Abstract
Thermal ablation has proven beneficial for hepatocellular carcinoma and possibly for colorectal liver metastases, but data is lacking for other liver metastases. Computer-assisted navigation can increase ablation efficacy and broaden its indications. We present our experience with percutaneous stereotactic image-guided microwave ablation (SMWA) for non-colorectal liver metastases (NCRLM), in form of a retrospective study including all SMWA for NCRLM from 2015 to 2017. Indication for SMWA was determined at a multidisciplinary tumorboard. End-points include recurrence, overall and liver-specific disease progression and complications. Twenty-three patients underwent 25 interventions for 40 lesions, including 17 neuroendocrine tumor, nine breast cancer, four sarcoma, two non-small cell lung cancer, three duodenal adenocarcinoma, one esophageal adenocarcinoma, one pancreatic adenocarcinoma, one ampullary carcinoma, one prostate carcinoma, and one renal cell carcinoma metastases. Median follow-up was 15 months (2–32). Incomplete ablation rate was 2.5% (1/40), local recurrence rate 10% (4/40). Three patients (12%) had minor complications. Overall disease progression was 73.9% (17/23), median disease-free survival 7 months (0–26) and overall survival 18 months (2–39). SIMWA is feasible, safe and minimally invasive for NCRLM in selected patients. While it might offer an alternative to resection or palliative strategies, the oncological benefit needs to be evaluated in a larger patient cohort.
Subject terms: Targeted therapies, Surgical oncology
Introduction
Ablation is an increasingly recognized alternative to surgery for liver tumors in patients with impaired liver function, associated extrahepatic disease, lesions inaccessible to surgical resection, extensive bilobar metastatic disease or concurrent medical conditions precluding an operation1.
Thermal Ablation techniques include radiofrequency ablation (RFA) and microwave ablation (MWA). The advantages of MWA are the potential to treat larger tumors faster, with a reduced perivascular heat sink effect2. MWA is well recognized for hepatocellular carcinoma (HCC), where it has been shown to be a safe, effective and minimally-invasive treatment option, that can be repeated in case of local recurrence3. It is also increasingly recognized for colorectal liver metastases (CRLM), where it is being investigated in the setting of prospective randomized clinical trials, comparing surgery to thermal ablation, such as the COLLISION Trial4–7.
Furthermore, the use of MWA for the treatment of other liver metastases with curative intent, such as is the case for hepatic neuroendocrine tumor (NET) metastases, is gaining popularity. When feasible, treatment with curative intent for hepatic NET metastases consists in (if necessary, repeated) liver resection, resulting in improved survival8–10. However, while current data show that RFA of NET liver metastases may be beneficial, there is still no conclusive evidence on the efficacy of MWA for such lesions11. Data is even scarcer on the treatment of other hepatic tumor entities using MWA and the benefit of such local treatment strategies remains unproven.
More recently, image-guided ablation is increasingly being combined with computer-assisted stereotactic navigation12. Computer-assisted stereotactic navigation is particularly interesting in the setting of very small or invisible “vanishing” lesions (targeting accuracy), very large lesions requiring multiple needle placements in order to achieve complete ablation, or difficult-to-reach or treat lesions (close proximity to major vessels/bile ducts, liver dome, segment I lesions). The use of computer-assisted stereotactic navigation has shown to improve the precision of the needle placement13–15, but data is lacking on complete ablation rates and long-term results.
The aim of our study is to analyze our cohort of patients with liver metastasis of non-colorectal, non-HCC origin treated with stereotactic image-guided microwave ablation (SMWA) between 2015 and 2017.
Methods
Between January 2015 and December 2017, 163 patients with 286 lesions were treated with SMWA in our institution, a certified cancer center. Most patients had HCC or CRLM. We performed a retrospective analysis of prospectively collected data from all patients undergoing SMWA for liver metastases of non-colorectal, non-HCC origin in this time period. SMWA was carried out as per recommendation by our multidisciplinary tumorboard. Obligatory participants include a radiologist, an interventional radiologist, a hepatologist, an oncologist, a radiooncologist, a pathologist and a hepatobiliary surgeon. Exclusion criteria were cross-sectional images (CT or MRI) indicative for, or biopsy proven HCC, CRLM or benign lesions. Clinical data was obtained from the electronic patient files.
End-points included local recurrence rate at the site of ablation, appearance of new intrahepatic or extrahepatic lesions, post-interventional complications according to the Clavien-Dindo Classification16 and hospital length of stay.
Follow-up consisted of 3-monthly clinical and radiological (CT scan or MRI) check-ups. Additional oncological follow-up was carried out at the discretion of and as recommended by the treating oncologist.
Incomplete ablation was defined as detectable tumor on the edge of the ablation zone on the first post-SMWA follow-up image. An initial imaging is carried out directly post-ablation while the patient is still in the CT suite, allowing for immediate validation of treatment efficacy. Local recurrence was defined as the presence of a detectable tumor within 10 mm from the edge of the ablation zone17.
The study protocol was approved by the Regional Ethical Review Boards in Bern (Kantonale Ethikkommission Bern, KEK-Nr. 2017-01038). General consent was obtained at time of hospitalisation, but specific consent was waived due to the retrospective study design. The study was performed in accordance with the relevant national guidelines and regulations.
Stereotactic image-ablation
All CT-guided interventions were performed by a dedicated interdisciplinary team of surgeons and radiologists, based on CT imaging. Trajectory planning, probe positioning and validation of treatment was conducted using a commercially available navigation system for interventional radiology (CAS-ONE, CASination AG, Bern, Switzerland). Microwave energy (Acculis MTA System, AngioDynamics, Latham, NY, USA) was used.
Interventions were performed under general anesthesia, and patients were ventilated using high frequency jet ventilation, ensuring minimal diaphragmatic movement during the procedure.
The procedure included the following steps: Planning of the trajectory of the ablation needle, navigated positioning of the ablation needles in the lesion, validation of their localisation in the liver and ablation of the lesion using 100 W energy. The duration of ablation was determined according to the size of the lesion, the proximity to blood vessels or bile ducts and the quality of the surrounding liver tissue. Finally, the pre- and post-ablation CT scans were overlayed to validate the ablation zone.
Statistical analysis
Descriptive statistics were used to present patient characteristics and results. Continuous data is presented as total number, percentage, mean and standard deviation or median and range. All analyses were performed using a commercially available software (SPSS version 25).
Results
Patient characteristics
In total, 23 patients required 25 interventions to treat 40 malignant liver lesions of non-colorectal, non-HCC origin.
Most patients were male, median age was 61 years and 74% of patients presented with significant comorbidities, ASA III or more (Table 1)18.
Table 1.
Patients characteristics (n = 23).
| Variable | |
|---|---|
| Mean age, years (range) | 58.4 (7–79) |
| Male gender, n (%) | 13 (56.5) |
| ASA classification, n (%) | |
| ASA II | 6 (26.1) |
| ASA III | 16 (69.6) |
| ASA IV | 1 (4.3) |
| Identification of liver metastases, n (%) | |
| Before primary tumor | 1 (4.3) |
| At the same time | 2 (8.7) |
| After primary tumor | 20 (87) |
| Median time between initial diagnosis and metastasis, months (range) | 19 (0–312) |
| Previous treatment for liver metastases, n (%) | 5 (21.7) |
| IRE | 1 (4.3) |
| Surgical resection only | 2 (8.7) |
| Surgical resection and open MWA | 1 (4.3) |
| Laparoscopic MWA | 1 (4.3) |
| Extra-hepatic disease at time of SMWA, n (%) | 3 (13) |
| Systemic therapy at time of SMWA, n (%) | 7 (30.4) |
ASA American Society for Anesthesia, IRE irreversible electroporation, MWA Microwaveablation.
In 87% of patients the liver metastases were diagnosed 3 to 312 months after initial diagnosis of the primary tumor. In two patients the liver metastases and the primary tumor were diagnosed at the same time. In one patient the liver metastasis was diagnosed prior to identification of the primary tumor. Five patients underwent previous local treatment for liver metastases, such as laparoscopic MWA or surgical resection. Three patients had stable extrahepatic disease at the time of SMWA, and almost one third of patients underwent SMWA while under systemic therapy.
Eight patients had liver metastases originating from a NET, four from breast cancer, three from a sarcoma, two from a non-small cell lung cancer (NSCLC), and one each from a duodenal adenocarcinoma, esophageal adenocarcinoma, pancreatic adenocarcinoma, renal clear cell carcinoma, ampullary carcinoma and prostate carcinoma (Fig. 1).
Figure 1.
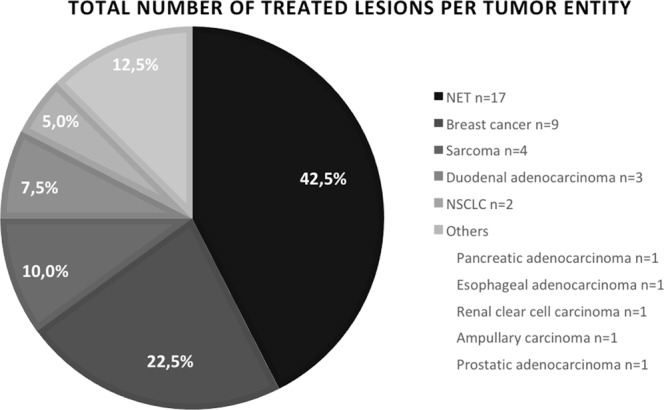
Origin and number of treated liver metastases. NET Neuroendocrine Tumor, NSCLC Non-small cell lung cancer.
The decision to use SMWA was always based on the multidisciplinary tumorboard recommendation. A detailed description can be found in Table 2.
Table 2.
Detailed results per patient.
| Patient | Tumor entity | Treatment before SMWA | Tumorboard decision | Systemic treatment at time of SMWA | Follow-up time, months |
Number of sessions | Number of treated lesions | Incomplete ablation | Local recurrence | Disease progression in the liver | Disease progression elsewhere |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NET | Resection of primary tumor, systemic Sandostatin | Ablation by adverse reaction to sandostatin treatment | none | 14 | 1 | 1 | ||||
| 2 | NET | Resection of primary tumor, systemic Sandostatin | Only two lesions, minimal progression under systemic therapy | Sandostatin | 17 | 1 | 2 | X | |||
| 3 | NET | Resection of primary tumor and Laparoscopic MWA | Single lesion on two separate occasions | none | 18 | 1 | 1 | ||||
| 4 | NET | Resection of primary tumor | Two clearly defined metastasis | none | 24 | 1 | 2 | ||||
| 5 | NET | Resection of primary tumor, systemic Sandostatin | Multiple liver metastasis, ablation of the biggest lesions (debulking) | Sandostatin | 19 | 1 | 4 | X | |||
| 6 | NET | Resection of primary tumor | Single metastasis in segment I, operative risk too high | none | 20 | 2 | 4 | X | X | ||
| 7 | NET | Metabolic therapy, resection primary tumor | Multiple liver metastasis, after systemic therapy only one remains | none | 22 | 1 | 1 | X | |||
| 8 | NET | Resection of primary tumor | To confirm diagnosis, biopsy and ablation of single lesion | none | 10 | 1 | 1 | X | X | ||
| 9 |
Breast cancer, Triple-positive |
Resection of primary tumor, radiotherapy, hormonal therapy | Single liver metastasis, biopsy indicated by previous rectum carcinoma, ablation in the same session | hormonal therapy | 29 | 1 | 2 | X | X | ||
| 10 |
Breast cancer, ER and HER-2 positive |
Resection of primary tumor, adjuvant chemotherapy, immunotherapy and radiotherapy | Two liver metastasis, good response under systemic therapy | immunotherapy | 15 | 1 | 2 | X | |||
| 11 |
Breast cancer, HER-2 positive |
Resection of primary tumor, adjuvant chemotherapy and immunotherapy | Two vanishing liver metastasis, biopsy and ablation indicated | adjuvant chemotherapy and immunotherapy | 14 | 1 | 1 | ||||
| 12 | Breast cancer, triple-negative | Resection of primary tumor, palliative chemotherapy | Single liver metastasis, young patient. 2nd ablation under chemotherapy, patient wishes | chemotherapy | 22 | 2 | 4 | X | X | ||
| 13 | Sarcoma | Resection of primary tumor, hemihepatectomy and MWA for two lesions in the left liver | No other metastasis, young patient, limited therapeutic options | none | 10 | 1 | 4 | X | |||
| 14 | Sarcoma | Resection of primary tumor | Ablation for single growing liver metastasis, with stable small pulmonary lesions | none | 22 | 1 | 1 | X | |||
| 15 | Sarcoma | Resection of primary tumor | Single liver lesion, biopsy indicated, concomitant ablation | none | 17 | 1 | 1 | X | X | ||
| 16 | NSCLC | Resection of primary tumor | Single liver lesion, biopsy and concomitant ablation indicated by DD HCC | none | 2 | 1 | 1 | X | |||
| 17 | NSCLC | Resection of primary tumor, adjuvant chemotherapy, Radiosurgery brain metastasis | Long oligometastatic course, systemic therapy contra-indicated, ablation for single lesion | none | 7 | 1 | 1 | X | X | ||
| 18 | Duodenum adenocarcinoma | Resection of primary tumor, adjuvant chemotherapy, palliative chemotherapy for local recurrence | Three isolated liver metastasis two years after end of chemotherapy, young patient | none | 10 | 1 | 3 | X | X | X | |
| 19 | Esophagus adenocarcinoma | Palliative chemotherapy (liver metastasis diagnosed before primary tumor) | Liver cirrhosis, liver lesion suspicious of HCC, biopsy and concomitant ablation indicated | none | 7 | 1 | 1 | X | X | X | |
| 20 | Pancreas adenocarcinoma | Palliative chemotherapy and radiotherapy | single lesion, atypical for metastasis from pancreatic adenocarcinoma, biopsy indicated, ablation at the same time | none | 7 | 1 | 1 | ||||
| 21 | Renal clear cell carcinoma | Resection of primary tumor | Single liver lesion, biopsy indicated, and concomitant ablation | none | 20 | 1 | 1 | ||||
| 22 | Prostatic carcinoma | Resection of primary tumor, antiresorbtive and antiandrogen therapy | Single metastasis, origin unclear by previous rectal carcinoma, biopsy and ablation indicated | antiresorbtive and antiandrogen therapy | 8 | 1 | 1 | X | |||
| 23 | Ampullary carcinoma | Resection of primary tumor, adjuvant chemotherapy | Single metastasis, stable months after end of chemotherapy | none | 6 | 1 | 1 | X | X |
MWA Microwave ablation, DD differential diagnosis, HCC Hepatocellular carcinoma.
Intervention data
Most patients underwent one SMWA session; two underwent a second SMWA session, one to treat an incomplete ablation and two new liver lesions, the other to treat a single new liver lesion. In 74% of patients (n = 17), a CT-guided biopsy of the lesion was performed during SMWA, proving the diagnosis in all but two patients, where histology was inconclusive. The smallest successfully biopsied lesion was 7 mm in diameter in this series. One to four lesions were treated per session, with all lesions being smaller than four centimeters in diameter (Table 3). One patient presented with incomplete ablation as diagnosed on the first post-SMWA imaging immediately after the intervention, and confirmed on the follow-up imaging at 3 months. The lesion had to be treated with special care initially, as it was in segment I and close to the vena cava, where the heat sink effect was higher. We successfully treated this incomplete ablation with an irreversible electroporation (IRE) session.
Table 3.
Results – Intervention.
| Variable | |
|---|---|
| Number of treated lesions, n | 40 |
| SMWA sessions, n | 25 |
| Median number of session per patient, n (range) | 1 (1–2) |
| Median number of treated lesions per session, n (range) | 2 (1–4) |
| Median lesion size, mm (range) | 13.5 (6–39) |
| Median duration of ablation, minutes (range) | 4.75 (1.25–18) |
| Intraoperative biopsy, n (%) | 17 (73.9) |
| Positive biopsy | 15 (88.2) |
| Incomplete ablation, number of lesions (%) | 1 (2.5) |
| Time to diagnosis of incomplete ablation, months | 3 |
| Re-Ablation (IRE), n | 1 |
IRE irreversible electroporation.
Lesion location did not limit treatment option, with any location within the liver amenable to SMWA. The majority of lesions were located at the liver dome (Segment VII and VIII) and in segment IV, two lesions were treated in segment I (Fig. 2).
Figure 2.
Distribution of treated liver lesions, per segment.
Post-interventional data
Length of hospital stay was two nights for the majority of patients (Table 4). There were no major complications. Three (12%) patients presented with minor complications (≤grade IIIa), including one pleuritis (grade I), one liver abscess requiring rehospitalisation and percutaneous drainage (grade IIIa), and one thrombosis of a branch of the left portal vein necessitating anticoagulation for three months (grade II).
Table 4.
Post-interventional data.
| Variable | |
|---|---|
| Median LOS, days (range) | 2 (2–14) |
| Number of patients with complications, n (%) | 3 (12) |
| Dindo-Clavien classification | |
| Grade I | 1 |
| Grade II | 1 |
| Grad IIIa | 1 |
| Median duration of follow-up, months (range) | 15 (2–32) |
| Local recurrence at ablation site, number of lesions (%) | 4 (10) |
| Time to diagnosis, months | 3 |
| Re-Ablation, n | 0 |
| Overall disease progression, n (%) | 17 (74) |
| Intrahepatic only, n (%) | 5 (29) |
| Extrahepatic only, n (%) | 4 (24) |
| Intra- and Extrahepatic, n (%) | 8 (47) |
| Deceased during follow-up, n (%) | 7 (30) |
| Disease-free survival, median in months (range) | 7 (0–26) |
| Overall survival, median in months (range) | 18 (2–39) |
LOS Length of stay.
Median follow-up was 15 months (2–32). We observed local recurrence in four patients, diagnosed on the first follow-up scan at three months. All four patients also presented with disease progression – intra- and/or extrahepatic - prompting a change in therapeutic strategy, so that no re-ablation was attempted. Overall disease progression was observed in 74% of patients (n = 17), at a median of seven months after SMWA (range 0–25). Isolated intrahepatic disease progression occurred in five patients.
Seven patients died of disease progression during the follow-up period, between 2 and 25 months after SMWA. The patient who died two months after SMWA had NSCLC, and cerebral metastases were diagnosed shortly after the intervention.
Discussion
In this study, we are able to demonstrate that stereotactic ablation is a very safe and technically feasible treatment for patients with malignant liver lesions. Using a navigated, percutaneous approach not only allows for the treatment of lesions otherwise not amenable to ablation (such as lesions located close to major vessels and bile ducts, subcapsular, subdiaphragmatic liver dome lesions, or lesion located in segment I). It also allows for simultaneous, diagnostic biopsy of very small (sub-centimetric) tumors within the liver. Being able to precisely biopsy and simultaneously ablate very small lesions is of particular interest in a patient population with a history of previous cancer or in the setting of concomitant tumors, where determining the origin of the liver metastases at a very early stage is a prerequisite for correct and rapid diagnosis and treatment. This concurs with currently available literature reports, which show that the use of a navigation system improves precision of needle placement and reduces multiple needle repositioning12–14.
SMWA can be safely performed in patients while under systemic therapy, without additional risks and without the need to stop the therapy. In this setting, SMWA may be seen as an adjunct to a systemic therapy or other local treatment strategies.
In contrast to more invasive local treatment options such as surgical tumor resection, SMWA is tolerated very well, with the vast majority of patients not suffering from any postoperative symptoms or complications, and a short hospital-stay. Postinterventional intrahepatic thrombosis have been reported in patients treated by thermal ablation close to a blood vessel19–21, and portal veins are particularly susceptible to thrombosis21, which could have serious consequences in patients with little hepatic reserve, due to underlying liver disease or previous interventions. Following this complication, we elected to treat all patients with prophylactic anticoagulation if the ablation had been performed close to a major blood vessel. One patient with a hepaticojejunostomy after a pancreaticoduodenectomy presented with an abscess in the ablation zone and was treated with antibiotics. This population has been identified as at higher risk for post-ablation abscesses22,23. Following this, we implemented a prophylactic antibiotic regimen in all patients undergoing SMWA. No further abscesses were seen. Further studies are needed to determine if this is necessary, or if prophylactic antibiotics should be reserved for high-risk patients. Other common complications include injury to the biliary tract, pleural effusion and liver dysfunction, which did not occur in this series24,25. Whether or not this can be attributed to the use of navigation technology needs to be further analyzed.
Cancer treatment is becoming increasingly personalized. In selected patients with oligometastatic disease in whom the classical systemic therapies fail to offer complete tumor response, SMWA may be an alternative treatment for local tumor control of single active tumor lesions. SMWA also allows for simultaneous acquisition of tissue for histopathological and molecular analyses, in particular in the context of now increasingly available tumor-targeted treatments26.
However, in our patient cohort, ultimately 3/4 of patients presented with some form of disease progression in the shorter or longer term. This stresses the importance of patient selection, so that the indication for SMWA or any other local treatment for hepatic metastases has to be made in the setting of a multidisciplinary tumorboard. Whether or not SMWA results in prolonged patient survival in the setting of stage IV cancer cannot be answered within this cohort.
Particularly in the setting of symptomatic hepatic NET metastases, SMWA offers a low-risk, tissue-sparing treatment option that can be repeated in case of tumor recurrence. At the moment, treatment with curative intent consists in liver resection, and has shown to improve survival9,10. Palliative therapeutic strategies for symptomatic disease also include surgical resection, sometimes as a debulking procedure. However, given the tendency for recurrence, a safe, minimally invasive solution is needed. Available data shows that RFA of NET liver metastases may be beneficial, but to this day, there is little evidence on the efficacy of SMWA of these lesions11. SMWA was performed in our patient series with stable disease, in symptomatic patients where systemic therapy was contra-indicated or when biopsy of a single lesion was needed for diagnostic purposes.
As for other, non-CRLM and non-NET liver metastasis, the benefit of resection remains unproven and there is currently few data on the oncological benefit of a local therapy for such patients10,27–32. Surgical resection might be beneficial in selected patients with well-controlled metastatic disease under systemic therapy, but with a significant morbidity27. In breast cancer for example, repeated histology becomes increasingly important for targeted therapy33. In this situation, adding local treatment to navigated biopsy of a liver metastasis is an interesting option.
Conclusion
Percutaneous SMWA is a safe and technically feasible treatment for NCRLM, which can be repeatedly performed in a low-risk minimally invasive setting and as an adjunct to systemic therapy, particularly if the lesion is unresectable or conventionally unablatable. SIMWA combines a precise biopsy (diagnostics), even for small, sub-centimetric lesions, with the actual ablation (treatment). Even in the increasingly important era of personalized and patient-oriented medicine, the indication for SMWA must always be determined by a multidisciplinary tumorboard.
Clearly, further studies are needed to validate these results and to analyze the long-term oncological and quality of life benefit for these patients.
Author Contributions
S.P., A.L., M.M. and V.B. collected the data, analyzed it and interpreted it. The study material and patients were provided by all authors (S.P., A.L., M.M., C.K.-F., D.C. and V.B.). The manuscript was written by S.P., A.L. and V.B. and edited and approved by all authors (S.P., A.L., M.M., C.K.-F., D.C. and V.B.).
Competing Interests
Stéphanie Perrodin, Anja Lachenmayer, Martin Maurer, Corina Kim-Fuchs and Vanessa Banz have no competing interests to disclose. Daniel Candinas is co-founder and shareholder in CAScination AG, manufacturer of the navigation technology under investigation in this study.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Martin RC, Scoggins CR, McMasters KM. Safety and efficacy of microwave ablation of hepatic tumors: a prospective review of a 5-year experience. Ann Surg Oncol. 2010;17:171–78. doi: 10.1245/s10434-009-0686-z. [DOI] [PubMed] [Google Scholar]
- 2.Lencioni R, de Baere T, Martin RC, Nutting CW, Narayanan G. Image-guided ablation of malignant liver tumors: recommendations for clinical validation of novel thermal and non-thermal technologies – a western perspective. Liver cancer. 2015;4:208–14. doi: 10.1159/000367747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zamora-Valdes D, Taner T, Nagorney DM. Surgical treatment of hepatocellular carcinoma. Cancer Control. 2017;24:1–15. doi: 10.1177/1073274817729258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engstrand J, et al. A multiple microwave ablation strategy in patients with initially unresectable colorectal cancer liver metastases – A safety and feasibility study of a new concept. EJSO. 2014;40:1488–93. doi: 10.1016/j.ejso.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Groeschl RT, et al. Recurrence after microwave ablation of liver malignancies: A single institution experience. HPB (Oxford). 2013;15:365–371. doi: 10.1111/j.1477-2574.2012.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groeschl RT, et al. Microwave ablation for hepatic malignancies, a multiinstitutional analysis. Ann Surg. 2014;259:1195–1200. doi: 10.1097/SLA.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 7.Pujik RS, et al. Colorectal liver metastases: surgery versus thermal ablation (COLLISION) – a phase III single-blind prospective randomized controlled trial. BMC Cancer. 2018;18:821. doi: 10.1186/s12885-018-4716-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurusamy, K. S., Ramamoorthy, R., Sharma, D. & Davidson, B. R. Liver resection versus other treatments for neuroendocrine tumours in patients with resectable liver metastases, Cochrane Database Syst Rev, 10.1002/14651858 (April 15, 2009). [DOI] [PMC free article] [PubMed]
- 9.Sarmiento JM, et al. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg. 2003;197:29–37. doi: 10.1016/S1072-7515(03)00230-8. [DOI] [PubMed] [Google Scholar]
- 10.Tan MC, Jarnagin WR. Surgical management of non-colorectal hepatic metastasis. J Surg Oncol. 2014;109:8–13. doi: 10.1002/jso.23462. [DOI] [PubMed] [Google Scholar]
- 11.Akyildiz HY, Mitchell J, Milas M, Siperstein A, Berber E. Laparoscopic radiofrequency thermal ablation of neuroendocrine hepatic metastases: Long‐term follow‐up. Surgery. 2010;148:1288–93. doi: 10.1016/j.surg.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Banz VM, et al. Intraoperative image-guided navigation system: development and applicability in 65 patients undergoing liver surgery. Langenbecks Arch Surg. 2016;401:495–502. doi: 10.1007/s00423-016-1417-0. [DOI] [PubMed] [Google Scholar]
- 13.Engstrand J, et al. Stereotactic CT-guided percutaneous microwave ablation of liver tumors with the use of high-frequency jet ventilation: an accuracy and procedural safety study. Am J Roentgenol. 2017;208:193–200. doi: 10.2214/AJR.15.15803. [DOI] [PubMed] [Google Scholar]
- 14.Beyer LP, et al. Stereotactically navigated percutaneous microwave ablation (MWA) compared to conventional MWA: a matched pair analysis. Int J Comput Assist radiol Surg. 2018;13:1991–1997. doi: 10.1007/s11548-018-1778-7. [DOI] [PubMed] [Google Scholar]
- 15.Widman G, Schullian MH, Haidu M, Bale R. Stereotactic Radiofrequency ablation (SFRA) of liver lesions: technique effectiveness, safety and interoperator performance. Cardiovasc Intervent Radiol. 2012;35:570–580. doi: 10.1007/s00270-011-0200-4. [DOI] [PubMed] [Google Scholar]
- 16.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survery. Ann Surg. 2004;240:205–2013. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tinguely P, et al. Laparoscopic image-based navigation for microwave ablation of liver tumors – a multi-center study. Surg Endosc. 2017;31:4315–24. doi: 10.1007/s00464-017-5458-4. [DOI] [PubMed] [Google Scholar]
- 18.Hurwitz EE, et al. Adding examples to the ASA-physical status classification improves correct assignment to patients. Anesthesiology. 2017;126:614–22. doi: 10.1097/ALN.0000000000001541. [DOI] [PubMed] [Google Scholar]
- 19.Kang TW, et al. Perivascular versus nonperivascular small HCC treated with percutaneous RF ablation: retrospective comparison of long-term therapeutic outcomes. Radiology. 2014;270:888–899. doi: 10.1148/radiol.13130753. [DOI] [PubMed] [Google Scholar]
- 20.Kojima Y, et al. Portal vein thrombosis caused by microwave coagulation therapy for hepatocellular carcinoma: report of a case. Surg Today. 2000;30:844–8. doi: 10.1007/s005950070071. [DOI] [PubMed] [Google Scholar]
- 21.Chang J, et al. Effects of microwave ablation on arterial and venous vasculature after treatment of hepatocellular carcinoma. Radiology. 2016;281:617–24. doi: 10.1148/radiol.2016152508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhatia SS, et al. Is antibiotic prophylaxis for percutaneous radiofrequency ablation (RFA) of primary liver tumors necessary? Results from a single-center experience. Cardiovasc Intervent Radiol. 2015;38:922–928. doi: 10.1007/s00270-014-1020-0. [DOI] [PubMed] [Google Scholar]
- 23.Ding J, et al. Complications of thermal ablation of hepatic tumours: comparison of radiofrequency and microwave ablative techniques. Clin Radiol. 2012;68:608–15. doi: 10.1016/j.crad.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Lahat E, et al. Complications after percutaneous ablation of liver tumors: a systematic review. Hepatobiliary Surg Nutr. 2014;3:317–23. doi: 10.3978/j.issn.2304-3881.2014.09.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calzadilla Bertot L, Sato M, Tateishi R, Yoshida H, Koike K. Mortality and complication rates of percutaneous ablative techniques for the treatment of liver tumors: a systematic review. Eur Radiol. 2011;21:2584–96. doi: 10.1007/s00330-011-2222-3. [DOI] [PubMed] [Google Scholar]
- 26.Hong V, Rice J, Sharma D, Martin RCG. The use of IRE in multi-modality treatment for oligometastatic pancreatic cancer. Am J Surg. 2018;216:106–110. doi: 10.1016/j.amjsurg.2018.01.037. [DOI] [PubMed] [Google Scholar]
- 27.Groeschl RT, et al. Hepatectomy for noncolorectal nonneuroendocrine metastatic cancer: a multi-institutional analysis. J Am Coll Surg. 2012;214:769–777. doi: 10.1016/j.jamcollsurg.2011.12.048. [DOI] [PubMed] [Google Scholar]
- 28.Yamanaka T, et al. Radiofrequency ablation for liver metastasis from gastrointestinal stromal tumor. J vasc Interv Radiol. 2013;24:341–46. doi: 10.1016/j.jvir.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 29.Golse N, Adam R. Liver metastases from breast cancer: what role for surgery? Indications and results. Clinical breast cancer. 2017;17:256–65. doi: 10.1016/j.clbc.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, et al. Radiofrequency ablation for liver metastasis from gastric cancer. Eur J Surg Oncol. 2013;39:701–706. doi: 10.1016/j.ejso.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 31.Guner A, et al. Liver-directed treatments for liver metastasis from gastric adenocarcinoma: comparison between liver resection and radiofrequency ablation. Gastric Cancer. 2016;19:951–960. doi: 10.1007/s10120-015-0522-z. [DOI] [PubMed] [Google Scholar]
- 32.Park JB, et al. Radiofrequency ablation of liver metastasis in patients with locally controlled pancreatic ductal adenocarcinoma. J Vasc Interv Radiol. 2012;29:635–41. doi: 10.1016/j.jvir.2012.01.080. [DOI] [PubMed] [Google Scholar]
- 33.Xiangying M, et al. Receptor conversion in metastatic breast cancer: a prognosticator of survival. Oncotarget. 2016;7:1887–1903. doi: 10.18632/oncotarget.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]



