Abstract
Objective
Kikyo-to (KKT) is a fixed combination of glycyrrhiza root and platycodon root extracts. It is an herbal medicine traditionally used in Japan for relieving sore throat associated with acute upper respiratory tract infection (URTI). No controlled studies have yet demonstrated its effect, however. We investigated the efficacy of KKT on sore throat associated with acute URTI.
Methods
Patients with sore throat who were diagnosed with URTI at the General Medicine Department Office, Akashi Medical Center Hospital, between December 2017 and May 2018 were enrolled. Participants were randomly assigned to two groups at a 1:1 ratio, with stratification by age and sore throat score on a Visual Analogue Scale (VAS), to receive 2.5 g of either KKT or a placebo. Participants and investigators were blinded to group allocation. The primary outcome was the change in sore throat score on VAS 10 minutes after KKT administration. Secondary outcomes were the impact of the sore throat on daily life (none, mild, moderate, and severe) at 10 minutes after administration.
Results
Thirty-five participants were assigned to each group (n=70, total). The difference in the mean change of sore throat score according to VAS within 10 minutes between the two groups was not statistically significant (KKT 14.40 vs. placebo 17.00; p=0.39). The proportion of patients with a moderate or greater impact of their sore throat on their daily life was also not significantly different between the groups (KKT 22.9% vs. placebo 40.0%; p=0.20). Patients reported no side effects.
Conclusion
KKT did not significantly relieve sore throat associated with acute URTI compared with placebo.
Keywords: kikyo-to, upper respiratory tract infection, sore throat
Introduction
A sore throat is one of the most commonly reported conditions (1). Upper respiratory tract infections (URTIs) characterized by sore throat are the most frequent acute illnesses (2), and most cases of sore throat are caused by viral infections (3). Despite symptomatic inefficacy and the implementation of Choosing Wisely campaigns promoting the reconsideration of antibiotic use due to the risk of antimicrobial resistance, antibiotics are still prescribed for patients with a sore throat (4). The financial burden of upper respiratory infection is high, costing an estimated $1.2 billion annually according to one report based in the United States (5). Most URTIs do not require treatment, so the major treatment goal for patients with a sore throat is to relieve pain and substantially reduce the inappropriate prescription of antibiotics.
Kikyo-to (KKT), a Japanese herbal medicine composed of glycyrrhiza root and platycodon root extracts, is used empirically to relieve sore throat associated with acute URTI and is often prescribed in primary care settings. The components are also used in China and Korea. KKT is inexpensive ($0.13) compared to the costs of other medications used for sore throat, such as lidocaine ($2/pill).
One extract of platycodon root, saponin, reduced the level of tumor necrosis factor (TNF)-alpha and prostaglandin E2 (PGE2) in a rat model (6). A case series reported the effect of KKT on aphthous stomatitis (7) and burning mouth syndrome (8) and the effect of sho-saiko-to-ka-kikyo-sekko, another herbal medicine including platycodon root, on chronic tonsillitis (9). Few studies, however, have described the effects of KKT on sore throat associated with acute URTI. One reason for the limited research is the unique method of administration of this medicine. In the case series mentioned above, KKT was gargled without swallowing the medication. Nevertheless, oral administration is recommended in the package inserts and is the commonly accepted dose method. In our previous study of URTI patients, we showed that treatment with KKT led to a significantly decreased sore throat severity at 10 minutes after the administration, and the effect continued beyond 30 minutes (10). The study lacked a control group, however, so the results may have been partially affected by a placebo effect from hot water.
To overcome these limitations, we conducted a double-blind, placebo-controlled randomized study to investigate the efficacy of oral administration of KKT for sore throat associated with acute URTI.
Materials and Methods
Design and participants
This was a stratified randomized, double-blind, placebo-controlled trial. URTI patients ≥20 and ≤65 years of age with a sore throat who visited the General Medicine Department at Akashi Medical Center between December 2017 and May 2018 were included in the study. This hospital is a local medical support center in Hyogo, Japan, with a primary care practice. In this study, URTI was defined as acute upper respiratory tract infection caused by viruses or bacteria, including influenza and acute streptococcal tonsillitis. Previous studies on sore throat also did not exclude streptococcal tonsillitis (11,12). All patients clinically diagnosed with URTI by office physicians were eligible, except those who met any of the following exclusion criteria: known allergy to KKT; lactose intolerance; current use of KKT; presence of aldosteronism, myopathy, or hypokalemia (contraindications for the use of KKT); pregnant or breast-feeding; admitted to the emergency ward; unable to participate in the study because of dementia or visual impairment; unable to answer or complete the study form.
Randomization and masking
An independent, automated web-based service at the Wakayama Medical University Clinical Study Support Centre randomly assigned participants to receive either KKT or a placebo on a 1:1 basis according to a computer-generated sequence, with stratified assignment according to age (<45 years, ≥45 years) and sore throat score using Visual Analogue Scale (VAS) (score <50, score of ≥50). As participants were enrolled and randomized, we assigned them allocated numbers with packs of blinded prerandomized medication. Patients in the intervention group received 1 dose of 2.5 g herbal extract preparation of KKT dissolved in a cup of hot water. KKT (2.5 g) contained 0.25 g extract from dried root of glycyrrhiza and 0.17 g extract from dried root of platycodon (Chinese bellflower). KKT was dissolved in a matrix containing magnesium stearate and lactose. The control group received 1 dose of 2.5 g placebo (lactose) also dissolved in a cup of hot water. Participants and investigators were blinded to group allocation for the duration of the study.
Procedures
Participants were recruited on the day of presentation of sore throat. Before the administration of KKT, data on the demographic characteristics of the patients, sore throat duration, sore throat score using the VAS, and impact of sore throat on daily activities were obtained using a patient-answered questionnaire. Body temperature was taken by nursing staff. Participants were randomized to the KKT group or placebo group, and the trial medication was prepared by the research assistant. Patients were then advised by the assistant to take the hot water containing the trial medication. The treatment group assignment was concealed to both participants and investigators. Primary outcome data were collected 10 minutes after administration.
Outcome measures
The primary outcome was the change in sore throat score by VAS at 10 minutes after administration. The secondary outcome was the impact of sore throat on daily life at 10 minutes after administration. Any adverse events were to be reported by patients. The sore throat score using the VAS was used to assess sore throat severity (scale from 0-100, wherein the value “0” indicates no pain and “100” indicates the highest severity of sore throat) before the administration of KKT and 10 minutes after its administration. We also assessed the impact of sore throat on daily activities and classified this into four grades based on patient interviews by office physicians: “none,” “mild,” “moderate,” and “severe.” This was based on a method used by Jackson et al. (1958) and taken before administration of KKT and again 10 minutes after administration (13). “Mild” impact was defined as unawareness of sore throat when the patient was busy with something, “moderate,” was defined as a continuous sensation of sore throat, and “severe” was defined as difficulties in daily life.
Sample size and statistical analyses
Based on clinical data from a previous before-after study of KKT, the between-group difference in the change in the mean VAS score from baseline was estimated to be 13.2 at 10 minutes after administration between the KKT and placebo groups (10). We assumed no change in the VAS score in the placebo group. Therefore, this study required a total of 62 patients to have a power of at least 80% at a significance level of 0.05 (two-sided). To allow for 10% dropout, we aimed to recruit at least 35 participants for each group. All efficacy analyses were conducted using the full analysis set (FAS), which comprised all patients in the treated set who received at least one dose of randomized treatment. Safety analyses were conducted using all treatment cases, which were defined as cases that received some or all of the protocol treatment.
The goal of the main analysis of this study was to determine whether or not the amount of change in the VAS for sore throat (major change in the VAS) was significantly reduced in the KKT group compared to the placebo group. If a significant difference was observed between the KKT group and the placebo group and the mean amount of change in the VAS for sore throat was greater in the KKT group, sore throat would be prove to be ameliorated by this therapy, indicating KKT to be a useful method of treatment. If the difference was not significant, KKT would be deemed to have no marked effect on sore throat. Differences between the treatment groups with regard to the primary outcome ‘the change in the sore throat score on VAS at 10 minutes after administration’ were tested using an analysis of covariance (ANCOVA) after adjusting for stratified factors with a 2-sided 5% significance level. The multiple regression model was constructed using dummy variables of stratification factors and treatment arms. A point estimate and a 95% confidence interval of the adjusted therapeutic effect were calculated.
Regarding the secondary outcome on effectiveness, the impact of sore throat on daily activities, the number of cases, and the proportion of each category of sore throat grade (none, mild, moderate, severe) at 10 minutes after administration were calculated based on the FAS. These four sore throat grades were divided into two groups: the none/mild group and the moderate/severe group. With the number of cases of FAS as a denominator, differences in the number of cases and proportion of moderate/severe cases within each treatment group were analyzed at 10 minutes after administration. Confidence intervals (CIs; 95%) of the proportion of patients in each treatment group were calculated using the Clopper-Pearson method. Differences between the treatment groups of assessed changes in the proportion of moderate/severe cases were analyzed using Fisher's exact, test and the odds ratio and its associated 95% CI in the KKT group compared with the placebo group were calculated. P value <0.05 was considered statistically significant for all analyses. All statistical analyses were performed using the JMP Pro 11.2.1 software program (SAS Institute, Cary, USA).
Ethical approval and registration
The study design was registered as a University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) Clinical Trial (UMIN trial ID: UMIN000030239) on 17 January 2018 (UMIN-CTR URL: http://www.umin.ac.jp/ctr/index.htm). This study was approved by the Akashi Medical Center Research Ethics Committee.
Results
Between December 2017 and May 2018, 70 patients were enrolled and entered the study, with 35 randomized to receive placebo and 35 to receive KKT. Fig. 1 shows the flow of participants. The FAS included 70 patients (placebo: n=35; 10 men and 25 women and KKT: n=35; 12 men and 23 women) who were treated and provided data for the primary outcome. Table 1 shows that the two groups were well balanced with regard to baseline demographics, clinical characteristics including sore throat VAS scores, impact of sore throat on daily activities, and the initial diagnosis. The characteristics of the two groups at baseline were found to be similar.
Figure 1.
Flow diagram for the KURI trial. Enrolled and entered into the study were 70 patients, with 35 randomized to receive a placebo and 35 to receive a KKT. The FAS included all 70 patients who were treated and provided data for the primary outcome. KKT: kikyo-to, FAS: full analysis set
Table 1.
Characteristics of the Study Subjects.
| KKT (n=35) | Placebo (n=35) | p value*1 | ||
|---|---|---|---|---|
| Sex | Male | 12 ( 34.3) | 10 ( 28.6) | 0.797 |
| Female | 23 ( 65.7) | 25 ( 71.4) | ||
| Age | Mean (SD) | 37.91 (12.13) | 37.89 (12.99) | 0.992 |
| Median [Range] | 35.00 [20.00, 63.00] | 35.00 [22.00, 65.00] | 0.860 | |
| Body temperature | Mean (SD) | 36.89 (0.77) | 37.05 (0.78) | 0.409 |
| Median [Range] | 36.80 [35.20, 39.30] | 36.90 [35.80, 39.30] | 0.514 | |
| Sore throat score on VAS at baseline | Mean (SD) | 46.63 (22.23) | 42.94 (22.20) | 0.490 |
| Median [Range] | 41.00 [9.00, 96.00] | 39.00 [10.00, 90.00] | 0.507 | |
| Impact of sore throat on daily activities | Mild | 10 ( 28.6) | 16 ( 45.7) | 0.400 |
| Moderate | 23 ( 65.7) | 18 ( 51.4) | ||
| Severe | 2 (5.7) | 1 (2.9) | ||
| Final diagnosis | Influenza | 4 (11.4) | 10 (28.6) | 0.192 |
| Pharyngitis | 27 (77.1) | 23 (65.7) | ||
| Tonsillitis | 3 (8.6) | 1 (2.9) | ||
| Sinusitis | 0 (0.0) | 1 (2.9) | ||
| Streptococcal tonsillitis | 1 (2.9) | 0 (0.0) | ||
| Stratified by age | <45 | 24 (68.6) | 25 (71.4) | 1.000 |
| ≥45 | 11 (31.4) | 10 (28.6) | ||
| Stratified by VAS | <50 | 21 (60.0) | 22 (62.9) | 1.000 |
| ≥50 | 14 (40.0) | 13 (37.1) | ||
*1: Categorical variables: Fisher’s exact test, mean: 2 sample t-test, median: Wilcoxon test
KKT: kikyo-to, VAS: Visual Analogue Scale
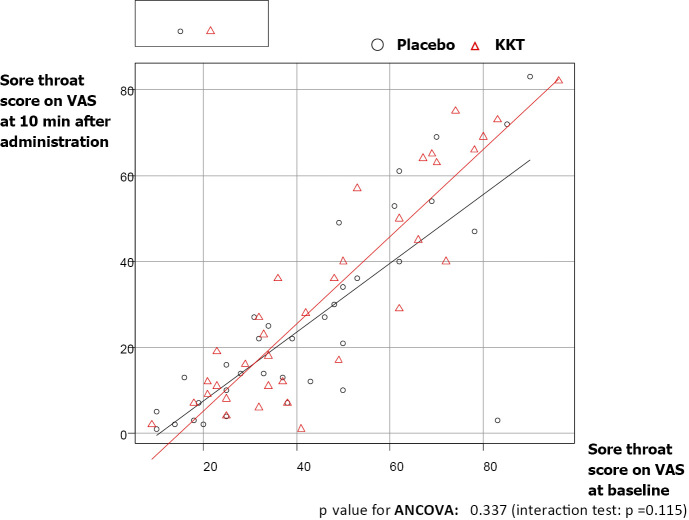
Table 2 shows the results of the ANCOVA for the outcome variables of the change in the sore throat score on VAS between baseline and 10 minutes after administration. Treatment with KKT decreased the mean sore throat score on VAS from 46.6 [standard deviation (SD): 22.2] to 32.2 (25.0) at 10 minutes after administration, whereas placebo decreased the mean sore throat score on VAS from 42.9 (22.2) to 25.9 (22.5) at 10 minutes after administration. The mean change in the sore throat score on VAS between the KKT and placebo groups was not statistically significant [14.40 (SD: 10.6), 95% CI (-4.00, 40.00) vs. 17.00 (SD: 14.5), 95% CI (0.00, 80.00); p=0.39]. Figure 2 shows the results of the ANCOVA for the sore throat score on VAS at 10 minutes after administration adjusted by the VAS sore throat score at baseline. A multiple regression analysis of the change in the sore throat score on VAS showed no significant differences in the therapeutic effect after adjusting for stratification factors and treatment arms (Table 3).
Table 2.
Change in Sore Throat Score on VAS between Baseline and at 10 Minutes after Administration.
| KKT (n=35) | Placebo (n=35) | p value | ||
|---|---|---|---|---|
| VAS at baseline | Mean (SD) | 46.63 (22.23) | 42.94 (22.20) | 0.490 |
| Median [Range] | 41.00 [9.00, 96.00] | 39.00 [10.00, 90.00] | 0.507 | |
| VAS at 10 min after administration | Mean (SD) | 32.23 (24.99) | 25.94 (22.54) | 0.273 |
| Median [Range] | 27.00 [1.00, 82.00] | 21.00 [1.00, 83.00] | 0.288 | |
| Change of VAS | Mean (SD) | 14.40 (10.55) | 17.00 (14.50) | 0.394 |
| Median [Range] | 12.00 [-4.00, 40.00] | 15.00 [0.00, 80.00] | 0.488 | |
KKT: kikyo-to, VAS: Visual Analogue Scale
Figure 2.
Results of the ANCOVA for the sore throat score on the VAS at 10 minutes after administration adjusted by the sore throat score on the VAS at baseline. p value for ANCOVA: 0.337 (interaction test: p=0.115). ANCOVA: analysis of covariance, VAS: Visual Analogue Scale
Table 3.
Multiple Regression Analysis of the Change in Sore Throat Score on VAS.
| Regression coefficient [95% CI] | p value | |
|---|---|---|
| Groups (KKT/Placebo) | -2.736 [-8.821, 3.349] | 0.373 |
| Age (<45/≥45) | 3.652 [-2.996, 10.299] | 0.277 |
| VAS at baseline(<50/≥50) | 1.096 [-5.162, 7.354] | 0.728 |
KKT: kikyo-to, VAS: Visual Analogue Scale
The proportion of patients suffering from a moderate or greater impact of their sore throat on their daily life at 10 minutes after administration was 22.9% in the KKT group compared with 40.0% in the placebo group [odds ratio (95% CI) =0.45 (0.14-1.40), p=0.20; Table 4]. The proportion of patients showing improvement of the impact of their sore throat on their daily life at 10 minutes after administration did not differ significantly between groups [45.7% in the KKT group and 37.1% in the placebo group; odds ratio (95% CI) =1.42 (0.43-4.13), p=0.63; Table 5]. No patients in either group reported adverse effects.
Table 4.
Number of Cases and Proportion of “Moderate” and “Severe” Impact of Sore Throat on Daily Life after 10 Minutes for Each Treatment Group.
| N | Moderate·Severe | Proportion [95%C.I.] | |
|---|---|---|---|
| KKT | 35 | 8 | 22.9% [10.4-40.1%] |
| Placebo | 35 | 14 | 40.0% [23.9-57.9%] |
Odds ratio [95%C.I.]=0.450 [0.136 - 1.404], p value=0.197
KKT: kikyo-to
Table 5.
Proportion of Patients Showing Improvement of Impact of Sore Throat on Daily Life after 10 Minutes for Each Treatment Group.
| N | Cases with improvement | Proportion [95%C.I.] | |
|---|---|---|---|
| KKT | 35 | 16 | 45.7% [28.8-63.4%] |
| Placebo | 35 | 13 | 37.1% [21.5-55.1%] |
Odds ratio [95%C.I.]=1.418 [0.433 - 4.132], p value=0.626
KKT: kikyo-to
Discussion
Summary of main findings
To our knowledge, this is the first randomized, double-blind, placebo-controlled trial to determine the efficacy of KKT on sore throat associated with acute URTI. We were unable to detect any rapid analgesic effect of KKT surpassing placebo in patients with acute URTI in the present trial. The mean change in the sore throat score on VAS and the proportion of patients suffering from a moderate or greater impact of sore throat on their daily life at 10 minutes after administration were not significantly different between the two groups.
One explanation for the unexpected placebo effect is the heterogeneity of the analgesic effect in the placebo group. As seen in Fig. 2, a single patient in the placebo group was plotted far from the diagonal line made using the sore throat score on VAS at 10 minutes after administration after adjusting for the score at baseline. However, the discrepancy due to this outlier did not disproportionately affect the impact of sore throat on the daily activities which were classified into four categories. Another factor that may explain the placebo effect is the means of treatment administration, as both KKT and placebo were offered diluted in a cup of hot water. Sanu et al. (2008) reported on the effect of hot water for sore throat relief (14), which may explain the augmentation of the placebo effect in the present study.
A comparison with existing literature
Our previous study was the only trial to investigate the clinical efficacy of oral administration of KKT for sore throat associated with acute URTI. The sore throat scores on VAS at baseline in the present study were similar to those of the participants in the previous study. Regarding the primary outcome, the change in the mean VAS score between baseline and 10 minutes after administration was 13.2 in the previous study, which was also similar to the change in the two treatment groups in the present trial. Furthermore, in the previous study, the proportion of patients suffering from a moderate or greater impact of sore throat on their daily life was 67.5%, which was reduced significantly to 37.5% at 10 minutes after administration. In the present trial, we confirmed a similar change in both treatment groups. In contrast to the previous study, however, we only conducted observations up to 10 minutes after administration. As we were focusing on the feasibility of the trial, we set the minimum follow-up period for achieving outcomes based on the presence of statistically significant differences seen in the previous trial. A further study with a longer observational period may help determine the actual therapeutic effects of KKT separate from the placebo effect.
In the present study, patients received 2.5 g of KKT based on the findings from our previous study of URTI patients, which demonstrated that 2.5 g of KKT was sufficient to significantly reduce sore throat (10). Kuwamura et al. (2016) used 5.0 g of KKT mixed with jelly perioperatively and demonstrated relief of postoperative sore throat (15). Those findings suggest the possible augmentation of the effect of KKT in association with the increment in its dosage.
With regard to the safety, treatment with KKT was found to be well tolerated in human subjects. No side effects were observed in the present study, similar to observations in our previous study.
Strengths and limitations
This was the first randomized, double-blind placebo-controlled trial to investigate the efficacy of KKT for treatment of sore throat. The results from this trial were robust, and the outcome results were similar to those in our previous study. Notably, the outcomes in the KKT group did not differ markedly from those of the placebo group. Several studies of symptomatic relief for sore throat in URTI have demonstrated the analgesic effect of lozenges (16-19), but those studies lacked clear documentation concerning allocation concealment. In the current trial, the treatment group assignment was concealed to both participants and investigators. Whereas most prior studies did not disclose the composition of the study placebo (20), we documented the placebo composition and precise usage of the study drugs.
However, several limitations associated with the present study warrant mention. First, participants were recruited from a single institution, which may lead to reduced generalization of data. Furthermore, although this study was conducted in a single institution, the location differed from that in our previous study. However, both study sites are community hospitals in primary-care settings, so our results may be applicable to such primary-care settings. Second, the palliative effect of KKT should be assessed for longer than 10 minutes. Third, our results are not applicable to children or elderly patients, as our study did not include these age groups.
To overcome these limitations and determine the analgesic effect of KKT for sore throat associated with acute URTI, we propose the performance of double-blind, randomized studies with longer observation periods in multiple facilities.
Implications for future research or clinical practice
Although KKT did not outperform the placebo with regard to relieving sore throat associated with acute URTI, KKT may have an analgesic effect similar to a placebo without any adverse effects. Ameliorating sore throat may play a key role in reducing the number of inappropriate prescriptions of antibiotics for what are actually self-curing URTIs.
Conclusion
The oral administration of KKT does not significantly relieve sore throat associated with acute URTI compared with placebo. Further research is needed to determine the analgesic effects of KKT for sore throat with a longer follow-up.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank Benjamin Phillis of Akashi Medical Center for proofreading and editing the manuscript.
References
- 1. Hannaford PC, Simpson JA, Bisset AF, Davis A, McKerrow W, Mills R. The prevalence of ear, nose and throat problems in the community: results from a national cross-sectional postal survey in Scotland. Fam Pract 22: 227-233, 2005. [DOI] [PubMed] [Google Scholar]
- 2. Goto M, Kawamura T, Shimbo T, et al. . Influence of loxoprofen use on recovery from naturally acquired upper respiratory tract infections: a randomized controlled trial. Intern Med 46: 1179-1186, 2007. [DOI] [PubMed] [Google Scholar]
- 3. Wong DM, Blumberg DA, Lowe LG. Guidelines for the use of antibiotics in acute upper respiratory tract infections. Am Fam Physician 74: 956-966, 2006. [PubMed] [Google Scholar]
- 4. Singer A. Choosing wisely Canada. Can Fam Physician 63: 372, 2017. [PMC free article] [PubMed] [Google Scholar]
- 5. Salkind AR, Wright JM. Economic burden of adult pharyngitis: the payer's perspective. Value Health 11: 621-627, 2008. [DOI] [PubMed] [Google Scholar]
- 6. Kim JY, Hwang YP, Kim DH, et al. . Inhibitory effect of the saponins derived from roots of Platycodon grandiflorum on carrageenan-induced inflammation. Biosci Biotechnol Biochem 70: 858-864, 2006. [DOI] [PubMed] [Google Scholar]
- 7. Yamaguchi K, Mukai H, Kawashima K, Kuniyoshi H, et al. . Four cases of aphthous stomatitis treated with Kampo Medicines. Itami to Kampo (Pain and Kampo Medicine) 18: 114-119, 2008(in Japanese, Abstract in English). [Google Scholar]
- 8. Yamaguchi K, Mukai H, Kawashima K, Kuniyoshi H, Sugihara K. Effect of Kampo Medicines on the burning mouth Syndrome. Itami to Kampo (Pain and Kampo Medicine) 17: 43-47, 2007(in Japanese, Abstract in English). [Google Scholar]
- 9. Goto F, Asama Y, Ogawa K. Sho-saiko-to-ka-kikyo-sekko as an alternative treatment for chronic tonsillitis to avoid surgery. Complement Ther Clin Pract 16: 216-218, 2010. [DOI] [PubMed] [Google Scholar]
- 10. Ishimaru N, Maeno T, Suzuki M. Rapid effects of Kikyo-to on sore throat pain associated with acute upper respiratory tract infection. J Complement Integr Med 11: 51-54, 2013. [DOI] [PubMed] [Google Scholar]
- 11. Hayward GN, Hay AD, Moore MV, et al. . Effect of oral dexamethasone without immediate antibiotics vs placebo on acute sore throat in adults: a randomized clinical trial. JAMA 317: 1535-1543, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Korb K, Scherer M, Chenot JF. Steroids as adjuvant therapy for acute pharyngitis in ambulatory patients: a systematic review. Ann Fam Med 8: 58-63, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jackson GG, Dowling HF, Spiesman IG, Boand AV. Transmission of the common cold to volunteers under controlled conditions. I. The common cold as a clinical entity. AMA Arch Intern Med 101: 267-278, 1958. [DOI] [PubMed] [Google Scholar]
- 14. Sanu A, Eccles R. The effects of a hot drink on nasal airflow and symptoms of common cold and flu. Rhinology 46: 271-275, 2008. [PubMed] [Google Scholar]
- 15. Kuwamura A, Komasawa N, Takahashi R, Tanaka M, Minami T. Preoperative oral administration of Kikyo-to, a Kampo medicine, alleviates postoperative sore throat: a prospective, double-blind, randomized study. The Journal of Alternative and Complementary Medicine 22: 294-297, 2016. [DOI] [PubMed] [Google Scholar]
- 16. Chenot J-F, Weber P, Friede T. Efficacy of Ambroxol lozenges for pharyngitis: a meta-analysis. BMC Fam Pract 15: 45, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McNally D, Simpson M, Morris C, Shephard A, Goulder M. Rapid relief of acute sore throat with AMC/DCBA throat lozenges: randomised controlled trial. Int J Clin Pract 64: 194-207, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wade AG, Morris C, Shephard A, Crawford GM, Goulder MA. A multicentre, randomised, double-blind, single-dose study assessing the efficacy of AMC/DCBA Warm lozenge or AMC/DCBA Cool lozenge in the relief of acute sore throat. BMC Fam Pract 12: 6, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shephard A, Smith G, Aspley S, Schachtel BP. Randomised, double-blind, placebo-controlled studies on flurbiprofen 8.75 mg lozenges in patients with/without group A or C streptococcal throat infection, with an assessment of clinicians' prediction of ‘strep throat’. Int J Clin Pract 69: 59-71, 2015. [DOI] [PubMed] [Google Scholar]
- 20. Golomb BA, Erickson LC, Koperski S, Sack D, Enkin M, Howick J. What's in placebos: who knows? Analysis of randomized, controlled trials. Ann Intern Med 153: 532-535, 2010. [DOI] [PubMed] [Google Scholar]