Abstract
Purpose
An older female predominance has been reported among chronic cough patients in Western countries, which is considered to be associated with a higher cough sensitivity in females. However, the characteristics of Chinese chronic cough patients remain unclear. This study aimed to explore the age and sex distribution as well as their relationship with cough reflex sensitivity to capsaicin in Chinese chronic cough patients.
Methods
We analyzed the demographic features of 1,882 consecutive chronic cough patients who attended our cough clinic in Guangzhou, China. Cough sensitivity to capsaicin, which was defined as the lowest concentration of capsaicin causing 5 coughs or more (C5), was measured in 539 of the 1,882 patients and 68 healthy volunteers.
Results
The mean age of the patients was 43.0 ± 13.7 years and patients aged <50 years accounted for more than two-thirds of the study population. Around 87% of the patients were never-smokers. The proportion of females (51.5%) was almost equal to that of males (48.5%). The pattern of the age and sex distribution was consistently reflected within most common causes of chronic cough, while a female predominance was shown in patients with cough-variant asthma and patients aged ≥50 years. Female patients had higher cough sensitivity to capsaicin than male patients (log C5: 1.58 ± 0.84 vs. 2.04 ± 0.84 μmol/L, P = 0.001), and patients aged ≥50 years had higher cough sensitivity to capsaicin than patients aged <50 years.
Conclusions
In China, patients with chronic cough have a roughly equal sex distribution and a middle-aged predominance, irrespective of a higher cough sensitivity to capsaicin in females and older patients.
Trial Registration
ClinicalTrials.gov Identifier: NCT02591550
Keywords: Age distribution, capsaicin, cough, sex distribution, TRPV1 receptor
INTRODUCTION
Chronic cough, defined as a cough lasting for more than 8 weeks, is a common presentation seen in general practice and respiratory specialist clinics. A recent international survey reported that the prevalence of chronic cough is 9.6% in the general population.1 Clinic-based studies have indicated that cough-variant asthma (CVA), upper airway cough syndrome (UACS), eosinophilic bronchitis (EB) and gastro-esophageal reflux related cough (GERC) are common causes of chronic cough.2,3,4 Patients with chronic cough suffer from a marked decrement in quality of life,5 which is comparable to those with lung cancer and end-stage chronic obstructive pulmonary disease (COPD).6,7 An older female predominance among patients with chronic cough has been reported by individual cough clinics.8,9 Recently, a worldwide survey of chronic cough confirmed that two-thirds of patients were female, and that the most common age for presentation was 60-69 years, with this profile being uniform across several Western countries.10 This female predominance was thought to be associated with higher cough sensitivity in females compared to males,10,11,12 and enhanced cough sensitivity to capsaicin in post-menopausal women compared to pre-menopausal women as well.13 In our practice in a cough clinic in Guangzhou, China, we have noticed neither an obvious sex difference nor a predominance of patients aged over 60 years.2,14 Because there has been no study on the age and sex distribution among patients with chronic cough in China, we investigated the demographic profiles of patients with chronic cough, along with their relationship with cough sensitivity to capsaicin in our clinic.
MATERIALS AND METHODS
Subjects
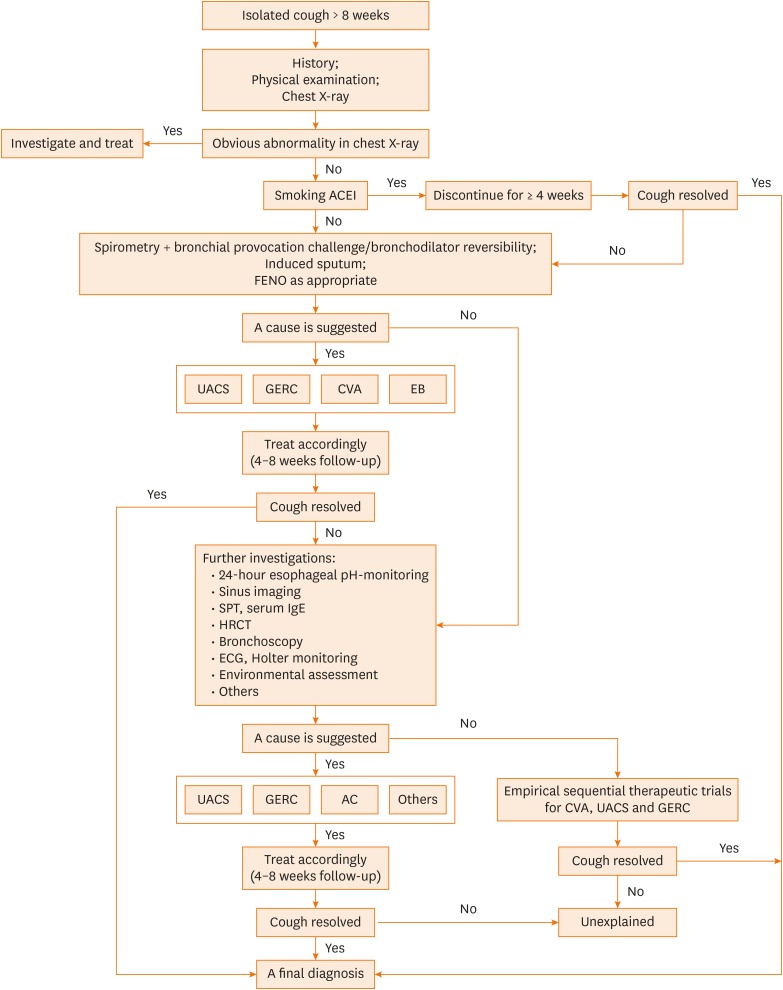
We retrospectively analyzed the demographic features of consecutive patients who attended our specialist chronic cough clinic with thorough investigations and etiological diagnosis from January 2003 to December 2017. Patients were managed using a uniform diagnostic algorithm (Fig. 1).15,16 The final diagnosis was determined when the cough was resolved. If the cough remained uncontrolled even after extensive investigation and treatment, it was considered unexplained chronic cough (UC). Details of diagnostic criteria and management are depicted in Appendix 1.
Fig. 1. Algorithm for the management of patients with chronic cough.
ACEI, angiotensin-converting enzyme inhibitor; FENO, fractional exhaled nitric oxide; UACS, upper airway cough syndrome; GERC, gastro-esophageal reflux related cough; CVA, cough-variant asthma; EB, eosinophilic bronchitis; SPT, skin prick test; IgE, immunoglobulin E; HRCT, high-resolution computed tomography; ECG, electrocardiogram; AC, atopic cough.
Capsaicin cough challenge
To examine the relationship between demographic features and cough sensitivity, cough sensitivity to capsaicin was reviewed in both patients with chronic cough and healthy volunteers. Data were obtained from cough challenge experiments conducted under ethics approvals granted by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (IRB No. 201518) and registered at ClinicalTrials.gov (NCT02591550). Inclusion criteria for participants were: age ≥18 years; had a ratio of forced expiratory volume in 1 second to forced vital capacity ≥0.7; without significant radiological abnormality as well as without active respiratory infection or malignancy. Healthy volunteers were lifetime non-smokers and patients with chronic cough should have a cough duration of >8 weeks. As previously described,17 capsaicin cough challenge test was undertaken with a compressed air-driven nebulizer controlled by a breath activated dosimeter (output, 0.025 mL per inhalation). Subjects were instructed to exhale to functional residual capacity and then take a single slow inhalation through a mouthpiece for 1 second. Increasing doubling concentrations (range, 1.95-1,000 μmol/L) of capsaicin solutions (Sigma-Aldrich, St. Louis, MO, USA) were administered with 3 placebo inhalations of 0.9% saline solution randomly interspersed. Coughs were counted by the cough challenge administrator in the first 30 seconds after inhalation and there was a 1-minute interval between individual inhalations. The challenge was terminated once the capsaicin induced 5 coughs and the lowest concentration of capsaicin causing 5 coughs or more (C5) was recorded as cough reflex sensitivity threshold.
Statistical analysis
Statistical analysis was performed using SPSS 22.0 (SPSS Inc., Chicago, IL, USA). The C5 values were logarithmically transformed (to base 10) for analysis. For comparisons of 2 groups, parametrically distributed data were analyzed using Student's t test and non-parametric data were analyzed using the Mann-Whitney test. For comparisons of more than 2 groups, parametrically distributed data were analyzed using 1-way independent analysis of variance. The χ2 test was used for categorical data. A P value of <0.05 was considered statistically significant.
RESULTS
Overall age and sex distribution of the patients
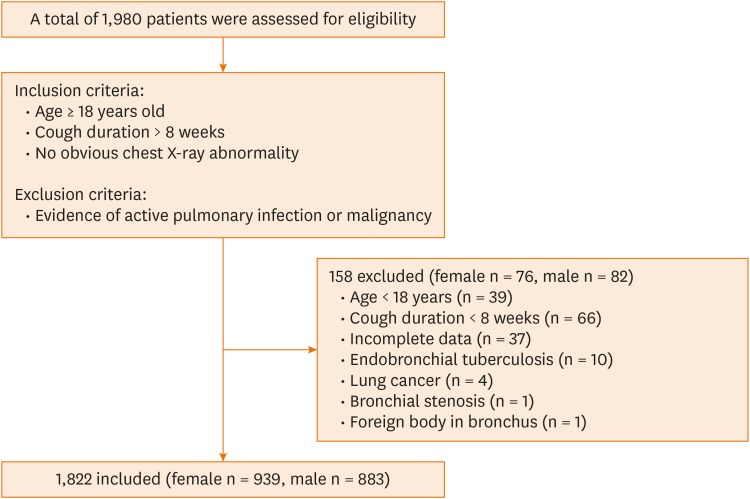
A total of 1,980 patients were screened and 158 (76 females) patients were excluded (Fig. 2). Of the 1,822 patients with chronic cough, the number of females (939, 51.5%) was roughly equal to that of males (883, 48.5%). The mean age of all patients was 43.0 ± 13.7 years. Males were significantly younger than females (40.8 ± 13.2 vs. 45.0 ± 13.8 years, P = 0.001). Around 75% of patients (73.7% females vs. 75.7% males, P = 0.360) had sought medical advice in primary healthcare institutions or secondary hospitals before visiting our cough clinic (Table 1).
Fig. 2. Summary of screening procedures.
Table 1. Clinical characteristics of patients with chronic cough.
| Characteristics | All | Female | Male | P value | |
|---|---|---|---|---|---|
| No. | 1,822 (100) | 939 (51.5) | 883 (48.5) | - | |
| Age (yr) | 43.0 ± 13.7 | 45.0 ± 13.8 | 40.8 ± 13.2 | 0.001 | |
| Cough duration (mon) | 24.0 (8.0–84.0) | 24.0 (6.0–72.0) | 36.0 (12.0–96.0) | 0.001 | |
| FEV1 % pred | 95.6 ± 17.0 | 95.8 ± 17.7 | 95.1 ± 15.6 | 0.433 | |
| FEV1/FVC | 81.2 ± 9.7 | 81.3 ± 9.7 | 81.1 ± 9.7 | 0.706 | |
| Sputum neutrophils (%) | 60.3 (37.5–84.5) | 65.0 (36.9–86.1) | 66.0 (38.0–84.0) | 0.951 | |
| Sputum eosinophils (%) | 1.0 (0.0–6.0) | 1.0 (0.0–5.5) | 1.5 (0.0–6.5) | 0.112 | |
| FENO (ppb) | 18.0 (11.0–31.5) | 15.0 (10.0–32.0) | 19.0 (13.0–31.0) | 0.001 | |
| Daytime cough scores | 3.0 (2.0–3.0) | 3.0 (2.0–4.0) | 3.0 (2.0–3.0) | 0.828 | |
| Night-time cough scores | 1.0 (1.0–2.0) | 2.0 (1.0–2.0) | 1.0 (1.0–2.0) | 0.001 | |
| Cough VAS (mm) | 60.0 (50.0–70.0) | 60.0 (50.0–80.0) | 60.0 (50.0–70.0) | 0.008 | |
| Primary care or secondary hospitals visit | 1,360 (74.6) | 692 (73.7) | 668 (75.7) | 0.360 | |
| Occupational or environmental exposure | 311 (17.1) | 143 (15.2) | 168 (19.0) | 0.031 | |
| Laryngeal paresthesia | 1,450 (79.6) | 769 (81.9) | 681 (77.1) | 0.020 | |
| Cough triggers | |||||
| Dust | 682 (37.4) | 381 (40.6) | 301 (34.1) | 0.006 | |
| Cigarette smoke | 778 (42.7) | 441 (47.0) | 337 (38.2) | 0.001 | |
| Cooking smell | 916 (50.3) | 508 (54.1) | 408 (46.2) | 0.001 | |
| Odours | 1,038 (57.0) | 561 (59.7) | 477 (54.0) | 0.020 | |
| Cold air | 920 (50.5) | 493 (52.5) | 427 (48.4) | 0.099 | |
| Talking | 527 (28.9) | 305 (32.5) | 222 (25.1) | 0.001 | |
Data are shown as mean ± standard deviation, median (interquartile range) or number (%). Comparisons were conducted between females and males.
FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; VAS, visual analogue scale; FENO, fractional exhaled nitric oxide; Occupational or environmental exposure, exposure to triggers which were known to be harmful to human health in workplace or at home environment; Laryngeal paresthesia, an abnormal sensation or a tickle in the throat when not exposed to a cough trigger.
To validate the representativeness of our data, the age and sex distribution was compared to a nationwide multicenter study which focused on causes of chronic cough in China.2 The present study had a similar sex distribution as the nationwide study (females: 51.5% vs. 55.3%, P = 0.1), and exhibited a significantly higher age than the nationwide study (43.0 ± 13.7 vs. 40.4 ± 12.8 years, P < 0.001).
Age and sex distribution of patients in different age groups
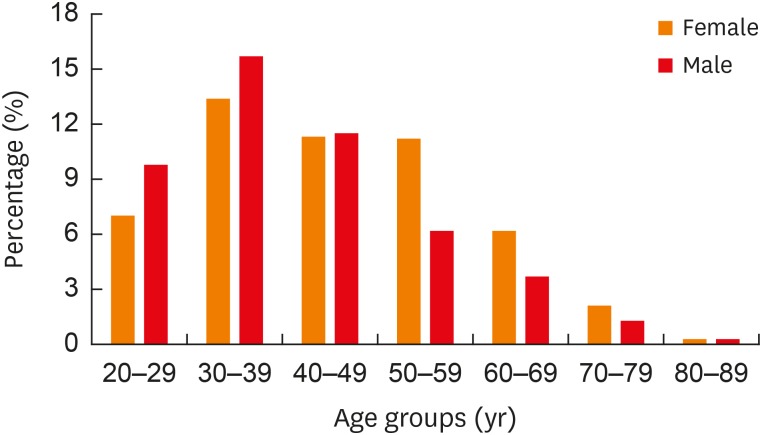
Subdividing patients into 7 groups from the second to the eighth decade, for both females and males, patients aged 30-39 years were most frequently seen, which accounted for 26.0% of females and 32.4% of males. Within the age range of 20-49 years, the number of males (675, 37.1%) was slightly larger than that of females (578, 31.7%). Conversely, within the age range of 50-89 years, the number of females (361, 19.8%) was 1.7-fold higher than that of males (208, 11.4%) (Fig. 3).
Fig. 3. Age and sex distribution of the whole study population. Percentage (%): ratios of the number of females or males to the total number of cough patients (n = 1,822).
There was a 50% reduction in the number of male patients from the age range of 50-59 years upwards, while the number of female patients fell by 50% or more from the later age range of 60-69 years.
Age and sex distribution of different causes
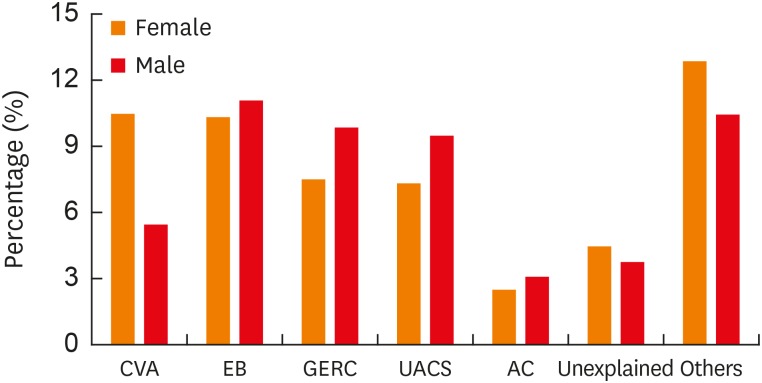
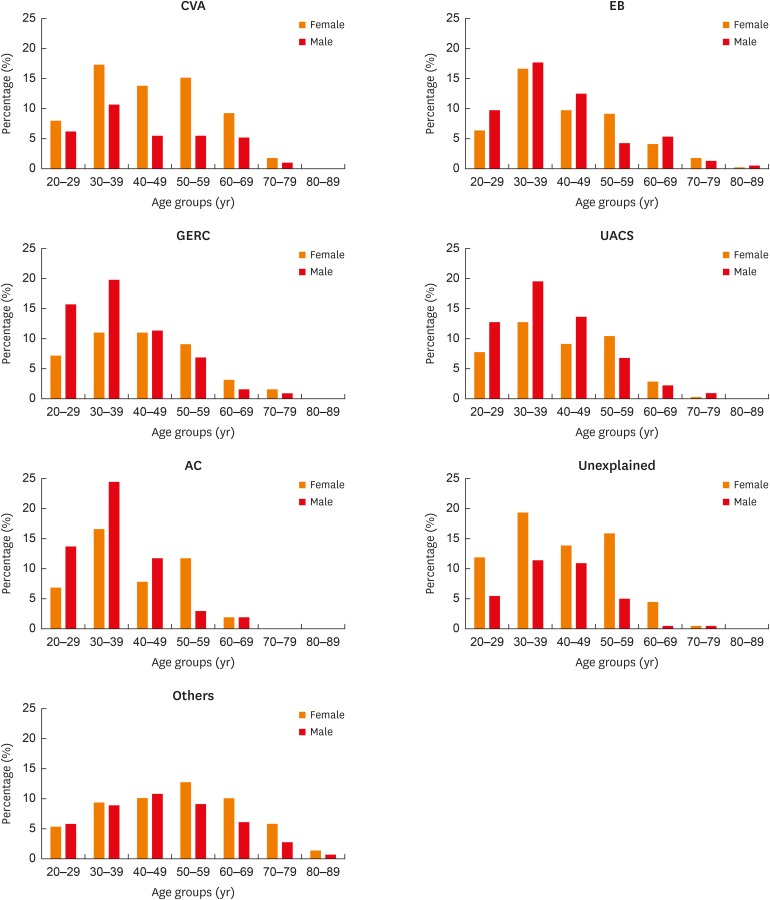
Of the 1,822 patients, dual causes were identified in 128 patients and triple causes were identified in 11 patients. CVA (288, 15.8%), EB (389, 21.4%), GERC (321, 17.6%) and UACS (313, 17.2%) were the most common causes of chronic cough, followed by atopic cough (AC) (102, 5.6%) (Supplementary Table S1). A slightly higher proportion of males was found in most of the common causes of chronic cough except for CVA, which displayed a female preponderance (Fig. 4).
Fig. 4. Sex distribution of different causes of chronic cough. Percentage (%): ratios of the number of females or males to the total cough patients (n = 1,822).
CVA, cough-variant asthma; EB, eosinophilic bronchitis; GERC, gastro-esophageal reflux related cough; UACS, upper airway cough syndrome; AC, atopic cough.
The mean age of the patients was largely uniform among CVA (44.4 ± 13.7 years), EB (41.9 ± 13.6 years), GERC (40.0 ± 13.0 years), UACS (40.0 ± 12.2 years), AC (39.1± 10.9 years) and UC (42.3 ± 12.3 years), while other causes displayed a relatively older age (48.9 ± 15.2 years) (Fig. 5).
Fig. 5. Age distribution of different causes of chronic cough. Percentage (%): ratios of the number of females or males to a specific cause.
CVA, cough-variant asthma; EB, eosinophilic bronchitis; GERC, gastro-esophageal reflux related cough; UACS, upper airway cough syndrome; AC, atopic cough.
Smoking status of patients with chronic cough
The majority of patients (1,583, 86.9%) were never smokers. Former smokers and current smokers were more common in males no matter in those aged 20-49 years or in those aged 50–89 years. In terms of never smokers, the number of males was roughly equal to that of females in the age group of 20-49 years, the number of females was 2.7-fold than males in the age group of 50-89 years (Table 2).
Table 2. Smoking status of patients with chronic cough.
| Smoking status | 20–49 years | 50–89 years | Total | ||||
|---|---|---|---|---|---|---|---|
| Female | Male | All | Female | Male | All | ||
| Never smokers | 568 (31.2) | 522 (28.6) | 1,090 (59.8) | 360 (19.8) | 133 (7.3) | 493 (27.1) | 1,583 (86.9) |
| Former smokers | 4 (0.2) | 91 (5.0) | 95 (5.2) | 1 (0.1) | 60 (3.3) | 61 (3.4) | 156 (8.6) |
| Current smokers | 6 (0.3) | 62 (3.4) | 68 (3.7) | 0 (0.0) | 15 (0.8) | 15 (0.8) | 83 (4.5) |
Data are presented as number (%). Patients who had no smoking history were defined as never smokers, those with a smoking history who had quit smoking for more than 1 month were defined as former smokers, otherwise they were defined as current smokers.
Cough sensitivity to capsaicin
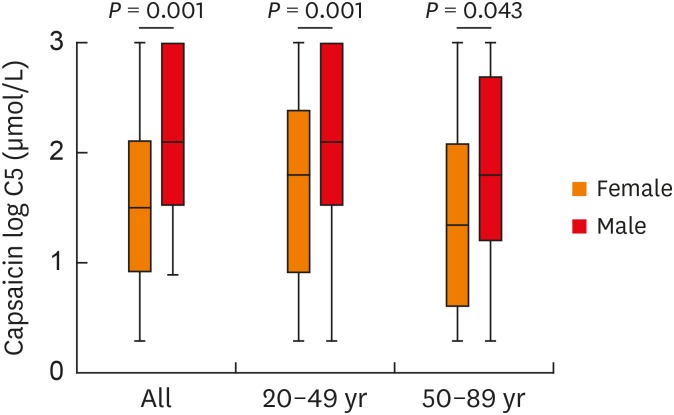
Data on cough sensitivity to capsaicin were available for 539 (269 females) of the 1822 chronic cough patients and 68 (32 females) healthy volunteers. There was no significant difference in age and sex distribution among the 1,822 patients, 539 patients who completed the capsaicin cough challenge test, and healthy volunteers (Supplementary Table S2). Female patients had a significantly heightened cough sensitivity compared to male patients overall (log C5: 1.58 ± 0.84 vs. 2.04 ± 0.84 μmol/L, P = 0.001), in the age range of 20-49 years (log C5: 1.60 ± 0.86 vs. 2.07 ± 0.84 μmol/L, P = 0.001), and in the age range of 50-89 years (log C5: 1.56 ± 0.82 vs. 1.94 ± 0.87 μmol/L, P = 0.038) (Fig. 6).
Fig. 6. Age and sex differences in cough reflex sensitivity measured as C5. The C5 values were logarithmically transformed (to base 10). Error bars indicate range, median and 25th and 75th percentiles.
C5, the concentration of capsaicin causing 5 coughs or more.
For males, patients aged 50-89 years had higher cough sensitivity than those aged 20-49 years (log C5: 1.94 ± 0.87 vs. 2.07 ± 0.84 μmol/L, P = 0.015). For females, patients aged 50-89 years exhibited an increasing tendency in cough sensitivity compared to those aged 20-49 years (log C5: 1.55 ± 0.82 vs. 1.60 ± 0.86 μmol/L, P = 0.205) (Fig. 6).
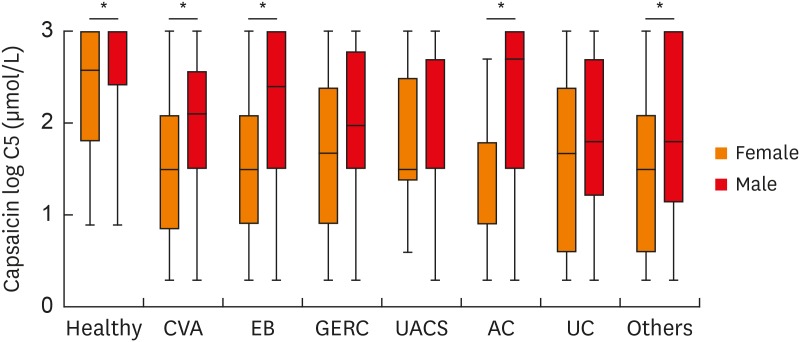
To evaluate cough sensitivity in patients with different causes, the evaluations of cough sensitivity to capsaicin were conducted in patients with a single cause. There was no significant difference in mean age and sex ratio between all patients with a single cause and those completed capsaicin cough challenge (Supplementary Table S3). Females had higher cough reflex (a lower log C5 value) compared to males (healthy volunteers: 2.31 ± 0.70 vs. 2.63 ± 0.59 μmol/L, P = 0.044; CVA: 1.50 ± 0.75 vs. 1.82 ± 0.75 μmol/L, P = 0.045; EB: 1.74 ± 0.77 vs. 2.24 ± 0.85 μmol/L, P = 0.001; GERC: 1.70 ± 0.90 vs. 1.97 ± 0.79 μmol/L, P = 0.061; UACS: 1.88 ± 0.71 vs. 2.29 ± 0.83 μmol/L, P = 0.110; AC: 1.33 ± 0.81 vs. 2.31 ± 1.02 μmol/L, P = 0.004; unexplained cough: 1.57 ± 0.92 vs. 1.91 ± 0.86 μmol/L, P = 0.167; other causes: 1.43 ± 0.88 vs. 2.06 ± 0.89 μmol/L, P = 0.026) (Fig. 7).
Fig. 7. Cough reflex sensitivity measured as C5 in different causes of chronic cough. The C5 values were logarithmically transformed (to base 10). Error bars indicate range, median and 25th and 75th percentiles.
C5, the concentration of capsaicin causing 5 coughs or more; CVA, cough-variant asthma; EB, eosinophilic bronchitis; GERC, gastro-esophageal reflux related cough; UACS, upper airway cough syndrome; AC, atopic cough; UC, unexplained chronic cough.
*Females had significantly heightened cough reflex sensitivity compared to males.
For both females and males, patients had higher cough sensitivity to capsaicin than healthy volunteers. However, no matter in females or males, there was no significant difference in cough sensitivity to capsaicin among patients with different causes of chronic cough (Fig. 7).
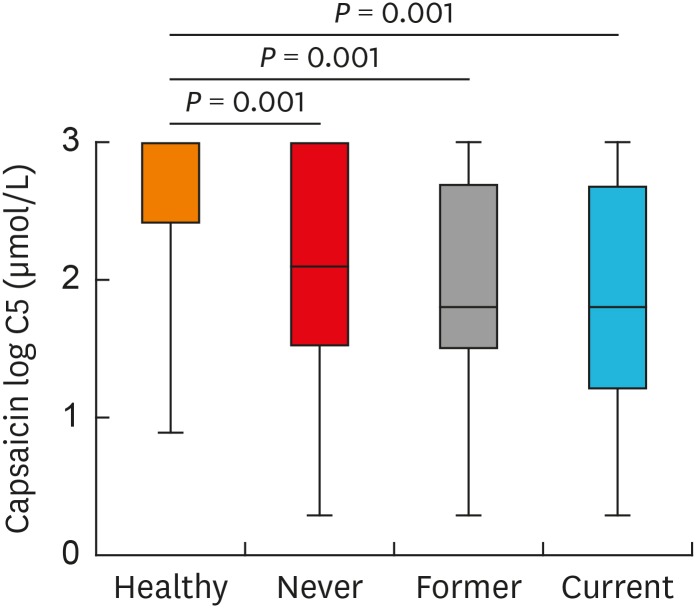
Since few female patients had a smoking history, we analyzed the influence of smoking on cough reflex in male patients. There was no significant difference in the ratio of never smokers, former smokers and current smokers as well as in the mean age between the 883 male patients and 270 who completed capsaicin cough challenge test (Supplementary Table S4). Patients with chronic cough had enhanced cough sensitivity compared to healthy volunteers (log C5: 2.63 ± 0.58 μmol/L), whereas no difference in cough sensitivity was found among patients who were never smokers (log C5: 2.06 ± 0.86 μmol/L), former smokers (log C5: 1.95 ± 0.76 μmol/L) and current smokers (log C5: 2.10 ± 0.89 μmol/L) (Fig. 8).
Fig. 8. Cough reflex sensitivity measured as C5 in male patients with different smoking status. The C5 values were logarithmically transformed (to base 10). Error bars indicate range, median and 25th and 75th percentiles.
C5, the concentration of capsaicin causing 5 coughs or more.
DISCUSSION
We evaluated almost 2,000 patients with chronic cough attending our Cough Clinic in Guangzhou, China over a period of 15 years. The results showed an overall younger age and a lack of female preponderance in Chinese chronic cough patients, despite a higher cough sensitivity to capsaicin in women and older patients as well as similar causes of chronic cough as Western countries.18 The study could, to some extent, expand upon data previously published by Morice et al.10 which displayed a strikingly homogeneous older female predominance of chronic cough patients worldwide.
Cough reflex sensitivity in female patients with chronic cough as well as in female healthy volunteers has been reported to be increased compared to male counterparts.12,13 It has been proposed that hypersensitivity of airway afferents and central-nervous system form the background of a higher prevalence of chronic cough in females.9,10 Capsaicin, a selective transient receptor potential vanilloid 1 (TRPV1) agonist, is most widely used to evaluate cough reflex sensitivity. Consistent with previous reports in Western countries, Chinese females were more sensitive to capsaicin compared to males despite the aetiology and age groups in this study. We found more females showed hypersensitivity to environmental cough triggers and talking, in addition to a greater prevalence of laryngeal paresthesia (Table 1), supporting the higher cough hypersensitivity found in females. Moreover, irrespective of sex, patients in the higher age range had enhanced cough sensitivity compared to the younger patients. It has previously been reported that ageing in adults is not associated with a decrease in cough sensitivity.19 Additionally, higher cough sensitivity to capsaicin in the post-menopausal women compared to the pre-menopausal women13 provides a possible mechanism underlying an older female predominance in other cough clinics, yet a higher cough sensitivity to capsaicin was not consistent with the age and sex distribution of chronic cough patients in the present study.
Smoking was identified as an important risk factor for chronic cough in community-based surveys.20 The vast majority of patients presenting to specialist cough clinics are lifetime non-smokers,21 which is a potential reason for the discrepant result could be that smokers with isolated cough symptom tend to ascribe their cough to tobacco and hence rarely seek medical advice specifically to combat this.22 In addition, smokers who developed COPD or lung cancer could be excluded by primary care because of typical radiographic findings.18 In our study, 86.9% of patients with chronic cough were never smokers. Although smoking was more prevalent in males, when patients with smoking history were excluded, a roughly equal sex distribution in the age range of 20-59 years and a female preponderance in the age range of 50-89 years were still present. Moreover, no significant difference in cough sensitivity was found among never smokers, former smokers and current smokers in male patients, while they all had higher cough sensitivity to capsaicin than healthy never smokers. Taken together, smoking was insufficient to explain the distinct age and sex distribution of chronic cough patients in our study.
Since China has no strict upward referral system, patients might directly attend specialist clinics without visiting primary care clinics. In the present study, of the 1,822 patients, only 25% were self-referrals without seeking any medical attention before visiting our cough clinic, while 75% had sought medical advice in primary health care institutions or secondary hospitals. Furthermore, EB, GERC, UACS and CVA were the top 4 causes, which were similar to previous reports from other specialist cough clinics.2,23 The total proportion (42.8%) of CVA, EB and AC was slightly higher in our study than in Western countries (10%-43%).4 When patients with CVA, EB and AC were excluded, the number of females (n = 516, 49.5%) was still similar as that of males (n = 527, 50.5%), and the mean age remained 43 years, which suggests that the higher proportion of asthma-related cough was not the main contributor to the distinct age and sex distribution.
Considering the diverse climate and geography in China, regional differences might occur in chronic cough population. A few years ago, a nationwide study focusing on causes of chronic cough was conducted in 8 large cities from 5 geographic areas of China. A homogeneous age (40.4 ± 12.8 years) was observed across the 5 geographic areas, while a similar sex distribution (mean percentage of females: 52.7%) was found in South, North, Northeast and West China, with the exception of East China (Shanghai), which revealed a female predominance (percentage of females: 63.3%).2 This study displayed a similar sex distribution as the nationwide study. Although the mean age of patients in the present study was 2.6 years older, we think it does not have clinical significance in this context, given that previous studies describing patients from Western countries reported a much older mean age (around 55 years).10,21,24 Taken together, we think our study could, to some extent, represent demographic features of the broader chronic cough population in China. Although Shanghai is the most Westernized city in China might be a contributor to a female predominance, further studies are needed to clarify it.
A few studies have reported a homogeneous age and sex distribution of chronic cough patients in Western populations and non-Chinese Asian populations.11,25,26 Song et al.11found a middle-aged female predominance (mean age: 52.8 ± 15.7 years and females 69.1%) of chronic cough patients referred to a tertiary cough clinic in Korea. Niimi et al.25 conducted a multicentre study in Japan and showed that the mean age of chronic cough patients was 51.8 ± 18.9 years and that 59.7% were female. In addition, Dicpinigaitis et al.27 has reported that no ethnic differences is found in cough reflex sensitivity among Caucasian, Indian and Chinese. Based on the above findings and the fact that a female predominance was shown in patients aged ≥50 years who accounted for merely 31.2% of the study population in this study, we speculate that the distinct demographic feature in China may be explained by the strikingly younger population with cough.
Rising levels of air pollution from industrial and traffic emissions have become a major threat to public health in China.28 It has been reported that pollutants can not only enhance cough reflex sensitivity, but also induce non-allergic eosinophilic airway inflammation.29,30 In China, males account for over 80% of industrial workers31 and traffic police,32 as well as 95% of professional taxi33 and bus drivers,34 whose mean age was 37.6-38.5 years. Additionally, it has been found that although there is a tendency toward greater fractional deposition of inhaled particles in females rather than in males, because males have 45% higher minute ventilations than females, the particles depositing per unit of time is 30% greater in males than in females.35 Moreover, more males (19.0%) had a history of occupational or home environment exposure than females (15.2%) in this study. Taken together, we speculate that because of the male predominance in pollution-intensive industries as well as the higher minute ventilations, younger males are more likely to suffer from occupational and environmental triggers. Further investigations are needed to confirm our speculation, given that transient receptor potential ankyrin-1 (TRPA1) rather than TRPV1 has been implicated in traffic-derived particulate matter eliciting cough.36,37 Furthermore, short-time exposure to high concentrations of particulate matter in Beijing increases citric acid cough reflex sensitivity in healthy tourists.29 In the present study, however, only capsaicin cough challenge was analyzed. It is noteworthy that in previous studies of patients with chronic cough and healthy subjects, females displayed enhanced cough reflex sensitivity to a selective TRPA1 agonist, allyl isothiocyanate17 and citric acid.24 These findings can be explained by 2 possibilities. One is that cough reflex response to tussigenic agents are inherently lower in males than in females, therefore, up-regulated cough reflex sensitivity inducing cough in male patients might still be lower when compared to increased cough reflex sensitivity in female patients. The other is that in some patients coughs are induced predominantly by continued exposure to endogenous inflammatory mediators or inhaled irritants rather than by reduced threshold for the stimulation of afferent neurons.
Our study has some limitations. First, it is a single-center survey. In fact, many patients in our cough clinic were from different areas around our country. And our data displayed a similar age and sex distribution as the previous nationwide study, which supports the representativeness of this study. It is noteworthy that both the nationwide study and this study only analyzed patients presenting for the evaluation of chronic cough; therefore, demographic features of overall chronic cough population throughout China might not be reflected. It is conceivable that the lack of a sex difference in this study may have not been attributed to the possibility that women are less able or willing to present to a physician for the evaluation of chronic cough. When patients were divided according to the causes of cough, a female predominance was shown in those with CVA, which is consistent with results of previous studies conducted in China.14,38,39 Secondly, given that capsaicin cough challenge is not a clinical routine test, it was performed only in a fraction of patients. Since there was no significant difference in age and sex distribution, between patients completed capsaicin cough challenge and in the broader group, it is believed that those completed capsaicin cough challenge were representative of the broader group. Thirdly, we could not determine which patients had been to a primary care center, a secondary care center or both, since we collected information by asking a question, “Have you ever been to a primary care center or a secondary care center for chronic cough?” However, when CVA, EB and AC, which are easily treated in a primary care and a secondary care center, were excluded, the age and sex distribution remained unchanged. Finally, due to the retrospective nature of this study, we could not clarify the effects of occupational and environmental exposures on the age and sex distribution of patients with chronic cough.
In summary, Chinese chronic cough patients had a roughly equal sex distribution and a middle-aged predominance, irrespective of a higher cough sensitivity to capsaicin in females and older patients. Further studies are needed to clarify the strikingly younger population with cough in China, considering a female predominance was shown in patients aged ≥50 years.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (Award No. 81270151), Incubative Project for Innovation Team of GMU (2017-159) and Guangzhou Municipal Science and Technology Key Project (2002Z2-E0091). The funders of the study had no role in the study design; the collection, analysis, and interpretation of data; the writing of the manuscript; and the decision to submit the manuscript for publication. Authors are indebted to all volunteers and patients who participated in this study. Special thanks to Mei Jiang of the First Affiliated Hospital of Guangzhou Medical University, for assistance in data analysis.
Appendix 1
Details of diagnostic criteria and management
CVA: Diagnosis was made based on the presence of bronchial hyper-responsiveness or reversible airflow obstruction along with a response to inhaled bronchodilators and inhaled corticosteroids therapy, or with the addition of leukotriene receptor antagonists and/or a short course of oral corticosteroid (10-20 mg/day, 3-5 days).
EB: Diagnosis was made based on the presence of eosinophil count ≥2.5% in induced sputum, without bronchial hyper-responsiveness or reversible airflow obstruction, and the cough respond to treatment with inhaled corticosteroids, or with the addition of oral corticosteroid (10-20 mg/day, 3-5 days)
UACS: Diagnosis was made based on the presence of upper airway abnormalities (not a necessary condition) and the cough respond to therapy targeted at underlying upper airway diseases. For example, nasal corticosteroids, antihistamines/decongestant, environmental trigger avoidance were used for the treatment of allergic rhinitis and allergic sinusitis; antimicrobial treatment and mucoactive agents were used for the treatment of bacterial sinusitis.
GERC: In addition to the presence of heartburn, acid regurgitation, belching, or an association between posture or eating and cough (not necessary conditions), along with DeMeester scores ≥12.70, and/or symptom associated probability ≥75% for patients who underwent oesophageal 24-h pH-monitoring, diagnosis was made based on the efficacy of lifestyle modification, proton pump inhibitor or H2-receptor antagonists combined with prokinetic agents.
AC: Diagnosis was made based on the presence of one or more of the following features, 1) history of atopy; 2) positive skin allergy and 3) elevated total IgE or specific IgE, along with normal spirometry and eosinophil count ˂2.5% in induced sputum, without bronchial hyper-responsiveness and without allergic rhinitis/sinusitis, and the cough respond to inhaled corticosteroids, antihistamines.
Unexplained chronic cough (UC): If the cough remained uncontrolled even after extensive investigation and treatment trials, the cough was categorised as UC.
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIALS
Causes of patients with chronic cough (n = 1,822, cases = 1,972)
Comparisons of 1,822 patients, patients completed capsaicin cough challenge test and healthy volunteers
Comparisons of patients with single cause and those completed capsaicin cough challenge test
Smoking status of patients
References
- 1.Song WJ, Chang YS, Faruqi S, Kim JY, Kang MG, Kim S, et al. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J. 2015;45:1479–1481. doi: 10.1183/09031936.00218714. [DOI] [PubMed] [Google Scholar]
- 2.Lai K, Chen R, Lin J, Huang K, Shen H, Kong L, et al. A prospective, multicenter survey on causes of chronic cough in China. Chest. 2013;143:613–620. doi: 10.1378/chest.12-0441. [DOI] [PubMed] [Google Scholar]
- 3.Morice AH, Fontana GA, Sovijarvi AR, Pistolesi M, Chung KF, Widdicombe J, et al. The diagnosis and management of chronic cough. Eur Respir J. 2004;24:481–492. doi: 10.1183/09031936.04.00027804. [DOI] [PubMed] [Google Scholar]
- 4.Chung KF, Pavord ID. Prevalence, pathogenesis, and causes of chronic cough. Lancet. 2008;371:1364–1374. doi: 10.1016/S0140-6736(08)60595-4. [DOI] [PubMed] [Google Scholar]
- 5.Young EC, Smith JA. Quality of life in patients with chronic cough. Ther Adv Respir Dis. 2010;4:49–55. doi: 10.1177/1753465809358249. [DOI] [PubMed] [Google Scholar]
- 6.French CL, Irwin RS, Curley FJ, Krikorian CJ. Impact of chronic cough on quality of life. Arch Intern Med. 1998;158:1657–1661. doi: 10.1001/archinte.158.15.1657. [DOI] [PubMed] [Google Scholar]
- 7.Dicpinigaitis PV, Tso R, Banauch G. Prevalence of depressive symptoms among patients with chronic cough. Chest. 2006;130:1839–1843. doi: 10.1378/chest.130.6.1839. [DOI] [PubMed] [Google Scholar]
- 8.Chamberlain SA, Garrod R, Douiri A, Masefield S, Powell P, Bücher C, et al. The impact of chronic cough: a cross-sectional European survey. Lung. 2015;193:401–408. doi: 10.1007/s00408-015-9701-2. [DOI] [PubMed] [Google Scholar]
- 9.Kavalcikova-Bogdanova N, Buday T, Plevkova J, Song WJ. Chronic cough as a female gender issue. Adv Exp Med Biol. 2016;905:69–78. doi: 10.1007/5584_2015_182. [DOI] [PubMed] [Google Scholar]
- 10.Morice AH, Jakes AD, Faruqi S, Birring SS, McGarvey L, Canning B, et al. A worldwide survey of chronic cough: a manifestation of enhanced somatosensory response. Eur Respir J. 2014;44:1149–1155. doi: 10.1183/09031936.00217813. [DOI] [PubMed] [Google Scholar]
- 11.Song WJ, Kim JY, Jo EJ, Lee SE, Kim MH, Yang MS, et al. Capsaicin cough sensitivity is related to the older female predominant feature in chronic cough patients. Allergy Asthma Immunol Res. 2014;6:401–408. doi: 10.4168/aair.2014.6.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kastelik JA, Thompson RH, Aziz I, Ojoo JC, Redington AE, Morice AH. Sex-related differences in cough reflex sensitivity in patients with chronic cough. Am J Respir Crit Care Med. 2002;166:961–964. doi: 10.1164/rccm.2109061. [DOI] [PubMed] [Google Scholar]
- 13.Fujimura M, Kasahara K, Kamio Y, Naruse M, Hashimoto T, Matsuda T. Female gender as a determinant of cough threshold to inhaled capsaicin. Eur Respir J. 1996;9:1624–1626. doi: 10.1183/09031936.96.09081624. [DOI] [PubMed] [Google Scholar]
- 14.Yi F, Chen R, Luo W, Xu D, Han L, Liu B, et al. Validity of fractional exhaled nitric oxide in diagnosis of corticosteroid-responsive cough. Chest. 2016;149:1042–1051. doi: 10.1016/j.chest.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Morice AH, McGarvey L, Pavord I British Thoracic Society Cough Guideline Group. Recommendations for the management of cough in adults. Thorax. 2006;61(Suppl 1):i1–i24. doi: 10.1136/thx.2006.065144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irwin RS, Madison JM. The diagnosis and treatment of cough. N Engl J Med. 2000;343:1715–1721. doi: 10.1056/NEJM200012073432308. [DOI] [PubMed] [Google Scholar]
- 17.Long L, Yao H, Tian J, Luo W, Yu X, Yi F, et al. Heterogeneity of cough hypersensitivity mediated by TRPV1 and TRPA1 in patients with chronic refractory cough. Respir Res. 2019;20:112. doi: 10.1186/s12931-019-1077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morice AH, Kastelik JA. Cough. 1: chronic cough in adults. Thorax. 2003;58:901–907. doi: 10.1136/thorax.58.10.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang AB, Widdicombe JG. Cough throughout life: children, adults and the senile. Pulm Pharmacol Ther. 2007;20:371–382. doi: 10.1016/j.pupt.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Çolak Y, Nordestgaard BG, Laursen LC, Afzal S, Lange P, Dahl M. Risk factors for chronic cough among 14,669 individuals from the general population. Chest. 2017;152:563–573. doi: 10.1016/j.chest.2017.05.038. [DOI] [PubMed] [Google Scholar]
- 21.Dicpinigaitis PV. Thoughts on one thousand chronic cough patients. Lung. 2012;190:593–596. doi: 10.1007/s00408-012-9420-x. [DOI] [PubMed] [Google Scholar]
- 22.Jorm LR, Shepherd LC, Rogers KD, Blyth FM. Smoking and use of primary care services: findings from a population-based cohort study linked with administrative claims data. BMC Health Serv Res. 2012;12:263. doi: 10.1186/1472-6963-12-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kastelik JA, Aziz I, Ojoo JC, Thompson RH, Redington AE, Morice AH. Investigation and management of chronic cough using a probability-based algorithm. Eur Respir J. 2005;25:235–243. doi: 10.1183/09031936.05.00140803. [DOI] [PubMed] [Google Scholar]
- 24.Kelsall A, Decalmer S, McGuinness K, Woodcock A, Smith JA. Sex differences and predictors of objective cough frequency in chronic cough. Thorax. 2009;64:393–398. doi: 10.1136/thx.2008.106237. [DOI] [PubMed] [Google Scholar]
- 25.Niimi A, Ohbayashi H, Sagara H, Yamauchi K, Akiyama K, Takahashi K, et al. Cough variant and cough-predominant asthma are major causes of persistent cough: a multicenter study in Japan. J Asthma. 2013;50:932–937. doi: 10.3109/02770903.2013.823444. [DOI] [PubMed] [Google Scholar]
- 26.Fujimura M, Abo M, Ogawa H, Nishi K, Kibe Y, Hirose T, et al. Importance of atopic cough, cough variant asthma and sinobronchial syndrome as causes of chronic cough in the Hokuriku area of Japan. Respirology. 2005;10:201–207. doi: 10.1111/j.1440-1843.2005.00686.x. [DOI] [PubMed] [Google Scholar]
- 27.Dicpinigaitis PV, Allusson VR, Baldanti A, Nalamati JR. Ethnic and gender differences in cough reflex sensitivity. Respiration. 2001;68:480–482. doi: 10.1159/000050554. [DOI] [PubMed] [Google Scholar]
- 28.Guan WJ, Zheng XY, Chung KF, Zhong NS. Impact of air pollution on the burden of chronic respiratory diseases in China: time for urgent action. Lancet. 2016;388:1939–1951. doi: 10.1016/S0140-6736(16)31597-5. [DOI] [PubMed] [Google Scholar]
- 29.Sato R, Gui P, Ito K, Kohzuki M, Ebihara S. Effect of short-term exposure to high particulate levels on cough reflex sensitivity in healthy tourists: a pilot study. Open Respir Med J. 2016;10:96–104. doi: 10.2174/1874306401610010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang Z, Huang C, Zhang JJ, Xie J, Dai S, Ge E, et al. Traffic-related air pollution induces non-allergic eosinophilic airway inflammation and cough hypersensitivity in guinea-pigs. Clin Exp Allergy. 2019;49:366–377. doi: 10.1111/cea.13308. [DOI] [PubMed] [Google Scholar]
- 31.Lin J, Wang H, Yan F, Tang K, Zhu H, Weng Z, et al. Effects of occupational exposure to noise and dust on blood pressure in Chinese industrial workers. Clin Exp Hypertens. 2018;40:257–261. doi: 10.1080/10641963.2017.1368534. [DOI] [PubMed] [Google Scholar]
- 32.Tang YX, Bloom MS, Qian ZM, Liu E, Jansson DR, Vaughn MG, et al. Association between ambient air pollution and hyperuricemia in traffic police officers in China: a cohort study. Int J Environ Health Res. 2019:1–9. doi: 10.1080/09603123.2019.1628926. [DOI] [PubMed] [Google Scholar]
- 33.Feng Z, Zhan J, Wang C, Ma C, Huang Z. The association between musculoskeletal disorders and driver behaviors among professional drivers in China. Int J Occup Saf Ergon. 2018:1–11. doi: 10.1080/10803548.2018.1482088. [DOI] [PubMed] [Google Scholar]
- 34.Wu X, Zhang H, Xiao W, Ning P, Schwebel DC, Hu G. Are bus company regulations associated with crash risk? Findings from a retrospective survey in four Chinese cities. Int J Environ Res Public Health. 2019;16:E1342. doi: 10.3390/ijerph16081342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bennett WD, Zeman KL, Kim C. Variability of fine particle deposition in healthy adults: effect of age and gender. Am J Respir Crit Care Med. 1996;153:1641–1647. doi: 10.1164/ajrccm.153.5.8630615. [DOI] [PubMed] [Google Scholar]
- 36.Robinson RK, Birrell MA, Adcock JJ, Wortley MA, Dubuis ED, Chen S, et al. Mechanistic link between diesel exhaust particles and respiratory reflexes. J Allergy Clin Immunol. 2018;141:1074–1084.e9. doi: 10.1016/j.jaci.2017.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deering-Rice CE, Romero EG, Shapiro D, Hughen RW, Light AR, Yost GS, et al. Electrophilic components of diesel exhaust particles (DEP) activate transient receptor potential ankyrin-1 (TRPA1): a probable mechanism of acute pulmonary toxicity for DEP. Chem Res Toxicol. 2011;24:950–959. doi: 10.1021/tx200123z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng-Jia C, Xin-Yan H, Geng-Peng L, Yang-Li L, Can-Mao X. Validity of fractional exhaled nitric oxide and small airway function indices in diagnosis of cough-variant asthma. J Asthma. 2018;55:750–755. doi: 10.1080/02770903.2017.1366509. [DOI] [PubMed] [Google Scholar]
- 39.Cao Y, Lin SH, Zhu D, Xu F, Chen ZH, Shen HH, et al. WeChat public account use improves clinical control of cough-variant asthma: a randomized controlled trial. Med Sci Monit. 2018;24:1524–1532. doi: 10.12659/MSM.907284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Causes of patients with chronic cough (n = 1,822, cases = 1,972)
Comparisons of 1,822 patients, patients completed capsaicin cough challenge test and healthy volunteers
Comparisons of patients with single cause and those completed capsaicin cough challenge test
Smoking status of patients