the CNS are commonly referred to as white matter (WM) [5–7]. Beside myelinating the axons and modifying their conduction velocity, OL have a number of supporting roles, which makes them an invaluable and irreplaceable partner for the axons. The latter rely on the OL also for their survival and integrity and deficits in such partnership play a role in neuropsychiatric and neurological disorders. As described in previous work, deficits in WM observed in some neurodevelopmental disorders are presumably a reflection of abnormalities in OL maturations or their ability to properly form and assemble myelin [3,4,8].
Oligodendrocyte progenitor cells (OPC) actively proliferate during brain development and are present in adulthood. OL lineage progression is probably the best characterized in the CNS (Fig. 1) and recent reviews are available [9,10]. Importantly, these are the last neural cells to mature, hence, this process occurs in an intricate environment under the influence of highly coordinated signals from the surrounding neural cells. Both cell intrinsic and extrinsic factors regulating OL maturation have been identified and extensively studied [11–13]. Many aspects of OL maturation are modulated by local extrinsic signals, including astrocytic and neuronal activity, as well as more global signals like hormones [13–15]. OPC and OL cells express a variety of neurotransmitter receptors and ion channels [16–17], and neural activity alters their maturation and consequently myelination of axons in both the developing and mature CNS. For example, some of the major neurotransmitters controlling arousal like Acetylcholine (ACh) and Norepinephrine (NE) regulate neurogenesis, but also OPC proliferation and survival [18–21]. Both neural activity and the release of neurotransmitters involved in the control of arousal (ACh, NE, etc) vary with a daily cycle and thus the circadian system is likely to influence OPC development, at least, through the regulation of cell extrinsic factors.
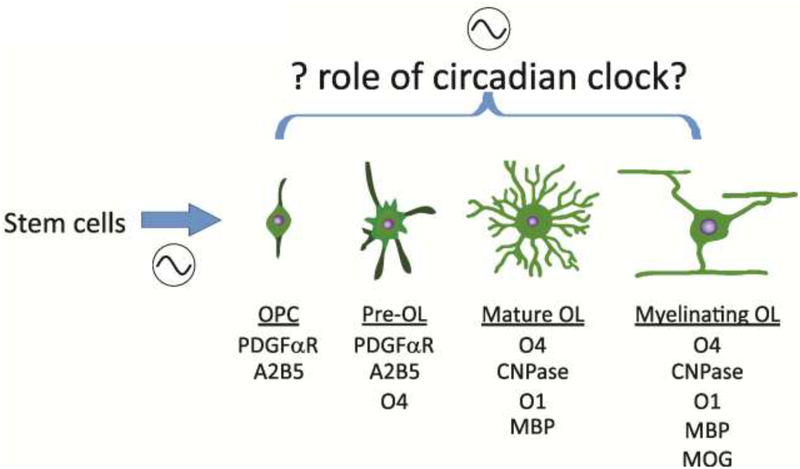
Fig. 1: Circadian regulation of OL maturation likely occurs at multiple points.
The circadian system (shown by the sine wave) likely gates the production of OPCs from stem cells [156] and modulates their lineage progression through its regulation of extrinsic factors like neural activity, secretion of neurotransmitters, levels of growth factors and hormones. Schematic representation of the developmental stages of the OL lineage, after Traiffort et al., 2016, [157], along with a list of some of the commonly used stage-specific markers.
Hence, we propose that the circadian system may also be involved in the regulation of the intrinsic factors controlling OL maturation. Several cell types possess an intrinsic clock that regulate their maturations and function(s) and is aligned and reset by internal and external environmental cues. Segments of the cell maturation processes, such as cell cycle and differentiation, are “timed”. OPC, like other precursor cells, will divide a specific number of times controlled and limited by this intrinsic timer, before exiting the cell cycle and terminally differentiate [22,23]. Remarkably, this “counting” seems to be maintained even in the absence of a cell cycle regulator, the cyclin-dependent kinase inhibitor p27 [24]. There is strong evidence for a cross talk between circadian rhythms and the cell cycle [25,26]. Perhaps, the circadian system regulates this timing mechanism and an important area for future work would be to see if clock mutants with long or short cycle lengths also exhibit corresponding changes in OL maturation.
Although developmental myelination is not complete in the cortex until early adulthood, there is abundant evidence that this process can continue until late in the adult brain. Adult myelination is important to allow remyelination in response to injury and to permit plasticity in function. Circadian rhythms and sleep are likely to be crucial regulators of the maturation of OPCs into myelinating OL in the adult. For example, Cirelli and colleagues [27] showed that OPC proliferation in the adult subventricular zone (SVZ) doubles during sleep but is disrupted by sleep deprivation. In the adult, levels of neural activity as well as of neurotransmitters, known to influence OL lineage progression at different “check-points”, do vary with the sleep/wake cycle (Table 1). Hence, to a first approximation the increase in neural activity and release of neurotransmitters, such as glutamate, during wake would inhibit OPC proliferation, while lower activity and reduced secretion during rest would allow for proliferation to occur. In agreement, it was reported that activation of glutamatergic receptors, AMPA-subtype, on OPC in culture as well as in vivo elicits a reversible blockade of proliferation and likely regulates their migration [21,28–32]. In contrast, activation of GABAB, but not GABAA receptors, stimulates cell proliferation and migration [28,33–35]. These observations are consistent with a highly plausible model in which the circadian system through direct regulation of arousal and neural activity would act in concert with sleep to regulate the temporal pattern of OPC proliferation and migration.
Table 1: Factors known to display circadian fluctuations have a role in OL maturation as well as myelination, another link?
The timing of sleep and arousal is controlled by the circadian timing system with a central clock located in the suprachiasmatic nucleus (SCN) of the hypothalamus. The SCN synchronizes independent circadian clocks located in each organ of the body to generate tissue specific rhythms. Light entrains the master pacemaker in the SCN, which in turn synchronizes extra-SCN central (brain) and peripheral clocks. Brain clock outputs include behavioural rhythms (i.e., sleep, feeding), while peripheral clock outputs include metabolic rhythms (i.e., glucose and lipid homeostasis). Among the rhythmically regulated SCN outputs important for oligodendrocytes are melatonin, glucocorticoids, arousal circuits mediated by NE and ACH.
| Additional factors and supporting evidence, not an exhaustive list | |
|---|---|
| Insulin | Roth et al. (1985) J Neurol Sci 71(2–3):339–50 Haroutunian et al (2014) Glia 2014 62(11):1856–77 Chirivella et al. (2017) Stem Cells 35(12):2403–2416 |
| Glucocorticoids | Chetty et al. (2014) Mol Psychiatry 19(12):1275–1283 Hinds et al. (2017) PLoS One 12(4):e0175075 |
| Thyroid hormone | Almazan et al. (1985) Dev Neurosci 7(l):45–54. Lee & Petratos (2016) Mol Neurobiol 53(9):6568–6583 Zhang et al. (2016) Mol Neurobiol 53(7):4406–16 |
| Melatonin | Wen et al. (2016) J Neuroimmune Pharmacol. ll(4):763–773 Ghareghani et al. (2017) Cell Mol Neurobiol. 37(7):1319–1324 |
| BDNF | Miyamoto et al. (2015) J Neurosci 35(41): 14002–8 Peckham et al. (2016) Glia 64(2):255–69 |
| Norepinephrine | Ghiani CAetal. (1999) Development 126(5):1077–90 Ghiani & Gallo (2001) J Neurosci 21(4):1274–82 Marinelli et al. (2016) Front Cell Neurosci. 10:27 |
| Acetylcholine | Cohen et al. (1996) Brain Res Mol Brain Res 43,193–201 Zhou et al. (2004) Cell Biol Int 28:63–67 De Angelis et al. (2012) Dev Neurobiol 72(5):713–28 Imamura et al. (2015) J Neurochem. 135,1086–1098 Marinelli et al. (2016) Front Cell Neurosci. 10:27 Fields et al. (2017) Glia 65(5):687–698 |
| Histamine | Chen et al. (2017) PLoS One 12(12):e0189380 Schwartzbach et al. (2017) J Neurol 264(2):304–315 |
| Glutamate | Gallo et al. (1996) J Neurosci 16(8):2659–70 Gallo & Ghiani (2000) Trends Pharmacol Sci 21(7):252–8 Fannon et al. (2015) Glia 63(6):1021–35 |
| GABA | Yuan et al. (1998) Development 125(15):2901–14 Luyt et al. (2007) J Neurochem 100(3):822–40 Hamilton et al. (2017) Glia 65(2):309–321. |
OL intracellular transcriptional dynamics vary with sleep/wake cycle.
OL adapt to changes in the brain and one of the most prominent changes in the CNS involves the daily sleep/wake cycle. Pioneering work by Cirelli [36,37] and colleagues has been exploring day/night differences in brain gene expression, and most importantly, how it is affected by sleep deprivation [38]. They reported a sleep-associated increase in the transcription of factors involved in OL maturation such as the insulin-like growth factor binding protein 2, as well as of OL genes encoding for myelin components and enzymes (Myelin Oligodendrocyte Basic Protein, Mobp; myelin-associated glycoprotein, Mag; plasmolipin, CD9, 2′:3′-cyclic nucleotide-3′-phosphodiesterase, CNPase). Furthermore, increased levels in the expression of genes involved in fatty acid synthesis and in the synthesis and transport of cholesterol, a major constituent of myelin and other membranes, were observed during sleep. Cirelli’s group continued this line of work by delineating the genome-wide mRNA profile in immature and mature OL as a function of sleep, wake, and acute sleep deprivation [27]. In this work, by specifically targeting mRNAs attached to ribosomes, so that the transcripts were more likely to be translated into proteins, they found that genes implicated OPC differentiation, as well as in apoptosis, cellular stress, and metabolism were upregulated during wake, whilst those involved in OPC proliferation, phospholipids synthesis, and myelination were preferentially transcribed during sleep. Crucially, sleep deprivation disrupted this temporal pattern of expression [38–40] and caused changes to myelin structure in adolescent mice [41]. This evidence advocates for the pivotal role of sleep/wake cycles during windows of rapid OL maturation and intense myelination, which bizarrely coincide with those periods in life when physiologically longer sleep times are natural, i.e. from birth to late adolescence. Given the well-documented restriction and problems with sleep in the present society, especially during vulnerable periods of brain development such as adolescence when myelination is an actively ongoing process, the authors understandably interpreted their work in the context of sleep regulation. However, anatomically distinct neural cell populations control sleep and circadian rhythms but work together to generate rhythms in sleep and rest. The temporal pattern of sleep is regulated by the circadian timing system [42], and these sleep/wake effects can, and should, be considered as the result of circadian regulation.
The Molecular Clock
At a cellular level, circadian rhythms (Fig. 2) are generated by the highly coordinated functional interaction of the core circadian clock genes, such as Circadian Locomotor Output Cycles Kaput (Clock), Brain and Muscle Aryl Hydrocarbon Receptor Nuclear Translocator-Like Protein 1 (Bmal1), Period (Per)1/2/3, and Cryptochrome (Cry) 1/2. This clockwork drives waves of transcription in most cells in the body, including neurons and astrocytes. The negative transcription-translation feedback loop is dependent upon the interaction of two heterodimer complexes: CLOCK/BMAL1 and PER/CRY, with PER2 levels being the limiting factor. The CLOCK/BMAL1 complex works as a transcriptional activator to initiate transcription of Per1/2/3 and Cry1/2 genes, then formed PER/CRY complexes will reach the stoichiometric levels to inhibit the transcriptional activity of CLOCK/BMAL1. The functional interactions of this heterodimer trigger the expression of the Per1/2/3 and Cry1/2 genes with a cycle length of approximately 24 hours. In addition to the core feedback loop, Retinoic Acid Receptor-Related Orphan Receptor (ROR) and Reverse ERB (REV-ERB)α/β activate and suppress Bmal1 transcription, respectively, to augment the 24-hour cycle. Phosphorylation of the negative regulators of the molecular clock (by kinases such as casein kinase I) can target these proteins for proteasomal degradation or increase the rate of nuclear translocation. CLOCK-controlled PAR-domain basic leucine zipper transcription factors DBP, TEF, and HLF are highly expressed in many cell populations with circadian rhythmicity [43,44]. Genome-wide analyses of the clock feedback loop revealed a global circadian control over processes involved in tissue-specific temporal regulation of functionally important pathways, such as transcription, and chromatin modifications and remodelling [43,44], critical players in OL maturation, regeneration and survival [45,46]. Broadly speaking, the targets of circadian clocks are intimately linked to the regulation of cell growth, maturation, metabolism, so, why not also in OL? (Fig. 2).
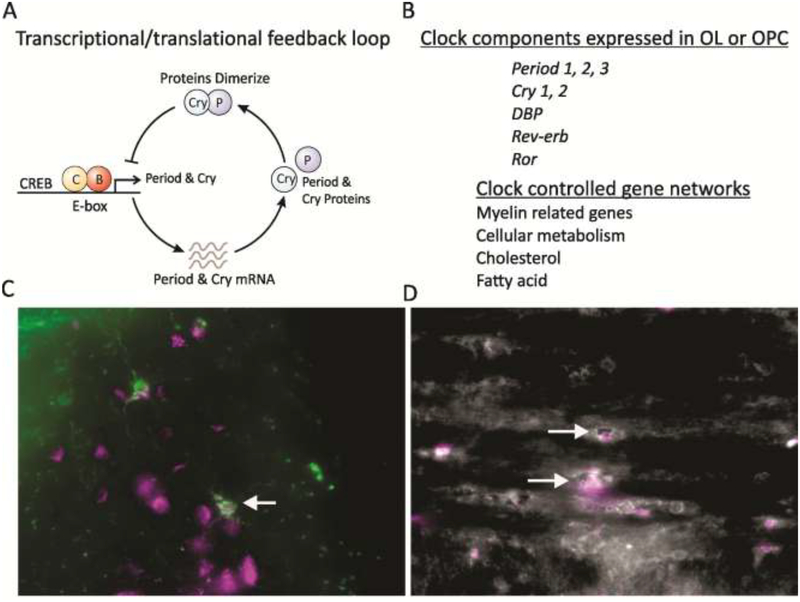
Fig. 2: Circadian timing system is likely to be active in OPC and OL.
A. schematic of the transcriptional/translational negative feedback loop that drives circadian rhythms in gene expression in most cells in our body. At the beginning of the cycle, CLOCK and BMAL1 protein complexes bind a specific promoter region (E-box) to activate the transcription of a family of genes including the Period (Per1/Per2/Per3) and Cryptochrome (Cry1/Cry2) genes. The levels of the transcripts for Per and Cry reach their peak during mid to late day, while the PER and CRY proteins peak in the early night. The PERs, CRYs, and other proteins form complexes in the cytoplasm that translocate back into the nucleus and turn off the transcriptional activity driven by CLOCK–BMAL1 with a delay (due to transcription, translation, dimerization, nuclear entry). The proteins are then degraded by ubiquitation allowing the cycle to begin again. In its simplest form, many cells contain this molecular feedback loop that regulates the rhythmic transcription of a number of genes. Additional feedback loops serve to contribute to the precision and robustness of this core oscillation. B. Microarray analysis indicates that OL express most of the genes that generate circadian oscillations [47]. The temporal profile of clock gene expression in OLs has not been established. A number of gene networks critical to OL function are known to be rhythmic and listed in this figure. C. O4 (left, green) and D. CNPase (right, white) positive OL in the white matter of adult C57bl/6j mice express PER2 (magenta). Arrows highlight OL co-expressing the markers. Mice were perfused at Zeitgeber Time (ZT) 6 and double-immunolabelling for O4 or CNPase and Per2 was performed as previously reported [158,159]. PER2 expression can be also appreciated in other neural cells surrounding the O4 positive OL. It should be noted that cells from different lineages will exhibit the peak of PER2 expression at different phases of the daily rhythm. The O4 hydridoma was a kind gift of Drs. Pfeiffer and Bansal, University of Connecticut [160,161].
Somewhat surprisingly, the presence of such a circadian clock in OL has not been documented as yet. Nevertheless, there are good reasons to assume that such timing system is present in this glial cell type. First, most cell populations contain a cell autonomous molecular clock that gates the transcription of genes important to the function of that cell population, including the other main macroglial cell type, the astrocytes (see below). Second, OL do express most of the genes that generate circadian oscillations as reported by the Barres group, who carried out gene profiling using Affymetrix GeneChip Arrays in fluorescent-activated cell sorted OL from S100β-GFP transgenic mice at postnatal day 1 and 30 [47]. This transcriptome database indicated that both the key positive elements Clock and Bmal1 as well as the negative elements Per1, Per2, Cry1 and Cry2 are expressed in mouse OL, and even some well-known clock-controlled genes such as D-box binding PAR bZIP transcription factor (Dbp). Third, a number of OL-enriched genes, such as platelet-derived growth factor receptor alpha (PDGFαR), myelin oligodendrocyte glycoprotein (Mog), Mag, myelin basic protein (MBP), CNPase, serum- and glucocorticoid-inducible kinase 1 (Sgk1) have been shown to be rhythmically regulated in the CNS (Fig. 3; please see: CircaDB: http://circadb.hogeneschlab.org/about & SCNseq: http://www.wgpembroke.com/shiny/SCNseq/; [48]). For instance, the expression of Sgk1 in rats OL and WM was shown to fluctuate accordingly with the diurnal variations of corticosterone, with a peak in the early night/active phase near the time for the peak of this steroid secretion [49]. Finally, there appear to be daily rhythms in the proliferation of OPCs in the adult hippocampus [50] and in the SVZ [27]. Therefore, while untested, it seems likely that the OPC and OL exhibit cell autonomous circadian rhythms and disrupting the circadian clock would impact OPC and OL, after all “they can count time”. The mechanisms through which the central clock in the hypothalamus would regulate cell autonomous oscillations in OPC/OL have still to be identified. However, it is worth emphasizing that a large number of factors known to display circadian fluctuations, also have a demonstrated role in OL maturation and their function, i.e. myelin biogenesis (Table 1). There is no shortage of candidate signalling molecules that could serve to link the central circadian clock with cell autonomous oscillations in OL and their progenitors.
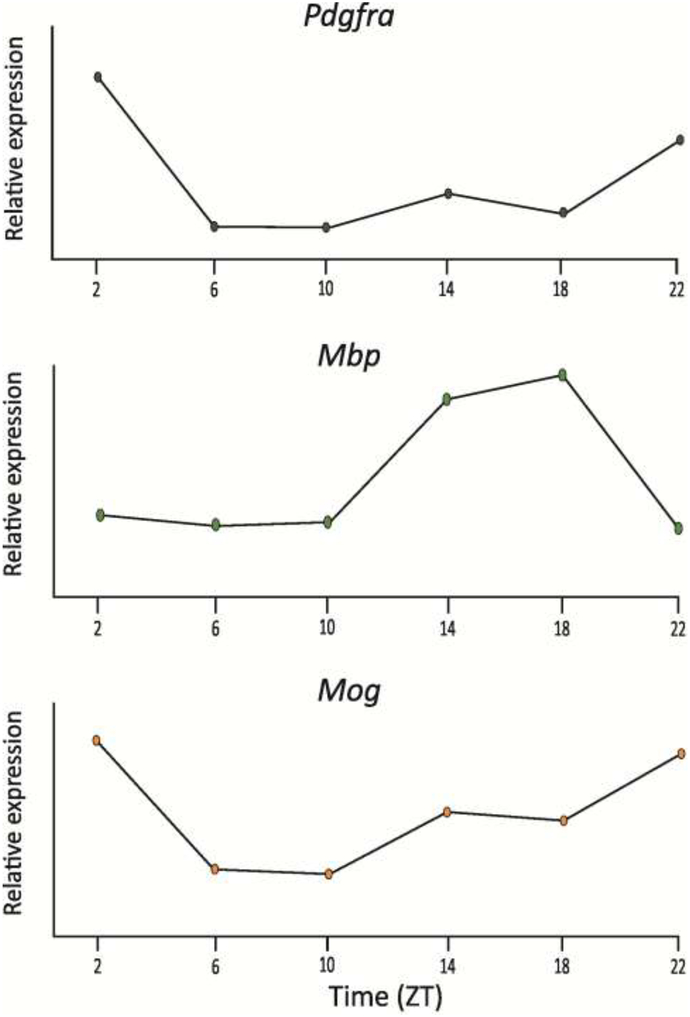
Fig. 3: Transcripts of OL specific genes are rhythmically expressed.
Search of publicly available databases indicates that a number of OL-enriched genes express a daily rhythm. Expression levels were measured by RNA-seq. A. Platelet-derived growth factor receptor alpha (Pdgfra), B. Myelin basic protein (Mbp), and C. Myelin oligodendrocyte glycoprotein (Mog). (SCNseq: http://www.wgpembroke.com/shiny/SCNseq/) [48]. Hitherto, the functions of these transcriptional rhythms are unknown.
Cell-type specific function of the molecular clock in OL, just a gossip?
We can only speculate about the function of the molecular clockwork in OL but, based on work done in other cells types, can expect at least three key intracellular processes to be rhythmically regulated in OL. Since the assembly of the myelin sheet requires high levels of lipid synthesis locally in the CNS as the blood brain barriers would largely prevent lipids originating in the liver from reaching the brain [51], one of the most obvious links would be the temporal control of cholesterol metabolism. The brain is the most cholesterol-rich organ, containing perhaps 20% of the whole body’s contents [52], and cholesterol metabolism is strongly regulated by the circadian system [53]. This rhythmicity is likely to temporally synchronize the consumption of cholesterol during wake to its metabolism and processing into cell membranes.
Next, fatty acid synthesis and β-oxidation are important for myelination and, at least in the liver, are tightly controlled by the circadian system [54]. Alteration of the liver circadian clock disrupts fatty acid biosynthesis. Mitochondrial acetyl CoA is exported to the cytoplasm, where ATP citrate lyase (ACLY) is a rate-limiting enzyme. The circadian peak of ACLY expression coincides with feeding. In addition, the rate of mitochondrial β oxidation is limited by the entry of fatty acyl groups into the mitochondria by carnitine palmitoyl transferase (CPT) 1 and 2. The levels of L-carnitine, CPT1, and CPT2 all show circadian rhythms. Such circadian- and feeding-mediated regulation generates a daily rhythm in fatty acid synthesis and oxidation, which peak during feeding and fasting, respectively.
Last, there is a growing body of data indicating that OL metabolically support the axons [8,55–57] and the circadian system controls the temporal pattern of mitochondrial function [58] (Fig. 4). The energy for the axon, in the form of ATP, would be generated from glucose in the neuronal cell bodies but it is likely that local energy is required to maintain axonal function along its long course. A detailed proteomic map of myelin has been drawn revealing a number of mitochondrial proteins [59], in conformity with early work [60,61], as well as more recent [62], that demonstrated the presence of mitochondria in the cytoplasmic veins of myelin-like membrane in both the peripheral and central NS. Furthermore, functional enzymes for the glycolytic and Kreb’s cycles are expressed in myelin [63]. More recent work examined the impact of a conditional loss of the mitochondrial complex 4 (COX) in OL [64], reporting, among other findings, no signs of demyelination or axonal degeneration, but increased brain lactate concentrations. The authors suggested that lactate originating from the OL is enough to “maintain” the axon under these low energy conditions. This work complements findings [65] that the lactate transporter monocarboxylate transporter 1 (MCT1, Slc16a1) is highly enriched within OL, and disruption of its functions can produce axonal damage and neuronal loss in both cell culture and mouse models. Notably, the Slc16a1 gene exhibits a strong circadian rhythm in the CNS but also in peripheral tissue (liver, heart, lung).
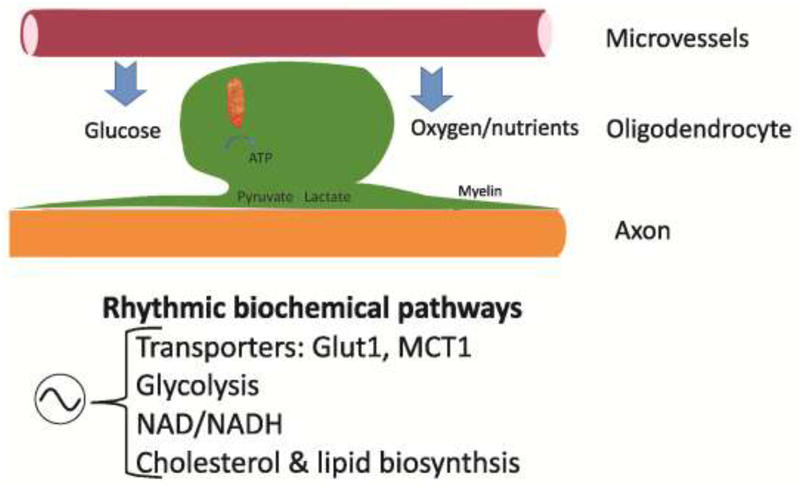
Fig. 4: Circadian clock likely to influence metabolic role of mature OL.
There is a growing body of data indicating that OL metabolically support axons [8,55,57]. The circadian system controls the temporal pattern of mitochondrial function [58] as well as the availability of glucose. Some of the key gene networks known to be regulated by the circadian system include the transporters Glut1 and MCT1, glycolysis, cholesterol as well as lipid biosynthesis, after Saab et al., 2016 [8] & Saab and Nave, 2017 [57].
Undoubtedly, the circadian timing system is intimately linked to metabolism at a cellular, molecular and system level [66]. One of the most dramatic daily rhythms in the body is the feeding/fasting cycle in which an organism has a number of hours with abundant glucose followed by hours without [54]. The circadian system regulates both ingestive behaviours and the metabolic systems by which the food is processed, and as mentioned above, also sleep. One of oldest theories explaining the function of sleep is to reduce activity during a time that it is not energetically advantageous. Thus, the circadian clock coordinates appropriate metabolic responses within peripheral tissues with the light-dark cycle. For example, the liver clock will promote gluconeogenesis and glycogenolysis during the sleep/fasting period, while fostering glycogen and cholesterol synthesis during the wake/feeding period.
To adapt to the daily feeding/fasting cycle, mitochondria are highly dynamic in form and function. Interestingly, recent studies have suggested that a viable circadian clock is required for the generation of new mitochondria and changes in their morphology. Furthermore, diurnal variations in mitochondrial respiration were shown in several organ tissues [58,67]. Electron carriers, also called electron shuttles, are small organic molecules that play key roles in cellular respiration such as nicotinamide adenine dinucleotide (NAD+). The rate-limiting enzyme in NAD+ biosynthesis, nicotinamide phosphoribosyltransferase (NAMPT), and NAD+ levels both exhibit circadian oscillations under the control of the core clock machinery, at least in mice. In particular, they are involved along with Sirtuin (SIRT) 1/CLOCK:BMAL1 in a feedback loop to promote oscillation of the clock gene Per2 [68]. Mice with a perturbed molecular clock displayed compromised mitochondrial rhythmicity and altered cellular respiration [69], which were restored by imposing a scheduled feeding time that coincided with the active phase of the animals [58]. A role for malfunctioning mitochondria and impaired metabolism has also been proposed in neurodegenerative disorders and dys/demyelinating diseases [69,70]. Children with mitochondrial disorders present with abnormal and delayed myelination [71]. Hence, it is possible that genetic and/or environmental disruption of the circadian system can contribute to perturb myelination by compromising the energy supplies, and so a, still to be proven, dysfunctional clock in OL would interfere with the reciprocal axonal-OL/myelin support.
Astrocytes are rhythmic
In contrast to the limited information about circadian rhythms in OL, compelling data indicate that astrocytes possess robust circadian rhythms in gene expression and that these rhythms are functionally significant. Optical reporters have helped to demonstrate that astrocytes exhibit a robust circadian clock and rhythmic gene expression [72], and not surprisingly, clock genes regulate astrocytic glutamate uptake and ATP release [73–75]. The fact that astrocytes can exhibit circadian rhythms in intracellular calcium has been long appreciated [76,77], and stunning new observations suggest that their circadian clock is essential for the rhythms expressed in the neural circuit within the central circadian clock in the suprachiasmatic nucleus (SCN) [78]. These data fit nicely with other work showing that disruption of the molecular clock by removing Bmal1 only in astrocytes altered daily rhythms in behaviour [69,79,80]. Brain-specific Bmal1 deletion weakened the blood-brain barrier by causing loss of pericytes [82], elicited astrogliosis, microglia activation and elevation of inflammatory gene expression mediated in part by suppression of glutathione-S-transferase signalling [69,81]. Functionally, loss of Bmal1 in astrocytes promoted neuronal death in vitro [82].
Implications for “broken” circadian rhythms in OPC and OL: 4 case studies to highlight the potential significance.
As detailed above, OPC/OL are likely to exhibit cell autonomous circadian rhythms and, in this section, we highlight some of the implications for these rhythms across different ages. In each of these cases, we would like to emphasize that an altered circadian clock can cause malfunctions of the immune system as well as metabolism. To date, it has not been possible to disentangle direct effects of circadian disruption on OPC/OL from those mediated by signalling from the neighbouring cell types.
Neonatal units and White Matter Injury
The duration of sleep that people need to be healthy varies with age with infants needing the most sleep, which is also, not surprisingly, the time in development when rapid OL maturation and myelination are occurring. This relationship could just be a coincidence but may also reflect a functional relationship if more sleep allows greater OPC proliferation and myelination as suggested by the work of Cirelli and colleagues [27,41]. This functional link is particularly relevant for neonatal intensive care units (NICU), which traditionally do not consider the importance of the light/dark cycle in the care of their patients. Many NICU keep their “isolettes” in constant light (LL) to facilitate the ability of the staff to monitor the infants. LL is particularly disruptive to the circadian timing system at a behavioural and system level [83], as it literally causes the single cell circadian oscillators to become desynchronised from each other [84]. Therefore, it should be perhaps no surprise that several studies on preterm infants revealed that imposing a rhythm to the NICU lighting conditions exerts beneficial acute effects, e.g. faster weight gain and recovery, shorter hospitalization [85–89]. Some benefits were found to be stronger than others, but all were encouraging. Lighting technologies are rapidly evolving, creating many opportunities for inexpensively improving the illumination of these facilities [90]. As far as we know, the long-term impact of the lighting conditions in the NICU on WM development in childhood and adult development/health has not been explored. However, this is an extremely important point as diffuse WM injury is extremely common in survivor preterm infants (23–32 weeks of gestation) and has burdensome consequence on their cognitive, sensory and behavioural functions. At this time, OL maturation is ongoing in the human brain and the WM is mainly populated by pre-myelinating OL, a stage highly sensitive to oxidative stress and ischemia/hypoxia-induced cell death, whereas OPC and mature OL are more resistant. These brains do present with hypomyelination, as, albeit OPC are present and proliferating post injury, pre-OL fail to progress along the lineage with consequent failure in myelination [4,10,91]. This raises the possibility that the constant light in the NICU could further endanger the, already, aberrant OL maturation by, perhaps, disturbing and desynchronising their internal rhythms. Mechanistically, it is not known if the benefits observed by imposing a light-dark cycle were mediated by sleep and circadian rhythms on OPC development, but this is an important area for future work.
Poor sleep in Adolescents with Intellectual and Developmental Disabilities (IDD)
Adolescence is a crucial window of brain development with actively ongoing myelination along with refinement and pruning of synapses in regions centrally involved in cognitive functions and profound behavioural changes. Environmental stressors at such sensitive period may trigger long lasting changes in brain wiring and the emergence of psychiatric syndromes, which will have a worse outcome in individuals rendered more susceptible by genetic predisposition [4,92,93].
A significant proportion of individual with IDD experiences disturbances in their daily sleep/wake cycles, which become particularly obvious during adolescence. Among the most common complaints are delayed bedtime and frequent nocturnal awakenings [94,95]. Perhaps because of this disrupted temporal pattern of sleep, individuals with IDD are more exposed to light via electronic screens during the night [96,97]. This nocturnal light exposure by itself has been shown to delay sleep in healthy young people [98–100]. These disruptions to sleep and circadian rhythms could also impact OL maturation and function. Several studies have found evidence for abnormalities in white matter connectivity in autism and other IDDs [101,102], although these findings are not universal [103]. Even in typically developing adolescence, there appears to be a relationship between white matter structures and cognitive abilities [104]. Intriguingly, sleep variability in adolescence has been associated with alterations in brain connectivity, reduced academic performance and increased risk-taking behaviour [105–107].
In general, adolescents are, already, particularly vulnerable to disruption of the circadian timing due to social influences [108,109]. The human circadian cycle varies with age and young adults have the longest cycle length. This translates to a natural tendency to stay up late and sleep in for many in this age group. Cross-cultural data suggests a typical mid-point of sleep to be between 5 and 5:30am for young adults [110]. This tendency runs into direct conflict with school start times that frequently befall at 7:30 in the morning. So that, to get to school on-time, many teens are forced to wake up between 5:30–6:00am, or just after the midpoint of their sleep cycle. Of course, additionally demanding loads of homework and extracurricular activities make the sleep problem even worse as does the use of light-emitting devices not only for homework, e.g. entertainment and socialisation. The net result is a population of sleep-deprived teens with altered circadian cycles. Later school start times have been associated with improved sleep and academic performance [111]. Given the links between circadian rhythms, sleep and OL maturation highlighted above, it seems likely that the early school start times would be negatively impacting the myelination in the brain.
Multiple sclerosis (MS)
MS is a demyelinating disease with an age of onset starting in the 20s and lasting through middle age [112]. Sleep disturbances characterised by sleep fragmentation, apnoea, and daytime sleepiness are common in MS patients [113,114]. The majority of these data come from patient surveys providing valuable, although subjective, insights, some of which were corroborated by more quantitative EEG-based, polysomnography analyses [115]. These data also indicated that there is a strong correlation between the individuals with the worse sleep pattern and the severity of their clinical symptoms [116–118]. These findings alone do not specifically implicate circadian dysfunctions. For instance, sleep apnoea is directly related to respiration, since obstructive sleep apnoea and central sleep apnoea (more common in MS) occur as a consequence of disrupted airflow through the throat and miscommunications between the brain and the muscle that control breathing, respectively. Even so, other clinical data support the possible dysregulation of the circadian system in MS patients. In fact, these individuals exhibit malfunctioning of two of the strongest outputs driven by the central circadian clock, the SCN, i.e. the circadian rhythm in the secretion of cortisol [119] and melatonin [120,121]. Together the rhythmic secretion of cortisol (peaks in the morning) and melatonin (peaks in the evening) provides vital temporal cues for the circadian clocks in peripheral organs and tissues, such as liver, heart, adipose tissue. So, the disruption of the hormonal rhythms is likely to have an impact on tissues throughout the body. Furthermore, the severity of MS symptoms, especially central fatigue, appears to vary with the daily cycle [122–125]. Finally, genetic polymorphisms in the circadian clock genes Per3, Bmal1 and Clock have been associated with MS [126,127]. These clinical associations support the use of sleep and circadian measurements as biomarkers for the disease progression [128] but, of course, do not establish causal relationships.
A clear missing piece in this story is the availability of data from animal models of MS to specifically test the involvement of the circadian system. The three most characterized animal models are (1) the experimental autoimmune/allergic encephalomyelitis (EAE), (2) the virally-induced chronic demyelinating disease, known as Theiler׳ s murine encephalomyelitis virus (TMEV) infection, which best mimic the autoimmune and inflammatory components as well as the “clinical manifestations” of MS, and (3) the cuprizone-induced demyelination [129], better suited to investigate myelin injury and repair. All of the models have advantages and disadvantages but, as far as we can tell, no work has been done on possible circadian dysfunction in the TMEV or neurotoxin-evoked models. In the EAE model, one study found a clear disruption in the diurnal (light-dark) rhythms in heart rate, blood pressure, corticosterone and leptin levels, along with abnormal rhythmic expression of PER2 in the liver [130]. A more recent study delineates a pathway through which the circadian timing system may affect EAE pathophysiology [131]. The authors reported diurnal rhythm in the accumulation and activation of various immune cells, which were dependent upon the circadian clock gene Bmal1. This gene is important for the maintenance of anti-inflammatory responses, and its loss in myeloid cells enhanced the inflammatory environment in the CNS through the expansion and infiltration of IL-1β-secreting monocytes. The result was elevated levels of activated T-cells in the absence of Bmal1 or at times of the day when BMAL1 levels are naturally low. This study revealed the importance of the molecular clock in the immune cells but did not explore the possible impact of EAE on the central circadian timing system. A critical test to determine the causal involvement of the circadian system would be to place the organism in constant darkness and determine if the rhythmicity and robustness of the wake/sleep cycles are preserved. This is difficult to do in humans and animal are critical to address this issue as well as exploring the underlying pathogenesis. Future work should examine validated animal models of MS to confirm that the genetic or environmental disruption of the circadian clock impacts myelination/remyelination but also to develop new treatments.
An intriguing example of the interactions between circadian disorders and MS comes from work on the interplay between the PPARγ and the WNT/β-Catenin signalling pathways [132]. PPARγ is a circadian transcription factor [133], know to regulate rhythmic metabolism, including glucose and lipid metabolism, and to have an anti-inflammatory effect by acting on the levels of NF-κB. Dysregulation of the circadian system results in the activation of NF-κB [134,135], which in turn leads to the upregulation of WNT/beta-catenin pathway. Impaired OPC differentiation and failure to remyelinate in MS and EAE are, at least in part, a consequence of overactivation of the WNT/β-Catenin signalling pathway. PPARγ absence aggravates EAE pathophysiology, whilst, its agonists have shown anti-inflammatory and neuroprotective effects, in addition to rendering the environment permissive to remyelination and ameliorating both EAE and MS symptoms [132]. Hence, PPARγ agonists appear a promising treatment to promote remyelination by abolishing the prohibitive effects of the WNT/beta-catenin pathway through regulation of NF-κB activity.
Huntington Disease (HD)
HD is a progressive neurodegenerative disorder caused by a CAG trinucleotide repeat expansion within the Huntingtin gene, and a typical middle age onset inversely correlated with the length of the repeats [136]. Recent evidence suggests that myelin loss and circadian dysregulation may be centrally involved in HD. The hallmark pathology in HD is loss of neurons in the striatum with consequent decline of motor functions. However, cognitive impairments along with altered sleep/wake cycles manifest much earlier in pre-symptomatic stages. Prior work has firmly established loss of white matter in HD patients [137–140]. Myelin deficits have, as well, been reported in mouse models of HD [141–143] along with altered levels of cholesterol in the striatum [144], gangliosides in the corpus callosum [145] and altered transcription of myelin-related genes [146]. In addition, sleep disorders are extremely common in HD patients and have detrimental effects on the daily functioning and quality of life of patients and their caregivers [147,148]. One of the first signs of the disease in HD patients is a phase delay in the nightly rise in melatonin [149] and, by the end of life, the central circadian clock (SCN) shows evidence of degeneration [150]. Mouse models of HD also exhibit a progressive and rapid breakdown of the circadian rest/activity cycle that closely mimics the condition observed in human patients. Phenotype includes loss of consolidated sleep, increased wakeful activity during the rest phase, and more sleep during the active phase [147,151–153]. Collectively this prior research supports the hypothesis that circadian dysfunction is an integral component of HD pathophysiology and could be contributing to the deficits in white matter. Recently, we have shown that some of the behavioural, physiological, and transcriptional deficits in HD animal models were improved by ‘re-aligning’ the circadian timing of these mice by imposing a daily feeding/fasting cycle [154,155]. We are presently determining whether restoration of the circadian rhythms would delay the loss of axonal and myelin integrity observed in these models and perhaps similar environmental manipulations could become regular practice in the preventive treatment of HD and similar neurodegenerative disorders.
Conclusions
In conclusion, although not proven, the findings presented and discussed in this review are consistent with the assumption that OL and their progenitors contain their own cell-autonomous circadian clock. The function of this clock would be to control the temporal pattern of gene expression of transcripts important for OL maturation and myelination during windows of rapid brain development plus additional critical functions in the adult CNS. The circadian clock is intimately tied to cellular metabolism and there is increasing evidence that the OL metabolically support the axons that they insulate. In the liver, the circadian system strongly regulates cholesterol and lipid metabolism: two biochemical processes also important in OL. These cellular clocks are normally synchronized by a neural circuit centred in the SCN. The SCN circuit synchronizes the rest of the CNS through control of centrally active hormones including glucocorticoids and melatonin as well as the driving of neural activity and secretion in arousal centres in the locus coeruleus (NE), Raphe nucleus (5HT) and basal ganglia cholinergic cell populations (ACh). Many of these hormones and neurotransmitters have been shown to alter OPC proliferation, migration and lineage progression, and now Cirelli and colleagues [27,41] have extensively documented the impact of sleep on these cells. Finally, many individuals in the present society exhibit disrupted sleep/wake cycles, including patients with IDD or neurological/neurodegenerative disorders, raising the possibility of underlying alterations of the circadian timing system in the aetiology of these disorders.
Contributor Information
Christopher S. Colwell, Email: cghiani@mednet.ucla.edu.
Cristina A. Ghiani, Email: ccolwell@mednet.ucla.edu.
References
- 1.Bercury KK, Macklin WB (2015) Dynamics and mechanisms of CNS myelination. Dev Cell 32(4):447–58. 10.1016/j.devcel.2015.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughes EG, Appel B (2016) The cell biology of CNS myelination. Curr Opin Neurobiol 39:93–100. 10.1016/j.conb.2016.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mount CW, Monje M. (2017) Wrapped to Adapt: Experience-Dependent Myelination. Neuron 95(4):743–756. 10.1016/j.neuron.2017.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forbes TA, Gallo V. (2017) All Wrapped Up: Environmental Effects on Myelination. Trends Neurosci 40(9):572–587 10.1016/j.tins.2017.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filley CM, Fields RD (2016) White matter and cognition: making the connection. J Neurophysiol 116(5):2093–2104. 10.1152/jn.00221.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porter A, Leckie R, Verstynen T (2018) White matter pathways as both a target and mediator of health behaviors. Ann N Y Acad Sci. 1428(1):71–88. 10.1111/nyas.13708 [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Olson IR (2018) The Original Social Network: White Matter and Social Cognition. Trends Cogn Sci 22(6):504–516. 10.1016/j.tics.2018.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saab AS, Nave KA (2017) Myelin dynamics: protecting and shaping neuronal functions. Curr Opin Neurobiol 47:104–112. 10.1016/j.conb.2017.09.013 [DOI] [PubMed] [Google Scholar]
- 9.Bergles DE, Richardson WD (2015) Oligodendrocyte Development and Plasticity Cold Spring Harb Perspect Biol 8(2):a020453 http://cshperspectives.cshlp.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Tilborg E, de Theije CGM, van Hal M, Wagenaar N, de Vries LS, Benders MJ, Rowitch DH, Nijboer CH (2018) Origin and dynamics of oligodendrocytes in the developing brain: Implications for perinatal white matter injury. Glia 66(2):221–238. 10.1002/glia.23256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sauvageot CM, Stiles CD (2002) Molecular mechanisms controlling cortical gliogenesis. Curr Opin Neurobiol 12(3):244–9. 10.1016/S0959-4388(02)00322-7 [DOI] [PubMed] [Google Scholar]
- 12.Zuchero JB, Barres BA (2013) Intrinsic and extrinsic control of oligodendrocyte development. Curr Opin Neurobiol 914–20. 10.1016/j.conb.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayoral SR, Chan JR (2016) The environment rules: spatiotemporal regulation of oligodendrocyte differentiation. Curr Opin Neurobiol 39:47–52. 10.1016/j.conb.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitew S, Hay CM, Peckham H, Xiao J, Koenning M, Emery B (2014) Mechanisms regulating the development of oligodendrocytes and central nervous system myelin. Neuroscience 276:29–47. 10.1016/j.neuroscience.2013.11.029 [DOI] [PubMed] [Google Scholar]
- 15.Almeida RG, Lyons DA (2017) On Myelinated Axon Plasticity and Neuronal Circuit Formation and Function. J Neurosci 37(42):10023–10034. 10.1523/JNEUROSCI.3185-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verkhratsky A, Steinhäuser C (2000) Ion channels in glial cells. Brain Res Brain Res Rev 32(2–3):380–412. 10.1016/S0165-0173(99)00093-4 [DOI] [PubMed] [Google Scholar]
- 17.Butt AM, Fern RF, Matute C (2014) Neurotransmitter signaling in white matter. Glia 62(11):1762–79. 10.1002/glia.22674 [DOI] [PubMed] [Google Scholar]
- 18.Ragheb F, Molina-Holgado E, Cui QL, Khorchid A, Liu HN, Larocca JN, Almazan G (2001) Pharmacological and functional characterization of muscarinic receptor subtypes in developing oligodendrocytes. J Neurochem. 77(5):1396–406. 10.1046/j.1471-4159.2001.00356.x [DOI] [PubMed] [Google Scholar]
- 19.De Angelis F, Bernardo A, Magnaghi V, Minghetti L, Tata AM (2012) Muscarinic receptor subtypes as potential targets to modulate oligodendrocyte progenitor survival, proliferation, and differentiation. Dev Neurobiol 72(5):713–28. 10.1002/dneu.20976 [DOI] [PubMed] [Google Scholar]
- 20.Ghiani CA, Eisen AM, Yuan X, DePinho RA, McBain CJ, Gallo V (1999) Neurotransmitter receptor activation triggers p27(Kip1) and p21(CIP1) accumulation and G1 cell cycle arrest in oligodendrocyte progenitors. Development 126(5):1077–90. [DOI] [PubMed] [Google Scholar]
- 21.Ghiani CA, Gallo V (2001) Inhibition of cyclin E-cyclin-dependent kinase 2 complex formation and activity is associated with cell cycle arrest and withdrawal in oligodendrocyte progenitor cells. J Neurosci 21(4):1274–82. 10.1523/JNEUROSCI.21-04-01274.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barres B, Raff M (1994) Control of oligodendrocyte number in the developing rat optic nerve. Neuron, 12:935–942. [DOI] [PubMed] [Google Scholar]
- 23.Gao F-B, Durand B, Raff M (1997) Oligodendrocyte precursor cells count time but not cell divisions before differentiation. Current Biology 7:152–155. [DOI] [PubMed] [Google Scholar]
- 24.Casaccia-Bonnefil P, Hardy RJ, Teng KK, Levine JM, Koff A, Chao MV (1999) Loss of p27Kip1 function results in increased proliferative capacity of oligodendrocyte progenitors but unaltered timing of differentiation. Development 126(18):4027–37. [DOI] [PubMed] [Google Scholar]
- 25.Draijer S, Chaves I, Hoekman MFM (2018) The circadian clock in adult neural stem cell maintenance. Prog Neurobiol pii: S0301–0082(18)30020–0. 10.1016/j.pneurobio.2018.05.007 [DOI] [PubMed] [Google Scholar]
- 26.Gaucher J, Montellier E, Sassone-Corsi P (2018) Molecular Cogs: Interplay between Circadian Clock and Cell Cycle. Trends Cell Biol 28(5):368–379. 10.1016/j.tcb.2018.01.006 [DOI] [PubMed] [Google Scholar]
- 27.Bellesi M, Pfister-Genskow M, Maret S, Keles S, Tononi G, Cirelli C (2013) Effects of Sleep and Wake on Oligodendrocytes and Their Precursors. J Neurosci 33:14288–14300. 10.1523/JNEUROSCI.5102-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallo V, Zhou JM, McBain CJ, Wright P, Knutson PL, Armstrong RC (1996) Oligodendrocyte progenitor cell proliferation and lineage progression are regulated by glutamate receptor-mediated K+ channel block. J Neurosci 16(8):2659–70. 10.1523/JNEUROSCI.16-08-02659.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallo V, Ghiani CA (2000) Glutamate receptors in glia: new cells, new inputs and new functions. Trends Pharmacol Sci 21(7):252–8. 10.1016/S0165-6147(00)01494-2 [DOI] [PubMed] [Google Scholar]
- 30.Fannon J, Tarmier W, Fulton D (2015) Neuronal activity and AMPA-type glutamate receptor activation regulates the morphological development of oligodendrocyte precursor cells. Glia 63(6):1021–35. 10.1002/glia.22799 [DOI] [PubMed] [Google Scholar]
- 31.Gudz TI, Komuro H, Macklin WB (2006) Glutamate stimulates oligodendrocyte progenitor migration mediated via an alphav integrin/myelin proteolipid protein complex. J Neurosci 26(9):2458–66. 10.1523/JNEUROSCI.4054-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harlow DE, Saul KE, Komuro H, Macklin WB (2015) Myelin Proteolipid Protein Complexes with αv Integrin and AMPA Receptors In Vivo and Regulates AMPA-Dependent Oligodendrocyte Progenitor Cell Migration through the Modulation of Cell-Surface GluR2 Expression. J Neurosci 35(34):12018–32. 10.1523/JNEUROSCI.5151-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan X, Eisen AM, McBain CJ, Gallo V (1998) A role for glutamate and its receptors in the regulation of oligodendrocyte development in cerebellar tissue slices. Development 125(15):2901–14. [DOI] [PubMed] [Google Scholar]
- 34.Luyt K, Slade TP, Dorward JJ, Durant CF, Wu Y, Shigemoto R, Mundell SJ, Váradi A, Molnár E (2007) Developing oligodendrocytes express functional GABA(B) receptors that stimulate cell proliferation and migration. J Neurochem 100(3):822–40. 10.1111/j.1471-4159.2006.04255.x [DOI] [PubMed] [Google Scholar]
- 35.Hamilton NB, Clarke LE, Arancibia-Carcamo IL, Kougioumtzidou E, Matthey M, Káradóttir R, Whiteley L, Bergersen LH, Richardson WD, Attwell D (2017) Endogenous GABA controls oligodendrocyte lineage cell number, myelination, and CNS internode length. Glia. 65(2):309–321. 10.1002/glia.23093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cirelli C, Tononi G. (2000) Gene expression in the brain across the sleep-waking cycle. Brain Res 885:303–321. 10.1016/S0006-8993(00)03008-0 [DOI] [PubMed] [Google Scholar]
- 37.Cirelli C, Gutierrez CM, Tononi G. (2004) Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron 41:35–43. 10.1016/S0896-6273(03)00814-6 [DOI] [PubMed] [Google Scholar]
- 38.Cirelli C, Faraguna U, Tononi G. (2006) Changes in brain gene expression after long-term sleep deprivation. J. Neurochem 98:1632–1645. 10.1111/j.1471-4159.2006.04058.x [DOI] [PubMed] [Google Scholar]
- 39.Terao A, Wisor JP, Peyron C, Apte-Deshpande A, Wurts SW, Edgar DM, Kilduff TS (2006) Gene expression in the rat brain during sleep deprivation and recovery sleep: an Affymetrix GeneChip® study. Neuroscience 137:593–605. 10.1016/j.neuroscience.2005.08.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vecsey CG, Peixoto L, Choi JHK, Wimmer M, Jaganath D, Hernandez PJ, Blackwell J, Meda K, Park AJ, Hannenhalli S, Abel T (2012) Genomic analysis of sleep deprivation reveals translational regulation in the hippocampus. Physiol Genomics 44:981–991. 10.1152/physiolgenomics.00084.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bellesi M, Haswell JD, de Vivo L, Marshall W, Roseboom PH, Tononi G, Cirelli C (2018) Myelin modifications after chronic sleep loss in adolescent mice. Sleep January;41(5). 10.1093/sleep/zsy034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blum ID, Bell B, Wu MN (2018) Time for Bed: Genetic Mechanisms Mediating the Circadian Regulation of Sleep. Trends Genet 34(5):379–388. 10.1016/j.tig.2018.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takahashi JS (2017) Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. 2017 Mar;18(3):164–179. 10.1038/nrg.2016.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB (2014) A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A 111:16219–16224. 10.1073/pnas.1408886111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hernandez M, Casaccia P (2015) Interplay between transcriptional control and chromatin regulation in the oligodendrocyte lineage. Glia. 63(8):1357–75. 10.1002/glia.22818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gregath A, Lu QR (2018) Epigenetic modifications-insight into oligodendrocyte lineage progression, regeneration, and disease. FEBS Lett 592(7):1063–1078. 10.1002/1873-3468.12999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA (2008) A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28:264–278. 10.1523/JNEUROSCI.4178-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pembroke WG, Babbs A, Davies KE, Ponting CP, Oliver PL (2015) Temporal transcriptomics suggest that twin-peaking genes reset the clock. Elife 4 pii: e10518. 10.7554/eLife.10518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hinds LR, Chun LE, Woodruff ER, Christensen JA, Hartsock MJ, Spencer RL (2017) Dynamic glucocorticoid-dependent regulation of Sgk1 expression in oligodendrocytes of adult male rat brain by acute stress and time of day. PLoS One 12(4):e0175075 10.1371/journal.pone.0175075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsumoto Y, Tsunekawa Y, Nomura T, Suto F, Matsumata M, Tsuchiya S, Osumi N Differential proliferation rhythm of neural progenitor and oligodendrocyte precursor cells in the young adult hippocampus. PLoS One. 2011;6(11):e27628 10.1371/journal.pone.0027628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chrast R, Saher G, Nave KA, Verheijen MH (2011) Lipid metabolism in myelinating glial cells: lessons from human inherited disorders and mouse models. J Lipid Res 52(3):419–34. http://www.jlr.org/content/early/2010/11/09/jlr.R009761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Björkhem I, Meaney S, Fogelman AM (2004) Brain cholesterol: long secret life behind a barrier. Arterioscler Thromb Vasc Biol 24:806–815. 10.1161/01.ATV.0000120374.59826.1b [DOI] [PubMed] [Google Scholar]
- 53.Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, Ellisman MH, Panda S (2012) Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab 15(6):848–60. 10.1016/j.cmet.2012.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Panda S (2016) Circadian physiology of metabolism. Science 354(6315):1008–1015. 10.1126/science.aah4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morrison BM, Lee Y, Rothstein JD (2013) Oligodendroglia: metabolic supporters of axons. Trends Cell Biol 23(12):644–51. 10.1016/j.tcb.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yin X, Kidd GJ, Ohno N, Perkins GA, Ellisman MH, Bastian C, Brunet S, Baltan S, Trapp BD. Proteolipid protein-deficient myelin promotes axonal mitochondrial dysfunction via altered metabolic coupling. J Cell Biol 2016. 215(4):531–542. 10.1083/jcb.201607099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saab AS, Tzvetavona ID, Trevisiol A, Baltan S, Dibaj P, Kusch K, Möbius W, Goetze B, Jahn HM, Huang W, Steffens H, Schomburg ED, Pérez-Samartín A, Pérez-Cerdá F, Bakhtiari D, Matute C, Löwel S, Griesinger C, Hirrlinger J, Kirchhoff F, Nave KA (2016) Oligodendroglial NMDA Receptors Regulate Glucose Import and Axonal Energy Metabolism. Neuron 91(1):119–32. 10.1016/j.neuron.2016.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neufeld-Cohen A, Robles MS, Aviram R, Manella G, Adamovich Y, Ladeuix B, Nir D, Rousso-Noori L, Kuperman Y, Golik M, Mann M, Asher G (2016) Circadian control of oscillations in mitochondrial rate-limiting enzymes and nutrient utilization by PERIOD proteins. Proc Natl Acad Sci USA 113:E1673–E1682. 10.1073/pnas.1519650113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taylor CM, Marta CB, Claycomb RJ, Han DK, Rasband MN, Coetzee T, Pfeiffer SE (2004) Proteomic mapping provides powerful insights into functional myelin biology. Proc Natl Acad Sci USA 101:4643–4648. 10.1073/pnas.0400922101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mugnaini E, Osen KK, Schnapp B,Friedrich VL Jr (1977) Distribution of Schwann cell cytoplasm and plasmalemmal vesicles (caveolae) in peripheral myelin sheaths. An electron microscopic study with thin sections and freeze-fracturing. J Neurocytol 6, 647–668. [DOI] [PubMed] [Google Scholar]
- 61.Bhat S, Pfeiffer SE (1985) Subcellular distribution and developmental expression of cholesterol ester hydrolases in fetal rat brain cultures. J Neurochem 45: 1356–1362. 10.1111/j.1471-4159.1985.tb07200.x [DOI] [PubMed] [Google Scholar]
- 62.Rinholm JE, Vervaeke K, Tadross MR, Tkachuk AN, Kopek BG, Brown TA, Bergersen LH, Clayton DA (2016) Movement and structure of mitochondria in oligodendrocytes and their myelin sheaths. Glia 64(5):810–25. 10.1002/glia.22965 [DOI] [PubMed] [Google Scholar]
- 63.Ravera S, Bartolucci M, Calzia D, Aluigi MG, Ramoino P, Morelli A, Panfoli I (2013) Tricarboxylic acid cycle-sustained oxidative phosphorylation in isolated myelin vesicles. Biochimie 95:1991–1998. 10.1016/j.biochi.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 64.Fünfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Möbius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave KA (2012) Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485:517–521. 10.1038/nature11007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, Pellerin L, Magistretti PJ, Rothstein JD (2012) Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487(7408):443–8. 10.1038/nature11314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bass J, Takahashi JS 2010. Circadian integration of metabolism and energetics. Science 330(6009):1349–54. 10.1126/science.1195027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Goede P, Wefers J, Brombacher EC, Schrauwen P, Kalsbeek A (2018) Circadian rhythms in mitochondrial respiration. J Mol Endocrinol 60(3):R115–R130 10.1530/JME-17-0196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, Takahashi JS, Imai S, Bass J (2009) Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 324(5927):651–4. 10.1126/science.1171641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Musiek ES, Lim MM, Yang G, Bauer AQ, Qi L, Lee Y, Roh JH, Ortiz-Gonzalez X, Dearborn JT, Culver JP, Herzog ED, Hogenesch JB, Wozniak DF, Dikranian K, Giasson BI, Weaver DR, Holtzman DM, Fitzgerald GA (2013) Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest 123(12):5389–400. 10.1172/JCI70317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ravera S, Panfoli I (2015) Role of myelin sheath energy metabolism in neurodegenerative diseases. Neural Regen Res 10(10):1570–1. http://www.nrronline.org/text.asp?2015/10/10/1570/167749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Valanne L, Ketonen L, Majander A, Suomalainen A, Pihko H (1998) Neuroradiologic findings in children with mitochondrial disorders. AJNR Am J Neuroradiol 19(2):369–77. [PMC free article] [PubMed] [Google Scholar]
- 72.Prolo LM, Takahashi JS, Herzog ED (2005) Circadian rhythm generation and entrainment in astrocytes. J. Neurosci 2005; 25: 404–408 10.1523/JNEUROSCI.4133-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beaulé C, Swanstrom A, Leone MJ, Herzog ED (2009) Circadian modulation of gene expression, but not glutamate uptake, in mouse and rat cortical astrocytes. PLoS ONE. 2009; 4: e7476 10.1371/journal.pone.0007476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Womac AD, Burkeen JF, Neuendorff N, Earnest DJ, Zoran MJ (2009) Circadian rhythms of extracellular ATP accumulation in suprachiasmatic nucleus cells and cultured astrocytes. Eur J Neurosci 30(5):869–76. 10.1111/j.1460-9568.2009.06874.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marpegan L, Swanstrom AE, Chung K, Simon T, Haydon PG, Khan SK, Liu AC, Herzog ED, Beaulé C (2011) Circadian regulation of ATP release in astrocytes. J Neurosci 31(23):8342–50. 10.1523/JNEUROSCI.6537-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van den Pol AN, Finkbeiner SM, Cornell-Bell AH (1992) Calcium excitability and oscillations in suprachiasmatic nucleus neurons and glia in vitro. J Neurosci 12(7):2648–64. 10.1523/JNEUROSCI.12-07-02648.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burkeen JF, Womac AD, Earnest DJ, Zoran MJ (2011) Mitochondrial calcium signaling mediates rhythmic extracellular ATP accumulation in suprachiasmatic nucleus astrocytes. J Neurosci 31(23):8432–40. 10.1523/JNEUROSCI.6576-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brancaccio M, Patton AP, Chesham JE, Maywood ES, Hastings MH (2017) Astrocytes Control Circadian Timekeeping in the Suprachiasmatic Nucleus via Glutamatergic Signaling. Neuron. 2017 March 22;93(6):1420–1435.e5. 10.1016/j.neuron.2017.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barca-Mayo O, Pons-Espinal M, Follert P, Armirotti A, Berdondini L, De Pietri Tonelli D (2017) Astrocyte dletion of Bmal1 alters daily locomotor activity and cognitive functions via GABA signalling. Nat Commun 8:14336 10.1038/ncomms14336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tso CF, Simon T, Greenlaw AC, Puri T, Mieda M, Herzog ED. Astrocytes Regulate Daily Rhythms in the Suprachiasmatic Nucleus and Behavior. Curr Biol. 2017. April 3;27(7):1055–1061. 10.1016/j.cub.2017.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nakazato R, Kawabe K, Yamada D, Ikeno S, Mieda M, Shimba S, Hinoi E, Yoneda Y, Takarada T (2017) Disruption of Bmal1 impairs blood-brain barrier integrity via pericyte dysfunction. J. Neurosci 2017; 37: 10052–10062. 10.1523/JNEUROSCI.3639-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lananna BV, Nadarajah CJ, Izumo M, Cedeño MR, Xiong DD, Dimitry J, Tso CF, McKee CA, Griffin P, Sheehan PW, Haspel JA, Barres BA, Liddelow SA, Takahashi JS, Karatsoreos IN, Musiek ES (2018) Cell-Autonomous Regulation of Astrocyte Activation by the Circadian Clock Protein BMAL1. Cell Rep. 2018 25(1):1–9.e5. 10.1016/j.celrep.2018.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lucassen EA, Coomans CP, van Putten M, de Kreij SR, van Genugten JH, Sutorius RP, de Rooij KE, van der Velde M, Verhoeve SL, Smit JW, Löwik CW, Smits HH, Guigas B, Aartsma-Rus AM, Meijer JH. (2016) Environmental 24-hr Cycles Are Essential for Health. Curr Biol 26(14):1843–53. 10.1016/j.cub.2016.05.038 [DOI] [PubMed] [Google Scholar]
- 84.Ohta H, Mitchell AC, McMahon DG (2006) Constant light disrupts the developing mouse biological clock. Pediatr Res. 2006 September;60(3):304–8. Epub 2006 Jul 20. 10.1203/01.pdr.0000233114.18403.66 [DOI] [PubMed] [Google Scholar]
- 85.Rivkees SA, Mayes L, Jacobs H, Gross I (2004) Rest-activity patterns of premature infants are regulated by cycled lighting. Pediatrics 113:833–9. 10.1542/peds.113.4.833 [DOI] [PubMed] [Google Scholar]
- 86.Guyer C, Huber R, Fontijn J, Bucher HU, Nicolai H, Werner H, Molinari L, Latal B, Jenni OG (2012) Cycled light exposure reduces fussing and crying in very preterm infants. Pediatrics 130:e145–51. 10.1542/peds.2011-2671 [DOI] [PubMed] [Google Scholar]
- 87.Vásquez-Ruiz S, Maya-Barrios JA, Torres-Narváez P, Vega-Martínez BR, Rojas-Granados A, Escobar C, Angeles-Castellanos M (2014) A light/dark cycle in the NICU accelerates body weight gain and shortens time to discharge in preterm infants. Early Hum Dev 90:535–40. 10.1016/j.earlhumdev.2014.04.015 [DOI] [PubMed] [Google Scholar]
- 88.Morag I, Ohlsson A (2016) Cycled light in the intensive care unit for preterm and low birth weight infants. Cochrane Database Syst Rev 8:CD006982 https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD006982.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brandon DH, Silva SG, Park J, Malcolm W, Kamhawy H, Holditch-Davis D (2017) Timing for the Introduction of Cycled Light for Extremely Preterm Infants: A Randomized Controlled Trial. Res Nurs Health 40(4):294–310. doi: 10.1002/nur.21797. https://dx.doi.org/10.1002%2Fnur.21797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rea MS, Figueiro MG (2016) The NICU Lighted Environment. Newborn Infant Nurs Rev 16(4):195–202. https://dx.doi.org/10.1053%2Fj.nainr.2016.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Back SA (2017) White matter injury in the preterm infant: pathology and mechanisms. Acta Neuropathol 134:331–349. 10.1007/s00401-017-1718-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paus T, Keshavan M, Giedd JN (2008) Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci 9(12):947–57. 10.1038/nrn2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Galván A (2017) Adolescence, brain maturation and mental health. Nat Neurosci 20(4):503–504. 10.1038/nn.4530 [DOI] [PubMed] [Google Scholar]
- 94.Mazurek MO, Sohl K (2016) Sleep and Behavioral Problems in Children with Autism Spectrum Disorder. J Autism Dev Disord 46: 1906–1915. 10.1007/s10803-016-2723-7 [DOI] [PubMed] [Google Scholar]
- 95.Robinson-Shelton A, Malow BA (2016) Sleep Disturbances in Neurodevelopmental Disorders. Curr Psychiatry Rep 18:6 10.1007/s11920-015-0638-1 [DOI] [PubMed] [Google Scholar]
- 96.Engelhardt CR, Mazurek MO, Sohl K (2013) Media use and sleep among boys with autism spectrum disorder, ADHD, or typical development. Pediatrics 132: 1081–1089. 10.1542/peds.2013-2066 [DOI] [PubMed] [Google Scholar]
- 97.Mazurek MO, Engelhardt CR, Hilgard J, Sohl K (2016) Bedtime Electronic Media Use and Sleep in Children with Autism Spectrum Disorder. J Dev Behav Pediatr 37(7):525–31. 10.1097/DBP.0000000000000314 [DOI] [PubMed] [Google Scholar]
- 98.Wood B, Rea MS, Plitnick B, Figueiro MG (2013) Light level and duration of exposure determine the impact of self-luminous tablets on melatonin suppression. Appl Ergon 44: 237–240. 10.1016/j.apergo.2012.07.008 [DOI] [PubMed] [Google Scholar]
- 99.Chang AM, Aeschbach D, Duffy JF, Czeisler CA (2015) Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci USA 112:1232–1237. 10.1073/pnas.1418490112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gronli J, Byrkjedal IK, Bjorvatn B, Nodtvedt O, Hamre B, Pallesen S (2016) Reading from an iPad or from a book in bed: the impact on human sleep. A randomized controlled crossover trial. Sleep Med 21: 86–92. 10.1016/j.sleep.2016.02.006 [DOI] [PubMed] [Google Scholar]
- 101.Kleinhans NM, Pauley G, Richards T, Neuhaus E, Martin N, Corrigan NM, Shaw DW, Estes A, Dager SR (2012) Age-related abnormalities in white matter microstructure in autism spectrum disorders. Brain Res 1479:1–16. 10.1016/j.brainres.2012.07.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ameis SH, Catani M (2015) Altered white matter connectivity as a neural substrate for social impairment in Autism Spectrum Disorder Cortex 62:158–81 10.1016/j.cortex.2014.10.014 [DOI] [PubMed] [Google Scholar]
- 103.Maricich SM, Azizi P, Jones JY, Morriss MC, Hunter JV, Smith EO, Miller G (2007) Myelination as assessed by conventional MR imaging is normal in young children with idiopathic developmental delay. AJNR Am J Neuroradiol 28(8):1602 10.3174/ajnr.A0602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Samara A, Feng K, Pivik RT, Jarratt KP, Badger TM, Ou X (2018) White Matter Microstructure Correlates with Memory Performance in Healthy Children: A Diffusion Tensor Imaging Study. J Neuroimaging. November 6 10.1111/jon.12580 [DOI] [PubMed] [Google Scholar]
- 105.Telzer EH, Fuligni AJ, Lieberman MD, Galván A (2013) The effects of poor quality sleep on brain function and risk taking in adolescence. Neuroimage 71:275–83. 10.1016/j.neuroimage.2013.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Telzer EH, Goldenberg D, Fuligni AJ, Lieberman MD, Gálvan A (2015) Sleep variability in adolescence is associated with altered brain development. Dev Cogn Neurosci 14:16–22. 10.1016/j.dcn.2015.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tashjian SM, Goldenberg D, Galván A (2017) Neural connectivity moderates the association between sleep and impulsivity in adolescents. Dev Cogn Neurosci 27:35–44. doi: 10.1016/j.dcn.2017.07.006. 10.1016/j.dcn.2017.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wittmann M, Dinich J, Merrow M, Roenneberg T (2006) Social jetlag: misalignment of biological and social time. Chronobiol Int 23(1–2):497–509. 10.1080/07420520500545979 [DOI] [PubMed] [Google Scholar]
- 109.Touitou Y, Touitou D, Reinberg A (2016) Disruption of adolescents’ circadian clock: The vicious circle of media use, exposure to light at night, sleep loss and risk behaviors. J Physiol Paris 110(4 Pt B):467–479. 10.1016/j.jphysparis.2017.05.001 [DOI] [PubMed] [Google Scholar]
- 110.Roenneberg T, Merrow M (2016) The Circadian Clock and Human Health. Curr Biol 26: R432–443. 10.1016/j.cub.2016.04.011 [DOI] [PubMed] [Google Scholar]
- 111.Dunster GP, de la Iglesia L, Ben-Hamo M, Nave C, Fleischer JG, Panda S, de la Iglesia HO (2018) Sleepmore in Seattle: Later school start times are associated with more sleep and better performance in high school students. Sci Adv 2018 December 12;4(12):eaau6200 https://dx.doi.org/10.1126%2Fsciadv.aau6200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Scalfari A, Lederer C, Daumer M, Nicholas R, Ebers GC, Muraro PA. (2016) The relationship of age with the clinical phenotype in multiple sclerosis. Mult Scler. 22(13):1750–1758. https://doi.org/10.1177%2F1352458516630396 [DOI] [PubMed] [Google Scholar]
- 113.Caminero A, Bartolomé M (2011) Sleep disturbances in multiple sclerosis. J Neurol Sci 309(1–2):86–91. 10.1016/j.jns.2011.07.015 [DOI] [PubMed] [Google Scholar]
- 114.Lunde HM, Bjorvatn B, Myhr KM, Bø L (2013) Clinical assessment and management of sleep disorders in multiple sclerosis: a literature review. Acta Neurol Scand Suppl (196):24–30. 10.1111/ane.12046 [DOI] [PubMed] [Google Scholar]
- 115.Chinnadurai SA, Gandhirajan D, Pamidimukala V, Kesavamurthy B, Venkatesan SA (2018) Analysing the relationship between polysomnographic measures of sleep with measures of physical and cognitive fatigue in people with multiple sclerosis. Mult Scler Relat Disord 24:32–37. 10.1016/j.msard.2018.05.016 [DOI] [PubMed] [Google Scholar]
- 116.Nociti V, Losavio FA, Gnoni V, Losurdo A, Testani E, Vollono C, Frisullo G, Brunetti V, Mirabella M, Della Marca G. (2017) Sleep and fatigue in multiple sclerosis: A questionnaire-based, cross-sectional, cohort study. J Neurol Sci. 372:387–392. 10.1016/j.jns.2016.10.040 [DOI] [PubMed] [Google Scholar]
- 117.Braley TJ, Kratz AL, Kaplish N, Chervin RD (2016) Sleep and Cognitive Function in Multiple Sclerosis. Sleep 39(8):1525–33. 10.5665/sleep.6012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vitkova M, Gdovinova Z, Rosenberger J, Szilasiova J, Mikula P, Stewart RE, Groothoff JW, van Dijk JP (2018) Is Poor Sleep Quality Associated With Greater Disability in Patients With Multiple Sclerosis? Behav Sleep Med 16(2):106–116. 10.1080/15402002.2016.1173555 [DOI] [PubMed] [Google Scholar]
- 119.Kern S, Krause I, Horntrich A, Thomas K, Aderhold J, Ziemssen T 2013. Cortisol awakening response is linked to disease course and progression in multiple sclerosis. PLoS One 8(4):e60647 10.1371/journal.pone.0060647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Akpinar Z, Tokgöz S, Gökbel H, Okudan N, Uğuz F, Yilmaz G (2008) The association of nocturnal serum melatonin levels with major depression in patients with acute multiple sclerosis. Psychiatry Res 161(2):253–7. 10.1016/j.psychres.2007.11.022 [DOI] [PubMed] [Google Scholar]
- 121.Damasceno A, Moraes AS, Farias A, Damasceno BP, dos Santos LM, Cendes F (2015) Disruption of melatonin circadian rhythm production is related to multiple sclerosis severity: A preliminary study. J Neurol Sci 353(1–2):166–8. 10.1016/j.jns.2015.03.040 [DOI] [PubMed] [Google Scholar]
- 122.Melamud L, Golan D, Luboshitzky R, Lavi I, Miller A (2012) Melatonin dysregulation, sleep disturbances and fatigue in multiple sclerosis. J Neurol Sci 314(1–2):37–40. 10.1016/j.jns.2011.11.003 [DOI] [PubMed] [Google Scholar]
- 123.Streckis V, Skurvydas A, Mamkus G (2014) Effect of the time of day on central and peripheral fatigue during 2-min maximal voluntary contractions in persons with multiple sclerosis: gender differences. J Electromyogr Kinesiol 24(5):601–6. 10.1016/j.jelekin.2014.06.001 [DOI] [PubMed] [Google Scholar]
- 124.Kratz AL, Murphy SL, Braley TJ (2017) Ecological Momentary Assessment of Pain, Fatigue, Depressive, and Cognitive Symptoms Reveals Significant Daily Variability in Multiple Sclerosis. Arch Phys Med Rehabil 98(11):2142–2150. 10.1016/j.apmr.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wens I, Hansen D (2017) Muscle Strength, But Not Muscle Oxidative Capacity, Varies Between the Morning and the Afternoon in Patients with Multiple Sclerosis: A Pilot Study. Am J Phys Med Rehabil 96(11):828–830. 10.1097/PHM.0000000000000703 [DOI] [PubMed] [Google Scholar]
- 126.Golalipour M, Maleki Z, Farazmandfar T, Shahbazi M (2017) PER3 VNTR polymorphism in Multiple Sclerosis: A new insight to impact of sleep disturbances in MS. Mult Scler Relat Disord 17:84–86. 10.1016/j.msard.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 127.Lavtar P, Rudolf G, Maver A, Hodžić A, Starčević Čizmarević N, Živković M, Šega Jazbec S, Klemenc Ketiš Z, Kapović M, Dinčić E, Raičević R, Sepčić J, Lovrečić L, Stanković A, Ristić S, Peterlin B (2018) Association of circadian rhythm genes ARNTL/BMAL1 and CLOCK with multiple sclerosis. PLoS One 13(1):e0190601 https://dx.doi.org/10.1371%2Fjournal.pone.0190601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wipfler P, Heikkinen A, Harrer A, Pilz G, Kunz A, Golaszewski SM, Reuss R, Oschmann P, Kraus J (2013) Circadian rhythmicity of inflammatory serum parameters: a neglected issue in the search of biomarkers in multiple sclerosis. J Neurol 260(1):221–7. 10.1007/s00415-012-6622-3 [DOI] [PubMed] [Google Scholar]
- 129.Procaccini C, De Rosa V, Pucino V, Formisano L, Matarese G (2015) Animal models of Multiple Sclerosis. Eur J Pharmacol 759:182–91. 10.1016/j.ejphar.2015.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Buenafe AC (2012) Diurnal rhythms are altered in a mouse model of multiple sclerosis. J Neuroimmunol. 243(1–2):12–7. 10.1016/j.jneuroim.2011.12.002 [DOI] [PubMed] [Google Scholar]
- 131.Sutton CE, Finlay CM, Raverdeau M, Early JO, DeCourcey J, Zaslona Z, O’Neill LAJ, Mills KHG, Curtis AM (2017) Loss of the molecular clock in myeloid cells exacerbates T cell-mediated CNS autoimmune disease. Nat Commun 8(1):1923 10.1038/s41467-017-02111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vallée A, Lecarpentier Y, Guillevin R, Vallée JN (2018) Demyelination in Multiple Sclerosis: Reprogramming Energy Metabolism and Potential PPARγ Agonist Treatment Approaches. Int J Mol Sci 19(4) pii: E1212. 10.3390/ijms19041212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Eckel-Mahan KL, Patel VR, de Mateo S, Orozco-Solis R, Ceglia NJ, Sahar S, Dilag-Penilla SA, Dyar KA, Baldi P, Sassone-Corsi P. Reprogramming of the circadian clock by nutritional challenge. Cell 155(7):1464–78. 10.1016/j.cell.2013.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, and Verma IM (2012) Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci USA 109 (31) 12662–12667. 10.1073/pnas.1209965109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Spengler ML, Kuropatwinski KK, Comas M, Gasparian AV Fedtsova N, Gleiberman AS, Gitlin II, Artemicheva NM, Deluca KA, Gudkov AV, and Antoch MP (2012) Core circadian protein CLOCK is a positive regulator of NF-κB–mediated transcription. Proc Natl Acad Sci USA 2012 109(37): E2457–E2465. 10.1073/pnas.1206274109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bates GP, Dorsey R, Gusella JF, Hayden MR, Kay C, Leavitt BR, Nance M, Ross CA, Scahill RI, Wetzel R, Wild EJ, Tabrizi SJ (2015) Huntington disease. Nat Rev Dis Primers 1:15005. doi: 10.1038/nrdp.2015.5. 10.1038/nrdp.2015.5 [DOI] [PubMed] [Google Scholar]
- 137.Bartzokis G, Lu PH, Tishler TA, Fong SM, Oluwadara B, Finn JP, Huang D, Bordelon Y, Mintz J, Perlman S (2007) Myelin breakdown and iron changes in Huntington’s disease: pathogenesis and treatment implications. Neurochem Res 32(10):1655–64. 10.1007/s11064-007-9352-7 [DOI] [PubMed] [Google Scholar]
- 138.Poudel GR, Stout JC, Domínguez DJF, Churchyard A, Chua P, Egan GF, Georgiou-Karistianis N (2015) Longitudinal change in white matter microstructure in Huntington’s disease: The IMAGE-HD study. Neurobiol Dis 74:406–12. 10.1016/j.nbd.2014.12.009 [DOI] [PubMed] [Google Scholar]
- 139.Faria AV, Ratnanather JT, Tward DJ, Lee DS, van den Noort F, Wu D, Brown T, Johnson H, Paulsen JS, Ross CA, Younes L, Miller MI; PREDICT-HD Investigators and Coordinators of the Huntington Study Group (2016) Linking white matter and deep gray matter alterations in premanifest Huntington disease. Neuroimage Clin 11:450–460. 10.1016/j.nicl.2016.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bourbon-Teles J, Bells S, Jones DK, Coulthard E, Rosser A, Metzler-Baddeley C (2017) Myelin breakdown in human Huntington’s disease: Multi-modal evidence from diffusion MRI and quantitative magnetization transfer. Neuroscience pii: S0306–4522(17)30376–7. 10.1016/j.neuroscience.2017.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xiang Z, Valenza M, Cui L, Leoni V, Jeong HK, Brilli E, Zhang J, Peng Q, Duan W, Reeves SA, Cattaneo E, Krainc D (2011) Peroxisome-proliferator-activated receptor gamma coactivator 1 α contributes to dysmyelination in experimental models of Huntington’s disease. J Neurosci 31(26):9544–53. 10.1523/JNEUROSCI.1291-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jin J, Peng Q, Hou Z, Jiang M, Wang X, Langseth AJ, Tao M, Barker PB, Mori S, Bergles DE, Ross CA, Detloff PJ, Zhang J, Duan W (2015) Early white matter abnormalities, progressive brain pathology and motor deficits in a novel knock-in mouse model of Huntington’s disease. Hum Mol Genet 24(9):2508–27. 10.1093/hmg/ddv016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Teo RT, Hong X, Yu-Taeger L, Huang Y, Tan LJ, Xie Y, To XV, Guo L, Rajendran R, Novati A, Calaminus C, Riess O, Hayden MR, Nguyen HP, Chuang KH, Pouladi MA (2016) Structural and molecular myelination deficits occur prior to neuronal loss in the YAC128 and BACHD models of Huntington disease. Hum Mol Genet 25(13):2621–2632. 10.1093/hmg/ddw122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shankaran M, Di Paolo E, Leoni V, Caccia C, Ferrari Bardile C, Mohammed H, Di Donato S, Kwak S, Marchionini D, Turner S, Cattaneo E, Valenza M (2017) Early and brain region-specific decrease of de novo cholesterol biosynthesis in Huntington’s disease: A cross-validation study in Q175 knock-in mice. Neurobiol Dis 98:66–76. 10.1016/j.nbd.2016.11.013 [DOI] [PubMed] [Google Scholar]
- 145.Di Pardo A, Amico E, Maglione V (2016) Impaired Levels of Gangliosides in the Corpus Callosum of Huntington Disease Animal Models. Front Neurosci 10:457 https://dx.doi.org/10.3389%2Ffnins.2016.00457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Huang B, Wei W, Wang G, Gaertig MA, Feng Y, Wang W, Li XJ, Li S (2015) Mutant huntingtin downregulates myelin regulatory factor-mediated myelin gene expression and affects mature oligodendrocytes. Neuron 85(6):1212–26. 10.1016/j.neuron.2015.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Morton AJ, Wood NI, Hastings MH, Hurelbrink C, Barker RA, Maywood ES (2005) Disintegration of the sleep-wake cycle and circadian timing in Huntington’s disease. J Neurosci 25(1):157–63. 10.1523/JNEUROSCI.3842-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Goodman AO, Rogers L, Pilsworth S, McAllister CJ, Shneerson JM, Morton AJ, Barker RA (2011) Asymptomatic sleep abnormalities are a common early feature in patients with Huntington’s disease. Curr Neurol Neurosci Rep 11(2):211–7. 10.1007/s11910-010-0163-x [DOI] [PubMed] [Google Scholar]
- 149.Kalliolia E, Silajdžić E, Nambron R, Hill NR, Doshi A, Frost C, Watt H, Hindmarsh P, Björkqvist M, Warner TT (2014) Plasma melatonin is reduced in Huntington’s disease. Mov Disord 29(12):1511–5. 10.1002/mds.26003 [DOI] [PubMed] [Google Scholar]
- 150.van Wamelen DJ, Aziz NA, Roos RA, Swaab DF (2014) Hypothalamic alterations in Huntington’s disease patients: comparison with genetic rodent models. J Neuroendocrinol 26(11):761–75. 10.1111/jne.12190 [DOI] [PubMed] [Google Scholar]
- 151.Kudo T, Schroeder A, Loh DH, Kuljis D, Jordan MC, Roos KP, Colwell CS (2011) Dysfunctions in circadian behavior and physiology in mouse models of Huntington’s disease. Exp Neurol 228(1):80–90. 10.1016/j.expneurol.2010.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Loh DH, Kudo T, Truong D, Wu Y, Colwell CS (2013) The Q175 mouse model of Huntington’s disease shows gene dosage- and age-related decline in circadian rhythms of activity and sleep. PLoS One 10.1371/journal.pone.006999330;8(7):e69993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kuljis DA, Gad L, Loh DH, MacDowell Kaswan Z, Hitchcock ON, Ghiani CA, Colwell CS (2016) Sex Differences in Circadian Dysfunction in the BACHD Mouse Model of Huntington’s Disease. PLoS One 11(2):e0147583 10.1371/journal.pone.0147583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang HB, Loh DH, Whittaker DS, Cutler T, Howland D, Colwell CS (2018) Time-Restricted Feeding Improves Circadian Dysfunction as well as Motor Symptoms in the Q175 Mouse Model of Huntington’s Disease. eNeuro 5(1). pii: ENEURO.0431–17.2017. https://dx.doi.org/10.1523%2FENEURO.0431-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Whittaker DS, Loh DH, Wang HB, Tahara Y, Kuljis D, Cutler T, Ghiani CA, Shibata S, Block GD, Colwell CS (2018) Circadian-based Treatment Strategy Effective in the BACHD Mouse Model of Huntington’s Disease. J Biol Rhythms 33(5):535–554. https://doi.org/10.1177%2F0748730418790401 [DOI] [PubMed] [Google Scholar]
- 156.Weger M, Diotel N, Dorsemans AC, Dickmeis T, Weger BD (2017) Stem cells and the circadian clock. Dev Biol 431(2):111–123. 10.1016/j.ydbio.2017.09.012 [DOI] [PubMed] [Google Scholar]
- 157.Traiffort E, Zakaria M, Laouarem Y, Ferent J (2016). Hedgehog: A Key Signaling in the Development of the Oligodendrocyte Lineage. J Develop Biol. 4(3). pii: E28. 10.3390/jdb4030028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nobuta H, Ghiani CA, Paez PM, Spreuer V, Dong H, Korsak RA, Manukyan A, Li J, Vinters HV, Huang EJ, Rowitch DH, Sofroniew MV, Campagnoni AT, de Vellis J, Waschek JA (2012) STAT3-mediated astrogliosis protects myelin development in neonatal brain injury. Ann Neurol 72(5):750–65. 10.1002/ana.23670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lee FY, Wang HB, Hitchcock ON, Loh DH, Whittaker DS, Kim YS, Aiken A, Kokikian C, Dell’Angelica EC, Colwell CS, Ghiani CA (2018) Sleep/Wake Disruption in a Mouse Model of BLOC-1 Deficiency. Front Neurosci. 12:759 10.3389/fnins.2018.00759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sommer I, Schachner M (1981) Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev Biol 83:311–327. 10.1016/0012-1606(81)90477-2 [DOI] [PubMed] [Google Scholar]
- 161.Bansal R, Warrington AE, Gard AL, Ranscht B, Pfeiffer SE (1989) Multiple and novel specificities of monoclonal antibodies O1, O4, and R-mAb used in the analysis of oligodendrocyte development. J Neurosci Res 24:548–557. 10.1002/jnr.490240413. [DOI] [PubMed] [Google Scholar]