Abstract
The aim of this study is to explore the expression of osteopontin (OPN) and its relationship with prognostic factors and survival in diffuse large B cell lymphoma (DLBCL). A tissue microarray was performed for immunohistochemical evaluation. Contingency tables were analyzed for trends; chi-square test was used to determine differences between groups. Univariate and multivariate Cox proportional hazards-regression analyses were performed to evaluate the impact of prognostic factors on survival. Expression of OPN was observed in 28%. It was different in non-germinal center DLBCL (P=0.04). The mean overall survival (OS) was lower in patients with positive OPN expression (19.762; CI 95% 14.269-25.255) it was not significant (P=0.123). It is not possible to establish a clear relationship between the expression by immunohistochemistry of osteopontin and a poor prognosis but it would be important to complement the analysis with other techniques as PCR or NGS that allow us to assess the influence of the isoforms and post-translational modifications of OPN on the biological behavior of DLBCL. Our findings indicate that OPN expression could be associated with a more aggressive variant of lymphoma: non-germinal center DLBCL.
Key words: osteopontin, lymphoma, prognosis
Introduction
Diffuse large B cell lymphoma (DLBCL) is a heterogeneous disease which prognosis depends on clinical and biological factors.1 Advanced age, low performance status, advanced Ann Arbor stage, elevated lactate dehydrogenase (LDH) and extranodal disease have shown to be predictors of survival.2 The origin of the neoplastic cell (germinal center vs. non-germinal center), MYC, BCL6 and BCL2 translocations (double-hit or triple hit lymphoma) also influence the prognosis.
Osteopontin (OPN) is a non-collagenous extracellular matrix (ECM) protein with cytokine activity, expressed by various cell types, and is involved in multiple biological processes, both physiological and pathological; different isoforms (a, b, c) can be produced by alternative splicing.3-7 The OPN was found intracellular and could also be secreted by an alternative translation mechanism and undergoes post-translational modifications (cleavage, glycosylation, etc.).8-11 OPN exerts its function binding to integrins and CD44.12,13 The biological function that OPN produces depend on the type of cell, isoforms and receptors that recognize the proteins.8 In cancer, OPN induces the inhibition of apoptosis, favors tumor invasion, metastasis, angiogenesis and deregulation of cellular energetics, avoiding immune destruction and tumorpromoting inflammation.14
The increase in the production of osteopontin in different types of neoplasias has been associated with tumor aggressiveness and poor prognosis.13,15-22
A retrospect cohort study was conducted among patients with DLBCL; the purpose was to evaluate the expression of osteopontin in order to analyze its association with known prognostic factors and its influence on the overall survival.
Materials and Methods
Patients and tissue specimens
The present study was approved by the IRB Committee (Rev/93/16), Instituto Nacional de Cancerologia Mexico (INCan). Data were obtained from DLBCL database at INCan, between November 2014 and March 2016. All patients included in the analysis meet the following criteria: (i) histologically diagnosed as DLBL; (ii) tumor specimens with available quality for tissue microarray construction; (iii) complete data parameters to calculate the IPI and NCCNIPI scales on diagnosis (age, performance status, Ann Arbor stage, LDH levels and extranodal disease); (iv) Patients who were followed-up at the INCan. Clinical stage was determined according to the Ann Arbor staging system. Cellular origin was determined according to the Hans algorithm.23 As a result, 80 cases met the inclusion criteria and were incorporated in our study. The survival time was measured from the date of diagnosis to the date of death, or last follow- up.
Immunohistochemistry detection of osteopontin
The paraffin-embedded primary tumor tissues of 80 patients were used to construct the DLBCL tissue microarray and were cut into 4 mm thick slices. For the IHC analysis, the slides were hydrated and antigenically reactivated using a citrate buffer (0.01 M citric acid, 0.01 M sodium citrate) for 10 min at 90°C. Endogenous peroxidase was blocked using Bloxall solution (Cat. SP6000. Vector), and after three washes with PBS 1X, nonspecific antigenic sites were blocked using 1% bovine serum albumin (BSA)/Triton X-100 0.1% dissolved PBS 1X during 30 min at 37°C. Blocked solution was discarded and samples were incubated with OPN 1:200 (Cat. Sc-21742. Santa Cruz Biotechnology) dissolved in BSA 0.1%/Triton X-100 0.01% overnight at 4°C. The slides were washed 3 times with PBS 1X, and the secondary antibody was used as specified by the manufacturer (mouse and rabbit specific HRP/AEC detection IHC kit, ab94705. Abcam). The slides were counterstained with Mayer’s haematoxylin (Cat. HK100-9K. Bio Genex) and were mounted using Aqua Mounter Solution (Cat. BSB0091). Digital images of tissue sections (40X) were captured using a light microscope and a color AxioCam MRc5 camera (Zeiss).
Three different pathologists, blinded to the clinical information, determined the immunoreactivity of OPN. To evaluate the immunohistochemical expression of OPN, we used a score corresponding to the sum of both staining intensities (0=negative; 1=weak; 2=intermediate; 3=strong) and the percentage of positive cells (0=0% positive cells; 1=25% positive cells; 2=26–50% positive cells; 3>50% positive cells). The sum of a+b reached a maximum score of 6. A score equal or greater than 3 represented a positive immunohistochemical survey.24
Statistical analysis
The SPSS statistical software package version 20 was used for data analysis. Contingency tables were analyzed for trends; chi-square test was used to determine the significance of differences between groups for categorical variables. Univariate survival analysis was based on the Kaplan-Meier product limit estimator. Univariate and multivariate Cox proportional hazards-regression analyses were performed to evaluate the impact of prognostic factors on survival.
Results
Patients
In total, 48 females and 32 males were enrolled in our study, with a mean age of 59.64 years (range, 22–88 years); 85% were treated with R-CHOP or CHOP-like chemotherapy. OPN was expressed in 43.8% of the cases, primarily presenting a nuclear and cytoplasmic staining pattern, however when applying the score only 28% were considered positive; in some cases, OPN also presented little expression in macrophages and interstitial tissue (Figure 1).
Correlation of OPN positivity with prognostic factors
Positivity of OPN was not significantly correlated with age, performance status, Ann Arbor stage, LDH levels and extranodal disease but was significantly different in cases of non-germinal center lymphoma. Table 1 summarizes the detailed information of the included cases.
Long-term survival
The mean OS time for the entire cohort of patients was 24.04 months (95% confidence interval [CI]: 21.4–26.6 months), with a 30-months OS rate of 75.8%; the median was not reached. Table 2 and Figure 2 summarize the survival details according to the expression of OPN.
Factors that impact survival
Univariate analysis, using the Cox proportional hazards regression model, demonstrated that performance status was the only factor that adversely affected OS (Table 3). Multivariate analysis did not identify any independent factor that adversely affected OS (Table 3).
Figure 1.
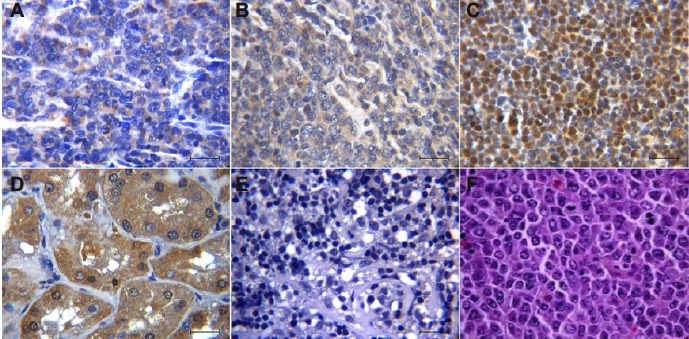
Osteopontin (OPN) expression immunohistochemistry in Diffuse Large B Cell Lymphoma (DLBCL). (A) Cytoplasmic OPN staining with positivity < 50%. (B) Cytoplasmic OPN staining with positivity > 50%. (C) Nuclear OPN staining. (D) Kidney sample was used as positive control of OPN. (E) Negative expression of OPN in DLBCL. (F) Hematoxylin and Eosin staining in a case of DLBCL. (A-F) Scale bar =50 μm. Immunostaining in tissues was visualized at a magnification 400x.
Discussion and Conclusions
DLBCL is an aggressive disease with a heterogeneous clinical behavior, while some patients may respond in a great way to treatment with immune-chemotherapy; however about 30% of cases do not achieve an adequate response. Although risk factors have been identified, it is important to identify targets that influence the prognosis and may be susceptible to treatment.
Osteopontin has been identified as a prognostic factor in hematologic and nonhematologic malignancies. Aggressive cases of DLBCL expressing osteopontin have been reported.25,26 In our study, we did not find a significant association between OPN expressions and known risk factors. But, we found a significant difference of OPN expression between germinal center and non-germinal center (NGC) lymphomas, that until today never has been reported, and this finding have clinical relevance since this last variant (NGC) is associated with a lower overall and progressionfree survival.
An association between the expression of osteopontin and extranodal disease has been also reported.27 OPN has been linked to the tropism of neoplastic B cells to the central nervous system (CNS) and it has been found that the OPN gene is up-regulated in the primary nervous system lymphoma. 28-30 Although the characteristics of our study do not allow us to identify a relationship between OPN expression and CNS involvement by lymphoma, we consider that it is important to deepen the investigations to know if the expression of OPN in the neoplastic cell can predict an infiltration, posterior to the central nervous system, as it would help to discern which patients with DLBCL would benefit from prophylaxis to prevent CNS involvement.
In our study, a decreased overall survival was found in patients with positive OPN expression; however, this was not significant. In the multivariate analysis, all the risk factors evaluated (age, PS, Ann Arbor stage, elevated LDH, extranodal disease, cell origin and OPN expression) confer an increased risk of mortality, but were not statistically significant, probably due to size of our cohort and the losses in the follow-up of the patients.
Figure 2.
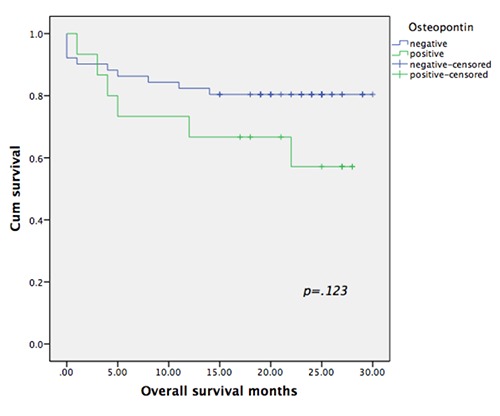
Survival curves of patients grouped according to the OPN expression.
Table 1.
Correlation of osteopontin expression with clinical-pathological characteristics.
| Characteristics | Total (%) | Positive (%) | Negative (%) | P-value |
|---|---|---|---|---|
| Age | 0.152 | |||
| <60y | 35 (43.8) | 6 (7.5) | 29 (36.3) | |
| >60y | 45 (56.2) | 14 (17.5) | 31 (31.7) | |
| ECOG PS | 0.199 | |||
| 0,1 | 57 (71.3) | 12(15) | 45 (56.3) | |
| >/=2 | 23 (28.7) | 8(10) | 15 (18.7) | |
| Ann Arbor stage | 0.330 | |||
| I,II | 25 (31.3) | 8(10) | 17 (21.3) | |
| III, IV | 55 (68.7) | 12(15) | 43 (53.7) | |
| LDH | 0.691 | |||
| Normal | 31 (38.7) | 7 (8.7) | 24(30) | |
| Increased | 49 (61.3) | 13 (16.3) | 36(45) | |
| Extranodal disease | 0.424 | |||
| Negative | 30 (37.5) | 6 (7.5) | 24(30) | |
| Positive | 50 (62.5) | 14 (17.5) | 36(45) | |
| Cell origin | 0.040 | |||
| Germinal center | 42 (59.2) | 8 (11.3) | 34 (47.9) | |
| Non-germinal center | 29 (40.8) | 12 (16.9) | 17 (23.9) |
Table 2.
Survival comparison between groups with positive and negative OPN expression.
| OPN | Mean (CI 95%) | Median (CI 95%) | P-value |
|---|---|---|---|
| Negative | 24.961 (22.098-27.823) | Not reached | 0.123 |
| Positive | 19.762 (14.269-25.255) | Not reached |
Table 3.
Univariate and multivariate Cox regression analysis prognostic factors for OS.
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| Hazard ratio (95% IC) | P-value | Hazard ratio (95% IC) | P-value | |
| Age>60y | 2.204 (0.765-6.345) | 0.143 | 3.275 (0.906-11.840) | 0.070 |
| ECOG>/=2 | 3.739 (1.385-10.096) | 0.009 | 2.081 (0.610-7.094) | |
| Ann Arbor stage III, IV | 3.364 (0.764-14.814) | 0.109 | 2.966 (0.572-15.378) | 0.195 |
| LDH increased | 2.730 (0.880-8.473) | 0.082 | 1.763 (0.465-6.677) | 0.404 |
| Extranodal disease | 2.338 (0.753-7.255) | 0.142 | 1.449 (0.422-4.969) | 0.555 |
| Non-germinal center | 1.307 (0.458-3.727) | 0.617 | 1.277 (0.370-4.408) | 0.699 |
| Osteopontin expression | 2.161 (0.785-5.952) | 0.136 | 2.337 (0.677-8.064) | 0.179 |
In other types of neoplasms, the identification of the different OPN isoforms has allowed to relate some of them to the prognosis, an example could be breast cancer, since the identification of the mRNA of the C isoform in tumor cells is associated to a poor prognosis, recurrence and decrease in disease-free survival, on the other hand, there is a decrease in the mRNA of isoform A as it increases the clinical stage by TMN; another example would be pancreatic cancer, since mRNA detection of isoform C is associated with metastatic disease while mRNA of isoform B is associated with decreased overall survival.18
In conclusion, the expression of osteopontin in DLBCL is relatively frequent, in our series it was observed in 28% of the cases, with a slight predominance in those cases of non-germinal center origin. Although it is not possible to establish a clear relationship between the expression by immunohistochemistry of osteopontin and a poor prognosis it would be important to complement the analysis with other techniques as PCR or NGS that allow us to assess the influence of the isoforms and post-translational modifications of OPN on the biological behavior of DLBCL.
Acknowledgments
We are grateful to Biologist Victor Hugo Olivera Rodríguez from Molecular Pathology and Immunohistochemistry Laboratory for her technical support in the construct of DLBCL tissue microarray.
To the Fundacion Carolina for the grant granted to GBL for the Pediatric Oncology and Adult Oncology research program BBVA.
Funding Statement
Funding: none.
References
- 1.Sehn LH, Gascoyne RD. Diffuse large B-cell lymphoma: optimizing outcome in the context of clinical and biologic heterogeneity. Blood 2015;125:22-32. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Z, Sehn LH, Rademaker AW, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 2015;123:837-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiefer MC, Bauer DM, Barr PJ. The cDNA and derived amino acid sequence for human osteopontin. Nucleic Acids Res 1989;17:3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rangaswami H, Bulbule A, Kundu GC. Osteopontin: Role in cell signaling and cancer progression. Trends Cell Biol 2006;16:79-87. [DOI] [PubMed] [Google Scholar]
- 5.Young MF, Kerr JM, Termine JD, et al. cDNA cloning, mRNA distribution and heterogeneity, chromosomal location, and RFLP analysis of human osteopontin (OPN). Genomics 1990;7:491-502. [DOI] [PubMed] [Google Scholar]
- 6.Wang KX, Denhardt DT. Osteopontin: Role in immune regulation and stress responses. Cytokine Growth Factor Rev 2008;19:333-45. [DOI] [PubMed] [Google Scholar]
- 7.Kahles F, Findeisen HM, Bruemmer D. Osteopontin: A novel regulator at the cross roads of inflammation, obesity and diabetes. Mol Metab 2014;3:384-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gimba ER, Tilli TM. Human osteopontin splicing isoforms: Known roles, potential clinical applications and activated signaling pathways. Cancer Lett 2013;331:11-7. [DOI] [PubMed] [Google Scholar]
- 9.Anborgh PH, Mutrie JC, Tuck AB, Chambers AF. Pre- and post-translational regulation of osteopontin in cancer. J Cell Commun Signal 2011;5:111-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue M, Shinohara ML. Intracellular osteopontin (iOPN) and immunity. Immunol Res 2011;49:160-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed M, Behera R, Chakraborty G, et al. Osteopontin: a potentially important therapeutic target in cancer. Expert Opin Ther Target 2011;15:1113-26. [DOI] [PubMed] [Google Scholar]
- 12.Lin J, Myers AL, Wang Z, et al. Osteopontin (OPN/SPP1) isoforms collectively enhance tumor cell invasion and dissemination in esophageal adenocarcinoma. Oncotarget 2015;6:22239-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chagan-Yasutan H, Tsukasaki K, Takahashi Y, et al. Involvement of osteopontin and its signaling molecule CD44 in clinicopathological features of adult T cell leukemia. Leuk Res 2011;35:1484-90. [DOI] [PubMed] [Google Scholar]
- 14.Shevde LA, Samant RS. Role of osteopontin in the pathophysiology of cancer. Matrix Biol 2014;37:131-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee CY, Tien HF, Hou HA, et al. Marrow osteopontin level as a prognostic factor in acute myeloid leukaemia. Br J Haematol 2008;141:736-9. [DOI] [PubMed] [Google Scholar]
- 16.Weber GF, Lett GS, Haubein NC. Osteopontin is a marker for cancer aggressiveness and patient survival. Br J Cancer 2010;103:861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minarik J, Pika T, Bacovsky J, et al. Prognostic Value of Hepatocyte Growth Factor, Syndecan-1, and Osteopontin in Multiple Myeloma and Monoclonal Gammopathy of Undetermined Significance. Sci World J 2012;2012: 356128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Briones MA, Avendaño SE, Aparicio DI, et al. Osteopontin splice variants and polymorphisms in cancer progression and prognosis. Biochim Biophys 2017;1868:93-108. [DOI] [PubMed] [Google Scholar]
- 19.Liersch R, Gerss J, Schliemann C, et al. Osteopontin is a prognostic factor for survival of acute myeloid leukemia patients. Blood 2012;119:5215-20. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Sun B, Pei B, et al. Osteopontin- Expressing Macrophages in Non-Small Cell Lung Cancer Predict Survival. Ann Thorac Surg 2015;99:1140-8. [DOI] [PubMed] [Google Scholar]
- 21.Zhang T, Zhang D, Zhao D, et al. Osteopontin expression is associated with platinum-based chemotherapy response and prognosis of patients with advanced non small cell lung cancer. J Buon 2014;19:742-8. [PubMed] [Google Scholar]
- 22.Rodrigues LR, Teixeira J, Schmitt FL, et al. The role of osteopontin in tumor progression and metastasis in breast cancer. Cancer Epidemiol Biomarkers Prev 2007;16:1087-97. [DOI] [PubMed] [Google Scholar]
- 23.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Neoplasia 2004;103:275-82. [DOI] [PubMed] [Google Scholar]
- 24.Shijubu N, Uede T, Kon S, et al. Vascular Endothelial Growth Factor and Osteopontin in Stage I Lung Adenocarcinoma. Am J Respir Crit Care Med 1999;160:1269-73. [DOI] [PubMed] [Google Scholar]
- 25.Starr JS, Jiang L, Li Z, et al. CD47 and osteopontin expression in diffuse large B-cell lymphoma with nodal and intravascular involvement. Clin Lymphoma Myeloma Leuk 2013;13: 597-601. [DOI] [PubMed] [Google Scholar]
- 26.Jiang L, Marlow LA, Cooper SJ, et al. Selective central nervous system tropism of primary central nervous system lymphoma. Int J Clin Exp Pathol 2010; 3:763-7. [PMC free article] [PubMed] [Google Scholar]
- 27.Pamuk GE, Uyanik MS, Pamuk ON, et al. Decreased dickkopf-1 levels in chronic lymphocytic leukemia and increased osteopontin levels in non- Hodgkin’s lymphoma at initial diagnosis: Could they be playing roles in pathogenesis? Hematology 2015;20: 267-71. [DOI] [PubMed] [Google Scholar]
- 28.Yuan J, Gu K, He J, Sharma S. Preferential up-regulation of osteopontin in primary central nervous system lymphoma does not correlate with putative receptor CD44v6 or CD44H expression. Hum Pathol 2013;44:606-11. [DOI] [PubMed] [Google Scholar]
- 29.Yushi Q, Li Z, Von Roemeling CA, et al. Osteopontin is a multi-faceted protumorigenic driver for central nervous system lymphoma. Oncotarget 2016;7: 32156-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strehlow F, Bauer S, Martus P, et al. Osteopontin in cerebrospinal fluid as diagnostic biomarker for central nervous system lymphoma. J Neurooncol 2016;129:165-71. [DOI] [PubMed] [Google Scholar]


