Abstract
Algae ponds used in industrial biomass production are susceptible to pathogen or grazer infestation, resulting in pond crashes with high economic costs. Current methods to monitor and mitigate unhealthy ponds are hindered by a lack of early indicators that precede culture crash. We used solid-phase microextraction (SPME) coupled with gas chromatography-mass spectrometry (GC-MS) to identify volatiles emitted from healthy and rotifer infested cultures of Microchloropsis salina. After 48 hours of algal growth, marine rotifers, Brachionus plicatilis, were added to the algae cultures and volatile organic compounds (VOC) were sampled from the headspace using SPME fibers. A GC-MS approach was used in an untargeted analysis of VOCs, followed by preliminary identification. The addition of B. plicatilis to healthy cultures of M. salina resulted in decreased algal cell numbers, relative to uninfected controls, and generated trans-β-ionone and β-cyclocitral, which were attributed to carotenoid degradation. The abundances of the carotenoid-derived VOCs increased with rotifer consumption of algae. Our results indicate that specific VOCs released by infected algae cultures may be early indicators for impending pond crashes, providing a useful tool to monitor algal biomass production and pond crash prevention.
Subject terms: Small molecules, Applied microbiology, Biodiesel
Introduction
As the energy needs of the world increase, dependence on non-renewable sources of energy remains a concern. Increased production of corn starch or sugarcane-based ethanol has resulted in increased atmospheric carbon dioxide levels, diversion of arable land from food production, and increased consumer cost for sugar and corn1. For these reasons, microalgae production systems are considered a promising avenue for biofuel production. Microalgal strains are capable of growth in a range of environments (e.g. freshwater, marine, hypersaline, highly acidic) including high-nutrient municipal wastewater systems2, allowing for simultaneous bioremediation and biofuel production. Microalgae’s ability for rapid growth in non-potable (brackish or marine) water sources using non-arable land, combined with their high capacity for fixation of atmospheric carbon dioxide, and high lipid-to-biomass ratios are significant advantages toward its use as a biofuel feedstock. The development of optimized systems for sustainable and dependable biofuel production through algal pond systems are necessary as the global energy strategies continue to evolve3 (for review, see Kayitar 2017).
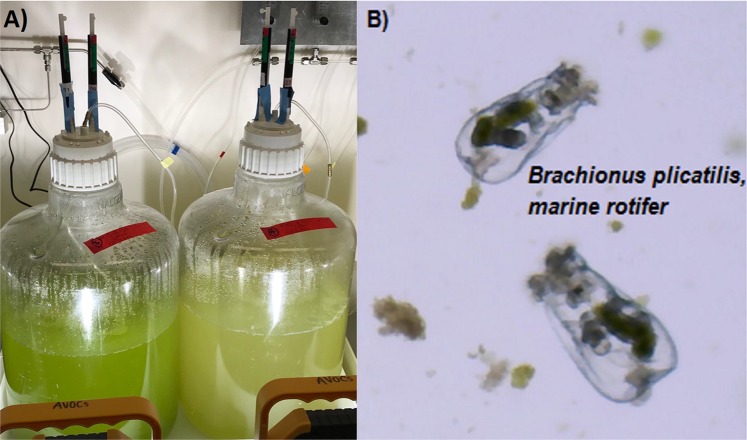
A major challenge faced in algal production are ‘pond crashes’, which are defined as devastating, often unpredictable losses of entire crops due to parasitism, grazing, weather, or many other factors. Closed photobioreactors are less likely to be susceptible to contamination with deleterious species (e.g. viruses, fungi, protozoans, detrimental microbes), but involve higher capital costs4 and, once contaminated, can be difficult to disinfect. Open algal ponds are less expensive to set up but are more likely to succumb to crashes (Fig. 1A) caused by grazing or parasitism5,6. Notably, a single adult marine rotifer, Brachionus plicatilis (Fig. 1B), can consume 200 microalgal cells per minute and double in population within 1–2 days7. It is estimated that pond crashes account for 30% loss of annualized algal production4 and represent a significant economic barrier to biofuel production8.
Figure 1.
(A) 15 L algal mass cultures (analgous to production ponds) with a healthy algal culture on the left compared with a crashed algal culture on the right. (B) Brachionus plicatilis (average length 160 µm), marine rotifer, in a field of microalgae, Microchloropsis salina.
Current pond crash mitigation strategies, both prophylactic and interdictive, are largely focused on chemical treatments, such as hypochlorite9, copper10,11, quinine sulfate12, rotenone13, additives that lower pH (to less than 3.0)14, and biocides, such as tossendanin15. The use of chemical countermeasures as a prophylactic strategy to prevent pond crashes is prohibitively expensive for most algal industry business models. Chemical additives can degrade from sun exposure and may need to be repeatedly added to cultures to maintain protection. In addition, frequent and repeated chemical application can be environmentally detrimental through the development of resistant pest species or through unacceptable off-target effects (e.g., pisicidal effects of rotenone). However, when applied early and in a targeted fashion after the detection of a deleterious species16, chemical additives can be highly effective at saving algal cultures [for review of crop protection strategies, see Fisher & Lane 2019].
In order to attain the production levels of 25 g m−2 d−1 ash free dry weight (or 2,500 gallons of biofuel per acre per year)17, deemed necessary for economic algal biofuel production, cost-effective pond monitoring strategies are necessary to reduce culture loss and increase annualized production. Currently, algal production facilities utilize light microscopy to identify contaminants, pathogens, and competing algal strains that could lead to the demise of the desired algal strain18. However, microscopy is slow, labor-intensive, and requires advanced operator training for differentiating various microbiota. Alternative methods involving automated and semi-automated technologies, such as FlowCAM imaging flow-cytometry19,20, polymerase chain reaction, and hybridization-based assays21,22 are under development to increase sensitivity and expedite analysis for daily algal culture monitoring.
Volatile organic compounds (VOCs) are carbon-containing molecules with high vapor pressures at ambient temperatures23. Within the field of chemical ecology, VOCs have been identified as secondary metabolites and include, but are not limited to, pheromones, semiochemicals, odorants, and phytohormones24,25. Algal VOC production has been associated with intra- and inter-species communication, allelopathy, semiochemical production, and predator deterrence26 [for review, see Zuo 2019]. A well characterized example of an algal volatile involves conversion of nonvolatile dimethylsulfoniopropionate (DMSP) to volatile dimethyl sulfide (DMS). In intact Emiliania huxleyi cells, conversion of DMSP to DMS by the enzyme DMSP lyase is minimal. However, during algal grazing, such as by the dinoflagellate Oxyrrhis marina, E. huxleyi is disrupted, releasing DMSP. Once in solution, DMSP lyases, including those from bacteria, catalyze conversion of DMSP to DMS27,28. DMS then acts not only as a deterrent against herbivory by Oxyrrhis marina29, but additionally as an attractant for other species such as birds and fish30.
The aims of this study were to 1) develop a methodology to detect VOCs from healthy algal cultures (Microchloropsis salina) as well as algal cultures in the presence of a grazer (M. salina cultures with marine rotifer Brachionus plicatilis) and 2) evaluate whether specific VOCs could serve as early indicators of an imminent culture crash. A setup based upon solid-phase microextraction (SPME) fibers coupled with gas chromatography-mass spectrometry (GC-MS) allowed for non-invasive monitoring of volatile emissions. Compounds present during the active grazing period of rotifers on algal cultures, but not produced in healthy controls, were deemed potential biomarkers of high stress conditions. We propose that these biomarker compounds are potential diagnostic tools for chemical monitoring systems in microalgal cultivation systems to enabling the early detection of culture stress for improved algal crop production.
Results
Cell counts of infected and control cultures
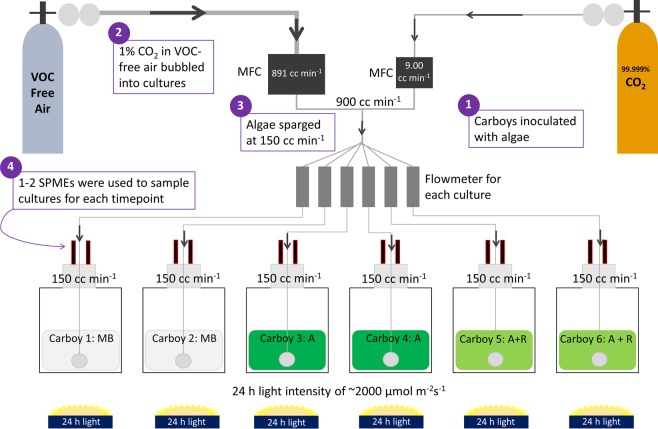
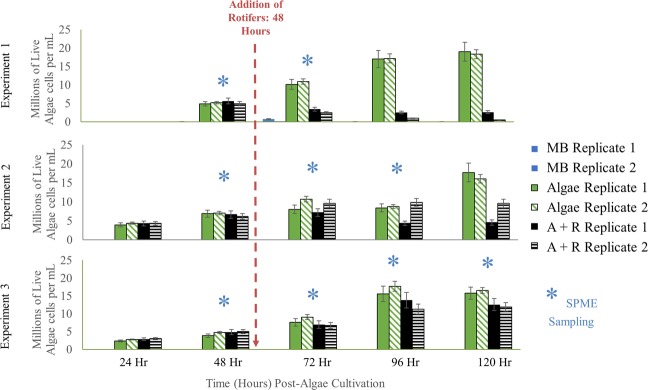
Our experimental setup (summarized in Fig. 2) facilitated headspace volatile monitoring of M. salina with and without the microalgal grazer, B. plicatilis. In each experiment, algal cell concentrations and VOC headspace samples were collected at various time points for M. salina alone (abbreviated Algae or A), M. salina and B. plicatilis (abbreviated Algae + Rotifer or A + R), and ESAW media blanks (abbreviated Media Blank or MB) (Fig. 3, Supplemental Fig. 1). At 48 hours after inoculation, algal concentrations across all cultures were similar, approaching the mid- to late- stages of logarithmic growth. At this time, B. plicatilis were added to two M. salina cultures, resulting in time-dependent decreases in algal density relative to controls (Fig. 3). Despite consistent growth conditions, 96 hours after the initial cultures were started and 48 hours after rotifers were added, the Algae + Rotifer cultures displayed different extents of algal biomass loss attributed to rotifer grazing (see Fig. 3). This variation in rates of biomass loss may arise from differences in rotifer lots.
Figure 2.
Schematic of experimental setup for growth M. salina (Algae, A) in the presence of B. plicatilis (Rotifer, R) for 5 d. Mass flow controllers (MFCs) mixed 1% CO2 with VOC-free air to sparge 15 L cultures at 150 cc min−1. One to two SPME fibers were used to sample the headspace of media blank (MB), Algae only (A), and Algae + Rotifer (A + R) carboys for 30–60 min each at various timepoints over 2–4 days.
Figure 3.
Algae concentration as determined by fluorescence measurements collected for three experiments. Similar coloring and patterns represent biological replicates of each condition: media blanks (MB), Algae (A), and Algae + Rotifer (A + R) cultures. Error bars represent standard deviation derived from duplicate measurements for each sample. Significance levels for conditions that exhibited p < 0.05 are in Supplemental Table 1. Blue asterisks (*) indicate the time points for headspace VOC sampling by SPME fibers.
Headspace VOC results
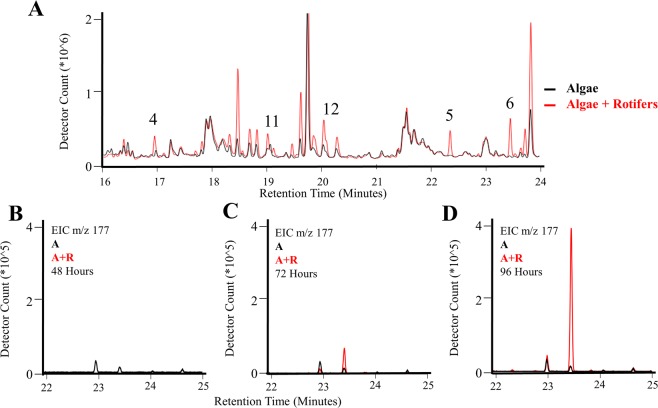
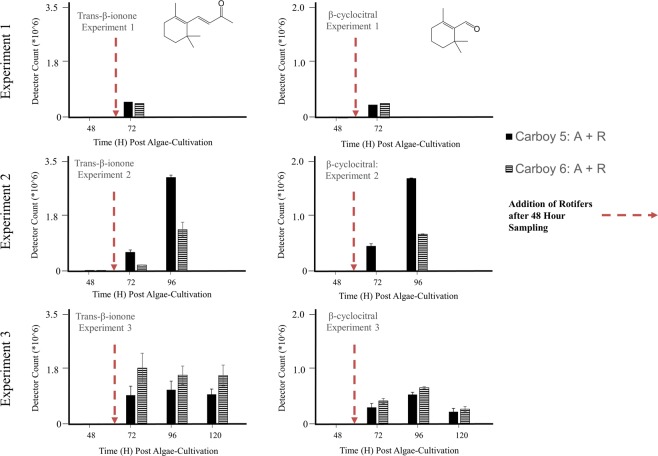
Headspace VOCs were sampled with SPME fibers for 30–60 min each at various time points (as indicated in Fig. 3) and analyzed by GC-MS. Qualitative and quantitative differences were observed in the VOC profiles of Algae + Rotifer cultures compared to the Algae cultures. Example total ion chromatograms for Algae and Algae + Rotifer cultures taken approximately 24 hours after addition of rotifers (Experiment #3) are shown in Fig. 4A. Several VOCs that differentiate the two culture conditions are enumeratedi (Fig. 4A, annotations in Table 1) and are potential early indicators of algal grazing or death. Extracted ion chromatograms were utilized to improve visualization of individual VOCs. Figure 4B–D demonstrate the increase in an VOC displaying a base peak m/z 177 and retention index 1495, observed over the time course of the experiment in Algae + Rotifer cultures. Although the Algae chromatogram for m/z 177 also displays a small peak at the same retention time, this VOC was not detected using the given experimental criteria for data processing.
Figure 4.
Example chromatograms for observed VOCs sampled from Algae (A) and Algae + Rotifer (A + R) cultures between 16 and 24 min, (A) Total ion chromatogram with indicated VOCs (Annotations See Table 1), (B–D) extracted ion chromatograms monitoring increase in compound 6 over time (m/z 177, RI 1495).
Table 1.
VOCs robustly and repeatedly detected from Algae and Algae + Rotifer experiments.
| Compound # | Mass | Tentative Compound Class* | NIST14 ID | NIST % Match | Experimental Retention Index | Theoretical Retention Index | Experiment # | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||||||
| VOCs detected in A+R cultures | 1 | 82 | Carotenoid | 2,2,6-trimethylcyclohexanone | 79 | 1021 | 1036 | X | X | |
| 2 | 107 | 1181 | X | X | X | |||||
| 3 | 121 | Phenol | 1191 | X | X | |||||
| 4 | 137 | Carotenoid | 2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde | 81 | 1209 | 1220 | X | X | X | |
| 5 | 121 | Carotenoid | 4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2-butanone | 76 | 1419 | 1433 | X | X | X | |
| 6 | 177 | Carotenoid | trans--ionone | 94 | 1495 | 1486 | X | X | X | |
| 7 | 57 | Alkane | 1691 | X | X | X | ||||
| VOCs detected in A+R and A cultures | 8 | 71 | 1039 | X | X | |||||
| 9 | 96 | Methyl Ester | 3-Nonenoic acid, methyl ester | 74 | 1134 | 1191 | X | X | X | |
| 10 | 341 | 1139 | X | X | ||||||
| 11 | 71 | 1293 | X | X | ||||||
| 12 | 138 | Terpene/Carotenoid | 1338 | X | X | |||||
| 13 | 73 | Fatty acid (Hexadecanoic acid) | 1983 | X | X | |||||
| 14 | 192 | 2197 | X | X | ||||||
*Tentative compound class for unknown compounds is based on fragmentation in averaged mass spectra determined via chromatographic deconvolution and alignment.
The number of compounds detected from deconvolution of chromatographic peaks varied with each sample. The analysis of a single sample commonly detected 100–200 chemical compounds, many of which were attributed to background (known from control measurements). Application of chromatographic peak alignment across the data from all samples and at every timepoint generated a list of more than 1800 compounds, consisting of both algal VOCs and extraneous signals from the experimental setup. Many compounds were attributed to known background or were not found reproducibly. Application of the filtering criteria based upon algal abundance and detection frequency across experimental replicates identified the most robust compounds as potential VOC biomarkers from either Algae or Algae + Rotifer cultures, removed irreproducible compounds, and narrowed the extensive list to ~50 compounds in any single experiment. Table 1 shows biomarkers that were only observed across multiple experiments. For a detailed list of the volatile biomarkers detected in each experiment, refer to Supplemental Table 1.
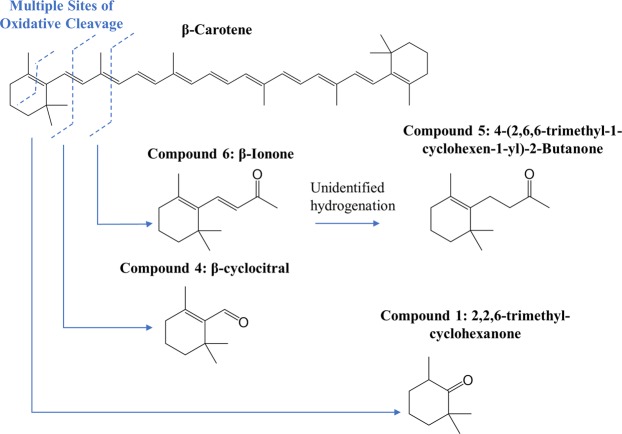
Comparison of three replicate experimental infections of both Algae and Algae + Rotifer cultures revealed several VOCs that were reproducibly observed in 1) Algae + Rotifer cultures and 2) both Algae and Algae + Rotifer cultures, represented in Table 1, despite the different rates in algal biomass loss. For example, Compound 6 monitored in Fig. 4B–D was identified with a 94% confidence score as trans-β-ionone using the NIST14 library. Confidence in this identification increases when considering the calculated experimental retention index (RI) of 1495 was within 5% of the literature theoretical value (1486, NIST 14 database). Within the Algae + Rotifer cultures, all of the discriminating VOCs were structurally-related ketones or aldehydes: (a) Compound 6: trans-β-ionone [IUPAC name: (E)-4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3-buten-2-one], (b) Compound 4: β-cyclocitral [IUPAC name: 2,6,6-trimethyl-1-cyclohexene-1- carboxaldehyde], (c) Compound 1: 2,2,6-trimethyl-cyclohexanone, and (d) Compound 5: 4-(2,6,6-trimethyl-1-cyclohexene-1-yl)-2-butanone. We propose these compounds as potential biomarkers indicative of algal stress or death. The VOCs observed in both Algae and Algae + Rotifer cultures were most likely algae-derived volatiles universally present in both conditions, with 3-nonenoic acid methyl ester having an adequate confidence for identification (74% spectral match, <5% RI deviation from theoretical value).
For those VOCs that could not be identified using the initial conservative thresholds, the observed spectra and experimental retention indices provided suggestions for their identification. The suggested compound classes for unknown compounds are provided in Table 1, and their experimental mass spectra are included in Supplemental Fig. 1. For example, the mass spectrum of Compound 12 has similar features to those of the identified carotenoids, suggesting a terpenoid structure with a molecular weight of 208 Da. The mass spectrum of Compound 13 contains characteristic ions of hexadecenoic acid including m/z 43, 60, 73, 129, 213, and 256 (M+), and its experimental RI is within 1% of the literature RI of hexadecanoic acid (1968).
Abundance of VOCs
In addition to the qualitative analysis, we examined the relationship between rotifer duration of feeding and the abundance of Algae + Rotifer distinguishing VOCs. Levels of Compounds 4 and 6 were compared for individual rotifer cultures (Carboys 5 and 6) across individual experiments (Fig. 5). While there was no detected signal from either compound before the addition of rotifers (48 hours), all detected signals after rotifer addition exceeded 2.0 × 105 counts, 2–3 orders of magnitude above detection threshold. Another carotenoid oxidation product, β-cyclocitral, appeared in Experiment 2 after 24 hours of rotifer feeding, with the signal increasing to more than 6.0 × 105 counts after 48 hours of rotifer feeding. Similar comparisons for Compounds 1–7 are included in Supplemental Fig. 2. Of note, comparison of Fig. 5 to Fig. 3 reveals several instances where these VOCs were detected in Algae + Rotifer cultures before biomass loss was apparent as compared to algae controls. For example, the second biological replicate of Algae + Rotifers in experiment 2 did not differ in algal density from the healthy controls at the 72 h and 96 h timepoints. However, the signals for Compounds 4 and 6 were already large (6.0 × 105 and 1.0 × 106 counts, respectively).
Figure 5.
Peak areas of extracted compound chromatograms for trans-β-ionone and β-cyclocitral across Experiments 1, 2, and 3, separated by biological replicates. Error bars represent standard deviation derived from duplicate measurements for each sample. The exposure time for SPME fibers was 30 minutes in Experiment 1 and 60 minutes in Experiments 2 and 3.
Discussion
Our SPME-GC-MS analysis has identified seven discriminating VOCs in M. salina cultures in the presence of actively-grazing B. plicatilis (Algae + Rotifer). The absence of these volatiles in the time-matched Algae control cultures (Fig. 3) suggests these chemicals are specific signals of algal grazing or algal death. Many of these chemicals were detected within 24 hours after rotifer addition and before algal cell densities changed substantially (Figs 2, 4). Specifically, Compounds 4, 5, 6, and 7 were identified as early and robust grazing signals observed in M. salina cultures containing rotifers. Several identified biomarkers – Compounds 1, 4, 5, 6 and 7 – were detected only during rotifer grazing and contained structural similarities, hinting at a shared metabolic pathway. Many of these compounds (Table 1) are known products of carotenoid oxidation31,32. Carotenoid-derived substances have been previously observed in microalgal volatile research, largely associated with investigations of flavor or smell components in food production. Carotenoids have important physiological functions as a component of photosystems – the light-harvesting complexes that transfer light energy to chlorophyll33. Oxidative cleavage of the carotenoid backbone can occur through enzymatic (carotenoid cleavage dioxygenases) or non-enzymatic (light, oxygen, temperature) mechanisms33. Potential pathways for oxidative cleavage of the carotenoid β-carotene are shown in Fig. 6. In our work, the carotenoid-derived VOCs could be generated from the oxidation of β-carotene released upon lysis of M. salina cells during the digestive process of B. plicatilis. This would be in agreement with studies of Arabidopsis plants exposed to reactive oxygen species resulting in the subsequent release of β-ionone and β-cyclocitral34. The results from vascular plants suggest that carotenoid degradation products may be more general indicators of stressed or wounded algae cultures, not solely limited to the interaction of algae with rotifers.
Figure 6.
VOCs identified from the headspace of Algae + Rotifer cultures formed from the oxidative cleavage of the carotenoid β-carotene. Only those oxidation cleavages relevant to this study are pictured, but all double bonds across the β-carotene backbone are cleaved.
Although there are no reports of such analyses of M. salina, a small number of studies have examined algae from the genus Nannochloropsis, of which M. salina is a close relative35. Van Durme et al.36 investigated the volatile composition of five microalgae species (Botryococcus braunii, Rhodomonas, Tetraselmis sp., Nannochloropsis oculata and Chlorella vulgaris) by heating samples (40 °C) to produce volatile signatures under heat stress conditions. Products of carotenoid oxidation, including α- or β- ionone and β-cyclocitral, were identified in all species tested. Interestingly, N. oculata contained a large abundance of ethanol, 2-hydroxy-2-butanone, and benzaldehyde, while only small amounts of β-cyclocitral and ionones were detected. Hosoglu37 likewise characterized the volatilomes of several microalgae species using SPME-GC-MS and GC-olfactometry for both chemical profiles and olfactory properties to benefit incorporation into food products and to minimize unpleasant smells. The species C. vulgaris, C. protothecoides, and T. chuii reportedly contain distinguishing amounts of the carotenoid degradation products, α- and β-ionone and 6-methyl-5-hepten-2-one, while expressing a woody smell.
While VOCs have been observed in analyses of chemical compositions of algae in destructive manners (i.e. heating, sonication, solvent extraction etc.), there are fewer reports of volatiles emitted from live, actively-growing cultures. A variety of live algae-derived volatiles (terpenoids, aldehydes, halogenated compounds, etc.) have been shown to influence the odor quality of water26. Zhou et al.38 investigated changes in the volatilome of intact algae over different growth phases (logarithmic, stationary, and decline phase) for six microalgae (Thalassiosira weissflogii, Nitzschia closterium, Chaetoceros calcitrans, Platymonas helgolandica, Nannochloropsis spp. (NMBluh014-1), and Dicrateria inornate). The Nannochloropsis volatilome was largely dominated by alkanes and alkenes and 8-heptadecene, but no carotenoid by-products were reported. Several functions for actively-released VOCs, including carotenoids, have been postulated, such as tolerance of light and oxidative stressors, signaling the presence of predators33, and transfer of information throughout algal colonies26. β-cyclocitral has previously been reported as a volatile emitted by the bloom-forming cyanobacterium Microcystis as a defense mechanism against grazing by Daphnia magna39. Similarly, our work focused on the volatiles generated from active grazing of algae, which will generate more rapid algal death compared to natural growth cycles. Here, we confirmed the importance of β-cyclocitral as an indication of algal cell damage due to grazing. Future work will determine if VOCs produced by M. salina in the presence of B. plicatilis has a similar role in algal-defense as observed previously.
Using a non-invasive, non-destructive sampling and analysis technique, we have demonstrated that VOCs from the headspace of algae cultures can distinguish between algae cultures with grazing rotifers present and uninfected algal cultures, and may serve as general indicators of algal cell death. In order to discover and validate additional diagnostic markers of grazer infection or other incipient crashes, more extensive study of emitted volatiles from microalgal species is required. For example, low levels of grazer-associated VOCs in healthy algal cultures may result from background rates of algal death. Such background signals are likely modulated by physiological state of the culture (e.g. exponential growth or stationary phase) or by nutrient limation. Thus, an improved understanding of the threshold biomarker concentrations that indicate the need for interdictive treatment is paramount.
It is also worth noting that the list of biomarkers reported in this analysis may be considered conservative owing to the stringency of our filtering criteria. Additional biomarkers may be observed with less stringent parameters for the filtration of detected VOC peaks in data processing. However, the identified carotenoid breakdown products served as indicators for algal crashes across all three experiments. Additionally, our SPME-GC-MS methodology is expected to have broader applications. Complex systems-level dynamics between algae, commensal bacteria, and various grazers will require more sophisticated sampling procedures alongside volatilomics data to include biological data sets, such as transcriptomics, metagenomics, and metabolomics. These systems-level analyses and bioinformatics analysis would be more likely to elucidate biological interactions or implications for the chemicals observed in the volatilome. Non-invasive and non-destructive VOC sampling is an attractive, analytical way to better understand and predict the health of microbial cultures.
Conclusions
Our work aims to increase the breadth and depth of reported algal and rotifer-specific VOCs, providing a tool to better define the physiological state of microalgal ponds and facilitate greater algal biomass production. A SPME-GC-MS methodology for non-invasive and non-destructive sampling of M. salina infected by B. plicatilis aided our discovery of seven putative culture crash biomarkers, including trans-β-ionone and β-cyclocitral, over several timepoints during active crashing of algal ponds. These biomarkers were not detected in cultures displaying natural background levels of cell death, suggesting that these signals are produced by high stress conditions, such as rotifer grazing. Finally, these biomarkers offer potential as diagnostic tools to signal the need for crash mitigation strategies, as several signals were detectable before cell death was evident from changes in cell density. Both VOC baselines and signatures from multiple healthy and infected cultures will be compiled in a data base. Early use of this technique would then include surveilling for the emergence of targeted VOC biomarkers of algal distress or injury above healthy baseline thresholds that are indicative of imminent culture failure.
We envision the use of VOC based monitoring in open ponds and we are in the process of extending this work to such systems. In such environments, one must take into account that the volatile “headspace” of open ponds may be influenced by external sources (e.g. VOCs from the environment, wind effects, particulates, etc.), creating a variable background that would require correction for the levels of biomarker compounds. It is possible to temporarily create closed “headspace” above an area of open pond (perhaps using a large funnel), during sample collection, which would serve to limit outside “noise”.
The SPME fibers used in this experiment are field deployable and can easily be adapted to an algal pond production system. Although SPME-GC-MS has proven powerful for untargeted discovery of algal volatile chemical signatures from healthy or grazed cultures, the cost of state-of-the art laboratory-based GC-MS systems and analyses efforts is prohibitive for using this method for continuous monitoring of industrial scale, open algal ponds. Knowledge gained and biomarkers annotated from our untargeted discovery efforts may guide development of targeted, lower-cost, field-deployable detectors capable of monitoring for changes in diagnostic chemical signatures and detecting volatile signals of infection in real-time to facilitate the timely deployment interdictive strategies to prevent pond crashes. Miniaturized GC-MS systems40,41 for field deployable detector systems is one such technology currently under development and optimization42 for this type of application.
Methods
Axenic microalgae culture
Microchloropsis salina (CCMP 1776) was obtained as an axenic stock culture (as determined by the supplier) from the National Center for Marine Algae and Microbiota (NCMA at Bigelow Laboratory, ME, USA). M. salina cultures were grown as previously described in Fisher et al., 201943. For volatilomics experiments, ESAW medium was modified to contain 7.5 mM NaNO3, 0.5 mM Na2PO4. Algal cultures were grown in 15-L of medium in 20-L polycarbonate carboys at room temperature (RT) of ~ 22 °C with 24-h light intensity of ~200 µmol m−2s−1 for 5 d. Carbon dioxide gas, research purity 99.999% (Matheson Tri-Gas, NJ, USA), and research grade air (70:30 N2/O2), VOC Free (Matheson Tri-Gas, NJ, USA) were supplied to all samples via two mass flow controllers (one for CO2 and one for air). The two mass flow controllers (Alicat, AZ, USA) were set to deliver 1% CO2 (9.00 cc min−1) and 99% air (891 cc min−1), for a total mass flow of 900 cc min−1 split equally across six culture vessels (150 cc min−1 sparging rate for each sample).
Xenic marine rotifers
Lots of 10–15 × 106 live, xenic, marine rotifers, Brachionus plicatilis, were obtained from Reed Mariculture, CA, USA, 1–2 days before each inoculation and were shipped overnight on ice. Upon arrival, B. plicatilis were kept at 4 °C until concentrated and inoculated into algal culture for each experiment.
Preparation of cultures
Experimental cultures were grown in in 20-L polycarbonate carboys (ThermoFisher Scientific, MA USA) containing 15 L nutrient enriched ESAW medium, described above. Cultures were initially inoculated with M. salina culture to a final concentration of 4–5 × 106 cells mL−1 in 15 L. Cultures were continuously sparged at of 150 cc min−1 with 1% CO2 in air through an air stone bubbler.
After 48 hours of M. salina growth and acclimation to culturing conditions, 1.32 × 106 live rotifers (final concentration of 88 rotifers mL−1) were added to two of the four algal cultures. Brachionus plicatilis were allowed to warm for 1–3 h to room temperature (22 °C), were gently concentrated using a 53 µm screen filter (Florida Aqua Farms, FL, USA) down to 100 mL of culture and rinsed twice with 200 mL of ESAW medium and resuspending in 100 mL ESAW medium. Rotifers were enumerated by direct counting using a Rafter counter.
Monitoring microalgal growth and rotifer cultures
Algal culture density was determined daily by chlorophyll fluorescence (430 nm excitation, 685 nm emission) using a Tecan i-control infinite 200Pro, version 1.11.1.0. Algal cell counts were obtained via direct enumeration with a Z2 Coulter Particle Count & Size Analyzer (Beckman Coulter, Pasadena CA).or derived by calculation via a standard curve correlating chlorophyll fluorescence with algal density. Duplicate cell counts and fluorescence measurements for each sample were averaged for each timepoint and then normalized to the final concentration measurements for the M. salina control in the absence of rotifers. Health and viability of rotifers within algal cultures was monitored daily via light microscopy. Significant differences between means of healthy or infected algal cultures were compared using two-way ANOVAs with Tukey’s HSD test.
SPME headspace sampling and GC-MS data acquisition
VOCs were sampled in duplicate from the headspaces of each culture and medium control vessel using portable field sampler SPME fibers, with 65 µm polydimethylsiloxane/divinylbenzene (PDMS/DVB) coatings (Supelco, Bellefonte, PA). As two vessels were prepared for each condition, duplicate SPME samplings generated four replicate measurements. For this work, we required a “portable field sampler” fiber design to facilitate sample collection, transport, and storage over the timecourse of the experiments. The bi-phasic coating (one of three commercially available field-portable options) was chosen for sampling a wide range of compounds, including polar analytes, semi-volatiles, and larger weight volatiles. SPME samples were obtained within 1–2 hours of the fluorescence measurements that were used to determine algae concentrations. SPME exposure times were shorter for Experiment 1 (30 min) compared to Experiments 2 and 3 (60 min). SPME fibers were stored in refrigerators at 2–4 °C after sampling. Unexposed SPME fibers served as “travel blanks” to account for extraneous volatiles arising from storage conditions. Samples were analyzed by GC-MS within 2 weeks of collection.
An untargeted GC-MS approach was used to analyze the collected VOCs with an Agilent 5975 T GC-MSD (Agilent Technologies, Santa Clara, CA) using an Agilent HP-5ms column (30 m × 250 µm × 0.25 µm) coupled to a single quadrupole mass analyzer with helium carrier gas at a constant flow rate of 1.2 mL/min. VOCs absorbed on the SPME fiber were desorbed in the heated GC inlet (280 °C) for 15 seconds using splitless injection. The column temperature was programmed, starting at 40 °C for 3 min, ramped at 5 °C/minute from 40 to 150 °C, ramped at 15 °C/min from 150 to 280 °C and held for 2 min. The total run time was 35.67 min. Ions were generated using electron ionization (70 eV) and acquired at 4 scans/s over m/z 35–450. Data acquisition was performed under control of ChemStation software (Agilent Technologies, version E.02.02). A commercial reference of 18 standard compounds (S-22329; AccuStandard, New Haven, CT) was used to evaluate day-to-day performance and to calculate retention indices.
GC-MS data processing
After GC-MS data acquisition, data processing procedures and criteria were applied to detect and identify individual biomarkers in each condition. All ChemStation data files (consisting of biological duplicates, media controls, and unexposed fibers) were translated for compatibility with Agilent’s MassHunter Software (MassHunter GC/MS Translator B.07.05). Chromatographic deconvolution and visualization were performed using MassHunter Qualitative (version B.07.00 SP2) using a Retention Time window size factor of 90.0, signal-to-noise ratio threshold of 2.00, absolute ion height filter of 1000 counts, and ≥5 ions required for compound detection (threshold of detection 5 × 103 counts per peak). An arbitrary small value of 1 was assigned to the signal value for compounds that were not detected.
Detected peaks were transferred into Mass Profiler Professional (MPP) 12.6.1 software and aligned across all samples in the data set using a retention time tolerance of 0.15 minutes, mass spectral match factor of 0.6 (of maximum 1.0), and a delta m/z tolerance of 0.2 Da. Putative identification of the aligned compounds was performed by searching spectra against the National Institute of Standards and Technology (NIST) mass spectral database, NIST14. Compounds with mass spectral matches ≥70% were subsequently annotated as the best match. Compounds that did not exceed the mass spectral match threshold were annotated using the base peak m/z and retention index (e.g. “Unknown m/z ##_RI ####”).
Two criteria were used to identify volatile biomarkers unique to the Algae or Algae + Rotifer conditions: (1) detection of the biomarker in at least three of the four replicates at each sampled timepoint and (2) a) the biomarker was present in the Algae or Algae + Rotifer condition and absent in the media blank or travel blank conditions; OR b) the biomarker was present in the Algae or Algae + Rotifer conditions at an abundance greater than 10x the abundance in the media blank or travel blank.
The peak areas of potential biomarkers passing the filter criteria were compared across the three performed experiments, with regards to both individual biomarkers and groups of biomarkers belonging to the same compound class. The presence or absence of these biomarkers in each experiment was determined, and the calculated peak areas were compared to algal density measurements.
Ethical approval and informed consent
No conflicts, informed consent, human or animal rights applicable.
Supplementary information
Acknowledgements
Thank you to our amazing team of Sandia interns, Frances Carcellar and Adison McLaggen, that contributed to this work. This work was performed, in part, at Sandia National Laboratories, a multi-mission laboratory managed and operated by National Technology and Engineering Solutions of Sandia, LLC., a wholly owned subsidiary of Honeywell International, Inc., for the U.S. Department of Energy’s National Nuclear Security Administration under contract DE-NA0003525. Algal culturing, sample collection, and materials for experiments at Sandia was supported by Sandia Lab Directed Research and Development (LDRD). GC-MS analysis was supported by the US Department of Energy’s Genomic Science Program under grant SCW1039. This work was performed, in part, under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52–07NA27344. We thank Roald Leif at the LLNL Forensic Science Center (FSC) for assisting with GC-MS instrumental setup and maintenance. We thank the LLNL FSC, in particular Deon Anex, for use of laboratory equipment and space. A.D.J. acknowledges support from Michigan AgBioResearch through the USDA National Institute of Food and Agriculture, Hatch project number MICL02474. This paper describes objective technical results and analysis. Any subjective views or opinions that might be expressed in the paper do not necessarily represent the views of the U.S. Department of Energy or the United States Government.
Author Contributions
K.L.R. implemented VOC sampling, collected and analyzed the VOC data, and drafted the manuscript; C.L.F. designed the experiments, analyzed the data, and drafted the manuscript, C.L.F., P.D.L., and J.D.J. set up the experiments and collected algal density data; M.W.M., A.D.J., M.F. and T.W.L. contributed to the analysis and interpretation of the data. All authors reviewed and edited the manuscript.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-50125-z.
References
- 1.Hannon M, Gimpel J, Tran M, Rasala B, Mayfield S. Biofuels from algae: challenges and potential. Biofuels. 2010;1:763–784. doi: 10.4155/bfs.10.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leite GB, Abdelaziz AEM, Hallenbeck PC. Algal biofuels: Challenges and opportunities. Bioresource technology. 2013;145:134–141. doi: 10.1016/j.biortech.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Katiyar, R., Kumar, A. & Gurjar, B. R. In Biofuels: Technology, Challenges and Prospects (eds Avinash Kumar Agarwal, Rashmi Avinash Agarwal, Tarun Gupta, & Bhola Ram Gurjar) 157–175 (Springer Singapore, 2017).
- 4.Richardson JW, et al. A financial assessment of two alternative cultivation systems and their contributions to algae biofuel economic viability. Algal Research. 2014;4:96–104. doi: 10.1016/j.algal.2013.12.003. [DOI] [Google Scholar]
- 5.Day JG, Gong Y, Hu Q. Microzooplanktonic grazers – A potentially devastating threat to the commercial success of microalgal mass culture. Algal Research. 2017;27:356–365. doi: 10.1016/j.algal.2017.08.024. [DOI] [Google Scholar]
- 6.Carney LT, Lane TW. Parasites in algae mass culture. Front Microbiol. 2014;5:278–278. doi: 10.3389/fmicb.2014.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirayama K, Ogawa S. Fundamental Studies on Physiology of Rotifer for its Mass Culture-I: Filter Feeding of Rotifer. Nippon Suisan Gakkaishi. 1972;38:1207–1214. doi: 10.2331/suisan.38.1207. [DOI] [Google Scholar]
- 8.McBride RC, et al. Contamination Management in Low Cost Open Algae Ponds for Biofuels Production. Industrial Biotechnology. 2014;10:221–227. doi: 10.1089/ind.2013.0036. [DOI] [Google Scholar]
- 9.Park S, et al. The Selective Use of Hypochlorite to Prevent Pond Crashes for Algae-Biofuel Production. Water environment research: a research publication of the Water Environment Federation. 2016;88:70–78. doi: 10.2175/106143015x14362865227670. [DOI] [PubMed] [Google Scholar]
- 10.Pradeep V, et al. Use of Copper to Selectively Inhibit Brachionus calyciflorus (Predator) Growth in Chlorella kessleri (Prey) Mass Cultures for Algae Biodiesel Production. International journal of molecular sciences. 2015;16:20674–20684. doi: 10.3390/ijms160920674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fott B. Phlyctidium scenedesmi spec. nova, a new chytrid destroying mass cultures of algae. Zeitschrift für allgemeine Mikrobiologie. 1967;7:97–102. doi: 10.1002/jobm.19670070203. [DOI] [PubMed] [Google Scholar]
- 12.Xu C, et al. The Use of the Schizonticidal Agent Quinine Sulfate to Prevent Pond Crashes for Algal-Biofuel Production. International journal of molecular sciences. 2015;16:27450–27456. doi: 10.3390/ijms161126035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Ginkel SW, et al. Taking advantage of rotifer sensitivity to rotenone to prevent pond crashes for algal-biofuel production. Algal Research. 2015;10:100–103. doi: 10.1016/j.algal.2015.03.013. [DOI] [Google Scholar]
- 14.Liu Z, Lu G. The sterilizing studies of flagellate and cilitate in marine unicellular algae liquid. Zhanjiang Aquacult. Coll. 1990;6:36–41. [Google Scholar]
- 15.Wang H, Zhang W, Chen L, Wang J, Liu T. The contamination and control of biological pollutants in mass cultivation of microalgae. Bioresource technology. 2013;128:745–750. doi: 10.1016/j.biortech.2012.10.158. [DOI] [PubMed] [Google Scholar]
- 16.Fisher Carolyn L., Ward Christopher S., Lane Pamela D., Kimbrel Jeffrey A., Sale Kenneth L., Stuart Rhona K., Mayali Xavier, Lane Todd W. Bacterial communities protect the alga Microchloropsis salina from grazing by the rotifer Brachionus plicatilis. Algal Research. 2019;40:101500. doi: 10.1016/j.algal.2019.101500. [DOI] [Google Scholar]
- 17.United States Department of Energy. Bioenergy Technologies office Multi-Year Program Plan: May, 2013. Washington, D.C., 2013.
- 18.Borowitzka, M. A. In Algal Culturing Techniques (ed Andersen R. A.) 205–218 (Elsevier Academic Press, 2005).
- 19.Day JG, Thomas NJ, Achilles-Day UE, Leakey RJ. Early detection of protozoan grazers in algal biofuel cultures. Bioresource technology. 2012;114:715–719. doi: 10.1016/j.biortech.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Castillo-Keller M, Eustance E, Sommerfeld M. Early detection and quantification of zooplankton grazers in algal cultures by FlowCAM. Algal. Research. 2017;21:98–102. doi: 10.1016/j.algal.2016.11.012. [DOI] [Google Scholar]
- 21.Carney Laura, McBride Robert, Smith Val, Lane Todd. Microalgal Production for Biomass and High-Value Products. 2016. Molecular Diagnostic Solutions in Algal Cultivation Systems; pp. 183–204. [Google Scholar]
- 22.Carney LT, et al. Pond Crash Forensics: Presumptive identification of pond crash agents by next generation sequencing in replicate raceway mass cultures of Nannochloropsis salina. Algal Research. 2016;17:341–347. doi: 10.1016/j.algal.2016.05.011. [DOI] [Google Scholar]
- 23.Achyuthan KE, Harper JC, Manginell RP, Moorman MW. Volatile Metabolites Emission by In Vivo Microalgae-An Overlooked Opportunity? Metabolites. 2017;7:39. doi: 10.3390/metabo7030039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rowan DD. Volatile Metabolites. Metabolites. 2011;1:41–63. doi: 10.3390/metabo1010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leach JE, Triplett LR, Argueso CT, Trivedi P. Communication in the Phytobiome. Cell. 2017;169:587–596. doi: 10.1016/j.cell.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 26.Zuo Z. Why Algae Release Volatile Organic Compounds-The Emission and Roles. Front Microbiol. 2019;10:491. doi: 10.3389/fmicb.2019.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolfe, G. & Steinke, M. Grazing-Activated Production of Dimethyl Sulfide (DMS) by two clones of Emiliania huxleyi. Vol. 41 (1996).
- 28.Wolfe GV, Sherr EB, Sherr BF. Release and consumption of DMSP from Emiliania huxleyi during grazing by Oxyrrhis marina. Mar. Ecol. Prog. Ser. 1994;111:111–119. doi: 10.3354/meps111111. [DOI] [Google Scholar]
- 29.Wolfe GV, Steinke M, Kirst GO. Grazing-activated chemical defence in a unicellular marine alga. Nature. 1997;387:894–897. doi: 10.1038/43168. [DOI] [Google Scholar]
- 30.Hay ME. Marine chemical ecology: chemical signals and cues structure marine populations, communities, and ecosystems. Annual review of marine science. 2009;1:193–212. doi: 10.1146/annurev.marine.010908.163708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benevides CMdJ, Veloso MCdC, de Paula Pereira PA, Andrade JBd. A chemical study of β-carotene oxidation by ozone in an organic model system and the identification of the resulting products. Food Chemistry. 2011;126:927–934. doi: 10.1016/j.foodchem.2010.11.082. [DOI] [Google Scholar]
- 32.Christaki E, Bonos E, Giannenas I, Florou-Paneri P. Functional properties of carotenoids originating from algae. Journal of the science of food and agriculture. 2013;93:5–11. doi: 10.1002/jsfa.5902. [DOI] [PubMed] [Google Scholar]
- 33.Havaux M. Carotenoid oxidation products as stress signals in plants. The Plant journal: for cell and molecular biology. 2014;79:597–606. doi: 10.1111/tpj.12386. [DOI] [PubMed] [Google Scholar]
- 34.Ramel F, et al. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5535–5540. doi: 10.1073/pnas.1115982109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fawley MW, Jameson I, Fawley KP. The phylogeny of the genus Nannochloropsis (Monodopsidaceae, Eustigmatophyceae), with descriptions of N. australis sp. nov. and Microchloropsis gen. nov. Phycologia. 2015;54:545–552. doi: 10.2216/15-60.1. [DOI] [Google Scholar]
- 36.Van Durme J, Goiris K, De Winne A, De Cooman L, Muylaert K. Evaluation of the volatile composition and sensory properties of five species of microalgae. Journal of agricultural and food chemistry. 2013;61:10881–10890. doi: 10.1021/jf403112k. [DOI] [PubMed] [Google Scholar]
- 37.Isleten Hosoglu M. Aroma characterization of five microalgae species using solid-phase microextraction and gas chromatography–mass spectrometry/olfactometry. Food Chemistry. 2018;240:1210–1218. doi: 10.1016/j.foodchem.2017.08.052. [DOI] [PubMed] [Google Scholar]
- 38.Zhou L, et al. Change of volatile components in six microalgae with different growth phases. Journal of the science of food and agriculture. 2017;97:761–769. doi: 10.1002/jsfa.7794. [DOI] [PubMed] [Google Scholar]
- 39.Juttner F, Watson SB, von Elert E, Koster O. beta-cyclocitral, a grazer defence signal unique to the cyanobacterium Microcystis. Journal of chemical ecology. 2010;36:1387–1397. doi: 10.1007/s10886-010-9877-0. [DOI] [PubMed] [Google Scholar]
- 40.Whiting JJ, et al. A high-speed, high-performance, microfabricated comprehensive two-dimensional gas chromatograph. Lab on a chip. 2019;19:1633–1643. doi: 10.1039/c9lc00027e. [DOI] [PubMed] [Google Scholar]
- 41.Lewis PR, et al. Recent advancements in the gas-phase MicroChemLab. IEEE Sensors Journal. 2006;6:784–795. doi: 10.1109/JSEN.2006.874495. [DOI] [Google Scholar]
- 42.Snyder DT, Pulliam CJ, Ouyang Z, Cooks RG. Miniature and Fieldable Mass Spectrometers: Recent Advances. Analytical chemistry. 2016;88:2–29. doi: 10.1021/acs.analchem.5b03070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fisher, C. & Lane T.W. Operational, Prophylactic, and Interdictive Technologies in Algal Crop Protection. Grand Challenges in Algae Biotechnology (2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.