Abstract
We herein report a 49-year-old woman with a perivascular epithelial cell tumor (PEComa) of the pancreas. Imaging studies demonstrated a relatively well-demarcated mass, measuring approximately 40 mm in diameter, located in the pancreatic tail. It was heterogeneously enhanced almost to the same degree as the surrounding pancreatic tissue in both the arterial and portal venous phases. We performed endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) using the AcquireⓇ 22-gauge needle and preoperatively obtained a definitive diagnosis with a sufficient sample. Clinicians should consider pancreatic PEComa in their differential diagnosis of patients with a pancreatic mass.
Keywords: perivascular epithelial cell tumor, pancreatic PEComa, EUS-FNA, AcquireⓇ
Introduction
Perivascular epithelioid cell tumors (PEComas) are a rare neoplasm derived from mesenchymal tissue with histological and immunohistochemical (IHC) characteristics of perivascular epithelioid cells (PECs) (1). PECs, which are distributed in the perivascular region in a radial pattern, show epithelioid features when near vessels but become spindle-shaped when distant from vessels. PECs are members of the PEComa tumor family, which includes tumors arising from the kidney and liver, such as angiomyolipoma, as well as clear-cell “sugar” tumors of the lung and extrapulmonary sites and lymphangioleiomyomatosis. On IHC staining, these neoplastic cells express melanocytic markers, including human melanoma black 45 (HMB-45) and melanoma antigen (Melan-A), as well as myogenic markers, such as α-smooth muscle actin (α-SMA) and desmin (1-6). PEComas can occur in any part of the body but tend to arise in bone, soft tissue, abdominopelvic organs, the gastrointestinal tract, and retroperitoneal organs. However, PEComas arising in the pancreas are extremely rare (7). Accordingly, the clinical and imaging manifestations of pancreatic PEComas have not been fully clarified.
The diagnosis of solid pancreatic masses using imaging modalities is sometimes difficult. To plan surgical resection of the pancreas, a histopathological examination is required preoperatively for a definitive diagnosis. It was reported previously that suspected pancreatic malignancy can be evaluated accurately using endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA), regardless of the size or location of the lesion, and the accuracy in all subgroups was >90% (8). Thus, it is possible to diagnose the majority of pancreatic lesions using EUS-FNA with considerably high accuracy. However, sufficient samples are necessary for a histopathological assessment, such as IHC staining, to obtain a correct diagnosis.
We herein report a rare case of PEComa of the pancreas diagnosed preoperatively by EUS-FNA.
Case Report
A 49-year-old woman was referred to our hospital for the further examination of a mass in the pancreatic tail that was incidentally discovered by a clinical survey using abdominal ultrasound. She had no remarkable medical history and did not consume alcohol. Her family history was not relevant to her current disorder. She did not have any symptoms, and her physical examination showed no significant abnormal findings.
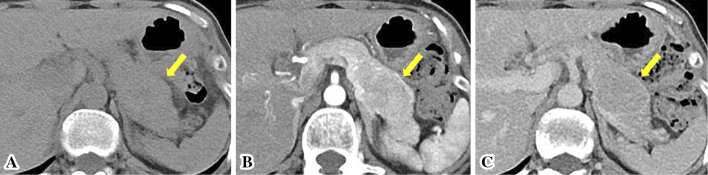
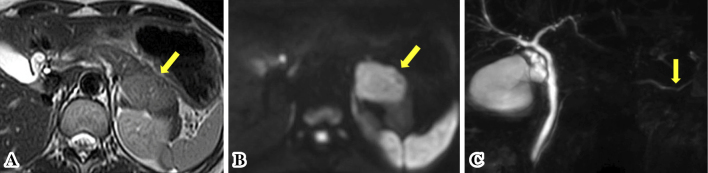
Laboratory tests, including hematological, serological, biochemical, and tumor marker analyses, revealed no significant abnormal findings. IgG4 was also within normal limit at 38.3 mg/dL (normal range, 4.5-117 mg/dL). Abdominal contrast-enhanced computed tomography (CT) demonstrated a relatively well-demarcated mass, measuring approximately 40 mm in diameter, located in the pancreatic tail (Fig. 1). It was heterogeneously enhanced almost to the same degree as the surrounding pancreatic tissue in both the arterial and portal venous phases. This mass was slightly hyperintense on T2-weighted magnetic resonance imaging (MRI) (Fig. 2A). Diffusion-weighted imaging (DWI) showed that the lesion was clearly hyperintense (Fig. 2B), and an apparent diffusion coefficient map demonstrated hypointensity on MRI. Magnetic resonance cholangiopancreatography (MRCP) revealed that the main pancreatic duct in the tail was translocated to the inferior side. Disruption and dilatation of the upstream main pancreatic duct were not found (Fig. 2C). There was no evidence of distant metastasis, direct invasion to adjacent organs, or extension of the lesion into the surrounding soft tissues. In addition, enlarged lymph nodes in the peri-pancreatic region were not detected by either CT or MRI. EUS revealed a well-defined hypoechoic mass region in the pancreatic tail, and contrast-enhanced harmonic EUS using SonazoidⓇ showed the tumor as iso-enhanced compared to the surrounding pancreatic tissue (Fig. 3A). Neuroendocrine neoplasm (NET), ductal adenocarcinoma, solid-pseudopapillary neoplasm (SPN), and pancreatic lymphoma were considered as differential diagnoses based on CT findings of heterogeneous enhancement of the pancreatic solid mass (9,10). However, as neither disruption nor dilatation of the upstream main pancreatic duct were revealed on MRCP, there was little possibility of pancreatic ductal adenocarcinoma. Tumor-forming pancreatitis was also suggested due to an isovascular pattern on contrast-enhanced harmonic EUS (11). Therefore, NET, SPN, tumor-forming pancreatitis, and other rare tumors of the pancreas were considered based on these preoperative imaging studies.
Figure 1.
Contrast-enhanced abdominal computed tomography revealed a relatively well-demarcated mass, measuring approximately 40 mm in diameter, located in the pancreatic tail (arrows). It was heterogeneously enhanced almost to the same degree as the surrounding pancreatic tissue in both the arterial and portal venous phases. (A) Plain scan. (B) Arterial phase. (C) Portal venous phase.
Figure 2.
The pancreatic mass was slightly hyperintense on T2-weighted magnetic resonance imaging (MRI) (arrows) (A). Diffusion-weighted imaging showed that the lesion was clearly hyperintense on MRI (arrows) (B). Magnetic resonance cholangiopancreatography showed that the main pancreatic duct was translocated to the inferior side (arrows). Disruption and dilatation of the upstream main pancreatic duct were not found (C).
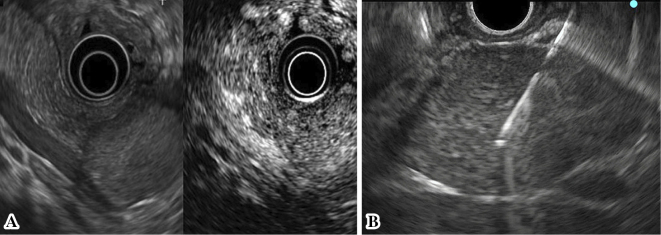
Figure 3.
EUS revealed a well-defined hypoechoic mass region in the pancreatic tail, and contrast-enhanced harmonic EUS using a Sonazoid® showed the tumor as iso-enhanced compared to the surrounding pancreatic tissue (A). The mass was relatively hard; however, the puncture was achieved with little effort, and the needle never bent during the procedures (B).
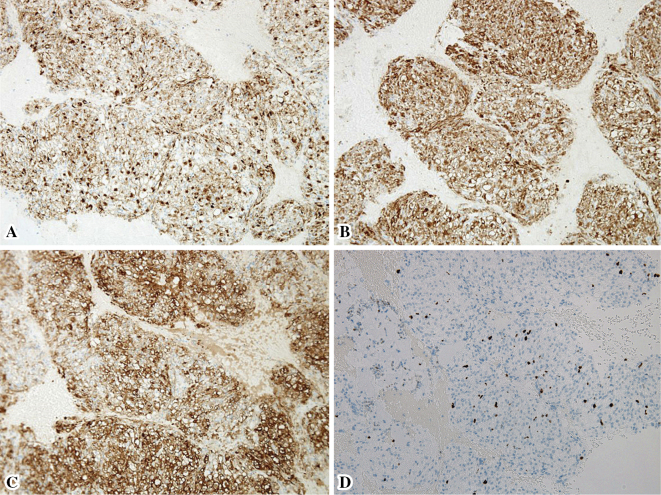
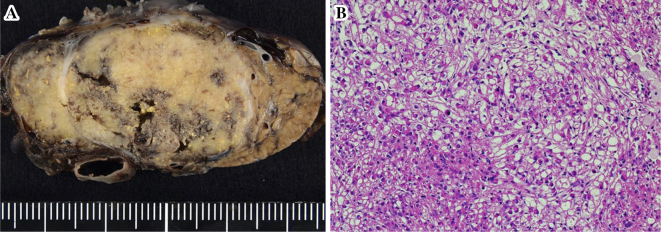
Since a malignant tumor could not be ruled out, the patient underwent EUS-FNA to establish a definitive diagnosis. We performed EUS-FNA with a total of three punctures using the AcquireⓇ device with a 22-gauge needle (Boston Scientific Corporation, Marlborough, USA), because a high rate of procuring a sample sufficient for a histological evaluation with a high diagnostic accuracy was reported with this device. The mass was relatively hard; however, the puncture was achieved with little effort, and the needle never bent during the procedures (Fig. 3B). We used the negative pressure suction technique with a 20-mL syringe and the fanning method, and the needle was moved back and forth approximately 30 times for each puncture. A rapid on-site evaluation by the Diff-Quick method revealed sufficient amount of specimen, and clusters of tumor cells were present with slightly hypertrophied nuclei. Histologically, the tumor was composed of epithelioid to spindle-shaped cells with abundant eosinophilic cytoplasm, round-to-oval nuclei, and indistinct small nucleoli proliferating in a sheet-like fashion (Fig. 4). Necrosis, calcification, atypia, and mitotic figures were not evident. The results of an IHC analysis are shown in Table 1. These tumor cells were positive for HMB-45, Melan-A, and α-SMA (Fig. 5A-C) but negative for a nervous system marker (S-100), epithelial markers (AE1/AE3 and CAM5.2), and endocrine markers (synaptophysin and chromogranin A). The Ki-67 index was <5% (Fig. 5D). The above features were consistent with a PEComa.
Figure 4.
Histological section of fragments of the pancreatic mass lesion obtained by EUS-FNA consisting of epithelioid to spindle-shaped cells with abundant eosinophilic cytoplasm, round-to-oval nuclei, and indistinct small nucleoli proliferating in a sheet-like fashion (Hematoxylin and Eosin staining ×200).
Table 1.
Results of Immunohistochemical Analysis.
| Antigen | Results |
|---|---|
| HMB-45 | + |
| Melan-A | + |
| cyclin-D1 | + |
| vimentin | + |
| α-SMA | + |
| β-Catenin | + |
| AE1/AE3 | - |
| CD10 | - |
| CD34 | - |
| CD56 | - |
| chromograninA | - |
| synaptophysin | - |
| c-kit | - |
| DOG1 | - |
| desmin | - |
| S-100 | - |
| Ki-67 | <5% |
Figure 5.
The results of immunohistochemical analyses. These tumor cells were positive for (A) human melanoma black 45 (HMB-45), (B) melanoma antigen (Melan-A), and (C) α-smooth muscle actin (α-SMA). (D) The Ki-67 labeling index was <5%. (×100)
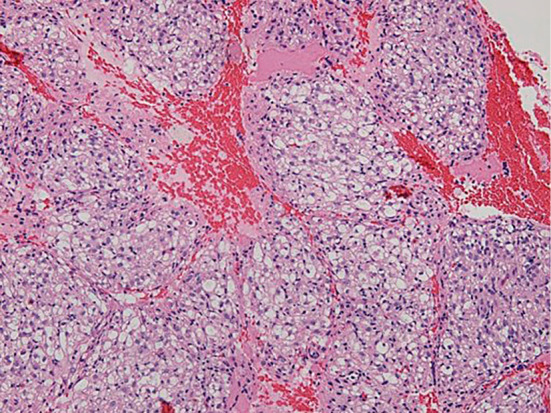
Subsequently, the patient underwent distal pancreatectomy. The surgical specimen showed a well-circumscribed, yellowish-white mass that measured 43 mm×30 mm and was surrounded by a complete fibrous capsule with a negative surgical margin (Fig. 6A). At the microscopic level, the tumor was composed of spindle cells possessing clear to focally granular eosinophilic cytoplasm without necrosis, atypia, or frequent mitoses (Fig. 6B). The IHC findings were as follows: HMB-45 (+), α-SMA (+), Melan A (+), AE1/AE3 (−), EMA (−), S-100 (−), CD34 (−), desmin (−), and TFE (−). The Ki-67 index was <2%. This histological and IHC examination showed similar features to those of specimens obtained by EUS-FNA. We therefore achieved a definitive diagnosis of PEComa of the pancreas.
Figure 6.
The surgical specimen showed a well-circumscribed, yellowish-white mass that measured 43mm×30mm and was surrounded by a complete fibrous capsule with a negative surgical margin (A). At the microscopic level, the tumor was composed of spindle-shaped cells possessing clear to focally granular eosinophilic cytoplasm without necrosis, atypia, or frequent mitoses (B) (Hematoxylin and Eosin staining ×200).
The patient was discharged on day 25 post-surgery and is currently undergoing outpatient follow-up, with no recurrence of the original tumor or distant metastasis observed at 12 months post-surgery.
Discussion
We herein report a case of PEComa of the pancreas diagnosed preoperatively by EUS-FNA. The World Health Organization defines PEComa as “a mesenchymal tumor composed of histologically and IHC distinctive PECs”. PECs are characterized by an epithelioid or spindle-shaped appearance with a clear eosinophilic or granular cytoplasm, a round-to-oval centrally located nucleus, and an inconspicuous nucleolus. On IHC, PECs express melanocytic and myogenic markers but not cytokeratins or endocrine markers (12), which concurred with the findings in our case. These tumors can arise in any part of the human body and at any age but are more predominant in women than in men. PEComa of the pancreas was first reported by Zamboni et al. in 1996 (13). We searched the MEDLINE database for papers in the English language from the year of database inception to May 2018 using the following terms: perivascular epithelial cell tumor, PEComa, pancreas, and pancreatic. After reviewing the titles and abstracts for eligibility, the literature search generated 24 cases from studies with available abstracts (2-6,13-32). The clinical characteristics of previously reported pancreatic PEComas are summarized in Table 2. The prognosis of pancreatic PEComa is generally good, with no local recurrence after surgical resection and a low rate of metastasis. However, two cases showed hepatic metastases during the observation period; one had multiple hepatic metastases at 27 months (31) and the other at 6 months after pancreatic resection (32). Folpe et al. previously described features predictive of the presence of tumor recurrence or metastasis in PEComa (12). These features included a large size (>5 cm), infiltrative growth, hypercellularity, high nuclear grade, high mitotic figures (>1/50 high-power field), and necrosis. Malignant PEComas show two or more of these worrisome features. In the present case, the tumor was approximately 40 mm in diameter, and none of the above features were identified, so this patient's prognosis is promising. As PEComa has malignant potential, surgery is considered the appropriate treatment choice, if possible. Therefore, the rapid and accurate assessment of pancreatic masses is warranted in order to direct patient management, and a correct preoperative diagnosis is crucial.
Table 2.
Previously Reported Cases of Pancreatic PEComas.
| Reference No. | Age (years) | Gender | Symptoms | FNA performed | Diagnosis by | Location | Size (mm) | Recurrenece or Metastasis |
|---|---|---|---|---|---|---|---|---|
| 13 | 60 | Female | Abdominal pain/discomfort (upper) | Yes | Surgery | Body | 20 | None |
| 15 | 74 | Female | Abdominal pain (right upper, right flank, right paraspinal), Early satiety, Dyspepsia | No | Surgery | Head | 45 | None |
| 16 | 31 | Female | Abdominal pain (right hypochondriac) | Yes | Surgery | Body | 15 | None |
| 17 | 46 | Female | Diarrhea | No | Surgery | Body | 17 | None |
| 18 | 47 | Female | Abdominal pain (lower) | No | Surgery | Head | 17 | None |
| 19 | 60 | Female | Abdominal bulge (right upper) | Yes | Surgery | Body | 32 | None |
| 20 | 49 | Male | Fever, Cough, Malaise | Yes | FNA | Head | 32 | None |
| 31 | 52 | Male | Abdominal pain | No | Surgery | Head | 40 | Liver |
| 21 | 58 | Female | Abdominal pain (upper), Dyspepsia | No | Surgery | Head | 22 | None |
| 22 | 62 | Female | None | Yes | Surgery | Head | 25 | None |
| 23 | 38 | Female | Abdominal pain/discomfort abdominal lump (upper) | No | Surgery | Tail | 140 | None |
| 24 | 38 | Female | Abdominal pain (upper) | Yes | FNA | Uncinate process | 18 | N/A |
| 3 | 43 | Female | Abdominal pain (central upper/left upper), Abdominal mass (central upper/left upper) | Yes | FNA | Body/Tail | 100 | None |
| 32 | 51 | Female | Abdominal pain (right upper)/Jaundice | Yes | FNA | Head | 60 | Liver |
| 25 | N/A | N/A | N/A | Yes | FNA | Head | N/A | N/A |
| 26 | 31 | Female | None | No | Surgery | Tail | 33 | None |
| 27 | 17 | Female | Anemia, Melena | Yes | Surgery | Head | 49 | None |
| 28 | 58 | Female | None | No | Surgery | Body | 20 | None |
| 4 | 50 | Female | None | Yes | Surgery | Head | 20 | None |
| 6 | 61 | Female | Abdominal pain (upper) | No | Surgery | Head/Body | 60 | None |
| 5 | 54 | Female | Abdominal pain (right upper) | Yes | FNA | Head/Body | 26 | None |
| 29 | 31 | Female | None | Yes | Surgery | Tail | 6/2/1.2 | N/A |
| 30 | 68 | Male | Abdominal pain (upper) | Yes | FNA | Head | 28 | None |
| 2 | 43 | Female | Abdominal pain (central upper) | No | Surgery | Head | 115 | None |
FNA: Fine-Needle Aspiration, N/A: not available
At present, the integration of CT, MRI, and ultrasound findings is the mainstay in the evaluation of pancreatic tumors. Pancreatic tumors include a heterogeneous group of primary lesions, such as adenocarcinoma, NET, SPN, pancreatoblastoma, pancreatic lymphoma, and rare miscellaneous neoplasms in general (9,10). Tan et al. reported the imaging characteristics of PEComa in 32 cases (33). Dynamic CT and MRI showed tumors that were hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging, although some were isodense with fat. The tumors usually had well-defined borders and a regular shape. Most tumors were significantly enhanced heterogeneously in the arterial and venous phases. Tumors were slightly hypodense on delayed-phase CT imaging, whereas some showed delayed enhancement. Our patient showed almost the same imaging findings as those described above. However, CT imaging provides insufficient sensitivity for the diagnosis of PEComa. Two previous reports described the respective rates of a correct preoperative PEComa diagnosis as being 15.7% and 31.3% using CT and 22.7% and 40% using MRI, with almost the same sensitivity (14,33). Therefore, imaging is not useful for differentiating pancreatic PEComa from other pancreatic mass lesions.
EUS-FNA is widely performed for the evaluation of pancreatic solid mass lesions. In the aforementioned 24 cases of pancreatic PEComa in the literature, 14 underwent EUS-FNA prior to surgery; however, 7 of these 14 cases did not obtain a clear diagnosis due to the limitations of EUS-FNA. The diagnostic accuracy of this method is affected by the availability of a rapid on-site evaluation, as a cytopathologist may not be widely available at all institutions. Furthermore, certain neoplasms, such as neuroendocrine tumors, lymphomas, and autoimmune pancreatitis, may be difficult to diagnose without IHC staining and intact histological architecture for an accurate pathological assessment. Core tissue is therefore needed in order to establish a definitive diagnosis. The AcquireⓇ needle is a device with Franseen geometry for EUS-FNA. Several studies have assessed the utility of the Franseen needle (34-36), and when using this needle we were able to successfully obtain sufficient core samples. However, there have been concerns about the potential for tumor seeding or dissemination of tumor cells via a puncture into the peritoneal cavity. Although preoperative EUS-FNA has not been shown to be associated with an increased risk of mortality concerning tumor cell dissemination along the needle track with resected pancreatic cancer (37), only a limited number of needle punctures should be performed, using a device such as a Franseen needle to facilitate the collection of a sufficient amount of specimen.
In summary, we encountered a case of PEComa of the pancreas diagnosed preoperatively with EUS-FNA. As obtaining a definitive diagnosis of pancreatic PEComa was difficult using only imaging, preoperative EUS-FNA was useful in this case. Clinicians should consider pancreatic PEComa in their differential diagnosis of patients with a pancreatic mass. The clinical manifestations of pancreatic PEComas have not been fully clarified, so long-term regular follow-up of this patient is essential.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Hornick JL, Fletcher CD. PEComa: what do we know so far? Histopathology 48: 75-82, 2006. [DOI] [PubMed] [Google Scholar]
- 2. Zhang S, Chen F, Huang X, et al. . Perivascular epithelial cell tumor (PEComa) of the pancreas: a case report and review of literature. Medicine (Baltimore) 96: e7050, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Okuwaki K, Kida M, Masutani H, et al. . A resected perivascular epithelioid cell tumor (PEComa) of the pancreas diagnosed using endoscopic ultrasound-guided fine-needle aspiration. Intern Med 52: 2061-2066, 2013. [DOI] [PubMed] [Google Scholar]
- 4. Jiang H, Ta N, Huang XY, et al. . Pancreatic perivascular epithelioid cell tumor: a case report with clinicopathological features and a literature review. World J Gastroenterol 22: 3693-3700, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Collins K, Buckley T, Anderson K, Karasik M, Ligato S. Perivascular epithelioid cell tumor (PEComa) of pancreas diagnosed preoperatively by endoscopic ultrasound-guided fine-needle aspiration: a case report and review of literature. Diagn Cytopathol 45: 59-65, 2017. [DOI] [PubMed] [Google Scholar]
- 6. Mizuuchi Y, Nishihara K, Hayashi A, et al. . Perivascular epithelial cell tumor (PEComa) of the pancreas: a case report and review of previous literatures. Surg Case Rep 2: 59, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martignoni G, Pea M, Reghellin D, Zamboni G, Bonetti F. PEComas: the past, the present and the future. Virchows Arch 452: 119-132, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Uehara H, Ikezawa K, Kawada N, et al. . Diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration for suspected pancreatic malignancy in relation to the size of lesions. J Gastroenterol Hepatol 26: 1256-1261, 2011. [DOI] [PubMed] [Google Scholar]
- 9. Scialpi M, Reginelli A, D'Andrea A, et al. . Pancreatic tumors imaging: an update. Int J Surg 28 (Suppl 1): S142-S155, 2016. [DOI] [PubMed] [Google Scholar]
- 10. Low G, Panu A, Millo N, Leen E. Multimodality imaging of neoplastic and nonneoplastic solid lesions of the pancreas. Radiographics 31: 993-1015, 2011. [DOI] [PubMed] [Google Scholar]
- 11. Sofuni A, Iijima H, Moriyasu F, et al. . Differential diagnosis of pancreatic tumors using ultrasound contrast imaging. J Gastroenterol 40: 518-525, 2005. [DOI] [PubMed] [Google Scholar]
- 12. Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, Weiss SW. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol 29: 1558-1575, 2005. [DOI] [PubMed] [Google Scholar]
- 13. Zamboni G, Pea M, Martignoni G, et al. . Clear cell “sugar” tumor of the pancreas. A novel member of the family of lesions characterized by the presence of perivascular epithelioid cells. Am J Surg Pathol 20: 722-730, 1996. [DOI] [PubMed] [Google Scholar]
- 14. Zizzo M, Ugoletti L, Tumiati D, et al. . Primary pancreatic perivascular epithelioid cell tumor (PEComa): a surgical enigma. A systematic review of the literature. Pancreatology 18: 238-245, 2018. [DOI] [PubMed] [Google Scholar]
- 15. Heywood G, Smyrk TC, Donohue JH. Primary angiomyolipoma of the pancreas. Pancreas 28: 443-445, 2004. [DOI] [PubMed] [Google Scholar]
- 16. Ramuz O, Lelong B, Giovannini M, et al. . “Sugar” tumor of the pancreas: a rare entity that is diagnosable on preoperative fineneedle biopsies. Virchows Arch 446: 555-559, 2005. [DOI] [PubMed] [Google Scholar]
- 17. Perigny M, Larochelle O, Hammel P, et al. . Pancreatic perivascular epithelioid cell tumor (PEComa). Ann Pathol 28: 138-142, 2008. [DOI] [PubMed] [Google Scholar]
- 18. Hirabayashi K, Nakamura N, Kajiwara H, et al. . Perivascular epithelioid cell tumor (PEComa) of the pancreas: immunoelectron microscopy and review of the literature. Pathol Int 59: 650-655, 2009. [DOI] [PubMed] [Google Scholar]
- 19. Baez JC, Landry JM, Saltzman JR, et al. . Pancreatic PEComa (sugar tumor): MDCT and EUS features. JOP 10: 679-682, 2009. [PubMed] [Google Scholar]
- 20. Zemet R, Mazeh H, Neuman T, et al. . Asymptomatic pancreatic perivascular epithelial cell tumor (PEComa) in a male patient: report and literature review. JOP 12: 55-58, 2011. [PubMed] [Google Scholar]
- 21. Xie FL, Song YN, Qu LJ, et al. . Clinicopathologic features of perivascular epithelioid cell tumor of the pancreas. Shijie Huaren Xiaohua Zazhi 19: 964-968, 2011. [Google Scholar]
- 22. Finzi G, Micello D, Wizemann G, et al. . Pancreatic PEComa: a case report with ultrastructural localization of HMB-45 within melanosomes. Ultrastruct Pathol 36: 124-129, 2012. [DOI] [PubMed] [Google Scholar]
- 23. Singh M, Saroha V, Wadhwa R, et al. . Solitary lymphangioleiomyoma of pancreas mimicking pancreatic pseudocyst-a case report. J Gastrointest Canc 43: 336-339, 2012. [DOI] [PubMed] [Google Scholar]
- 24. Al-Haddad M, Cramer HM, Muram T, et al. . Perivascular epithelioid cell tumor: an unusual pancreatic mass diagnosed by EUS-FNA. Gastrointest Endosc 78: 165-167, 2013. [DOI] [PubMed] [Google Scholar]
- 25. Tummala P, Rao S, Agarwal B. Differential diagnosis of focal non-cystic pancreatic lesions with and without proximal dilation of pancreatic duct noted on CT scan. Clin Transl Gastroenterol 4: e42, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim JY, Song JS, Park H, et al. . Primary mesenchymal tumors of the pancreas: single-center experience over 16 years. Pancreas 43: 959-968, 2014. [DOI] [PubMed] [Google Scholar]
- 27. Petrides C, Neofytou K, Khan AZ. Pancreatic perivascular epithelioid cell tumour presenting with upper gastrointestinal bleeding. Case Rep Oncol Med 431215, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wei J, Liu X, Fu Y, Zhang Z, et al. . Primary perivascular epithelioid cell tumor of the pancreas: a case report. Pancreatology S30, 2016. [Google Scholar]
- 29. Hartley CP, Kwiatkowski DJ, Hamieh L, et al. . Pancreatic PEComa is a novel member of the family of tuberous sclerosis complex-associated tumors: case report and review of the literature. Virchows Arch 469: 707-710, 2016. [DOI] [PubMed] [Google Scholar]
- 30. Zizzo M, Castro Ruiz C, Ugoletti L, et al. . Primary pancreatic PEComa: is surgery always the best choice? JOP 18: 92-94, 2017. [Google Scholar]
- 31. Nagata S, Yuki M, Tomoeda M, et al. . Perivascular epithelioid cell neoplasm (PEComa) originating from the pancreas and metastasizing to the liver. Pancreas 40: 1155-1157, 2011. [DOI] [PubMed] [Google Scholar]
- 32. Mourra N, Lazure T, Colas C, Arrive L, de Gramont A. Perivascular epithelioid cell tumor: the first malignant case report in the pancreas. Appl Immunohistochem Mol Morphol 21: e1-e4, 2013. [DOI] [PubMed] [Google Scholar]
- 33. Tan Y, Zhang H, Xiao EH. Perivascular epithelioid cell tumour: dynamic CT, MRI and clinicopathological characteristics-analysis of 32 cases and review of the literature. Clin Radiol 68: 555-561, 2013. [DOI] [PubMed] [Google Scholar]
- 34. Bang JY, Hebert-Magee S, Navaneethan U, Hasan MK, Hawes R, Varadarajulu S. Randomized trial comparing the Franseen and Fork-tip needles for EUS-guided fine-needle biopsy sampling of solid pancreatic mass lesions. Gastrointest Endosc 87: 1432-1438, 2018. [DOI] [PubMed] [Google Scholar]
- 35. Mitri RD, Rimbas M, Attili F, et al. . Performance of a new needle for endoscopic ultrasound-guided fine-needle biopsy in patients with pancreatic solid lesions: a retrospective multicenter study. Endosc Ultrasound 7: 329-334, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bang JY, Hebert-Magee S, Hasan MK, Navaneethan U, Hawes R, Varadarajulu S. Endoscopic ultrasonography-guided biopsy using a Franseen needle design: Initial assessment. Dig Endosc 29: 338-346, 2017. [DOI] [PubMed] [Google Scholar]
- 37. Ngamruengphong S, Swanson KM, Shah ND, Wallace MB. Preoperative endoscopic ultrasound-guided fine needle aspiration does not impair survival of patients with resected pancreatic cancer. Gut 64: 1105-1110, 2015. [DOI] [PubMed] [Google Scholar]