Abstract
AminoIndex Cancer Screening (AICS) is a novel cancer screening test based on plasma free amino acid (PFAA) levels. This system categorises subjects as rank A, B, or C in order of increasing probability of each cancer incidence. The current study aimed to validate the potential of AICS for cancer detection. AICS values were determined from the PFAA levels in subjects examined at Chiba Cancer Center Cohort, Mitsui Memorial Hospital, and Saihaku Hospital, and the cancer incidence was investigated. The sensitivities of rank C for cancer diagnosis within 1 year after AICS examination were 83.3% (10/12) for gastric, 50.0% (2/4) for lung, 46.2% (6/13) for colorectal, 50.0% (8/16) for prostate, 43.8% (7/16) for breast, and 50.0% (1/2) for uterine/ovarian cancer. The total cancer detection rate via AICS was 0.33% (34/10,245). The sensitivities during the maximum follow-up period of 6.2 years were 51.7% (15/29) for gastric, 18.2% (2/11) for lung, 28.6% (8/28) for colorectal, 36.4% (8/22) for prostate, 29.0% (9/31) for breast, and 33.3% (2/6) for uterine/ovarian cancers. In conclusion, AICS is a more useful method for evaluating the probability of cancer incidence than for predicting onset, suggesting that annual AICS should be recommended to detect any malignancy.
Subject terms: Computational biology and bioinformatics, Diagnostic markers
Introduction
Cancer mortality has been constantly increasing in Japan, and cancer has been the leading cause of death since 19811. In 2016, 28.5% of Japanese people died of cancer, and this is attributed to prolonged life expectancies and changes in dietary habits. As such, the prevention and early detection and treatment of cancer are becoming increasingly important. Cancer screening is known to be among the most effective strategies for early detection of cancer. Currently, the Japanese government distributes vouchers and provides free consultation services to encourage their citizens to undergo cancer screening. Despite these efforts, one of the current problems of cancer screening in Japan is a low medical examination rate because medical examinations for individual cancer types are time consuming and cause a physical burden. Moreover, some screening tests have low accuracy. Thus, a more convenient screening method is expected to increase the examination rate.
Metabolomics is one of the approaches intensively investigated for cancer diagnosis2–5. Particularly, because amino acids play essential physiological roles both as basic metabolites and metabolic regulators, plasma free amino acids (PFAAs) are promising biomarkers. PFAA profiles are known to be influenced by metabolic variations induced by specific diseases such as various cancers, liver failure, renal failure, or inflammatory bowel disease6–9.
As for cancer screening, changes in PFAA profiles have been examined in various cancers and has led to the development of AminoIndex Cancer Screening (AICS) test. AICS was developed using the “AminoIndex Technology,” which scores the probability of disease occurrence via multivariate analysis with PFAA concentration as a variable10,11. Currently, AICS is used in clinical practice in Japan to screen for gastric cancer, lung cancer, colon cancer, pancreatic cancer, prostate cancer, breast cancer, and uterine/ovarian cancer12–15.
Specifically, AICS was developed based on a multivariate analysis of PFAA concentrations compared with approximately 2,900 patients with various cancer and approximately 13,000 healthy controls through a case-control study. AICS (gastric), AICS (lung), AICS (colorectal), AICS (pancreatic), AICS (prostate), and AICS (breast) are screening markers for the corresponding cancer. Because PFAA changes were similar in cervical, endometrial, and ovarian cancer, AICS(uterine/ovarian) was developed for comprehensive screening of these three cancer types. From the amino acid data, the probability of each cancer incidence is expressed as a numerical AICS value of 0.0 to 10.0. As a criterion of the probability of each cancer, rank A is ranged from 0.0 to 4.9, rank B is ranged from 5.0 to 7.9, and rank C is ranged from 8.0 to 10.016–20. The higher AICS value, the higher the probability of each cancer incidence. In this study, we aimed to validate the clinical usefulness of AICS for cancer detection.
Results
Subject characteristics
The cohort comprised 10, 245 individuals who underwent AICS in Chiba Cancer Center cohort (N = 2,886, research cohort spanning urban area to rural area), Mitsui Memorial Hospital (N = 4,967, medical check-up in urban area), and Saihaku Hospital (N = 2,392, medical check-up in rural area). The male-to-female ratio was 1: 1.1. The mean age ± standard deviation of all 10,245 subjects (4,819 men and 5,426 women) at the time of the blood sampling was 59 ± 11 (range 24–93) years. The distribution of subjects by sex and age in each facility are shown in Table 1. The rank distribution of each AICS is shown in Fig. 1. All 10,245 subjects were examined using AICS (gastric), AICS (lung), and AICS (colorectal), whereas 4,819 men were examined using AICS (prostate), and 5,426 women were examined using AICS (breast) and AICS (uterine/ovarian). In total, 16.2%, 9.9%, 8.8%, 15.2%, 11.2%, and 9.0% of patients were categorised as rank C in AICS (gastric), AICS (lung), AICS (colorectal), AICS (prostate), AICS (breast), and AICS (uterine/ovarian),respectively. In total, 127 patients were diagnosed with cancer within the follow-up period (gastric cancer, 29; lung cancer, 11; colorectal cancer, 28; prostate cancer, 22; breast cancer, 31; endometrial cancer, 4; and ovarian cancer, 2) (Table 2). Of these, approximately 50% (63 patients) were diagnosed within 1 year after AICS examination. The maximum follow-up period was 6.2 years. Regarding cancer patients, 83.3%, 50.5%, 46.2%, 50.2%, 43.8%, and 50.0% of the corresponding cancer patients were categorised as rank C in AICS (gastric), AICS (lung), AICS (colorectal), AICS (prostate), AICS (breast), and AICS (uterine/ovarian), respectively (Fig. S1).
Table 1.
Subject characteristics.
| Chiba Cancer Center Cohort | Mitsui Hospital | Saihaku Hospital | Total | |
|---|---|---|---|---|
| Number of subjects | 2,886 | 4,967 | 2,392 | 10,245 |
| Age (average ± SD) | 58 ± 11 | 58 ± 11 | 63 ± 11 | 59 ± 11 |
| Sex (Male/Female) | 1,033/1,853 | 2,757/2,210 | 1,029/1,363 | 4,819/5,426 |
| Age: 20–29 years | — | 14/18 | — | 14/18 |
| Age: 30–39 years | 71/146 | 133/131 | 9/10 | 213/287 |
| Age: 40–49 years | 162/327 | 401/392 | 130/197 | 693/916 |
| Age: 50–59 years | 169/490 | 738/612 | 201/308 | 1,108/1,410 |
| Age: 60–69 years | 467/732 | 1,002/724 | 395/481 | 1,864/1,937 |
| Age: 70–79 years | 164/158 | 412/291 | 228/288 | 804/737 |
| Age: 80–89 years | — | 57/42 | 63/74 | 120/116 |
| Age: 90 + years | — | — | 3/5 | 3/5 |
Figure 1.
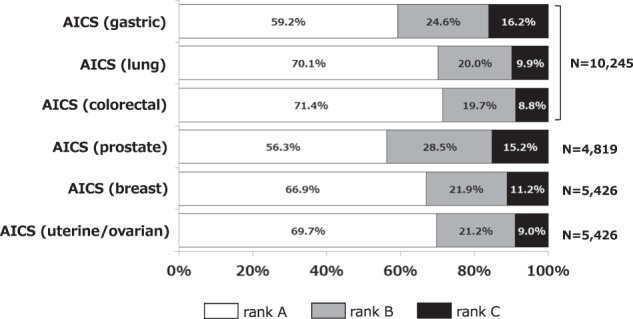
AICS rank distribution. White, grey and black parts show the distribution of rank A, B and C, respectively.
Table 2.
Number of subjects diagnosed with cancer at the different time periods.
| Type of cancer | All cases | Within 1 year | After more than 1 year | |
|---|---|---|---|---|
| Gastric cancer | 29 | 12 | 17 | |
| Lung cancer | 11 | 4 | 7 | |
| Colorectal cancer | 28 | 13 | 15 | |
| Prostate cancer | 22 | 16 | 6 | |
| Breast cancer | 31 | 16 | 15 | |
| Uterine/ovarian cancer | Cervical cancer | 0 | 0 | 0 |
| Endometrial cancer | 4 | 1 | 3 | |
| Ovarian cancer | 2 | 1 | 1 | |
| Total | 127 | 63 | 64 | |
Sensitivity
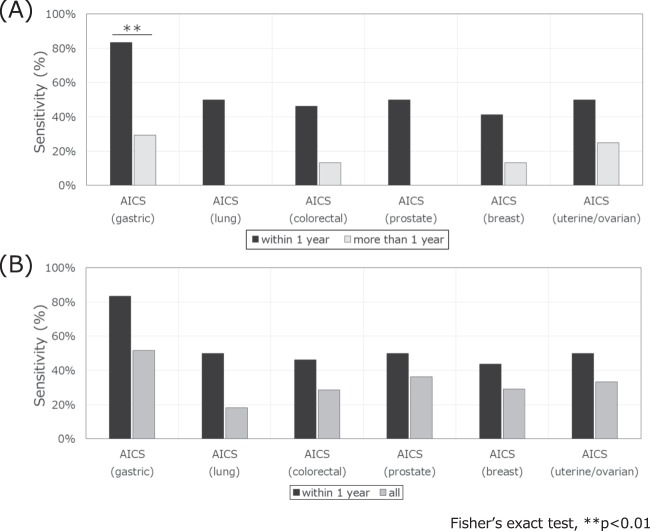
The sensitivities of the corresponding cancer in rank C within 1 year after AICS examination were 83.3% (10/12) for AICS (gastric), 50.0% (2/4) for AICS (lung), 46.2% (6/13) for AICS (colorectal), 50.0% (8/16) for AICS (prostate), 43.8% (7/16) for AICS (breast), and 50.0% (1/2) for AICS (uterine/ovarian). One patient who developed ovarian cancer was classified as rank C in AICS (uterine/ovarian). Meanwhile, the sensitivities of the corresponding cancer in rank C at more than 1 year after AICS examination were 29.4% (5/17) for AICS (gastric), 0% (0/7) for AICS (lung), 13.3% (2/15) for AICS (colorectal), 0% (0/6) for AICS (prostate), 13.3% (2/15) for AICS (breast), and 25.0% (1/4) for AICS (uterine/ovarian) (Fig. 2A, Table S1). The sensitivity of AICS (gastric) was significantly higher within 1 year than that at more than 1 year (Fisher exact test: P < 0.01). The sensitivities of AICS for other cancers were also higher within 1 year than that at more than 1 year, but the differences were not significant.
Figure 2.
Sensitivity of AICS for each type of cancer in rank C. (A) Comparison of sensitivity within 1 year (black bars) and at more than 1 year (light grey bars) after AICS examination. (B) Comparison of sensitivity within 1 year (black bars) and all follow-up periods (dark grey bars) after AICS examination. Significant difference between groups (p < 0.01, Fisher’s exact test).
The sensitivities during the follow-up periods were 51.7% (15/29) for AICS (gastric), 18.2% (2/11) for AICS (lung), 28.6% (8/28) for AICS (colorectal), 36.4% (8/22) for prostate, 29.0% (9/31) for AICS (breast), and 33.3% (2/6) for AICS (uterine/ovarian) (Fig. 2B, Table S1). The sensitivities of AICS were higher within 1 year than those at all follow-up periods, but the differences were not significant.
The sensitivities of AICS in this study compared to those in developmental case-control study13 were shown in Figs S2, S3. The sensitivity within 1 year after AICS examination was equal to or higher than that in a developmental case-control study.
Positive predictive values
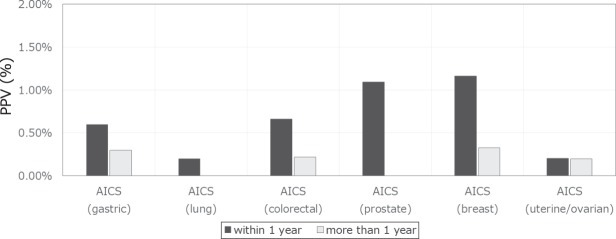
The positive predictive values (PPVs) in rank C within 1 year after AICS examination were 0.60% (10/1,660) for AICS (gastric), 0.20% (2/1,015) for AICS (lung), 0.66% (6/905) for AICS (colorectal), 1.09% (8/732) for AICS (prostate), 1.16% (7/606) for AICS (breast), and 0.20% (1/490) for AICS (uterine/ovarian). Meanwhile, the PPVs for cancer diagnosis at more than 1 year after AICS examination were 0.30% (5/1,650) for AICS (gastric), 0% (0/1,013) for AICS (lung), 0.22% (2/899) for AICS (colorectal), 0% (0/724) for AICS (prostate), 0.33% (2/599) for AICS (breast), and 0.20% (1/489) for AICS (uterine/ovarian) (Fig. 3, Table S2).
Figure 3.
Positive predictive value of AICS for each type of cancer in rank C. The black and grey bars represent the positive predictive value within and after more than 1 year after AICS examination.
The PPVs of AICS in this study compared to those in developmental case-control study were shown in Figs S4, S5. PPVs within 1 year after AICS examination, those of AICS (gastric), AICS (lung), AICS (colorectal), and AICS (uterine/ovarian) were lower than those reported in a previous case-control study, while those of AICS (prostate) and AICS (breast) were higher.
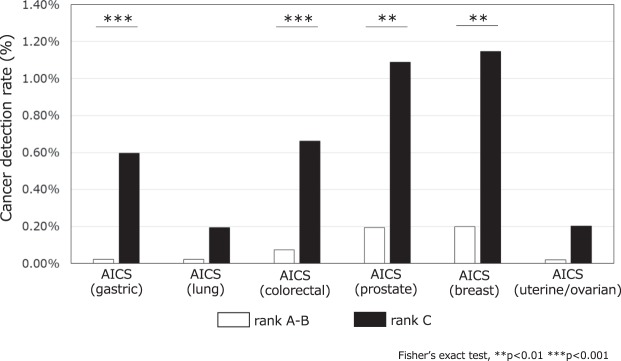
Meanwhile, the PPVs for cancer detection rates in rank A and rank B were 0.02% (2/8,585) for AICS (gastric), 0.02% (2/9,230) for AICS (lung), 0.07% (7/9,340) for AICS (colorectal), 0.20% (8/4,087) for AICS (prostate), 0.19% (9/4,820) for AICS (breast), and 0.02% (1/4,936) for AICS (uterine/ovarian) (Fig. 4). In AICS (gastric), AICS (colorectal), AICS (prostate), and AICS (breast), the cancer detection rate (PPVs) in rank C were significantly higher than those in rank A and B (AICS (gastric), P < 0.001; AICS (lung), P = 0.051; AICS (colorectal), P < 0.001; AICS (prostate), P = 0.001; AICS (breast), P = 0.001; and AICS (uterine/ovarian), P = 0.172).
Figure 4.
Cancer detection rate by each AICS rank. The white bars represent the cancer detection rate in AICS rank A and rank B, while the black bars indicate the rate in AICS rank C.
Discussion
In this study, we validated the usefulness of AICS as a cancer screening tool through a large multicentre prospective study of 10,245 subjects in Japan. We found that AICS is a more useful method for evaluating the probability of cancer incidence than for prediction of cancer onset.
Both the incidence and mortality rates of cancer are increasing worldwide21. Therefore, the implementation of methods for the prevention, early detection, and treatment of cancers is crucial to reduce mortality. Various screening methods have been established for cancers, but it can be expensive, time consuming, and physically and/or mentally taxing to undergo each screening examination separately. Blood cancer screening tests are useful modalities with a low physical burden, whereas most of the existing tumour markers are not adequate for early cancer detection22.
As previously reported, AICS was developed as a novel blood test for simultaneous screening of gastric, lung, colorectal, pancreatic, prostate, breast, and uterine/ovarian cancers based on the differences in PFAA in each cancer patient and healthy control12–15. These previous case-control studies reported that statistically significant changes in specific amino acid concentrations were observed even in the early stage of each cancer. Also, AICS has been reported to be a more useful tool for the early detection of various cancers compared to the existing tumour markers23.
In this study, the capability of AICS for cancer detection was prospectively investigated in various populations. AICS rank distribution was 56.3–71.4%, 19.7–28.5% and 8.8–16.2% for rank A, rank B and rankC, respectively. Sensitivities in rank C within 1 year after AICS examination were 83.3% 50.0%, 46.2%, 50.0%, 43.8%, and 50.0% for AICS (gastric), AICS (lung), AICS (colorectal), AICS (prostate), AICS (breast), and AICS (uterine/ovarian), respectively. The sensitivities and PPVs in rank C at more than 1 year were lower than those within 1 year. AICS was confirmed to be a more useful cancer screening tool for evaluating the probability of cancer incidence at the time of examination or within 1 year after AICS examination than for predicting cancer onset. Thus, AICS is recommended to be conducted annually for early detection of various cancers. Also, the results of AICS was suggested to reflect the cancer-bearing condition. We previously reported that AICS values decreased significantly after curative resection of various cancers24–26, suggesting that PFAA changes in AICS indicate cancer as a tumour marker.
Various possible mechanisms for PFAA profile changes in patients with cancer have been reported, including involvement of the metabolic changes in local cancer, induction of remote organ metabolic changes, or involvement of the immune system27–29. However, several points regarding the mechanisms behind changes in PFAA profiles in patients with cancer remain unclear, and thus further research is needed to clarify these issues.
In the current study, the sensitivity within 1 year after AICS examination was equal to or higher than that in a developmental case-control study13 (Figs S1, S2). The total cancer detection rate by AICS was 0.33% (34/10,245), which was superior to the 0.26% reported in the results of a national survey conducted by the Japan Society of Ningen Dock in 201530.
Regarding PPVs within 1 year after AICS examination, those of AICS (gastric), AICS (lung), AICS (colorectal), and AICS (uterine/ovarian) were lower than those reported in a previous case-control study13, while those of AICS (prostate) and AICS (breast) were higher (Figs S3, S4).
In the previous case-control study, the PPV was calculated by estimating the morbidity according to nationwide age class in Japan (0.10% for gastric, 0.09% for lung, 0.13% for colorectal, 0.33% for prostate, 0.13% for breast, and 0.09% for uterine/ovarian cancer)31. In this study, the cancer incidence within 1 year after AICS examination was 0.12% for gastric, 0.04% for lung, 0.13% for colorectal, 0.33% for prostate, 0.29% for breast, and 0.04% for uterine/ovarian cancer. For accurate comparison of PPVs between different studies, the number of cancer incidence in each group should be considered.
Significantly higher cancer detection rates in rank C than those in rank A and B were observed, suggesting the usefulness of AICS to identify the population with the highest probability of cancer incidence, allowing for detailed examinations of each cancer.
In conclusion, AICS was validated to be a useful cancer screening tool to evaluate the risk of various cancer in a large scale multicentre prospective study. This indicates that annual AICS should be recommended to detect any malignancy.
Methods
Ethical considerations
This prospective observational study was performed in accordance of the Declaration of Helsinki, and the study’s protocol was approved by the ethics committees of Chiba Cancer Center, Mitsui Memorial Hospital, and Saihaku Hospital. All participants provided written informed consent for inclusion in the study. All data were analysed anonymously throughout the study.
Subjects
The study enrolled individuals who underwent AICS in Chiba Cancer Center or Mitsui Memorial Hospital or Saihaku Hospital between January 2010 and December 2015.
Measurement of plasma amino acid concentrations
Blood samples (5 mL) were collected from a forearm vein in the morning after an overnight fasting, using tubes containing ethylenediaminetetraacetic acid (Termo, Tokyo, Japan). These were immediately placed on ice. Plasma was prepared via centrifugation at 3,000 rpm (15 min at 4 °C) and was stored at −80 °C until the analysis. The plasma samples were deproteinised before the measurements using acetonitrile at a final concentration of 80%, and the PFAA concentrations were measured using high-performance liquid chromatography/electrospray ionization tandem mass spectrometry with pre-column derivatization. These analytical methods have been described previously32–34.
Calculating AICS values and ranks
AICS values were classified as rank A, B, or C based on a previous report13. AICS values range from 0.0 to 10.0; a value of 5.0 and 8.0 has 80% and 95% specificity, respectively13,17–20. Higher values are associated with a greater probability of each cancer. For determining the probability of each cancer based on AICS value, the values were categorised into 3 as follows: rank A, 0.0–4.9; rank B, 5.0–7.9; and rank C, 8.0–10.0. In this study, the clinical performance of AICS (gastric), AICS(lung), AICS(colorectal), AICS(prostate), AICS(breast) and AICS(uterine/ovarian) were validated, while AICS (pancreatic) was excluded because because it was not commercially available.
Sensitivity and positive predictive value
The sensitivity in rank C of each AICS was calculated as the ratio of the number of patients with cancer categorised as rank C to the number of patients in the overall cohort with the corresponding cancer type. The PPV in rank C was calculated as the ratio of the number of patients with cancer categorised as rank C to the total cohort categorised as rank C. At the Chiba Cancer Center, cancer incidence was calculated from collated data from the regional cancer registry (death certificate notification: 8.8%; death certificate only: 3.4%). In Mitsui Memorial Hospital and Saihaku Hospital, detailed examinations were performed for rank C subjects, and information on cancer incidence was collected from the results of these detailed examinations. Also, subjects in Saihaku Hospital were tracked based on regional follow-up surveillance. Information on cancer incidence in rank A and rank B subjects was collected from the results in the health check-up records.
Statistical analysis
All statistical analyses were performed using Fisher’s exact test. All analyses were performed using R software (version 3.2.2; The R Foundation for Statistical Computing, Vienna, Austria). P < 0.05 was considered significant.
Supplementary information
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Author Contributions
Study concept and design: H.M., O.K., Y.N. and M.Y.; data acquisition and analysis: H.M., O.K., H.Y., S.K., Y.K., T.A., and M.Y.; drafting of the manuscript: H.M. All authors have reviewed and approved the final manuscript.
Data Availability
Requests for data and materials should be addressed to the corresponding author.
Competing Interests
This study was supported by Grants-in-Aid for Scientific Research for Priority Areas of Cancer (No. 17015018) and Innovative Areas (No. 221S0001) and by JSPS KAKENHI Grants (No. 16H06277) from the Japanese Ministry of Education, Culture, Sports, Science and Technology and also funded by Ajinomoto Co., Inc. and authors HY, TA, and SK are employees of Ajinomoto, Co., Inc. However, this does not alter the authors’ adherences to all of the journal policies.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-50304-y.
References
- 1.Foundation for Promotion of Cancer Research. In: Cancer statics in Japan-2017. The Editorial Board of the Cancer Statics in Japan, editor. Tokyo: FPCR c/o National Cancer Center; pp. 1–135 (2018).
- 2.Li Y, Kefeng L, Xiaoye Z. Next-generation metabolomics in lung cancer diagnosis, treatment and precision medicine: mini review. Oncotarget. 2017;8(70):115774–115786. doi: 10.18632/oncotarget.22404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang F, et al. Metabolomics for biomarker discovery in the diagnosis, prognosis, survival and recurrence of colorectal cancer: a systematic review. Oncotarget. 2017;8(21):35460–35472. doi: 10.18632/oncotarget.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly RS, Vander Heiden MG, Giovannucci E, Mucci LA. Metabolomic biomarkers of prostate cancer: prediction, diagnosis, progression, prognosis and recurrence. Cancer Epidemiol Biomarkers Prev. 2016;25(6):887–906. doi: 10.1158/1055-9965.EPI-15-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fahrmann Johannes F, Bantis Leonidas E, Capello Michela, Scelo Ghislaine, Dennison Jennifer B, Patel Nikul, Murage Eunice, Vykoukal Jody, Kundnani Deepali L, Foretova Lenka, Fabianova Eleonora, Holcatova Ivana, Janout Vladimir, Feng Ziding, Yip-Schneider Michele, Zhang Jianjun, Brand Randall, Taguchi Ayumu, Maitra Anirban, Brennan Paul, Max Schmidt C, Hanash Samir. A Plasma-Derived Protein-Metabolite Multiplexed Panel for Early-Stage Pancreatic Cancer. JNCI: Journal of the National Cancer Institute. 2018;111(4):372–379. doi: 10.1093/jnci/djy126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer JE, et al. The effect of normalization of plasma amino acids on hepatic encephalopathy in man. Surgery. 1976;80:77–91. [PubMed] [Google Scholar]
- 7.Holm E, Sedlaczek O, Grips E. Amino acid metabolism in liver disease. Curr Opin Clin Nutr Metab Care. 1999;2:47–53. doi: 10.1097/00075197-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Hong SY, Yang DH, Chang SK. The relationship between plasma homocysteine and amino acid concentrations in patients with end-stage renal disease. J Ren Nutr. 1998;8:34–39. doi: 10.1016/S1051-2276(98)90035-8. [DOI] [PubMed] [Google Scholar]
- 9.Hisamatsu T, et al. Novel, objective, multivariate biomarkers composed of plasma amino acid profiles for the diagnosis and assessment of inflammatory bowel disease. PLoS One. 2012;7:e31131. doi: 10.1371/journal.pone.0031131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noguchi Y, et al. Network analysis of plasma and tissue amino acids and the generation of an amino index for potential diagnostic use. Am. J. Clin. Nutr. 2006;83:513S–519S. doi: 10.1093/ajcn/83.2.513S. [DOI] [PubMed] [Google Scholar]
- 11.Kimura T, Noguchi Y, Shikata N, Takahashi M. Plasma amino acid analysis for diagnosis and amino acid-based metabolic networks. Curr. Opin. Clin. Nutr. Metab. Care. 2009;12:49–53. doi: 10.1097/MCO.0b013e3283169242. [DOI] [PubMed] [Google Scholar]
- 12.Miyagi Y, et al. Plasma free amino acid profiling of five types of cancer patients and its application for early detection. PLoS One. 2011;6:e24143. doi: 10.1371/journal.pone.0024143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okamoto N. Use of “AminoIndex technology” for cancer screening. Ningen Dock. 2012;26:911–922. [Google Scholar]
- 14.Miyagi E, et al. Diagnostic performance and clinical utility of novel gynecologic cancer screening method based on “AminoIndex technology”. Ningen Dock. 2012;26:749–755. [Google Scholar]
- 15.Fukutake N, et al. A novel multivariate index for pancreatic cancer detection based on the plasma free amino acid profile. PLoS One. 2015;10:e0132223. doi: 10.1371/journal.pone.0132223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yatabe J, Yatabe MS, Ishibashi K, Nozawa Y, Sanada H. Early detection of colon cancer by amino acid profiling using AminoIndex Technology: a case report. Diagn Pathol. 2013;8:203. doi: 10.1186/1746-1596-8-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamakado M, et al. Study on Usefulness of AminoIndexTM Cancer Screening as a Novel Cancer Screening Method – The First Report - Ningen Dock. 2013;28:673–677. [Google Scholar]
- 18.Yamakado M, et al. Study on Usefulness of AminoIndexTM Cancer Screening as a Novel Cancer Screening Method: The Second Report. Ningen Dock. 2014;28:763–767. [Google Scholar]
- 19.Yamakado M, et al. Study on Usefulness of AminoIndex® Cancer Screening as a Novel Cancer Screening Method - Third Report - Ningen Dock. 2017;31:681–688. [Google Scholar]
- 20.Kimura O, et al. Cancer risk screening based on AminoIndex –its application to health examinination for community residents-: The second report. The Journal of the Tottori Medical Association. 2015;43:94–101. [Google Scholar]
- 21.Fitzmaurice C, et al. Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018;4(11):1553–1568. doi: 10.1001/jamaoncol.2018.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagpal M, et al. Tumor markers: A diagnostic tool. J. Natl. Maxillofac. Surg. 2016;7:17–20. doi: 10.4103/0975-5950.196135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyagi Y, et al. Clinical Utility of AminoIndex Cancer Screening (AICS) for Early Detection of Various Cancers in Comparison with Detection Using Tumor Markers. Ningen Dock. 2014;29:585–591. [Google Scholar]
- 24.Katayama K, et al. Perioperative dynamics and significance of plasma-free amino acid profiles in colorectal cancer. BMC Surg. 2018;18(1):11. doi: 10.1186/s12893-018-0344-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anayama T, et al. Post-operative AICS status in completely resected lung cancer patients with pre-operative AICS abnormalities: predictive significance of disease recurrence. Sci Rep. 2018;8(1):12378. doi: 10.1038/s41598-018-30685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki Y, et al. Normalization of abnormal plasma amino acid profile-based indexes in patients with gynecological malignant tumors after curative treatment. BMC Cancer. 2018;18(1):973. doi: 10.1186/s12885-018-4875-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaelin WG, Thompson CB. Q&A: Cancer: clues from cell metabolism. Nature. 2010;465(7298):562–564. doi: 10.1038/465562a. [DOI] [PubMed] [Google Scholar]
- 28.Luo Y, et al. Cancer usurps skeletal muscle as an energy repository. Cancer Res. 2014;74:330–340. doi: 10.1158/0008-5472.CAN-13-1052. [DOI] [PubMed] [Google Scholar]
- 29.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J. Clin. Invest. 2007;117(5):1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Japan Society of Ningen Dock: Outline of Ningen Dock. https://www.ningen-dock.jp/other/release.
- 31.Center for Cancer Control and Information Services, National Cancer Center, Japan: Cancer Information Services. https://ganjoho.jp/en/professional/statistics/brochure/index.html.
- 32.Shimbo K, et al. Automated precolumn derivatization system for analyzing physiological amino acids by liquid chromatography/mass spectrometry. Biomed. Chromatogr. 2010;24:683–691. doi: 10.1002/bmc.1346. [DOI] [PubMed] [Google Scholar]
- 33.Shimbo K, Oonuki T, Yahashi A, Hirayama K, Miyano H. Precolumn derivatization reagents for high-speed analysis of amines and amino acids in biological fluid using liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2009;23:1483–1492. doi: 10.1002/rcm.4026. [DOI] [PubMed] [Google Scholar]
- 34.Shimbo K, Yahashi A, Hirayama K, Nakazawa M, Miyano H. Multifunctional and highly sensitive precolumn reagents for amino acids in liquid chromatography/tandem mass spectrometry. Anal. Chem. 2009;81:5172–5179. doi: 10.1021/ac900470w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Requests for data and materials should be addressed to the corresponding author.