Abstract
Renal disease is a common complication of rheumatoid arthritis (RA) and can occur secondary to RA or be induced by therapeutic agents. Recently, glomerular deposition of galactose-deficient IgA1 (Gd-IgA1) was identified as a feature of primary IgA vasculitis with nephritis (IgA-VN). We herein report a case of IgA-VN in an RA patient whose disease activity was controlled by treatment with etanercept. To distinguish between primary IgA-VN and secondary IgA-VN caused by RA or etanercept, we performed immunostaining of renal biopsy sections with the Gd-IgA1-specific antibody KM55. Positive KM55 staining confirmed the diagnosis of primary IgA-VN in a patient with RA.
Keywords: IgA vasculitis with nephritis, anti-galactose-deficient IgA1 antibody, nephrotic syndrome, rheumatoid arthritis
Introduction
Although renal involvement is common in a number of rheumatic diseases, the underlying pathophysiology varies. For example, high rates of renal disease are observed in both systemic lupus erythematosus (SLE) and microscopic polyangiitis, but it presents as immune complex-mediated glomerulonephritis in SLE patients and as pauci-immune glomerulonephritis (lacking immune complex and complement deposition) in microscopic polyangiitis patients (1). Renal involvement is also relatively common in patients with rheumatoid arthritis (RA), an autoimmune disease characterized by persistent synovitis (2,3).
Recently, Makino et al. analyzed renal biopsy specimens from 100 Japanese RA patients (4) and reported that the most common complicating kidney disease was membranous nephropathy (including that induced by disease-modifying anti-rheumatic drugs), followed by mesangial proliferative glomerulonephritis. Another complication of RA is secondary renal amyloidosis, which can lead to nephrotic syndrome and end-stage renal disease (5). The treatment of RA has changed significantly in the past couple of decades; in particular, biological agents have been in routine use since 2000. As a result, the pathological form and prevalence of kidney disease complicating RA has also changed (6). For example, tumor necrosis factor-α (TNF-α) inhibitors, such as etanercept, are used to treat a number of autoimmune diseases, including RA (2). However, emerging evidence indicates that these agents can themselves induce autoimmunity, such as vasculitis and SLE-like symptoms (7,8).
IgA nephropathy (IgA-N) and IgA vasculitis with nephritis (IgA-VN) have both been reported to be associated with RA. However, differentiation between primary IgA-VN and secondary IgA-VN caused by RA itself or by therapeutic agents, including biological agents, can be difficult based on traditional renal biopsy findings. In recent years, galactose-deficient IgA1 (Gd-IgA1) has been identified as a key effector molecule in the pathogenesis of IgA-N and IgA-VN (9). Consequently, immunostaining of renal biopsies with a Gd-IgA1-specific monoclonal antibody, KM55, has proven useful for distinguishing between primary IgA-VN and secondary IgA-VN caused by RA or agents used to treat RA (9).
We herein report a case of primary IgA-VN in a patient with RA, which was diagnosed by immunostaining with KM55.
Case Report
A 48-year-old woman was admitted to the Department of Rheumatology at our hospital in X-24 year and was diagnosed with RA based on morning stiffness, bilateral symmetric arthritis of the hands, and a positive test for serum rheumatoid factor. She had no remarkable history of medical problems. At the time of the diagnosis of RA, treatment with methotrexate and a small amount of prednisolone (5-10 mg/day) was initiated at X-24 year and continued until X-8 year, at which point the patient was started on etanercept. Because her RA disease activity had stabilized, prednisolone was discontinued at X-6 year, and treatment with methotrexate 6-8 mg/week and etanercept 25 mg/week was continued. However, despite stable RA disease activity, the patient developed sudden-onset purpura at X-28 day.
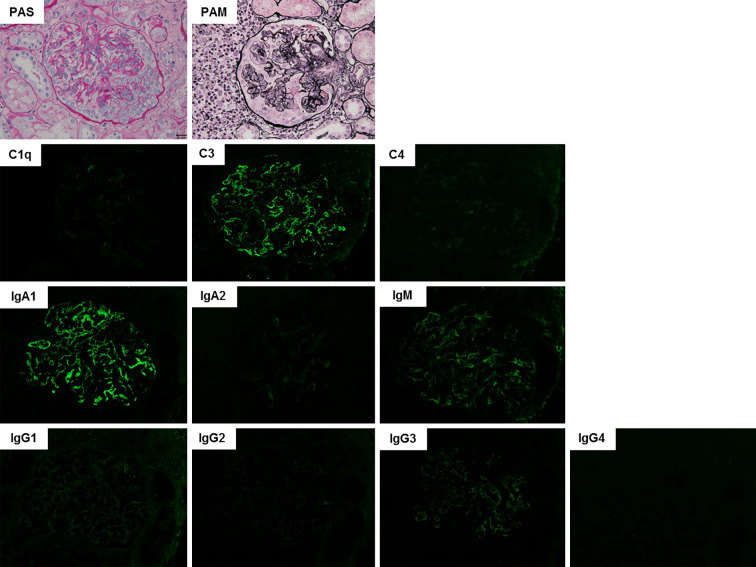
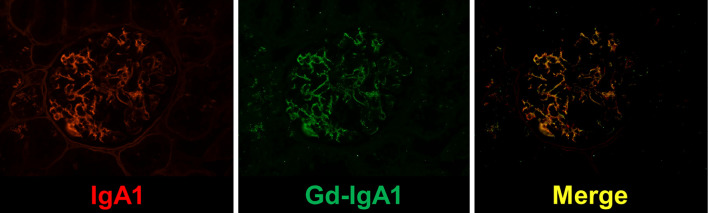
Immunostaining of a skin biopsy showed C3 deposition in the blood vessel wall in addition to leukocytoclastic vasculitis. Vasculitis associated with infection or caused by etanercept was suspected, and etanercept was discontinued. One month after the appearance of purpura, urine occult blood was 3+, proteinuria was 16.2 g/g Cr, serum creatinine was 0.95 mg/dL, and nephrotic syndrome and acute kidney injury developed. Antinuclear antibodies, perinuclear and cytoplasmic anti-neutrophil cytoplasmic antibodies, anti-glomerular basement membrane antibodies, and cryoglobulins were not detected. Electrophoresis of serum and urine proteins revealed no monoclonal Ig (M-protein) spike and no Bence Jones protein. A renal biopsy was performed, and sections were subjected to periodic acid-Schiff, periodic acid-methenamine-silver, and immunofluorescence staining. Light microscopy showed mesangial hypercellularity with mesangial matrix expansion. A cellular crescent was detected in several glomeruli. Immunofluorescence staining revealed global glomerular capillary wall and mesangial staining of IgA1, IgG, IgM, and complement C3 (Fig. 1). Congo red staining for amyloid was negative. Based on these findings, we diagnosed her with IgA-VN International Study of Kidney Diseases in Children classification grade III. Notably, immunostaining with KM55 was positive and co-localized with IgA1, confirming the presence of Gd-IgA1 (Fig. 2).
Figure 1.
Histological findings in a renal biopsy performed immediately before initiation of prednisolone treatment. Upper row: Light microscopy images of sections subjected to periodic acid-Schiff (PAS) or periodic acid-methenamine-silver (PAM) staining. Lower rows: Immunofluorescence microscopy images of sections stained for the indicated complement proteins or antibody isotypes. Original magnification ×400
Figure 2.
Detection of IgA1 and galactose-deficient IgA1. Immunofluorescence microscopy images of renal biopsy specimens stained with anti-human IgA1 or KM55. Original magnification ×400
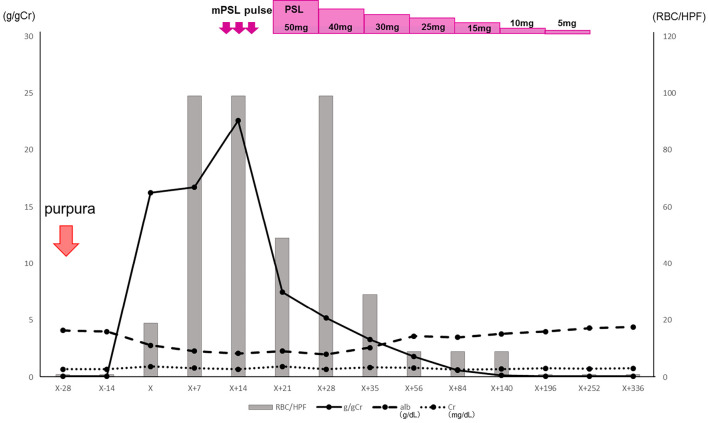
The patient was administered intravenous methylprednisolone pulse therapy (500 mg/day for 3 days), and oral prednisolone was then started at a dose of 50 mg/day (0.8 mg/kg/day). Six months later, complete remission of nephrotic syndrome was achieved. Based on the urinary findings, prednisolone was tapered to about 5-10 mg/day over 2 years and then discontinued (Fig. 3). The patient has remained in complete remission (proteinuria <0.3 g/g Cr) since then (10). As treatment for RA, when prednisolone was tapered to 7 mg/day, we resumed methotrexate at 8 mg/week. Currently, the patient's RA is being treated with only methotrexate at 12 mg/week without prednisolone or etanercept, and the disease activity of RA is stable.
Figure 3.
Patient clinical course. After the initiation of prednisolone treatment, the urinary protein concentration decreased, and complete remission of nephrotic syndrome was achieved. Alb: albumin, Cr: creatinine, m-PSL: methylprednisolone, PSL: prednisolone
Discussion
IgA vasculitis, also referred to as Henoch-Schönlein purpura, is characterized by IgA1-dominant immune deposits affecting small vessels and often involves the skin, gastrointestinal tract, joints, and kidneys (11). Mesangial proliferative glomerulonephritis typical of IgA-N is a common histopathological finding in RA (4,12) and is also observed in IgA-VN. Until recently, mesangial proliferative glomerulonephritis in RA patients was considered to result from binding of IgA, IgM, IgA-rheumatoid factor, and/or IgM-rheumatoid factor with high affinity for the mesangium (13,14). The chronic inflammatory conditions associated with RA are considered to result in virtually continuous B cell stimulation and antibody production. Furthermore, RA-related autoantibodies are thought to stimulate the proliferation of IgA-producing plasmablasts (15). These observations suggested that IgA-VN may occur as a secondary complication of RA itself. Such “rheumatoid vasculitis” is defined as a clinicopathologic manifestation of RA characterized by tissue damage or ischemia verified pathologically by vasculitis. Leukocytoclastic vasculitis is the most recognized finding in rheumatoid vasculitis, and it can occur in RA when disease activity is high or poorly controlled (16,17). However, our patient developed IgA-VN when she was being treated with etanercept and the RA disease activity was stable. Despite their usefulness for RA treatment, TNF-α inhibitors have been reported to induce autoimmune disease symptoms, such as vasculitis (including leukocytoclastic vasculitis) and SLE-like symptoms, but there have been few reports of IgA-VN induced by a TNF-α inhibitor (18). Drug-induced vasculitis, including IgA-VN, can be influenced by a number of factors, such as the duration of drug treatment and high RA disease activity (6-8). From the pattern of vasculitis onset in the patient described here, we suspected primary IgA-VN rather than secondary IgA-VN caused by RA or its treatment.
Recent work has shown that renal Gd-IgA1 staining is observed only in primary IgA-N and IgA-VN, and not in other forms of glomerular disease (9). This finding allows a clear distinction between primary IgA-VN and secondary causes of IgA deposition in the glomeruli, which is a crucial step in ensuring the most appropriate treatment. Gd-IgA1 is secreted by antibody-producing B cells and plasma cells located predominantly in the germinal centers of the tonsils and lymph nodes (19), suggesting that tonsillectomy may be a potential treatment of primary IgA-VN. In contrast, secondary IgA-VN may be better treated by controlling the underlying RA and/or discontinuing therapy with biological agents. In the case described here, KM55 staining of renal sections enabled an accurate diagnosis of primary IgA-VN, and with the correct treatment, the patient achieved remission.
Conclusion
Differentiation between primary and secondary IgA-VN in patients with RA is difficult. However, KM55-mediated staining of Gd-IgA1 renal deposits is useful as a discriminatory diagnostic tool and enables selection of an effective treatment. KM55 staining should be performed routinely when encountering mesangial proliferative glomerulonephritis in an RA patient.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Mittal T, Rathi M. Rheumatological diseases and kidneys: a nephrologist's perspective. Int J Rheum Dis 17: 834-844, 2014. [DOI] [PubMed] [Google Scholar]
- 2. Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet 376: 1094-1108, 2010. [DOI] [PubMed] [Google Scholar]
- 3. Kronbichler A, Mayer G. Renal involvement in autoimmune connective tissue diseases. BMC Med 11: 95, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Makino H, Yoshinaga Y, Yamasaki Y, Morita Y, Hashimoto H, Yamamura M. Renal involvement in rheumatoid arthritis: analysis of renal biopsy specimens from 100 patients. Mod Rheumatol 12: 148-154, 2002. [DOI] [PubMed] [Google Scholar]
- 5. Okuda Y, Yamada T, Ueda M, Ando Y. First nationwide survey of 199 patients with amyloid A amyloidosis in Japan. Intern Med 57: 3351-3355, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bateman J, Penfold R, Rigby SP. Comment on: kidney disease in RA patients: prevalence and implication on RA-related drugs management: the MATRIX study. Rheumatology (Oxford) 47: 1259 (author reply 1259-1260), 2008. [DOI] [PubMed] [Google Scholar]
- 7. Sokumbi O, Wetter DA, Makol A, Warrington KJ. Vasculitis associated with tumor necrosis factor-α inhibitors. Mayo Clin Proc 87: 739-745, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramos-Casals M, Brito-Zerón P, Muñoz S, et al. . Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore) 86: 242-251, 2007. [DOI] [PubMed] [Google Scholar]
- 9. Suzuki H, Yasutake J, Makita Y, et al. . IgA nephropathy and IgA vasculitis with nephritis have a shared feature involving galactose-deficient IgA1-oriented pathogenesis. Kidney Int 93: 700-705, 2018. [DOI] [PubMed] [Google Scholar]
- 10. Japanese Society of Nephrology [Guidelines for the treatment of nephrotic syndrome]. Nihon Jinzo Gakkai Shi 53: 78-122, 2011(in Japanese). [PubMed] [Google Scholar]
- 11. Heineke MH, Ballering AV, Jamin A, Ben Mkaddem S, Monteiro RC, Van Egmond M. New insights in the pathogenesis of immunoglobulin A vasculitis (Henoch-Schönlein purpura). Autoimmun Rev 16: 1246-1253, 2017. [DOI] [PubMed] [Google Scholar]
- 12. Helin HJ, Korpela MM, Mustonen JT, Pasternack AI. Renal biopsy findings and clinicopathologic correlations in rheumatoid arthritis. Arthritis Rheum 38: 242-247, 1995. [DOI] [PubMed] [Google Scholar]
- 13. Marinchev L, Atanasova S, Robeva R, Todorov T. Diffuse mesangial IgA glomerulonephritis in a patient with rheumatoid arthritis: a possible extra-articular manifestation in rheumatoid arthritis. BMJ Case Rep 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Korpela M, Mustonen J, Teppo AM, Helin H, Pasternack A. Mesangial glomerulonephritis as an extra-articular manifestation of rheumatoid arthritis. Br J Rheumatol 36: 1189-1195, 1997. [DOI] [PubMed] [Google Scholar]
- 15. Kinslow JD, Blum LK, Deane KD, et al. . Elevated IgA plasmablast levels in subjects at risk of developing rheumatoid arthritis. Arthritis Rheumatol 68: 2372-2383, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bartels CM, Bridges AJ. Rheumatoid vasculitis: vanishing menace or target for new treatments? Curr Rheumatol Rep 12: 414-419, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Genta MS, Genta RM, Gabay C. Systemic rheumatoid vasculitis: a review. Semin Arthritis Rheum 36: 88-98, 2006. [DOI] [PubMed] [Google Scholar]
- 18. Duffy TN, Genta M, Moll S, Martin PY, Gabay C. Henoch Schönlein purpura following etanercept treatment of rheumatoid arthritis. Clin Exp Rheumatol 24 (Suppl 41): S106, 2006. [PubMed] [Google Scholar]
- 19. Muto M, Manfroi B, Suzuki H, et al. . Toll-like receptor 9 stimulation induces aberrant expression of a proliferation-inducing ligand by tonsillar germinal center B cells in IgA nephropathy. J Am Soc Nephrol 28: 1227-1238, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]