Abstract
Objectives
To investigate the sensitivity and specificity of a temporal artery biopsy (TAB) in the diagnosis of giant cell arteritis (GCA) in a single-center retrospective cohort in Japan.
Methods
A retrospective chart review was performed on consecutive patients who visited our hospital between April 2009 and October 2018 and underwent a TAB. The sensitivity and specificity were calculated for the three pathological standards for a TAB, predetermined according to the pathological criterion of the 1990 American College of Rheumatology (ACR) criteria: A) vasculitis characterized by predominant mononuclear cell infiltration; B) vasculitis with granulomatous inflammation; and C) vasculitis with multinucleated giant cells. We also analyzed the clinical parameters predicting the diagnosis of GCA and the impact of a diagnostic delay of ≥3 months on cardiovascular complications of GCA.
Results
Our study population was 16 cases in the GCA group and 13 in the non-GCA group. The sensitivity and specificity for Standard A of a TAB were 81% and 85%, respectively, while those for stricter Standards B or C were identical, at 75% and 100%, respectively. These pathological standards, but not any other parameters, significantly predicted the diagnosis. A diagnostic delay tended to cause cardiovascular complications (p=0.057).
Conclusion
The sensitivity and specificity of the pathological standards of a TAB were favorable in our cohort and were the only predictors for the diagnosis of GCA. Considering the possible impact of a diagnostic delay on cardiovascular complications, the early recognition and prompt initiation of glucocorticoid therapy is needed, even in Japan, where GCA is uncommon.
Keywords: temporal artery biopsy, giant cell arteritis, sensitivity, specificity, Japan
Introduction
A temporal artery biopsy (TAB) has remained the gold standard for the diagnosis of giant cell arteritis (GCA). A British guideline suggested that a TAB should be considered whenever a diagnosis of GCA is suspected and also noted that a biopsy should not delay the prompt initiation of high-dose glucocorticoid therapy (1). The aim of early management of this disease is to prevent cardiovascular complications of GCA, such as cerebral infarction (CI), acute myocardial infarction (AMI) and visual loss, which are often present at or shortly after the diagnosis (1-4). The results of a TAB are negative in some GCA patients, but if a typical clinical picture and response to steroids are apparent, the patient should be treated as having biopsy-proven GCA. However, if clinical suspicion remains low in the case of a negative TAB result, physicians must search for an alternative diagnosis and rapidly taper the steroid dose. As the premise of this strategy, the sensitivity of a TAB ideally should be high enough that a negative result could sufficiently lower the possibility of GCA.
The sensitivity of a TAB is reported to range between 32% and 85% (4-11). These values may be influenced by the length of the artery biopsy or preceding use of glucocorticosteroids (7,12); these values are largely related to the experience of the surgeon or pathologist in performing a TAB. However, the number of Japanese institutions with adequate experience in performing a TAB may be limited, as relatively few studies have investigated this disease (13-15). Furthermore, the diagnostic performance of a TAB has not been evaluated appropriately in Japan.
Therefore, in the present study, we investigated for the first time the sensitivity and specificity of a TAB in Japan, where GCA is uncommon. We also investigated whether or not clinical parameters, particularly the TAB findings, predicted the diagnosis of GCA and whether or not a diagnostic delay had any influence on cardiovascular complications of GCA.
Materials and Methods
Patients
We screened consecutive patients who visited our hospital between April 2009 and October 2018 and received a TAB. The inclusion criteria for the GCA group were 1) new-onset cases with a clinical diagnosis of GCA and 2) no change in the diagnosis of GCA until the final observation. All of the cases that did not meet these criteria were defined as the non-GCA group.
Data extraction
A retrospective chart review was performed in both groups. The following clinical parameters were extracted: final diagnosis; indication for a TAB; duration from the onset to referral, referral to the TAB, TAB to therapy and TAB to reporting of results; length of the biopsied artery (in millimeters); presence of headache, a fever (body temperature >37℃), malaise, weight loss, polymyalgia rheumatica (PMR), visual disturbance, vertigo/dizziness, jaw claudication, arm claudication, cough, dyspnea, dilatation of the temporal artery (TA), tenderness of the TA, decreased pulse of the TA, tenderness or decreased pulse of the TA, any abnormality of the TA, leukocytosis (white blood cell count >8,000 /μL), anemia (hemoglobin <13 g/dL for men, <12 g/dL for women), thrombocytosis (platelet >450,000 /μL) and high C-reactive protein (CRP) level (≥10 mg/dL), high erythrocyte sedimentation rate (ESR ≥50 mm/h); and meeting ≥3 of the 1990 American College of Rheumatology (ACR) classification criteria (16). Pathological parameters included three standard findings, predetermined according to the pathological description of the 1990 ACR criteria as follows: Standard A, vasculitis characterized by a predominance of mononuclear cell infiltration (in practical terms, meeting the pathological criterion of the 1990 ACR criteria); Standard B, vasculitis with granulomatous inflammation; and Standard C, vasculitis with multinucleated giant cells (Standards B and C, which are also part of the ACR criteria, are more specific but not necessary for the diagnosis). In principle, these standards were extracted from the formal pathological report, and for missing data, we consulted one of the authors (K.I.), who once again judged each pathological finding from the specimens. Granulomatous inflammation was regarded as aggregations of several histiocytes, with or without multinucleated giant cells.
Statistical analyses
First, we calculated the sensitivity and specificity of the pathological standards (Standards A, B, and C), based on the findings from the initial unilateral TAB, as our primary outcome. We then analyzed those clinical parameters predicting the diagnosis of GCA, all of which were categorical variables. These parameters were compared between the GCA and non-GCA groups using Fisher's exact test, based on evaluable cases. If a significant difference was detected between the groups, the parameters were again judged with missing data assumed to be unlikely to produce a significance difference. We then investigated the timing of glucocorticoid therapy and its impact on the TAB result, based on case descriptions. Finally, to determine whether or not a diagnostic delay contributed to cardiovascular complications of GCA, we analyzed the association of a diagnostic delay, defined as ≥3 months between the onset and initiation of therapy, with cardiovascular complications of GCA that developed after therapy, using Fisher's exact test.
The threshold for statistical significance was set at p <0.05. All analyses were conducted using the EZR software program (17). This study was approved by the institutional review boards of our hospital.
Results
A total of 29 patients received a TAB, including 16 who met the inclusion criteria for the GCA group and 13 who met the criterion for the non-GCA group. There were no GCA cases who did not receive a TAB, as we considered any clinical suspicion of GCA as the indication of the biopsy. None of non-GCA group developed GCA during the follow-up period. Patient characteristics of the groups are shown in Table 1. As soon as possible after our clinical examination, each case was referred to the surgeons handling the TAB, who scheduled operations. Twenty-five of the 29 cases underwent a TAB in the Department of Plastic Surgery at our hospital, although the early cases (Cases 1-3, 17) underwent a TAB in another department or institution.
Table 1.
Clinical Characteristics of Patients in the GCA and Non-GCA Groups.
| Age/ sex |
Final diagnosis | Indication of TAB* | Onset to referral (months) | Referral to TAB (days) | TAB to therapy (days) | TAB to reporting (days) | LVV on CECT | Standard A | Standard B | Standard C | FN | Complications of GCA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 84 F | GCA | H, NP | 0.33 | 11 | -10 | 6 | (+) | (+) | (+) | (+) | (-) | none |
| 2 | 82 M | GCA | H | 7.5 | 6 | 16 | 5 | NA | (-) | (-) | (-) | (-) | AMI |
| 3 | 75 F | GCA | Anemia | 8 | 9 | 2 | 6 | (+) | (+) | (+) | (+) | (-) | none |
| 4 | 77 M | GCA | H, cough | 4 | 12 | 2 | 2 | (+) | (+) | (+) | (+) | (-) | CI |
| 5 | 82 F | GCA | H | 0.53 | 12 | 2 | 7 | (-) | (+) | (+) | (+) | (-) | none |
| 6 | 65 | GCA | FUO, LVV | 0.4 | 3 | -6 | 6 | (+) | (-) | (-) | (-) | (-) | none |
| 7 | 76 F | GCA | NP | 2 | 12 | 15 | 7 | (-) | (+) | (+) | (+) | (-) | none |
| 8 | 77 F | GCA | H | 2 | 7 | 1 | 15 | (+) | (+) | (+) | (+) | (-) | none |
| 9 | 73 F | GCA | H | 1 | 10 | 1 | 6 | (+) | (+) | (+) | (+) | (-) | none |
| 10 | 81 F | GCA | H | 0.17 | 6 | 1 | 10 | (-) | (+) | (+) | (+) | (+) | none |
| 11 | 80 F | GCA | H | 1.8 | 11 | 4 | 6 | (-) | (+) | (+) | (+) | (-) | none |
| 12 | 73 M | GCA | Anorexia, malaise | 3.6 | 8 | 1 | 6 | (+) | (+) | (+) | (+) | (-) | none |
| 13 | 64 M | GCA | H | 1.9 | 6 | 1 | 5 | (+) | (+) | (+) | (+) | (-) | none |
| 14 | 76 M | GCA | H | 0.67 | 7 | 1 | 6 | (-) | (+) | (-) | (-) | (-) | none |
| 15 | 83 F | GCA | H | 0.3 | 0 | 0 | 3 | (-) | (+) | (+) | (+) | (+) | none |
| 16 | 66 M | GCA | H, LVV | 5.1 | 3 | -2 | 6 | (+) | (-) | (-) | (-) | (-) | CI |
| 17 | 66 M | FUO | H | 0.63 | 2 | No therapy | 6 | (-) | (-) | (-) | (-) | (-) | - |
| 18 | 83 M | PMR | PMR | 1 | 5 | 6† | 5 | (-) | (-) | (-) | (-) | (-) | - |
| 19 | 80 F | PMR | H, PMR | 0.67 | 3 | 1† | 5 | NA | (-) | (-) | (-) | (-) | - |
| 20 | 72 M | ALCL | H | 0.5 | 6 | 2 | 8 | (-) | (-) | (-) | (-) | (-) | - |
| 21 | 87 M | HUE | H | 12 | 5 | No therapy | 3 | (-) | (-) | (-) | (-) | (-) | - |
| 22 | 84 M | FUO | H | 0.2 | 12 | No therapy | 6 | (-) | (-) | (-) | (-) | (-) | - |
| 23 | 58 F | PAN | H | 0.67 | 14 | 4 | 3 | (-) | (+) | (-) | (-) | (+) | - |
| 24 | 84 F | S/o LCH | H | 9 | 7 | No therapy | 3 | (-) | (-) | (-) | (-) | (-) | - |
| 25 | 80 F | NAION, RPE | Vision loss | 0.77 | 5 | 4‡ | 2 | NA | (-) | (-) | (-) | (-) | - |
| 26 | 74 M | FUO | FUO | 0.67 | 5 | No therapy | 3 | (-) | (+) | (-) | (-) | (-) | - |
| 27 | 63 M | IAAA | FUO | 0.53 | 6 | 5†† | 7 | (-) | (-) | (-) | (-) | (-) | - |
| 28 | 52 F | TAK | H, LVV | 0.43 | 6 | -6 | 5 | (+) | (-) | (-) | (-) | (-) | |
| 29 | 91 F | ALA | H | 12 | 12 | No therapy | 5 | NA | (-) | (-) | (-) | (-) | - |
LVV on CECT: large vessel vasculitis on contrast-enhanced computed tomography, Standard A: Mononuclear cell infiltration, Standard B: granulomatous inflammation, Standard C: Multinucleated giant cells, FN: Fibrinoid necrosis, GCA: giant cell arteritis, FUO: fever of unknown origin, PMR: polymyalgia rheumatica, ALCL: aplastic large cell lymphoma, HUE: headache of unknown etiology, PAN: polyarteritis nodosa, LCH: Langerhans cell histiocytosis, NAION: nonarteritic anterior ischemic optic neuropathy, RPE: rheumatoid pleural effusion, IAAA: inflammatory abdominal aortic aneurysm, TAK: Takayasu arteritis, ALA: AL amyloidosis, H: headache, NP: neck pain, AMI: acute myocardial infarction, CI: cerebral infarction
†Two cases with PMR improved after low-dose prednisolone (15 mg/day). ‡This patient with rheumatoid arthritis suffering from fever and visual loss also developed aseptic pleuritis, which was successfully treated with moderate-dose prednisolone (20 mg/day). ††This case involved persistent fever after endovascular aneurysm repair for impending rupture of abdominal aortic aneurysm, which improved with moderate-dose prednisolone (20 mg/day) for a clinical diagnosis of IAAA.
The median duration between the onset and referral for the GCA and non-GCA groups was 1.85 months [interquartile range (IQR), 0.52-3.7] and 0.67 months (0.53-1.0), respectively. The median duration between the referral and the TAB for the GCA and non-GCA groups was 7.5 days (IQR, 6-11) and 6 days (5-7), respectively. For the GCA group, the median duration between the TAB and initiation of therapy was 1 day (IQR, 0.75-2 days), while that between the TAB and reporting of results was 6 days (IQR, 5.75-6.25 days). The mean (±standard deviation) length of the biopsied artery in the 8 evaluable cases for both groups was 20.0±6.8 mm.
Clinical characteristics of patients in the GCA and non-GCA groups
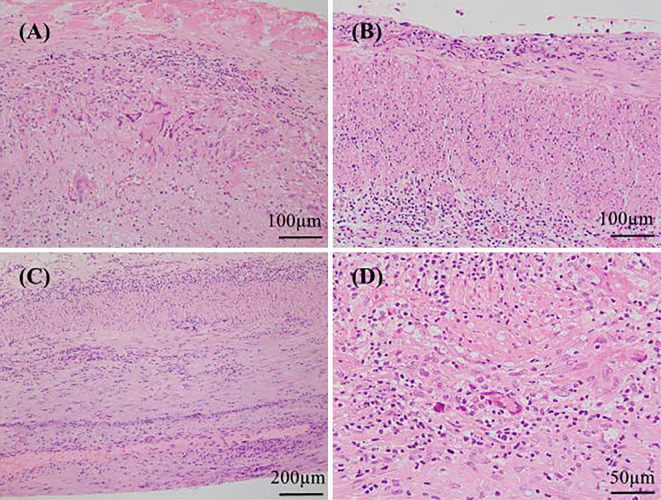
In the GCA group, a TAB was indicated for headache (n=12), fever of unknown origin (FUO) (n=1), anemia of chronic inflammation (n=1), posterior neck pain (n=1) and appetite loss (n=1). Typical TAB findings with granulomatous inflammation and multinucleated giant cells invading the internal elastic lamina (Case 4) are shown in Fig. 1A. Large-vessel involvement was found in 9 (60%) of 15 evaluable cases in the GCA group, compared with 1 (7.7%) of 13 in the non-GCA group, while only 1 case with inflammatory abdominal aortic aneurysm (Case 27) in both groups had an aortic aneurysm.
Figure 1.
A) Granulomatous inflammation and multinucleated giant cells invading the internal elastic lamina, typical of the pathological diagnosis of GCA (Case 4). B) Mononuclear cell infiltration without granulomatous inflammation or giant cells in the arterial wall in a case (Case 26) with fever of unknown origin. C) Mononuclear cell infiltration without granulomatous inflammation or giant cells in the arterial wall in one GCA case (Case 14). D) Granulomatous inflammation and multinucleated giant cell in the arterial wall in the TAB specimen of a GCA case obtained 10 days after high-dose steroid therapy (Case 1).
In the non-GCA group, a TAB was indicated for headache (n=9), FUO (2), PMR (1) and loss of vision (1). Even in the non-GCA group, a TAB directly contributed to a different diagnosis in two cases: one case (Case 23) with a fever, polyarthritis and a tender TA was considered to have polyarteritis nodosa (PAN) based on TAB results showing arteritis of all layers with fibrinoid necrosis, which was more consistent with a diagnosis of PAN than that of GCA (18,19). The other case (Case 29) with renal failure and a tender and swollen TA was diagnosed with amyloid light-chain (AL) amyloidosis based on the presence of amyloid deposition in the arterial wall on a TAB in addition to the findings of a bone marrow examination.
Importantly, negative results from a TAB indirectly contributed to a different diagnosis in 4 cases: PMR (n=2), nonarteritic anterior ischemic optic neuropathy (n=1) and Takayasu arteritis (TAK) (n=1). In two cases of FUO, the symptoms eventually subsided after the TAB.
Sensitivity and specificity of a TAB for the diagnosis of GCA
When using the pathological Standard A (i.e., vasculitis characterized by predominant mononuclear cell infiltration or meeting the pathological criterion of the 1990 ACR criteria), three false-negative case (Case 2, 6 and 16) and two false-positive cases (Cases 23 and 26) were identified. In one false-negative case (Case 2), a contralateral TAB showed vasculitis with granulomatous inflammation and giant cells after a negative result on the initial TAB. In the remaining two false-negative cases (Case 6 and 16), large vessel vasculitis on contrast-enhanced CT suggested a diagnosis of GCA. In one false-positive case with PAN (Case 23), a TAB showed mononuclear cell infiltration without granulomatous inflammation or giant cells. The other false-positive case (Case 26) presented with a persistent fever, bilateral thigh pain and tender and enlarged TA. A TAB showed mild vasculitis with mononuclear cell infiltration in the vessel wall (Fig. 1B), but the symptoms improved five days later without the need for steroid therapy. Accordingly, the patient was considered as having had an unexplained fever but no clinically significant disease. The sensitivity and specificity for Standard A of a TAB were therefore calculated as 81% (13/16) and 85% (11/13), respectively.
For Standard B, an additional case of GCA (Case 14) in which a TAB showed mononuclear inflammation without granulomatous inflammation or giant cells was considered as showing a false-negative result (Fig. 1C). Accordingly, the sensitivity of Standard B of a TAB was 75% (12/16), whereas the specificity was 100% (13/13) because none of the non-GCA group had granulomatous inflammation in their TAB specimens. Similarly, the sensitivity and specificity of Standard C were also 75% (12/16) and 100% (13/13), respectively.
Clinical parameters predicting the diagnosis of GCA
Among the clinical parameters (Table 2), the large vessel vasculitis (LVV) on contrast-enhanced computed tomography (CECT) and weight loss was significantly more frequent in the GCA group than in the non-GCA group; however, neither of these differences reached significance after the assumption of missing data. A high CRP level of ≥10 mg/dL was frequently found in the non-GCA group. As is obvious, each pathological standard of a TAB (Standards A-C) more strongly predicted the diagnosis of GCA than any other parameters (p<0.001 for Standard A; p<0.0001 for both Standards B and C). In contrast, meeting the 1990 ACR criteria (≥3 items) did not significantly contribute to the diagnosis of GCA (88% vs. 69% in the GCA and non-GCA groups, respectively).
Table 2.
Clinical Parameters Predicting the Diagnosis of GCA.
| GCA | non-GCA | p value | |
|---|---|---|---|
| positive/negative/missing | positive/negative/missing | ||
| Headache | 12/4/0 | 9/4/0 | 1.0 |
| Fever >37°C | 12/4/0 | 11/1/1 | 0.355 |
| Malaise | 11/4/1 | 10/1/2 | 0.317 |
| Weight loss | 10/5/1 | 2/9/2 | 0.0214 |
| #Weight loss | 10/6 | 4/9 | 0.139 |
| PMR | 3/13/0 | 2/10/1 | 1.0 |
| Visual disturbance | 1/15/0 | 1/11/1 | 1.0 |
| Vertigo/dizziness | 1/14/1 | 1/7/5 | 1.0 |
| Jaw claudication | 6/9/1 | 3/8/2 | 0.683 |
| Arm claudication | 1/13/2 | 0/9/4 | 1.0 |
| Cough | 5/11/0 | 0/12/1 | 0.0525 |
| Dyspnea | 1/15/0 | 0/12/1 | 1.0 |
| Dilatation of TA | 14/2/0 | 7/6/0 | 0.0923 |
| Tenderness of TA | 8/7/1 | 6/7/0 | 1.0 |
| Decreased pulse of TA | 5/10/1 | 1/8/4 | 0.351 |
| Tenderness or decreased pulse of TA | 11/5/0 | 6/4/3 | 0.692 |
| Any abnormality of TA | 14/2/0 | 9/2/2 | 1.0 |
| leukocytosis | 10/6/0 | 11/2/0 | 0.238 |
| Anemia | 14/2/0 | 11/2/0 | 1.0 |
| thrombocytosis | 2/14/0 | 3/10/0 | 0.632 |
| high CRP (≥ 10 mg/dL) | 5/11/0 | 10/3/0 | 0.0253 |
| high ESR (≥ 50 mm/h) | 13/3/0 | 9/4/0 | 0.667 |
| Meeting ACR criteria (≥ 3 items) | 14/2/0 | 9/4/0 | 0.364 |
| LVV on CECT | 9/6/1 | 1/9/3 | 0.0177 |
| #LVV on CECT | 9/7 | 4/9 | 0.264 |
| TAB; vasculitis characterized by predominance of mononuclear cell infiltration | 13/3/0 | 2/11/0 | <0.001 |
| TAB; vasculitis with granulomatous inflammation | 12/4/0 | 0/13/0 | <0.0001 |
| TAB; vasculitis with multinucleated giant cells | 12/4/0 | 0/13/0 | <0.0001 |
PMR: polymyalgia rheumatica, TA: temporal artery, CRP: C-reactive protein, ESR: erythrocyte sedimentation rate, ACR: American College of Rheumatology, LVV on CECT: large vessel vasculitis on contrast-enhanced computed tomography, TAB: temporal artery biopsy, GCA: giant cell arteritis
# Missing data were assumed to be unlikely to produce any significance difference.
Timing of glucocorticosteroid therapy and its impact on the TAB findings
Glucocorticoid therapy for GCA was initiated prior to a TAB in 4 of 16 GCA cases (Fig. 2, Table 1). In 1 of these patients (Case 1), therapy with prednisolone (0.8 mg/kg/day) was initiated 11 days prior to the TAB, which nonetheless revealed granulomatous inflammation with multinucleated giant cells (Fig. 1D). Another positive result on a TAB was seen in a case (Case 11) who had taken low-dose prednisolone (10 mg/day) for PMR for 7 weeks before the referral. The TAB showed granulomatous arteritis with giant cells, although the degree of inflammation was not as intense as in other GCA cases with steroid-naïve TAB findings. In the remaining 2 cases (Case 6 and 16), prednisolone at 0.5 mg/kg/day and 1.0 mg/day was initiated 6 and 2 days before TABs, respectively, with normal pathological findings noted in both. These two cases also had normal temporal arteries on a physical examination and LVV on contrast-enhanced CT: one without cranial symptoms was considered to have large-vessel GCA, while another presenting with vertebrobasilar infarction, typical of GCA (3), was regarded as having cranial GCA without temporal arteritis.
Figure 2.
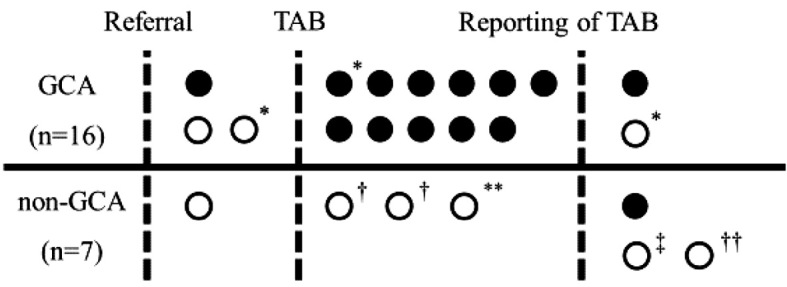
Timing of glucocorticoid therapy in association with the referral, TAB and its reporting. Closed and open circles indicate positive and negative results for Standard A (or the pathological criterion of a TAB according to the 1990 ACR criteria), respectively. For the non-GCA group, glucocorticoid therapy was started in seven cases. *cardiovascular complication, †polymyalgia rheumatica, **aplastic large cell lymphoma; ‡rheumatoid pleural effusion; ††inflammatory abdominal aortic aneurysm. GCA: giant cell arteritis, TAB: temporal artery biopsy
In contrast, 1 of 13 cases in the non-GCA group started therapy prior to a TAB. That case (Case 28) presented with neck pain, headache and a fever and showed large-vessel vasculitis on contrast-enhanced CT. Under a provisional diagnosis of either TAK or GCA, prednisolone (1 mg/kg/day) was initiated at the timing of the referral. A TAB performed six days after starting therapy showed normal findings. The patient was eventually considered to have TAK because of carotidynia and tender carotid arteries, both of which are characteristic of TAK (20).
Association of a diagnostic delay with the development of cardiovascular complications of GCA
Cardiovascular complications of GCA, AMI or CI developed in three cases, but visual loss was not seen in any patients. The first patient (Case 2) had noticed temporal headache 7.5 months before the referral. High-dose prednisolone (1 mg/kg/day) was started 22 days after the referral. Subsequently, the patient developed AMI 30 days after the initiation of therapy. The second patient (Case 4) suffered from cough and headache for 4 months, and high-dose prednisolone (0.8 mg/kg/day) was initiated 14 days after the referral. Five days after starting therapy, the patient developed Wallenberg syndrome. The third patient (Case 16) initially presented with infarction of the bilateral cerebellum and right occipital lobe five months before the referral. Following the onset of the CI, he was found to have jaw claudication and malaise with an unexplained elevation in his CRP levels. Fortunately, all of these cases recovered without irreversible sequelae.
When analyzing the impact of a diagnostic delay on cardiovascular complications of GCA, we excluded the third patient with a diagnostic delay of five months because his initial symptom was CI. Cardiovascular complications developed in 2 of the 4 GCA patients with a diagnostic delay of ≥3 months compared with 0 of the 11 patients without such a long delay; this difference was nearly, but not actually, significant (p=0.0571).
Discussion
This study is the first to describe the diagnostic performance of a TAB for the diagnosis of GCA in Japan. We considered our cohort to be a good sample for calculating the sensitivity of a TAB because all of our cases with GCA underwent a TAB, which eliminates potential selection bias. The sensitivity and specificity were calculated based on consecutive patients who underwent a TAB. Three pathological standards for TAB findings were applied: Standard A, vasculitis characterized by a predominance of mononuclear cell infiltration, which is practically identical to the pathological criterion of the 1990 ACR criteria; Standard B, vasculitis with granulomatous inflammation; and Standard C, vasculitis with multinucleated giant cells. The sensitivity and specificity for Standard A (or the pathological criterion of the ACR criteria) were 81% and 85%, respectively, compared to 75% and 100%, respectively, for the stricter Standards B and C. Each of these appeared clinically useful, as clinically differentiating false-positive cases from true-positive cases with GCA was not difficult. However, given that other types of vasculitis or clinically irrelevant arterial inflammation can mimic the modest histological findings of GCA (mononuclear cell infiltration), vasculitis with granulomatous inflammation or with giant cells may thus be an important finding for classifying untreated GCA cases. Unsurprisingly, these standards significantly predicted the diagnosis, with no other parameters-including the ACR criteria-found to be useful.
The high sensitivities of 81% and 75% for a TAB in this study may have been associated with the low rate of steroid use prior to the TAB. In a retrospective cohort of 70 GCA patients investigated by Narváez et al., the sensitivity of a TAB decreased with the preceding use of glucocorticoids, being 78% for 1-14 days of steroid use, 65% for 15-28 days and 40% for >28 days (21). Another pathological analysis of 35 steroid-treated patients showed that giant cells were found in 53% of patients with <6 days of steroid therapy, 36% of those with 4-11 days of therapy and 22% of those with >14 days of therapy (12). Importantly, all 9 cases treated for >14 days had inflammation limited to the media-adventitia junction. This may suggest that experienced pathologists from institutions that achieved a high sensitivity with TABs were able to detect limited inflammation in TAB samples even after steroid therapy. In two of our four cases in which glucocorticoid therapy preceded the TABs, the specimens showed typical findings of GCA, although inflammation was mild in one. For the remaining two cases, we did not consider glucocorticoid therapy of <7 days' duration to have significantly influenced the pathological findings, as neither of the arterial specimens of these cases showed any inflammation.
Another extremely important finding was that cardiovascular events developed in 19% (3/16) of our GCA cohort, and a diagnostic delay of ≥3 months tended to cause such complications. Two cases with cardiovascular complications that developed shortly after starting therapy had a prolonged duration between the onset and referral of 4-7.5 months, and it took a further 14-22 days before therapy was started; the complications in these cases may have been able to be prevented if we had initiated steroid therapy promptly after the referral. Considering the short median duration between the referral and the TAB in the GCA group [7.5 days (IQR, 6-11)], even if we had started glucocorticoid therapy immediately at the initial evaluation, the sensitivity of the TAB would not have been decreased markedly by such short-term therapy, as reported in the abovementioned study (21). Immediate steroid therapy in patients with a high index of suspicion for GCA may therefore be acceptable in institutions with a high TAB sensitivity, even in Japan.
Unexpectedly, a TAB contributed to the diagnosis directly or indirectly in nearly half (6/13) of non-GCA cases. Thus, negative TAB results for GCA in our institution may not only reduce the possibility of GCA but also suggest alternative diagnoses. In addition, the pretest probability of GCA of 55% (16/29) among our cohort may be relatively low for Japan, meaning that the indication of a TAB may be limited to cases with a very high suspicion of GCA in Japan. However, large-scale studies in other countries have provided similar or even lower pretest probabilities of 51% (156/305) to 40% (54/134) (7,5). Attempting to screen almost all GCA patients among all individuals clinically suspected of having GCA may be difficult without some TABs eventually proving to be unnecessary. We therefore believe that the pretest probability of around 50% in these studies may be reasonable to avoid overlooking this disease, which is associated with serious complications.
Given the retrospective nature of our study and the small sample size, our results are at risk of potential bias. Furthermore, the length of the biopsied artery is considered to have a marked influence on the sensitivity of TABs, but these data were often unavailable in our study. However, our results were based on the pathological findings of consecutive cases seen during a nine-year period in a hospital in Japan, a region where GCA is uncommon.
In conclusion, our study showed that the sensitivity and specificity of a TAB were sufficiently high to allow for early management of GCA in Japan. The sensitivity of a TAB was found to be 81% for vasculitis characterized by mononuclear cell infiltration, 75% for vasculitis with granulomatous inflammation and 75% for vasculitis with multinucleated giant cells, with specificities of 85%, 100% and 100%, respectively. These TAB findings were the only predictors for the diagnosis of GCA. Given the favorable diagnostic performance of TABs in this study as well as the potential adverse influence of diagnostic delay on cardiovascular complications, we concluded that steroid therapy prior to a TAB for patients with clinically suspected GCA would be reasonable in order to prevent potentially irreversible complications of the disease, even in Japan, as is recommended in Western countries.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Dasgupta B, Borg FA, Hassan N, et al. . BSR and BHPR guidelines for the management of giant cell arteritis. Rheumatology (Oxford) 49: 1594-1597, 2010. [DOI] [PubMed] [Google Scholar]
- 2. Tomasson G, Peloquin C, Mohammad A, et al. . Risk for cardiovascular disease early and late after a diagnosis of giant-cell arteritis: a cohort study. Ann Intern Med 160: 73-80, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Boysson H, Liozon E, Larivière D, et al. . Giant cell arteritis-related stroke: a retrospective multicenter case-control study. J Rheumatol 44: 297-303, 2017. [DOI] [PubMed] [Google Scholar]
- 4. Kobayashi S, Yano T, Matsumoto Y, et al. . Clinical and epidemiologic analysis of giant cell (temporal) arteritis from a nationwide survey in 1998 in Japan: the first government-supported nationwide survey. Arthritis Rheum 49: 594-598, 2003. [DOI] [PubMed] [Google Scholar]
- 5. Hall S, Persellin S, Lie JT, O'Brien PC, Kurland LT, Hunder GG. The therapeutic impact of temporal artery biopsy. Lancet 2: 1217-1220, 1983. [DOI] [PubMed] [Google Scholar]
- 6. Ray-Chaudhuri N, Kiné DA, Tijani SO, et al. . Effect of prior steroid treatment on temporal artery biopsy findings in giant cell arteritis. Br J Ophthalmol 86: 530-532, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Breuer GS, Nesher R, Nesher G. Effect of biopsy length on the rate of positive temporal artery biopsies. Clin Exp Rheumatol 27 (1 Suppl 52): S10-S13, 2009. [PubMed] [Google Scholar]
- 8. Diamantopoulos AP, Haugeberg G, Hetland H, Soldal DM, Bie R, Myklebust G. Diagnostic value of color Doppler ultrasonography of temporal arteries and large vessels in giant cell arteritis: a consecutive case series. Arthritis Care Res (Hoboken) 66: 113-119, 2014. [DOI] [PubMed] [Google Scholar]
- 9. Rhéaume M, Rebello R, Pagnoux C, et al. . High-resolution magnetic resonance imaging of scalp arteries for the diagnosis of giant cell arteritis: results of a prospective cohort study. Arthritis Rheumatol 69: 161-168, 2017. [DOI] [PubMed] [Google Scholar]
- 10. Roth AM, Milsow L, Keltner JL. The ultimate diagnoses of patients undergoing temporal artery biopsies. Arch Ophthalmol 102: 901-903, 1984. [DOI] [PubMed] [Google Scholar]
- 11. Saedon H, Saedon M, Goodyear S, Papettas T, Marshall C. Temporal artery biopsy for giant cell arteritis: retrospective audit. JRSM Short Rep 3: 73, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Font RL, Prabhakaran VC. Histological parameters helpful in recognising steroid-treated temporal arteritis: an analysis of 35 cases. Br J Ophthalmol 91: 204-209, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Imai N, Kuroda R, Konishi T, Serizawa M, Kobari M. Giant cell arteritis: clinical features of patients visiting a headache clinic in Japan. Intern Med 50: 1679-1682, 2011. [DOI] [PubMed] [Google Scholar]
- 14. Morinobu A, Tsuji G, Kasagi S, et al. . Role of imaging studies in the diagnosis and evaluation of giant cell arteritis in Japanese: report of eight cases. Mod Rheumatol 21: 391-396, 2011. [DOI] [PubMed] [Google Scholar]
- 15. Suematsu E, Miyamura T, Nakamura M, et al. . Clinical study of giant cell arteritis in our hospital. Nihon Rinsho Meneki Gakkai Kaishi 38: 466-472, 2015(in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 16. Hunder GG, Bloch DA, Michel BA, et al. . The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum 33: 1122-1128, 1990. [DOI] [PubMed] [Google Scholar]
- 17. Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 48: 452-458, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lie JT. Illustrated histopathologic classification criteria for selected vasculitis syndromes. American College of Rheumatology Subcommittee on Classification of Vasculitis. Arthritis Rheum 33: 1074-1087, 1990. [DOI] [PubMed] [Google Scholar]
- 19. Généreau T, Lortholary O, Pottier MA, et al. . Temporal artery biopsy: a diagnostic tool for systemic necrotizing vasculitis. French Vasculitis Study Group. Arthritis Rheum 42: 2674-2681, 1999. [DOI] [PubMed] [Google Scholar]
- 20. Valsakumar AK, Valappil UC, Jorapur V, Garg N, Nityanand S, Sinha N. Role of immunosuppressive therapy on clinical, immunological, and angiographic outcome in active Takayasu's arteritis. J Rheumatol 30: 1793-1798, 2003. [PubMed] [Google Scholar]
- 21. Narváez J, Bernad B, Roig-Vilaseca D, et al. . Influence of previous corticosteroid therapy on temporal artery biopsy yield in giant cell arteritis. Semin Arthritis Rheum 37: 13-19, 2007. [DOI] [PubMed] [Google Scholar]