Abstract
BACKGROUND
The Baveno VI criteria for predicting esophageal varices, i.e., liver stiffness measurement (LSM) < 20 kPa and platelet (PLT) count > 150 × 109/L, identify patients who can safely avoid gastroscopy screening. However, they require further refinement.
AIM
To evaluate the utility of LSM and serum markers of liver fibrosis in ruling out high-risk varices (HRV) in patients who do not meet Baveno VI criteria.
METHODS
Data from 132 patients with hepatitis B virus (HBV)-related compensated liver cirrhosis who did not meet the Baveno VI criteria were retrospectively reviewed. MedCalc 15.8 was used to calculate receiver operating characteristic (ROC) curves, and the accuracy of LSM, PLT count, aspartate aminotransferase (AST)-to-PLT ratio index, Fibrosis-4, and the Lok index in predicting HRV were evaluated according to the area under each ROC curve (AUROC). The utility of LSM, PLT, and serum markers of liver fibrosis stratified by alanine transaminase (ALT) and total bilirubin (TBil) levels was evaluated for ruling out HRV.
RESULTS
In all patients who did not meet the Baveno VI criteria, the independent risk factors for HRV were LSM and ALT. Only the AUROC of Lok index was above 0.7 for predicting HRV, and at a cutoff value of 0.4531 it could further spare 24.2% of gastroscopies without missing HRVs. The prevalence of HRV was significantly lower in patients with ALT or TBil ≥ 2 upper limit of normal (ULN) (14.3%) than in patients with both ALT and TBil < 2 ULN (34.1%) (P = 0.018). In the 41 patients with ALT and TBil < 2 ULN, LSM had an AUROC for predicting HRV of 0.821. LSM < 20.6 kPa spared 39.0% of gastroscopies without missing HRVs. In the 91 patients with ALT or TBiL ≥ 2 ULN, the Lok index and PLT had AUROCs of 0.814 and 0.741, respectively. Lok index ≤ 0.5596 or PLT > 100 × 109/L further spared 39.6% and 43.9% of gastroscopies, respectively, without missing HRVs.
CONCLUSION
In HBV-related compensated cirrhosis patients who do not meet Baveno VI criteria, the LSM, PLT, or Lok index cutoff stratified by ALT and TBil accurately identifies more patients without HRV.
Keywords: Baveno VI, Esophageal varices, Liver cirrhosis, Liver stiffness measurement, Serum markers of liver fibrosis
Core tip: In patients with hepatitis B virus (HBV)-related compensated cirrhosis who did not meet the Baveno VI criteria, the prevalence of high-risk varices among patients with alanine transaminase (ALT) or total bilirubin (TBil) ≥ 2 upper limit of normal (ULN) was significantly lower compared to patients with ALT and TBil < 2 ULN. In the 41 patients with ALT and TBil < 2 ULN, liver stiffness measurement (LSM) < 20.6 kPa spared 39.0% of gastroscopies without missing high-risk varices (HRVs). In the 91 patients with ALT or TBiL ≥ 2 ULN, Lok index ≤ 0.5596 or platelet (PLT) > 100 × 109/L further spared 39.6% and 43.9% of gastroscopies, respectively, without missing HRVs.
INTRODUCTION
About 30% of cases of liver cirrhosis worldwide result from chronic hepatitis B virus (HBV) infection[1]. Esophageal varices (EV) and esophagogastric variceal bleeding (EVB) are major complications among patients with liver cirrhosis and are associated with high morbidity and mortality[2]. Six-week mortality rates range between 15% and 25% in patients with EVB[3,4]. High-risk varices (HRV) are medium or large EV or small EV with red wale signs. Prophylactic therapy with beta-blockers or elastic band ligation benefits patients with HRV and is the standard of care in patients with cirrhosis to identify those with HRV[5,6].
Gastroscopy is the gold standard for diagnosing EV and assessing bleeding risk[7]. However, gastroscopy is an invasive and expensive procedure with associated risks[6]. In the last 10 years, evidence has accumulated regarding the usefulness of non-invasive methods for stratifying EV risk in patients with compensated advanced chronic liver disease[8]. The 2015 Baveno VI consensus workshop recommended that patients with liver stiffness measurement (LSM) < 20 kPa and platelet (PLT) count > 150 × 109/L could safely avoid gastroscopy screening[3,4]. These criteria were verified in several clinical studies and can safely spare 20%-30% of liver cirrhosis patients from undergoing gastroscopy[9,10]. The American Association for the Study of Liver Disease recently recommended using the Baveno VI criteria to stratify EV risk in patients with liver cirrhosis[3]. However, up to 40% of gastroscopies are still unnecessary[11]. It is therefore imperative to finding a new strategy to identify more patients without HRV.
Several studies reported that adjusting the LSM and PLT thresholds could spare more patients from undergoing gastroscopy. The expanded Baveno VI criteria (PLT > 110 × 109/L and LSM < 25 kPa) further spare 19% of gastroscopies compared to the original Baveno VI criteria, with a risk of missing 1.6% of HRV cases[12]. Jangouk et al[9] recently reported a 12% increase in spared gastroscopies (with no additional HRV cases missed) by expanding the Baveno VI criteria to include a Model for End-Stage Liver Disease (MELD) score of 6. Using cutoff values of LSM ≤ 25 kPa and PLT ≥ 100 × 109/L, Ding et al[13] found that 21% of gastroscopies were spared. However, these criteria have not been confirmed.
Because liver inflammation can significantly increase LSM, it is recommended that the interpretation of LSM is based on the levels of alanine transaminase (ALT) and total bilirubin (TBil)[14,15]. Clinically, most patients with HBV-related liver cirrhosis have concomitant liver inflammation. However, it remains unknown whether adjusting the LSM and PLT cutoffs according to ALT and TBiL levels would increase the number of spared gastroscopies among patients who do not meet the Baveno VI criteria.
Serum markers of liver fibrosis such as PLT count, aspartate aminotransferase (AST)-to-PLT ratio index (APRI), Fibrosis-4 (FIB-4), and the Lok index are useful in predicting severe liver fibrosis or cirrhosis[16-18], EV risk, and variceal bleeding risk in patients with HBV infection[19]. It remains unknown whether these serum markers of liver fibrosis can identify patients without HRV among those who do not meet the Baveno VI criteria. In this study, we aimed to evaluate the utilities of LSM, PLT count, APRI, FIB-4, and the Lok index stratified by ALT and TBil levels for ruling out HRV in patients with HBV-related compensated cirrhosis who did not meet the Baveno VI criteria.
MATERIALS AND METHODS
Study population
From September 2016 to June 2018, we applied the Baveno VI criteria to patients with compensated liver cirrhosis who were admitted to the Affiliated Hospital of Zunyi Medical University and Suining Central Hospital. All the patients with compensated liver cirrhosis who did not meet the Baveno VI criteria underwent gastroscopy screening during this period. We retrospectively reviewed records of 183 hospitalized patients with HBV-related compensated liver cirrhosis who underwent a FibroScan procedure and gastroscopy within 6 months and had complete clinical, laboratory, and imaging data. A total of 132 patients were included in the study. The remaining 51 were excluded from the study because they had the following concomitant conditions: 19, alcoholic liver disease; 15, hepatocellular carcinoma; 6, invalid LSM; 4, human immunodeficiency virus infection; 3, cardiovascular disease; 3, splenectomy; and 1, primary biliary cholangitis.
Diagnostic criteria for HBV-related compensated liver cirrhosis
The diagnosis of cirrhosis was based on previous liver biopsy findings or a composite of clinical signs and findings provided by laboratory tests, gastroscopy, radiologic imaging, and FibroScan procedures. Decompensated liver cirrhosis was defined as the presence of one of the following: New onset of hepatic encephalopathy, EVB, or ascites[2].
Two professionally trained operators performed each transient elastography (TE) measurement using a FibroScan device (Echosens, Paris, France). The M probe was used in all measurements. Only cases with 10 valid measurements obtained with a success rate ≥ 60% and an interquartile range-to-median ratio ≤ 30% were considered valid. The median valid LSM value is expressed in kPa.
Grading criteria for EV
Gastroscopy procedures were performed by two experienced endoscopists who were unaware of the LSM results. EV stage was classified as none (no veins above the esophageal mucosal surface; F0), small (minimally elevated veins above the esophageal mucosal surface; F1), medium (large tortuous veins occupying < 1/3 of the lumen; F2), or large (large coil-shaped veins occupying ≥ 1/3 of the lumen; F3). HRV was defined as F2/F3 EV or F1 EV with red wale signs[20].
Candidate predictor variables
Laboratory parameters included white blood cell (WBC) count, PLT count, ALT, AST, gamma-glutamyl transpeptidase (GGT), TBil, albumin (ALB), globulin (GLB), prothrombin (PT), prothrombin time activity (PTA), international normalized ratio (INR), HBV DNA, serum sodium (Na+), blood urea nitrogen (BUN), creatinine (Cr), and alpha-fetoprotein (AFP). For patients with multiple laboratory parameter measurements, we used the results obtained nearest in time to the TE procedure.
The serum markers of liver fibrosis were calculated according to the following formulas: APRI = [AST/upper limit of normal (ULN)] × 100/PLT; FIB-4 = (age × AST)/(PLT × square root of ALT); Lok index = exp (log odds)/[1 + exp(log odds)], log odds = -5.56 - 0.0089 × PLT + 1.26 × (AST/ALT) + 5.27 × INR. The following formula was used for calculating the MELD scores: MELD score = 3.8 × ln[TBil (mg/dL)] + 11.2 × ln(INR) + 9.6 × ln[Cr (mg/dL)] + 6.4 × (constant for liver disease etiology: 0 if cholestatic or alcoholic, otherwise 1).
Ethics statement
The protocol conformed to the provisions of the Declaration of Helsinki and was approved by the Human Ethical Committee of the Affiliated Hospital of Zunyi Medical University and Suining Central Hospital. All patients were informed in writing regarding the potential use of their data for clinical research purposes, and all accepted.
Statistical analysis
Statistical analyses were performed using SPSS 19.0 (SPSS, Chicago, IL, USA) and MedCalc® 15.8 (MedCalc Software BVBA, Ostend, Belgium). Patient characteristics were compared between patients with and without HRV, using χ2 tests for categorical variables, t-tests for variables with normal distributions, and Mann–Whitney U tests for variables with non-normal distributions. Logistic regression analysis was used for univariate and multivariate analyses. MedCalc 15.8 was used to calculate receiver operating characteristic (ROC) curves, and the accuracy of each diagnostic criterion was evaluated according to the area under each ROC curve (AUROC). Given that the aim of this study was to identify patients without HRV, we defined the cutoff values of LSM, PLT, MELD score, and serum markers of liver fibrosis based on the values corresponding to a negative predictive value (NPV) of 100%. The main results calculated were sensitivity, specificity, positive predictive value (PPV), and positive likelihood ratio (PLR) as well as the number of unnecessary gastroscopy procedures. Since we set the cutoff values based on an NPV of 100%, we did not calculate the negative likelihood ratio (NLR). P < 0.05 was considered statistically significant.
RESULTS
Patient characteristics and HRV prevalence
Among the 132 patients enrolled in the study, 59 (44.7%) had EV. Among them, 32 (24.2%) had small EV without red wale signs and 27 (20.5%) had HRV (medium or large EV). Regarding Child-Pugh class, 95 and 37 patients had classes A and B, respectively. Of the 132 patients, 99 (26 with HRV) did not meet the Baveno VI criteria due to having both LSM ≥ 20 kPa and PLT ≤ 150 × 109/L; 11 (1 with HRV) due to having only LSM ≥ 20 kPa; and 22 (0 with HRV) due to having only PLT ≤ 150 × 109/L. As shown in Table 1, PT, INR, LSM, and MELD score were significantly higher in patients with HRV than in those without, whereas ALT, AST, GGT, PLT, PTA, and HBV DNA levels were significantly lower.
Table 1.
Biochemical characteristics of patients with hepatitis B virus-related liver cirrhosis with and without high-risk varices
| Variable | Without high-risk varices (n = 105) | With high-risk varices (n = 27) | P-value |
| Age | 46.32 ± 11.83 | 46.74 ± 11.17 | 0.8691 |
| Male | 88 (83.81) | 25 (92.59) | 0.2462 |
| BMI (kg/m2) | 24.6 ± 3.53 | 23.8 ± 2.96 | 0.9121 |
| HBeAg: Positive | 59 (56.19) | 11 (40.74) | 0.1512 |
| HBV DNA (log 10, copies/mL) | 6.27 ± 1.43 | 5.08 ± 1.46 | 0.0021 |
| Na+ (mmol/L) | 137.56 ± 13.80 | 139.12 ± 3.07 | 0.5601 |
| ALT (U/L) | 177.0 (64.0-318.0) | 54.0 (33.0-92.0) | 0.0003 |
| AST (U/L) | 144.0 (55.5-266.5) | 60.0 (37.0-136.9) | 0.0013 |
| GGT (U/L) | 105.0 (68.4-198.0) | 60.0 (35.0-93.0) | 0.0003 |
| TBil (μmol/L) | 24.4 (16.75-37.5) | 27.8 (15.5-48.2) | 0.6763 |
| ALB (g/L) | 37.35 ± 5.74 | 36.07 ± 5.74 | 0.3051 |
| GLB (g/L) | 31.4 (28.55-35.65) | 34.6 (28.6-39.1) | 0.1013 |
| AFP (ng/mL) | 16.47 (6.66-74.73) | 13.6 (7.00-30.1) | 0.4223 |
| BUN (mmol/L) | 4.64 ± 1.28 | 4.71 ± 1.29 | 0.8011 |
| Cr (μmol/L) | 73.0 (62.0-80.5) | 73.0 (69.0-90.0) | 0.2773 |
| PT (s) | 13.0 (11.8-14.4) | 15.4 (12.5-16.3) | 0.0023 |
| INR | 1.08 (0.98-1.17) | 1.23 (1.04-1.38) | 0.0033 |
| PTA (%) | 85.05 ± 21.33 | 74.52 ± 20.25 | 0.0221 |
| WBC (109/L) | 4.21 (3.28-5.34) | 3.64 (2.68-4.50) | 0.0523 |
| PLT (109/L) | 98.35 ± 46.49 | 67.00 ± 28.29 | 0.0001 |
| LSM (kPa) | 28.93 ± 12.41 | 35.42 ± 14.44 | 0.0211 |
| Child-Pugh score | 5.00 (5.00-7.00) | 6.00 (5.00-7.00) | 0.5083 |
| MELD | 8.83 (7.50-11.76) | 11.33 (8.81-13.18) | 0.0253 |
Data are presented as the mean ± SD, or median (interquartile range).
t-test results;
Chi square test results;
Mann–Whitney U test results.
P < 0.05 was statistically significant. AFP: Alpha-fetoprotein; ALB: Albumin; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; BMI: Body mass index; BUN: Urea nitrogen; Cr: Creatinine; GGT: Glutamine transpeptidase; GLB: Globulin; HBeAg: Hepatitis B e-antigen; HBV DNA: Hepatitis B virus DNA; INR: International normalized ratio; LSM: Liver stiffness measurement; MELD: Model for End-Stage Liver Disease; Na+: Sodium; PLT: Platelet; PT: Prothrombin time; PTA: Prothrombin activity; TBil: Total bilirubin; WBC: White blood cell.
Independent risk factors for HRV and performance of LSM and serum markers of liver fibrosis for ruling out HRV
As shown in Table 2, the risk factors for HRV in patients who did not meet the Baveno VI criteria included PT, PLT, ALT, GGT, LSM, and MELD score. Independent risk factors for HRV were LSM and ALT. We then explored whether adjusting the LSM and PLT cutoff values and using serum markers of liver fibrosis could exclude more patients without HRV among those who did not meet the Baveno VI criteria. As shown in Table 3, the Lok index had an AUROC of 0.753 [95% confidence interval (CI): 0.671-0.824]. The AUROCs of LSM, APRI, FIB-4, and MELD score were all < 0.7. The Lok index cutoff value of 0.4531 could further spare 32/132 (24.2%) of gastroscopies without missing HRVs. Although PLT had an AUROC of 0.712 (95%CI: 0.627-0.787), PLT > 151 × 109/L could only spare 10/132 (7.6%) of gastroscopies without missing HRVs. The AUROC of LSM was 0.637 (95%CI: 0.548-0.718), and LSM < 20.9 kPa could spare 31/132 (23.5%) of gastroscopies without missing HRVs.
Table 2.
Univariate and multivariate analyses of risk factors associated with high-risk varices in patients who did not meet the 2015 Baveno VI criteria
| Variable |
Univariate analysis |
Multivariate analysis |
||||||
| β | OR | 95%CI | P-value | β | OR | 95%CI | P-value | |
| PT | 0.289 | 1.335 | 1.117-1.595 | 0.001 | ||||
| PLT | -0.025 | 0.975 | 0.960-0.990 | 0.001 | ||||
| ALT | -0.008 | 0.992 | 0.987-0.997 | 0.003 | -0.007 | 0.993 | 0.987-0.999 | 0.029 |
| GGT | -0.013 | 0.987 | 0.979-0.996 | 0.003 | ||||
| LSM | 0.035 | 1.035 | 1.004-1.068 | 0.025 | 0.077 | 1.080 | 1.030-1.132 | 0.001 |
| MELD | 0.091 | 1.096 | 1.002-1.198 | 0.045 | ||||
ALT: Alanine aminotransferase; CI: Confidence interval; GGT: Glutamine transpeptidase; LSM: Liver stiffness measurement; MELD: Model for End-Stage Liver Disease; OR: Odds ratio; PLT: Platelet; PT: Prothrombin time. P < 0.05 was statistically significant.
Table 3.
Performance of liver stiffness measurement and serum markers of liver fibrosis in predicting high-risk varices in patients who did not meet the 2015 Baveno VI criteria
| Variable | AUROC (95%CI) | P-value | Cutoff value | Se (%) | Sp (%) | PPV (%) | PLR | Spared gastroscopies, n = 132, (%) |
| LSM | 0.637 (0.548-0.718) | 0.0141 | 20.9 | 100 | 29.52 | 26.7 | 1.42 | 31/132 (23.5) |
| PLT | 0.712 (0.627-0.787) | < 0.0001 | 151.0 | 100 | 9.52 | 22.1 | 1.11 | 10/132 (7.6) |
| Lok index | 0.753 (0.671-0.824) | < 0.0001 | 0.4531 | 100 | 30.48 | 27.0 | 1.44 | 32/132 (24.2) |
| MELD | 0.644 (0.556-0.726) | 0.0122 | 6.0 | 100 | 6.67 | 21.6 | 1.07 | 7/132 (5.3) |
| APRI | 0.586 (0.497-0.671) | 0.1453 | ||||||
| FIB-4 | 0.585 (0.496-0.670) | 0.1281 |
APRI: Aspartate aminotransferase-to-platelet ratio index; AUROC: Area under the receiver operating characteristic curve; CI: Confidence interval; MELD: Model for End-Stage Liver Disease; PLT: Platelet; PLR: Positive likelihood ratio; PPV: Positive predictive value; Se: Sensitivity; Sp: Specificity. P < 0.05 was statistically significant.
If the expanded Baveno VI criteria (LSM < 25 kPa with a PLT count > 110 × 109/L) were applied to patients who did not meet the Baveno VI criteria, they only spared 8 (6.1%) gastroscopies without missing HRVs. In our study, only 7 patients had an MELD score of 6, and a stepwise strategy using PLT > 150 × 109/L and MELD = 6 only led to 7/132 (5.3%) additional patients avoiding gastroscopies without missing HRVs. Using cutoff values of LSM ≤ 25 kPa and PLT ≥ 100 × 109/L, as suggested by Ding et al[13], only led to 16/132 (12.1%) more patients avoiding gastroscopies without missing HRVs.
Effects of ALT and TBil on LSM in patients who did not meet the Baveno VI criteria
LSM in patients with ALT < 2 ULN (26.77 ± 12.08 kPa) was significantly lower than that in patients with ALT ≥ 2 ULN (32.59 ± 13.25 kPa) (P = 0.011). The mean LSM value in patients with TBil < 2 ULN (26.40 ± 8.66 kPa) was also significantly lower than that in patients with TBil ≥ 2 ULN (38.80 ± 16.77 kPa) (P = 0.000). The LSM in patients with both ALT and TBil < 2 ULN (23.66 ± 8.44 kPa) was significantly lower than that in patients with ALT or TBil ≥ 2 ULN (33.23 ± 13.71 kPa) (P = 0.000).
LSM and serum markers of liver fibrosis for ruling out HRV in patients with ALT and TBil < 2 ULN
To explore the possibility of increasing the number of patients spared gastroscopy by adjusting the cutoff values of LSM and PLT according to ALT and TBil levels, we divided them into patients with ALT and TBil < 2 ULN (n = 41) and patients with ALT or TBil ≥ 2 ULN (n = 91). Among the 41 patients with both ALT and TBIL < 2 ULN, 14 (34.1%) had HRV. As shown in Table 4, LSM had an AUROC of 0.821 (95%CI: 0.670-0.923, P < 0.0001). At a cutoff value of 20.6 kPa, LSM further spared 16/41 (39.0%) of gastroscopies without missing HRVs. PLT, Lok index, MELD score, APRI, FiB-4, and PLT had no predictive value for HRV.
Table 4.
Performance of liver stiffness measurement and serum markers of liver fibrosis in predicting high-risk varices in patients with alanine aminotransferase and total bilirubin < 2 upper limit of normal
| Variable | AUROC (95%CI) | P -value | Cutoff value | Se (%) | Sp (%) | PPV (%) | PLR | Spared gastroscopies, n = 41, (%) |
| LSM | 0.821 (0.670-0.923) | < 0.0001 | 20.60 | 100 | 66.7 | 60.9 | 3.00 | 16/41 (39.0) |
| PLT | 0.628 (0.464-0.774) | 0.1928 | ||||||
| Lok index | 0.638 (0.473-0.782) | 0.1407 | ||||||
| MELD | 0.644 (0.479-0.787) | 0.1449 | ||||||
| APRI | 0.569 (0.405- 0.722) | 0.4667 | ||||||
| FIB-4 | 0.608 (0.444-0.757) | 0.2474 |
APRI: Aspartate aminotransferase-to-platelet ratio index; AUROC: Area under the receiver operating characteristic curve; CI: Confidence interval; MELD: Model for End-Stage Liver Disease; PLT: Platelet; PLR: Positive likelihood ratio; PPV: Positive predictive value; Se: Sensitivity; Sp: Specificity. P < 0.05 was statistically significant.
LSM and serum markers of liver fibrosis for ruling out HRV in patients with ALT or TBil ≥ 2 ULN
Among the 91 patients with ALT or TBil ≥ 2 ULN, the prevalence of HRV (13/91, 14.3%) was significantly lower than that among patients with ALT and TBil < 2 ULN (14/41, 34.1%, P < 0.05). As shown in Table 5, only the AUROC of the Lok index for identifying HRV was > 0.800. The AUROC of LSM was significantly reduced to 0.672, which was lower than those for the Lok index (0.814), PLT (0.741), and MELD score (0.735).
Table 5.
Performance of liver stiffness measurement and serum markers of liver fibrosis in predicting high-risk varices in patients with alanine aminotransferase or total bilirubin ≥ 2 upper limit of normal
| Variable | AUROC (95%CI) | P-value | Cutoff value | Se (%) | Sp ( %) | PPV (%) | PLR | Spared gastroscopies, n = 91, (%) |
| LSM | 0.672 (0.565-0.766) | 0.0508 | ||||||
| PLT | 0.741 (0.639-0.827) | < 0.0001 | 100.0 | 100 | 51.28 | 25.5 | 2.05 | 40/91 (43.9) |
| Lok index | 0.814 (0.718-0.888) | < 0.0001 | 0.5596 | 100 | 47.44 | 24.1 | 1.90 | 36/91 (39.6) |
| MELD | 0.735 (0.632-0.822) | 0.0003 | 7.0 | 100 | 12.82 | 16.0 | 1.15 | 10/91 (11.0) |
| APRI | 0.532 (0.424-0.637) | 0.7256 | ||||||
| FIB-4 | 0.576 (0.468-0.679) | 0.3329 |
APRI: Aspartate aminotransferase-to-platelet ratio index; AUROC: Area under the receiver operating characteristic curve; CI: Confidence interval; MELD: Model for End-Stage Liver Disease; PLT: Platelet; PLR: Positive likelihood ratio; PPV: Positive predictive value; Se: Sensitivity; Sp: Specificity. P < 0.05 was statistically significant.
Using a Lok index cutoff ≤ 0.5596 could spare 36/91 (39.6%) of gastroscopies without missing HRVs. Using PLT > 100 × 109/L could spare 40/91 (43.9%) of gastroscopies without missing HRVs. An MELD score ≤ 7 could only spare 10/91 (11.0%) of gastroscopies without missing HRVs. These results suggested that the Lok index and PLT performed satisfactorily in identifying patients without HRV among patients with ALT or TBil ≥ 2 ULN.
DISCUSSION
Although many studies have validated the accuracy of the Baveno VI criteria for ruling out HRV[9,21-23], studies have also found that the criteria are conservative and need to be refined to accurately identify more patients without HRV[10,24,25]. In this study, we focused on patients with HBV-related compensated liver cirrhosis who did not meet the 2015 Baveno VI criteria because the prevalence of HRV is relatively high in this population and the possible HRV risk factors may be different from those for patients who fulfill the 2015 Baveno VI criteria[3,26]. We found that only 20.5% of the patients had HRV. Moreover, among the 91 patients with ALT or TBil ≥ 2 ULN, only 14.3% had HRV. Thus, our results demonstrated that many unnecessary gastroscopies are conducted in patients with HBV-related compensated liver cirrhosis despite excluding patients at low risk for EV using the 2015 Baveno VI criteria, especially among patients with ALT or TBil ≥ 2 ULN.
Although LSM was still one of the independent factors associated with HRV, it only had an AUROC of 0.637 for predicting HRV among patients who did not meet the Baveno VI criteria. This result indicated that LSM was not a good predictor of HRV in the overall cohort of patients who did not meet the Baveno VI criteria.
We also found that ALT was independently negatively associated with HRV, because most patients in our study had concomitant liver inflammation. Previous studies showed that LSM can be elevated by liver inflammation and cholestasis[27,28]. Our results suggested that concomitant liver inflammation might be an important factor that interfered with the predictive accuracy of LSM in patients with HBV-related compensated liver cirrhosis who did not meet the Baveno VI criteria.
It was previously unknown whether adjusting the LSM cutoff value according to ALT and TBil levels could improve its utility for predicting HRV among patients with compensated liver cirrhosis and obvious liver inflammation. We found that LSM could accurately identify patients with HRV in those with ALT and TBil < 2 ULN. By slightly increasing the LSM cutoff to 20.6 kPa, LSM further spared 16/41 (39.0%) of gastroscopies without missing HRVs. PLT, Lok index, MELD score, APRI, FiB-4, and PLT had no value for predicting HRV. However, in the patients with ALT or TBil ≥ 2 ULN, LSM had no HRV predictive value.
Previous studies have found that serum markers of liver fibrosis have moderate value for predicting liver cirrhosis in patients with HBV infection[29,30]. However, there is disagreement on the performance of serum markers of liver fibrosis in EV or HRV prediction[8,19,31]. In this study, we found that APRI and FIB-4 could not predict HRV. We also found that PLT and the Lok index performed better in predicting HRV in patients with ALT or TBil ≥ 2 ULN compared with ALT and TBil < 2 ULN. As previous studies found that the Lok index could better predict liver fibrosis in patients with a slightly higher ALT level compared with a normal ALT level[16,32], it is reasonable that the Lok index performed better in predicting HRV in patients with ALT or TBil ≥ 2 ULN. However, it is difficult to explain the different performances of PLT in patients with ALT or TBil ≥ 2 ULN vs < 2 ULN. The different prevalence rates of HRV in these two groups may be a possible explanation, as the prevalence in the patients with ALT and TBil < 2 ULN (34.1%) was significantly higher compared to that in patients with ALT or TBil ≥ 2 ULN (14.3%). Indeed, previous studies reported that the utility of serum markers of liver fibrosis in predicting EV or HRV is greatly affected by the prevalence[29,33,34]. Because patients with ALT or TBil ≥ 2 ULN had obvious liver inflammation, which could elevate LSM, they were difficult to fulfill the Baveno VI criteria, and as a result, the prevalence rate of HRV in patients with ALT or TBil ≥ 2 ULN was lower than that in patients with ALT and TBil < 2 ULN.
Our study had several limitations. First, it was a two-center, retrospective study based on LSM assessed and gastroscopies performed by different operators, although all were experienced. Second, because we only included the patients with HBV-related compensated liver cirrhosis who did not meet the Baveno VI criteria, the number of patients in this study was small, and the cutoff values were not tested in a validation cohort. Further studies are required to verify our findings.
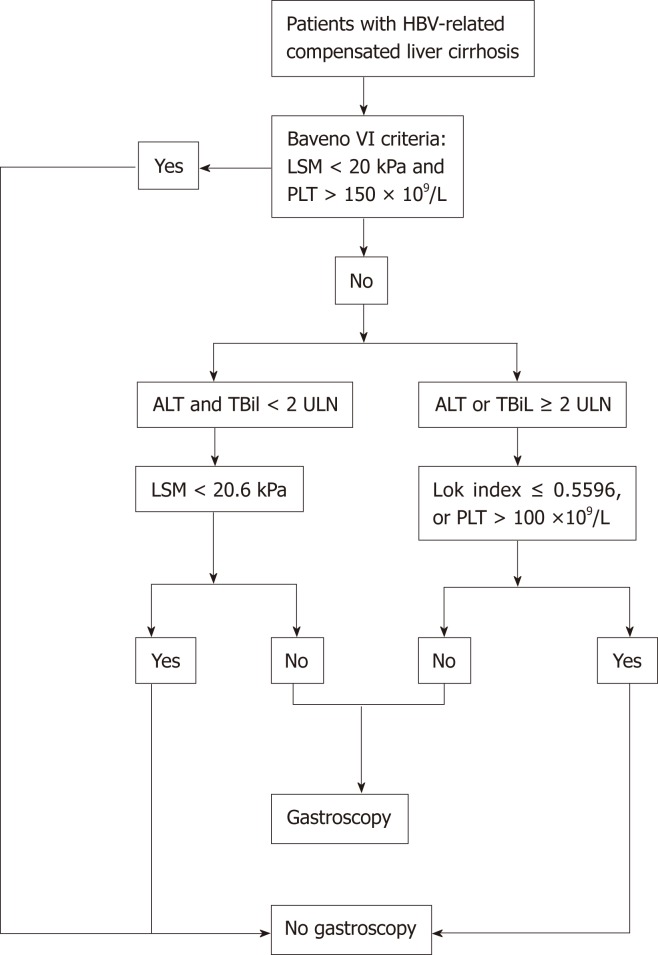
In conclusion, our study found that in patients with HBV-related compensated cirrhosis who did not meet the Baveno VI criteria, using the cutoff value of LSM, PLT, or the Lok index stratified by ALT and TBil levels accurately identified more patients without HRV. An algorithm-based screening process for HRV in patients with HBV-related compensated liver cirrhosis is shown in Figure 1.
Figure 1.
Algorithm-based screening process for high-risk varices in patients with HBV-related compensated liver cirrhosis. ALT: Alanine aminotransferase; HBV: Hepatitis B virus; LSM: Liver stiffness measurement; PLT: Platelet; TBil: Total bilirubin; ULN: Upper limit of normal.
ARTICLE HIGHLIGHTS
Research background
The 2015 Baveno VI consensus workshop recommended that patients with liver stiffness measurement (LSM) < 20 kPa and platelet (PLT) count >150 × 109/L could safely avoid gastroscopy screening. However, studies have also found that such criteria are conservative and need to be refined to accurately identify more patients without high-risk varices (HRV).
Research motivation
It is recommended that the interpretation of LSM is based on the levels of alanine transaminase (ALT) and total bilirubin (TBil), but it remains unknown whether the LSM, PLT and serum markers of liver fibrosis stratified by ALT and TBil levels can identify more patients without HRV among those who do not meet the Baveno VI criteria.
Research objectives
To evaluate the utility of LSM and serum markers of liver fibrosis in ruling out high-risk varices (HRV) in patients who do not meet Baveno VI criteria.
Research methods
Data from 132 patients with hepatitis B virus (HBV)-related compensated liver cirrhosis who did not meet the Baveno VI criteria were retrospectively reviewed. The accuracy of LSM, PLT count, aspartate aminotransferase (AST)-to-PLT ratio index, Fibrosis-4, and the Lok index in predicting HRV was evaluated. The utility of LSM, PLT, and serum markers of liver fibrosis stratified by ALT and TBil levels was evaluated for ruling out HRV.
Research results
The prevalence of HRV was significantly lower in patients with ALT or TBil ≥ 2 upper limit of normal (ULN) (14.3%) than in patients with both ALT and TBil < 2 ULN (34.1%) (P = 0.018). In the 41 patients with ALT and TBil < 2 ULN, LSM < 20.6 kPa spared 39.0% of gastroscopies without missing HRVs. In the 91 patients with ALT or TBiL ≥ 2 ULN, Lok index ≤ 0.5596 or PLT > 100 × 109/L further spared 39.6% and 43.9% of gastroscopies, respectively, without missing HRVs.
Research conclusions
In HBV-related compensated cirrhosis patients who do not meet Baveno VI criteria, the LSM, PLT, or Lok index cutoff stratified by ALT and TBil accurately identified more patients without HRV.
Research perspectives
Our study found that in patients with HBV-related compensated cirrhosis who did not meet the Baveno VI criteria, using the cutoff value of LSM, PLT or the Lok index stratified by ALT and TBil levels accurately identified more patients without HRV.
Footnotes
Institutional review board statement: This study was approved by the Institutional Review Board of Affiliated Hospital of Zunyi Medical University, Guizhou Province, China.
Informed consent statement: All patients were informed in writing of the use of their data for clinical research purposes and accepted.
Conflict-of-interest statement: The authors declare that they have no conflicts of interest in this study.
STROBE statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Peer-review started: May 30, 2019
First decision: July 21, 2019
Article in press: July 21, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Karaksy H, Fouad YM, Thomopoulos K S-Editor: Yan JP L-Editor: Wang TQ E-Editor: Ma YJ
Contributor Information
Hong Zhou, Department of Infectious Diseases, Affiliated Hospital of Zunyi Medical University, Zunyi 563003, Guizhou Province, China; Department of Infectious Diseases, Suining Central Hospital, Suining 629000, Sichuan Province, China.
Jun Long, Department of Infectious Diseases, Affiliated Hospital of Zunyi Medical University, Zunyi 563003, Guizhou Province, China.
Han Hu, Department of Infectious Diseases, Affiliated Hospital of Zunyi Medical University, Zunyi 563003, Guizhou Province, China.
Cai-Yun Tian, Department of Infectious Diseases, Affiliated Hospital of Zunyi Medical University, Zunyi 563003, Guizhou Province, China.
Shi-De Lin, Department of Infectious Diseases, Affiliated Hospital of Zunyi Medical University, Zunyi 563003, Guizhou Province, China. linshide6@zmu.edu.cn.
References
- 1.Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS, Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310–335. doi: 10.1002/hep.28906. [DOI] [PubMed] [Google Scholar]
- 4.de Franchis R Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 5.Fukui H, Saito H, Ueno Y, Uto H, Obara K, Sakaida I, Shibuya A, Seike M, Nagoshi S, Segawa M, Tsubouchi H, Moriwaki H, Kato A, Hashimoto E, Michitaka K, Murawaki T, Sugano K, Watanabe M, Shimosegawa T. Evidence-based clinical practice guidelines for liver cirrhosis 2015. J Gastroenterol. 2016;51:629–650. doi: 10.1007/s00535-016-1216-y. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922–938. doi: 10.1002/hep.21907. [DOI] [PubMed] [Google Scholar]
- 7.de Franchis R Baveno V Faculty. Revising consensus in portal hypertension: Report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762–768. doi: 10.1016/j.jhep.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Sebastiani G, Tempesta D, Fattovich G, Castera L, Halfon P, Bourliere M, Noventa F, Angeli P, Saggioro A, Alberti A. Prediction of oesophageal varices in hepatic cirrhosis by simple serum non-invasive markers: Results of a multicenter, large-scale study. J Hepatol. 2010;53:630–638. doi: 10.1016/j.jhep.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 9.Jangouk P, Turco L, De Oliveira A, Schepis F, Villa E, Garcia-Tsao G. Validating, deconstructing and refining Baveno criteria for ruling out high-risk varices in patients with compensated cirrhosis. Liver Int. 2017;37:1177–1183. doi: 10.1111/liv.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sousa M, Fernandes S, Proença L, Silva AP, Leite S, Silva J, Ponte A, Rodrigues J, Silva JC, Carvalho J. The Baveno VI criteria for predicting esophageal varices: Validation in real life practice. Rev Esp Enferm Dig. 2017;109:704–707. doi: 10.17235/reed.2017.5052/2017. [DOI] [PubMed] [Google Scholar]
- 11.Silva MJ, Bernardes C, Pinto J, Loureiro R, Duarte P, Mendes M, Calinas F. Baveno VI Recommendation on Avoidance of Screening Endoscopy in Cirrhotic Patients: Are We There Yet? GE Port J Gastroenterol. 2017;24:79–83. doi: 10.1159/000452693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Augustin S, Pons M, Maurice JB, Bureau C, Stefanescu H, Ney M, Blasco H, Procopet B, Tsochatzis E, Westbrook RH, Bosch J, Berzigotti A, Abraldes JG, Genescà J. Expanding the Baveno VI criteria for the screening of varices in patients with compensated advanced chronic liver disease. Hepatology. 2017;66:1980–1988. doi: 10.1002/hep.29363. [DOI] [PubMed] [Google Scholar]
- 13.Ding NS, Nguyen T, Iser DM, Hong T, Flanagan E, Wong A, Luiz L, Tan JY, Fulforth J, Holmes J, Ryan M, Bell SJ, Desmond PV, Roberts SK, Lubel J, Kemp W, Thompson AJ. Liver stiffness plus platelet count can be used to exclude high-risk oesophageal varices. Liver Int. 2016;36:240–245. doi: 10.1111/liv.12916. [DOI] [PubMed] [Google Scholar]
- 14.Shiha G, Ibrahim A, Helmy A, Sarin SK, Omata M, Kumar A, Bernstien D, Maruyama H, Saraswat V, Chawla Y, Hamid S, Abbas Z, Bedossa P, Sakhuja P, Elmahatab M, Lim SG, Lesmana L, Sollano J, Jia JD, Abbas B, Omar A, Sharma B, Payawal D, Abdallah A, Serwah A, Hamed A, Elsayed A, AbdelMaqsod A, Hassanein T, Ihab A, GHaziuan H, Zein N, Kumar M. Asian-Pacific Association for the Study of the Liver (APASL) consensus guidelines on invasive and non-invasive assessment of hepatic fibrosis: A 2016 update. Hepatol Int. 2017;11:1–30. doi: 10.1007/s12072-016-9760-3. [DOI] [PubMed] [Google Scholar]
- 15.Singh S, Muir AJ, Dieterich DT, Falck-Ytter YT. American Gastroenterological Association Institute Technical Review on the Role of Elastography in Chronic Liver Diseases. Gastroenterology. 2017;152:1544–1577. doi: 10.1053/j.gastro.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Dong M, Wu J, Yu X, Li J, Yang S, Qi X, Mao R, Zhang Y, Yu J, Zhu H, Yang F, Qin Y, Zhang J. Validation and comparison of seventeen noninvasive models for evaluating liver fibrosis in Chinese hepatitis B patients. Liver Int. 2018;38:1562–1570. doi: 10.1111/liv.13688. [DOI] [PubMed] [Google Scholar]
- 17.Lok AS, Ghany MG, Goodman ZD, Wright EC, Everson GT, Sterling RK, Everhart JE, Lindsay KL, Bonkovsky HL, Di Bisceglie AM, Lee WM, Morgan TR, Dienstag JL, Morishima C. Predicting cirrhosis in patients with hepatitis C based on standard laboratory tests: Results of the HALT-C cohort. Hepatology. 2005;42:282–292. doi: 10.1002/hep.20772. [DOI] [PubMed] [Google Scholar]
- 18.Ma J, Jiang Y, Gong G. Evaluation of seven noninvasive models in staging liver fibrosis in patients with chronic hepatitis B virus infection. Eur J Gastroenterol Hepatol. 2013;25:428–434. doi: 10.1097/MEG.0b013e32835cb5dd. [DOI] [PubMed] [Google Scholar]
- 19.Rockey DC, Elliott A, Lyles T. Prediction of esophageal varices and variceal hemorrhage in patients with acute upper gastrointestinal bleeding. J Investig Med. 2016;64:745–751. doi: 10.1136/jim-2015-000047. [DOI] [PubMed] [Google Scholar]
- 20.Beppu K, Inokuchi K, Koyanagi N, Nakayama S, Sakata H, Kitano S, Kobayashi M. Prediction of variceal hemorrhage by esophageal endoscopy. Gastrointest Endosc. 1981;27:213–218. doi: 10.1016/s0016-5107(81)73224-3. [DOI] [PubMed] [Google Scholar]
- 21.Marot A, Trépo E, Doerig C, Schoepfer A, Moreno C, Deltenre P. Liver stiffness and platelet count for identifying patients with compensated liver disease at low risk of variceal bleeding. Liver Int. 2017;37:707–716. doi: 10.1111/liv.13318. [DOI] [PubMed] [Google Scholar]
- 22.Stafylidou M, Paschos P, Katsoula A, Malandris K, Ioakim K, Bekiari E, Haidich AB, Akriviadis E, Tsapas A. Performance of Baveno VI and Expanded Baveno VI Criteria for Excluding High-Risk Varices in Patients With Chronic Liver Diseases: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2019;17:1744–1755. e11. doi: 10.1016/j.cgh.2019.04.062. [DOI] [PubMed] [Google Scholar]
- 23.Augustin S, Pons M, Genesca J. Validating the Baveno VI recommendations for screening varices. J Hepatol. 2017;66:459–460. doi: 10.1016/j.jhep.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 24.Colecchia A, Ravaioli F, Marasco G, Colli A, Dajti E, Di Biase AR, Bacchi Reggiani ML, Berzigotti A, Pinzani M, Festi D. A combined model based on spleen stiffness measurement and Baveno VI criteria to rule out high-risk varices in advanced chronic liver disease. J Hepatol. 2018;69:308–317. doi: 10.1016/j.jhep.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 25.Moctezuma Velázquez C, Abraldes JG. Non-invasive diagnosis of esophageal varices after Baveno VI. Turk J Gastroenterol. 2017;28:159–165. doi: 10.5152/tjg.2017.16744. [DOI] [PubMed] [Google Scholar]
- 26.Maurice JB, Brodkin E, Arnold F, Navaratnam A, Paine H, Khawar S, Dhar A, Patch D, O'Beirne J, Mookerjee R, Pinzani M, Tsochatzis E, Westbrook RH. Validation of the Baveno VI criteria to identify low risk cirrhotic patients not requiring endoscopic surveillance for varices. J Hepatol. 2016;65:899–905. doi: 10.1016/j.jhep.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 27.Wong GL, Wong VW, Choi PC, Chan AW, Chim AM, Yiu KK, Chan FK, Sung JJ, Chan HL. Increased liver stiffness measurement by transient elastography in severe acute exacerbation of chronic hepatitis B. J Gastroenterol Hepatol. 2009;24:1002–1007. doi: 10.1111/j.1440-1746.2009.05779.x. [DOI] [PubMed] [Google Scholar]
- 28.Millonig G, Reimann FM, Friedrich S, Fonouni H, Mehrabi A, Büchler MW, Seitz HK, Mueller S. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718–1723. doi: 10.1002/hep.22577. [DOI] [PubMed] [Google Scholar]
- 29.Leung JC, Loong TC, Pang J, Wei JL, Wong VW. Invasive and non-invasive assessment of portal hypertension. Hepatol Int. 2018;12:44–55. doi: 10.1007/s12072-017-9795-0. [DOI] [PubMed] [Google Scholar]
- 30.Tan YW, Zhou XB, Ye Y, He C, Ge GH. Diagnostic value of FIB-4, aspartate aminotransferase-to-platelet ratio index and liver stiffness measurement in hepatitis B virus-infected patients with persistently normal alanine aminotransferase. World J Gastroenterol. 2017;23:5746–5754. doi: 10.3748/wjg.v23.i31.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Feng Y, Ma X, Wang G, Wu H, Xie X, Zhang C, Zhu Q. Diagnostic efficacy of noninvasive liver fibrosis indexes in predicting portal hypertension in patients with cirrhosis. PLoS One. 2017;12:e0182969. doi: 10.1371/journal.pone.0182969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong XQ, Wu Z, Zhao H, Wang GQ China HepB-Related Fibrosis Assessment Research Group. Evaluation and comparison of thirty noninvasive models for diagnosing liver fibrosis in chinese hepatitis B patients. J Viral Hepat. 2019;26:297–307. doi: 10.1111/jvh.13031. [DOI] [PubMed] [Google Scholar]
- 33.Paternostro R, Reiberger T, Bucsics T. Elastography-based screening for esophageal varices in patients with advanced chronic liver disease. World J Gastroenterol. 2019;25:308–329. doi: 10.3748/wjg.v25.i3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calès P, Buisson F, Ravaioli F, Berger A, Carboni C, Marasco G, Festi D. How to clarify the Baveno VI criteria for ruling out varices needing treatment by noninvasive tests. Liver Int. 2019;39:49–53. doi: 10.1111/liv.13945. [DOI] [PubMed] [Google Scholar]