Abstract
Objective: The POSEIDON criteria are used to stratify patients with low prognosis after assisted reproductive technology (ART) treatment. Since its introduction, there has been no large study about the prognosis of the POSEIDON population. We used the POSEIDON criteria in Chinese women who underwent repeated ART treatment and analyzed the association between POSEIDON criteria and the cumulative live-birth rate (CLBR).
Methods: This was a retrospective cohort study of 62,749 women (97,388 cycles) who underwent ART treatment at the Reproductive and Genetic Hospital of CITIC-XIANGYA between January 2014 and June 2017. Among them, 19,781 (31.52%) women fulfilled the POSEIDON criteria, including 26,697 cycles. The optimal and conservative CLBRs within a complete IVF/ICSI treatment cycle were calculated, as well as the CLBRs following repeated ovarian stimulation cycles.
Results: In POSEIDON groups 1, 2, 3, and 4, the optimal and conservative CLBRs of three complete consecutive in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) cycles were 83.87 and 66.06%, 53.67 and 37.72%, 44.24 and 27.98%, and 14.20 and 9.68%, respectively. The POSEIDON stratification [group 2: odds ratio (OR) = 2.319, 95% confidence interval (CI): 2.131–2.525, P < 0.001; group 3: OR = 1.356, 95% CI: 1.005–1.828, P = 0.046; group 4: OR = 3.525, 95% CI: 2.774–4.479, P < 0.001; all vs. group 1] and ovarian stimulation protocol [gonadotropin-releasing hormone (GnRH) antagonist protocol: OR = 1.856, 95% CI: 1.640–2.100, P < 0.001; other protocols: OR = 1.651, 95% CI: 1.155–2.361, P = 0.006; both vs. long GnRH agonist protocol] were associated with live birth in the first stimulation cycle. For the second stimulation cycle, the POSEIDON stratification (except POSEIDON group 3) and ovarian stimulation protocol were associated with live birth. A change in ovarian stimulation protocol was not associated with an improvement in the live birth rate.
Conclusions: More than 30% of women who undergo IVF/ICSI treatment may be classified as low prognosis. Different reproductive outcomes were observed among the four POSEIDON groups. The most optimal outcomes after three successive cycles of IVF/ICSI treatment were observed in groups 1, 2, and 3.
Keywords: assisted reproductive technology, poor ovarian response, POSEIDON stratification, low prognosis, cumulative live-birth rate
Introduction
Management of patients with diminished ovarian reserve (DOR) or poor ovarian response (POR) is a challenge in reproductive medicine. Most women with DOR will ultimately require in vitro fertilization (IVF) to be pregnant (1). POR limits the success of assisted reproductive technology (ART) (2). In POR, the number of oocytes, but not their quality, limits the success of ART in these patients (3). A comprehensive evaluation of the ovarian reserve and ovarian response is essential for individualized therapeutic strategy in order to optimize the success rate of ART. Many and inconsistent definitions of POR have been used, preventing the direct comparisons among studies, as well as the generalizability and applicability of the results (4). Unfortunately, despite many efforts, there are currently no tests that can reliably predict the ovarian response in all women undergoing ART treatment (2, 5).
After the Bologna criteria for POR were proposed in 2011 (6), the universal definition of POR helped investigators enroll more homogeneous populations when conducting studies in patients undergoing IVF/intracytoplasmic sperm injection (ICSI). Although the Bologna criteria were thought initially to characterize a homogeneous population, subsequent research showed that this was not the fact, and that the Bologna criteria describe a heterogeneous population with different reproductive outcomes, mainly because the effect of age on oocyte quality was not taken into consideration (7, 8). Thus, the Bologna criteria can be considered as a useful mathematical model, but the criteria did not provide enough clinical recommendations for managing this type of patient (9–11). Recently, in an effort to further refine the Bologna criteria, the Patient-Oriented Strategies Encompassing IndividualizeD Oocyte Number (POSEIDON) was proposed. These criteria stratify patients according to age (and therefore the expected euploidy rate), ovarian biomarkers, and ovarian response if a previous stimulation has been performed (7, 8). Moreover, the concept of POR was changed to low prognosis in order to better reflect the clinical difference between POR and DOR (12, 13). The aim of the POSEIDON stratification was not only to help clinicians counsel and set patient expectation, but also to establish a working plan to reduce the time to pregnancy (7, 8, 12, 13).
The primary goal for a woman undergoing ART is a live birth. The majority of patients will undergo repeated stimulation cycles, especially if they belong to the POSEIDON group of low prognosis. Therefore, the cumulative live-birth rate (CLBR) is a very meaningful parameter of efficacy and reproductive success. Until now no study explored the POSEIDON stratification in a large study population. At our institute, >28,000 IVF/ICSI cycles are performed each year.
In the present study, we retrospectively applied the POSEIDON stratification to patients who underwent repeated IVF/ICSI treatment to better determine the CLBRs according to each POSEIDON subgroups in a real world setting. The results could provide evidence to physicians regarding the potential reproductive prognosis of these patients and also indicate whether changes in ART treatment strategies could result in better outcomes.
Methods
Study Design and Patients
This was a retrospective study of women who underwent ART at the Reproductive and Genetic Hospital of CITIC-XIANGYA between January 2014 and June 2017. Women ≥18 years old who underwent IVF/ICSI were included. The exclusion criteria were: (1) women who underwent their first ovarian stimulation treatment before 2014; (2) women with adequate ovarian reserve [antral follicle count (AFC) ≥ 5 and anti-Müllerian hormone (AMH) ≥ 1.2 ng/ml] and optimal ovarian response (>9 oocytes retrieved in the first stimulation cycle); (3) women who had adequate ovarian reserve, but did not receive a standard ovarian stimulation protocol [long gonadotropin-releasing hormone (GnRH) agonist protocol or GnRH antagonist protocol] during their first stimulation cycle; or (4) women who received preimplantation genetic screening or preimplantation genetic diagnosis. This study was approved by the Ethics Committee of the Reproductive and Genetic Hospital of CITIC-XIANGYA (LL-SC-2018-033). The need for individual consent was waived by the committee due to the retrospective character of the study.
POSEIDON Stratification
The POSEIDON stratification was applied retrospectively to the patients according to the age when the patients received their first ART treatment, and based on AFC, AMH, and the number of oocytes retrieved during the first stimulation cycle (7, 8). The POSEIDON groups 1 and 2 included patients with AFC ≥ 5, AMH ≥ 1.2 ng/ml, and ≤9 oocytes retrieved after standard ovarian stimulation. Younger patients (age < 35 years) were included in POSEIDON group 1, while older patients (age ≥ 35 years) were included in POSEIDON groups 2. The POSEIDON groups 3 and 4 consisted of patients with AFC < 5 or AMH < 1.2 ng/ml. Younger patients (age < 35 years) were included in POSEIDON group 3, while older patients (age ≥ 35 years) were included in POSEIDON groups 4.
Controlled Ovarian Stimulation Protocol
Standard ovarian stimulation protocol referred to long GnRH agonist protocol or GnRH antagonist protocol. For the long GnRH agonist protocol, on day 20 of the patient's menstrual cycle, 1.5–1.875 mg of GnRH agonist were injected intramuscularly. After 13–20 days, after confirmation of pituitary-ovarian suppression, 112.5–300 IU/d of recombinant follicular-stimulating hormone (rFSH; Gonal-F or Puregon, Merck Serono S.A., Coinsins, Switzerland) were administered for 4–5 days.
For the GnRH antagonist protocol, ovarian stimulation started with 112.5–300 IU of rFSH from day 3 of the menstrual cycle. The dosage of rFSH was adjusted according to the ovarian response, which was assessed by ultrasound and serum hormone levels. A daily dose of 0.25 mg GnRH antagonist (Cetrotide, Serono, Switzerland) was initiated when a lead follicle reached a mean diameter of 14 mm; the dose was continued until the day of human chorionic gonadotropin (hCG) administration.
For both standard ovarian stimulation protocols, hCG was injected after confirmation of adequate follicle stimulation by ultrasound and serum hormone levels.
Embryo Transfer Policy
Oocyte retrieval was performed 35–36 h after trigger injection. Drugs and techniques for oocyte aspiration, oocyte, and embryo culture, insemination, ICSI, assisted hatching, and embryo transfer were performed routinely (ISO 9001 Certification) (14). No more than three embryos were transferred on day 3–5 after oocyte collection. The luteal phase was supported with vaginal progesterone (Crinone 8%; Merck Serono) in all patients.
For the frozen embryo transfer cycle, no more than three embryos were transferred to each patient. Embryos were warmed using a commercially available warming solution (Kitazato Biopharma), according to the manufacturer's instruction (15). After warming, the embryos were transferred to G1.5/G2.5 medium and cultured for 2–6 h. Only embryos in the cleavage stage that exhibited >50% intact blastomeres or blastocysts that re-expanded after warming were considered as suitable for transfer. The cleavage stage embryos or blastocysts were transferred 3 or 5 days after ovulation in a natural cycle or 3 or 5 days after progesterone supplementation in hormone replacement treatment cycle. Luteal support was applied when the dominant follicle disappeared in a natural cycle or satisfactory endometrial development (thickness ≥ 8 mm, confirmed by ultrasound examination) in hormone replacement treatment cycle.
Data Collection
Infertility is defined as the inability to conceive after 1 year of unprotected sexual intercourses (16). Duration of infertility was defined as the time from the start of regular, unprotected sexual intercourse, to enrollment. All demographic and clinical data were obtained from the database of our hospital. Follicular-stimulating hormone (FSH) levels, estradiol levels, luteinizing hormone (LH) levels, AMH levels, and AFC were measured before the first ART treatment. Serum AMH levels were measured routinely using an enzyme-linked immunosorbent assay (ELISA) kit, according to the manufacturer's instructions (KR-AMH-001; Kangrun biotech, Guangzhou, China; the antibody was from Ansh Labs, USA). The minimum detectable concentration for AMH was 0.06 ng/mL. The intra-assay coefficients of variation were <8%. AFC was defined as the number of follicles of 2–9 mm in diameter, counted at 2–5 days during menstruation using B-mode ultrasound. The maximum time between AMH assessment and the first ovarian stimulation cycle was 1 year.
Live birth was defined as a neonate showing any sign of life, irrespective of gestational age, as defined by the World Health Organization (WHO) (17).
An ovarian stimulation cycle encompassed the initiation of ovarian stimulation, aspiration, insemination, and all resulting separate fresh or frozen embryo transfers. A cycle with no oocytes retrieved after ovarian stimulation or with no embryo transfer was also considered as a stimulation cycle. Cycles canceled before oocyte retrieval were not included in the analysis.
The CLBR within one complete IVF/ICSI treatment cycle was defined as the probability of a live birth from an ovarian stimulation, including all embryo transfers (fresh and frozen) from that stimulation.
The CLBR of repeated ovarian stimulation cycles (during the study period) was defined as the probability of a live birth from all cycles during the study period. Finally, a woman who had a live birth after an ovarian stimulation cycle, regardless of fresh or frozen embryo transfer, was considered as a new patient if she underwent a new ART cycle (18, 19).
Dealing With the Discontinuation of Assisted Reproduction Technology Treatment
Infertile women may discontinue ART treatment for physical, psychological reasons, and/or relationship problems (20). Nevertheless, CLBR assumptions (both optimal and conservative) have to take into account the rate of those who discontinued would they have pursued ART treatments. The optimal estimate is based on the observed data and assumes that the CLBR in women who discontinue ART treatment without live-birth would be equal to the rate in those who continue (21). The conservative estimate assumes that those who discontinue ART treatment would have had a live-birth rate of zero (21).
Statistical Analysis
Continuous variables are expressed as means ± standard deviation, and were compared using one-way analysis of variance (ANOVA) and the post-hoc Bonferroni test. Categorical variables are expressed as frequencies (percentages), and were compared using the chi-square test or Fisher's exact test. The CLBRs within a complete IVF/ICSI treatment cycle and across all cycles, as well as the optimal and conservative estimates, were calculated. The estimated CLBR depends on the estimate of the live birth rate within each aspiration cycle (p) (22). It is calculated as CLBR = 1-(1-p1)(1-p2)…(1-pt). The optimal estimate is calculated as pt = xt/nt. The conservative estimate is calculated as (x1+x2…+xt)/n1 (23). Univariable and multivariable logistic regression analyses were used to analyze the factors associated with live birth in the first and second stimulation cycles; odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. “No live birth” was considered as the event in the logistic regression; therefore, variables with OR > 1 were risk factors (i.e., detrimental to live births) while variables with OR < 1 were protective factors (i.e., improved live birth rates). POSEIDON group 1 was taken as the reference group for CLBR prognosis. P < 0.05 was considered statistically significant.
Results
Patients
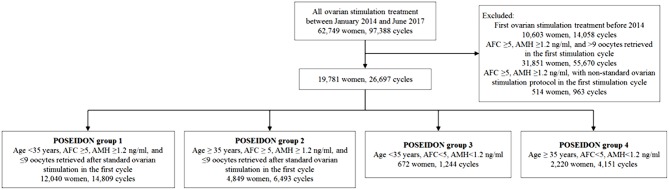
Between January 2014 and June 2017, 62,749 women underwent ovarian stimulation at the Reproductive and Genetic Hospital of CITIC-XIANGYA. Among them, 19,781 (31.52%) fulfilled the POSEIDON criteria and were included in the study. Figure 1 presents the patient flowchart.
Figure 1.
Flowchart of patient recruitment. Between January 2014 and June 2017, 62,749 women underwent ovarian stimulation. Among them, 19,781 (31.52%) fulfilled the POSEIDON criteria and were included in the study. AFC, antral follicular count; AMH, anti-Müllerian hormone.
Table 1 shows that age, BMI, duration of infertility, baseline FSH levels, and baseline estradiol levels increased with the POSEIDON group (all P < 0.001), while baseline LH levels, baseline AMH levels, baseline AFC, and the frequency of ovarian hyperstimulation syndrome (OHSS) during the study period decreased with the POSEIDON group (all P < 0.001). The major cause of infertility in the four groups was tubal factor infertility, accounting for more than 56% of the women. Regarding the ART outcomes, the number of ovarian stimulation cycles increased with the POSEIDON grade, the number of embryo transfer cycles decreased, the number of retrieved oocytes decreased, and the number of transferred embryos decreased (all P < 0.001).
Table 1.
Characteristics of the patients.
| Variable |
POSEIDON group 1 (n = 12,040) |
POSEIDON group 2 (n = 4,849) |
POSEIDON group 3 (n = 672) |
POSEIDON group 4 (n = 2,220) |
P |
|---|---|---|---|---|---|
| Age (years), mean ± SD | 29.2 ± 3.1 | 38.0 ± 2.6a | 30.6 ± 2.9ab | 40.6 ± 3.3abc | <0.001 |
| BMI (kg/m2), n (%) | <0.001* | ||||
| <18.5 | 1,172 (9.73) | 183 (3.77)a | 65 (9.67)b | 56 (2.52)abc | |
| 18.5–25 | 9,249 (76.82) | 3,749 (77.31) | 523 (77.83) | 1,778 (80.09)a | |
| >25 | 1,619 (13.45) | 917 (18.91)a | 84 (12.50)b | 386 (17.39)ac | |
| Duration of infertility (years), mean ± SD | 4.06 ± 2.66 | 6.65 ± 5.00a | 4.61 ± 3.02ab | 7.29 ± 5.77ac | <0.001 |
| Cause of infertility, n (%) | <0.001# | ||||
| Tubal | 6,769 (56.22) | 2,925 (60.32)a | 382 (56.85) | 1,338 (60.27)a | |
| Ovulation disorder | 1 (0.01) | 0 (0) | 0 (0) | 0 (0) | |
| Endometriosis | 98 (0.81) | 40 (0.82) | 15 (2.23)ab | 12 (0.54)c | |
| Male | 599 (4.98) | 125 (2.58)a | 17 (2.53)a | 67 (3.02)a | |
| Others | 7 (0.06) | 4 (0.08) | 2 (0.30) | 5 (0.23) | |
| Multiple causes | 4,289 (35.62) | 1,573 (32.44)a | 234 (34.82) | 688 (30.99)a | |
| Unknown | 277 (2.30) | 182 (3.75)a | 22 (3.27) | 110 (4.95)a | |
| Baseline FSH (mIU/mL), mean ± SD | 6.18 ± 1.80 | 6.61 ± 1.98a | 10.25 ± 6.44ab | 10.5 ± 6.54abc | <0.001 |
| Baseline LH (mIU/mL), mean ± SD | 4.34 ± 2.84 | 3.63 ± 1.79a | 3.85 ± 3.27a | 4.15 ± 2.92abc | <0.001 |
| Baseline E2 (pmol/L), mean ± SD | 38.02 ± 24.05 | 40.23 ± 32.32a | 51.08 ± 55.37a | 52.79 ± 81.90ab | <0.001 |
| Baseline AMH (ng/ml), mean ± SD | 4.89 ± 3.83 | 2.97 ± 2.23a | 0.56 ± 0.64ab | 0.52 ± 0.64ab | <0.001 |
| Baseline AFC, mean ± SD | 20.04 ± 10.72 | 12.69 ± 6.91a | 3.01 ± 1.00ab | 2.83 ± 1.05ab | <0.001 |
| OHSS during the study period, n (%) | 18 (0.15) | 2 (0.04) | 0 (0) | 0 (0) | 0.076# |
| Number of ovarian stimulation cycles, mean ± SD | 1.23 ± 0.49 | 1.34 ± 0.65a | 1.85 ± 1.22ab | 1.87 ± 1.31ab | <0.001 |
| Number of embryo transfer cycles, mean ± SD | 1.08 ± 0.46 | 1.07 ± 0.57 | 0.78 ± 0.68ab | 0.72 ± 0.70abc | <0.001 |
| Total number of ovarian stimulation cycles | 14,809 | 6,493 | 1,244 | 4,151 | |
| Ovarian stimulation protocol, n (% per cycle) | <0.001 | ||||
| Long GnRH agonist protocol | 12,722 (85.91) | 3,984 (61.36)a | 28 (2.25)ab | 51 (1.23)abc | |
| GnRH antagonist protocol | 1,925 (13.00) | 2,247 (34.61)a | 512 (41.16)ab | 1,589 (38.28)ab | |
| Others | 162 (1.09) | 262 (4.03)a | 704 (56.59)ab | 2,511 (60.49)abc | |
| Number of oocytes retrieved, mean ± SD | 7.14 ± 2.94 | 5.92 ± 2.85a | 2.07 ± 2.16ab | 1.65 ± 1.76abc | <0.001 |
| Method of fertilization, n (% per cycle) | <0.001 | ||||
| IVF | 10,920 (73.74) | 4,907 (75.57)a | 862 (69.29)ab | 2,992 (72.08)ab | |
| ICSI | 3,889 (26.26) | 1,586 (24.43)a | 382 (30.71)ab | 1,159 (27.92)ab | |
| Total number of embryo transfer events | 15,159 | 6,159 | 560 | 1,695 | <0.001 |
| Number of embryos transferred, mean ± SD | 1.86 ± 0.35 | 1.81 ± 0.42a | 1.60 ± 0.50ab | 1.52 ± 0.52abc | <0.001 |
Data are shown as number of patients (percentage) or mean ± SD. SD, standard deviation; BMI, body mass index; FSH, follicular-stimulating hormone; LH, luteinizing hormone; E2, estradiol; P, progesterone; PRL, prolactin; AMH, anti-Müllerian hormone; AFC, antral follicular count; OHSS, ovarian hyperstimulation syndrome; GnRH, gonadotropin-releasing hormone; IVF, in vitro fertilization; ICSI, intracytoplasmic sperm injection.
P < 0.05, vs. POSEIDON group 1;
P < 0.05, vs. POSEIDON group 2;
P < 0.05, vs. POSEIDON group 3;
Chi-square test;
Fisher exact test.
Cumulative Live Birth Rates
In POSEIDON groups 1, 2, 3, and 4, the CLBRs after one complete cycle were 56.04, 30.85, 14.73, and 6.58%, respectively. After three cycles, the optimal and conservative CLBR were (from group 1 to group 4) 83.87 and 66.06%, 53.67 and 37.72%, 44.24 and 27.98%, 14.20 and 9.68%, respectively, in the four groups (Table 2). This indicates that women with adequate ovarian reserve had a better prognosis than those with low reserves. In addition, the impact of age is seen in both groups of ovarian reserve.
Table 2.
Cumulative live birth rates according to the POSEIDON stratification.
| Cycle | Number of patients | Live birth, n (%) | CLBR across all cycles (%) | |
|---|---|---|---|---|
| Optimal estimatea | Conservative estimateb | |||
| POSEIDON GROUP 1 | ||||
| First cycle | 12,040 | 6,747 (56.04) | 56.04 | 56.04 |
| Second cycle | 2,452 | 1,123 (45.80) | 76.17 | 65.37 |
| Third cycle | 277 | 84 (30.32) | 83.87 | 66.06 |
| Fourth cycle | 32 | 5 (15.63) | 86.39 | 66.10 |
| Fifth cycle | 6 | 3 (50.00) | 93.20 | 66.13 |
| ≥Sixth cycle | 1 | 0 (0) | 93.20 | 66.13 |
| POSEIDON GROUP 2 | ||||
| First cycle | 4,849 | 1,496 (30.85) | 30.85 | 30.85 |
| Second cycle | 1,299 | 301 (23.17) | 46.87 | 37.06 |
| Third cycle | 250 | 32 (12.80) | 53.67 | 37.72 |
| Fourth cycle | 64 | 9 (14.06) | 60.17 | 37.90 |
| Fifth cycle | 19 | 0 (0) | 60.17 | 37.90 |
| ≥Sixth cycle | 9 | 0 (0) | 60.17 | 37.90 |
| POSEIDON GROUP 3 | ||||
| First cycle | 672 | 99 (14.73) | 14.73 | 14.73 |
| Second cycle | 331 | 63 (19.03) | 30.91 | 24.11 |
| Third cycle | 135 | 26 (19.26) | 44.24 | 27.98 |
| Fourth cycle | 51 | 11 (21.57) | 56.29 | 29.61 |
| Fifth cycle | 24 | 1 (4.17) | 58.12 | 29.76 |
| ≥Sixth cycle | 16 | 0 (0) | 58.12 | 29.76 |
| POSEIDON GROUP 4 | ||||
| First cycle | 2,220 | 146 (6.58) | 6.58 | 6.58 |
| Second cycle | 1,062 | 55 (5.18) | 11.46 | 9.05 |
| Third cycle | 457 | 14 (3.06) | 14.20 | 9.68 |
| Fourth cycle | 202 | 11 (5.45) | 18.83 | 10.18 |
| Fifth cycle | 94 | 3 (3.19) | 21.43 | 10.32 |
| ≥Sixth cycle | 53 | 4 (7.55) | 25.91 | 10.50 |
Data are shown as number of patients and percentages. CLBR, cumulative live-birth rate.
The optimal estimate assumes that the CLBR in women who discontinue assisted reproductive technology treatment without live-birth would be equal to the rate in those who continue.
The conservative estimate assumes that those who discontinue assisted reproductive technology treatment would have had a live-birth rate of zero.
Figure 2 shows that the CLBR increased from cycle 1 to cycle 3 in all POSEIDON groups, plateauing thereafter, which is expected from multiple attempts at pregnancy. The optimal estimated CLBR of POSEIDON groups 1 and 2 differed by 30% after three ART cycles. Women in POSEIDON group 3 are young but with low ovarian reserve. If they continue ART treatment, the optimal estimated CLBR could be comparable to that of older women with adequate ovarian reserve (POSEIDON group 2). Although the CLBR of women receiving more than three cycles could not be analyzed due to the small sample size, there was such a trend observed during the first three cycles, which needs to be further studied. Taken together, significant differences in CLBR were observed among the four POSEIDON groups. Among all women, 2,841 (23.60%), 2,054 (42.36%), 242 (36.01%), and 1,012 (45.59%) discontinued ART treatment in POSEIDON groups 1, 2, 3, and 4 if they did not have a live birth after the first cycle. In addition, the number of women with two, three, and four cycles decreased, even when accounting for those with successful cycle. For women in POSEIDON groups 1, 2, and 3, the prognosis was favorable after three successive cycles of IVF/ICSI treatment.
Figure 2.
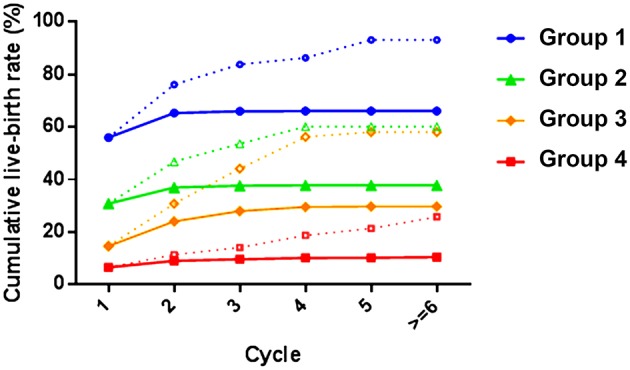
Cumulative live-birth rates according to the POSEIDON stratification. The POSEIDON groups 1 and 2 included patients with antral follicle count (AFC) ≥ 5, anti-Müllerian hormone (AMH) ≥ 1.2 ng/ml, and ≤9 oocytes retrieved after standard ovarian stimulation [long gonadotropin-releasing hormone (GnRH) agonist protocol or GnRH antagonist protocol]. Younger patients (age < 35 years) were included in POSEIDON group 1, while older patients (age ≥ 35 years) were included in POSEIDON groups 2. The POSEIDON groups 3 and 4 consisted of patients with AFC < 5 or AMH < 1.2 ng/ml. Younger patients (age < 35 years) were included in POSEIDON group 3, while older patients (age ≥ 35 years) were included in POSEIDON groups 4. The full lines are the conservative estimated live-birth rate and the dashed lines are the optimal estimated live-birth rate.
Factors Associated With Live Birth in the First Two Cycles
The multivariable analysis for the first and second stimulation cycles are shown in Tables 3, 4, respectively. Table 3 shows that the POSEIDON stratification (group 2: OR = 2.319, 95% CI: 2.131–2.525, P < 0.001; group 3: OR = 1.356, 95% CI: 1.005–1.828, P = 0.046; group 4: OR = 3.525, 95% CI: 2.774–4.479, P < 0.001; all vs. group 1), ovarian stimulation protocol (GnRH antagonist protocol: OR = 1.856, 95% CI: 1.640–2.100, P < 0.001; other ovarian stimulation protocols: OR = 1.651, 95% CI: 1.155–2.361, P = 0.006; both vs. long GnRH agonist protocol), number of embryos transferred (2: OR = 0.514, 95% CI: 0.468–0.565, P < 0.001), baseline LH and estradiol levels, duration of infertility, and fresh and frozen embryo transfer were independently associated with live birth in the first stimulation cycle.
Table 3.
Logistic regression based on the occurrence of live birth in the first stimulation cycle.
| Variable | Non-live birth (n = 11,293) | Live birth (n = 8,488) | Univariable logistic regression | Multivariable logistic regression | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |||
| Grouping, n (%) | ||||||
| POSEIDON group 1 | 5,293 (43.96) | 6,747 (56.04) | Reference | Reference | ||
| POSEIDON group 2 | 3,353 (69.15) | 1,496 (30.85) | 2.857 (2.662, 3.067) | <0.001 | 2.319 (2.131, 2.525) | <0.001 |
| POSEIDON group 3 | 573 (85.27) | 99 (14.73) | 7.378 (5.943, 9.16) | <0.001 | 1.356 (1.005, 1.828) | 0.046 |
| POSEIDON group 4 | 2,074 (93.42) | 146 (6.58) | 18.104 (15.249, 21.493) | <0.001 | 3.525 (2.774, 4.479) | <0.001 |
| Baseline FSH, mean ± SD | 7.37 ± 3.89 | 6.3 ± 2.36 | 1.150 (1.136, 1.165) | <0.001 | 1.017 (1.0, 1.034) | 0.053 |
| Baseline LH, mean ± SD | 4.01 ± 2.56 | 4.28 ± 2.79 | 0.962 (0.952, 0.973) | <0.001 | 0.979 (0.965, 0.993) | 0.003 |
| Baseline E2, mean ± SD | 42.8 ± 45.87 | 37.83 ± 25.61 | 1.006 (1.005, 1.007) | <0.001 | 1.002 (1.001, 1.004) | 0.001 |
| BMI, n (%) | ||||||
| <18.5 | 757 (6.70) | 719 (8.47) | 0.786 (0.706, 0.875) | <0.001 | 1.014 (0.891, 1.154) | 0.831 |
| 18.5–25 | 8,759 (77.56) | 6,540 (77.05) | Reference | Reference | ||
| >25 | 1,777 (15.74) | 1,229 (14.48) | 1.080 (0.997, 1.169) | 0.059 | 1.033 (0.938, 1.138) | 0.505 |
| Duration of infertility, mean ± SD | 5.57 ± 4.47 | 4.42 ± 3.25 | 1.079 (1.071, 1.088) | <0.001 | 1.023 (1.013, 1.033) | <0.001 |
| Cause of infertility, n (%) | ||||||
| Tubal cause | 10,309 (91.29) | 7,762 (91.45) | 0.98 (0.886, 1.083) | 0.692 | ||
| Ovulation disorder | 58 (0.51) | 83 (0.98) | 0.523 (0.373, 0.732) | <0.001 | 1.049 (0.722, 1.524) | 0.803 |
| Endometriosis | 1,078 (9.55) | 777 (9.15) | 1.047 (0.95, 1.154) | 0.351 | ||
| Male cause | 3,368 (29.82) | 2,816 (33.18) | 0.856 (0.806, 0.909) | <0.001 | 0.991 (0.920, 1.068) | 0.817 |
| Others | 193 (1.71) | 133 (1.57) | 1.092 (0.874, 1.364) | 0.44 | ||
| Multiple causes | 10,911 (96.62) | 8,279 (97.54) | 0.721 (0.608, 0.856) | <0.001 | 0.932 (0.758, 1.146) | 0.502 |
| Method of fertilization, n (%) | ||||||
| IVF | 8,853 (78.39) | 6,712 (79.08) | Reference | |||
| ICSI | 2,440 (21.61) | 1,776 (20.92) | 1.042 (0.972, 1.116) | 0.247 | ||
| Ovarian stimulation protocol, n (%) | ||||||
| Long GnRH agonist protocol | 7,192 (63.69) | 7,773 (91.58) | Reference | Reference | ||
| GnRH antagonist protocol | 2,630 (23.29) | 653 (7.69) | 4.353 (3.972, 4.77) | <0.001 | 1.856 (1.640, 2.100) | <0.001 |
| Others | 1,471 (13.03) | 62 (0.73) | 25.642 (19.848, 33.128) | <0.001 | 1.651 (1.155, 2.361) | 0.006 |
| Fresh embryo/frozen embryo, n (%) | ||||||
| Fresh embryo transfer | 7,510 (66.50) | 6,927 (81.61) | Reference | Reference | ||
| Fresh and frozen embryo transfer | 3,783 (33.50) | 1,561 (18.39) | 2.235 (2.089, 2.39) | <0.001 | 2.772 (2.567, 2.993) | <0.001 |
| Number of embryos transferred, n (%) | ||||||
| 0 | 3,998 (35.4) | 0 (0) | >999.999 (0, >999.999) | 0.903 | >999.999 (0, >999.999) | 0.973 |
| 1 | 1,938 (17.16) | 945 (11.13) | Reference | Reference | ||
| 2 | 5,296 (46.9) | 7,503 (88.4) | 0.344 (0.316, 0.375) | <0.001 | 0.514 (0.468, 0.565) | <0.001 |
| 3 | 61 (0.54) | 40 (0.47) | 0.744 (0.495, 1.116) | 0.153 | 0.747 (0.483, 1.155) | 0.190 |
Data are shown as number of patients (percentage) or mean ± SD. OR, odd ratio; CI, confidence interval; BMI, body mass index; FSH, follicular-stimulating hormone; SD, standard deviation; LH, luteinizing hormone; E2, estradiol; P: progesterone; PRL, prolactin; AMH, anti-Müllerian hormone; AFC, antral follicular count; OHSS, ovarian hyperstimulation syndrome; GnRH, gonadotropin-releasing hormone; IVF, in vitro fertilization; ICSI, intracytoplasmic sperm injection.
Table 4.
Logistic regression based on the occurrence of live birth in the second stimulation cycle.
| Variable | Non-live birth (n = 2,085) | live birth (n = 1,542) | Univariable logistic regression | Multivariable logistic regression | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |||
| Grouping, n (%) | ||||||
| POSEIDON group 1 | 1,328 (36.88) | 1,123 (72.83) | Reference | Reference | ||
| POSEIDON group 2 | 998 (27.71) | 301 (19.52) | 2.804 (2.41, 3.262) | <0.001 | 2.098 (1.762, 2.497) | <0.001 |
| POSEIDON group 3 | 268 (7.44) | 63 (4.09) | 3.597 (2.703, 4.787) | <0.001 | 0.952 (0.649, 1.397) | 0.803 |
| POSEIDON group 4 | 1,007 (27.96) | 55 (3.57) | 15.478 (11.666, 20.536) | <0.001 | 3.887 (2.726, 5.541) | <0.001 |
| Baseline FSH, mean ± SD | 8.02 ± 4.59 | 6.57 ± 2.5 | 1.143 (1.117, 1.17) | <0.001 | 1.011 (0.984, 1.038) | 0.440 |
| Baseline LH, mean ± SD | 4.03 ± 2.56 | 4.14 ± 2.49 | 0.983 (0.961, 1.006) | 0.145 | ||
| Baseline E2, mean ± SD | 44.89 ± 41.83 | 38.23 ± 25.85 | 1.007 (1.005, 1.01) | <0.001 | 1.004 (1.001, 1.006) | 0.012 |
| BMI, n (%) | ||||||
| <18.5 | 207 (5.75) | 105 (6.81) | 0.846 (0.663, 1.079) | 0.178 | ||
| 18.5–25 | 2,961 (82.23) | 1,270 (82.36) | Reference | |||
| >25 | 433 (12.02) | 167 (10.83) | 1.112 (0.919, 1.345) | 0.274 | ||
| Duration of infertility, mean ± SD | 5.86 ± 4.59 | 4.79 ± 3.53 | 1.078 (1.062, 1.094) | <0.001 | 1.022 (1.003, 1.041) | 0.025 |
| Cause of infertility, n (%) | ||||||
| Tubal cause | 3,204 (88.98) | 1,344 (87.16) | 1.189 (0.991, 1.426) | 0.062 | ||
| Ovulation disorder | 6 (0.17) | 4 (0.26) | 0.642 (0.181, 2.277) | 0.492 | ||
| Endometriosis | 282 (7.83) | 149 (9.66) | 0.794 (0.645, 0.978) | 0.03 | 0.828 (0.645, 1.063) | 0.139 |
| Male cause | 819 (22.74) | 401 (26.01) | 0.838 (0.73, 0.962) | 0.012 | 0.956 (0.813, 1.123) | 0.582 |
| Others | 71 (1.97) | 21 (1.36) | 1.456 (0.892, 2.379) | 0.133 | ||
| Multiple causes | 3,412 (94.75) | 1,462 (94.81) | 0.988 (0.755, 1.292) | 0.929 | ||
| Method of fertilization, n (%) | ||||||
| IVF | 2,205 (61.23) | 909 (58.95) | Reference | |||
| ICSI | 1,396 (38.77) | 633 (41.05) | 0.909 (0.805, 1.027) | 0.125 | ||
| Ovarian stimulation protocol, n (%) | ||||||
| Long GnRH agonist protocol | 866 (24.05) | 833 (54.02) | Reference | Reference | ||
| GnRH antagonist protocol | 1,672 (46.43) | 611 (39.62) | 2.632 (2.305, 3.006) | <0.001 | 1.761 (1.416, 2.189) | <0.001 |
| Others | 1,063 (29.52) | 98 (6.36) | 10.434 (8.309, 13.102) | <0.001 | 1.767 (1.252, 2.494) | 0.001 |
| Change of ovarian stimulation protocol, n (%) | ||||||
| No | 1,866 (51.82) | 928 (60.18) | Reference | Reference | ||
| Yes | 1,735 (48.18) | 614 (39.82) | 1.405 (1.245, 1.586) | <0.001 | 0.876 (0.715, 1.074) | 0.204 |
| Fresh embryo/frozen embryo, n (%) | ||||||
| Fresh embryo transfer | 2,126 (59.04) | 895 (58.04) | Reference | |||
| Fresh and frozen embryo transfer | 1,475 (40.96) | 647 (41.96) | 0.96 (0.85, 1.083) | 0.505 | ||
| Number of embryos transferred, n (%) | ||||||
| 0 | 1,516 (42.1) | 0 (0) | >999.999 (0, >999.999) | 0.917 | >999.999 (0, >999.999) | 0.984 |
| 1 | 643 (17.86) | 225 (14.59) | Reference | Reference | ||
| 2 | 1,439 (39.96) | 1,313 (85.15) | 0.384 (0.324, 0.454) | <0.001 | 0.518 (0.432, 0.621) | <0.001 |
| 3 | 3 (0.08) | 4 (0.26) | 0.262 (0.058, 1.182) | 0.093 | 0.255 (0.052, 1.257) | 0.093 |
Data are shown as number of patients (percentage) or mean ± SD. OR, odd ratio; CI, confidence interval; BMI, body mass index; FSH, follicular-stimulating hormone; SD, standard deviation; LH, luteinizing hormone; E2, estradiol; P, progesterone; PRL, prolactin; AMH, anti-Müllerian hormone; AFC, antral follicular count; OHSS, ovarian hyperstimulation syndrome; GnRH, gonadotropin-releasing hormone; IVF, in vitro fertilization; ICSI, intracytoplasmic sperm injection.
Table 4 shows that the POSEIDON stratification (group 2: OR = 2.098, 95% CI: 1.762–2.497, P < 0.001; group 4: OR = 3.887, 95% CI: 2.726–5.541, P < 0.001; both vs. group 1), ovarian stimulation protocol (GnRH antagonist protocol: OR = 1.761, 95% CI: 1.416–2.189, P < 0.001; other ovarian stimulation protocols: OR = 1.767, 95% CI: 1.252–2.494, P = 0.001; both vs. long GnRH agonist protocol), number of embryos transferred (2: OR = 0.518, 95% CI: 0.432–0.621, P < 0.001), baseline estradiol levels, and duration of infertility were independently associated with live birth in the second stimulation cycle. Change of ovarian stimulation protocol was not independently associated with improvement in live birth rate.
Discussion
The POSEIDON criteria are used to stratify women with low prognosis after ART treatment. To the best of our knowledge, this is the first large population study that analyzed the CLBRs of women according to POSEIDON groups. The conservative CLBR was the highest for POSEIDON group 1, followed by groups 2, 3, and 4. Unsurprisingly, the results show that it is indeed more difficult for older women with POR to achieve a live birth after IVF/ICSI compared with younger women. Age is the key factor influencing CLBR after ART (24–26).
In women with POR according to the Bologna criteria, the CLBR within one cycle was previously reported to be poor (27), and the overall prognosis was improved after three cycles (conservative CLBR: from 10.5 to 14.3; optimal CLBR: from 10.5 to 23.7) (26). When using the Bologna criteria, it is difficult to distinguish POR women with differences in reproductive prognosis. In contrast, the POSEIDON criteria were specifically designed to solve the heterogeneity observed in the Bologna criteria (7, 8, 12, 13).
POSEIDON groups 1 and 2 are characterized by hypo-response to ovarian stimulation, which can be caused by among others environmental contaminants, polymorphisms, and drugs. The mechanisms are still far from being understood, but studies suggest some genetic causes (28–30). A study showed that intrafollicular concentrations of benzene were associated with the outcomes of ART treatment, suggesting that environmental or occupational exposure to pollutants affects the reproductive outcomes (31). Women in POSEIDON groups 1 and 2 have an adequate ovarian reserve, but unexpectedly the ovarian response to stimulation is either poor or suboptimal, defined as <4 oocytes and ≤9 oocytes, respectively. Dose adjustments of FSH in the subsequent cycles and possible supplementation with recombinant LH might help these patients produce more follicles and oocytes (32, 33). Regarding POSEIDON groups 3 and 4, the low AFC and the expected decrease in the number of euploid embryos for transfer are the main causes of poor outcomes. The retrieval of a large number of oocytes (which will increase the likelihood of having at least one euploid embryo) is difficult in POSEIDON 3 and 4 women (34). In POSEIDON groups 3 and 4, the reasons for poor response include poor ovarian reserve, asynchronous development, and genetic polymorphisms in FSH receptor, LH receptor, and the possible presence of variant LH-β. The clinical management include down-regulation with a long GnRH agonist protocol, stimulation with recombinant FSH with or without recombinant LH, possible pre-treatment with androgens, fresh embryo transfer, or oocyte/embryo accumulation and frozen embryo transfer (34). Specifically for POSEIDON group 4, recombinant LH can be added to increase circulating androgens (which are decreased in older patients and play an important role for optimal folliculogenesis stimulation) (34).
Based on the present study, for women with an adequate ovarian reserve, but initial poor response, and who underwent three ART cycles, the optimal estimated CLBR can be up to 83.9 and 53.7%, respectively in POSEIDON groups 1 and 2. This should encourage POSEIDON groups 1 and 2 women to pursue cycles when unsuccessful. Unfortunately, the number of women who received more than three cycles was too small to perform any reliable analysis. Nevertheless, Gu et al. (35) and Smith et al. (23) previously showed that CLBR increases with repeated ART in younger women.
In the present study, CLBR was not only associated with the POSEIDON stratification, but also with clinical factors such as duration of infertility and ovarian stimulation protocols. This is generally consistent with the literature (36, 37). Interestingly, compared with the first stimulation cycle, changing the ovarian stimulation protocol in the second cycle did not seem to be associated with an improvement in live birth. Due to the retrospective nature of this study, results need to be confirmed by prospective studies.
It is quite clear that natural cycle IVF results in a very low CLBR (1.2%) in women with POR according to the Bologna criteria (at least two of the following criteria: ≥40 years of age; history of <3 oocytes with a conventional ovarian stimulation method; and AFC < 5.7) (26). In contrast, ovarian stimulation with exogenous gonadotropins results in a significantly higher live birth rate after one stimulation with fresh embryo transfer when using the long GnRH agonist protocol (11.7%) (38) or the GnRH antagonist protocol (15–20%) (39–41).
The long GnRH agonist protocol was used for most women in POSEIDON groups 1 and 2, while the GnRH antagonist protocol was used for women in POSEIDON groups 3 and 4. Although the long GnRH agonist protocol is used to a lower extent in other countries (42, 43), it is still used as the preferred protocol in China (44–46). In the present study, although changing the ovarian stimulation protocol during the second cycle did not seem to improve the live birth rate, selecting different ovarian stimulation protocols were associated with live birth. Further studies should be conducted to explore the impact of different ovarian stimulation protocols in the POSEIDON population. Considering the poor baseline condition of these women, individualized treatment in all steps of ART, including the choice of gonadotropin type and dose, ovulation trigger, and the possible use of adjuvant therapies should be considered (34). Importantly, the most optimal protocol for POSEIDON groups 3 and 4 still remains a clinical challenge.
A limitation of this study is its retrospective nature. Nevertheless, this is the largest study so far (26,697 cycles) assessing the prevalence, management, and effect of the POSEIDON classification in a Chinese IVF population. In addition, Humaidan et al. (7) showed that the POSEIDON criteria can be applied retrospectively to structured databases to determine the POSEIDON groups. Of course, the treatment that the patient ultimately received was not tailored according to the POSEIDON groups because of the retrospective determination of grouping, and the subsequent reproductive outcomes in relation to POSEIDON must be taken with caution. These results will have to be confirmed prospectively. Furthermore, there might be ethnic differences, which could impair the generalizability of the present study to other populations. Nevertheless, our data are in line with previous smaller reports from other regions of the world (12, 13). Future prospective studies in different populations may be necessary to validate the results. Secondly, we stratified patients according to the baseline data only, and a re-stratification of patients during the subsequent stimulation cycles was not performed. Finally, no data about the dose and type of gonadotropins used for ART treatments were collected. Further studies are essential to explore whether adjustment should be performed and whether this would further improve the outcome of the POSEIDON patient.
In conclusion, our study showed that more than 30% of Chinese women undergoing IVF/ICSI treatment can be classified into one of the four POSEIDON groups, and that significant differences in reproductive prognosis are observed between the four POSEIDON groups. Patients with low prognosis may increase their chances of live birth by repeated ART treatments. For women in POSEIDON groups 1, 2, and 3, the prognosis was favorable after three successive cycles of IVF/ICSI treatment. Future randomized controlled trials should investigate whether the CLBR of the low prognosis patient can be improved by individualized ART treatment according to the suggestions made by the POSEIDON stratification group.
Data Availability Statement
All datasets generated for this study are included in the manuscript/supplementary files.
Author Contributions
YL conceived and coordinated the study, designed, performed, and analyzed the experiments, and wrote the paper. XL, XY, and SC carried out the data collection and data analysis. GLu and GLi designed and guided the study. PH supervised and revised the paper. FG designed and guided the study. All authors reviewed the results and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to thank all staff involved in IVF/ICSI treatment for their support and help which made this large data analysis feasible.
Footnotes
Funding. This study was funded by the National Science Foundation of China (81501328) and Wu Jieping Medical Foundation (320.6750.15259).
References
- 1.Jirge PR. Poor ovarian reserve. J Hum Reprod Sci. (2016) 9:63–9. 10.4103/0974-1208.183514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badawy A, Wageah A, El Gharib M, Osman EE. Prediction and diagnosis of poor ovarian response: the dilemma. J Reprod Infertil. (2011) 12:241–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Morin SJ, Patounakis G, Juneau CR, Neal SA, Scott RT, Jr, Seli E. Diminished ovarian reserve and poor response to stimulation in patients <38 years old: a quantitative but not qualitative reduction in performance. Hum Reprod. (2018) 33:1489–98. 10.1093/humrep/dey238 [DOI] [PubMed] [Google Scholar]
- 4.Polyzos NP, Devroey P. A systematic review of randomized trials for the treatment of poor ovarian responders: is there any light at the end of the tunnel? Fertil Steril. (2011) 96:1058–61 e7. 10.1016/j.fertnstert.2011.09.048 [DOI] [PubMed] [Google Scholar]
- 5.Vaiarelli A, Cimadomo D, Ubaldi N, Rienzi L, Ubaldi FM. What is new in the management of poor ovarian response in IVF? Curr Opin Obstetr Gynecol. (2018) 30:155–62. 10.1097/GCO.0000000000000452 [DOI] [PubMed] [Google Scholar]
- 6.Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L, et al. ESHRE consensus on the definition of ‘poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. (2011) 26:1616–24. 10.1093/humrep/der092 [DOI] [PubMed] [Google Scholar]
- 7.Humaidan P, Alviggi C, Fischer R, Esteves SC. The novel POSEIDON stratification of ‘Low prognosis patients in Assisted Reproductive Technology' and its proposed marker of successful outcome. F1000Res. (2016) 5:2911. 10.12688/f1000research.10382.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poseidon G, Alviggi C, Andersen CY, Buehler K, Conforti A, De Placido G, et al. A new more detailed stratification of low responders to ovarian stimulation: from a poor ovarian response to a low prognosis concept. Fertil Steril. (2016) 105:1452–3. 10.1016/j.fertnstert.2016.02.005 [DOI] [PubMed] [Google Scholar]
- 9.Boots CE, Bernardi LA. Bologna criteria: clinically or academically relevant? Fertil Steril. (2018) 109:59–60. 10.1016/j.fertnstert.2017.10.022 [DOI] [PubMed] [Google Scholar]
- 10.Younis JS, Ben-Ami M, Ben-Shlomo I. The Bologna criteria for poor ovarian response: a contemporary critical appraisal. J Ovarian Res. (2015) 8:76. 10.1186/s13048-015-0204-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boza A, Oguz SY, Misirlioglu S, Yakin K, Urman B. Utilization of the Bologna criteria: a promise unfulfilled? A review of published and unpublished/ongoing trials. Fertil Steril. (2018) 109:104–9 e2. 10.1016/j.fertnstert.2017.09.024 [DOI] [PubMed] [Google Scholar]
- 12.Conforti A, Esteves SC, Picarelli S, Iorio G, Rania E, Zullo F, et al. Novel approaches for diagnosis and management of low prognosis patients in assisted reproductive technology: the POSEIDON concept. Panminerva Med. (2019) 61:24–9. 10.23736/S0031-0808.18.03511-5 [DOI] [PubMed] [Google Scholar]
- 13.Esteves SC, Roque M, Bedoschi GM, Conforti A, Humaidan P, Alviggi C. Defining low prognosis patients undergoing assisted reproductive technology: POSEIDON criteria-the why. Front Endocrinol. (2018) 9:461. 10.3389/fendo.2018.00461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alper MM, Brinsden PR, Fischer R, Wikland M. Is your IVF programme good? Hum Reprod. (2002) 17:8–10. 10.1093/humrep/17.1.8 [DOI] [PubMed] [Google Scholar]
- 15.Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology. (2007) 67:73–80. 10.1016/j.theriogenology.2006.09.014 [DOI] [PubMed] [Google Scholar]
- 16.Lindsay TJ, Vitrikas KR. Evaluation and treatment of infertility. Am Fam Phys. (2015) 91:308–14. [PubMed] [Google Scholar]
- 17.World Health Organization International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Vol. 2 Geneva: World Health Organization; (1993). [Google Scholar]
- 18.Patil M, Shah D. Maximum number of embryos to be transferred in assisted reproductive technologies cycle: ethics opinion. J Hum Reprod Sci. (2018) 11:93–5. 10.4103/jhrs.JHRS_66_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhillon RK, McLernon DJ, Smith PP, Fishel S, Dowell K, Deeks JJ, et al. Predicting the chance of live birth for women undergoing IVF: a novel pretreatment counselling tool. Hum Reprod. (2016) 31:84–92. 10.1093/humrep/dev268 [DOI] [PubMed] [Google Scholar]
- 20.Gameiro S, Boivin J, Peronace L, Verhaak CM. Why do patients discontinue fertility treatment? A systematic review of reasons and predictors of discontinuation in fertility treatment. Hum Reprod Update. (2012) 18:652–69. 10.1093/humupd/dms031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luke B, Brown MB, Wantman E, Lederman A, Gibbons W, Schattman GL, et al. Cumulative birth rates with linked assisted reproductive technology cycles. N Engl J Med. (2012) 366:2483–91. 10.1056/NEJMoa1110238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The international glossary on infertility and fertility care, 2017. Fertil Steril. (2017) 108:393–406. 10.1016/j.fertnstert.2017.06.005 [DOI] [PubMed] [Google Scholar]
- 23.Smith A, Tilling K, Nelson SM, Lawlor DA. Live-birth rate associated with repeat in vitro fertilization treatment cycles. JAMA. (2015) 314:2654–62. 10.1001/jama.2015.17296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goswami SK, Das T, Chattopadhyay R, Sawhney V, Kumar J, Chaudhury K, et al. A randomized single-blind controlled trial of letrozole as a low-cost IVF protocol in women with poor ovarian response: a preliminary report. Hum Reprod. (2004) 19:2031–5. 10.1093/humrep/deh359 [DOI] [PubMed] [Google Scholar]
- 25.Tan TY, Lau SK, Loh SF, Tan HH. Female ageing and reproductive outcome in assisted reproduction cycles. Singapore Med J. (2014) 55:305–9. 10.11622/smedj.2014081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu B, Chen Y, Geerts D, Yue J, Li Z, Zhu G, et al. Cumulative live birth rates in more than 3,000 patients with poor ovarian response: a 15-year survey of final invitro fertilization outcome. Fertil Steril. (2018) 109:1051–9. 10.1016/j.fertnstert.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 27.Busnelli A, Papaleo E, Del Prato D, La Vecchia I, Iachini E, Paffoni A, et al. A retrospective evaluation of prognosis and cost-effectiveness of IVF in poor responders according to the Bologna criteria. Hum Reprod. (2015) 30:315–22. 10.1093/humrep/deu319 [DOI] [PubMed] [Google Scholar]
- 28.Alviggi C, Conforti A, Santi D, Esteves SC, Andersen CY, Humaidan P, et al. Clinical relevance of genetic variants of gonadotrophins and their receptors in controlled ovarian stimulation: a systematic review and meta-analysis. Hum Reprod Update. (2018) 24:599–614. 10.1093/humupd/dmy019 [DOI] [PubMed] [Google Scholar]
- 29.Alviggi C, Conforti A, Esteves SC, Vallone R, Venturella R, Staiano S, et al. Understanding ovarian hypo-response to exogenous gonadotropin in ovarian stimulation and its new proposed marker-the follicle-to-oocyte (FOI) index. Front Endocrinol. (2018) 9:589. 10.3389/fendo.2018.00589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alviggi C, Pettersson K, Longobardi S, Andersen CY, Conforti A, De Rosa P, et al. A common polymorphic allele of the LH beta-subunit gene is associated with higher exogenous FSH consumption during controlled ovarian stimulation for assisted reproductive technology. Reprod Biol Endocrinol. (2013) 11:51. 10.1186/1477-7827-11-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alviggi C, Guadagni R, Conforti A, Coppola G, Picarelli S, De Rosa P, et al. Association between intrafollicular concentration of benzene and outcome of controlled ovarian stimulation in IVF/ICSI cycles: a pilot study. J Ovarian Res. (2014) 7:67. 10.1186/1757-2215-7-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drakopoulos P, Santos-Ribeiro S, Bosch E, Garcia-Velasco J, Blockeel C, Romito A, et al. The effect of dose adjustments in a subsequent cycle of women with suboptimal response following conventional ovarian stimulation. Front Endocrinol. (2018) 9:361. 10.3389/fendo.2018.00361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alviggi C, Conforti A, Esteves SC, Andersen CY, Bosch E, Buhler K, et al. Recombinant luteinizing hormone supplementation in assisted reproductive technology: a systematic review. Fertil Steril. (2018) 109:644–64. 10.1016/j.fertnstert.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 34.Haahr T, Esteves SC, Humaidan P. Individualized controlled ovarian stimulation in expected poor-responders: an update. Reprod Biol Endocrinol. (2018) 16:20. 10.1186/s12958-018-0342-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu F, Ruan S, Xu Y, Zhou G. Cumulative live-birth rate with repeat in vitro fertilization treatment cycles of chinese advanced age women. Fertil Steril. (2017) 108:e343 10.1016/j.fertnstert.2017.07.1007 [DOI] [Google Scholar]
- 36.Gnoth C, Maxrath B, Skonieczny T, Friol K, Godehardt E, Tigges J. Final ART success rates: a 10 years survey. Hum Reprod. (2011) 26:2239–46. 10.1093/humrep/der178 [DOI] [PubMed] [Google Scholar]
- 37.Hodgson I, Plummer ML, Konopka SN, Colvin CJ, Jonas E, Albertini J, et al. A systematic review of individual and contextual factors affecting ART initiation, adherence, and retention for HIV-infected pregnant and postpartum women. PLoS ONE. (2014) 9:e111421. 10.1371/journal.pone.0111421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Humaidan P, Schertz J, Fischer R. Efficacy and safety of pergoveris in assisted reproductive technology–ESPART: rationale and design of a randomised controlled trial in poor ovarian responders undergoing IVF/ICSI treatment. BMJ Open. (2015) 5:e008297. 10.1136/bmjopen-2015-008297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pu D, Wu J, Liu J. Comparisons of GnRH antagonist versus GnRH agonist protocol in poor ovarian responders undergoing IVF. Hum Reprod. (2011) 26:2742–9. 10.1093/humrep/der240 [DOI] [PubMed] [Google Scholar]
- 40.Baker VL, Brown MB, Luke B, Smith GW, Ireland JJ. Gonadotropin dose is negatively correlated with live birth rate: analysis of more than 650,000 assisted reproductive technology cycles. Fertil Steril. (2015) 104:1145–52 e1–5. 10.1016/j.fertnstert.2015.07.1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Humaidan P, Bungum L, Bungum M, Hald F, Agerholm I, Blaabjerg J, et al. Reproductive outcome using a GnRH antagonist (cetrorelix) for luteolysis and follicular synchronization in poor responder IVF/ICSI patients treated with a flexible GnRH antagonist protocol. Reprod Biomed Online. (2005) 11:679–84. 10.1016/S1472-6483(10)61685-9 [DOI] [PubMed] [Google Scholar]
- 42.Shrestha D, La X, Feng HL. Comparison of different stimulation protocols used in in vitro fertilization: a review. Ann Transl Med. (2015) 3:137. 10.3978/j.issn.2305-5839.2015.04.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lambalk CB, Banga FR, Huirne JA, Toftager M, Pinborg A, Homburg R, et al. GnRH antagonist versus long agonist protocols in IVF: a systematic review and meta-analysis accounting for patient type. Hum Reprod Update. (2017) 23:560–79. 10.1093/humupd/dmx017 [DOI] [PubMed] [Google Scholar]
- 44.Huang MC, Tzeng SL, Lee CI, Chen HH, Huang CC, Lee TH, et al. GnRH agonist long protocol versus GnRH antagonist protocol for various aged patients with diminished ovarian reserve: a retrospective study. PLoS ONE. (2018) 13:e0207081. 10.1371/journal.pone.0207081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang R, Lin S, Wang Y, Qian W, Zhou L. Comparisons of GnRH antagonist protocol versus GnRH agonist long protocol in patients with normal ovarian reserve: A systematic review and meta-analysis. PLoS ONE. (2017) 12:e0175985. 10.1371/journal.pone.0175985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ho CH, Chen SU, Peng FS, Chang CY, Lien YR, Yang YS. Prospective comparison of short and long GnRH agonist protocols using recombinant gonadotrophins for IVF/ICSI treatments. Reprod Biomed Online. (2008) 16:632–9. 10.1016/S1472-6483(10)60476-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets generated for this study are included in the manuscript/supplementary files.