Abstract
Background
Randomized controlled trials (RCTs) have yielded conflicting results regarding the ability of beta‐blockers to influence perioperative cardiovascular morbidity and mortality. Thus routine prescription of these drugs in an unselected population remains a controversial issue. A previous version of this review assessing the effectiveness of perioperative beta‐blockers in cardiac and non‐cardiac surgery was last published in 2018. The previous review has now been split into two reviews according to type of surgery. This is an update, and assesses the evidence in non‐cardiac surgery only.
Objectives
To assess the effectiveness of perioperatively administered beta‐blockers for the prevention of surgery‐related mortality and morbidity in adults undergoing non‐cardiac surgery.
Search methods
We searched CENTRAL, MEDLINE, Embase, CINAHL, Biosis Previews and Conference Proceedings Citation Index‐Science on 28 June 2019. We searched clinical trials registers and grey literature, and conducted backward‐ and forward‐citation searching of relevant articles.
Selection criteria
We included RCTs and quasi‐randomized studies comparing beta‐blockers with a control (placebo or standard care) administered during the perioperative period to adults undergoing non‐cardiac surgery. If studies included surgery with different types of anaesthesia, we included them if 70% participants, or at least 100 participants, received general anaesthesia. We excluded studies in which all participants in the standard care control group were given a pharmacological agent that was not given to participants in the intervention group, studies in which all participants in the control group were given a beta‐blocker, and studies in which beta‐blockers were given with an additional agent (e.g. magnesium). We excluded studies that did not measure or report review outcomes.
Data collection and analysis
Two review authors independently assessed studies for inclusion, extracted data, and assessed risks of bias. We assessed the certainty of evidence with GRADE.
Main results
We included 83 RCTs with 14,967 participants; we found no quasi‐randomized studies. All participants were undergoing non‐cardiac surgery, and types of surgery ranged from low to high risk. Types of beta‐blockers were: propranolol, metoprolol, esmolol, landiolol, nadolol, atenolol, labetalol, oxprenolol, and pindolol. In nine studies, beta‐blockers were titrated according to heart rate or blood pressure. Duration of administration varied between studies, as did the time at which drugs were administered; in most studies, it was intraoperatively, but in 18 studies it was before surgery, in six postoperatively, one multi‐arm study included groups of different timings, and one study did not report timing of drug administration. Overall, we found that more than half of the studies did not sufficiently report methods used for randomization. All studies in which the control was standard care were at high risk of performance bias because of the open‐label study design. Only two studies were prospectively registered with clinical trials registers, which limited the assessment of reporting bias. In six studies, participants in the control group were given beta‐blockers as rescue therapy during the study period.
The evidence for all‐cause mortality at 30 days was uncertain; based on the risk of death in the control group of 25 per 1000, the effect with beta‐blockers was between two fewer and 13 more per 1000 (risk ratio (RR) 1.17, 95% confidence interval (CI) 0.89 to 1.54; 16 studies, 11,446 participants; low‐certainty evidence). Beta‐blockers may reduce the incidence of myocardial infarction by 13 fewer incidences per 1000 (RR 0.72, 95% CI 0.60 to 0.87; 12 studies, 10,520 participants; low‐certainty evidence). We found no evidence of a difference in cerebrovascular events (RR 1.65, 95% CI 0.97 to 2.81; 6 studies, 9460 participants; low‐certainty evidence), or in ventricular arrhythmias (RR 0.72, 95% CI 0.35 to 1.47; 5 studies, 476 participants; very low‐certainty evidence). Beta‐blockers may reduce atrial fibrillation or flutter by 26 fewer incidences per 1000 (RR 0.41, 95% CI 0.21 to 0.79; 9 studies, 9080 participants; low‐certainty evidence). However, beta‐blockers may increase bradycardia by 55 more incidences per 1000 (RR 2.49, 95% CI 1.74 to 3.56; 49 studies, 12,239 participants; low‐certainty evidence), and hypotension by 44 more per 1000 (RR 1.40, 95% CI 1.29 to 1.51; 49 studies, 12,304 participants; moderate‐certainty evidence).
We downgraded the certainty of the evidence owing to study limitations; some studies had high risks of bias, and the effects were sometimes altered when we excluded studies with a standard care control group (including only placebo‐controlled trials showed an increase in early mortality and cerebrovascular events with beta‐blockers). We also downgraded for inconsistency; one large, well‐conducted, international study found a reduction in myocardial infarction, and an increase in cerebrovascular events and all‐cause mortality, when beta‐blockers were used, but other studies showed no evidence of a difference. We could not explain the reason for the inconsistency in the evidence for ventricular arrhythmias, and we also downgraded this outcome for imprecision because we found few studies with few participants.
Authors' conclusions
The evidence for early all‐cause mortality with perioperative beta‐blockers was uncertain. We found no evidence of a difference in cerebrovascular events or ventricular arrhythmias, and the certainty of the evidence for these outcomes was low and very low. We found low‐certainty evidence that beta‐blockers may reduce atrial fibrillation and myocardial infarctions. However, beta‐blockers may increase bradycardia (low‐certainty evidence) and probably increase hypotension (moderate‐certainty evidence). Further evidence from large placebo‐controlled trials is likely to increase the certainty of these findings, and we recommend the assessment of impact on quality of life. We found 18 studies awaiting classification; inclusion of these studies in future updates may also increase the certainty of the evidence.
Plain language summary
Beta‐blockers to prevent death or serious events after surgery not involving the heart
This review assessed evidence from randomized controlled trials (RCTs) on whether beta‐blockers reduce deaths or other serious events when given to people undergoing surgery other than heart surgery. The findings for heart surgery are covered in another review.
Background
Surgery increases stress in the body, which responds by releasing the hormones adrenaline and noradrenaline. Stress from surgery can lead to death or other serious events such as heart attacks, stroke, or an irregular heartbeat. For surgery that does not involve the heart, an estimated 8% of people may have injury to their heart around the time of surgery. Beta‐blockers are drugs that block the action of adrenaline and noradrenaline on the heart. Beta‐blockers can slow down the heart, and reduce blood pressure, and this may reduce the risk of serious events. However, beta‐blockers may lead to a very low heart rate or very low blood pressure which could increase the risk of death or a stroke. Prevention of early complications after surgery is important, but using beta‐blockers to prevent these complications is controversial.
Study characteristics
The evidence is current to 28 June 2019. We included 83 RCTs with 14,967 adults who were undergoing different types of surgery other than heart surgery. Eighteen studies are awaiting classification (because we did not have enough details to assess them), and three studies are ongoing. The types of beta‐blockers used in the studies were: propranolol, metoprolol, esmolol, landiolol, nadolol, atenolol, labetalol, oxprenolol, and pindolol. Studies compared these beta‐blockers with either a placebo (disguised to look like a beta‐blocker but containing no medicine) or with standard care.
Key results
Beta‐blockers may make little or no difference to the number of people who die within 30 days of surgery (16 studies, 11,446 participants; low‐certainty evidence), have a stroke (6 studies, 9460 participants; low‐certainty evidence), or experience ventricular arrhythmias (irregular heartbeat rhythms, starting in the main chambers of the heart, that are potentially life‐threatening and may need immediate medical treatment; 5 studies, 476 participants; very low‐certainty evidence). We found that beta‐blockers may reduce atrial fibrillation (an irregular heartbeat, starting in the atrial chambers of the heart, that increases the risk of stroke if untreated; 9 studies, 9080 participants; low certainty‐evidence), and the number of people who have a heart attack (12 studies, 10,520 participants; low‐certainty evidence). However, taking beta‐blockers may increase the number of people who experience a very low heart rate (49 studies, 12,239 participants; low‐certainty evidence), or very low blood pressure (49 studies, 12,304 participants; moderate‐certainty evidence), around the time of surgery.
In a few studies, we also found little or no difference in the number of people who died after 30 days, who died because of a heart problem, or had heart failure. We found no evidence of whether beta‐blockers alter the length of time in hospital.
No studies assessed whether people who were given beta‐blockers had a better quality of life after heart surgery.
Certainty of the evidence
The certainty of the evidence in this review was limited by including some studies that were at high risk of bias, and we noticed that some of our findings were different if we only included placebo‐controlled studies or studies that reported how participants were randomized. We also found one large, well‐conducted, international study that had different findings to the smaller studies. It showed a reduction in heart attacks and an increase in stroke and all‐cause mortality when beta‐blockers were used, whilst the other studies did not show a clear effect. We were also less certain of the findings for outcomes with few studies, such as for ventricular arrhythmias.
Conclusion
Although beta‐blockers may make little or no difference to the number of people who die within 30 days, have a stroke, or have ventricular arrhythmias, they may reduce atrial fibrillation and heart attacks. Taking beta‐blockers may increase the number of people with a very low heart rate or very low blood pressure around the time of surgery. Further evidence from large, placebo‐controlled trials is likely to increase the certainty of these findings, and we recommend the assessment of impact on quality of life.
Summary of findings
Summary of findings for the main comparison. Perioperative beta‐blockers compared to placebo or standard care for preventing surgery‐related mortality and morbidity in adults undergoing non‐cardiac surgery.
| Perioperative beta‐blockers compared to placebo or standard care for preventing mortality and morbidity in adults undergoing non‐cardiac surgery | |||||
| Population: adults undergoing non‐cardiac surgery under general anaesthesia (to include: low‐risk, medium‐risk, and high‐risk surgeries) Setting: hospitals in: Argentina, Australia, Bangladesh, Brazil, Canada, China, Columbia, Cuba, Denmark, Egypt, Equador, El Salvador, Finland, France, Ghana, Greece, Hong Kong, Hungary, India, Italy, Japan, South Korea, Malaysia, Mexico, Nepal, New Zealand, Norway, Peru, Singapore, Spain, Sweden, Switzerland, Thailand, Taiwan, Turkey, UK, USA Intervention: beta‐blockers (to include: propranolol, metoprolol, esmolol, landiolol, nadolol, atenolol, labetalol, oxprenolol, and pindolol) Comparison: placebo or standard care | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with placebo or standard care | Risk with beta‐blockers | ||||
|
Early all‐cause mortality (within 30 days) |
Study population | RR 1.17 (0.89 to 1.54) | 11,446 (16 studies) | ⊕⊕⊝⊝ Lowa |
|
| 25 per 1000 | 29 per 1000 (22 to 38) | ||||
|
Acute myocardial infarction (within 30 days) |
Study population | RR 0.72
(0.60 to 0.87) NNTB: 74 (52 to 160) |
10,520 (12 studies) | ⊕⊕⊝⊝ Lowb |
|
| 48 per 1000 | 35 per 1000 (29 to 42) | ||||
|
Cerebrovascular events (within 30 days) |
Study population | RR 1.65 (0.97 to 2.81) |
9460 (6 studies) | ⊕⊕⊝⊝ Lowc |
|
| 5 per 1000 | 8 per 1000 (5 to 14) | ||||
|
Ventricular arrhythmias (within 30 days) |
Study population | RR 0.72 (0.35 to 1.47) | 476 (5 studies) | ⊕⊝⊝⊝ Very lowd |
|
| 101 per 1000 | 73 per 1000 (35 to 149) | ||||
|
Atrial fibrillation or atrial flutter, or both (within 30 days) |
Study population | RR 0.41
(0.21 to 0.79) NNTB: 39 (29 to 108) |
9080 (9 studies) | ⊕⊕⊝⊝ Lowe |
|
| 44 per 1000 | 18 per 1000 (9 to 35) | ||||
|
Bradycardia (within 30 days; as defined by study authors, minimum heart rate < 60 beats per minute or requiring medication) |
Study population | RR 2.49
(1.74 to 3.56) NNTH: 18 (11 to 37) |
12,239 (49 studies) | ⊕⊕⊝⊝ Lowf |
|
| 37 per 1000 | 92 per 1000 (65 to 132) | ||||
|
Hypotension (within 30 days; as defined by study authors, minimum systolic blood pressure < 90 mmHg or requiring medication) |
Study population | RR 1.40
(1.29 to 1.51) NNTH: 23 (18 to 31) |
12,304 (49 studies) | ⊕⊕⊕⊝ Moderateg |
|
| 110 per 1000 | 154 per 1000 (142 to 166) | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; NNTB: number needed to treat for an additional beneficial outcome; NNTH: number needed to treat for an additional harmful outcome | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aWe downgraded by two levels: one for study limitations because we assessed 10 studies to be at high risk of bias in at least one domain, and the effect estimate was not robust when we excluded studies of poorer methodological standards in sensitivity analysis; and one for inconsistency because we noted that one large study demonstrated an increase in mortality that was not replicated in the remaining smaller studies. bWe downgraded by two levels: one level for study limitations because we assessed eight studies to be at high risk of bias in at least one domain; and one level for inconsistency because we noted that one large study demonstrated a protective effect that was not replicated in the remaining smaller studies. cWe downgraded by two levels. We downgraded by one level for inconsistency because we noted differences in the effect between studies; in particular, one large study demonstrated an increase in cerebrovascular events that was not replicated in the remaining smaller studies. We also downgraded by one level for study limitations; the effect estimate was not robust when we excluded studies with poorer methodological standards in sensitivity analysis. dWe downgraded by three levels: one level for imprecision because the evidence was from too few participants and few single centre studies, one level for inconsistency because we noted a moderate level of statistical heterogeneity that we were unable to explain, and one level for study limitations because we judged several studies to be at high or unclear risk of bias. eWe downgraded by two levels: one level owing to inconsistency because we were unable to effectively assess or explain the substantial statistical heterogeneity that we noted for this outcome, and one level for study limitations because the effect estimate was not robust when we excluded studies with poorer methodological standards in sensitivity analysis. fWe downgraded by two levels: one level for study limitations because we assessed several studies to be at high or unclear risk of bias, and one level for inconsistency owing to substantial statistical heterogeneity that we were unable to explain from subgroup analyses. gWe downgraded by one level for study limitations because we assessed several studies to be at high or unclear risk of bias.
Background
Description of the condition
Cardiovascular mortality and morbidity are prevalent and costly in people undergoing cardiac and non‐cardiac surgery. Worldwide, major perioperative complications are responsible for a third of deaths during the perioperative period (Devereaux 2015). Complications include myocardial infarction and myocardial injury (Sellers 2018), and in non‐cardiac surgery, up to 8% of patients may show signs of myocardial injury during the perioperative period (VISION 2014). Postoperative arrhythmias, such as atrial fibrillation, occur in 3% of people (Sellers 2018). Prevention of postoperative complications remains a major issue (Jørgensen 2018).
Description of the intervention
There are various pharmacological agents that may be used to protect people undergoing surgery against adverse cardiovascular events (Lewis 2018), and alpha‐2 adrenergic agents have also been evaluated for their use perioperatively (Duncan 2018). In addition, the choice of anaesthetic drugs and techniques can also affect cardiovascular outcomes (Hristovska 2017).
This review, however, evaluates the effectiveness of beta‐adrenoceptor blocking agents, or beta‐blockers, for this purpose. These pharmacological agents block the actions of the stress hormones epinephrine (adrenaline) and norepinephrine (noradrenaline). They are typically used to manage abnormal heart rhythms, heart failure, coronary heart disease, and their effectiveness has been assessed for hypertension (Wiysonge 2017) and for secondary prevention of stroke (De Lima 2014).
How the intervention might work
Cardiac adverse events appear to be related to the persistently exaggerated sympathetic response that is associated with substantial increases in heart rate and myocardial oxygen consumption. Drugs that block beta‐adrenergic receptors, and thus the sympathetic response, are capable of preventing cardiac complications in people with acute myocardial infarction, silent ischaemia and heart failure (Jørgensen 2018). There is thus a pharmacological rationale to support the use of beta‐blockers in the perioperative period (Oprea 2019).
Why it is important to do this review
Although several trials have provided encouraging findings demonstrating a reduced perioperative incidence of death from cardiac causes and non‐fatal cardiovascular complications (Mangano 1990; Wallace 1998), the routine administration of beta‐blocking agents in an unselected population before major surgery is still discussed as a controversial topic, especially after the results of the POISE trial were published (POISE 2008), which demonstrated an increase of all‐cause mortality and stroke with the use of beta‐blockers. Until recently, recommendations were largely based on the results of four randomized trials (DECREASE‐IV 2009; POISE 2008; Poldermans 1999; Wallace 1998). After the DECREASE (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echo) trial family, which showed beneficial effects of beta‐blockers on mortality and on prevention of myocardial infarction in the perioperative setting, was discredited in 2011 (Bouri 2014; Chopra 2012), perioperative use of beta‐blockers became once again a matter of controversy.
The previous version of this review, which assessed the effectiveness of beta‐blockers in both cardiac and non‐cardiac surgery, excluded one retracted study but did not include an updated search (Blessberger 2018). The previous version has now been split into two reviews according to type of surgery. This is an update, and incorporates new evidence in non‐cardiac surgery only. Evidence for cardiac surgery is reported in (Blessberger 2019).
Objectives
To assess the effectiveness of perioperatively administered beta‐blockers for the prevention of surgery‐related mortality and morbidity in adults undergoing non‐cardiac surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) and quasi‐randomized studies in which investigators used methods to allocate participants to groups such as hospital record number or date of birth.
Types of participants
We included studies that assessed the effects of beta‐blockers on adults who were 18 years of age or older and who were undergoing non‐cardiac surgery under general anaesthesia. If studies included surgery under different types of anaesthesia, we included the study if more than 100 randomly assigned participants received general anaesthesia, or if more than 70% of participants received general anaesthesia.
We excluded trials investigating procedures that required local or regional anaesthesia only.
Types of interventions
We included studies in which beta‐adrenoceptor‐blockers (beta‐blockers) were administered during the perioperative period; we defined the perioperative period as 30 days before surgery to 30 days after surgery. Beta‐blockers could be started before surgery, during surgery or at the latest by the end of the first day after surgery. Beta‐blockers were given intravenously, orally or via a feeding tube, and were compared with a control (placebo or standard care). We excluded studies in which all participants in the standard care control group were given a pharmacological agent that was not given to participants in the intervention group; similarly, we excluded studies in which all participants in the control group were given a beta‐blocker.
We excluded studies (or intervention groups within a multi‐arm study) in which the beta‐blocker was given with a supplementary agent (e.g. magnesium) unless the agent was given in both groups as part of standard care management.
Types of outcome measures
We excluded studies that did not measure review outcomes (see Differences between protocol and review). Except for long‐term all‐cause mortality, length of hospital stay, and quality of life, we aimed to collect outcome data that were measured within 30 days postoperatively or before hospital discharge (whichever occurred later). We removed some outcomes in this update (see Differences between protocol and review).
Primary outcomes
Early all‐cause mortality
Secondary outcomes
Long‐term all‐cause mortality, occurring later than 30 days postoperatively
Death due to cardiac causes
Acute myocardial infarction, as defined by study authors. We included only non‐fatal myocardial infarctions if a distinction was possible.
Cerebrovascular events: transient ischaemic attack, prolonged reversible ischaemic neurological deficit, or stroke, as defined by study authors. We included only non‐fatal cerebrovascular events if a distinction was possible.
Ventricular arrhythmias: ventricular tachycardias and ventricular fibrillation
Atrial fibrillation or atrial flutter (or both)
Bradycardia, as defined by study authors (minimum criteria: below 60 beats per minute or requiring medical intervention)
Hypotension, as defined by study authors (minimum criteria: below 90 mmHg systolic blood pressure or requiring medical intervention)
Congestive heart failure, as defined by study authors
Length of hospital stay
Quality of life, as defined by study authors
Search methods for identification of studies
Electronic searches
We identified RCTs through literature searching with systematic and sensitive search strategies, as outlined in Chapter 6.4 of theCochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). We applied no restrictions to language or publication status. We searched the following databases for relevant trials:
Cochrane Central Register of Controlled Trials (CENTRAL; 2019; Issue 6) via the Cochrane Library (searched on 28 June 2019);
MEDLINE (Ovid SP; 1946 to 28 June 2019);
Embase (Ovid SP; 1974 to 28 June 2019);
CINAHL (EBSCOhost: 1981 to 28 June 2019)
Biosis Previews (1969 to 28 June 2019)
Web of Science (SCI‐EXPANDED; 1900 to 28 June 2019)
Conference Proceedings Citation Index‐Science (CPCI‐S; 1990 to 28 June 2019)
In consultation with the Information Specialist for Cochrane Anaesthesia, we developed a subject‐specific and sensitive search strategy in MEDLINE and other listed databases. We subtracted the previous review's search results (up to and including 2012) from the new search. Search strategies can be found in: Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7.
We scanned the following clinical trials registers for ongoing and unpublished trials:
World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp/en/) on 22 March 2019;
ClinicalTrials.gov (www.clinicaltrials.gov) on 22 March 2019.
Searching other resources
We carried out forward‐citation searching of identified included studies published since 2012 in Web of Science on 22 March 2019 (apps.webofknowledge.com). We conducted a search of grey literature using Opengrey on 5 April 2019 (www.opengrey.eu/). In addition, we scanned reference lists of relevant systematic reviews published since 2015.
Data collection and analysis
Two review authors (SL, and LF or MP) independently selected studies and extracted data from new included studies. We compared decisions at each stage. In cases of disagreement, we reassessed the respective studies to reach consensus, and if necessary included a third review author (HB) for resolution.
Selection of studies
We used reference management software to collate the results of searches and to remove duplicates (Endnote). We used Covidence 2019 software to screen results of the search of titles and abstracts and to identify potentially relevant studies. We sourced the full texts of all potentially relevant studies and considered whether they met the inclusion criteria (see Criteria for considering studies for this review). We reviewed abstracts at this stage and included them in the review only if they provided sufficient information and relevant results that included denominator figures for the intervention and control groups. We recorded the number of papers retrieved at each stage and report this information using a PRISMA flow chart (see Figure 1). We report in the review brief details of closely related but excluded papers.
Data extraction and management
We used a data extraction form to collect information and outcome data from studies (Appendix 8). We collected the following information.
Methods: type of study design, setting, dates of study, funding sources and study author declarations of interest
Participants: number randomized to each group; number of losses; number analysed in each group and whether intention‐to‐treat analysis was used; baseline characteristics (age, gender, American Society of Anesthesiologists (ASA) grade of other measure of health status, type of surgery, history of coronary heart disease, myocardial infarction, hypertension, reduced ejection fraction, chronic obstructive pulmonary disease, preoperative use of beta‐blockers).
Intervention: details of beta‐blocker (type; dose; time; duration; route of administration; goal‐directed or fixed‐dose), and details of control (placebo or standard care)
Outcomes: data for all reported review outcomes, including study author definitions, measurement tools, and time points.
We considered the applicability of information from individual studies and generalizability of the data to our intended study population (i.e. the potential for indirectness in our review).
In multi‐arm studies, we did not collect data on intervention agents that were not eligible for inclusion in the review.
Assessment of risk of bias in included studies
We assessed study quality, study limitations, and the extent of potential bias using the Cochrane 'Risk of bias' tool (Higgins 2017). We considered the following domains.
Sequence generation (selection bias);
Allocation concealment (selection bias);
Blinding of participants, personnel, and outcomes assessors (performance and detection bias);
Incomplete outcome data (attrition bias);
Selective outcome reporting (reporting bias);
Other potential risks of bias.
For each domain, two review authors (SRL, and MP or LF) judged whether study authors made sufficient attempts to minimize bias in their study design. We made judgements using three measures ‐ high, low, or unclear risk of bias. We recorded this in 'Risk of bias' tables and present a 'Risk of bias' graph and a summary 'Risk of bias' figure (see Figure 2 and Figure 3).
For other potential risks of bias, we considered the effect of beta‐blockers given as 'rescue therapy' to treat specified conditions. We judged studies to have a high risk of other bias if administration of such 'rescue therapy' had the potential to influence outcome data.
Measures of treatment effect
We collected dichotomous data for mortality outcomes, acute myocardial infarction, cerebrovascular events, ventricular arrhythmias, atrial fibrillation and atrial flutter, bradycardia, hypotension, and congestive heart failure. We collected continuous data for length of hospital stay.
We report dichotomous data as risk ratios (RRs) to compare groups, and continuous data as mean differences (MDs). We report 95% confidence intervals (CI).
Unit of analysis issues
For multi‐arm studies, which included different types of beta‐blockers or different doses of beta‐blockers, we combined dichotomous data to create a single beta‐blocker group, and we used these composite data in the primary analysis. During subgroup analysis by type of beta‐blocker, we included data separately for each type of beta‐blocker, and used the 'halving method' with data in the control group to avoid a unit of analysis error (Deeks 2017).
If multi‐arm studies had included continuous data for length of stay, we planned to calculate combined mean and standard deviation values according to the formula provided in Chapter 7.7.3.8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
If information on both study group allocation and respective outcomes was available, we re‐included withdrawn participants in keeping with the intention‐to‐treat principle. If information was not available, we performed an available case analysis. We did not perform imputation techniques.
We excluded continuous data that assessed length of stay, if a range of dispersion (standard deviation or standard error) was not provided along with mean values. When both measures of spread (standard deviation and standard error) were presented, we used the standard deviation as the measure of choice. We did not apply imputation techniques.
We attempted contact with some study authors for additional information; we report this information in the Notes section of the Characteristics of included studies.
Assessment of heterogeneity
We assessed whether evidence of inconsistency was apparent in our results by considering heterogeneity. We assessed clinical and methodological heterogeneity by comparing similarities in our included studies between study designs, participants, interventions, and outcomes, and used the data collected from the full‐text reports (as stated in Data collection and analysis). We explored clinical and methodological heterogeneity through subgroup analysis. We assessed statistical heterogeneity by calculating the ChI2 test or I2 statistic (Higgins 2003), and judged any heterogeneity above an I2 statistic value of 40% and a Chi2 P value of 0.05 or less to indicate moderate to substantial statistical heterogeneity (Deeks 2017). We did not conduct meta‐regression to explore heterogeneity in this updated review (see Differences between protocol and review).
As well as looking at statistical results, we considered point estimates and overlap of CIs. If CIs overlap, then results are more consistent. However, combined studies may show a large consistent effect but with significant heterogeneity. We, therefore, planned to interpret heterogeneity with caution (Guyatt 2011a).
Assessment of reporting biases
We attempted to source published protocols for each of our included studies by using clinical trials registers. We planned to compare published protocols with published study results, to assess the risk of selective reporting bias. In addition, we appraised reporting bias through visual assessment of funnel plots (Egger 1997). We only included figures of funnel plots in the review in which we identified possible reporting bias based on visual assessment.
Data synthesis
We presented a statistical summary of treatment effects in the absence of significant clinical or methodological heterogeneity. We used the statistical calculator in Review Manager 5 (RevMan 5) to perform meta‐analysis (Review Manager 2014).
For dichotomous outcomes, we used the Mantel‐Haenszel random‐effects model to account for potential variability in participant conditions between studies (Borenstein 2010). For continuous outcomes, we used inverse variance using a random‐effects model.
We calculated CIs at 95%, and used a P value of 0.05 or less to judge whether a result was statistically significant; for statistically significant results, we also reported the number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH). We considered imprecision in the results of analyses by assessing the CI around effect measures; a wide CI would suggest a higher level of imprecision in our results. A small number of studies may also reduce precision (Guyatt 2011b).
Subgroup analysis and investigation of heterogeneity
In subgroup analysis, we evaluated the following factors that may influence the results.
The effect of the start of beta‐blocker therapy (i.e. before surgery, during surgery, or after surgery)
The type of beta‐blocking agent
The degree of surgical risk (low‐ and medium‐risk procedures versus high‐risk procedures). We determined degree of risk using Kristensen 2014 as a guide.
In multi‐arm studies of more than one type of beta‐blocking agent, we compared each type of beta‐blocker using the 'halving method' for the control group data (Higgins 2011b); thus, we avoided a unit of analysis error.
We planned to complete subgroup analysis in which we found more than 10 studies (Deeks 2017), for the following outcomes.
Early all‐cause mortality
Acute myocardial infarction
Cerebrovascular events
Ventricular arrhythmias
Atrial fibrillation or atrial flutter, or both
Bradycardia
Hypotension
Sensitivity analysis
We explored the potential effect of decisions made as part of the review process. In each sensitivity analysis, we compared the effect estimate with the main analysis. We reported these effect estimates only if they indicated a difference in interpretation of the effect. We performed the following sensitivity analyses.
We excluded studies in which the control group was standard care rather than placebo.
We excluded studies that we judged at high or unclear risk of selection bias.
We excluded studies that we judged to have high risk of attrition bias because of missing data which were a loss of more than 10% participants, were unbalanced between groups, or which were unexplained.
In addition to sensitivity analyses reported in an earlier version of the review (Blessberger 2018), we also used sensitivity analysis to explore the potential effect of studies in which the control group were given beta‐blockers as a 'rescue therapy'. The review included several studies published before 2000, in which clinical management may differ from current standards. We, therefore, made a post‐hoc decision to explore the potential effect of these early studies on the outcomes; in sensitivity analysis, we excluded studies published before 2000.
We calculated RRs using a random‐effects model for all analyses in the review. Although the random‐effects model accounted for potential variation in the population, this statistical tool did not account for outcomes with rare events. In sensitivity analysis, we evaluated the effect of outcomes with events fewer than 1% using Peto odds ratio (Higgins 2011).
We assessed the effect of these potential biases on the following outcomes.
Early all‐cause mortality
Acute myocardial infarction
Cerebrovascular events
Ventricular arrhythmias
Atrial fibrillation or flutter, or both
Bradycardia
Hypotension
'Summary of findings' table and GRADE
One review author (SL) used the GRADE system to assess the certainty of the body of evidence and construct a 'Summary of findings' table associated with the following outcomes (Guyatt 2008).
Early all‐cause mortality
Acute myocardial infarction
Cerebrovascular events
Ventricular arrhythmias
Atrial fibrillation or atrial flutter, or both
Bradycardia
Hypotension
The GRADE approach appraises the certainty of a body of evidence based on the extent to which we can be confident that an estimate of effect or association reflects the item being assessed. Evaluation of the certainty of a body of evidence considers within‐study risk of bias, directness of the evidence, heterogeneity of the data, precision of the effect estimates, and risk of publication bias. We constructed a 'Summary of findings' table using GRADEpro GDT software (gradepro.org).
We used the GRADE approach to appraise the certainty of the body of evidence for the remaining outcomes, but we did not construct a 'Summary of findings' table for these outcomes.
Results
Description of studies
Results of the search
After the removal of duplicates from the search results, we screened 5972 titles and abstracts for this update, which included forward‐ and backward‐citation searches, clinical trials registers and grey literature. We sourced 312 full‐text reports to assess eligibility. See Figure 1.
1.
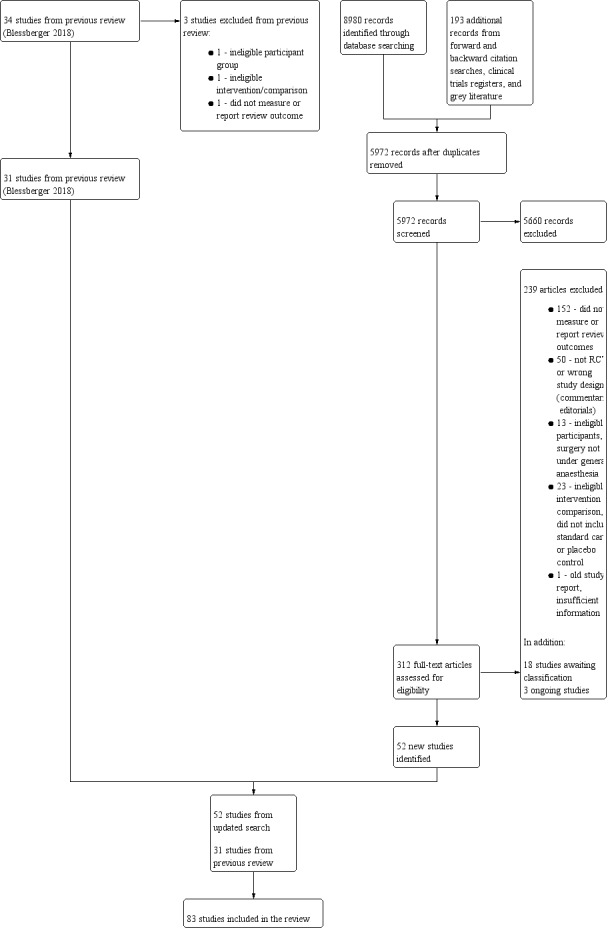
Study flow diagram for updated search on 28 June 2019
Included studies
See Characteristics of included studies.
We included 83 RCTs with 14,967 participants (Ali 2015; Alkaya 2014; Aoyama 2016; Apipan 2010; Bayliff 1999; Bhattacharjee 2016; Burns 1988; Ceker 2015; Chung 1992; Cucchiara 1986; DIPOM 2006; Do 2012; El‐Shmaa 2016; Gibson 1988; Goyagi 2005; Gupta 2011; Harasawa 2006; Helfman 1991; Horikoshi 2017; Inada 1989; Inoue 2010; Jakobsen 1986; Jakobsen 1992; Jakobsen 1997; Jangra 2016; Kao 2017; Kawaguchi 2010; Kindler 1996; Lai 2006; Lee 1994; Lee 2010; Lee 2015; Lee 2017; Lim 2000; Liu 1986; Liu 2006; Louizos 2007; Magnusson 1986; Mallon 1990; Meftahuzzaman 2014; Menigaux 2002; Mikawa 1991; Miller 1990; Miller 1991; Miyazaki 2009; Moon 2011; Neary 2006; Ohri 1999; Ojima 2017; Oxorn 1990; Park 2009; POBBLE 2005; POISE 2008; PRESAGE 2016; Raby 1999; Safwat 1984; Shailaja 2013; Sharma 1996; Sharma 2018; Shrestha 2011; Shukla 2010; Singh 1995; Singh 2010; Singh 2012; Srivastava 2015; Stone 1988; Sugiura 2007; Tendulkar 2017; Ugur 2007; Unal 2008; Urias 2016; Van den Berg 1998; Verma 2018; Wajima 2011; Wallace 1998; Ward‐Booth 1983; White 2003; Whitehead 1980; Yamazaki 2005; Yang 2006; Yang 2008; Yoshida 2017; Zaugg 1999). We found no quasi‐randomized studies. We included one study for which we could only source the abstract and this limited the details of study characteristics that we were able to extract (Urias 2016). We sourced the full text of all remaining studies.
This review included 52 new non‐cardiac surgery studies (Ali 2015; Alkaya 2014; Aoyama 2016; Bhattacharjee 2016; Ceker 2015; Chung 1992; Do 2012; El‐Shmaa 2016; Goyagi 2005; Harasawa 2006; Helfman 1991; Horikoshi 2017; Inoue 2010; Jakobsen 1986; Jangra 2016; Kao 2017; Kindler 1996; Lee 1994; Lee 2015; Lee 2017; Lim 2000; Louizos 2007; Mallon 1990; Meftahuzzaman 2014; Menigaux 2002; Mikawa 1991; Miyazaki 2009; Ohri 1999; Ojima 2017; Park 2009; PRESAGE 2016; Safwat 1984; Shailaja 2013; Sharma 1996; Sharma 2018; Shrestha 2011; Singh 1995; Singh 2010; Singh 2012; Srivastava 2015; Sugiura 2007; Tendulkar 2017; Ugur 2007; Unal 2008; Urias 2016; Van den Berg 1998; Verma 2018; Wajima 2011; Ward‐Booth 1983; White 2003; Yamazaki 2005; Yoshida 2017). The remaining studies were previously included in Blessberger 2018.
Study population
Participants were scheduled for the surgery, which we categorized as high risk, medium risk, and low risk (Kristensen 2014). We categorized studies in which study authors did not specify types of surgery as medium risk.
Twenty‐two studies included high‐risk surgeries which were as follows.
Thoracic surgery: segmentectomy, lobectomy, or pneumonectomy (Aoyama 2016); pneumectomy, lobectomy, oesophagectomy (Bayliff 1999); oesophagectomy (Horikoshi 2017; Lai 2006; Ojima 2017; Yoshida 2017); thoracotomy for lung resection (Jakobsen 1997); lung cancer surgery (PRESAGE 2016)
Vascular surgery: infrarenal vascular surgery (POBBLE 2005); aortic aneurysm repair, infrainguinal arterial bypass, carotid endarterectomy (Raby 1999); abdominal aortic surgery, infrainguinal or extra‐anatomical revascularization (Yang 2006)
Neurosurgery (Gibson 1988; Gupta 2011; Kawaguchi 2010; Srivastava 2015); intracranial surgery (Lim 2000); craniotomy (Alkaya 2014)
Burns surgery (Ali 2015)
Mixed: intracranial or maxillofacial surgery (Harasawa 2006); lung resection, oesophagectomy, gastrectomy (Liu 2006); emergency surgery ‐ gastrointestinal resection surgery, major limb amputation, arterial reconstruction, orthopaedic procedures (Neary 2006); major vascular, intra‐abdominal, orthopaedic, neurosurgical, and other (Wallace 1998)
Forty‐eight studies included medium‐risk surgeries, which were as follows.
Vascular surgery: carotid endarterectomy (Cucchiara 1986); peripheral vascular surgery (Miller 1990)
Abdominal surgery: major lower abdominal (Shukla 2010); major abdominal (Yang 2008); laparoscopic cholecystectomy (Bhattacharjee 2016; Urias 2016); cholecystectomy, herniorrhaphy (Magnusson 1986); laparoscopic appendectomy (Lee 2010); lower and upper abdominal (Wajima 2011); non‐specified abdominal (Sharma 1996)
Gynaecological surgery: general gynaecological (Burns 1988; Chung 1992; Inoue 2010; Kindler 1996; Moon 2011; White 2003); hysterectomy (Jakobsen 1992; Oxorn 1990); hysterectomy and gynaecological surgery (Liu 1986);
Lumbar disc surgery (Unal 2008);
Nephrectomy (Verma 2018);
Mixed: abdominal, peripheral, and vascular surgery (Stone 1988); major non‐cardiac ‐ orthopaedic, neurological, vascular, gynaecological, thoracic, intra‐abdominal and other operations (DIPOM 2006); orthopaedic or gynaecological surgery (Miyazaki 2009); vascular, intraperitoneal, orthopaedic and other (POISE 2008); major abdominal surgery, hip replacement, intrathoracic surgery (Zaugg 1999); general to include open thyroid surgery, endoscopic thyroidectomy, or orthopaedic surgery (Lee 2015); cancer (stomach, large intestine, uterine, ovarian) or middle ear infection (myringoplasty and tympanoplasty; Do 2012);
Not specified: Ceker 2015; El‐Shmaa 2016; Goyagi 2005; Helfman 1991; Inada 1989; Lee 1994; Mallon 1990; Meftahuzzaman 2014; Menigaux 2002; Mikawa 1991; Miller 1991; Ohri 1999; Safwat 1984; Shailaja 2013; Sharma 2018; Shrestha 2011; Singh 1995; Singh 2010; Singh 2012; Tendulkar 2017; Ugur 2007; Yamazaki 2005.
Eleven studies included low‐risk surgeries, which were as follows.
Oral and maxillofacial surgery: orthognathic surgery (Apipan 2010); wisdom teeth removal (Whitehead 1980); dental surgery (Lee 2017); oral surgery (Ward‐Booth 1983)
Ear, nose and throat: middle ear or nasal septum surgery (Jakobsen 1986); endoscopic sinus surgery (Jangra 2016); microlaryngeal surgery (Louizos 2007).
Thyroidectomy (Park 2009)
Cataract extraction (Van den Berg 1998)
Minor breast surgery (Kao 2017)
Not specified minor surgery (Sugiura 2007)
All trials included participants under general anaesthesia only, except four studies, which included participants who received other types of anaesthesia (DIPOM 2006; POISE 2008; Raby 1999; Yang 2006); at least 100 participants, or 70% of participants in each group received general anaesthesia. Study authors in these four trials did not report subgroup data according to type of anaesthesia.
We collected data from study reports on additional risk factors for included participants; we used information reported in the baseline characteristics tables and in the study inclusion and exclusion criteria. We summarized the most commonly reported factors in a table (Appendix 9).
Thirty studies excluded participants who were taking beta‐blockers preoperatively (Alkaya 2014; Bayliff 1999; DIPOM 2006; Gibson 1988; Horikoshi 2017; Inada 1989; Jangra 2016; Lai 2006; Lee 2015; Lim 2000; Liu 2006; Mallon 1990; Menigaux 2002; Miller 1990; Neary 2006; Oxorn 1990; POBBLE 2005; POISE 2008; PRESAGE 2016; Safwat 1984; Sharma 2018; Singh 2010; Singh 2012; Srivastava 2015; Sugiura 2007; Unal 2008; Verma 2018; Yang 2006; Yang 2008; Zaugg 1999). Whilst no studies specifically included participants who were already taking beta‐blockers, four studies reported in baseline characteristics tables that at least some participants were taking beta‐blockers pre‐operatively (Helfman 1991; Raby 1999; Van den Berg 1998; Wallace 1998); the remaining studies did not report this information.
Six studies included only participants who had hypertension (Ceker 2015; Magnusson 1986; Shailaja 2013; Sharma 1996; Stone 1988; Sugiura 2007), whilst 12 studies excluded participants who had a history of hypertension (Bhattacharjee 2016; Chung 1992; El‐Shmaa 2016; Goyagi 2005; Lee 2015; Liu 1986; Meftahuzzaman 2014; Menigaux 2002; Sharma 2018; Singh 2012; Srivastava 2015; Verma 2018). Seventeen studies reported in baseline characteristics tables that at least some participants had a history of hypertension (Bayliff 1999; DIPOM 2006; Gibson 1988; Helfman 1991; Horikoshi 2017; Inada 1989; Kawaguchi 2010; Lai 2006; Lim 2000; Miller 1991; Ojima 2017; POISE 2008; PRESAGE 2016; Safwat 1984; Van den Berg 1998; Wallace 1998; Zaugg 1999).
In addition, we noted that one study included only participants with diabetes (DIPOM 2006), and one study included only participants who were cigarette smokers (Louizos 2007). Four studies specified inclusion of only elderly participants (Lai 2006; Liu 2006; Miyazaki 2009; Zaugg 1999), and one included only participants described as middle‐aged to elderly (Van den Berg 1998). However, because the surgical population in this review was mixed, we noted that some studies also typically included an elderly population.
Study setting
All studies were conducted in a hospital setting. Studies did not always report whether they were conducted in a single centre or in multiple centres. Six studies reported that they were multi‐centre studies (Cucchiara 1986; DIPOM 2006; Miller 1991; POBBLE 2005; POISE 2008; Yang 2006), and we assumed that the remaining studies were all single‐centre studies.
Interventions and comparisons
Twenty‐two studies were multi‐arm studies and included:
more than one type of beta‐blocker (Inoue 2010; Singh 2010; Stone 1988);
different doses of the same beta‐blocker (Harasawa 2006; Inada 1989; Kindler 1996; Lee 1994; Lee 2017; Lim 2000; Louizos 2007; Mallon 1990; Mikawa 1991; Miller 1990; Miller 1991; Oxorn 1990; Sharma 1996; Sugiura 2007; Unal 2008; Yamazaki 2005);
different groups according to whether participants had hypertension (Miyazaki 2009);
different timings of the intervention agent (Safwat 1984; Zaugg 1999).
Types of beta‐blockers assessed were:
propranolol versus a placebo (Apipan 2010; Bayliff 1999; Safwat 1984), or standard care (Ali 2015);
metoprolol versus a placebo (DIPOM 2006; Jakobsen 1986; Jakobsen 1992; Jakobsen 1997; Magnusson 1986; POBBLE 2005; POISE 2008; Urias 2016; Ward‐Booth 1983; Whitehead 1980; Yang 2006), or standard care (Lai 2006; Liu 2006; PRESAGE 2016; Yang 2008);
esmolol versus a placebo (Alkaya 2014; Bhattacharjee 2016; Ceker 2015; Cucchiara 1986; Gibson 1988; Helfman 1991; Inoue 2010; Jangra 2016; Kao 2017; Kindler 1996; Lee 2010; Lee 2015; Lee 2017; Lim 2000; Liu 1986; Louizos 2007; Mallon 1990; Menigaux 2002; Miller 1990; Miller 1991; Moon 2011; Oxorn 1990; Park 2009; Raby 1999; Shailaja 2013; Sharma 2018; Shrestha 2011; Shukla 2010; Singh 1995; Singh 2010; Singh 2012; Srivastava 2015; Ugur 2007; Unal 2008; Van den Berg 1998; Verma 2018; White 2003), or standard care (Ohri 1999; Tendulkar 2017);
landiolol versus a placebo (Aoyama 2016; Goyagi 2005; Harasawa 2006; Horikoshi 2017; Inoue 2010; Miyazaki 2009; Ojima 2017; Sugiura 2007; Wajima 2011; Yamazaki 2005; Yoshida 2017), or standard care (Kawaguchi 2010);
nadolol with a placebo (Burns 1988);
atenolol with a placebo (Gupta 2011; Neary 2006; Wallace 1998), or standard care (Stone 1988; Zaugg 1999);
labetolol with a placebo (Chung 1992; Do 2012; El‐Shmaa 2016; Inada 1989; Lee 1994; Meftahuzzaman 2014; Singh 2010), or standard care (Stone 1988);
oxprenolol with standard care (Stone 1988);
pindolol with a placebo (Mikawa 1991).
In nine studies, beta‐blockers were titrated according to heart rate or blood pressure (Ali 2015; DIPOM 2006; Harasawa 2006; Kawaguchi 2010; POBBLE 2005; Raby 1999; Wallace 1998; Yang 2008; Zaugg 1999). In the remaining studies, beta‐blockers were given at a fixed dose.
In 18 studies, administration was started before surgery, and we defined this time point as any time up to 15 minutes before the induction of anaesthesia (Ali 2015; Apipan 2010; Bayliff 1999; Burns 1988; DIPOM 2006; Gupta 2011; Jakobsen 1986; Jakobsen 1992; Jakobsen 1997; Magnusson 1986; POBBLE 2005; POISE 2008; Shrestha 2011; Shukla 2010; Ward‐Booth 1983; Whitehead 1980; Yang 2006; Yang 2008). In six studies, administration was started after surgery (Harasawa 2006; Ojima 2017; Park 2009; PRESAGE 2016; Raby 1999; Wallace 1998). Urias 2016 did not report time of administration, and Zaugg 1999 was a multi‐arm study that included administration pre‐ and postoperatively and administration intraoperatively. In the remaining studies, administration was during surgery; we defined this time point as starting immediately before, and up to 15 minutes before, induction of anaesthesia until emergence from anaesthesia. Duration of administration varied across studies.
Outcomes
All studies included at least one review outcome as this formed part of the inclusion criteria for this review. However, we found that we could not use outcome data in 15 studies because they had not clearly reported data (Alkaya 2014; Chung 1992; Do 2012; Gibson 1988; Helfman 1991; Jakobsen 1986; Jangra 2016; Kindler 1996; Oxorn 1990; Sharma 1996; Singh 2010; Van den Berg 1998; Verma 2018; Wajima 2011; White 2003). We reported numbers of studies for each outcome in Effects of interventions.
Funding
We found that most studies did not report sources of support or conflicts of interest. Seven studies reported support from pharmaceutical companies (Inada 1989; Jakobsen 1986; Lee 2017; Mallon 1990; Miller 1991; Raby 1999; Whitehead 1980). Twenty‐four studies reported departmental or other sources of funding, which we assumed to be independent (Ali 2015; Bayliff 1999; Kao 2017; Kawaguchi 2010; Liu 1986; Magnusson 1986; Menigaux 2002; Neary 2006; POBBLE 2005; Wajima 2011; Wallace 1998; White 2003; Yang 2006), or that they received no funding (Alkaya 2014; Bhattacharjee 2016; El‐Shmaa 2016; Gupta 2011; Horikoshi 2017; Jangra 2016; PRESAGE 2016; Sharma 2018; Singh 2010; Ugur 2007; Verma 2018). Three studies declared support from both pharmaceutical and independent sources (DIPOM 2006; Helfman 1991; POISE 2008).
Excluded studies
We excluded 239 articles following assessment of full texts (Figure 1). It was not practical to report the details of all 239 excluded articles in the review, and therefore, we report the details of only seven studies, which we consider to be most closely related to our review criteria (Chae 1990; Ryder 1973; Sezai 2015; Taenaka 2013; Tan 2002; Vucevic 1992; Zmora 2016).
In summary, the reasons for exclusion were because studies did not measure or report review outcomes (152 studies); articles were the wrong design (such as commentaries or editorials) or were not RCTs (50 studies); studies had an ineligible population (13 studies), this included studies in which surgical procedures were not conducted under general anaesthesia and one study in which participants were as young as 10 years of age (Ryder 1973); studies had an ineligible intervention or comparison (23 studies), this includes studies that did not have a placebo or standard care group, participants in the intervention group were given an additional agent that was not a beta‐blocker (Chae 1990; Tan 2002; Zmora 2016), all participants were given beta‐blockers after surgery during the study follow‐up period (Sezai 2015), and administration was started up to seven days after surgery (Taenaka 2013). One old study did not report the number of participants in each group, and contact author detail was insufficient (Vucevic 1992).
In addition, we re‐evaluated studies included in the previous version of the review (Blessberger 2018), and excluded three of these: in one study, participants were undergoing a non‐surgical procedure (Sandler 1990); in one study, the control group included an additional intervention (dobutamine echocardiography), which was not equivalent to standard care practices in other control groups (Marwick 2009); and in one study, a change to the review outcomes meant that one study no longer measured or reported review outcomes (Coleman 1980).
See Characteristics of excluded studies for the 10 cited studies. This review does not include studies that were previously excluded; details of previous exclusions can be found elsewhere (Blessberger 2014; Blessberger 2018).
Studies awaiting classification
We found 18 studies awaiting classification (ACTRN12605000639628; ACTRN12615000889; Birbicer 2007; Boussofara 2001; Boussofara 2004; Gong 1999; Hornamand 2017; Inada 2002; Itani 2013; Joo 2010; Kajiura 2013; Kawano 2005; NCT02466542; Tangoku 2016; UMIN000024040; Wang 1994; Wang 1999; Yuan 1994). Three studies were published only as abstracts, with insufficient detail to assess eligibility (Itani 2013; Kajiura 2013; Tangoku 2016), and we were unable to access the full text of seven studies (Birbicer 2007; Boussofara 2001; Boussofara 2004; Gong 1999; Inada 2002; Wang 1994; Yuan 1994). Four studies are awaiting translation in order to assess study eligibility (Hornamand 2017; Joo 2010; Kawano 2005; Wang 1999). Four studies were described as completed in a clinical trials register but results are not yet published (ACTRN12605000639628; ACTRN12615000889; NCT02466542; UMIN000024040). See Characteristics of studies awaiting classification.
Ongoing studies
We found three ongoing studies (EUCTR2010‐021844‐17; NCT01555554; NCT03138603). One study compares esmolol with a placebo in people undergoing arterial vascular surgery (EUCTR2010‐021844‐17), one compares propranolol with a placebo given to veterans with post‐traumatic stress disorder (PTSD), who are scheduled for any type of surgical procedure under general anaesthesia (NCT01555554), and one compares metoprolol with a placebo in participants who have or are at risk of coronary artery disease and are scheduled for major non‐cardiac surgery (NCT03138603). See Characteristics of ongoing studies.
Risk of bias in included studies
See Characteristics of included studies, Figure 2 and Figure 3.
2.
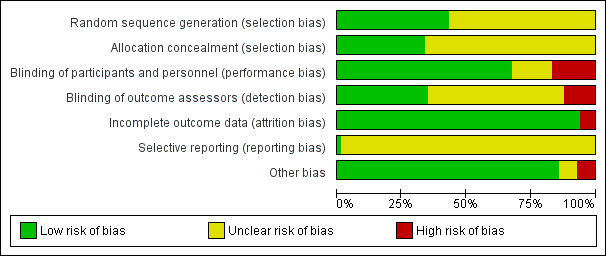
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies
3.
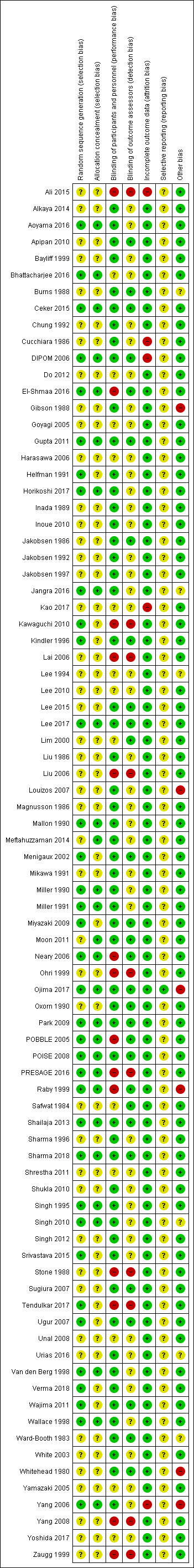
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
Overall, we found 26.5% studies were at high risk of bias in at least one domain.
Allocation
For random sequence generation, we found 36 studies that reported sufficient methods to randomize participants to groups, and we judged these studies to be at low risk of selection bias (Aoyama 2016; Bhattacharjee 2016; Ceker 2015; DIPOM 2006; El‐Shmaa 2016; Gupta 2011; Helfman 1991; Horikoshi 2017; Jangra 2016; Kawaguchi 2010; Kindler 1996; Lee 2017; Mallon 1990; Menigaux 2002; Miller 1990; Miller 1991; Miyazaki 2009; Neary 2006; Ojima 2017; Park 2009; POBBLE 2005; POISE 2008; PRESAGE 2016; Raby 1999; Shailaja 2013; Sharma 2018; Singh 1995; Singh 2010; Srivastava 2015; Tendulkar 2017; Ugur 2007; Van den Berg 1998; Verma 2018; Wajima 2011; Wallace 1998; Yang 2006). We judged the risk of selection bias for random sequence generation in the remaining studies to be unclear.
For allocation concealment, we judged 28 studies to be at low risk of bias (Aoyama 2016; Bhattacharjee 2016; Ceker 2015; DIPOM 2006; El‐Shmaa 2016; Gupta 2011; Horikoshi 2017; Jangra 2016; Lee 2017; Mallon 1990; Meftahuzzaman 2014; Miller 1990; Miller 1991; Moon 2011; Neary 2006; Ojima 2017; Park 2009; POBBLE 2005; POISE 2008; PRESAGE 2016; Raby 1999; Shailaja 2013; Sharma 2018; Singh 1995; Singh 2010; Van den Berg 1998; Wallace 1998; Yang 2006). We judged the risk of selection bias for allocation concealment in the remaining studies to be unclear, because of inadequate reporting.
Blinding
Some of the studies in this review compared a beta‐blocker with standard care, and because it was not feasible to blind personnel to the intervention, we judged all studies with a standard care control group to be at high risk of performance bias because of this open‐label study design (Ali 2015; Kawaguchi 2010; Lai 2006; Liu 2006; Ohri 1999; PRESAGE 2016; Stone 1988; Tendulkar 2017; Yang 2008; Zaugg 1999). In addition, we judged four placebo‐controlled trials to be at high risk of performance bias because personnel were aware, or appeared to be aware, of group allocation (El‐Shmaa 2016; Neary 2006; POBBLE 2005; Raby 1999). In 13 studies, study authors reported insufficient information to ascertain whether anaesthetists or clinicians were blinded and we judged risk of performance bias in these studies to be unclear (Bhattacharjee 2016; Do 2012; Goyagi 2005; Harasawa 2006; Kao 2017; Lee 1994; Lee 2010; Lim 2000; Safwat 1984; Shrestha 2011; Unal 2008; Yamazaki 2005; Yoshida 2017). We judged the remaining studies to be at low risk of bias.
Twenty‐nine studies reported that outcome assessors were blinded and we judged these studies to be at low risk of detection bias (Apipan 2010; Burns 1988; Ceker 2015; DIPOM 2006; El‐Shmaa 2016; Gupta 2011; Kindler 1996; Jakobsen 1986; Lee 2015; Lee 2017; Lim 2000; Menigaux 2002; Miyazaki 2009; Moon 2011; Neary 2006; Ojima 2017; Oxorn 1990; Park 2009; POBBLE 2005; POISE 2008; Raby 1999; Safwat 1984; Shailaja 2013; Sharma 2018; Sugiura 2007; Ugur 2007; Wajima 2011; Ward‐Booth 1983; Whitehead 1980). We judged studies with a standard care control group to be at high risk of detection bias if study authors did not describe whether outcome assessors were blinded (Ali 2015; Kawaguchi 2010; Lai 2006; Liu 2006; Ohri 1999; PRESAGE 2016; Stone 1988; Tendulkar 2017; Yang 2008; Zaugg 1999). Study authors did not describe whether outcome assessors were blinded in the remaining studies and we judged the risk of bias to be unclear.
Incomplete outcome data
Most studies did not report losses, and therefore we assumed that there were no losses and we used the numbers of randomized participants to inform the assumed number of analysed participants. In addition, most studies did not report whether they used intention‐to‐treat (ITT) analysis and when losses were reported and study authors did not state use of ITT analysis, we assumed that study investigators used per‐protocol analysis.
We judged five studies to have a high risk of attrition bias (Ali 2015; Cucchiara 1986; DIPOM 2006; Kao 2017; Yang 2006); these studies lost more than 10% of participants, or loss of participants was imbalanced between groups. We judged the remaining studies to have a low risk of attrition bias because study authors reported no losses or losses were fewer than 10% and we did not expect the loss to influence outcome data.
Selective reporting
Only two studies were prospectively registered with a clinical trials register (Lee 2017; Ojima 2017). We judged Ojima 2017 to be at low risk of reporting bias but, because the clinical trials register documents listed primary and secondary outcomes that were not consistent with the published study report, we judged the risk of reporting bias in Lee 2017 to be unclear. Seven studies reported clinical trial registration that was retrospective, and therefore, we could not feasibly assess risk of bias from these registration documents (DIPOM 2006; Horikoshi 2017; Kawaguchi 2010; Lee 2015; POBBLE 2005; POISE 2008; PRESAGE 2016). We judged these, and all other studies, to have an unclear risk of reporting bias because we could not assess this domain without access to published protocols or prospectively registered clinical trial documents.
Other potential sources of bias
Six studies reported use of beta‐blockers as rescue therapy in the control group (Gibson 1988; Louizos 2007; Ojima 2017; Raby 1999; Whitehead 1980; Yang 2006). We believed that this introduced considerable bias to the data and we judged all these studies to be at high risk of bias.
Three studies had limited information in the report and it was also not feasible to effectively assess risks of other bias in these studies (Burns 1988; Urias 2016; Ward‐Booth 1983). We used the information reported in the English abstract and tables in one study written in Korean and, similarly, we were unable to effectively assess other risks of bias from this report (Lee 1994). We noted a difference in the time of administration of two different blockers in Singh 2010, which were either before or after tracheal intubation, and we were uncertain whether this difference in study design might have influenced outcome data. Study authors in Jangra 2016 highlighted differences between groups and we were similarly uncertain whether this difference might influence outcome data. We judged these six studies to be at unclear risk of bias. We did not identify any other sources of bias in the remaining study reports.
Effects of interventions
See: Table 1
Early all‐cause mortality at 30 days
Sixteen studies reported mortality data (Ali 2015; Aoyama 2016; Bayliff 1999; DIPOM 2006; Kawaguchi 2010; Lai 2006; Miller 1990; Miller 1991; Neary 2006; Ojima 2017; POBBLE 2005; POISE 2008; PRESAGE 2016; Wallace 1998; Yang 2006; Yang 2008). We noted that six of these studies had no deaths in either group (Aoyama 2016; Lai 2006; Miller 1990; Miller 1991; Ojima 2017; PRESAGE 2016).
Based on the risk of death in the control group of 25 per 1000, the effect with beta‐agonists was between 2 fewer and 13 more deaths per 1000 (risk ratio (RR) 1.17, 95% confidence interval (CI) 0.89 to 1.54; I2 = 5%; 16 studies, 11,446 participants; low‐certainty evidence; Analysis 1.1). See Figure 4.
1.1. Analysis.
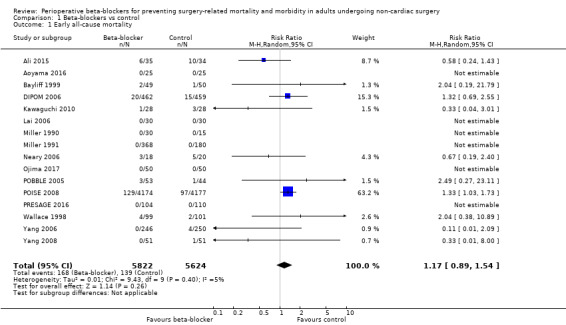
Comparison 1 Beta‐blockers vs control, Outcome 1 Early all‐cause mortality.
4.
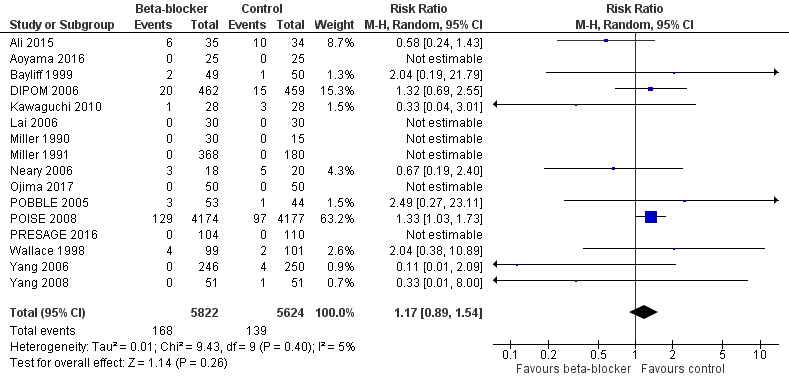
Forest plot of comparison 1. Beta‐blockers vs control, outcome: 1.1 Early all‐cause mortality
We used GRADE to downgrade the certainty of the evidence for all‐cause mortality by two levels: one for study limitations because we assessed 10 studies to be at high risk of bias in at least one domain, and the effect estimate was not robust when we excluded studies with poorer methodological standards in sensitivity analysis; and one for inconsistency because from visual inspection of the data, we noted that one large international study represented 63.2% of the weighting and showed an increase in mortality when beta‐blockers were used, which was not replicated in the remaining smaller studies. See Table 1.
From visual inspection of a funnel plot, we note the possibility of publication bias for this outcome; however, we did not explore this observation further (Figure 5).
5.
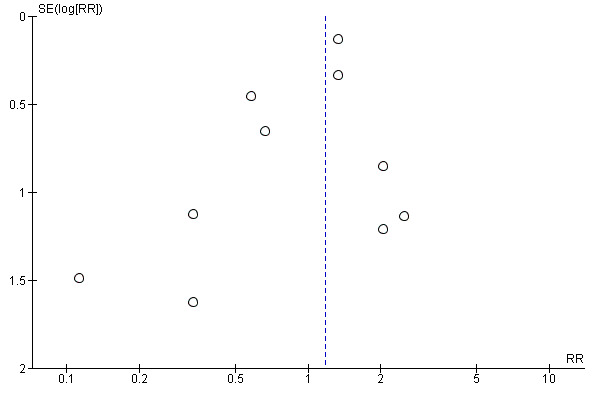
Funnel plot of comparison 1. Beta‐blockers vs control, outcome: 1.1 Early all‐cause mortality
Long‐term mortality
Five studies measured long‐term mortality (DIPOM 2006; Kawaguchi 2010; Neary 2006; Wallace 1998; Yang 2006). Time points of measurement were at three months (Kawaguchi 2010), at six months (DIPOM 2006; Yang 2006), and at two years (Neary 2006; Wallace 1998). We were unable to include Yang 2006 in analysis because data were not clearly reported.
We found little or no difference in long‐term mortality according to whether perioperative beta‐blockers were administered (RR 0.82, 95% CI 0.58 to 1.17; I2 = 20%; 5 studies, 1215 participants; low‐certainty evidence; Analysis 1.2). We used GRADE to downgrade the certainty of the evidence by two levels: one level for study limitations because we assessed three studies to be at high risk of bias in at least one domain; and one level for imprecision because, for this outcome, evidence was from few studies with few participants.
1.2. Analysis.
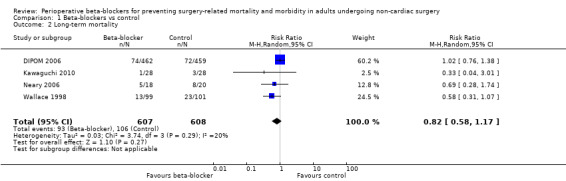
Comparison 1 Beta‐blockers vs control, Outcome 2 Long‐term mortality.
Death due to cardiac causes
Three studies reported how many participants died because of cardiac causes (POISE 2008; Wallace 1998; Yang 2006). We found little or no difference in the number of deaths according to whether perioperative beta‐blockers were administered (RR 1.25, 95% CI 0.90 to 1.75; I2 = 0%; 3 studies, 9047 participants; low‐certainty evidence; Analysis 1.3). We used GRADE to downgrade the certainty of the evidence by two levels; one level for study limitation because we assessed one of the three studies to be at high risk of bias in one domain; and one level for imprecision because studies were few, despite a large sample size owing to one large international study (97.1% weighting).
1.3. Analysis.
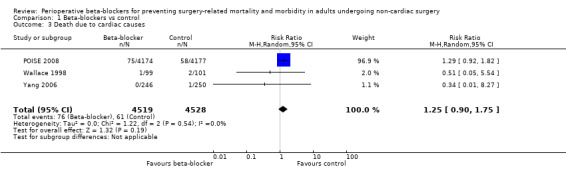
Comparison 1 Beta‐blockers vs control, Outcome 3 Death due to cardiac causes.
Acute myocardial infarction
Twelve studies reported whether participants had a myocardial infarction (DIPOM 2006; Jakobsen 1997; Lai 2006; Miller 1990; POBBLE 2005; POISE 2008; Raby 1999; Stone 1988; Wallace 1998; Yang 2006; Yang 2008; Zaugg 1999). Three of these studies reported no events in either group (Lai 2006; Miller 1990; Stone 1988).
Beta‐blockers may reduce the occurrence of myocardial infarctions (RR 0.72, 95% CI 0.60 to 0.87; I2 = 0%; 12 studies, 10,520 participants; low‐certainty evidence; Analysis 1.4); the number needed to treat for an additional beneficial outcome (NNTB) was 74 (95% CI 52 to 160). We used GRADE to downgrade the certainty of the evidence by two levels: one level for study limitations because we assessed eight studies to be at high risk of bias in at least one domain; and one level for inconsistency because from visual inspection of the data, we noted that one large international study represented 87.9% weighting in the primary analysis and demonstrated a protective effect of beta‐blockers, which was not replicated in the remaining smaller studies. See Table 1.
1.4. Analysis.
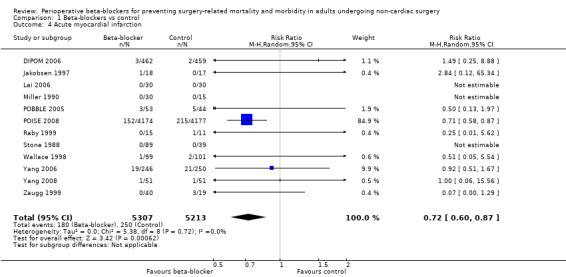
Comparison 1 Beta‐blockers vs control, Outcome 4 Acute myocardial infarction.
Cerebrovascular events
Six studies reported cerebrovascular events (POBBLE 2005; POISE 2008; PRESAGE 2016; Wallace 1998; Yang 2006; Yang 2008).
We found no evidence of a difference in cerebrovascular events when beta‐blockers were used (RR 1.65, 95% CI 0.97 to 2.81; I2 = 0%; 6 studies, 9460 participants; low‐certainty evidence; Analysis 1.5). We used GRADE to downgrade the certainty of the evidence by one level for inconsistency. From visual inspection of the data, we noted that one large international study showed an increase in cerebrovascular events when beta‐blockers were used, but this effect was not replicated in the smaller studies and we found too few studies in order to explore this difference effectively through subgroup analysis. We also downgraded the evidence by one level for study limitations; we found that the effect was not robust when we excluded studies with poorer methodological standards during sensitivity analyses. See Table 1.
1.5. Analysis.
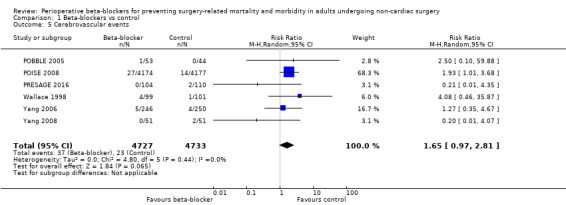
Comparison 1 Beta‐blockers vs control, Outcome 5 Cerebrovascular events.
Ventricular arrhythmias
Five studies reported ventricular arrhythmias (Bayliff 1999; Jakobsen 1997; Mallon 1990; POBBLE 2005; Wallace 1998).
We found no evidence of a difference to ventricular arrhythmias when beta‐blockers were used (RR 0.72, 95% CI 0.35 to 1.47; I2 = 35%; 5 studies, 476 participants; very low‐certainty evidence; Analysis 1.6). We used GRADE to downgrade the certainty of the evidence by three levels: one level for imprecision because the evidence was from too few participants and few single‐centre studies; one level for inconsistency because we noted a moderate level of statistical heterogeneity that we were unable to explain, and one level for study limitations because we judged several studies to be at high or unclear risk of bias. See Table 1.
1.6. Analysis.
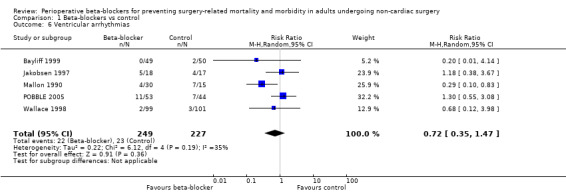
Comparison 1 Beta‐blockers vs control, Outcome 6 Ventricular arrhythmias.
Atrial fibrillation or atrial flutter, or both
Nine studies reported atrial fibrillation or atrial flutter (Aoyama 2016; Bayliff 1999; Horikoshi 2017; Lai 2006; Ojima 2017; POISE 2008; PRESAGE 2016; Urias 2016; Yoshida 2017).
Beta‐blockers may reduce atrial fibrillation (RR 0.41, 95% CI 0.21 to 0.79; I2 = 67%; 9 studies, 9080 participants; low‐certainty evidence; Analysis 1.7); the NNTB was 39 (95% CI 29 to 108). We used GRADE to downgrade the certainty of the evidence by one level owing to inconsistency; we were unable to effectively assess or explain the substantial statistical heterogeneity that we noted for this outcome. We also downgraded one level for study limitations; the effect estimate was not robust when we excluded studies with poorer methodological standards in sensitivity analysis. See Table 1.
1.7. Analysis.
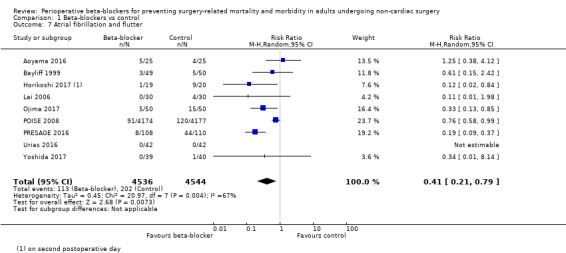
Comparison 1 Beta‐blockers vs control, Outcome 7 Atrial fibrillation and flutter.
Bradycardia
Sixty‐five studies reported bradycardia (Ali 2015; Alkaya 2014; Apipan 2010; Bayliff 1999; Bhattacharjee 2016; Burns 1988; Ceker 2015; Chung 1992; Cucchiara 1986; Do 2012; El‐Shmaa 2016; Gibson 1988; Goyagi 2005; Gupta 2011; Harasawa 2006; Helfman 1991; Horikoshi 2017; Inada 1989; Inoue 2010; Jakobsen 1986; Jakobsen 1992; Jakobsen 1997; Kao 2017; Kawaguchi 2010; Kindler 1996; Lee 1994; Lee 2015; Lee 2017; Lim 2000; Liu 1986; Liu 2006; Louizos 2007; Magnusson 1986; Mallon 1990; Meftahuzzaman 2014; Menigaux 2002; Mikawa 1991; Miller 1991; Miyazaki 2009; Moon 2011; Neary 2006; Ojima 2017; Oxorn 1990; Park 2009; POBBLE 2005; POISE 2008; Safwat 1984; Shailaja 2013; Sharma 2018; Shrestha 2011; Shukla 2010; Singh 2010; Srivastava 2015; Stone 1988; Tendulkar 2017; Ugur 2007; Van den Berg 1998; Verma 2018; Wajima 2011; Wallace 1998; Ward‐Booth 1983; Whitehead 1980; Yamazaki 2005; Yang 2006; Yoshida 2017). Of these, four studies reported data unclearly (Alkaya 2014; Jakobsen 1986; Oxorn 1990; Van den Berg 1998), and 12 studies did not clearly report whether bradycardia was measured in all groups (Chung 1992; Do 2012; Gibson 1988; Helfman 1991; Kindler 1996; Neary 2006; Shrestha 2011; Singh 2010; Tendulkar 2017; Verma 2018; Wajima 2011; Yoshida 2017); we did not include these 16 studies in analysis. We note that 21 studies reported no events in either group (Ceker 2015; El‐Shmaa 2016; Harasawa 2006; Horikoshi 2017; Inada 1989; Inoue 2010; Jakobsen 1997; Kao 2017; Lee 1994; Lee 2015; Lee 2017; Louizos 2007; Menigaux 2002; Mikawa 1991; Moon 2011; Ojima 2017; Safwat 1984; Sharma 2018; Srivastava 2015; Ugur 2007; Yamazaki 2005).
Perioperative beta‐blockers probably increase incidences of bradycardia (RR 2.49, 95% CI 1.74 to 3.56; I2 = 68%; 49 studies, 12,239 participants; low‐certainty evidence; Analysis 1.8); the number needed to treat for an additional harmful outcome (NNTH) was 18 (95% CI 11 to 37). We used GRADE to downgrade the certainty of the evidence for this outcome by one level for inconsistency owing to substantial statistical heterogeneity which we were unable to explain from subgroup analyses. We also downgraded by one level for study limitations because we assessed several studies to be at high or unclear risk of bias. See Table 1.
1.8. Analysis.
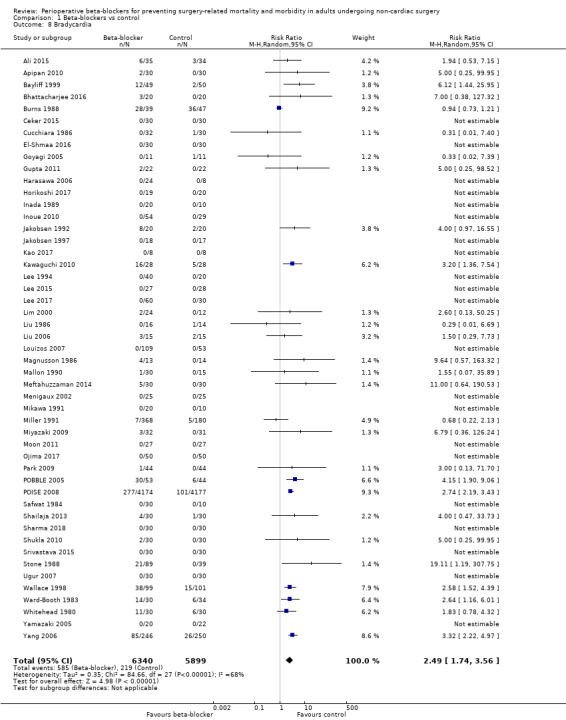
Comparison 1 Beta‐blockers vs control, Outcome 8 Bradycardia.
Hypotension
Sixty‐two studies reported hypotension (Ali 2015; Alkaya 2014; Bayliff 1999; Bhattacharjee 2016; Ceker 2015; Chung 1992; Cucchiara 1986; Do 2012; El‐Shmaa 2016; Gibson 1988; Goyagi 2005; Gupta 2011; Harasawa 2006; Helfman 1991; Horikoshi 2017; Inada 1989; Inoue 2010; Jakobsen 1992; Jakobsen 1997; Jangra 2016; Kao 2017; Kawaguchi 2010; Kindler 1996; Lee 1994; Lee 2015; Lee 2017; Liu 1986; Louizos 2007; Magnusson 1986; Mallon 1990; Menigaux 2002; Mikawa 1991; Miller 1990; Miller 1991; Moon 2011; Neary 2006; Ohri 1999; Ojima 2017; Oxorn 1990; Park 2009; POBBLE 2005; POISE 2008; PRESAGE 2016; Safwat 1984; Shailaja 2013; Sharma 1996; Sharma 2018; Shrestha 2011; Shukla 2010; Singh 1995; Singh 2012; Stone 1988; Sugiura 2007; Tendulkar 2017; Unal 2008; Verma 2018; Wajima 2011; Wallace 1998; Whitehead 1980; Yamazaki 2005; Yang 2006; Yoshida 2017). Of these, two studies reported data unclearly (Alkaya 2014; Oxorn 1990), and 11 studies did not clearly report whether hypotension was measured in all groups (Chung 1992; Do 2012; Gibson 1988; Helfman 1991; Jangra 2016; Kindler 1996; Neary 2006; Sharma 1996; Verma 2018; Wajima 2011; Yoshida 2017); we did not include these 13 studies in analysis. We note that 22 studies reported no events in either group (Goyagi 2005; Gupta 2011; El‐Shmaa 2016; Harasawa 2006; Horikoshi 2017; Inada 1989; Inoue 2010; Kao 2017; Lee 1994; Lee 2015; Lee 2017; Louizos 2007; Menigaux 2002; Mikawa 1991; Moon 2011; Ojima 2017; Safwat 1984; Sharma 2018; Shrestha 2011; Singh 2012; Tendulkar 2017; Yamazaki 2005).
Perioperative beta‐blockers probably increase incidences of hypotension (RR 1.40, 95% CI 1.29 to 1.51; I2 = 0%; 49 studies, 12,304 participants; moderate‐certainty evidence; Analysis 1.9); the NNTH was 23 (95% CI 18 to 31). See Figure 6.
1.9. Analysis.
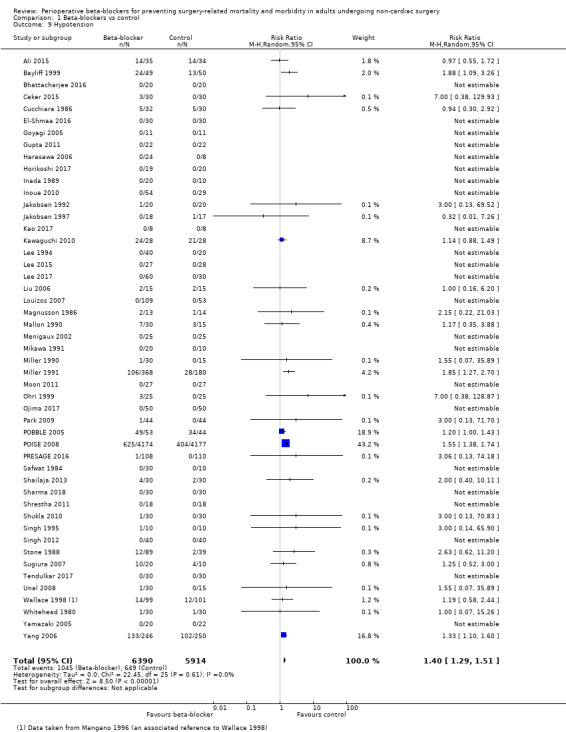
Comparison 1 Beta‐blockers vs control, Outcome 9 Hypotension.
6.
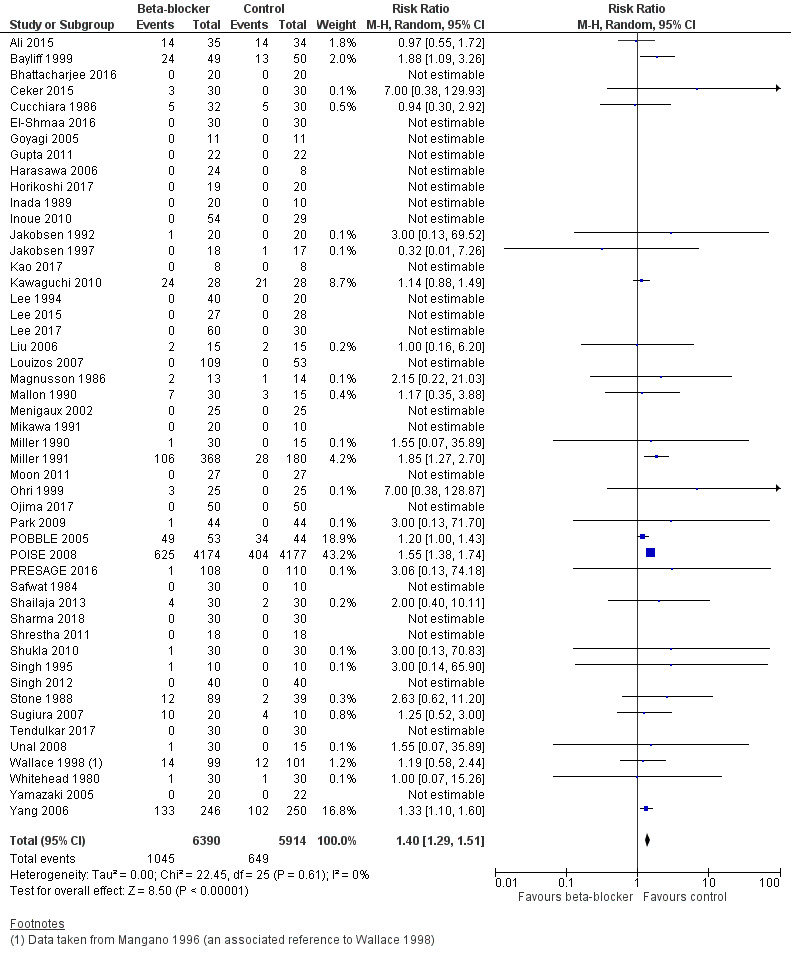
Forest plot of comparison: 1 Beta‐blockers vs control, outcome: 1.9 Hypotension
We used GRADE to downgrade the certainty of the evidence for hypotension by one level for study limitations because we assessed several studies to be at high or unclear risk of bias. See Table 1.
Congestive heart failure
Six studies reported congestive heart failure (Bayliff 1999; Horikoshi 2017; Magnusson 1986; POISE 2008; Wallace 1998; Yang 2006). One study reported no events in either group (Horikoshi 2017).
We found little or no difference in the number of people who had congestive heart failure according to whether perioperative beta‐blockers were administered (RR 1.18, 95% CI 0.94 to 1.48; I2 = 0%; 6 studies, 9212 participants; low‐certainty evidence; Analysis 1.10). We used GRADE to downgrade the certainty of the evidence by one level owing to study limitations because we assessed some studies to be at high risk of bias in at least one domain, and one level for imprecision because studies were few, despite a large sample size owing to one large international study (86.9% weighting).
1.10. Analysis.
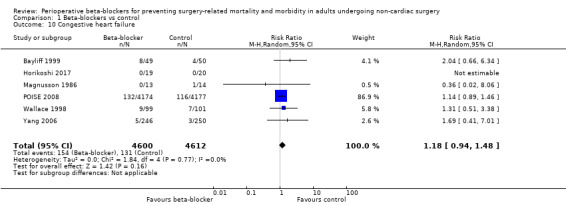
Comparison 1 Beta‐blockers vs control, Outcome 10 Congestive heart failure.
Length of hospital stay
Seven studies reported length of hospital stay (Bayliff 1999; Horikoshi 2017; Lee 2010; POBBLE 2005; POISE 2008; PRESAGE 2016; White 2003). We did not include three studies in analysis because they did not report data using equivalent values (Bayliff 1999; POISE 2008; White 2003); study authors reported little or no difference between groups.
In the remaining studies, we found little or no difference in hospital length of stay according to whether perioperative beta‐blockers were administered (mean difference (MD) −1.21 days, 95% CI −2.75 to 0.33; I2 = 66%; 404 participants; very low‐certainty evidence; Analysis 1.11). We used GRADE to downgrade the certainty of the evidence by three levels: one level for study limitation because we assessed some studies to be at high risk of bias in at least one domain; one level for imprecision because the evidence was from few studies with few participants, and one level for inconsistency because we noted a moderate level of statistical heterogeneity.
1.11. Analysis.
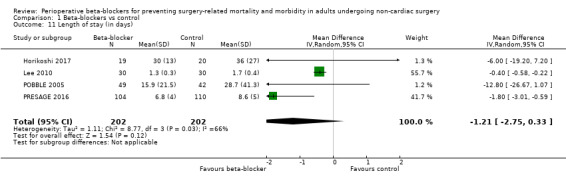
Comparison 1 Beta‐blockers vs control, Outcome 11 Length of stay (in days).
Quality of life
No studies reported outcome data for quality of life.
Subgroup analysis
We did not complete subgroup analysis for cerebrovascular events, ventricular arrhythmias, and atrial fibrillation or flutter because we found fewer than 10 studies for these outcomes.
Type of beta‐blocker
Two studies were multi‐arm studies with more than one type of beta‐blocker (Inoue 2010; Stone 1988); in subgroup analysis, we divided the control group data evenly in order to avoid a unit of analysis error.
All‐cause mortality: we found no evidence of a difference between subgroups (P = 0.31, I2 = 17.1%; Analysis 2.1). Most types of agent showed little or no difference in early all‐cause mortality, which was consistent with our primary analysis: propranolol (RR 0.68, 95% CI 0.30 to 1.58; I2 = 0%; 2 studies, 168 participants); landiolol (RR 0.33, 95% CI 0.04 to 3.01; 3 studies, 206 participants); and atenolol (RR 1.02, 95% CI 0.35 to 2.98; I2 = 9%; 2 studies, 238 participants). However, evidence for metoprolol, which included the large multi‐centre study POISE 2008, showed an increase in mortality with beta‐blocker use (RR 1.31, 95% CI 1.03 to 1.66; I2 = 0%; 7 studies, 10,241 participants). Evidence for esmolol was from studies with no event data.
Acute myocardial infarction: we found no evidence of a difference between subgroups (P = 0.40, I2 = 0%; Analysis 2.2). Most studies compared metoprolol and analysis of this subgroup was consistent with our primary analysis for acute myocardial infarction (RR 0.73, 95% CI 0.61 to 0.88; I2 = 0%; 7 studies, 10,062 participants). For oxprenolol and labetalol, evidence was from a study with no events in either group (Stone 1988). For esmolol, only one study had event data with little or no difference between groups (RR 0.25, 95% CI 0.01 to 5.62; 2 studies, 71 participants). For atenolol, only two studies had event data with little or no difference between groups (RR 0.23, 95% CI 0.03 to 1.57; I2 = 9%; 3 studies, 302 participants).
Bradycardia: we noted a difference between subgroups (P < 0.00001, I2 = 87.7%; Analysis 2.3). Analysis for four agents was largely consistent with the primary analysis and showed evidence of an increase in bradycardia in the beta‐blocker group: propranolol (RR 3.39, 95% CI 1.35 to 8.50; I2 = 0%; 4 studies, 268 participants); metoprolol (RR 2.87, 95% CI 2.40 to 3.42; 0%; 9 studies, 9200 participants); atenolol (RR 2.76, 95% CI 1.66 to 4.61; I2 = 0%; 3 studies, 287 participants); and labetalol (RR 8.13, 95% CI 1.10 to 60.07; I2 = 0%; 5 studies, 252 participants). Analysis of landiolol showed only slightly more incidences of bradycardia in the beta‐blocker group (RR 2.76, 95% CI 0.98 to 7.75; I2 = 11%; 8 studies, 395 participants); we noted that most studies in this subgroup reported zero event data. Data for oxprenolol and nadolol were both from single studies with little or no difference between groups for each agent (RR 4.06, 95% CI 0.23 to 70.46; 43 participants; and RR 0.94, 95% CI 0.73 to 1.21; 86 participants, respectively), and data for pindolol were from a single study with no events in either group. Esmolol, which was the largest subgroup, showed little or no difference in bradycardia according to whether beta‐blockers were administered (RR 1.29, 95% CI 0.60 to 2.75; I2 = 0%; 20 studies, 1678 participants).
Hypotension: the test for subgroup differences showed no difference (P = 0.51; I2 = 0%; Analysis 2.4). The two largest groups of agents were consistent with the primary analysis: metoprolol (RR 1.38, 95% CI 1.24 to 1.55; I2 = 12%; 9 studies, 9354 participants); and esmolol (RR 1.78, 95% CI 1.29 to 2.45; I2 = 0%; 23 studies, 1828 participants). In subgroup analysis of the remaining agents, which had fewer studies, we found little or no difference according to whether beta‐blockers were administered: propranolol (RR 1.36, 95% CI 0.71 to 2.61; I2 = 63%; 3 studies, 208 participants); landiolol (RR 1.15, 95% CI 0.90 to 1.48; I2 = 0%; 8 studies, 362 participants; we noted that only two of the seven landiolol studies had events); atenolol (RR 1.20, 95% CI 0.61 to 2.38; I2 = 0%; 3 studies, 287 participants); oxprenolol (RR 2.26, 95% CI 0.12 to 44.02; 1 study; 43 participants), and labetalol (RR 3.14, 95% CI 0.43 to 22.97; 4 studies, 192 participants). One study of pindolol had no events in either group.
2.1. Analysis.
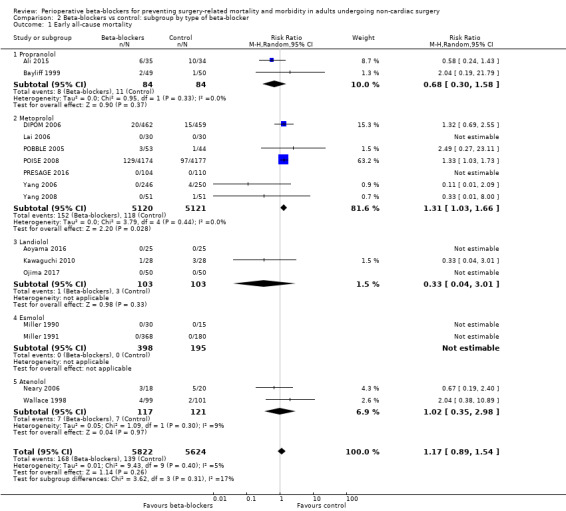
Comparison 2 Beta‐blockers vs control: subgroup by type of beta‐blocker, Outcome 1 Early all‐cause mortality.
2.2. Analysis.
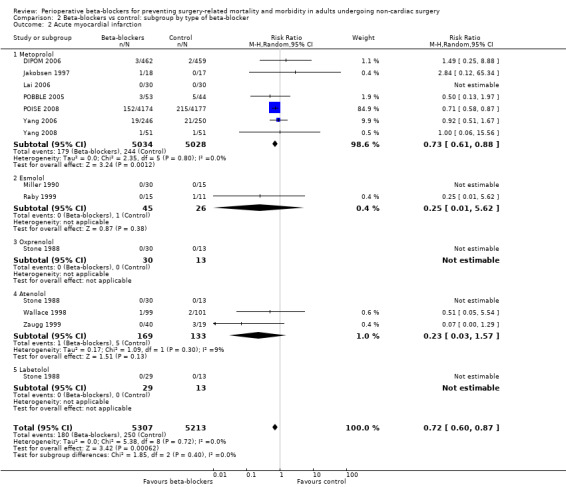
Comparison 2 Beta‐blockers vs control: subgroup by type of beta‐blocker, Outcome 2 Acute myocardial infarction.
2.3. Analysis.
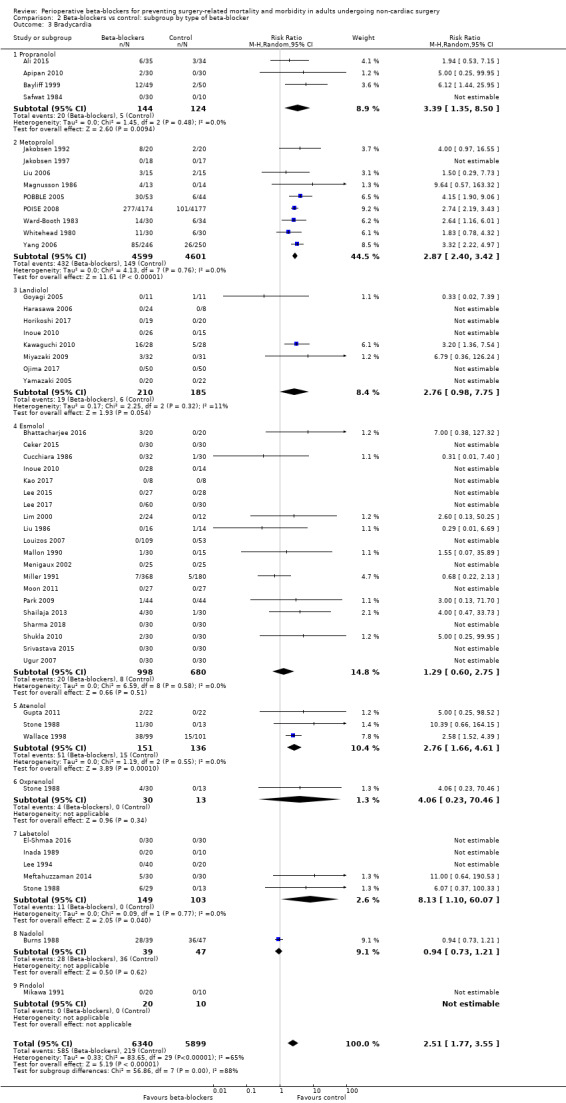
Comparison 2 Beta‐blockers vs control: subgroup by type of beta‐blocker, Outcome 3 Bradycardia.
2.4. Analysis.
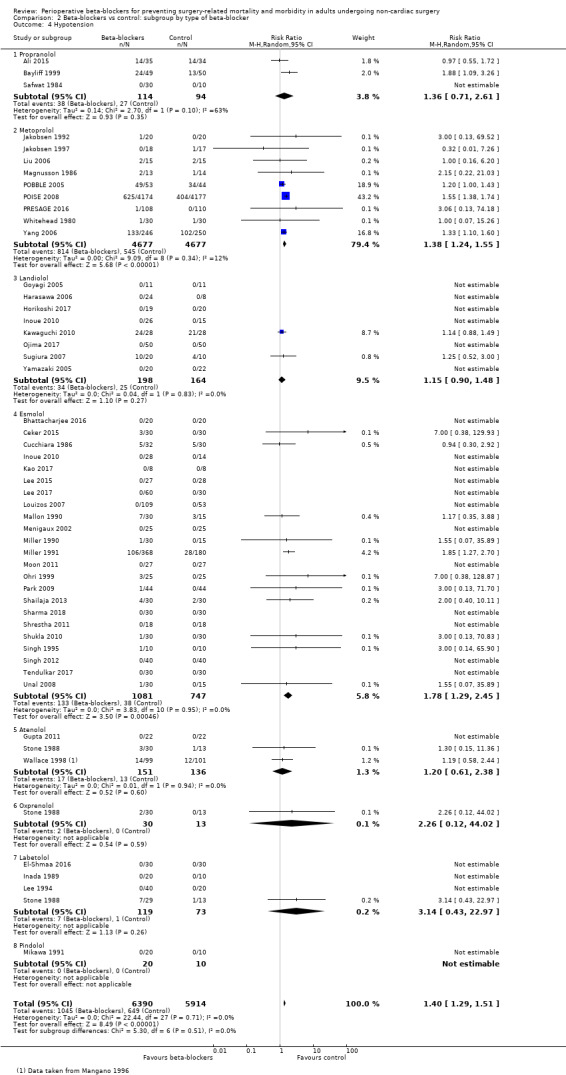
Comparison 2 Beta‐blockers vs control: subgroup by type of beta‐blocker, Outcome 4 Hypotension.
Start of beta‐blocker therapy
One study included two intervention groups in which beta‐blockers were given pre‐ and postoperatively, and intraoperatively (Zaugg 1999); for subgroup analysis, we included only outcome data for the group in which beta‐blockers were given intraoperatively.
All‐cause mortality (30 days): we found no evidence of a difference between subgroups (P = 0.36, I2 = 3.2%; Analysis 3.1). Each analysis by time of administration showed little or no difference according to whether beta‐blockers were administered, which was consistent with our primary analysis: before surgery (RR 1.17, 95% CI 0.83 to 1.64; I2 = 12%; 7 studies, 10,135 participants); during surgery (RR 0.56, 95% CI 0.18 to 1.69; I2 = 0%; 6 studies, 797 participants); and after surgery (RR 2.04, 95% CI 0.38 to 10.89; 3 studies, 514 participants).
Acute myocardial infarction: we found no evidence of a difference between subgroups (P = 0.43, I2 = 0%; Analysis 3.2). Evidence for before surgery was from the largest group of studies and this was consistent with the primary analysis (RR 0.73, 95% CI 0.61 to 0.88; I2 = 0%; 6 studies, 10,002 participants). Of four studies in which beta‐blockers were given during surgery, only one study had outcome data with little or no difference between groups (RR 0.14, 95% CI 0.01 to 2.47; 4 studies, 272 participants). Although the effect for administration after surgery showed little or no difference between groups, this analysis included only two small studies (RR 0.39, 95% CI 0.06 to 2.60; I2 = 0%; 2 studies, 226 participants).
Bradycardia: we found no evidence of a difference between subgroups (P = 0.59, I2 = 0%; Analysis 3.3). Analysis by time of administration at each time point was consistent with our primary analysis and showed an increase in bradycardia with beta‐blocker use: before surgery (RR 2.89, 95% CI 1.70 to 4.91; I2 = 84%; 13 studies, 9528 participants); during surgery (RR 1.98, 95% CI 1.17 to 3.35; I2 = 14%; 32 studies, 2291 participants); and after surgery (RR 2.59, 95% CI 1.54 to 4.37; I2 = 0%; 4 studies, 420 participants).
Hypotension: we found no evidence of a difference between subgroups (P = 0.99, I2 = 0%; Analysis 3.4). Analysis for administration of beta‐blockers at two of the time points showed an increase in hypotension with beta‐blocker use, which was consistent with our primary analysis: before surgery (RR 1.37, 95% CI 1.19 to 1.58; I2 = 31%; 11 studies, 9354 participants); and during surgery (RR 1.36, 95% CI 1.12 to 1.65; I2 = 0%; 33 studies, 2312 participants). Analysis for administration after surgery was from only four studies, with event data in three of these studies; analysis showed little or no difference according to whether beta‐blockers were given postoperatively (RR 1.30, 95% CI 0.65 to 2.58; I2 = 0%; 5 studies, 638 participants).
3.1. Analysis.
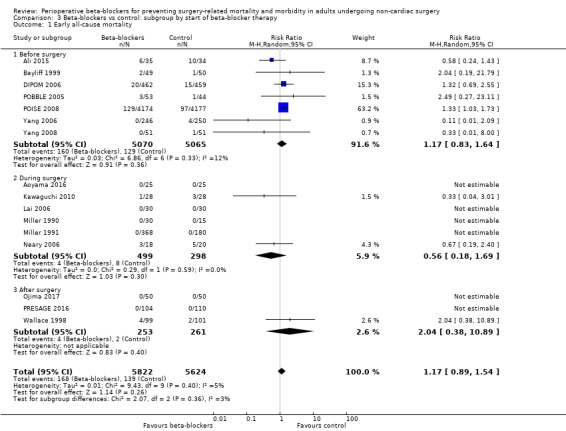
Comparison 3 Beta‐blockers vs control: subgroup by start of beta‐blocker therapy, Outcome 1 Early all‐cause mortality.
3.2. Analysis.
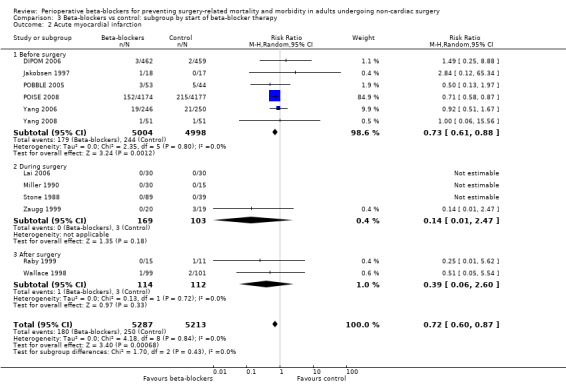
Comparison 3 Beta‐blockers vs control: subgroup by start of beta‐blocker therapy, Outcome 2 Acute myocardial infarction.
3.3. Analysis.
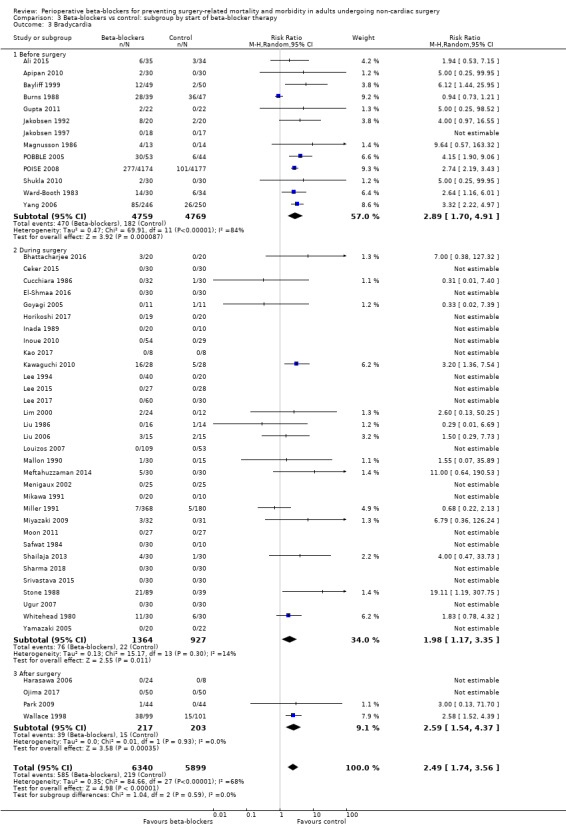
Comparison 3 Beta‐blockers vs control: subgroup by start of beta‐blocker therapy, Outcome 3 Bradycardia.
3.4. Analysis.
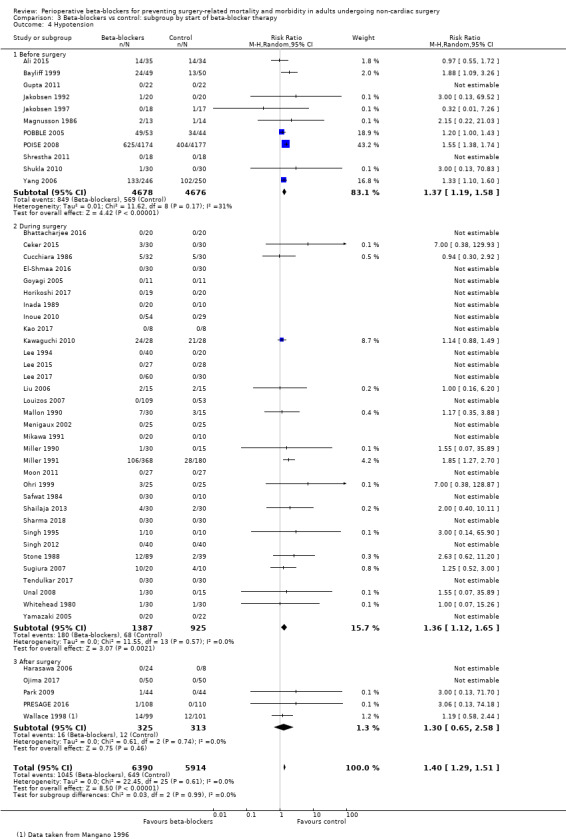
Comparison 3 Beta‐blockers vs control: subgroup by start of beta‐blocker therapy, Outcome 4 Hypotension.
Surgical risk factors
All‐cause mortality (30 days): we found no evidence of a difference between subgroups (P = 0.08, I2 = 67.2%; Analysis 4.1). However, evidence in low‐ to medium‐risk surgeries showed an increase in mortality with beta‐blockers (RR 1.32, 95% CI 1.04 to 1.68; I2 = 0%; 5 studies, 9967 participants). Only three studies of low‐ or medium‐risk surgery had outcome data in this analysis and one was the large multi‐centre study POISE 2008. For high‐risk surgery, we found little or no difference between groups (RR 0.75, 95% CI 0.42 to 1.35; I2 = 0%; 11 studies, 1479 participants)
Acute myocardial infarction: whilst we found no evidence of a difference between subgroups (P = 0.67, I2 = 0%; Analysis 4.2), the effect for each subgroup differed from the primary analysis. We noted little or no difference between groups in the number of myocardial infarctions for both low‐ to medium‐risk surgery (RR 0.71, 95% CI 0.47 to 1.07; I2 = 5%; 6 studies, 9606 participants) and high‐risk surgery (RR 0.82, 95% CI 0.49 to 1.37; I2 = 0%; 6 studies, 914 participants).
Bradycardia: we found no evidence of a difference between subgroups (P = 0.24, I2 = 26.9%; Analysis 4.3). Subgroup analysis according to surgical risk was consistent with the primary analysis, showing an increase in bradycardia when beta‐blockers were used: in low‐ and medium‐risk surgery (RR 2.19, 95% CI 1.28 to 3.74; I2 = 74%; 35 studies, 10,846 participants); and high‐risk surgery (RR 3.13, 95% CI 2.40 to 4.07; I2 = 0%; 14 studies, 1393 participants). The substantial statistical heterogeneity that we noted in the primary analysis was between the studies with the low‐ and medium‐risk surgeries, rather than between studies with high‐risk surgeries.
Hypotension: whilst the test for subgroup differences showed a statistically significant difference (P = 0.003, I2 = 89.1%; Analysis 4.4), we noted that overall subgroup analysis according to each surgical risk group was consistent with the primary analysis, showing an increase in hypotension when beta‐blockers were used: in low‐ and medium‐risk surgery (RR 1.57, 95% CI 1.41 to 1.75; I2 = 0%; 36 studies, 10,789 participants); and high‐risk surgery (RR 1.24, 95% CI 1.11 to 1.38; I2 = 0%; 13 studies; 1515 participants).
4.1. Analysis.
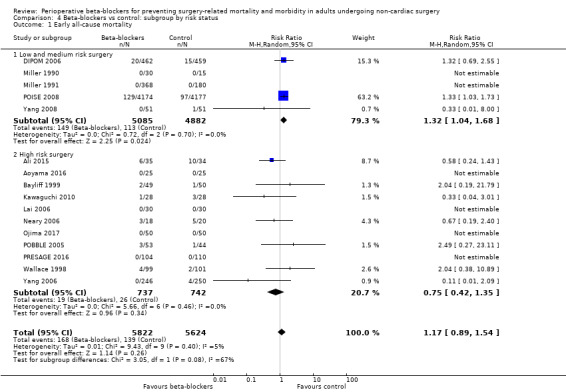
Comparison 4 Beta‐blockers vs control: subgroup by risk status, Outcome 1 Early all‐cause mortality.
4.2. Analysis.
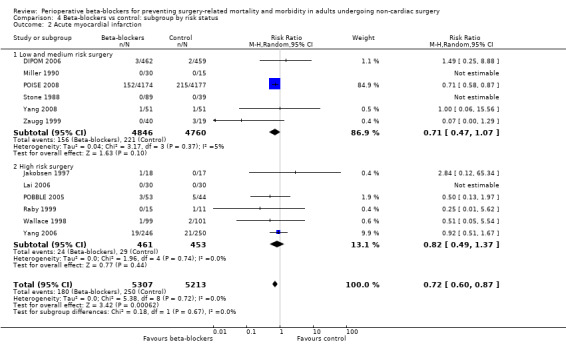
Comparison 4 Beta‐blockers vs control: subgroup by risk status, Outcome 2 Acute myocardial infarction.
4.3. Analysis.
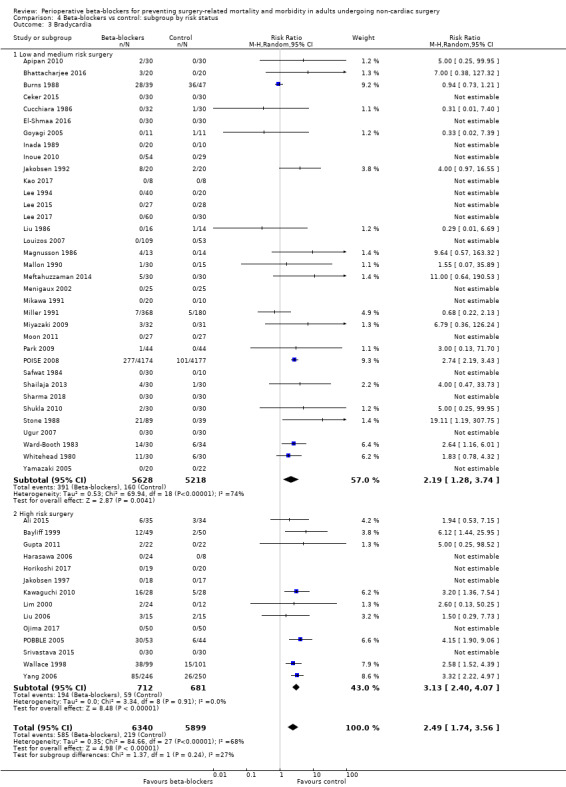
Comparison 4 Beta‐blockers vs control: subgroup by risk status, Outcome 3 Bradycardia.
4.4. Analysis.
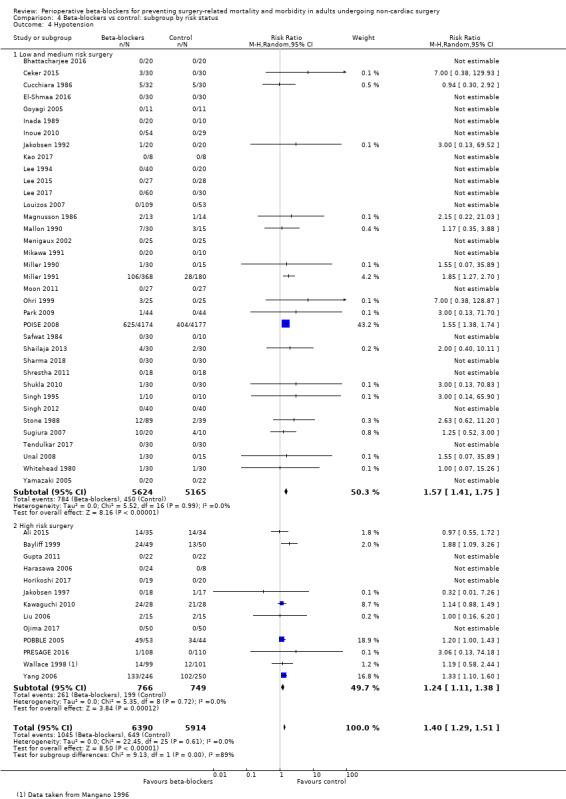
Comparison 4 Beta‐blockers vs control: subgroup by risk status, Outcome 4 Hypotension.
Sensitivity analysis
Standard care control
All‐cause mortality (30 days): we excluded five studies from analysis in which the control group comparison was standard care (Ali 2015; Kawaguchi 2010; Lai 2006; PRESAGE 2016; Yang 2008). Including only placebo‐controlled trials in this analysis, we found that mortality was slightly increased when beta‐blockers were given (RR 1.30, 95% CI 1.03 to 1.65; I2 = 0%; 11 studies, 10,945 participants); this differed from our primary analysis in which we found little or no difference between groups.
Acute myocardial infarction: we excluded four studies from analysis in which the control group comparison was standard care (Lai 2006; Stone 1988; Yang 2008; Zaugg 1999). This did not alter interpretation of the effect for this outcome.
Cerebrovascular events: we excluded two studies from analysis in which the control group comparison was standard care (PRESAGE 2016; Yang 2008). Including only placebo‐controlled trials in this analysis, we found that cerebrovascular events were increased with beta‐blocker use (RR 1.89, 95% CI 1.09 to 3.28; I2 = 0%; 4 studies, 9144 participants); this differed from our primary analysis in which we found little or no difference between groups.
Ventricular arrhythmias: the primary analysis included only placebo‐controlled trials.
Atrial fibrillation or atrial flutter, or both: we excluded two studies from analysis in which the control group comparison was standard care (Lai 2006; PRESAGE 2016). This did not alter interpretation of the effect for this outcome.
Bradycardia: we excluded four studies from analysis in which the control group comparison was standard care (Ali 2015; Kawaguchi 2010; Liu 2006; Stone 1988). This did not alter interpretation of the effect for this outcome.
Hypotension: we excluded seven studies from analysis in which the control group comparison was standard care (Ali 2015; Kawaguchi 2010; Liu 2006; Ohri 1999; PRESAGE 2016; Stone 1988; Tendulkar 2017). This did not alter interpretation of the effect for this outcome.
Risk of selection bias
All‐cause mortality (30 days): we excluded five studies with an unclear risk of selection bias (Ali 2015; Bayliff 1999; Lai 2006; Neary 2006; Yang 2008). Without these studies, analysis showed a slight increase in mortality when beta‐blockers were used (RR 1.31, 95% CI 1.03 to 1.66; I2 = 0%; 11 studies, 11,078 participants).
Acute myocardial infarction: we excluded five studies with an unclear risk of selection bias (Jakobsen 1997; Lai 2006; Stone 1988; Yang 2008; Zaugg 1999), This did not alter interpretation of the effect for this outcome.
Cerebrovascular events: we excluded Yang 2008, which had an unclear risk of selection bias. Without this study, analysis showed a slight increase in cerebrovascular events when beta‐blockers were used (RR 1.77, 95% CI 1.03 to 3.03; I2 = 0%; 5 studies, 9358 participants).
Ventricular arrhythmias: we excluded two studies with an unclear risk of selection bias (Bayliff 1999; Jakobsen 1997). This did not alter interpretation of the effect for this outcome.
Atrial fibrillation or atrial flutter, or both: we excluded four studies with an unclear risk of selection bias (Bayliff 1999; Lai 2006; Urias 2016; Yoshida 2017). This did not alter interpretation of the effect for this outcome.
Bradycardia: we excluded 28 studies with an unclear risk of selection bias (Ali 2015; Apipan 2010; Bayliff 1999; Burns 1988; Cucchiara 1986; Goyagi 2005; Harasawa 2006; Inada 1989; Inoue 2010; Jakobsen 1992; Jakobsen 1997; Kao 2017; Lim 2000; Lee 1994; Lee 2015; Liu 1986; Liu 2006; Louizos 2007; Magnusson 1986; Meftahuzzaman 2014; Mikawa 1991; Moon 2011; Safwat 1984; Shukla 2010; Stone 1988; Ward‐Booth 1983; Whitehead 1980; Yamazaki 2005). This did not alter interpretation of the effect for this outcome.
Hypotension: we excluded 27 studies with an unclear risk of selection bias (Ali 2015; Bayliff 1999; Cucchiara 1986; Goyagi 2005; Harasawa 2006; Inada 1989; Inoue 2010; Jakobsen 1992; Jakobsen 1997; Kao 2017; Lee 1994; Lee 2015; Liu 2006; Louizos 2007; Magnusson 1986; Mikawa 1991; Moon 2011; Ohri 1999; Safwat 1984; Shrestha 2011; Shukla 2010; Singh 2012; Stone 1988; Sugiura 2007; Unal 2008; Whitehead 1980; Yamazaki 2005). This did not alter interpretation of the effect for this outcome.
Risk of attrition bias
All‐cause mortality (30 days): we excluded three studies with a high risk of attrition bias (Ali 2015; DIPOM 2006; Yang 2006). Without these studies, analysis showed a slight increase in mortality when perioperative beta‐blockers were used (RR 1.29, 95% CI 1.01 to 1.65; I2 = 0%; 13 studies, 9960 participants).
Acute myocardial infarction: we excluded two studies with a high risk of attrition bias (DIPOM 2006; Yang 2006). This did not alter interpretation of the effect for this outcome.
Cerebrovascular events: we excluded Yang 2006, which had a high risk of attrition bias for this outcome. This did not alter interpretation of the effect for this outcome.
Ventricular arrhythmias: we included no studies with a high risk of attrition bias in analysis of this outcome.
Atrial fibrillation or atrial flutter, or both: we included no studies with a high risk of attrition bias in analysis of this outcome.
Bradycardia: we excluded four studies with a high risk of attrition bias (Ali 2015; Cucchiara 1986; Kao 2017; Yang 2006). This did not alter interpretation of the effect for this outcome.
Hypotension: we excluded four studies with a high risk of attrition bias (Ali 2015; Cucchiara 1986; Kao 2017; Yang 2006). This did not alter interpretation of the effect for this outcome.
Beta‐blockers given as rescue therapy
All‐cause mortality (30 days): we excluded two studies because participants in the control group of this study were given beta‐blockers as rescue therapy (Yang 2006; Ojima 2017). This did not alter interpretation of the effect for this outcome.
Acute myocardial infarction: we excluded two studies in which participants in the control group were given beta‐blockers as rescue therapy (Raby 1999; Yang 2006). This did not alter interpretation of the effect for this outcome.
Cerebrovascular events: we excluded Yang 2006 because participants in the control group of this study were given beta‐blockers as rescue therapy. This did not alter interpretation of the effect for this outcome.
Ventricular arrhythmias: for this outcome we included no studies in which study authors reported that participants in the control group were given additional beta‐blockers.
Atrial fibrillation and atrial flutter: we excluded one study in which participants in the control group were given beta‐blockers as rescue therapy. this did not alter interpretation of the effect.
Bradycardia: we excluded four studies in which participants in the control group were given beta‐blockers as rescue therapy (Louizos 2007; Ojima 2017; Whitehead 1980; Yang 2006). This did not alter interpretation of the effect for this outcome.
Hypotension: we excluded four studies in which participants in the control group were given beta‐blockers as rescue therapy (Louizos 2007; Ojima 2017; Whitehead 1980; Yang 2006). This did not alter interpretation of the effect for this outcome.
Studies published before 2000
All‐cause mortality (30 days): we excluded four studies published before 2000 (Bayliff 1999; Miller 1990; Miller 1991; Wallace 1998).This did not alter interpretation of the effect for this outcome.
Acute myocardial infarction: we excluded six studies published before 2000 (Jakobsen 1997; Miller 1990; Raby 1999; Stone 1988; Wallace 1998; Zaugg 1999).This did not alter interpretation of the effect for this outcome.
Cerebrovascular events: we excluded one study published before 2000 (Wallace 1998). This did not alter interpretation of the effect for this outcome.
Ventricular arrhythmias: for this outcome, we included only one study published after 2000 (POBBLE 2005). The effect estimate for this single study was consistent with the meta‐analysis which included studies published before 2000.
Atrial fibrillation and atrial flutter: we excluded one study published before 2000 (Bayliff 1999). This did not alter interpretation of the effect for this outcome.
Bradycardia: we 17 excluded studies published before 2000 (Bayliff 1999; Burns 1988; Cucchiara 1986; Inada 1989; Jakobsen 1992; Jakobsen 1997; Lee 1994; Liu 1986; Magnusson 1986; Mallon 1990; Mikawa 1991; Miller 1991; Safwat 1984; Stone 1988; Wallace 1998; Ward‐Booth 1983; Whitehead 1980). This did not alter interpretation of the effect for this outcome.
Hypotension: we excluded studies published before 2000 (Bayliff 1999; Cucchiara 1986; Inada 1989; Jakobsen 1992; Jakobsen 1997; Lee 1994; Magnusson 1986; Mallon 1990; Mikawa 1991; Miller 1990; Miller 1991; Ohri 1999; Safwat 1984; Singh 1995; Stone 1988; Wallace 1998; Whitehead 1980). This did not alter interpretation of the effect for this outcome.
Outcomes with rare events
In studies reporting cerebrovascular events, fewer than 1% participants had an event. In sensitivity analysis, we used Peto odds ratio. This did not alter the interpretation of the effect.
Discussion
Summary of main results
We found 83 studies in which beta‐blockers were given during the perioperative period to adults undergoing non‐cardiac surgery. We also identified 18 studies awaiting classification (three were reported only as abstracts; we were unable to access the full text of seven; four are completed trials without full‐text reports; and four require translation), and three ongoing studies.
The evidence for early all‐cause mortality was uncertain; based on the risk of death in the control group of 25 per 1000, the effect of beta‐blockers was between two fewer and 13 more per 1000. We found no evidence of a difference in cerebrovascular events (low‐certainty evidence), and ventricular arrhythmias (very low‐certainty evidence). We found low‐certainty evidence that beta‐blockers may reduce the incidence of myocardial infarction and atrial fibrillation or flutter. However, we also found low‐certainty evidence that beta‐blockers may increase bradycardia, and moderate‐certainty evidence that beta‐blockers probably increase hypotension.
We found little or no difference in long‐term mortality, death due to cardiac causes, and in congestive heart failure, depending on whether beta‐blockers were given; we assessed the certainty of the evidence for these outcomes to be low. We found no evidence of a difference in length of hospital stay, but we did not explore reasons for moderate statistical heterogeneity in this effect, and we assessed the certainty of the evidence to be very low. No studies assessed quality of life.
Overall completeness and applicability of evidence
We identified 83 studies with 14,967 participants, of which more than half were identified in the most recent search.
All studies included adult participants undergoing non‐cardiac surgery. Only one study assessed beta‐blockers for emergency surgery (Neary 2006); the remaining surgeries were elective. We anticipated that studies for this review would include a wide range of types of surgery, with different degrees of risk. The evidence included studies of surgery that were: thoracic; vascular; neuro; burns; abdominal; oral and maxillofacial; ear, nose and throat; gynaecological; orthopaedic; mixed surgeries; and some elective surgeries that were not specified by study authors. We categorized surgical risk according to Kristensen 2014, and found 26.5% studies included high‐risk surgeries and 13.3% included low‐risk surgeries. Although we categorized most studies as medium risk, we included in this category studies in which the type of surgery was not specified. We used subgroup analysis to explore whether beta‐blockers had a different effect according to the surgical risk. Whilst we found no evidence of a difference between subgroups overall, we noted the effect of POISE 2008 in the data for all‐cause mortality. For this subgroup analysis, we noted that studies of low‐ or medium‐risk surgeries showed an increase in mortality with beta‐blockers, but there was no evidence of a difference in effect in high‐risk surgeries; POISE 2008 recruited participants undergoing various surgeries to include vascular, intraperitoneal, and orthopaedic surgery and we categorized this as medium risk.
We included in the review several studies published prior to 2000, and these studies may have used clinical management strategies that differ from current practice. We explored this in sensitivity analysis, and found no changes to the interpretation of the effect for the main review outcomes.
Quality of the evidence
We used GRADE to downgrade the certainty of the evidence. In our risk of bias assessments, we judged 26.5% of studies to be at high risk of bias in at least one domain, and we downgraded evidence for all outcomes owing to study limitations. In sensitivity analyses, we explored the effect of including only studies with more robust study designs. We excluded open‐label studies in which comparisons had been made with standard care, and studies with risks of selection bias or attrition bias. We noted some differences in effect during some sensitivity analyses and we, therefore, also considered these sensitivity analyses, downgrading the certainty of the evidence owing to study limitations for all‐cause mortality, cerebrovascular events, and atrial fibrillation or flutter.
We noted that one large, international, and methodologically robust study did not report results that were consistent with smaller studies (POISE 2008). We downgraded the evidence for all‐cause mortality and cerebrovascular events because POISE 2008 showed an increase in events when beta‐blockers were used, whilst the remaining smaller studies showed evidence of neither an increase or decrease in events. Similarly, we downgraded the evidence for myocardial infarction because POISE 2008 showed a reduction in events when beta‐blockers were used, whilst the remaining smaller studies showed evidence of neither an increase or decrease in events.
Evidence for ventricular arrhythmias was from few studies with few participants, and we downgraded this outcome owing to imprecision.
Overall, we graded the evidence for perioperative beta‐blockers as low certainty, very low certainty, and moderate certainty, which means there is the possibility that the true estimate may be substantially different
Potential biases in the review process
Our previous review assessed beta‐blockers in both cardiac and non‐cardiac surgery (Blessberger 2018); we split this review into two reviews according to type of surgery. This version incorporates data from studies of non‐cardiac surgery and includes an updated and refined search strategy. We conducted a thorough search in the update and used two review authors to assess study eligibility, extract data, and assess risk of bias in included studies; therefore, we reduced potential bias in the review process.
During the updating process, we made changes to the review to meet current Cochrane standards. This included minor clarifications to the inclusion criteria of the review, and changes to wording of the sections of the methods within Data collection and analysis. In particular, this version of the review includes fewer outcomes. Data for myocardial ischaemia, supraventricular arrhythmias (except atrial fibrillation), bronchospasm and cost of care are reported only in the previous version (Blessberger 2018). In reducing the number of outcomes, our intention was to improve the usability of the review; therefore, we selected outcomes that we considered to be most important to users of the review. We used the work of Myles and colleagues to support this decision making process (Myles 2016), and sought advice from a Cochrane Editor before agreeing to a reduction in outcomes.
In addition, we did not include meta‐regression as a method to explore heterogeneity in this review. We explored heterogeneity only through subgroup analysis, which was pre‐specified in the review protocol. We conducted analyses using a random‐effects model, rather than choosing the effects‐model based on statistical heterogeneity (Borenstein 2010); we evaluated in sensitivity analysis the effect of using Peto odds ratio for rare events, which uses a fixed‐effect model. Again, we made these decisions following advice from the Cochrane Editorial team.
We included only trials that investigated at least one outcome specified in the Methods section. Because of the vast scope of the literature search and the large number of search results and included trials, this approach was justified in order to improve the management of the review. Whilst this may have introduced a source of bias, we judged the impact of potentially missing studies as small because the available set of data was derived from a large number of trials. As explained in previous versions of the review, we included studies in which some participants may not have received general anaesthesia, which differed from our original protocol. Whilst we attempted to limit the number of participants not undergoing general anaesthesia, we could not be certain of the effect of including a mixed population.
Furthermore, we could not contact all study authors to gather further information about trial design or data analysis (e.g. performance of an ITT analysis) because of the large number of studies. However, it is likely that study authors, if contacted, may over‐report trial design quality criteria.
Agreements and disagreements with other studies or reviews
In the previous version of the review we found no clear evidence of a difference in all‐cause mortality (Blessberger 2018). When analysis was restricted to placebo‐controlled studies, we continued to find an increase in all‐cause mortality with the use of beta‐blockers. However, our subgroup analysis continued to be led by one large multi‐centre, and well‐conducted, study in which metoprolol given to participants undergoing medium risk surgeries showed an increase in mortality with beta‐blocker use (POISE 2008). The effect of POISE 2008 was also evident in the analyses for myocardial infarction (POISE 2008 showed a reduction in myocardial infarction) and cerebrovascular events (POISE 2008 showed an increase in stroke), and these results were also inconsistent with the remaining smaller studies. The review by Bouri and colleagues endeavoured to re‐evaluate the evidence for perioperative beta‐blockers without studies that were at risk of including fictitious data. Bouri 2014 assessed only preoperative beta‐blocker use, therefore including POISE 2008. Their analyses showed an increase in mortality and stroke with beta‐blocker use, alongside a reduction in myocardial infarction.
It should be noted that our primary analyses include evidence from a variety of different beta‐blockers, and doses of beta‐blockers, which were started before, during or after surgery of different risk statuses. We cannot ignore the results from POISE 2008, which indicate an increased risk of mortality and stroke; however, results of POISE 2008 may be influenced specifically by the use of a relatively high dose of metoprolol administered if the pre‐operative heart rate was above 50 beats per minute (not titrated to a specific heart rate), and was given just before surgery (two to four hours). We have allowed for this inconsistency when downgrading the certainty of the evidence in this review.
Our findings for all‐cause mortality and myocardial infarction were consistent with an early systematic review, which included POISE 2008 (Bangalore 2008); although we noted that Bangalore 2008 found an increase in cerebrovascular events with beta‐blockers. Hajibandeh 2017 found little or no difference in effect for all‐cause mortality and myocardial infarction. The systematic review included both observational studies and RCTs and was limited to vascular and endovascular surgery; its findings were consistent with our subgroup analyses results for high‐risk surgery.
Authors' conclusions
Implications for practice.
In non‐cardiac surgery, the evidence for early all‐cause mortality was uncertain. We found no evidence of a difference in cerebrovascular events or ventricular arrhythmias; however, the certainty of this evidence was low and very low. We found low‐certainty evidence that beta‐blockers may reduce atrial fibrillation and myocardial infarctions. However, perioperative beta‐blockers may increase incidences of bradycardia and probably increase hypotension. We found 18 studies awaiting classification; inclusion of these studies in future updates may also increase the certainty of the evidence.
Implications for research.
We continued to find some uncertainty in the evidence, demonstrated by inconsistency between study results in smaller trials and one large trial. Further research is needed in the field of non‐cardiac surgery so that firm conclusions can be drawn on the use of perioperative beta‐blockers.
Furthermore, timing of beta‐blocker application may have an important influence on their effect (for example, the possible effect of plaque stabilization, and haemodynamic adaptation). More large placebo‐controlled trials with robust methodology are needed to explore this effect in unselected surgical populations.
We found no studies measuring the effect on quality of life. This important outcome can be measured effectively through validated questionnaires, and we recommend that this outcome is incorporated into all future studies in this field.
The review assessed only direct comparisons. Given the different types of beta‐blockers, and continuing research that we expect to contribute to this field, a network meta‐analysis should be considered in future review updates. A network meta‐analysis would explore indirect comparisons, as well as direct comparisons, and may be useful to clinicians by providing a more comprehensive analysis of the effects of each type of beta‐blocker, and further improve the precision of the estimates.
What's new
| Date | Event | Description |
|---|---|---|
| 30 August 2019 | New citation required but conclusions have not changed |
|
| 30 August 2019 | New search has been performed |
|
History
Review first published: Issue 9, 2019
| Date | Event | Description |
|---|---|---|
| 22 February 2018 | New citation required but conclusions have not changed | Removal of retracted study (Suttner 2009); conclusions unchanged. |
| 22 February 2018 | Amended | Following publication of the first version of this systematic review, a previously included study was retracted (Suttner 2009). This trial has now been excluded and all relevant analyses and results have been amended accordingly. All NNTB/Hs were recalculated according to the formula in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Guideline recommendations were updated in the Discussion section. |
Notes
None.
Acknowledgements
We would like to thank the authors involved in previous versions of the review (Danyel Azar, Martin Schillinger, Franz Wiesbauer), people who offered editorial support or advice in preparation of the protocol (M Müllner, L Richard, K Schroeder and MJ Zeitler), or editorial support in previous versions of the review (Jane Cracknell, Managing Editor; Harald Herkner, Content Editor; Cathal Walsh, Statistical Editor; Janet Wale, Consumer Editor). We thank peer review support in previous versions of the review (G Guyatt, F Botto, G Lurati, M Mrkobrada, P Foex and J Wetterslev), assistance with translation of articles in Chinese (Y Wang; formerly of Linz General Hospital, Austria), and researchers who provided additional information by email (G Hamilton, University College London Medical School; and P Rahimzadeh Iran University of Medical Sciences).
We would like to thank Anna Lee (Content Editor), Susanne Schmitz (Statistical Editor), Ben Gibbison (Peer Reviewer), Janet Wale (Consumer Editor), Liz Bickerdike (Associate Editor, Acute and Emergency Care Network), Toby Lasserson (Senior Editor, Review Production and Quality Unit), Jane Cracknell and Teo Quay (Managing Editors), and Andrew Smith (Co‐ordinating Editor) for their help and editorial advice during the production of this review.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Adrenergic beta‐Antagonists] explode all trees
#2 MeSH descriptor: [Bendroflumethiazide] explode all trees
#3 (acebutolol* or adimolol* or afurolol* or alpha hydroxymetoprolol* or alprenolol* or amosulalol* or arotinolol* or atenolol* or befunolol* or bendroflumethiazide* or betaxolol* or bevantolol* or bfe 55 or bisoprolol* or bopindolol* or bornaprolol* or brefonalol* or bromoacetylalprenololmenthane* or bucindolol* or bucumolol* or bufetolol* or bufuralol* or bunitrolol* or bunolol* or bupranolol* or butofilolol* or butoxamine* or carazolol* or carteolol* or carvedilol* or celiprolol* or cetamolol* or cloranolol* or cyanoiodopindolol* or cyanopindolol* or deacetylmetipranolol* or diacetolol* or dihydroalprenolol* or dilevalol* or epanolol* or esmolol* or exaprolol* or falintolol* or flestolol* or flusoxolol* or hydroxybenzylpindolol* or indenolol* or iodocyanopindolol* or iodopindolol* or iprocrolol* or isamoltane* or isoxaprolol* or labetalol* or landiolol* or levobunolol* or levomoprolol* or medroxalol* or mepindolol* or mercuderamide* or metipranolol* or metoprolol* or moprolol* or nadolol* or nebivolol* or nifenalol* or nipradilol* or oxprenolol* or pafenolol* or pamatolol* or penbutolol* or pindolol* or practolol* or primidolol* or prizidilol* or pronetalol* or propranolol* or proxodolol* or ridazolol* or salcardolol* or sandoz 204545 or soquinolol* or sotalol* or spirendolol* or talinolol* or tertatolol* or tienoxolol* or tilisolol* or timolol* or tolamolol* or toliprolol* or trasitensin* or trepress* or tribendilol* or viskaldix* or xibenolol* or zolertine or adaprolol* or bamosiran* or bendacalol* or dramedilol* or ersentilide* or oberadilol* or procinolol* or zoleprodolol*):ti,ab,kw
#4 (beta* near (adrenergic* or blocker* or blockade* or blocking)):ti,ab,kw
#5 (#1 OR #2 OR #3 OR #4)
#6 MeSH descriptor: [Premedication] explode all trees
#7 MeSH descriptor: [Preanesthetic Medication] explode all trees
#8 MeSH descriptor: [Intraoperative Period] explode all trees
#9 MeSH descriptor: [Intraoperative Complications] explode all trees
#10 MeSH descriptor: [Perioperative Period] explode all trees
#11 MeSH descriptor: [Postoperative Complications] explode all trees
#12 MeSH descriptor: [Postoperative Period] explode all trees
#13 MeSH descriptor: [Preoperative Period] explode all trees
#14 (intraoperat* or perioperat* or peroperat* or postoperat* or preoperat* or premedicat* or intra operat* or peri operat* or per operat* or post operat* or pre operat* or pre medicat*):ti,ab,kw or ((during or before or prior or undergo* or following or after) NEAR/3 (surg* or operat* or infusion* or procedur* or bypass or intubat* or anaesth* or anesth*)):ti,ab,kw
#15 (#6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14)
#16 MeSH descriptor: [Specialties, Surgical] explode all trees
#17 MeSH descriptor: [Anesthesia, General] explode all trees
#18 MeSH descriptor: [Coronary Artery Bypass] explode all trees
#19 (surg* or operat* or anesth* or anaesth* or bypass):ti,ab,kw
#20 (#16 OR #17 OR #18 OR #19)
#21 (#5 AND #15 AND #20)
#22 #21 in Trials
Appendix 2. MEDLINE search strategy
exp Adrenergic beta‐Antagonists/ or exp Bendroflumethiazide/ or (acebutolol* or adimolol* or afurolol* or alpha hydroxymetoprolol* or alprenolol* or amosulalol* or arotinolol* or atenolol* or befunolol* or bendroflumethiazide* or betaxolol* or bevantolol* or bfe 55 or bisoprolol* or bopindolol* or bornaprolol* or brefonalol* or bromoacetylalprenololmenthane* or bucindolol* or bucumolol* or bufetolol* or bufuralol* or bunitrolol* or bunolol* or bupranolol* or butofilolol* or butoxamine* or carazolol* or carteolol* or carvedilol* or celiprolol* or cetamolol* or cloranolol* or cyanoiodopindolol* or cyanopindolol* or deacetylmetipranolol* or diacetolol* or dihydroalprenolol* or dilevalol* or epanolol* or esmolol* or exaprolol* or falintolol* or flestolol* or flusoxolol* or hydroxybenzylpindolol* or indenolol* or iodocyanopindolol* or iodopindolol* or iprocrolol* or isamoltane* or isoxaprolol* or labetalol* or landiolol* or levobunolol* or levomoprolol* or medroxalol* or mepindolol* or mercuderamide* or metipranolol* or metoprolol* or moprolol* or nadolol* or nebivolol* or nifenalol* or nipradilol* or oxprenolol* or pafenolol* or pamatolol* or penbutolol* or pindolol* or practolol* or primidolol* or prizidilol* or pronetalol* or propranolol* or proxodolol* or ridazolol* or salcardolol* or sandoz 204545 or soquinolol* or sotalol* or spirendolol* or talinolol* or tertatolol* or tienoxolol* or tilisolol* or timolol* or tolamolol* or toliprolol* or trasitensin* or trepress* or tribendilol* or viskaldix* or xibenolol* or zolertine or adaprolol* or bamosiran* or bendacalol* or dramedilol* or ersentilide* or oberadilol* or procinolol* or zoleprodolol* or (beta* adj3 (adrenergic* or blocker* or blockade* or blocking))).ti,ab,kf.
exp Premedication/ or Preanesthetic Medication/ or Intraoperative Period/ or Intraoperative Complications/ or Perioperative Period/ or Postoperative Complications/ or Postoperative Period/ or Preoperative Period/ or (intraoperat* or perioperat* or peroperat* or postoperat* or preoperat* or premedicat* or intra operat* or peri operat* or per operat* or post operat* or pre operat* or pre medicat*).ti,ab,hw,kf. or ((during or before or prior or undergo* or following or after) adj3 (surg* or operat* or infusion* or intubat* or an?esth* or procedur* or bypass)).ti,ab,kf.
exp Specialties, Surgical/ or General Surgery/ or coronary artery bypass/ or su.xs. or (surg* or operat* or bypass).ti,ab,hw,kf. or exp Anesthesia, General/ or an?esth*.ti,ab,hw,kf.
((randomized controlled trial or controlled clinical trial).pt. or random*.ab. or placebo.ab. or clinical trials as topic.sh. or random allocation.sh. or trial.ti.) not (exp animals/ not humans.sh.)
1 and 2 and 3 and 4
Appendix 3. Embase search strategy
exp beta adrenergic receptor blocking agent/ or bendroflumethiazide/ or (acebutolol* or adimolol* or afurolol* or alpha hydroxymetoprolol* or alprenolol* or amosulalol* or arotinolol* or atenolol* or befunolol* or bendroflumethiazide* or betaxolol* or bevantolol* or bfe 55 or bisoprolol* or bopindolol* or bornaprolol* or brefonalol* or bromoacetylalprenololmenthane* or bucindolol* or bucumolol* or bufetolol* or bufuralol* or bunitrolol* or bunolol* or bupranolol* or butofilolol* or butoxamine* or carazolol* or carteolol* or carvedilol* or celiprolol* or cetamolol* or cloranolol* or cyanoiodopindolol* or cyanopindolol* or deacetylmetipranolol* or diacetolol* or dihydroalprenolol* or dilevalol* or epanolol* or esmolol* or exaprolol* or falintolol* or flestolol* or flusoxolol* or hydroxybenzylpindolol* or indenolol* or iodocyanopindolol* or iodopindolol* or iprocrolol* or isamoltane* or isoxaprolol* or labetalol* or landiolol* or levobunolol* or levomoprolol* or medroxalol* or mepindolol* or mercuderamide* or metipranolol* or metoprolol* or moprolol* or nadolol* or nebivolol* or nifenalol* or nipradilol* or oxprenolol* or pafenolol* or pamatolol* or penbutolol* or pindolol* or practolol* or primidolol* or prizidilol* or pronetalol* or propranolol* or proxodolol* or ridazolol* or salcardolol* or sandoz 204545 or soquinolol* or sotalol* or spirendolol* or talinolol* or tertatolol* or tienoxolol* or tilisolol* or timolol* or tolamolol* or toliprolol* or trasitensin* or trepress* or tribendilol* or viskaldix* or xibenolol* or zolertine or adaprolol* or bamosiran* or bendacalol* or dramedilol* or ersentilide* or oberadilol* or procinolol* or zoleprodolol* or (beta* adj3 (adrenergic* or blocker* or blockade* or blocking))).ti,ab,kw,hw.
premedication/ or intraoperative period/ or peroperative complication/ or perioperative period/ or postoperative complication/ or postoperative period/ or preoperative period/ or (intraoperat* or perioperat* or peroperat* or postoperat* or preoperat* or premedicat* or intra operat* or peri operat* or per operat* or post operat* or pre operat* or pre medicat*).ti,ab,hw,kw. or ((during or before or prior or undergo* or following or after) adj3 (surg* or operat* or infusion* or intubat* or an?esth* or procedur* or bypass)).ti,ab,kw.
exp surgery/ or exp general anesthesia/ or coronary artery bypass graft/ or (surg* or operat* or bypass or an?esth*).ti,ab,hw,kw.
(placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab.) not (animals not (humans and animals)).sh.
1 and 2 and 3 and 4
Appendix 4. CINAHL search strategy
S1 (MH "Adrenergic Beta‐Antagonists+") OR TX ( acebutolol* or adimolol* or afurolol* or alpha hydroxymetoprolol* or alprenolol* or amosulalol* or arotinolol* or atenolol* or befunolol* or bendroflumethiazide* or betaxolol* or bevantolol* or bfe 55 or bisoprolol* or bopindolol* or bornaprolol* or brefonalol* or bromoacetylalprenololmenthane* or bucindolol* or bucumolol* or bufetolol* or bufuralol* or bunitrolol* or bunolol* or bupranolol* or butofilolol* or butoxamine* or carazolol* or carteolol* or carvedilol* or celiprolol* or cetamolol* or cloranolol* or cyanoiodopindolol* or cyanopindolol* or deacetylmetipranolol* or diacetolol* or dihydroalprenolol* or dilevalol* or epanolol* or esmolol* or exaprolol* or falintolol* or flestolol* or flusoxolol* or hydroxybenzylpindolol* or indenolol* or iodocyanopindolol* or iodopindolol* or iprocrolol* or isamoltane* or isoxaprolol* or labetalol* or landiolol* or levobunolol* or levomoprolol* or medroxalol* or mepindolol* or mercuderamide* or metipranolol* or metoprolol* or moprolol* or nadolol* or nebivolol* or nifenalol* or nipradilol* or oxprenolol* or pafenolol* or pamatolol* or penbutolol* or pindolol* or practolol* or primidolol* or prizidilol* or pronetalol* or propranolol* or proxodolol* or ridazolol* or salcardolol* or sandoz 204545 or soquinolol* or sotalol* or spirendolol* or talinolol* or tertatolol* or tienoxolol* or tilisolol* or timolol* or tolamolol* or toliprolol* or trasitensin* or trepress* or tribendilol* or viskaldix* or xibenolol* or zolertine or adaprolol* or bamosiran* or bendacalol* or dramedilol* or ersentilide* or oberadilol* or procinolol* or zoleprodolol*) OR TX ( (beta* and (adrenergic* or blocker* or blocking or blockade*)))
S2 ((MH "Premedication") OR (MH "Intraoperative Period") OR (MH "Postoperative Period") OR (MH "Intraoperative Complications") OR (MH "Preoperative Period") OR (MH "Postoperative Complications")) OR TX ( intraoperat* or perioperat* or peroperat* or postoperat* or preoperat* or premedicat* or intra operat* or peri operat* or per operat* or post operat* or pre operat* or pre medicat*) or TX ((during or before or prior or undergo* or following or after) N3 (surg* or operat* or infusion* or intubat* or anaesth* or anesth* or procedur* or bypass))
S3 (MH "Specialties, Surgical+") OR (MH "Anesthesia, General+") OR (MH "Coronary Artery Bypass+") OR TX (surg* or operat* or bypass or an*esth*)
S4 S1 AND S2 AND S3
S5 ((MH "Randomized Controlled Trials") OR (MH "Clinical Trials+") OR (MH "Random Assignment") OR (MH "Prospective Studies+") OR (MH "Clinical Trial Registry") OR (MH "Double‐Blind Studies") OR (MH "Single‐Blind Studies") OR (MH "Triple‐Blind Studies") OR (MH "Multicenter Studies") OR (MH "Placebos")) OR TX (random* or placebo* or trial*)
S6 S4 AND S5
Appendix 5. Biosis Previews search strategy
#1 TS=(acebutolol* or adimolol* or afurolol* or alpha hydroxymetoprolol* or alprenolol* or amosulalol* or arotinolol* or atenolol* or befunolol* or bendroflumethiazide* or betaxolol* or bevantolol* or bfe 55 or bisoprolol* or bopindolol* or bornaprolol* or brefonalol* or bromoacetylalprenololmenthane* or bucindolol* or bucumolol* or bufetolol* or bufuralol* or bunitrolol* or bunolol* or bupranolol* or butofilolol* or butoxamine* or carazolol* or carteolol* or carvedilol* or celiprolol* or cetamolol* or cloranolol* or cyanoiodopindolol* or cyanopindolol* or deacetylmetipranolol* or diacetolol* or dihydroalprenolol* or dilevalol* or epanolol* or esmolol* or exaprolol* or falintolol* or flestolol* or flusoxolol* or hydroxybenzylpindolol* or indenolol* or iodocyanopindolol* or iodopindolol* or iprocrolol* or isamoltane* or isoxaprolol* or labetalol* or landiolol* or levobunolol* or levomoprolol* or medroxalol* or mepindolol* or mercuderamide* or metipranolol* or metoprolol* or moprolol* or nadolol* or nebivolol* or nifenalol* or nipradilol* or oxprenolol* or pafenolol* or pamatolol* or penbutolol* or pindolol* or practolol* or primidolol* or prizidilol* or pronetalol* or propranolol* or proxodolol* or ridazolol* or salcardolol* or sandoz 204545 or soquinolol* or sotalol* or spirendolol* or talinolol* or tertatolol* or tienoxolol* or tilisolol* or timolol* or tolamolol* or toliprolol* or trasitensin* or trepress* or tribendilol* or viskaldix* or xibenolol* or zolertine or adaprolol* or bamosiran* or bendacalol* or dramedilol* or ersentilide* or oberadilol* or procinolol* or zoleprodolol* or (beta* NEAR (adrenergic* or blocker* or blocking)))
#2 TS=(intraoperat* or perioperat* or peroperat* or postoperat* or preoperat* or premedicat* or “intra operat*” or “peri operat*” or “per operat*” or “post operat*” or “pre operat*” or “pre medicat*”) or TS= ((during or before or prior or undergo* or following or after) NEAR/3 (surg* or operat* or infusion* or procedur* or bypass or intubat* or anaesth* or anesth*))
#3 TS=(surg* or operat* or anesth* or anaesth* or bypass)
#4 TS=(placebo* or random* or control* or prospectiv* or volunteer* or (clin* NEAR/3 trial*) or (compar* NEAR/3 stud*) or ((singl* or doubl* or trebl* or tripl*) NEAR/3 (blind* or mask*)))
#5 #4 AND #3 AND #2 AND #1
Appendix 6. Web of Science search strategy
#1 TS=(acebutolol* or adimolol* or afurolol* or alpha hydroxymetoprolol* or alprenolol* or amosulalol* or arotinolol* or atenolol* or befunolol* or bendroflumethiazide* or betaxolol* or bevantolol* or bfe 55 or bisoprolol* or bopindolol* or bornaprolol* or brefonalol* or bromoacetylalprenololmenthane* or bucindolol* or bucumolol* or bufetolol* or bufuralol* or bunitrolol* or bunolol* or bupranolol* or butofilolol* or butoxamine* or carazolol* or carteolol* or carvedilol* or celiprolol* or cetamolol* or cloranolol* or cyanoiodopindolol* or cyanopindolol* or deacetylmetipranolol* or diacetolol* or dihydroalprenolol* or dilevalol* or epanolol* or esmolol* or exaprolol* or falintolol* or flestolol* or flusoxolol* or hydroxybenzylpindolol* or indenolol* or iodocyanopindolol* or iodopindolol* or iprocrolol* or isamoltane* or isoxaprolol* or labetalol* or landiolol* or levobunolol* or levomoprolol* or medroxalol* or mepindolol* or mercuderamide* or metipranolol* or metoprolol* or moprolol* or nadolol* or nebivolol* or nifenalol* or nipradilol* or oxprenolol* or pafenolol* or pamatolol* or penbutolol* or pindolol* or practolol* or primidolol* or prizidilol* or pronetalol* or propranolol* or proxodolol* or ridazolol* or salcardolol* or sandoz 204545 or soquinolol* or sotalol* or spirendolol* or talinolol* or tertatolol* or tienoxolol* or tilisolol* or timolol* or tolamolol* or toliprolol* or trasitensin* or trepress* or tribendilol* or viskaldix* or xibenolol* or zolertine or adaprolol* or bamosiran* or bendacalol* or dramedilol* or ersentilide* or oberadilol* or procinolol* or zoleprodolol* or (beta* NEAR (adrenergic* or blocker* or blocking)))
#2 TS=(intraoperat* or perioperat* or peroperat* or postoperat* or preoperat* or premedicat* or “intra operat*” or “peri operat*” or “per operat*” or “post operat*” or “pre operat*” or “pre medicat*”) or TS= ((during or before or prior or undergo* or following or after) NEAR/3 (surg* or operat* or infusion* or procedur* or bypass or intubat* or anaesth* or anesth*))
#3 TS=(surg* or operat* or anesth* or anaesth* or bypass)
#4 TS=(placebo* or random* or control* or prospectiv* or volunteer* or (clin* NEAR/3 trial*) or (compar* NEAR/3 stud*) or ((singl* or doubl* or trebl* or tripl*) NEAR/3 (blind* or mask*)))
#5 #4 AND #3 AND #2 AND #1
Appendix 7. Conference Proceedings Citation Index‐Science search strategy
#1 TS=(acebutolol* or adimolol* or afurolol* or alpha hydroxymetoprolol* or alprenolol* or amosulalol* or arotinolol* or atenolol* or befunolol* or bendroflumethiazide* or betaxolol* or bevantolol* or bfe 55 or bisoprolol* or bopindolol* or bornaprolol* or brefonalol* or bromoacetylalprenololmenthane* or bucindolol* or bucumolol* or bufetolol* or bufuralol* or bunitrolol* or bunolol* or bupranolol* or butofilolol* or butoxamine* or carazolol* or carteolol* or carvedilol* or celiprolol* or cetamolol* or cloranolol* or cyanoiodopindolol* or cyanopindolol* or deacetylmetipranolol* or diacetolol* or dihydroalprenolol* or dilevalol* or epanolol* or esmolol* or exaprolol* or falintolol* or flestolol* or flusoxolol* or hydroxybenzylpindolol* or indenolol* or iodocyanopindolol* or iodopindolol* or iprocrolol* or isamoltane* or isoxaprolol* or labetalol* or landiolol* or levobunolol* or levomoprolol* or medroxalol* or mepindolol* or mercuderamide* or metipranolol* or metoprolol* or moprolol* or nadolol* or nebivolol* or nifenalol* or nipradilol* or oxprenolol* or pafenolol* or pamatolol* or penbutolol* or pindolol* or practolol* or primidolol* or prizidilol* or pronetalol* or propranolol* or proxodolol* or ridazolol* or salcardolol* or sandoz 204545 or soquinolol* or sotalol* or spirendolol* or talinolol* or tertatolol* or tienoxolol* or tilisolol* or timolol* or tolamolol* or toliprolol* or trasitensin* or trepress* or tribendilol* or viskaldix* or xibenolol* or zolertine or adaprolol* or bamosiran* or bendacalol* or dramedilol* or ersentilide* or oberadilol* or procinolol* or zoleprodolol* or (beta* NEAR (adrenergic* or blocker* or blocking)))
#2 TS=(intraoperat* or perioperat* or peroperat* or postoperat* or preoperat* or premedicat* or “intra operat*” or “peri operat*” or “per operat*” or “post operat*” or “pre operat*” or “pre medicat*”) or TS= ((during or before or prior or undergo* or following or after) NEAR/3 (surg* or operat* or infusion* or procedur* or bypass or intubat* or anaesth* or anesth*))
#3 TS=(surg* or operat* or anesth* or anaesth* or bypass)
#4 TS=(placebo* or random* or control* or prospectiv* or volunteer* or (clin* NEAR/3 trial*) or (compar* NEAR/3 stud*) or ((singl* or doubl* or trebl* or tripl*) NEAR/3 (blind* or mask*)))
#5 #4 AND #3 AND #2 AND #1
Appendix 8. Data extraction form
| Completed by: | |
| Date completed: | |
| Study ID | |
| Methods | |
| Participants |
Total number of randomized participants: Inclusion criteria: Exclusion criteria: Type of surgery: Baseline characteristics Intervention group
Control group
Country: Setting: |
| Interventions | Intervention group
Control group
|
| Outcomes |
Outcomes measured/reported by study authors: Outcomes relevant to the review: |
| Notes |
Funding/declarations of interest: Study dates: Notes: |
Outcome data
(Complete tables for all available relevant outcome data)
| Name of outcome: | |
| Time point of measurement: | |
| Intervention group | |
| Number of events | Total number of participants in the group |
| Control group | |
| Number of events | Total number of participants in the group |
| Name of outcome: | Length of stay | |
| Intervention group | ||
| Mean | SD | Total number of participants in the group |
| Control group | ||
| Mean | SD | Total number of participants in the group |
Risk of bias table
| Domain |
High/Low/ Unclear |
Judgement |
| Random sequence generation (selection bias) | ||
|
Allocation concealment (selection bias) |
||
| Blinding of participants and personnel (performance bias) | ||
| Blinding of outcome assessors (detection bias) | ||
| Incomplete outcome data (attrition bias) | ||
|
Selective reporting (reporting bias) |
||
| Other bias |
Appendix 9. Summary of risk factors in included studies
| Study ID | Age in years, mean (SD)a,b |
Gender, M/F, nb |
Previous MI, %a,b |
History of hypertension, %b |
Congestive heart failure, or coronary artery disease, %b |
Preoperative beta‐blockers, %b |
| Ali 2015 | I: 41 (± 14) C: 38 (± 16) |
I: 29/6 C: 30/4 |
NR | NR | NR | NR |
| Alkaya 2014 | I: 39.40 (± 10.76) C: 45.07 (± 13.27) |
I: 9/6 C:10/5 |
NR | NR | NR | Overall: 0 |
| Aoyama 2016 | I: 67.4 (± 8.7) C: 66.9 (± 8.9) |
I: 14/11 C: 17/8 |
Overall (recent history): 0 | NR | Overall: 0 | NR |
| Apipan 2010 | I: 24.63 (± 3.71) C: 26.30 (± 4.69) |
I: 19/11 C: 19/11 |
NR | NR | NR | NR |
| Bayliff 1999 | I: 63.3 (± 9.3) C: 61.5 (± 11.3) |
I: 31/18 C: 30/20 |
I: 8 C: 4 |
I: 8 C: 16 |
Overall: 0 | Overall: 0 |
| Bhattacharjee 2016 | I: 28.4 (± 5.12) C: 30.4 (± 5.24) |
I: 8/12 C: 8/12 |
NR | Overall: 0 | NR | NR |
| Burns 1988 | Overall: 34.2 | All F | NR | NR | NR | NR |
| Ceker 2015 | I: 57 (± 6.9) C: 57.9 (± 6.8) |
I: 23/7 C: 21/9 |
NR | Overall: 100 | Overall: 0 | NR |
| Chung 1992 | I: 40 (± 12) C: 41 (± 8) |
All F | NR | Overall: 0 | Overall: 0 | NR |
| Cucchiara 1986 | NR | NR | Overall (within 6 months): 0 | NR | Overall: 0 | NR |
| DIPOM 2006 | I: 64.9 (± 11.1) C: 64.8 (NR) |
I: 271/191 C: 268/191 |
I: 7.8 C: 7.4 |
I: 55 C: 62.7 |
Overall: 0 | Overall: 0 |
| Do 2012 | I: 41.89 (± 9.71) C: 41.26 (± 10.45) |
I: 5/22 C: 6/21 |
NR | NR | NR | NR |
| El‐Shmaa 2016 | I: 47.5 (± 6.8) C: 47.6 (± 8.3) |
I: 17/13 C: 14/16 |
NR | Overall: 0 | NR | NR |
| Gibson 1988 | I: 51.2 (± 17.5) C: 51.7 (± 15.7) |
I: 15/6 C: 8/11 |
NR | I: 8 C: 6 |
Overall: 0 | Overall: 0 |
| Goyagi 2005 | I: 51 (± 16) C: 55 (± 18) |
I: 4/7 C: 5/6 |
NR | Overall: 0 | Overall: 0 | NR |
| Gupta 2011 | NR | NR | NR | NR | Overall: 0 | NR |
| Harasawa 2006 | I: 51 (± 16) I: 44 (± 14) I: 45 (± 17) C: 48 (± 14) |
I: 4/4 I: 4/4 I: 4/4 C: 4.4 |
NR | NR | Overall: 0 | NR |
| Helfman 1991 | Overall range: 46 to 53 | NR | NR | I: 10 C: 10 |
Overall: 0 | I: 15 C: 15 |
| Horikoshi 2017 | I: 67 (± 7) C: 63 (± 8) |
I: 15/4 C: 18/2 |
Overall (recent): 0 | I: 31.6 C: 40 |
Overall: 0 | Overall: 0 |
| Inada 1989 | I: 52 (± 5) I: 53 (± 5) C: 49 (± 5) |
I: 5/5 I: 4/6 C: 5/5 |
I: 0 I: 0 C: 20 |
I: 30 I: 20 C: 10 |
Overall: 0 | Overall: 0 |
| Inoue 2010 | I: 62 (± 14) I: 56 (± 13) C: 57 (± 14) |
All F | NR | NR | Overall: 0 | NR |
| Jakobsen 1986 | I: 29.4 (± 3.8) C: 36.4 (± 3.4) |
NR | NR | NR | NR | NR |
| Jakobsen 1992 | I: 43 (± 15) C: 39 (± 2.2) |
All F | NR | NR | NR | NR |
| Jakobsen 1997 | I: 60.3 (± 8.6) C: 60.6 (± 8.4) |
I: 13/5 C: 10/7 |
NR | NR | NR | NR |
| Jangra 2016 | I: 26.7 (± 8.3) C: 31.9 (± 9.0) |
I: 3/7 C: 6/4 |
NR | NR | NR | Overall: 0 |
| Kao 2017 | I: 39.9 (± 9.4) C: 40.4 (± 8.1) |
All F | NR | NR | NR | |
| Kawaguchi 2010 | I: 57.1 (± 10.3) C: 57.3 (± 11.8) |
I: 11/17 C: 7/21 |
NR | I: 39.3 C: 50 |
I: 3.6 C: 3.6 |
NR |
| Kindler 1996 | I: 37 (SEM ± 3) I: 43 (SEM ± 3) C: 41 (SEM ± 3) |
All F | NR | NR | NR | NR |
| Lai 2006 | Median I: 66 C: 67 |
I: 24/6 C: 25/5 |
NR | I: 23.3 C: 16.7 |
Overall: 0 | Overall: 0 |
| Lee 1994 | I: 32 (± 9) I: 29 (± 10) C: 34 (± 9) |
I: 11/9 I: 10/10 C: 8/12 |
NR | NR | NR | NR |
| Lee 2010 | I: 36.3 (± 7.7) C: 34.5 (± 6.1) |
I: 12/18 C: 14/16 |
NR | NR | NR | NR |
| Lee 2015 | I: 41 (± 10) C: 48 9 (± 10) |
I: 4/23 C: 4/24 |
NR | Overall: 0 | NR | Overall: 0 |
| Lee 2017 | I: 42.8 (± 17.5) I: 42.8 (± 17.5) C: 43.5 (± 15.2) |
I: 13/17 I: 12/18 C: 13/17 |
NR | NR | NR | NR |
| Lim 2000 | Median (range) I: 35 (27‐67) I: 28 (21‐67) C: 57 (25‐74) |
I: 8/4 I: 5/7 C: 4/8 |
NR | I: 16.7 I: 16.7 C: 25 |
Overall: 0 | Overall: 0 |
| Liu 1986 | I: 45.6 (± 2.8) C: 44.9 (± 3.3) |
I: 2/14 C: 1/13 |
NR | Overall: 0 | Overall: 0 | Overall: 0 |
| Liu 2006 | I: 70 (± 5) C: 69 (± 8) |
I: 9/6 C: 7/8 |
NR | NR | NR | Overall: 0 |
| Louizos 2007 | I: 44 (± 8) I: 43 (± 11) C: 41 (± 8) |
I: 28/26 I: 26/29 C: 26/27 |
NR | NR | NR | NR |
| Magnusson 1986 | I: 56 (± 9) C: 59 (± 7) |
I: 3/10 C: 7/7 |
Overall: 0 | Overall: 100 | Overall: 0 | NR |
| Mallon 1990 | Overall: 44.7 (± 13) | Overall: 14/13 | NR | NR | Overall: 0 | Overall: 0 |
| Meftahuzzaman 2014 | I: 38.5 (± 11.23) C: 35.33 (± 10.41) |
I: 15/15 C: 15/15 |
NR | Overall: 0 | Overall: 0 | NR |
| Menigaux 2002 | I: 37 (range 19‐53) C: 36 (range 18‐54) |
I: 15/10 C: 18/7 |
NR | Overall: 0 | NR | Overall: 0 |
| Mikawa 1991 | I: 44.7 (range 26‐58) I: 45.1 (range 24‐57) C: 43.4 (range 25‐57) |
I: 3/7 I: 4/6 C: 3/7 |
NR | NR | NR | NR |
| Miller 1990 | I: 59 (± 9) I: 60 (± 9) C: 58 (± 8) |
I: 12/3 I: 13/2 C: 13/2 |
I: 0 I: 1 C: 20 |
NR | Overall: 100 | Overall: 0 |
| Miller 1991 | I: 56 (± 16) I: 55 (± 16) C: 56 (± 16) |
I: 90/97 I: 88/93 C: 85/95 |
Overall (within 3 months): 0 | I:28.3 I: 23.2 C: 33.9 |
I: 12.8 I: 11.1 C: 8.9 |
NR |
| Miyazaki 2009 | I: 74 (± 4) I: 73 (± 3) C: 75 (± 4) C: 76 (± 5) |
NR | NR | NR | Overall: 0 | NR |
| Moon 2011 | I: 37.9 (± 11.7) C: 40.9 (± 11.0) |
All F | NR | NR | NR | NR |
| Neary 2006 | NR | NR | NR | NR | NR | Overall: 0 |
| Ohri 1999 | I: 42.32 (± 8.58) C: 42.8 (± 12.39) |
I: 6/19 C: 2/23 |
NR | NR | Overall: 0 | NR |
| Ojima 2017 | Median (range) I: 68 (31‐85) C: 69 (45‐83) |
I: 36/14 C: 41/9 |
I: 4 C: 2 |
I: 44 C: 48 |
NR | NR |
| Oxorn 1990 | I: 43.9 (± 13.8) C: 43.9 (± 13.8) |
All F | Overall (within 3 months): 0 | NR | NR | Overall: 0 |
| Park 2009 | I: 48 (± 11) C: 45 (± 11) | I: 8/34 C: 11/32 |
NR | NR | NR | NR |
| POBBLE 2005 | Median (IQR) I: 73 (61‐79) C: 74 (66‐76) |
I: 40/13 C: 35/9 |
NR | NR | NR | Overall: 0 |
| POISE 2008 | I: 68.9 (± 10.5) C: 69.1 (± 10.4) |
I: 2625/1549 C: 2668/1509 |
NR | I: 63.1 C: 62.9 |
I: 43.2 C: 42.7 |
Overall: 0 |
| PRESAGE 2016 | I: 62 (± 11) C: 62 (± 10) |
I: 55/49 C: 59/51 |
NR | I: 15.4 C: 11.8 |
I: 5.8 C: 3.6 |
Overall: 0 |
| Raby 1999 | I: 69 C: 67 |
I: 8/7 C: 4/7 |
I: 40 C: 36.4 |
NR | NR | I: 33.3 C: 36.4 |
| Safwat 1984 | I: 33 (± 2.3) I: 37 (± 5.0) I: 36 (± 3.3) C: 33 (± 3.6) |
I: 5/5 I: 5/5 I: 6/4 C: 3/7 |
NR | I: 0 I: 10 I: 0 C: 10 |
Overall: 0 | Overall: 0 |
| Shailaja 2013 | I: 61.6 (± 9.09) C: 57.9 (± 11.2) |
I: 20/10 C: 19/11 |
NR | Overall: 100 | NR | I: 10 C: 3.3% |
| Sharma 1996 | I: 54.9 (± 1.6) I: 56.6 (± 2.0) C: 50.8 (± 1.2) |
I: 4/11 I: 5/10 C: 5/10 |
NR | Overall: 100 | NR | NR |
| Sharma 2018 | I: 30.86 (± 8.65) C: 31.46 (± 9.0) |
I: 18/12 C: 21/9 |
NR | Overall: 0 | NR | Overall: 0 |
| Shrestha 2011 | I: 31.11 (± 12.26) C: 32.11 (± 9.74) |
NR | NR | NR | NR | NR |
| Shukla 2010 | I: 44.1 (± 11.8) C: 43.1 (± 10.4) |
I: 14/16 C: 16/14 |
NR | NR | Overall: 0 | NR |
| Singh 1995 | I: 53 (± 15) C: 53 (± 11) |
All M | NR | NR | NR | NR |
| Singh 2010 | I: 31.08 I: 30.56 C: 30.28 |
I: 15/10 I: 15/10 C: 17/8 |
NR | NR | NR | Overall: 0 |
| Singh 2012 | I: 40.8 (± 13.7) C: 41.8 ( 12.7) |
I: 16/24 C: 11/29 |
NR | Overall: 0 | Overall: 0 | Overall: 0 |
| Srivastava 2015 | I: 50.8 (± 9.2) C: 53.4 (± 9.7) |
I: 20/10 C: 23/7 |
NR | Overall: 0 | NR | Overall; 0 |
| Stone 1988 | I: 63 (± 3) I: 65 (± 2) I: 65 (± 3) C: 68 (± 2) |
I: 21/8 I: 19/11 I: 22/8 C: 28/11 |
I: 10.3 I: 10 I: 3.3 C: 12.8 |
Overall: 100 | Overall: 0 | NR |
| Sugiura 2007 | I: 60 (± 3) I: 55 (± 7) C: 57 (± 4) |
I: 6/4 I: 6/4 C: 7/3 |
NR | Overall: 100 | NR | Overall: 0 |
| Tendulkar 2017 | I: 43.76 (± 13.33) C: 39.86 (± 12.59) |
I: 19/11 C: 13/17 |
NR | NR | NR | NR |
| Ugur 2007 | I: 41.76 (± 12.6) C: 36.96 (± 13.3) |
I: 10/20 C: 9/21 |
NR | NR | NR | NR |
| Unal 2008 | I: 50.3 (± 17.1) I: 48.1 (± 16.2) C: 50.3 (± 12.2) |
I: 7/8 I: 7/8 C: 8/7 |
NR | NR | Overall: 0 | Overall: 0 |
| Urias 2016 | NR | NR | NR | NR | NR | NR |
| Van den Berg 1998 | I: 67 (± 11) C: 65 (± 11) |
I: 27/13 C: 21/19 |
NR | I: 22.5 C: 10 |
NR | I: 10 C: 5 |
| Verma 2018 | I: 48.9 (± 13.4) C: 42.7 (± 17.1) |
I: 26/14 C: 23/17 |
NR | Overall: 0 | NR | Overall: 0 |
| Wajima 2011 | I: 42.6 (± 8.6) C: 41.5 (± 7.9) |
All F | NR | NR | NR | NR |
| Wallace 1998 | I; 68 (± 8.6) C: 67 (± 10.2) |
NR | I: 18.2 C: 25.7 |
I: 71.7 I: 59.4 |
I: 60.6 C: 41.6 |
I: 18.2 C: 7.9 |
| Ward‐Booth 1983 | Overall: 26.5 (range 16‐65) | NR | NR | NR | NR | NR |
| White 2003 | I: 41 (± 11) C: 37 (± 6) |
All F | NR | NR | NR | NR |
| Whitehead 1980 | I: 27 C: 22 |
NR | NR | NR | NR | NR |
| Yamazaki 2005 | I: 49.0 (± 16.4) I: 47.4 (± 14.5) C: 50.5 (± 16.4) |
NR | NR | NR | NR | NR |
| Yang 2006 | I: 66.4 (± 10) C: 65.9 (± 10) |
I: 193/53 C:184/66 |
I: 15 C: 12 |
NR | Overall: 0 | Overall: 0 |
| Yang 2008 | I: 71 (± 8) C: 71 (± 9) |
I: 21/30 C: 21/30 |
NR | NR | Overall (at risk or high risk): 100 | NR |
| Yoshida 2017 | Median (range) I: 64 (48‐79) C: 62 (45‐82) |
I: 35/4 C:32/8 |
NR | I: 30.8 C: 27.5 |
I: 2.6 C: 2.5 |
NR |
| Zaugg 1999 | I: 76 (± 7) I: 75 (± 7) C: 73 (± 6) |
I: 9/11 I: 15/5 C: 13/6 |
I: 45 I: 25 C: 31.6 |
I: 70 I: 85 C: 68.4 |
Overall: 0 | Overall: 0 |
| C: control group; I: intervention group; ID: identification; IQR: interquartile range;M/F: male/female; MI: myocardial infarction; NR: not reported; SD: standard deviation; SEM: standard error of the mean | ||||||
aUnless otherwise stated. bData were collected from baseline characteristics tables, or overall data were taken from study inclusion or exclusion criteria.
Data and analyses
Comparison 1. Beta‐blockers vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Early all‐cause mortality | 16 | 11446 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.89, 1.54] |
| 2 Long‐term mortality | 4 | 1215 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.58, 1.17] |
| 3 Death due to cardiac causes | 3 | 9047 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.90, 1.75] |
| 4 Acute myocardial infarction | 12 | 10520 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.60, 0.87] |
| 5 Cerebrovascular events | 6 | 9460 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.97, 2.81] |
| 6 Ventricular arrhythmias | 5 | 476 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.35, 1.47] |
| 7 Atrial fibrillation and flutter | 9 | 9080 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.21, 0.79] |
| 8 Bradycardia | 49 | 12239 | Risk Ratio (M‐H, Random, 95% CI) | 2.49 [1.74, 3.56] |
| 9 Hypotension | 49 | 12304 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.29, 1.51] |
| 10 Congestive heart failure | 6 | 9212 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.94, 1.48] |
| 11 Length of stay (in days) | 4 | 404 | Mean Difference (IV, Random, 95% CI) | ‐1.21 [‐2.75, 0.33] |
Comparison 2. Beta‐blockers vs control: subgroup by type of beta‐blocker.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Early all‐cause mortality | 16 | 11446 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.89, 1.54] |
| 1.1 Propranolol | 2 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.30, 1.58] |
| 1.2 Metoprolol | 7 | 10241 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.03, 1.66] |
| 1.3 Landiolol | 3 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 3.01] |
| 1.4 Esmolol | 2 | 593 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Atenolol | 2 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.35, 2.98] |
| 2 Acute myocardial infarction | 12 | 10520 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.60, 0.87] |
| 2.1 Metoprolol | 7 | 10062 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.61, 0.88] |
| 2.2 Esmolol | 2 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.01, 5.62] |
| 2.3 Oxprenolol | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Atenolol | 3 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.03, 1.57] |
| 2.5 Labetolol | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Bradycardia | 49 | 12239 | Risk Ratio (M‐H, Random, 95% CI) | 2.51 [1.77, 3.55] |
| 3.1 Propranolol | 4 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 3.39 [1.35, 8.50] |
| 3.2 Metoprolol | 9 | 9200 | Risk Ratio (M‐H, Random, 95% CI) | 2.87 [2.40, 3.42] |
| 3.3 Landiolol | 8 | 395 | Risk Ratio (M‐H, Random, 95% CI) | 2.76 [0.98, 7.75] |
| 3.4 Esmolol | 20 | 1678 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.60, 2.75] |
| 3.5 Atenolol | 3 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 2.76 [1.66, 4.61] |
| 3.6 Oxprenolol | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 4.06 [0.23, 70.46] |
| 3.7 Labetolol | 5 | 252 | Risk Ratio (M‐H, Random, 95% CI) | 8.13 [1.10, 60.07] |
| 3.8 Nadolol | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.73, 1.21] |
| 3.9 Pindolol | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Hypotension | 49 | 12304 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.29, 1.51] |
| 4.1 Propranolol | 3 | 208 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.71, 2.61] |
| 4.2 Metoprolol | 9 | 9354 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [1.24, 1.55] |
| 4.3 Landiolol | 8 | 362 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.90, 1.48] |
| 4.4 Esmolol | 23 | 1828 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [1.29, 2.45] |
| 4.5 Atenolol | 3 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.61, 2.38] |
| 4.6 Oxprenolol | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 2.26 [0.12, 44.02] |
| 4.7 Labetolol | 4 | 192 | Risk Ratio (M‐H, Random, 95% CI) | 3.14 [0.43, 22.97] |
| 4.8 Pindolol | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Beta‐blockers vs control: subgroup by start of beta‐blocker therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Early all‐cause mortality | 16 | 11446 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.89, 1.54] |
| 1.1 Before surgery | 7 | 10135 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.83, 1.64] |
| 1.2 During surgery | 6 | 797 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.18, 1.69] |
| 1.3 After surgery | 3 | 514 | Risk Ratio (M‐H, Random, 95% CI) | 2.04 [0.38, 10.89] |
| 2 Acute myocardial infarction | 12 | 10500 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.60, 0.87] |
| 2.1 Before surgery | 6 | 10002 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.61, 0.88] |
| 2.2 During surgery | 4 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.47] |
| 2.3 After surgery | 2 | 226 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.06, 2.60] |
| 3 Bradycardia | 49 | 12239 | Risk Ratio (M‐H, Random, 95% CI) | 2.49 [1.74, 3.56] |
| 3.1 Before surgery | 13 | 9528 | Risk Ratio (M‐H, Random, 95% CI) | 2.89 [1.70, 4.91] |
| 3.2 During surgery | 32 | 2291 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [1.17, 3.35] |
| 3.3 After surgery | 4 | 420 | Risk Ratio (M‐H, Random, 95% CI) | 2.59 [1.54, 4.37] |
| 4 Hypotension | 49 | 12304 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.29, 1.51] |
| 4.1 Before surgery | 11 | 9354 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [1.19, 1.58] |
| 4.2 During surgery | 33 | 2312 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.12, 1.65] |
| 4.3 After surgery | 5 | 638 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.65, 2.58] |
Comparison 4. Beta‐blockers vs control: subgroup by risk status.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Early all‐cause mortality | 16 | 11446 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.89, 1.54] |
| 1.1 Low and medium risk surgery | 5 | 9967 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [1.04, 1.68] |
| 1.2 High risk surgery | 11 | 1479 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.42, 1.35] |
| 2 Acute myocardial infarction | 12 | 10520 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.60, 0.87] |
| 2.1 Low and medium risk surgery | 6 | 9606 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.47, 1.07] |
| 2.2 High risk surgery | 6 | 914 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.49, 1.37] |
| 3 Bradycardia | 49 | 12239 | Risk Ratio (M‐H, Random, 95% CI) | 2.49 [1.74, 3.56] |
| 3.1 Low and medium risk surgery | 35 | 10846 | Risk Ratio (M‐H, Random, 95% CI) | 2.19 [1.28, 3.74] |
| 3.2 High risk surgery | 14 | 1393 | Risk Ratio (M‐H, Random, 95% CI) | 3.13 [2.40, 4.07] |
| 4 Hypotension | 49 | 12304 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.29, 1.51] |
| 4.1 Low and medium risk surgery | 36 | 10789 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [1.41, 1.75] |
| 4.2 High risk surgery | 13 | 1515 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.11, 1.38] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ali 2015.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 73 Inclusion criteria: > 18 years of age; burns wounds ≥ 30% TBSA; treatment with at least 1 surgical skin grafting procedure; consent to participate Exclusion criteria: not reported Type of surgery: skin graft surgery (burns) Baseline characteristics Intervention group (propranolol)
Control group (standard care)
Country: USA Setting: single centre; hospital (burns unit) |
|
| Interventions |
Intervention group (propranolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: mean dose requirement, cardiac function (HR, tachycardia), wound healing, blood loss, fluid balance, bradycardia (HR < 60 bpm), bradypnea, hypotension (SBP < 90 mmHg), ischaemia (MAP < 60 mmHg), mortality, length of stay (does not state whether hospital or burns unit length of stay) Outcomes relevant to the review: bradycardia, hypotension, mortality (time point not reported) |
|
| Notes |
Funding/declarations of interest: supported by grants from: Wound Healing Society Foundation 3M Fellowship Award; National Institute for Disabilities and Rehabilitation Research, National Institutes of Health, Shriners Hospitals for Children; Claude D Perpper Older Americans Independence Center Pilot. Funders had no role in the study design or collection, interpretation or analysis of data. Study authors declared no conflicts of interest Study dates: November 2004‐January 2014 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study; not feasible to blind personnel to treatment group |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss of 4 participants in the intervention group, because no treatment was given. Study authors do not report reasons for no treatment. ITT analysis not used. Although overall loss is < 10%, all losses are in the intervention group |
| Selective reporting (reporting bias) | Unclear risk | Prospective clinical trial registration or prepublished protocol not reported; not feasible to assess risk of reporting bias |
| Other bias | Low risk | Study authors noted that participants in the propranolol group had a higher percentage of TBSA; however, because of other burn measurements, study authors believed that severity of burns was equivalent between groups |
Alkaya 2014.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 30 Inclusion criteria: 20‐65 years of age; ASA status I‐II; scheduled for elective craniotomy Exclusion criteria: significant cardiac, pulmonary, renal, hepatic, and neuropsychiatric disease, chronic alcohol or drug use, a family history of allergy to the drugs used, HR < 50 bpm or > 100 bpm; arterial BP < 96/60 mmHg or > 180/100 mmHg; using beta‐blockers, sympathomimetic agents, calcium channel blockers, or monoamine oxidase inhibitors Type of surgery: elective craniotomy Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: Turkey Setting: single centre; hospital |
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; quality of extubation; hypertension; hypotension (SAP < 100 mmHg; treated with ephedrine); bradycardia (HR < 50 bpm; treated with atropine) Outcomes relevant to the review: hypotension; bradycardia |
|
| Notes |
Funding/declarations of interest: no financial support. Study authors declare no conflicts of interest Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double‐blinded, and we assumed that the anaesthetists were unaware of group allocation |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or a prepublished protocol. It is not feasible to assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Aoyama 2016.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 50 Inclusion criteria: adults of either gender; 20‐90 years of age; ASA I or II; scheduled to undergo elective segmentectomy, lobectomy, or pneumonectomy of the lung under GA with thoracic epidural anaesthesia Exclusion criteria: participant refusal; inability to communicate in Japanese; contraindications to epidural anaesthesia; recent history or evidence of acute MI; and supraventricular arrhythmia (under treatment or requiring treatment); 2nd‐ or 3rd‐degree atrioventricular block; severe heart failure; left ventricular ejection fraction ≤ 35%; prohibition from beta‐blocker; hypotension (≤ 90/60 mmHg); bradycardia (≤ 50 bpm) Type of surgery: elective segmentectomy, lobectomy, or pneumonectomy of the lung Baseline characteristics Intervention group (landiolol hydrochloride)
Control group (placebo)
Country: Japan Setting: single‐centre; hospital |
|
| Interventions |
Intervention group (landiolol hydrochloride)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: AF (in 7 days postoperatively); predictive bio‐markers of AF (serum magnesium, IL6 etc.); adverse events (not specified); mortality (time point not specified) Outcomes relevant to the review: AF; mortality |
|
| Notes |
Funding/declarations of interest: not reported. Study authors declare that they have no conflicts of interest Study dates: September 2009‐November 2010 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of computer‐generated randomization table |
| Allocation concealment (selection bias) | Low risk | Use of sealed, non‐transparent envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double‐blinded, placebo controlled. We have assumed that participants were blinded |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses reported. Study authors planned to use ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Apipan 2010.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 60 Inclusion criteria: people scheduled for bimaxillary surgery; ASA status I Exclusion criteria: people with renal, hepatic, cardiac, asthmatic, haematologic, or endocrine disease Type of surgery: orthognathic surgery Baseline characteristics Intervention group (propranolol)
Control group (placebo)
Country: Thailand Setting: single centre; hospital |
|
| Interventions |
Intervention group (propranolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: intraoperative HR; amount of sodium nitroprusside; intraoperative blood loss; bradycardia (HR < 50 bpm) Outcomes relevant to the review: bradycardia |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The anaesthesiologist and patients were kept unaware of which group the patient was in" |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors reported no losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Bayliff 1999.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 99 Inclusion criteria: > 18 years of age, undergoing major thoracic operations (pneumectomy, lobectomy, oesophagectomy) Exclusion criteria: history of asthma, congestive heart failure, ≥ 2nd‐degree heart block, history of SVTs, or were receiving any of the following drugs: digoxin, oral beta‐blocker, quinidine, procainamide, amiodarone, diltiazem, verapamil Type of surgery: major thoracic operations (pneumectomy, lobectomy, oesophagectomy) Baseline characteristics Intervention group (propranolol)
Control group
Country: Canada Setting: hospital, single centre |
|
| Interventions |
Intervention group (propranolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: arrhythmias requiring treatment (until 3 days following surgery, detected by Holter ECG); overall arrhythmia rate, myocardial ischaemia, mortality, bradycardia, hypotension (SBP < 100 mmHg), length of hospital stay, congestive heart failure, bronchospasm Outcomes relevant to the review: mortality, ventricular arrhythmias, AF, bradycardia (HR ≤ 60 bpm), hypotension, congestive heart failure, length of hospital stay (see notes below), |
|
| Notes |
Funding/declarations of interest: grant from Victoria Hospital Research Foundation and the Canadian Society of Hospital Pharmacists Research Foundation Study dates: February 1994‐October 1995 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization in blocks of 4, exact method of sequence generation unclear |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes. No additional details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We assumed from the information in the study report that all participants and personnel were blinded to the intervention |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | See below |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Bhattacharjee 2016.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 40 Inclusion criteria: ASA status I or II; 20‐60 years of age; undergoing elective laparoscopic cholecystectomy under GA Exclusion criteria: pre‐existing hypertension; bronchial asthma; diabetes; sinus bradycardia; severe hepatic, renal, endocrine and cardiac dysfunction. Participants in whom surgery could not be completed laparoscopically or had open cholecystectomy were excluded Type of surgery: elective laparoscopic cholecystectomy Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: India Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; adverse events (to include bradycardia, hypotension, and hypertension) during postoperative period in the PACU; recovery times Outcomes relevant to the review: bradycardia (HR < 60 bpm); hypotension (MAP < 60 mmHg) |
|
| Notes |
Funding/declarations of interest: no sources of funding and authors declare no conflicts Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of a computer‐derived random‐number sequence |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study is described as single‐blinded. It is not clear if the anaesthetists are blinded to treatment groups |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Burns 1988.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 86 Inclusion criteria: scheduled for laparoscopic gynaecological procedures Exclusion criteria: not reported Type of surgery: laparoscopy (gynaecological surgery) Baseline characteristics not reported Overall mean age: 34.2 years Country: UK Setting: single centre; hospital |
|
| Interventions |
Intervention group (nadolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: all types of arrhythmias (bradycardia, atrial and ventricular tachyarrhythmias, supraventricular ectopics, ventricular ectopics) Outcomes relevant to the review: bradycardia (HR < 60 bpm) |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, placebo‐controlled, double‐blind trial, We have therefore assumed that participants and personnel were blinded |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | ECG tracings were interpreted by 2 observers blinded to study group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Unclear risk | Short report with limited detail, and we could not assess other potential risks of bias |
Ceker 2015.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 60 Inclusion criteria: people with hypertension controlled by ACE inhibitors; 20‐65 years of age; undergoing elective surgery Exclusion criteria: unstable angina; conduction disorder or severe arrhythmia; COPD; heart failure; valvular heart disease; use of drugs that prolong QT‐interval; electrolyte disturbances or coagulation disorders; known hypersensitivity to trial drugs; pregnant women; difficult airways Type of surgery: elective surgery (type not specified ‐ we assumed non‐cardiac) Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: Turkey Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: corrected‐QT interval; haemodynamic variables; bradycardia (HR < 50 bpm); hypotension (MAP < 55 mmHg); arrhythmias; ventricular extrasystoles Outcomes relevant to the review: hypotension; bradycardia |
|
| Notes |
Funding/declarations of interest: study authors declare no conflicts Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | To ensure blinding, drugs were prepared by an anaesthetist not involved in the care of participants |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | ECG readings were evaluated by a cardiologist who was blind to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Chung 1992.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 36 Inclusion criteria: ASA I or II; scheduled for elective gynaecological procedures. All participants were free of any cardiac congestive heart failure, hypertension, or asthma Exclusion criteria: not reported Type of surgery: elective gynaecological surgery Baseline characteristics Intervention group (labetalol)
Control group (placebo)
Country: USA Setting: single centre; hospital |
|
| Interventions |
Intervention group (labetalol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic parameters, hypotension and bradycardia Outcomes relevant to the review: hypotension; bradycardia (see notes) |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double‐blinded, placebo‐controlled |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Cucchiara 1986.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 74 Inclusion criteria: scheduled for elective carotid endarterectomy Exclusion criteria: pregnant, < 21 years of age, AF or atrial flutter, atrioventricular conduction block > 1st degree, acute MI within 6 months, SBP < 100 mmHg or DBP < 50 mmHg, renal or hepatic failure, cardiac conditions that reduce interpretability of haemodynamic variables, congestive heart failure, bronchospasm or bronchial asthma, drug allergy or idiosyncracy to beta‐adrenergic drugs, experimental drugs within 2 weeks, adrenergic augmenting or depleting drugs, receipt of IV calcium channel blockers or beta‐blockers within 4 half‐lives Type of surgery: carotid endarterectomy Baseline characteristics: not fully reported (quote: "There were no significant differences between esmolol and placebo groups in sex, race, age, ASA classification") Country: USA Setting: multi‐centre; hospitals |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables, myocardial ischaemia, bradycardia (not defined), hypotension (not defined), bronchospasm Outcomes relevant to the review: bradycardia, hypotension |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, placebo‐controlled, double‐blind trial |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12 participants were excluded from analysis because of protocol violations |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
DIPOM 2006.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 921 Inclusion criteria: scheduled for major non‐cardiac surgery (expected to last > 1 h); > 39 years of age; with diabetes Exclusion criteria: systemic treatment with beta‐blockers; condition indicating beta‐blocker treatment; NYHA IV congestive heart failure; 3rd‐degree atrioventricular block; pregnancy or breast‐feeding; allergic to study drugs; previously included in DIPOM trial Type of surgery: orthopaedic, neurological, vascular, gynaecological, thoracic, intra‐abdominal and other operations (major non‐cardiac surgery) Baseline characteristics Intervention group (metoprolol)
Control group (placebo)
Country: Denmark Setting: multi‐centre; 9 hospitals |
|
| Interventions |
Intervention group (metoprolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: all‐cause mortality (within 30 days; at 6‐month follow‐up), MI, unstable angina or new congestive heart failure, cardiac mortality, non‐fatal cardiac morbidity; adverse events Outcomes relevant to the review: mortality (early and long‐term), MI |
|
| Notes |
Funding/declarations of interest: study drugs supplied by AstraZeneca who were also involved in study design and inclusion phase. Funding from AstraZeneca, Danish Heart Foundation, Danish Diabetes Foundation, Copenhagen Hospital Corporation's Research Council, Danish Medical Research Council, Copenhagen Hospital Corporation. Study authors declare no competing interests Study dates: July 2000‐July 2002 Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation sequence |
| Allocation concealment (selection bias) | Low risk | Participants were centrally randomly assigned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The DIPOM trial is an investigator initiated and controlled, randomized placebo controlled, multicentre trial with central randomisation and blinding of all parties in all phases. The code was broken when analyzes were completed and a conclusion formulated" |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | High risk | We noted a very high number of losses. Although study authors used ITT analysis, we should be wary of this analysis because of the high participant loss |
| Selective reporting (reporting bias) | Unclear risk | Retrospective registration with clinical trial register (ISRCTN58485613). Not feasible to assess risk of reporting bias from these documents |
| Other bias | Low risk | Not detected |
Do 2012.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 54 Inclusion criteria: ASA status I; 20‐60 years of age Exclusion criteria: cardiovascular, respiratory, or central nervous system disease; difficulty in maintaining the airway upon induction of anaesthesia; BP ≥ 140/90 mmHg at arrival for surgery and following a 10‐min rest. People with bradycardia (HR < 45 bpm), or severe hypotension on inducing anesthesia were also excluded Type of surgery: cancer (stomach, large intestine, uterine, ovarian) or middle ear infection (myringoplasty and tympanoplasty) Baseline characteristics Intervention group
Control group
Country: South Korea Setting: hospital; single centre |
|
| Interventions |
Intervention group (labetalol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; end tidal concentration of anaesthetic gas (desflurane); hypertension; tachycardia (not defined); hypotension (SBP ≤ 80 mmHg or decrease of > 30% from baseline); bradycardia (≤ 45 bpm); arrhythmia; laryngospasm; bronchospasm Outcomes relevant to the review: hypotension; bradycardia |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
El‐Shmaa 2016.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 60 Inclusion criteria: ASA status I ‐II; 20‐60 years of age; scheduled for elective surgery under GA Exclusion criteria: ≤ 20 years of age; known allergy to the anaesthetic agents; history of major psychiatric disorder; history of substance abuse and current opioid use; compromized renal, pulmonary, and cardiac status; diabetes; anticipated difficult intubation; hypertension; compensatory tachycardia; baseline pulse < 60 bpm; baseline SBP < 100 mmHg; taking medication with cardiovascular effects Type of surgery: type not specified (we assumed non‐cardiac) Baseline characteristics Intervention group (labetalol)
Control group (placebo)
Country: Egypt Setting: hospital; single centre |
|
| Interventions |
Intervention group (labetalol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; anaesthetic consumption; hypertension; tachycardia (HR > 25% baseline); bradycardia (HR < 50 bpm); hypotension (SBP < 25% baseline value or 90 mmHg) Outcomes relevant to the review: bradycardia (measured during study period of 15 min; see notes below); hypotension |
|
| Notes |
Funding/declarations of interest: no funding; no conflicts of interest Study dates: May 2011‐March 2013 Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Low risk | Quote: "The group assignment numbers were sealed in an envelope and kept by the study supervisor. After the written consent was signed, the opaque envelope was unsealed to determine which drug would be used" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Anaesthestists were aware of group allocation |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | Observer‐blinded trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Gibson 1988.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 40 Inclusion criteria: neurosurgical patients who exhibited at least 20% increase in SBP above average ward pressure on emergence from anaesthesia Exclusion criteria: unsuitable for beta‐blocker treatment, HR < 60 bpm, atrioventricular block, sick sinus syndrome, prior evidence of congestive heart failure, history of bronchospasm or asthma, impaired renal or hepatic function, receiving beta‐blockers or calcium channel blockers within 4 h of entry into study Type of surgery: different neurosurgical interventions Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: USA Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables, hypertension, hypotension (not defined), bradycardia (not defined); nausea and vomiting Outcomes relevant to the review: bradycardia and hypotension (see notes below) |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, placebo‐controlled, double‐blind trial |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | High risk | An antihypertensive agent (hydralazine or labetalol) was given intraoperatively to participants in either group to control BP. It is unclear how many participants in the control group received labetalol, which may influence outcome data |
Goyagi 2005.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 22 Inclusion criteria: ASA status I or II; 20‐60 years of age Exclusion criteria: unstable coronary artery disease or heart failure; atrial or ventricular tachyarrythmia, 2nd‐ or 3rd‐degree atrioventricular block; controlled and uncontrolled hypertension; and those who were pregnant Type of surgery: non‐cardiac surgery (type not specified) Baseline characteristics Intervention group (landiolol)
Control group (placebo)
Country: Japan Setting: single centre; hospital |
|
| Interventions |
Intervention group (landiolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic parameters; sinus tachycardia; hypertension; bradycardia (< 50 bpm); hypotension (SPB < 90 mmHg); bronchospasm Outcomes relevant to the review: bradycardia, hypotension |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "via sealed envelope assignment". Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes were used; however, no additional information is specfied |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Use of placebo agent, but not specified whether personnel were blinded |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Gupta 2011.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 44 Inclusion criteria: people undergoing elective sublabial rhinoseptal trans‐sphenoidal hypophysectomy; ASA status I or II; 18‐65 years of age Exclusion criteria: people with altered state of consciousness; evident intracranial hypertension; history of coronary artery disease; uncontrolled hypertension or diabetes mellitus; autonomic dysfunction; acromegaly; already taking atenolol and clonidine medication. Participants were excluded if they had bradycardia (HR < 40 bpm) or hypotension (MAP < 30% or baseline) needing intervention, or surgery was prolonged or delayed after administration of study drug Type of surgery: neurosurgery Baseline characteristics not reported. Study authors report "no difference in demographic variables among the groups" Country: India Setting: single centre; hospital |
|
| Interventions |
Intervention group (atenolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic parameters; intraoperative consumption of fentanyl and propofol; sedation scores; bradycardia (HR < 40 bpm); hypotension (MAP < 30% or baseline) Outcomes relevant to the review: bradycardia, hypotension |
|
| Notes |
Funding/declarations of interest: no sources of support, and study authors declare no conflicts Study dates: February 2008‐March 2009 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to 3 study groups according to a computer‐generated randomization schedule |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study described as double‐blinded, and medication and placebo were identical |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | Quote: "An independent anaesthesia registrar, blinded to study group allocation and not involved in anaesthesia management, observed changes in heart rate, mean arterial pressure,...and sedation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | We re‐included 2 excluded participants, because the reasons for their exclusion contributed to relevant review outcome data |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Harasawa 2006.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 32 Inclusion criteria: undergoing surgery for intracranial or maxillofacial tumours under GA; ASA I or II; intraoperative HR > 90 bpm for > 5 min and SBP > 100 mmHg Exclusion criteria: > 75 years of age; heart disease including arrhythmias; bronchial asthma; receiving preoperative cardiovascular medications Type of surgery: surgery for intracranial or maxillofacial tumours Baseline characteristics Intervention group (landiolol 0.1 mg/kg)
Intervention group (landiolol 0.2 mg/kg)
Intervention group (landiolol 0.3 mg/kg)
Control group (placebo)
Country: Japan Setting: single centre; university hospital |
|
| Interventions |
Intervention group (landiolol 0.1 mg/kg)
Intervention group (landiolol 0.2 mg/kg)
Intervention group (landiolol 0.3 mg/kg)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; hypotension (SBP < 80 mmHg); bradycardia (HR < 50 bpm); ischaemic changes on ECG Outcomes relevant to the review: hypotension; bradycardia |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as placebo‐controlled, but blinding is not reported |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Helfman 1991.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 40 Inclusion criteria: > 21 years of age; ASA status II, III, or IV; scheduled for non‐cardiac surgery Exclusion criteria: atrioventricular conduction blocker > 1st‐degree; congestive heart failure; cardiac arrhythmias; asthma; use of beta‐blockers within 24 h preceding surgery Type of surgery: elective non‐cardiac surgery Baseline characteristics Overall age range: 46‐53 years Intervention group (esmolol)
Control group (placebo)
Country: USA Setting: hospital; single centre |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; adverse events ‐ bradycardia (HR < 50 bpm), hypotension (SBP < 90 mmHg), bronchospasm, seizures, rigidity Outcomes relevant to the review: bradycardia; hypotension |
|
| Notes |
Funding/declarations of interest: supported in part by the VA Medical Center, University of Miami School of Medicine, Department of Anesthesiology, and in part by Dupon Pharmaceuticals Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study personnel were blinded to group allocation; study drugs were given in comparable volumes of solution |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Some losses (10%), which were not clearly reported, however we were not concerned by these losses and, additionally, we were not able to use available outcome data |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Horikoshi 2017.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 40 Inclusion criteria: ASA I or II; scheduled to undergo oesophagectomy for oesophageal cancer Exclusion criteria: people with cardiac disease (e.g. arrhythmias including AF, conduction abnormalities, antiarrhthymic medications including beta‐blockers, recent angina pectoris, and MI); pulmonary or renal disease; thyroid dysfunction Type of surgery: elective oesophagectomy Baseline characteristics Intervention group (landiolol)
Control group (placebo)
Country: Japan Setting: single centre; university hospital |
|
| Interventions |
Intervention group (landiolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: AF (reported after surgery, on 1st postoperative day, and 2nd postoperative day), sinus tachycardia; haemodynamic variables; plasma cytokines concentration in blood; bradycardia (not defined); hypotension (not defined); heart failure; cardiogenic shock; length of hospital stay Outcomes relevant to the review: AF (2nd postoperative day), hypotension; bradycardia; length of hospital stay; congestive heart failure |
|
| Notes |
Funding/declarations of interest: no external funding sources, and study authors declare no conflicts Study dates: April 2012‐January 2015 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Anaesthesiologists and surgeons were blinded to group assignment |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specfied |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of participant, ITT analysis not used |
| Selective reporting (reporting bias) | Unclear risk | Retrospective clinical trial registration (UMIN000020238). Not feasible to assess risk of reporting bias from these documents |
| Other bias | Low risk | Not detected |
Inada 1989.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 30 Inclusion criteria: adults, 21‐71 years of age, scheduled for elective surgery under GA with tracheal intubation Exclusion criteria: congestive heart failure; unstable angina; bronchospastic disease on bronchodilators; atrioventricular block; severe hepatic dysfunction; ASA physical status IV; concurrent alpha‐ or beta‐adrenergic drug administration Type of surgery: elective surgery (type not specified ‐ we assumed non‐cardiac) Baseline characteristics Intervention group (labetalol 5 mg)
Intervention group (labetalol 10 mg)
Control group (placebo)
Country: USA Setting: single centre; hospital |
|
| Interventions |
Intervention group (labetalol 5 mg)
Intervention group (labetalol 10 mg)
Control group
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic response; dysrhythmias (ventricular begeminy, ventricular extrasystoles); hypotension (not defined); bradycardia (not defined Outcomes relevant to the review: hypotension, bradycardia |
|
| Notes |
Funding/declarations of interest: drug preparation supported by funds from Shering Corporation Study dates: not reported Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, placebo‐controlled, double‐blind trial. Participants received study drugs from "identical syringes" intravenously: content of syringes not predictable in advance |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Inoue 2010.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 90 Inclusion criteria: ASA I to II; scheduled for gynaecological procedures in the supine position Exclusion criteria: symptomatic ischaemic heart disease, hepatic or renal disease; coagulopathy; administered vasodilator medications; unable to measure tympanic temperature; contraindications to beta‐blockers Type of surgery: elective gynaecological procedures Baseline characteristics Intervention group (landiolol)
Intervention group (esmolol)
Control group (placebo)
Country: Japan Setting: single centre; hospital |
|
| Interventions |
Intervention group (landiolol)
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: temperature changes; haemodynamic variables; bradycardia (not defined); hypotension (not defined); bronchospasm Outcomes relevant to the review: bradycardia; hypotension |
|
| Notes |
Funding/declarations of interest: funding sources not reported. Study authors declare no conflicts Study dates: not reported Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly assigned (closed envelope technique)" Comment: insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Use of "closed" envelopes; however, no additional information is specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Attending anesthesiologists were blinded to the test drugs" |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Small number of losses with reasons reported |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Jakobsen 1986.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 20 Inclusion criteria: scheduled for middle ear or nasal septum surgery, without evidence of cardiopulmonary disease Exclusion criteria: not reported Type of surgery: middle ear or nasal septum surgery Baseline characteristics Intervention group (metoprolol)
Control group (placebo)
Country: Denmark Setting: single centre; hospital |
|
| Interventions |
Intervention group (metoprolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: doses and concentration of anaesthetic agents; haemodynamic variables; blood loss; recovery times; PONV, and other side effects in the PACU Outcomes relevant to the review: bradycardia (see notes) |
|
| Notes |
Funding/declarations of interest: study drugs provided by Hässle, Denmark Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Use of an envelope system to randomize participants. Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Anaesthetists and surgeons were unaware of group allocation |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | Group allocation was concealed until after study results were available, and therefore we assumed that outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of 1 participant because of protocol violation (with premedication) |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Jakobsen 1992.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 40 Inclusion criteria: healthy women undergoing elective hysterectomy under GA Exclusion criteria: not reported Type of surgery: hysterectomy Baseline characteristics Intervention group (metoprolol)
Control group (placebo)
Country: Denmark Setting: single centre; hospital |
|
| Interventions |
Intervention group (metoprolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: changes in catecholamine levels, haemodynamic variables, arrhythmias (type not specified), and perioperative blood loss, bradycardia (HR < 50 bpm), hypotension (MAP < 60 mmHg) Outcomes relevant to the review: hypotension, bradycardia |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, double‐blind, placebo‐controlled trial; we therefore assumed that personnel were blinded |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Jakobsen 1997.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 36 Inclusion criteria: people without previous or present cardiovascular history undergoing elective thoracotomy for lung cancer Exclusion criteria: not reported Type of surgery: elective thoracotomy for lung resection (GA with thoracic epidural blockade) Baseline characteristics Intervention group (metoprolol)
Control group (placebo)
Country: Denmark Setting: single centre; hospital |
|
| Interventions |
Intervention group (metoprolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: perioperative haemodynamic effects, surgical stress response, ventricular and supraventricular arrhythmias, MI, bradycardia (HR < 50 bpm), hypotension (MAP < 60 mmHg) Outcomes relevant to the review: ventricular arrhythmias, MI, bradycardia, hypotension |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, placebo‐controlled, double‐blind trial |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant was withdrawn from the placebo group because an epidural catheter could not be accomplished; it is unclear to which group this participant belonged. No other apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Jangra 2016.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 20 Inclusion criteria: ASA I or II; scheduled for endoscopic sinus surgery Exclusion criteria: people on beta‐blockers and cardiovascular active drugs; major hepatic, renal or cardiac disease; haematological disorders; allergic to magnesium sulfate; history of neromuscular disorder; diabetic neuropathy; pregnancy; prior treatment with opioids or anticoagulants Type of surgery: elective endoscopic sinus surgery Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: India Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: hypotension (MAP < 50 mmHg); hypertension; need for additional hypotensive agents or vasopressors; reflex tachycardia and arrhythmias; bradycardia (not defined, and data not reported); bronchospasm (data not reported); arrhythmias; recovery times; blood loss; assessment of surgical field for bleeding Outcomes relevant to the review: hypotension (see notes below) |
|
| Notes |
Funding/declarations of interest: no sources of support, and study authors declare no conflicts Study dates: July 2007‐October 2008 Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of computerized programme for randomization |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and surgeons were blinded to group allocation. However, study authors do not report whether anaesthetists were blinded to group allocation |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Unclear risk | Study authors noted that the duration of surgery and anaesthesia were less in the esmolol group. It is unclear whether this may influence results |
Kao 2017.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 20 Inclusion criteria: ASA status I female participants; 20‐50 years of age; undergoing minor breast surgery. Participants were free of known systemic diseases, not receiving medication, had no improper drug or alcohol misuse, and BMI between 18.5 and 24.9 kg/m² Exclusion criteria: not reported Type of surgery: minor breast surgery Baseline characteristics Intervention group (esmolol)
Control group (saline)
Country: Taiwan Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol)
Control group
|
|
| Outcomes |
Outcomes measured/reported by study authors: hypotension (drop from 30% or baseline SBP); bradycardia (HR < 50 bpm); HR variability Outcomes relevant to the review: hypotension; bradycardia |
|
| Notes |
Funding/declarations of interest: grants from Chang Gung Memorial Hospital, Taiwan, and the Ministry of Science and Technology, Taiwan. Study authors declare no conflicts Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Use of a sealed envelope technique. Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not clear whether the control group was a placebo agent, and whether the anaesthetists were aware of group allocation |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Small sample size with 2 participant losses in each group (20% loss) |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Kawaguchi 2010.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 56 Inclusion criteria: subarachnoid haemorrhage due to a ruptured aneurysm; 20‐70 years of age; operation started within 48 h of onset of subarachnoid haemorrhage; HR ≥ 90 bpm at preoperative assessment Exclusion criteria: people with cardiac failure with hypotension < 90 mmHg SAP for > 30 min; asthma; diabetes ketosis or metabolic acidosis; 2nd‐ or 3rd‐degree atrioventricular block or sick sinus syndrome; pregnant; coma on arrival at hospital; renal failure or were receiving haemodialysis therapy, or both; severe liver disease; taking immunosuprressive agents; infectious disease of hepatitis B or C or HIV; dexmedetomidine was administered before surgery Type of surgery: neurosurgery Baseline characteristics Intervention group (landiolol)
Control group (standard care)
Country: Japan Setting: hospital; multi‐centre (10 centres) |
|
| Interventions |
Intervention group (landiolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic parameters, tissue injury markers, mortality (measured during hospital stay and as part of GOS at 3 months), bradycardia (< 60 bpm), hypotension (SBP < 80 mmHg), bronchospasm, myocardial ischaemia, pneumonia, hyponatremia, heaptic and renal disorders Outcomes relevant to the review: early mortality, late mortality, bradycardia, hypotension |
|
| Notes |
Funding/declarations of interest: departmental sources at Nara Medical University, Yamaguchi Unversity Graduate School of Medicine, Hachioji Medical Center, Tokyo Medical University Study dates: June 2005‐May 2008 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization list |
| Allocation concealment (selection bias) | Unclear risk | Envelope method, but no additional details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Retrospective clinical trial registration (UMIN000000945). Not feasible to assess risk of reporting bias from these documents |
| Other bias | Low risk | Not detected |
Kindler 1996.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 45 Inclusion criteria: normotensive; ASA status I or II; scheduled for elective gynaecological procedures under GA Exclusion criteria: not reported Type of surgery: elective gynaecological procedures Baseline characteristics Intervention group (esmolol 1 mg/kg)
Intervention group (esmolol 2 mg/kg)
Control group (placebo)
Country: Switzerland Setting: hospital; single centre |
|
| Interventions |
Intervention group (esmolol 1 mg/kg)
Intervention group (esmolol 2 mg/kg)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; arrhythmias (not defined); bradycardia (HR < 50 bpm); hypotension; bronchospasm Outcomes relevant to the review: bradycardia; hypotension |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | To ensure double‐blinding, a nurse‐anaesthetist who was not involved in the study prepared the study drugs |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | Independent observers recorded outcome data |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Lai 2006.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 60 Inclusion criteria: oesophageal cancer, > 65 years of age, scheduled for oesophagectomy under GA; HR > 55 bpm; 2nd‐ or 3rd‐degree atrioventricular block Exclusion criteria: 2nd‐ or 3rd‐degree atrioventricular block; congestive heart failure; bronchial asthma; ischaemic heart disease; hepatic and renal dysfunction; diabetes; hyptertension; preoperative beta‐blocker use Type of surgery: oesophagectomy Baseline characteristics Intervention group (metoprolol)
Control group (standard care)
Country: China Setting: single centre; hospital |
|
| Interventions |
Intervention group (metoprolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic parameters, serum levels of troponin T, MI, AF, mortality, myocardial ischaemia Outcomes relevant to the review: MI, AF, mortality |
|
| Notes |
Funding/declarations of interest: not reported Study dates: January 2004‐August 2004 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants were withdrawn from the study after randomization. Exact point of time and study group allocation were unclear, however loss was < 10% |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Lee 1994.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 60 Inclusion criteria: adults; ASA status I or II Exclusion criteria: not reported in English abstract Type of surgery: type of surgery not specified ‐ we assumed non‐cardiac Baseline characteristics Intervention group (labetalol 0.3 mg/kg)
Intervention group (labetalol 0.6 mg/kg)
Control group (placebo)
Country: South Korea Setting: hospital; single centre |
|
| Interventions |
Intervention group (labetalol 0.3 mg/kg)
Intervention group (labetalol 0.6 mg/kg)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; hypotension (not defined); significant bradycardia (not defined); bronchospasm; ECG changes Outcomes relevant to the review: hypotension; bradycardia |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified in English abstract |
| Allocation concealment (selection bias) | Unclear risk | Not specified in English abstract |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified in English abstract |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified in English abstract |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Unclear risk | It is not feasible to assess risk of other biases because most information was collected only from the English abstract |
Lee 2010.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 60 Inclusion criteria: undergoing laparoscopic appendectomy; ASA status I or II; 18‐45 years of age Exclusion criteria: side effects or hypersensitivity to esmolol; cardiovascular diseases; metabolic disaese; renal diseases; liver diseases; neurological disorders or pregnancy; breastfeeding women; had taken anti‐emetics within 48 h of start of study Type of surgery: laparoscopic appendectomy Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: Korea Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: pain, PONV, haemodynamic variables; length of hospital stay Outcomes relevant to the review: length of hospital stay |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants "were randomly placed into 2 groups" |
| Allocation concealment (selection bias) | Unclear risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Lee 2015.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 56 Inclusion criteria: 20‐65 years of age; ASA status I or II; scheduled to undergo elective general surgery Exclusion criteria: conditions that pose a risk to tracheal intubation, including BMI > 30 kg/m² or < 16.5 kg/m²; chronic preoperative beta‐blocker treatment; history of hypertension, neuromuscular disease, or renal disease Type of surgery: elective general surgery (such as open thyroid surgery, endoscopic thyroidectomy, or orthopaedic surgery) Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: South Korea Setting: hospital; single centre |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: intubation scores; haemodynamic variables; hypotension (not defined); bradycardia (not defined); rescue treatments; recall Outcomes relevant to the review: hypotension; bradycardia |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation using a "sealed envelope technique"; insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | To ensure blinding, a nurse who did not participate in the study prepared the study drugs and diluted esmolol in saline to equivalent volume as the placebo group drug. An anaesthetist who initiated treatment was blinded to group allocation. |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | An anaesthetist blinded to group allocation collected outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of one participant in the esmolol group; unlikely to influence results |
| Selective reporting (reporting bias) | Unclear risk | Clinical trial registration (KCT0001177). However, study was registered retrospectively and it is not feasible to use these documents to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Lee 2017.
| Methods | RCT. Parallel design | |
| Participants |
Total number of randomized participants: 90 Inclusion criteria: 18‐70 years of age; ASA status I or II; scheduled for elective dental surgery under GA Exclusion criteria: cardiac, neurologic, or psychiatric problems; people who had analgesic or sedative agents within 24 h before surgery; or requiring a rapid sequence induction Type of surgery: elective dental surgery Baseline characteristics Intervention group (esmolol 0.5 mg/kg)
Intervention group (esmolol 1 mg.kg)
Control group (placebo)
Country: Korea Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol 0.5 mg/kg)
Intervention group (esmolol 1 mg/kg)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: pain, reactions to emergence from anaesthesia to include bradycardia (not defined) and hypotension (not defined) Outcomes relevant to the review: bradycardia and hypotension |
|
| Notes |
Funding/declarations of interest: supported by a grant from Glaxo, Inc. Study authors declare no conflicts Study dates: not reported Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of computer‐generated randomization |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study nurses who were not involved in care of participants prepared coded syringes |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | Blinded anaesthesiologists measured outcome data |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | Prospective clinical trial registration (NCT01885364). Main outcome (pain) reported as specified in clinical trial register documents. Reactions to emergence from anaesthesia were not included in these documents |
| Other bias | Low risk | Not detected |
Lim 2000.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 36 Inclusion criteria: ASA status I or II; scheduled for elective craniotomy under GA Exclusion criteria: history of congestive cardiac failure; heart block; bronchial asthma; already taking beta‐blockers Type of surgery: intracranial surgery Baseline characteristics Intervention group (esmolol 100 µg/kg)
Intervention group (esmolol 200 µg/kg)
Control group (placebo)
Country: Singapore Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol 100 µg/kg)
Intervention group (esmolol 200 µg/kg)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; bronchospasm; bradycardia (requiring discontinution of drug and treatment with atropine); severe tachycardia (not defined by type of tachycardia); hypertension Outcomes relevant to the review: bradycardia |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo‐controlled trial, but study authors did not report whether anaesthetists were blinded |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | Observations made by a nurse not involved in drug administration |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Liu 1986.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 30 Inclusion criteria: ASA status I; no history of asthma, palpitations, hypertension, cardiac conduction defects, congestive heart failure or preoperative use of beta‐blockers Exclusion criteria: not reported Type of surgery: mainly hysterectomy and gynaecological procedures Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: USA Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; dysrhythmias (bradycardia, ventricular ectopic beats) during surgery; myocardial ischaemia (reported as ST depression); supraventricular arrhythmias Outcomes relevant to the review: bradycardia |
|
| Notes |
Funding/declarations of interest: supported by a grant from American Critical Care Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, placebo‐controlled, double‐blind trial |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Liu 2006.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 30 Inclusion criteria: elderly participants, 60‐75 years of age, undergoing elective non‐cardiac surgery Exclusion criteria: pre‐operative HR < 55 bpm; 2nd‐ or 3rd‐degree atrioventricular block; congestive heart failure; bronchospasm and people taking beta‐blockers Type of surgery: non‐cardiac surgery (lung resection, oesophagectomy, gastrectomy) Baseline characteristics Intervention group (metoprolol)
Control group (standard care)
Country: China Setting: single centre; university hospital |
|
| Interventions |
Intervention group (metoprolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic parameters, myocardial ischaemia, troponin levels, hypotension (not defined), bradycardia (not defined) Outcomes relevant to the Review: hypotension, bradycardia |
|
| Notes |
Funding/declarations of interest: not reported in abstract Study dates: not reported in abstract
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trials registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Louizos 2007.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 162 Inclusion criteria: cigarette smokers; ASA I‐III; undergoing elective microlaryngeal surgery under GA; having smoked an average of 20‐30 cigarettes daily for > 10 years Exclusion criteria: average in‐hospital HR < 55 bpm or SBP < 100 mmHg; COPD; sick sinus syndrome, conduction abnormalities on the ECG; cardiac failure; receive monoamine oxidase inhibitors or reserpine Type of surgery: elective microlaryngeal surgery Baseline characteristics Intervention group (esmolol 1 mg/kg)
Intervention group (esmolol 2 mg/kg)
Control group (placebo)
Country: Greece Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol 1 mg/kg)
Intervention group (esmolol 2 mg/kg)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; tachycardia (type of tachycardia not defined); bronchospasm, bradycardia, hypotension, hypertension, volume of rescue esmolol Outcomes relevant to the review: hypotension, bradycardia |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specfied |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Anaesthetists were blinded to the type of drug |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants were withdrawn before initiation of study intervention. No other apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | High risk | Participants in either group were treated with esmolol in the event of hypertension, arterial blood pressure and tachycardia. This may influence outcome data for some control group participants |
Magnusson 1986.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 30 Inclusion criteria: scheduled for cholecystectomy, herniorrhaphy, with hypertension Exclusion criteria: obstructive lung disease, previous MI, congestive heart failure, 2nd‐ or 3rd‐degree atrioventricular block Type of surgery: cholecystectomy, herniorrhaphy Baseline characteristics Intervention group (metoprolol)
Control group (placebo)
Country: Sweden Setting: single centre; hospital |
|
| Interventions |
Intervention group (metoprolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: multiple haemodynamic parameters, heart failure, ventricular extrasystoles, bradycardia (HR < 40 bpm), hypotension (SAP < 70 mmHg) Outcomes relevant to the review: bradycardia, hypotension |
|
| Notes |
Funding/declarations of interest: supported by university funding Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization was not specified. We noted a difference between groups in the number of participants who were previously taking anti‐hypertensive treatment (which included beta‐blockers); we could not be certain whether this difference was caused by insufficient randomization |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, double‐blind, placebo‐controlled trial |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Two patients in the treatment group and one patient in the placebo group were subsequently excluded because of complications during treatment". Comment: loss of < 10% |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Mallon 1990.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 45 Inclusion criteria: ASA I‐III; scheduled for non‐cardiac surgery under GA; men or postmenopausal women or non‐pregnant women; 18‐65 years of age Exclusion criteria: concurrent use of beta‐blockers or calcium channel blockers; cardiac conduction abnormality; congestive heart failure; unstable angina; asthma; cor pulmonale Type of surgery: elective non‐cardiac surgery Baseline characteristics not reported by group. Study authors report that "demographic variables were comparable among groups" Overall:
Country: Canada Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol 100 mg)
Intervention group (esmolol 200 mg)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic parameters; use of vasopressors; adverse events to include: bradycardia (HR < 50 bpm); hypertension; hypotension (MAP < 20% baseline); tachycardia (type of tachycardia not defined); ventricular dysrhythmia; ischaemia (ST depression); wheezing Outcomes relevant to the review: bradycardia; hypotension; ventricular dysrrhythmia |
|
| Notes |
Funding/declarations of interest: supported by a grant from Dupont Pharmaceuticals Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind placebo‐controlled trial. We assumed that anaesthetists were blinded to study drugs |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Meftahuzzaman 2014.
| Methods | RCT, parallel group | |
| Participants |
Total number of randomized participants: 60 Inclusion criteria: male or female; weighing 35‐60 kg; 15‐55 years of age; ASA I or II; scheduled for elective surgical procedures Exclusion criteria: predicted difficult intubation; hypertension; ischaemic heart disease; compensatory tachycardia; baseline HR < 60 bpm or SBP < 100 mmHg; chronic obstructive airway disease; taking medication with cardiovascular effects Type of surgery: elective surgery (type not specified; we assumed non‐cardiac) Baseline characteristics Intervention group (labetalol)
Control group (placebo)
Country: Bangladesh Setting: single centre; hospital |
|
| Interventions |
Intervention group (labetalol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; bradycardia (HR < 50 bpm) Outcomes relevant to the review: bradycardia |
|
| Notes |
Funding/declarations of interest: not reported Study dates: January 2012‐June 2013 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Low risk | Use of sequentially, numbered, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as placebo‐controlled double‐blinded study, and therefore we assumed that personnel were blinded |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | No specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Menigaux 2002.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 50 Inclusion criteria: 18‐70 years of age; ASA status I or II; scheduled for elective non‐cranial surgery Exclusion criteria: neurological, cardiac, or metabolic disease; chronic hypertension; asthma or reactive airway disease; cardiovascular or beta‐blocker medication; routine use of analgesics or hypnotic medication; drug or alcohol abuse; obesity Type of surgery: elective non‐cranial surgery Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: France Setting: hospital; single centre |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: BIS values; haemodynamic variables; response to laryngoscopy and intubation; hypotension (MAP < 60 mmHg); bradycardia (HR < 50 bpm) Outcomes relevant to the review: bradycardia; hypotension |
|
| Notes |
Funding/declarations of interest: supported by NIH grant, the Joseph Down Foundation, and the Commonwealth of Kentucky Research Challenge Trust Fund. Authors declare no financial interests in the research Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients and personnel involved in patient management and data collection were unaware of the group allocation" Comment: to ensure blinding, hospital pharmacists prepared syringes |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | Quote: "Patients and personnel involved in patient management and data collection were unaware of the group allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Mikawa 1991.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 30 Inclusion criteria: normotensive; ASA status I; undergoing elective surgery Exclusion criteria: not reported Type of surgery: type of surgery not specified (we assumed it was non‐cardiac) Baseline characteristics Intervention group (pindolol 2 µg/kg)
Intervention group (pindolol 4 µg/kg)
Control group (placebo)
Country: Japan Setting: single centre; hospital |
|
| Interventions |
Intervention group (pindolol 2 µg/kg)
Intervention group (pindolol 4 µg/kg)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; changes to ECG; ventricular extrasystoles; bradycardia (< 50 bpm and requiring treatment); hypotension; adverse respiratory events Outcomes relevant to the review: bradycardia and hypotension |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The investigator and the patients were blinded to the identity of the experimental treatment" |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Miller 1990.
| Methods | RCT, parallel | |
| Participants |
Total number of randomized participants: 45 Inclusion criteria: scheduled for peripheral vascular surgery, < 75 years of age, with coronary artery disease (based on a history of angina or MI), or with at least 2 risk factors for coronary artery disease Exclusion criteria: average ward HR < 60 bpm, average SBP < 110 mmHg, disturbance on preoperative ECG, history of bronchospasm, heart failure, MI within 6 months, on chronic beta‐blocker therapy Type of surgery: elective peripheral vascular surgery Baseline characteristics Intervention group (esmolol 1.5 mg/kg)
Intervention group (esmolol 3.0 mg/kg)
Control group (placebo)
Country: Canada Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol 1.5 mg/kg)
Intervention group (esmolol 3.0 mg/kg)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables, death, MI, myocardial ischaemia, hypotension, bronchospasm, ventricular premature beats Outcomes relevant to the review: death, MI, hypotension |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization schedule |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, placebo‐controlled, double‐blind trial |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Miller 1991.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 548 Inclusion criteria: ≥ 18 years of age, scheduled for non‐cardiac surgery under GA. Study authors reported that in at least 6 of the 12 centres, participants had a history of coronary artery disease, or at least 2 risk factors Exclusion criteria: HR < 60 bpm, SBP < 110 mmHg, diagnosis of sick sinus syndrome or conduction abnormality on preoperative ECG, congestive heart failure or MI within 3‐6 months, or history of bronchospasm, receiving adrenergic‐augmenting and depleting drugs. Study authors report that in 3 of the 12 centres, participants were allowed to continue taking beta‐adrenergic blocking agents; in remaining 9 centres, participants were not included if they had taken these agents within 24 h (or for nadolol, in previous 4 days) Type of surgery: elective non‐cardiac surgery Baseline characteristics Intervention group (esmolol 100 mg)
Intervention group (esmolol 200 mg)
Control group (placebo)
Country: Canada Setting: multi‐centre; 12 university‐affiliated hospitals |
|
| Interventions |
Intervention group (esmolol 100 mg)
Intervention group (esmolol 200 mg)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables, use of analgesics, bradycardia, hypotension, bronchospasm, mortality Outcomes relevant to the review: bradycardia, hypotension, mortality |
|
| Notes |
Funding/declarations of interest: supported by a grant from DuPont Pharmaceuticals Study dates: start date October 1987 Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization in blocks of 45 for each centre." Comment: we assumed that computer‐generated randomization was used, as was the case by the same study authors in Miller 1990 |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, placebo‐controlled, double‐blind trial |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Miyazaki 2009.
| Methods | RCT, parallel group | |
| Participants |
Total number of randomized participants: 63 Inclusion criteria: elderly (> 70 years of age); with or without hypertension; scheduled for surgery under GA Exclusion criteria: ASA status III, IV or V; atrioventricular block > 1st degree; history of drug allergy; asthma; bronchospasm; COPD; coronary artery disease; HR < 50 bpm; SBP < 80 mmHg 1 min before administration of landiolol Type of surgery: orthopaedic or gynaecological surgery Baseline characteristics Intervention group (landiolol ‐ hypertensive participants)
Intervention group (landiolol ‐ normotensive participants)
Control group (placebo ‐ hypertensive participants)
Control group (placebo ‐ normotensive participants)
Country: Japan Setting: single centre; hospital |
|
| Interventions |
Intervention group (landiolol ‐ hypertensive and normotensive participants)
Control group (placebo ‐ hypertensive and normotensive participants)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic parameters; bradycardia (HR < 50 bpm) Outcomes relevant to the review: bradycardia |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of a random‐number table |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All persons present during the study were blinded to the identity of the infusion being administered" |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Moon 2011.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 54 Inclusion criteria: women, 20‐60 years of age; ASA status I or II; scheduled for elective laparoscopic gynaecological surgery of < 2 h duration Exclusion criteria: cardiovascular disease (hypertension, arrhythmia or myocardial ischaemia); haemodynamic instability; asthma or COPD; allergy to study drug; BMI < 16 kg/m² or > 30 kg/m² Type of surgery: laparoscopic gynaecological surgery Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: Korea Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: consumption of sevoflurane and fentanyl; duration of stay in the PACU; pain; haemodynamic parameters (to include bradycardia, hypotension) Outcomes relevant to the review: bradycardia (HR < 50 bpm), hypotension (SBP < 80 mmHg) |
|
| Notes |
Funding/declarations of interest: funding sources not reported. Study authors declare no conflicts Study dates: December 2009‐May 2010 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Low risk | Quote: "Anaesthesiologists not involved in the patients' anaesthetic management prepared the covered syringe pump for esmolol or placebo solutions and held the randomization codes until the end of the study" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study drugs were masked to attending anaesthetists |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | Intraoperative haemodynamic variables were assessed by an anaesthesiologist blinded to study allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Neary 2006.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 38 Inclusion criteria: non‐elective, non‐cardiac emergency surgery, high risk of cardiac complications (previous MI or ischaemia, typical or atypical angina, stroke or transcient cerebral ischaemia) or minor risk (≥ 65 years of age, hypertension, current smoker, serum cholesterol concentration > 6.2 mmol/L, diabetes mellitus) Exclusion criteria: already on beta‐blockers, bradycardia, chronic obstructive airways disease or asthma, history of beta‐blocker intolerance, 2nd‐ or 3rd‐degree heart block, cardiovascular collapse or uncorrected hypovolaemia, anaesthetist does not think that participant should be given beta‐blocker Type of surgery: non‐elective, non‐cardiac emergency surgery (gastrointestinal resection surgery, major limb amputation, arterial reconstruction, orthopaedic procedures) Baseline characteristics not reported Country: UK Setting: single centre, hospital |
|
| Interventions |
Intervention group (atenolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: mortality and non‐fatal cardiac events until 30 days after surgery, bradycardia (not defined), hypotension (not defined), MI. Long‐term follow‐up was also performed (2 years) Outcomes relevant to the review: mortality (30 days and at 2 years), bradycardia, hypotension |
|
| Notes |
Funding/declarations of interest: grant from Gloucester Vascular Research Team Study dates: not reported Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study medication was provided in 1 box per participant containing placebo, as well as the active drug, in similar looking batches. The anaesthesiologist randomly chose 1 pack at the induction of anaesthesia. We classed this as a random method |
| Allocation concealment (selection bias) | Low risk | Quote: "The box also contained a sealed envelope with details of which packs contained the active drug in case of an emergency." Comment: because the sealed envelope was inside the box, we assumed that allocation was adequately concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Anaesthetist had access to treatment allocation and was able to decide not to give beta‐blocking agents to a randomized participant |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | ECGs and lab reports were evaluated by "blinded cardiology staff" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses after randomization |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Ohri 1999.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 50 Inclusion criteria: ASA status I or II; either gender; undergoing various types of elective surgery Exclusion criteria: pregnancy; 2nd‐ or 3rd‐ degree atrioventricular block; sick sinus syndrome without pacemaker; hypotension; NYHA class III and IV; congestive heart failure; COPD; use of beta‐blockers; bronchial asthma or bronchospasm; intolerance to beta‐blockers; concurrent alcohol or drug abuse; severe renal or hepatic dysfunction Type of surgery: elective surgery (types not specified ‐ we assumed non‐cardiac) Baseline characteristics Intervention group (esmolol)
Control group (standard care)
Country: India Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: dose and duration of pancuronium; hypotension (not defined) Outcomes relevant to the review: hypotension |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Ojima 2017.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 100 Inclusion criteria: pathologically confirmed thoracic oesophageal carcinoma; planned right transthoracic oesophagectomy with 2‐ or 3‐field lymph node dissection; clinical stage I, II, III or IV according to the UICC TNM classification; Eastern Cooperative Oncology Group performance status 0‐1; 20‐85 years of age; normal major organ function Exclusion criteria: resting HR < 50 bpm, 2nd‐ or 3rd‐degree atrioventricular block; history of AF. In addition, at randomization, participants were excluded if they were receiving dopamine, SBP < 80 mmHg or > 160 mmHg, HR < 50 bpm, or with arrhythmias or requiring ventilator assistance Type of surgery: oesophagectomy Baseline characteristics Intervention group (landiolol)
Control group (placebo)
Country: Japan Setting: hospital; single centre |
|
| Interventions |
Intervention group (landiolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: AF (between days 1‐7); rate of occurrence of AF in hospital; postoperative complications; haemodynamic parameters; change in inflammatory markers; mobility scores; side effects (to include hypotension and bradycardia); mortality Outcomes relevant to the review: AF, hypotension, bradycardia; mortality |
|
| Notes |
Funding/declarations of interest: funding not reported. Study authors declare no conflicts of interest Study dates: March 2013‐January 2016 Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization at a central registry |
| Allocation concealment (selection bias) | Low risk | Organized at a central registry |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients, care providers and investigators were blinded to the treatments." Quote: "The appearance and labelling of the dosage were indistinguishable between landiolol and placebo" |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | As above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses |
| Selective reporting (reporting bias) | Low risk | Prospective clinical trial registration (UMIN000010648). Outcomes are reported according to clinical trial documents |
| Other bias | High risk | Participants in either group who had AF were treated with landiolol |
Oxorn 1990.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 48 Inclusion criteria: ASA I and II, scheduled for hysterectomy, ≥ 18 years of age Exclusion criteria: treatment with beta‐blockers or calcium channel blockers within previous 24 h, ward or baseline HR < 70 bpm and SBP < 100 mmHg, P‐R interval > 0.24 seconds, 2nd‐ or 3rd‐degree heart block, sick sinus syndrome, right or left ventricular failure, treatment with adrenergic augmenting or depleting drugs, MI within previous 3 months, reactive airways disease, treatment with other experimental drugs within 14 days Type of surgery: hysterectomy Baseline characteristics Intervention group (esmolol 100 mg)
Intervention group (esmolol 200 mg)
Control group (placebo)
Country: Canada Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol 100 mg)
Intervention group (esmolol 200 mg)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables, perioperative hypotension (not defined), bradycardia (not defined), pain on injection, tachycardia, ventricular extrasystoles, myocardial ischaemia Outcomes relevant to the review: hypotension and bradycardia (see notes below) |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, triple‐blind, placebo‐controlled trial |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | Cardiologist assessing the ECG was blinded to study group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Park 2009.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 88 Inclusion criteria: 18‐70 years of age; ASA status I or II; scheduled for total thyroidectomy under GA Exclusion criteria: cardiovascular disorders; pulmonary disorder; diabetes Type of surgery: elective total thyroidectomy Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: Korea Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; bradycardia (HR < 45 bpm); hypotension (SBP < 85 mmHg or mean BP < 60 mmHg) Outcomes relevant to the review: bradycardia; hypotension |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using computer‐generated codes that were kept in sealed opaque envelopes" |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "An anesthetic nurse unaware of treatment assignments prepared study drugs as 20 ml solutions in identical black syringes" |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | Quote: "Anesthesiologists and investigators who collected postoperative data at postanesthesia care unit (PACU) and wards were also unaware of randomization details and study drugs allocations" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Small number of losses with reasons explained |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
POBBLE 2005.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 103 Inclusion criteria: scheduled for infrarenal vascular surgery under GA Exclusion criteria: already taking beta‐blockers, if giving beta‐blockers would be dangerous (e.g. because of intolerance), receiving treatment for asthma, aortic stenosis, bradycardia, hypotension, perioperative beta‐blockade had already been shown to be beneficial, unstable angina or angina with a positive dobutamine stress test Type of surgery: elective infrarenal vascular surgery Baseline characteristics Intervention group (metoprolol)
Control group (placebo)
Country: UK Setting: multi‐centre; 4 hospitals |
|
| Interventions |
Intervention group (metoprolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: fatal and non‐fatal cardiovascular events (MI, unstable angina, ventricular tachycardia, or stroke) within 30 days of operation, length of hospital stay, hypotension, bradycardia Outcomes relevant to the review: fatal and non‐fatal cardiovascular events (MI, ventricular tachycardia, or stroke), length of hospital stay (see notes below, mortality (at 30 days), hypotension (decrease in SBP > 25%), bradycardia (HR < 50 bpm) |
|
| Notes |
Funding/declarations of interest: supported by The British Heart Foundation Study dates: July 2001‐March 2004 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomization via Web page |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Anesthesiologists were unblinded to the drug allocation because those involved in the trial at participating centers would have immediately identified the active treatment and, for safety reasons, refused to collaborate in a blinded fashion" |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | Holter‐ECG results were coded centrally |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Small loss of participants (< 10%) because of cancellation of surgery, and death |
| Selective reporting (reporting bias) | Unclear risk | Study was retrospectively registered with a clinical trial register (ISRCTN13072628). It was not feasible to effectively assess risk of reporting bias from this report |
| Other bias | Low risk | Not detected |
POISE 2008.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 8351 Inclusion criteria: undergoing non‐cardiac surgery with, or at risk of atherosclerotic disease; ≥ 45 years of age; expected length of hospital stay of ≥ 24 h; fulfilled any of the following: history coronary artery disease, peripheral vascular disease, stroke, hospitalization for congestive heart failure within previous 3 years, undergoing major vascular surgery, or any 3 of 7 risk criteria (undergoing intrathoracic or intraperitoneal surgery, history of congestive heart failure, TIA, diabetes, serum creatinine > 175 µmol/L, > 70 years of age, undergoing emergent or urgent surgery) Exclusion criteria: HR < 50 bpm; 2nd‐ or 3rd‐degree heart block; asthma; receiving beta‐blocker or physician had planned start of beta‐blocker treatment perioperatively; prior adverse reaction to beta‐blockers; CABG surgery in preceding 5 years and no cardiac ischaemia since; low‐risk surgical proceures; on verapamil; previous enrolment in POISE study Type of surgery: non‐cardiac surgery (vascular, intraperitoneal, orthopaedic and other) Baseline characteristics Intervention group (metoprolol)
Control group (placebo)
Country: Argentina, Australia, Brazil, Canada, China, Columbia, Cuba, Equador, El Salvador, Finland, Hong Kong, Hungary, India, Malaysia, Mexico, New Zealand, Norway, Peru, Singapore, Spain, Sweden, Thailand, UK Setting: multi‐centre; 190 hospitals in 23 countries |
|
| Interventions |
Intervention group (metoprolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: cardiovascular death, non‐fatal MI, non‐fatal cardiac arrest, all‐cause mortality, hypotension (not defined), bradycardia (not defined), stroke, congestive heart failure, new‐onset AF, length of hospital stay Outcomes relevant to the review: death due to cardiac causes, non‐fatal MI, all‐cause mortality, hypotension, bradycardia, stroke, congestive heart failure, new‐onset AF, length of hospital stay (see notes below) |
|
| Notes |
Funding/declarations of interest: Canadian Institutes of Health Research; Commonwealth Government of Australia's National Health and Medical Research Council; Instituto de Salud Carlos III, Spain; British Heart Foundation; AstraZeneca. No funders had any role in trial design, conduct, data collection, analyses, or data interpretation. Two authors declared receipt of research funds or fees or honoraria from AstraZeneca Study dates: October 2002‐July 2007 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized randomization phone service |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, placebo‐controlled, triple‐blind multi‐centre trial |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | Outcome adjudicators were masked to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few losses and use of ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Retrospective clinical trial registration (NCT00182039). Not feasible to use these registration documents to assess risk of reporting bias |
| Other bias | Low risk | Not detected |
PRESAGE 2016.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 218 Inclusion criteria: scheduled for lung cancer surgery; ≥ 18 years of age; evidence of elevated NT‐proBNP value Exclusion criteria: hypersensitivity or intolerance to metoprolol or losartan; history of heart failure; left ventricular ejection fraction < 50%; permanent AF; current therapy with antiarrhythmics, beta‐blockers, ARBs and ACE inhibitors; SBP < 95 mmHg in first 12 h after surgery; history of sick sinus syndrome; atrioventricular block ≥ grade II; HR < 65 bpm in first 12 h after surgery; history of bronchial asthma; severe bronchopneumopathy; evidence of bronchospasm; prolonged mechanical ventilation (> 12 h) Type of surgery: elective lung cancer surgery Baseline characteristics Intervention group (metoprolol)
Control group (standard care)
Country: Italy Setting: single centre; hospital |
|
| Interventions |
Intervention group (metoprolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: postoperative AF; bronchospasm and hypotension (participants excluded for these events); acute pulmonary oedema; anaemia; in‐hospital mortality; TIA or stroke; acute coronary syndrome; cardiac arrest; reintervention; sepsis; need for CPAP; acute kidney injury; length of hospital stay Outcomes relevant to the review: postoperative AF; hypotension (not defined); in‐hospital mortality; cerebrovascular events (TIA or stroke); length of hospital stay |
|
| Notes |
Funding/declarations of interest: study authors report no external funding, and no conflicts Study dates: January 2009‐June 2013 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of 4 participants in the intervention group, which we were able to re‐include for some outcome data |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered with clinical trial register (NCT01281787). Not feasible to assess risk of reporting bias from these documents |
| Other bias | Low risk | Not detected |
Raby 1999.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 26 Inclusion criteria: undergoing aortic aneurysm repair, infrainguinal arterial bypass, carotid endarterectomy, having myocardial ischaemia 1‐12 days before surgery Exclusion criteria: receiving digitalis therapy, baseline left bundle branch block or left ventricular hypertrophy, baseline ST‐T changes that would preclude accurate interpretation of Holter monitor for ischaemia Type of surgery: aortic aneurysm repair, infrainguinal arterial bypass, carotid endarterectomy Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: USA Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: perioperative myocardial ischaemia, MI, unstable angina Outcomes relevant to the review: MI |
|
| Notes |
Funding/declarations of interest: supported in part by a grant from Ohmeda Inc. Study dates: not reported (study duration 2 years) Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin flipping |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Despite the double‐blind study design, a "significantly higher use of alternative beta‐blockers in the placebo group compared with the esmolol group" was noted. The study authors suggested that "managing clinicians recognized patients receiving the active drug or placebo despite blinding" |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | Holter monitoring was interpreted "by a technician blinded to patient characteristics and randomization" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "No patient in our study had beta‐blocker therapy suspended because of unacceptable side effects" |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | High risk | Quote: "The use of alternative beta‐blocker therapy, calcium channel blocker therapy, nitrates, and various formas of analgesia were permitted per the judgment of the independent managing physicians in both groups, without a specific protocol". Comment: we noted that more participants in the placebo group had alternative postoperative beta‐blockers. |
Safwat 1984.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 40 Inclusion criteria: ASA status I‐III; 21‐60 years of age; scheduled for elective surgery Exclusion criteria: pregnancy; history of asthma and hay fever; history of drug abuse; treatment with tricyclic antidepressants or reserpine; atrioventricular conduction abnormalities and bradycardia; coronary artery disease Type of surgery: elective surgery (type not specified ‐ we assumed non‐cardiac) Baseline characteristics Intervention group (propranolol: 2 min)
Intervention group (propranolol: 5 min)
Intervention group (propranolol: 8 min)
Control group (placebo)
Country: USA Setting: hospital; single centre |
|
| Interventions |
Intervention group (propranolol: 2 min)
Intervention group (propranolol: 5 min)
Intervention group (propranolol: 8 min)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; bradycardia (not defined); hypotension (not defined); extrasystoles; serum propranolol levels Outcomes relevant to the review: bradycardia; hypotension |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | Outcome assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Shailaja 2013.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 60 Inclusion criteria: people with hypertension; ASA status II; either gender; 35‐70 years of age; weighing ≤ 70 kg; SBP ≥ 140 mmHg; DBP ≥ 90 mmHg; scheduled for surgery under GA Exclusion criteria: ASA status ≥ III; anticipated difficult airway; pregnancy; cardiac and neurosurgical procedures; intubation requiring 2nd attempt and intubation prolonged for ≥ 25 seconds Type of surgery: elective surgery (type not specified ‐ we assumed non‐cardiac) Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: India Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; bradycardia (HR < 60 bpm); hypotension (< 20% baseline); hypertension; tachycardia (not defined); pain on injection; arrhythmia (not defined) Outcomes relevant to the review: bradycardia; hypotension |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random‐number table |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes, which were opened by anaesthetists who were not involved in study procedures |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Anaesthestists were blinded to group allocation; drug preparations were made by an anaesthetist who was not involved in the care of participants |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | Outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Sharma 1996.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 45 Inclusion criteria: adults with mild to moderate essential hypertension; receiving antihypertensive treatment with non‐beta‐blocking agents or diuretics; ASA status II; scheduled for elective abdominal surgery Exclusion criteria: HR < 70 BPM, SBP < 120 mmHg, chronic obstructive lung disease (especially bronchial asthma), past history of angina or MI in the last 3 months, heart blocks and congestive cardiac failure Type of surgery: elective abdominal surgery Baseline characteristics Intervention group (esmolol 100 mg)
Intervention group (esmolol 200 mg)
Control group (placebo)
Country: India Setting: hospital; single centre |
|
| Interventions |
Intervention group (esmolol 100 mg)
Intervention group (esmolol 200 mg)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; side effects attributable to esmolol (ECG changes, bronchospasm, hypotension) Outcomes relevant to the review: hypotension (see notes, below) |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All patients received 20 ml solution iv in a double blind manner" |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Sharma 2018.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 60 Inclusion criteria: either gender; 20‐50 years of age; ASA status I or II; scheduled for elective surgery under GA Exclusion criteria: anticipated difficult airway; laryngoscopy time > 20 seconds; history of hypertension, diabetes, hepatic disease or renal disease; on preoperative beta‐blockers; pregnant or lactating women Type of surgery: elective general surgery (types not specified ‐ we assumed non‐cardiac) Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: India Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; myocardial insult (not defined); hypotension (not defined); bradycardia (not defined) Outcomes relevant to the review: hypotension; bradycardia |
|
| Notes |
Funding/declarations of interest: no funding sources and study authors report no conflicts Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization, completed by anaesthetist who was not involved in drug administration or outcome assessment |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled trial, anaesthetists not aware of group allocation |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | Recordings made by a separate anaesthetist blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Shrestha 2011.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 36 Inclusion criteria: ASA status I or II; weighing 40‐70 kg; 18‐65 years of age; scheduled for elective surgery Exclusion criteria: pre‐existing cardiopulmonary disease; contraindications or known hypersensitivity to study drug; on antihypertensive medication or drugs with effect on central nervous system Type of surgery: elective surgery (type not specified ‐ we assumed non‐cardiac) Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: Nepal Setting: single centre; university teaching hospital |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; adverse effects ‐ nausea and vomiting, respiratory depression, dizziness, somnolence, ataxia, headache, bradycardia (HR 40 bpm); hypotension (requiring treatment with mephentermine) Outcomes relevant to the review: bradycardia; hypotension |
|
| Notes |
Funding/declarations of interest: not reported Study dates: January‐March 2011 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Use of sealed envelope method. Insufficient detail |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although study is placebo‐controlled, it is unclear whether anaesthetists were blinded to type of drug |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Shukla 2010.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 60 Inclusion criteria: adults of either gender, ASA status I or II, undergoing major lower abdominal surgeries under GA Exclusion criteria: ischaemic heart disease; heart block; pulmonary diseases; hepatic disease; known allergy to opioids; regular analgesic use for 3 days prior to surgery Type of surgery: major lower abdominal surgery Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: India Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables, intraoperative morphine consumption, sedation level, nausea, emesis, pruritus, respiratory depression, postoperative pain and morphine consumption, hypotension (MAP < 50 mmHg; treated with ephedrine); bradycardia (< 40 bpm; treated with atropine) Outcomes relevant to the review: hypotension; bradycardia |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly divided; no additional details |
| Allocation concealment (selection bias) | Unclear risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study described as double‐blinded; we therefore assumed that personnel were blinded |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Singh 1995.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 20 Inclusion criteria: normotensive; ASA I or II; adult males; undergoing elective surgery under GA Exclusion criteria: not reported Type of surgery: elective surgery (type not specified, we have assumed non‐cardiac) Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: USA Setting: single centre; university medical centre |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; cardiac arrythmias (not defined further); myocardial ischaemia (ischaemic changes on ECG); hypotension (MAP < 70 mmHg) Outcomes relevant to the review: hypotension |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double‐blind, placebo‐controlled trial. We assumed that anaesthetists were blinded to study groups |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Singh 2010.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 75 Inclusion criteria: ASA status I and II; 18‐45 years of age; undergoing elective surgery under GA requiring intubation Exclusion criteria: cardiovascular, plumonary, hepatic, and renal disease; people on beta‐blockers; difficult airways; laryngoscopy and intubation time > 30 seconds, or requiring > 2 attempts Type of surgery: elective surgery (type not specified, we assumed non‐cardiac) Baseline characteristics Intervention group (esmolol)
Intervention group (labetalol)
Control group (placebo)
Country: India Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol)
Intervention group (labetalol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; atrial ectopics; bradycardia (HR < 50 bpm) Outcomes relevant to the review: bradycardia (see notes below) |
|
| Notes |
Funding/declarations of interest: no sources of funding. Study authors report no conflicts Study dates: March 2006‐August 2007 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Low risk | Use of sequentially numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind placebo‐controlled trial. We assumed that anaesthetists were blinded to group allocation |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Unclear risk | Timings of administration of esmolol and labetolol differed between groups. We did not know whether this may influence results |
Singh 2012.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 80 Inclusion criteria: ASA I or II undergoing various elective surgeries; 18‐65 years of age Exclusion criteria: history of hypertension; diabetes; cardiac diseases; bronchial asthma and those on beta‐blockers Type of surgery: elective surgery (type not specified ‐ we assumed non‐cardiac) Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: Ghana Setting: single centre; teaching hospital |
|
| Interventions |
Intervention group (esmolol)
Control group (control)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic parameters; arrhythmias (type not defined; ventricular ectopics; dropped beats; hypotension (not defined) Outcomes relevant to the review: hypotension |
|
| Notes |
Funding/declarations of interest: not reported Study dates: November 2011‐May 2012 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double‐blinded; we therefore assumed that the anaesthetists were blinded |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Srivastava 2015.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 60 Inclusion criteria: 20‐60 years of age; ASA status I or II; either gender; scheduled for elective neurosurgical procedures Exclusion criteria: people with predicted difficult intubation; laryngoscopy and intubation time > 20 seconds; > 1 attempt at intubation; taking beta‐blocker therapy preoperatively; systemic illness such as hypertension, diabetes, hepatic failure, and renal failure Type of surgery: elective neurosurgical procedures Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: India Setting: hospital; single centre |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; bradycardia (HR < 50 bpm) Outcomes relevant to the review: bradycardia |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random‐number tables |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study drugs were prepared in identical syringes by an independent anaesthetist who was not involved in the study |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Stone 1988.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 128 Inclusion criteria: scheduled for major surgery necessitating GA; untreated hypertension (BP measurement between 160/90 mmHg and 200/100 mmHg at 1 h before surgery; not having received antihypertensive medication for at least a year) Exclusion criteria: people with angina pectoris, ECG changes indicative of active coronary artery disease, left bundle branch block, or left ventricular hypertrophy and strain Type of surgery: abdominal, peripheral, and vascular surgery (no further specification) Baseline characteristics Intervention group (labetalol)
Intervention group (atenolol)
Intervention group (oxprenolol)
Control group (standard care)
Country: UK Setting: single centre; hospital |
|
| Interventions |
Intervention group (labetalol)
Intervention group (atenolol)
Intervention group (oxprenolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: myocardial ischaemia (from interpretation of ECG recordings, based on ST‐segment depression values); MI; bradycardia (< 45 bpm), hypotension (SBP < 70 mmHg), ventricular extrasystoles Outcomes relevant to the review: MI; bradycardia (< 45 bpm), hypotension (SBP < 70 mmHg) |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Assessment of ECG recordings by a cardiologist who was unaware of group assignment. Study authors did not report whether assessors of other outcomes were blinded and we assumed not because of the open‐label study design |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Sugiura 2007.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 34 Inclusion criteria: people with mild to moderate hypertension scheduled to undergo minor surgery under GA; 20‐69 years of age Exclusion criteria: taking beta‐blockers before surgery; beta‐blockers, propofol, vecuronium, and fentanyl were contraindicated; heart rhythm other than sinus rhythm; uncontrolled diabetes mellitus; valvular disease Type of surgery: minor surgery Baseline characteristics Intervention group (landiolol: low dose)
Intervention group (landiolol: high dose)
Control group (standard care)
Country: Japan Setting: hospital; single centre |
|
| Interventions |
Intervention group (landiolol: low dose)
Intervention group (landiolol: high dose)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; hypotension (> 30% decrease from baseline; reported at 2 time points, which were: before intubation, and 5 min after intubation) Outcomes relevant to the review: hypotension (before intubation) |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This study was performed in a randomised, double‐blind fashion...using the envelope method" Comment: no additional details |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Anaesthetists were blinded to group allocation |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | Independent observers were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few losses (< 10%) |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Tendulkar 2017.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 60 Inclusion criteria: scheduled for surgery under GA; normotensive; ASA status I or II; 20‐70 years of age; either sex Exclusion criteria: ≥ ASA status III; pregnant; severe left ventricular dysfunction; bronchial asthma or COPD; high risk for stroke; pre‐exisiting AF or high‐degree atroventricular block; pacemaker dependency Type of surgery: type of surgery not specified (we assumed non‐cardiac) Baseline characteristics Intervention group (esmolol)
Control group (standard care)
Country: India Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic parameters; bradycardia (HR < 60 bpm; treated with atropine); hypotension (SBP < 80 mmHg; treated with mephentermine); coughing response to extubation; sedation scores Outcomes relevant to the review: bradycardia (see notes below); hypotension |
|
| Notes |
Funding/declarations of interest: not reported Study dates: July 2014‐August 2016 Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Ugur 2007.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 60 Inclusion criteria: 20‐50 years of age; ASA status I or II; undergoing elective surgery under GA Exclusion criteria: history of cardiovascular disease, diabetes mellitus, COPD, renal or hepatic failure; taking drugs that affect the cardiovascular system; SBP < 100 mmHg or > 160 mmHg; DBP < 50 mmHg or > 110 mmHg; HR < 50 bpm or > 120 bpm Type of surgery: elective surgery (type of surgery not specified ‐ we assumed non‐cardiac) Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: Turkey Setting: hospital; single centre |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; bradycardia (HR < 50 bpm); bronchospasm; arrhythmia (not defined); abnormal ST segments on ECG Outcomes relevant to the review: bradycardia |
|
| Notes |
Funding/declarations of interest: no funding; study authors declare no conflicts of interest Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization chart |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded randomized trial |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | Participants, and anaesthetists who recorded outcome data, were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Unal 2008.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 45 Inclusion criteria: ASA status I or II; scheduled for lumbar disc surgery Exclusion criteria: pregnant women; HR < 60 bpm; SBP < 100 mmHg; serious hepatic, renal and cardiovascular diseases; congestive heart failure; atrioventricular block; sick sinus syndrome; drug allergy and past history of beta‐blocker intolerance; bronchospasm; asthma; COPD; haemotological disorders; receiving beta‐blockers and calcium channel blockers Type of surgery: lumbar disc surgery Baseline characteristics Intervention group (esmolol 0.1 mg/kg/min)
Intervention group (esmolol 0.2 mg/kg/min)
Control group (placebo)
Country: Turkey Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol 0.1 mg/kg/min)
Intervention group (esmolol 0.2 mg/kg/min)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables, hypotension, hypertension Outcomes relevant to the review: hypotension |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Esmolol and SP (serum physiological) solutions were prepared without the knowledge of the person who would keep the records" Comment: it was unclear whether anaesthetists were blinded to the type of drug |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Urias 2016.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 84 Inclusion criteria: scheduled for laparoscopic cholecystectomy; without contraindications to the use of metoprolol Exclusion criteria: not reported Type of surgery: laparoscopic cholecystectomy Baseline characteristics not reported. Study authors reported that, "In demographic variables such as sex, age, there were no differences in the study" Country: Mexico Setting: single centre; hospital |
|
| Interventions |
Intervention group (metoprolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: HR, ischaemic heart disease; AF; isolated ventricular premature complexes Outcomes relevant to the review: AF |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specifed |
| Allocation concealment (selection bias) | Unclear risk | Not specifed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double‐blinded placebo‐controlled trial, and we assumed that the anaesthetists were blinded to study groups |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specifed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Unclear risk | Not feasible to assess other risks of bias from the short abstract report |
Van den Berg 1998.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 80 Inclusion criteria: ASA I‐III; middle‐aged to elderly healthy people; receiving treatment for diabetes, hypertension, ischaemic heart disease, or a combination of these diseases; scheduled for cataract extraction under GA because they were considered unsuitable for peribulbar analgesia due to senility, language barrier, chronic cough, renal failure, or an estimated inability to remain immobile in the supine position during surgery Exclusion criteria: asthma Type of surgery: cataract extraction under GA Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: Saudi Arabia Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic parameters; bradycardia (HR < 50 bpm and treated with atropine) Outcomes relevant to the review: bradycardia (see notes below) |
|
| Notes |
Funding/declarations of interest: not reported Study dates: January 1995‐April 1996 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of block randomization |
| Allocation concealment (selection bias) | Low risk | Use of sealed envelopes, and preparation of syringes by anaesthetists were blinded to treatment groups |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Anaesthetists were blinded, use of coded syringes |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Verma 2018.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 80 Inclusion criteria: adult, ASA status I and non‐hypertensive ASA status II; 20‐60 years of age; undergoing laparoscopic transperitoneal nephrectomy for poorly functional kidneys Exclusion criteria: hypertension, obesity; cardiovascular and pulmonary disease; renal insufficiency; known allergy to study drugs or taking any beta‐blocker or calcium channel blocker Type of surgery: laparoscopic nephrectomy Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: India Setting: hospital; single centre |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; bradycardia (HR < 50 bpm; treated with atropine); hypotension (MAP < 20% baseline; treated with mephentermine); hypertension Outcomes relevant to the review: bradycardia and hypotension (see notes below) |
|
| Notes |
Funding/declarations of interest: no funding, and study authors reported no conflicts of interest Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Anaesthetists were blinded to study medication |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Wajima 2011.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 42 Inclusion criteria: ASA status I and II, female adults; scheduled to undergo elective abdominal procedures Exclusion criteria: history of cardiac, pulmonary, or renal disease; oesophageal reflux or hiatal hernia; taking medications known to influence anaesthetic or analgesic requirements; taking antidepressants; drug or alcohol abuse; significant obesity; contraindication to facemask induction of anaesthesia Type of surgery: lower and upper abdominal surgery Baseline characteristics Intervention group (landiolol)
Control group (placebo)
Country: Japan Setting: university hospital; single centre |
|
| Interventions |
Intervention group (landiolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: concentration of anaesthetic agent; adverse efects (severe bradycardia or hypotension; not defined) Outcomes relevant to the review: bradycardia and hypotension (see notes below) |
|
| Notes |
Funding/declarations of interest: department funding only Study dates: not reported Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Anaesthetists were blinded to study group assignments |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | Outcome assessor was blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Wallace 1998.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 200 Inclusion criteria: scheduled for non‐cardiac surgery; definite coronary artery disease, or presence of risk factor of previous or current vascular surgery, or presence of at least 2 risk factors (≥ 65 years of age, hypertension, current smoking, serum cholesterol level ≥ 240 mg/dL, or diabetes mellitus) Exclusion criteria: left bundle branch block; cardiac pacemaker dependency; marked resting ST‐T wave abnormalities that preclude ECG interpretation Type of surgery: non‐cardiac surgery (major vascular, intra‐abdominal, orthopaedic, neurosurgical, other) Baseline characteristics Intervention group (atenolol)
Control group (placebo)
Country: USA Setting: single centre; hospital |
|
| Interventions |
Intervention group (atenolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: dysrhythmias, haemodynamic parameters, creatinine phosphokinase, death from cardiac causes, unstable angina, congestive heart failure, ventricular tachycardia, myocardial ischaemia, hypotension (SBP < 90 mmHg), bradycardia (HR < 50 bpm), mortality from all causes during the 2 years after discharge Outcomes relevant to the review: all‐cause mortality (30 days, and 2 years), death from cardiac causes, MI, ventricular tachycardia, stroke, bradycardia, hypotension, congestive heart failure |
|
| Notes |
Funding/declarations of interest: supported by Ischemia Research and Education Foundation Study dates: not reported
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer generated, randomized list" |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, placebo‐controlled, triple‐blind trial |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Cause of death data analysed by blinded pathologist. It is not clearly reported whether other outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Small number of participants were not followed up |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Ward‐Booth 1983.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 64 Inclusion criteria: healthy people undergoing simple oral procedures under GA; 16‐65 years of age Exclusion criteria: not reported Type of surgery: oral surgery Baseline characteristics not reported by group Overall age, mean (range): 26.5 (16‐65) years Country: UK Setting: hospital; single centre |
|
| Interventions |
Intervention group (metoprolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: ventricular arrhythmia; bradycardia (< 60 bpm) Outcomes relevant to the review: bradycardia |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded, placebo‐controlled trial. We assumed that the anaesthetists were blinded |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | Outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Unclear risk | Short report. It was not feasible to assess other risks of bias from this publication |
White 2003.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 30 Inclusion criteria: ASA status I or II; scheduled to undergo gynaecological procedures under GA Exclusion criteria: women with clinically significant cardiovascular, pulmonary, renal, hepatic, and neurologic diseases; body weight > 100% above the ideal; history of alcohol or drug abuse Type of surgery: gynaecological procedures Baseline characteristics Intervention group (esmolol)
Control group (placebo)
Country: USA Setting: single centre; hospital |
|
| Interventions |
Intervention group (esmolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; premature ventricular contractions (short run); transient nodal rhythm; adverse cardiovascular events (not defined); BIS; recovery times; length of hospital stay (min); postoperative analgesics and antiemetic medication; intraoperative recall Outcomes relevant to the review: length of hospital stay (see notes below) |
|
| Notes |
Funding/declarations of interest: supported by department funding and endowment funds (Margaret Milam McDermott Distinguished Chair in Anesthesiology) Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as placebo‐controlled, double‐blinded study; we therefore assumed that anaesthetists were blinded to study drugs |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Whitehead 1980.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 60 Inclusion criteria: scheduled for removal of wisdom teeth under GA Exclusion criteria: cardiac insufficiency, chronic obstructive airway disease, receiving routine medication, diabetes, severe hepatic, renal, or haematological disease Type of surgery: removal of wisdom teeth Baseline characteristics Intervention group (metoprolol)
Control group (placebo)
Country: UK Setting: single centre; hospital |
|
| Interventions |
Intervention group (metoprolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: cardiac arrhythmias (nodal rhythm and ventricular extrasystoles), bradycardia (HR < 50 bpm); hypotension (SBP < 100 mmHg) Outcomes relevant to the review: bradycardia (HR < 50 bpm); hypotension (SBP < 100 mmHg) |
|
| Notes |
Funding/declarations of interest: study drugs provided by Messrs Ciba‐Geigy Pharmaceuticals Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, placebo‐controlled, double‐blind trial. Ampoules of study drugs were visually similar |
| Blinding of outcome assessors (detection bias) All outcomes | Low risk | Anaesthesiologist evaluating ECG and haemodynamic parameters was blinded to study group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | High risk | Short report with baseline characteristics reported with limited detail. Not feasible to assess other risks of bias from this report. In addition, we noted that participants in either group were given metoprolol to manage ventricular dysrrhythmia. This may influence outcome data in the control group |
Yamazaki 2005.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 64 Inclusion criteria: undergoing elective non‐cardiac surgery under GA Exclusion criteria: history of cardiovascular disease; diabetes mellitis; disorders known to affect autonomic function; people taking medications known to affect cardiovascular function Type of surgery: type not specified Baseline characteristics Intervention group (landiolol 0.1 mg/kg)
Intervention group (landiolol 0.3 mg/kg)
Control group (placebo)
Country: Japan Setting: hospital; single centre |
|
| Interventions |
Intervention group (landiolol 0.1 mg/kg)
Intervention group (landiolol 0.3 mg/kg)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables; bradycardia and hypotension (not defined) Outcomes relevant to the review: bradycardia, hypotension |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Saline is given in the control group. However, study authors did not report whether anaesthetists were blinded to the agent |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Yang 2006.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 496 Inclusion criteria: scheduled for abdominal aortic surgery, infra‐inguinal or extra‐anatomical revascularization, ASA status ≤ III Exclusion criteria: current or recent beta‐blocker use, current amiodarone use, airflow obstruction requiring treatment, history of congestive heart failure, history of atrioventricular block, previous adverse drug reactions to beta‐blockers, previous participation in the MaVS study Type of surgery: abdominal aortic surgery, infrainguinal or extra‐anatomical revascularization Baseline characteristics Intervention group (metoprolol)
Control group (placebo)
Country: Canada Setting: multi‐centre; hospital |
|
| Interventions |
Intervention group (metoprolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: MI (non‐fatal), unstable angina, new congestive heart failure, dysrhythmias, all‐cause mortality (30 days and 6 months), death due to cardiac causes, hypotension (SBP < 90 mmHg), bradycardia (HR < 50 bpm), cerebrovascular event, bronchospasm Outcomes relevant to the review: MI (non‐fatal), new congestive heart failure, all‐cause mortality (30 days), death due to cardiac causes, hypotension, bradycardia, cerebrovascular event |
|
| Notes |
Funding/declarations of interest: funded by the Heart and Stroke Foundation of Canada Study dates: 1999‐2002 Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was constructed in blocks of four by the study statistician and study medication preparations by the pharmacists" |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomized, placebo‐controlled, double‐blind trial |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | High risk | We could not be certain of loss of participants (study authors reported discontinuation of treatment in 12% placebo group participants, and 13% metoprolol group participants). Study authors reported use of ITT |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | High risk | Although use of beta‐blockers was discouraged, study authors reported that 24 participants in the control group and 14 participants in the intervention group were given additional beta‐blockers. This may influence outcome data for this review |
Yang 2008.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 102 Inclusion criteria: non‐cardiac surgery (major abdominal) with risk or at high risk of coronary artery disease Exclusion criteria: chronic metoprolol use Type of surgery: non‐cardiac surgery (major abdominal) Baseline characteristics Intervention group (metoprolol)
Control group (standard care)
Country: China Setting: single centre; hospital |
|
| Interventions |
Intervention group (metoprolol)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: changes in HR, levels of creatine kinase (CK‐MB), mortality, acute MI, stroke Outcomes relevant to the review: mortality, acute MI, stroke |
|
| Notes |
Funding/declarations of interest: not reported Study dates: January 2006‐October 2007
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Yoshida 2017.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 80 Inclusion criteria: ≥ 20 years of age; pathologically confirmed oesophageal cancer not previously treated by surgery Exclusion criteria: cardiac shock; metabolic acidosis; diabetic mellitus‐related ketoacidosis; 2nd‐degree atrioventricular block; pulmonary hypertension‐related right heart failure; congestive heart failure; untreated phaeochromocytoma; hypersensitivity to components of landiolol hydrochloride Type of surgery: transthoracic oesophagectomy Baseline characteristics Intervention group (landiolol)
Control group (placebo)
Country: Japan Setting: single centre; hospital |
|
| Interventions |
Intervention group (landiolol)
Control group (placebo)
|
|
| Outcomes |
Outcomes measured/reported by study authors: AF, tachycardia (not defined as ventricular or supraventricular); postoperative complications; adverse events ‐ hypotension (SBP < 80 mmHg) or bradycardia (< 50 bpm) Outcomes relevant to the review: AF; bradycardia and hypotension (see notes below) |
|
| Notes |
Funding/declarations of interest: not reported Study dates: July 2009‐March 2014 Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo‐controlled trial. However, it is not clear whether anaesthetists were blinded to treatment |
| Blinding of outcome assessors (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of 1 participant in the intervention group |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
Zaugg 1999.
| Methods | RCT, parallel design | |
| Participants |
Total number of randomized participants: 63 Inclusion criteria: ≥ 65 years of age, scheduled for elective major noncardiac surgery under GA Exclusion criteria: preoperative treatment with beta‐adrenergic agonists, beta‐adrenergic antagonists, or glucocorticoids; 2nd‐ or 3rd‐degree heart block; nonsinus rhythm seen on an ECG; clinically significant congestive heart failure or bronchospasm; systemic infection; surgery within the previous month; neurologic disorders and current use of anticonvulsant or other psychoactive medication Type of surgery: non‐cardiac surgery (major abdominal surgery, hip replacement, intrathoracic surgery) Baseline characteristics Intervention group (atenolol: pre‐ and postoperative administration)
Intervention group (atenolol: intra‐operative administration)
Control group (standard care)
Country: USA Setting: single centre; hospital |
|
| Interventions |
Intervention group (atenolol: pre‐ and postoperative administration)
Intervention group (atenolol: intraoperative administration)
Control group (standard care)
|
|
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic variables, stress hormone levels, MI, myocardial ischaemia (from evaluation of ECGs) Outcomes relevant to the review: MI |
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessors (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors report loss of 4 participants with reasons provided. Loss of < 10% |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report prospective clinical trial registration or publication of a protocol. It was not feasible to effectively assess risk of reporting bias |
| Other bias | Low risk | Not detected |
ACE inhibitor: angiotensin‐converting‐enzyme inhibitor; AF: atrial fibrillation; AMI: acute myocardial infarction; ARB: angiotensin II receptor blockers; ASA: American Society of Anesthesiologists; BIS: bispectral index; BMI: body mass index; bpm: beats per minute; BP: blood pressure; CABG: coronary artery bypass graft; COPD: chronic obstructive pulmonary disease; CPAP: continuous positive airway pressure; DBP: diastolic blood pressure; ECG: electrocardiogram; GA: general anaesthesia; GOS: Glasgow Outcome Scale; HIV: human immunodeficiency virus; HR: heart rate; ITT: intention‐to‐treat; IQR: interquartile range; IV: intravenous(ly); LMA: laryngeal mask airway; MAP: mean arterial pressure; M/F: male/female; MI: myocardial infarction; NIH: National Institutes of Health; NT‐proBNP: N‐terminal pro‐brain natriuretic peptide; NYHA: New York Heart Association; PACU: post anaesthesia care unit; PONV: postoperative nausea and vomiting;P‐R interval: a period between waves on an electrocardiogram; QT: interval measurement on an electrocardiogram; RCT: randomized controlled trial; SAP: systolic arterial pressure; SBP: systolic blood pressure; SD: standard deviation; ST segment and ST‐T: a period between waves on an electrocardiogram; SVT: supraventricular tachycardia; TBSA: total burn surface area; TIA: transient ischaemic attack
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chae 1990 | RCT, participants with hypertension undergoing surgery. We excluded this study because participants in the intervention group (propranolol) were all given verapamil as an additional agent |
| Coleman 1980 | RCT, participants undergoing elective general surgery. Multi‐arm study: 2 mg metoprolol vs 4 mg metoprolol vs placebo. This study was included in previous versions of the review. We re‐evaluated this study following a change to the number of review outcomes; we excluded this study because study authors did not measure or report the review outcomes. |
| Marwick 2009 | RCT, bisoprolol vs stratified beta‐blocker therapy. The control group underwent dobutamine echocardiography to guide whether beta‐blockers were used and we excluded this study because the control group was not equivalent to 'standard care' practices in other studies. This study was included in previous versions of the review, and we re‐assessed its eligibility during the current update. |
| Ryder 1973 | RCT, practolol vs atropine vs placebo, given preoperatively before dental surgery under GA. We excluded this study because it included children from 10 years of age, and data were not reported separately for adult participants |
| Sandler 1990 | RCT, esmolol vs control. This study was included in previous versions of the review. We re‐evaluated eligibility of this study during the update and excluded it because the procedure (rigid bronchoscopy) did not involve surgery |
| Sezai 2015 | RCT, landiolol vs control. We excluded this study because we noted that participants in both groups were given an oral beta‐blocker as soon as oral treatment was tolerated which continued during period of outcome assessment, and therefore standard treatment included beta‐blocker treatment |
| Taenaka 2013 | RCT, participants undergoing highly invasive surgery who developed tachycardia within 7 days postoperatively. Multi‐arm study ‐ landiolol (low‐ to medium‐dose or medium‐ to high‐dose) vs placebo. We excluded this study because beta‐blocker treatment started up to 7 days after surgery |
| Tan 2002 | RCT, participants undergoing non‐cardiac surgery. We excluded this study because participants in the intervention group (esmolol) were all given nicardipine as an additional agent |
| Vucevic 1992 | RCT, participants undergoing elective abdominal surgery. We excluded this study because the report included no information on the sample size (randomized or analysed); the report is old, with insufficient author contact details. |
| Zmora 2016 | RCT, participants undergoing cancer surgery. We excluded this study because participants in the intervention group (propranolol) were all given a COX‐2 inhibitor as an additional agent. |
RCT: randomized controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
ACTRN12605000639628.
| Methods | RCT, parallel design |
| Participants |
Estimated number of randomized participants: 200 Inclusion criteria: undergoing major surgery under GA with at least 2 risk factors for coronary artery disease: diabetes; hypertension; current smoker; cholesterol > 6.2 mmol/L Exclusion criteria: known coronary artery disease; long term beta‐blocker use; poorly compensated CCF, 3rd‐degree heart block; unstable asthma Country: Australia Setting: hospital; single centre |
| Interventions | Atenolol: given preoperatively and for 7 days postoperatively Placebo: given the same as the intervention group |
| Outcomes | Intended outcome measures: mortality; cardiovascular morbidity |
| Notes | Study is completed. Awaiting publication of study results in order to assess eligibility for the review |
ACTRN12615000889.
| Methods | RCT, parallel design |
| Participants |
Estimated number of randomized participants: 64
Inclusion criteria: written, informed consent; female participants with histologically‐confirmed breast cancer, who will undergo surgical excision at least 7 days after enrolment; 18‐80 years of age; WHO ECOG performance status 0 or 1 Exclusion criteria: pregnant or breast feeding; absolute or relative contraindications to propranolol: sick sinus syndrome; sinus bradycardia; 1st‐, 2nd‐ or 3rd degree atrioventricular block; resting BP < 100/60 mmHg; untreated phaeochromocytoma; untreated thyroid disorder; receiving calcium channel blocking agents; severe peripheral vascular disease; receiving anti‐arrhythmic agents; renal impairment; liver impairment; receiving clonidine, digoxin, rizatriptan, cimetidine, hydralazine, guanethidine or ergotamine; episodes of major depression; breast resection within 6 months of study entry; received neoadjuvant chemotherapy prior to the planned breast cancer resection; histologically demonstrated ductal carcinoma in situ; non‐English speaking women; using regular (daily) pre‐operative anti‐inflammatory agents; using regular anxiolytics, alpha‐receptor adrenergic agonists; use of selective or non‐selective beta‐adrenergic inhibitors in the last three months; history of stroke; moderate or severe asthma; other medical conditions considered prohibitive by the treating physician (including frailty) Country: Australia Setting: hospital; single centre |
| Interventions | Propranolol: 40 mg propranolol, given orally, for 7 days preoperatively including day of surgery, then titrated down to discontinuation in 2 days postoperatively Placebo: given same as the intervention group |
| Outcomes | Intended outcome measures: primary tumour gene expression; tumour markers of inflammation; leukocyte gene expression; inflammation‐relevant cytokines; Beck Anxiety Inventory; hypotension; BP; bronchial hyper‐reactivity; bradycardia |
| Notes | Study is completed. Awaiting publication of study results in order to assess eligibility for the review |
Birbicer 2007.
| Methods | RCT, parallel design |
| Participants |
Total number of randomized participants: 60 Inclusion criteria: undergoing GA |
| Interventions | Esmolol vs placebo |
| Outcomes | Outcomes reported in the abstract: haemodynamic variables; ECG measurements; possibly AF |
| Notes | We were unable to access the full‐text of this article from British library sources; this full‐text may be available in future updates of the review |
Boussofara 2001.
| Methods | RCT, parallel design |
| Participants |
Total number of randomized participants:29 Inclusion criteria: ASA status II or III; undergoing GA |
| Interventions | Esmolol vs placebo |
| Outcomes | Outcomes reported in the abstract: haemodynamic variables |
| Notes | We were unable to access the full‐text of this article from British library sources; this full‐text may be available in future updates of the review |
Boussofara 2004.
| Methods | RCT, parallel design |
| Participants |
Total number of randomized participants: 32 Inclusion criteria: undergoing micro ENT surgery |
| Interventions | Esmolol vs placebo. Study included 2 additional groups (nicardipine; and lidocaine), which we will not include in the review |
| Outcomes | Outcomes reported in the abstract: haemodynamic variables |
| Notes | We were unable to access the full‐text of this article from British library sources; this full‐text may be available in future updates of the review |
Gong 1999.
| Methods | RCT, parallel design |
| Participants | Total number of randomized participants: 35 (number includes an additional group that is not eligible for the review |
| Interventions | Esmolol vs standard care. Study includes an additional group (alfentanil) which we will not include in the review |
| Outcomes | Outcomes reported in the abstract: haemodynamic variables; catecholamine responses |
| Notes | We were unable to access the full‐text of this article from British library sources; this full‐text may be available in future updates of the review |
Hornamand 2017.
| Methods | RCT, parallel design |
| Participants |
Total number of randomized participants: 105 (number includes participants in an additional group that is not eligible for the review Inclusion criteria: scheduled for surgery with laryngoscopy |
| Interventions | Labetolol vs normal saline. Study included an additional group (remifentanil), which we will not include in the review |
| Outcomes | Outcomes reported in the abstract: bradycardia; tachycardia; hypotension; hypertension |
| Notes | Awaiting translation from Persian to assess full eligibility; number of participants in each group is not reported. We identified another publication by the same author team (Hornamand 2016) and it is possible that these are associated publications of the same study. |
Inada 2002.
| Methods | RCT, parallel design |
| Participants | Inclusion criteria: people undergoing GA |
| Interventions | Esmolol vs placebo |
| Outcomes | Outcomes reported in the abstract: haemodynamic variables; side effects (to include hypotension) |
| Notes | We were unable to access the full‐text of this article from British library sources; this full‐text may be available in future updates of the review |
Itani 2013.
| Methods | RCT, parallel design |
| Participants |
Total number of randomized participants: 17 Inclusion criteria: people undergoing hepatectomy |
| Interventions | Landiolol vs saline |
| Outcomes | Outcomes reported in the abstract: haemodynamic variables; concentrations of epinephrine and norepinephrine; possible measurement of tachycardia |
| Notes | Study currently only published as an abstract; we need to assess the full‐text for eligibility |
Joo 2010.
| Methods | RCT, parallel design |
| Participants | Inclusion criteria: people undergoing ambulatory surgery |
| Interventions | Esmolol (0.25 mg/kg; 0.50 mg/kg; or 1 mg/kg) vs control (not defined), given during anaesthesia |
| Outcomes | Outcomes reported in the abstract: haemodynamic variables |
| Notes | Awaiting translation from Korean in order to fully assess eligibility. Unable to ascertain from the English abstract whether any outcomes relevant to the review are measured or reported |
Kajiura 2013.
| Methods | RCT, parallel design |
| Participants | Inclusion criteria: people undergoing lobectomy |
| Interventions | Landiolol infusion for 24 h from the start of surgery vs control (type of control not specified) |
| Outcomes | Outcomes reported in the abstract: hypotension, bradycardia, AF |
| Notes |
Study dates: June 2010‐April 2013 Study published only as an abstract. Appears to be eligible but insufficient information available to extract outcome data. We await publication of the full report in order to include this study in the review |
Kawano 2005.
| Methods | RCT, parallel design |
| Participants |
Total number of randomized participants: 40 Inclusion criteria: ASA status I |
| Interventions | Landiolol vs placebo |
| Outcomes | Outcomes reported in the abstract: haemodynamic variables; BIS during tracheal intubation |
| Notes | Awaiting translation from Korean in order to fully assess eligibility. Unable to ascertain from the English abstract whether any outcomes relevant to the review are measured or reported |
NCT02466542.
| Methods | RCT, parallel design |
| Participants |
Estimated number of randomized participants: 60 Inclusion criteria: women; 18‐75 years of age; scheduled for elective mastectomy; ASA I or II Exclusion criteria: unstable angina; poorly controlled asthma; substance abuse; sinus bradycardia; heart failure > 1st degree atrioventricular block; pregnant; allergy to dipyrone, morphine; chronic pain; severe hepatic disease; severe kidney disease; neurological disease; in other clinical studies; refusal to participate in the study; any other condition that, in the opinion of the investigator, may pose a risk to the participants or interfere with the study objectives |
| Interventions | Esmolol vs placebo |
| Outcomes | Intended outcome measures: pain scores; analgesic requirement; adverse events (incidence of tachycardia, hypertension, hypotension; bradycardia, use of vasopressors, recovery times, PONV, pruritis, urinary retention, drowsiness) |
| Notes | Study is completed. Awaiting publication of study results in order to assess eligibility for the review |
Tangoku 2016.
| Methods | RCT, parallel design |
| Participants |
Total number of randomized participants: 80 Inclusion criteria: scheduled for oesophagectomy |
| Interventions | Landiolol 5 g/kg/min after induction of anaesthesia and continued for 24 h vs control group (not defined in the abstract) |
| Outcomes | Outcomes reported in the abstract: tachycardia; AF; adverse event leading to discontinuation of treatment |
| Notes |
Study dates: July 2009‐March 2014 Study published only as an abstract. Appears to be eligible but insufficient information available to extract outcome data. We await publication of the full report in order to include this study in the review |
UMIN000024040.
| Methods | RCT, parallel design |
| Participants |
Estimated number of randomized participants: 188 Inclusion criteria: men or women; histologically or cytologically confirmed oesophageal cancer; 20‐80 years of age; scheduled for arthroscopic oesophagus resection Exclusion criteria: history of arrhythmias; preoperative bradycardia; thyroid dysfunction; preoperative beta‐blocker use; history of asthma; hospitalization for heart failure, left ventricular ejection fraction < 30%; untreated pheochromocytoma Country: Japan Setting: single centre; hospital |
| Interventions | Landiolol vs placebo |
| Outcomes | Intended outcome measures: AF |
| Notes | Study status described as 'no longer recruiting'. Awaiting publication of study results in order to assess eligibility for the review |
Wang 1994.
| Methods | RCT, parallel design |
| Participants |
Total number of randomized participants: 200 Inclusion criteria: undergoing elective non‐cardiac surgery |
| Interventions | Esmolol (20 mg; 40 mg; and 60 mg) vs placebo |
| Outcomes | Outcomes reported in the abstract: haemodynamic variables; tachycardia; hypertension |
| Notes | We were unable to access the full‐text of this article from British library sources; this full‐text may be available in future updates of the review |
Wang 1999.
| Methods | RCT, parallel design |
| Participants | Total number of randomized participants: 1830 |
| Interventions | Esmolol (1 mg/kg; and 2 mg/kg) vs placebo |
| Outcomes | Outcomes reported in the abstract: haemodynamic variables; tachycardia; hypotension; bradycardia |
| Notes | Awaiting translation from Chinese in order to assess eligibility |
Yuan 1994.
| Methods | RCT, parallel design |
| Participants |
Total number of randomized participants: 45 Inclusion criteria: undergoing elective non‐cardiac surgery |
| Interventions | Esmolol (100 mg; and 200 mg) vs placebo |
| Outcomes | Outcomes reported in the abstract: haemodynamic variables; tachycardia; hypotension; bradycardia |
| Notes | We were unable to access the full‐text of this article from British library sources; this full‐text may be available in future updates of the review |
AF: atrial fibrillation;ASA: American Society of Anesthesiologists;BP: blood pressure;BIS: bispectral index; CCF: congestive cardiac failure; ECG: electrocardiography; ENT: ear, nose, and throat; GA: general anaesthesia; IV: intravenous(ly); PONV: postoperative nausea and vomiting; RCT: randomized controlled trial: WHO ECOG: World Health Organization Eastern Cooperative Oncology Group
Characteristics of ongoing studies [ordered by study ID]
EUCTR2010‐021844‐17.
| Trial name or title | Perioperative esmolol infusion for haemodynamic stability during major vascular surgery |
| Methods | RCT, parallel design |
| Participants |
Estimated number of recruited participants: 260 Inclusion criteria: scheduled for arterial vascular surgery, including AAA repair, abdominal aortic stenosis surgery, lower limb arterial reconstruction or carotid artery stenosis repair Exclusion criteria: active bleeding; untreated left main disease; active cardiac condition; preoperative positive troponin T; contraindication to esmolol use; previous allergy or intolerance to esmolol; cancer with life expectancy < 6 months; excessive alcohol use; pregnancy or planning to become pregnant; failure to provide informed consent; failure to monitor HR with continuous 12‐lead ECG because of surgery or baseline ECG abnormalities Country: Netherlands Setting: hospital; single centre |
| Interventions | Esmolol hydrochloride IV Placebo |
| Outcomes |
Outcomes measured/reported by study authors: haemodynamic stability; myocardial ischaemia; TIA or stroke; bradycardia Outcomes relevant to the review: TIA or stroke; bradycardia |
| Starting date | Unknown, registered in July 2010 |
| Contact information | Erasmus Medical Centre |
| Notes |
NCT01555554.
| Trial name or title | Perioperative propranolol in patients with post traumatic stress disorder (PTSD) |
| Methods | RCT, parallel design |
| Participants |
Estimated number of recruited participants: 110 Inclusion criteria: veterans with PTSD; scheduled for any surgical procedure under GA or combined general‐regional anaesthesia, with the exception of open‐heart or intracranial surgery; anticipated postoperative hospital admission (defined as at least 1 overnight hospital stay) Exclusion criteria: on beta‐blocker therapy at the time of the preoperative baseline assessment; sensitivity or allergies to propranolol, or a history of PTSD exacerbation with prior propranolol therapy; veterans who fulfil the AHA/ACC level I recommendation criteria for perioperative beta‐blocker therapy (e.g. metoprolol, atenolol) and should not be randomized to placebo group; medical exclusions criteria: high‐grade heart block without pacemaker (2nd‐ and 3rd degree heart block), marked resting bradycardia (heart rate ≤ 55 bpm), BP < 100 mmHg, uncompensated congested heart failure, severe hyperactive airway disease, and Raynaud's disease; pregnancy; current use of medication that may involve potentially dangerous interaction with propranolol; circumstances that, in the opinion of the principal investigator, would preclude participation in a study of this type (e.g. medical concerns or difficulty in long‐term follow‐up); open‐heart surgery and intracranial surgery Country: USA Setting: hospital; single centre |
| Interventions | Propranol orally, started on day of surgery, continued for 14 days Placebo, taken same as the propranolol group |
| Outcomes |
Outcomes measured/reported by study authors: ICU length of stay; hospital length of stay; postoperative delirium; postoperative renal dysfunction; perioperative complications; pain intensity; pain unpleasantness; analgesics use; length of intubation and mechanical ventilation; PTSD symptomatology; quality of life; functional status; sleep quality; depression symptoms; postoperative neurocognitive dysfunction score; 30‐day, 3‐months, and 1‐year mortality; postoperative complications Outcomes relevant to the review: early and late mortality; length of hospital stay |
| Starting date | May 2012 |
| Contact information | Principal Investigator: Marek Brzezinski, MD, PhD, University of California, San Francisco |
| Notes |
NCT03138603.
| Trial name or title | Metoprolol to reduce perioperative myocardial injury |
| Methods | RCT, parallel design |
| Participants |
Estimated number of recruited participants: 600 Inclusion criteria: ≥ 50 years of age; beta‐blocker naive (30 days prior to surgery); previously diagnosed coronary artery disease; history of peripheral vascular disease or chronic kidney disease; positive stress test; at risk for coronary artery disease; scheduled for major non‐cardiac, elective surgery under general anaesthesia Exclusion criteria: history of stroke, or TIA; previously diagnosed carotid disease; HR ≤ 55 bpm; congestive heart failure; severe valvular regurgitation; 2nd‐ or 3rd‐degree atrioventricular block without pacemaker; active asthma or COPD; anaemia; allergy to beta‐blockade drugs; unwilling or unable to give consent for participation; undergoing any carotid endarterectomy, endovascular, endoscopic, superficial, or ambulatory procedures; pregnancy or lactating women; prisoners Country: USA Setting: hospital; single centre |
| Interventions | Metoprolol: up to 3 IV doses of IV metoprolol tartrate 5 mg prior to extubation, and subsequently an oral dose 25 mg metoprolol tartrate in the PACU, and then oral dosing at approximately every 8 h thereafter up to postoperative day 3 Placebo: given same as the intervention group |
| Outcomes |
Outcomes measured/reported by study authors: myocardial injury; MACE; myocardial ischaemia (ST‐depression/elevation duration); stroke; cumulative vasopressor requirements in PACU; incidence rate bradycardia duration (HR < 50/min); cumulative rate bradycardia duration (HR < 50/min); unplanned ICU admission; length of hospital stay; length of ICU stay Outcomes relevant to the review: stroke; length of hospital stay |
| Starting date | December 2016 |
| Contact information | Principal Investigator: Peter Nagele, MD, Washington University School of Medicine |
| Notes |
AAA: abdominal aortic aneurysm; AHA/ACC: American Heart Association/American College of Cardiology; BP: blood pressure; bpm: beats per minute; COPD: chronic obstructive pulmonary disorder; ECG: electrocardiography; GA: general anaesthesia; HR: heart rate; ICU: intensive care unit; IV: intravenous(ly); MACE: major adverse cardiovascular event; PTSD: post‐traumatic stress disorder; PACU: post‐anaesthesia care unit; RCT: randomized controlled trial; TIA: transient ischaemic attack
Differences between protocol and review
Differences between the previous review and the updated review
The previous version of the review assessed the effectiveness of perioperative beta‐blockers in cardiac and non‐cardiac surgery (Blessberger 2018). The previous version has now been split into two reviews according to type of surgery. This review assesses the evidence in non‐cardiac surgery only; we made appropriate changes throughout the review to reflect evidence for only this type of surgery. Data for cardiac surgery can be found in (Blessberger 2019). Whilst updating the review, we made changes to ensure that it met current Cochrane standards (MECIR), adding additional subheadings where necessary. In addition, we made the following changes.
Authors: we added three new review authors (Sharon Lewis, Michael Pritchard, Lizzy Fawcett), and we removed three review authors that had been involved in previous updates (Danyel Azar, Martin Schillinger, Franz Wiesbauer).
Objectives: we re‐worded these to account for the inclusion of only non‐cardiac surgery.
Types of studies: we clarified the inclusion of quasi‐randomized studies.
Types of interventions: we clarified the routes by which beta‐blockers could be given; previously, we had stated 'any route', but we did not intend to include beta‐blockers that were administered topically. We clarified the exclusion of studies (or study arms within a multi‐arm study) in which a supplementary agent was given with a beta‐blocker; we used this criteria in the previous version of the review. We added an exclusion criteria for the standard care control group, and excluded studies in which participants were given an agent that was not given to those in the intervention or in which all participants in the control group were given a beta‐blockers; this was because we expected such comparison groups could introduce too much contamination in the results.
Types of outcome measures: in order to increase the usability, manageability, and focus of the review, we clarified the exclusion of studies that did not measure the review outcomes (this was a change from protocol made in the previous version of the review). We removed some outcomes from this update (myocardial ischaemia, supraventricular arrhythmias (except for atrial fibrillation), ventricular extrasystoles, bronchospasm, and cost of care). We used the work of Myles and colleagues as a guide to select outcomes that we considered to be most important to the user of the review (Myles 2016), and we sought advice from the Cochrane editorial team to approve this change. Data from these outcomes are available in the previous publication of this review (Blessberger 2018). We included atrial fibrillation and atrial flutter as a distinct outcome; this was previously reported with data for supraventricular arrhythmias.
Search methods: we made changes to the search strategies to include terms for beta‐blockers. We did not search additional databases as part of a search of other resources, nor did we search the National Research Register or the Meta register of controlled trials.
Data extraction and management: we used an amended template for collecting data, which was more familiar to the new review authors who were responsible for data extraction in this review. In order to improve transparency, we added additional detail to the tables in Characteristics of included studies, and we created a summary table of the participant risk factors (Appendix 9).
Unit of analysis issues: we clarified that we used the 'halving method' for the control group in multi‐arm studies during subgroup analysis by the type of beta‐blocker, if necessary.
Assessment of heterogeneity: we did not use meta‐regression to explore heterogeneity. Previously effect modifiers were identified and assessed in meta‐regression. We could not be certain of our confidence in the findings from so many effect‐modifiers, and felt that this additional analysis detracted from the primary analysis. In the review, we explained that we considered clinical and methodological difference between studies as well as consideration of statistical heterogeneity. We collected clinical differences between study participants and presented this in a table. In addition, we expanded the information reported in the Characteristics of included studies tables to present information to the readers of possible effect modifiers.
Data synthesis: we used a random‐effects model, which allowed for the potential variation between participants (Borenstein 2010).
Sensitivity analysis: we evaluated the decision to include studies published prior to 2000; these studies may use clinical management practices that are not consistent with current standards. We evaluated the decision to use risk ratio with a random‐effects model for effects in which events were rare; in sensitivity analysis, for cerebrovascular events, we used the Peto odds ratio.
'Summary of findings' table: we changed the outcomes, replacing supraventricular arrhythmias with atrial fibrillation.
We re‐evaluated all studies that were previously included in the review, and decided to exclude three studies. One study no longer included relevant outcomes (Coleman 1980), one study included an additional intervention that was not equivalent to standard care practices in other control groups (Marwick 2009), and in one study, participants were undergoing non‐surgical procedures (Sandler 1990). We merged two references that were previously reported separately into Wallace 1998; these articles both described the same study with the same study participants.
Differences between protocol and previous review versions
Changes relevant to Blessberger 2014 and Blessberger 2018.
Change to inclusion criteria regarding general anaesthesia. Review included studies if more than 100 randomly assigned participants were operated on under general anaesthesia, or more than 70% of participants received general anaesthesia.
Bronchospasm and cost of care were added as secondary outcomes. These outcomes have been removed in the current version (see above)
Meta‐regression was conducted to evaluate potential effect modifiers. This was not completed in the current version (see above).
Trial sequential analysis was conducted. This was not completed in the current version (see above).
Risk ratio was used, rather than the odds ratio.
Data were reported separately for cardiac and non‐cardiac surgeries.
Contributions of authors
Contributions made in previous versions of the review can be found in Blessberger 2014 and Blessberger 2018.
Hermann Blessberger (HB), Sharon Lewis (SL), Michael Pritchard (MP), Lizzy Fawcett (LF), Juergen Kammler (JK), Hans Domanovits (HD), Oliver Schlager (OS), Brigitte Wildner (BW), Clemens Steinwender (CS)
Conceiving the review: previous review author F Wiesbauer
Co‐ordinating the review: SL
Undertaking manual searches: SL, BW, Janne Vendt (Information Specialist, Cochrane Anaesthesia Review Group)
Screening search results: SL, MP
Organizing retrieval of papers: SL, MP
Screening retrieved papers against inclusion criteria: SL, MP, HB
Appraising quality of papers: SL, MP, HB
Abstracting data from papers: SL, MP, LF
Managing data for the review: SL
Entering data into Review Manager 5 (Review Manager 2014): SL, MP, LF
Analysing Review Manager 5 statistical data: SL, HB
Interpreting data: SL, HB, JK, CS, OS, HD
Making statistical inferences: SL, HB, JK, CS
Writing the review: SL, HB
Securing funding for the review: Cochrane Anaesthesia
Performing previous work that was the foundation of the present study: E. Villanueva, R. Johnston and A. Rauli (preparation of first protocol draft)
Serving as guarantor for the review (one author): HB
Taking responsibility for reading and checking the review before submission: HB
Sources of support
Internal sources
None, Other.
External sources
NIHR Cochrane Incentive Awards Scheme, 2018, UK.
Declarations of interest
Hermann Blessberger: none known
Sharon Lewis: none known
Michael Pritchard: none known
Lizzy Fawcett: none known
Hans Domanovits: none known
Oliver Schlager: none known
Brigitte Wildner: none known
Juergen Kammler: none known
Clemens Steinwender: I have received speaker's honoraria from MSD, Sanofi‐Aventis, Boehringer‐Ingelheim, Bayer, Medtronic, Biotronic, Abbott, St. Jude Medical and Boston Scientific. Sanofi‐Aventis, Boehringer‐Ingelheim Europe, Medtronic, Biotronik, Bayer Austria, Abbott Vascular, St. Jude Medical and Boston Scientific do not produce, market or distribute any of the studied drug entities. MSD (timolol) have a beta‐blocker in their portfolio.
New
References
References to studies included in this review
Ali 2015 {published data only}
- Ali A, Herndon DN, Mamachen A, Hasan S, Andersen CR, Grogans RJ, et al. Propranolol attenuates hemorrhage and accelerates wound healing in severely burned adults. Critical Care 2015;19(1):217. [PUBMED: 25936635] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alkaya 2014 {published data only}
- Alkaya MA, Saracoglu KT, Pehlivan G, Eti Z, Gogus FY. Effects of esmolol on the prevention of haemodynamic responses to tracheal extubation after craniotomy operations. Turkish Journal of Anaesthesiology and Reanimation 2014;42(2):86‐90. [PUBMED: 27366396] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aoyama 2016 {published data only}
- Aoyama H, Otsuka Y, Aoyama Y. Landiolol infusion during general anesthesia does not prevent postoperative atrial fibrillation in patients undergoing lung resection. General Thoracic and Cardiovascular Surgery 2016;64(12):735‐41. [PUBMED: 27553581] [DOI] [PubMed] [Google Scholar]
Apipan 2010 {published data only}
- Apipan B, Rummasak D. Efficacy and safety of oral propranolol premedication to reduce reflex tachycardia during hypotensive anesthesia with sodium nitroprusside in orthognathic surgery: a double‐blind randomized clinical trial. Journal of Oral and Maxillofacial Surgery 2010;68(1):120‐4. [PUBMED: 20006165] [DOI] [PubMed] [Google Scholar]
Bayliff 1999 {published data only}
- Bayliff CD, Massel DR, Inculet RI, Malthaner RA, Quinton SD, Powell FS, et al. Propranolol for the prevention of postoperative arrhythmias in general thoracic surgery. Annals of Thoracic Surgery 1999;67(1):182‐6. [PUBMED: 10086546 ] [DOI] [PubMed] [Google Scholar]
Bhattacharjee 2016 {published data only}
- Bhattacharjee DP, Saha S, Paul S, Roychowdhary S, Mondal S, Paul S. A comparative study of esmolol and dexmedetomidine on hemodynamic responses to carbon dioxide pneumoperitoneum during laparoscopic surgery. Anaesthesia, Essays and Researches 2016;10(3):580‐4. [PUBMED: 27746555] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burns 1988 {published data only}
- Burns JM, Hart DM, Hughes RL, Kelman AW, Hillis WS. Effects of nadolol on arrhythmias during laparoscopy performed under general anaesthesia. British Journal of Anaesthesiology 1988;61(3):345‐6. [PUBMED: 2972306] [DOI] [PubMed] [Google Scholar]
Ceker 2015 {published data only}
- Ceker Z, Takmaz SA, Battaci B, Basar H. The effect of esmolol on corrected‐QT interval, corrected‐QT interval dispersion changes seen during anesthesia induction in hypertensive patients taking an angiotensin‐converting enzyme inhibitor. Revista Brasileira de Anestesiologia 2015;65(1):34‐40. [PUBMED: 25497747] [DOI] [PubMed] [Google Scholar]
Chung 1992 {published data only}
- Chung KS, Sinatra RS, Chung JH. The effect of an intermediate dose of labetalol on heart rate and blood pressure responses to laryngoscopy and intubation. Journal of Clinical Anesthesia 1992;4(1):11‐5. [PUBMED: 1540362] [DOI] [PubMed] [Google Scholar]
Cucchiara 1986 {published data only}
- Cucchiara RF, Benefiel DJ, Matteo RS, DeWood M, Albin MS. Evaluation of esmolol in controlling increases in heart rate and blood pressure during endotracheal intubation in patients undergoing carotid endarterectomy. Anesthesiology 1986;65(5):528‐31. [PUBMED: 2877599 ] [DOI] [PubMed] [Google Scholar]
DIPOM 2006 {published and unpublished data}
- Juul AB, Wetterslev J, Gluud C, Kofoed‐Enevoldsen A, Gorm J, Callesen T, et al. Effect of perioperative β blockade in patients with diabetes undergoing major non‐cardiac surgery: randomised placebo controlled, blinded multicentre trial. BMJ 2006;332(7556):1482. [PUBMED: 16793810] [DOI] [PMC free article] [PubMed] [Google Scholar]
Do 2012 {published data only}
- Do H‐S, Kim SY, Heo S‐J, Park S‐J. The effect of intravenous labetalol administration on hemodynamic responses during desflurane inhalation. Korean Journal of Anesthesiology 2012;62(3):245‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
El‐Shmaa 2016 {published data only}
- El‐Shmaa NS, El‐Baradey GF. The efficacy of labetalol vs dexmedetomidine for attenuation of hemodynamic stress response to laryngoscopy and endotracheal intubation. Journal of Clinical Anesthesia 2016;31:267‐73. [PUBMED: 27185725] [DOI] [PubMed] [Google Scholar]
Gibson 1988 {published data only}
- Gibson BE, Black S, Maass L, Cucchiara RF. Esmolol for the control of hypertension after neurologic surgery. Clinical Pharmacologic Therapy 1988;44(6):650‐3. [PUBMED: 2904310] [DOI] [PubMed] [Google Scholar]
Goyagi 2005 {published data only}
- Goyagi T, Tanaka M, Nishikawa T. Landiolol attenuates the cardiovascular response to tracheal intubation. Journal of Anesthesia 2005;19(4):282‐6. [PUBMED: 16261464] [DOI] [PubMed] [Google Scholar]
Gupta 2011 {published data only}
- Gupta D, Srivastava S, Dubey RK, Prakash PS, Singh PK, Singh U. Comparative evaluation of atenolol and clonidine premedication on cardiovascular response to nasal speculum insertion during trans‐sphenoid surgery for resection of pituitary adenoma: a prospective, randomised, double‐blind, controlled study. Indian Journal of Anaesthesia 2011;55(2):135‐40. [PUBMED: 21712869] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harasawa 2006 {published data only}
- Harasawa R, Hayashi Y, Iwasaki M, Kamibayashi T, Mashimo T. Bolus administration of landiolol, a short‐acting, selective beta‐blocker, to treat tachycardia during anesthesia: a dose‐dependent study. Journal of Cardiothoracic and Vascular Anesthesia 2006;20(6):793‐5. [PUBMED: 17138082] [DOI] [PubMed] [Google Scholar]
Helfman 1991 {published data only}
- Helfman SM, Gold MI, DeLisser EA, Herrington CA. Which drug prevents tachycardia and hypertension associated with tracheal intubation: lidocaine, fentanyl, or esmolol?. Anesthesia and Analgesia 1991;72(4):482‐6. [PUBMED: 1672488] [DOI] [PubMed] [Google Scholar]
Horikoshi 2017 {published data only}
- Horikoshi Y, Goyagi T, Kudo R, Kodama S, Horiguchi T, Nishikawa T. The suppressive effects of landiolol administration on the occurrence of postoperative atrial fibrillation and tachycardia, and plasma IL‐6 elevation in patients undergoing esophageal surgery: a randomized controlled clinical trial. Journal of Clinical Anesthesia 2017;38:111‐6. [PUBMED: 28372647] [DOI] [PubMed] [Google Scholar]
Inada 1989 {published data only}
- Inada E, Cullen DJ, Nemeskal AR, Teplick R. Effect of labetalol or lidocaine on the hemodynamic response to intubation: a controlled randomized double‐blind study. Journal of Clinical Anesthesiology 1998;1(3):207‐13. [PUBMED: 2697239] [DOI] [PubMed] [Google Scholar]
Inoue 2010 {published data only}
- Inoue S, Abe R, Kawaguchi M, Kobayashi H, Furuya H. Beta blocker infusion decreases the magnitude of core hypothermia after anesthesia induction. Minerva Anestesiologica 2010;76(12):1002‐9. [PUBMED: 20838372] [PubMed] [Google Scholar]
Jakobsen 1986 {published data only}
- Jakobsen CJ, Grabe N, Christensen B. Metoprolol decreases the amount of halothane required to induce hypotension during general anaesthesia. British Journal of Anaesthesia 1986;58(3):261‐6. [PUBMED: 3511931] [DOI] [PubMed] [Google Scholar]
Jakobsen 1992 {published data only}
- Jakobsen CJ, Blom L. Effect of pre‐operative metoprolol on cardiovascular and catecholamine response and bleeding during hysterectomy. European Journal of Anaesthesiology 1992;9:209‐15. [PUBMED: 1600972] [PubMed] [Google Scholar]
Jakobsen 1997 {published data only}
- Jakobsen CJ, Bille S, Ahlburg P, Rybro L, Pedersen KD, Rasmussen B. Preoperative metoprolol improves cardiovascular stability and reduces oxygen consumption after thoracotomy. Acta Anaesthesiologica Scandinavica 1997;41(10):1324‐30. [PUBMED: 9422300] [DOI] [PubMed] [Google Scholar]
Jangra 2016 {published data only}
- Jangra K, Malhotra SK, Gupta A, Arora S. Comparison of quality of the surgical field after controlled hypotension using esmolol and magnesium sulfate during endoscopic sinus surgery. Journal of Anaesthesiology, Clinical Pharmacology 2016;32(3):325‐8. [PUBMED: 27625479] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kao 2017 {published data only}
- Kao MC, Tzeng IS, Chan HL. Esmolol pretreatment attenuates heart rate increase and parasympathetic inhibition during rapid increases in desflurane concentration. Medicine 2017;96(42):e8340. [PUBMED: 29049251] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kawaguchi 2010 {published data only}
- Kawaguchi M, Utada K, Yoshitani K, Uchino H, Takeda Y, Masui K, et al. Effects of a short‐acting β1 receptor antagonist landiolol on hemodynamics and tissue injury markers in patients with subarachnoid hemorrhage undergoing intracranial aneurysm surgery. Journal of Neurosurgical Anesthesiology 2010;22(3):230‐9. [PUBMED: 20118792] [DOI] [PubMed] [Google Scholar]
Kindler 1996 {published data only}
- Kindler CH, Schumacher PG, Schneider MC, Urwyler A. Effects of intravenous lidocaine and/or esmolol on hemodynamic responses to laryngoscopy and intubation: a double‐blind, controlled clinical trial. Journal of Clinical Anesthesia 1996;8(6):491‐6. [PUBMED: 8872690] [DOI] [PubMed] [Google Scholar]
Lai 2006 {published data only}
- Lai R, Xu M, Huang W, Wang X, Zeng W, Lin W. Beneficial effects of metoprolol on perioperative cardiac function of elderly esophageal cancer patients. Chinese Journal of Cancer 2006;25(5):609‐13. [PUBMED: 16687084] [PubMed] [Google Scholar]
Lee 1994 {published data only}
- Lee HY, Yim CH, Chung CW, Kim HY. The effect of labetalol on the hemodynamic response to endotracheal intubation. Korean Journal of Anesthesiology 1994;27(11):1611‐9. [Google Scholar]
Lee 2010 {published data only}
- Lee SJ, Lee JN. The effect of perioperative esmolol infusion on the postoperative nausea, vomiting and pain after laparoscopic appendectomy. Korean Journal of Anesthesiology 2010;59(3):179‐84. [PUBMED: 20877702] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2015 {published data only}
- Lee JH, Kim Y, Lee KH, Rim SK, Lee JY, Lee C. The effects of nicardipine or esmolol on the onset time of rocuronium and intubation conditions during rapid sequence induction: a randomized double‐blind trial. Journal of Anesthesia 2015;29(3):403‐8. [PUBMED: 25374138] [DOI] [PubMed] [Google Scholar]
Lee 2017 {published data only}
- Lee M, Kwon T, Kim S, Kim S, Park K, Jeon Y. Comparative evaluation of the effect of remifentanil and 2 different doses of esmolol on pain during propofol injection: a double‐blind, randomized clinical consort study. Medicine 2017;96(10):e6288. [PUBMED: 28272252] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lim 2000 {published data only}
- Lim SH, Chin NM, Tai HY, Wong M, Lin TK. Prophylactic esmolol infusion for the control of cardiovascular responses to extubation after intracranial surgery. Annals of the Academy of Medicine, Singapore 2000;29(4):447‐51. [PUBMED: 11056773] [PubMed] [Google Scholar]
Liu 1986 {published data only}
- Liu PL, Gatt S, Gugino LD, Mallampati SR, Covino BG. Esmolol for control of increases in heart rate and blood pressure during tracheal intubation after thiopentone and succinylcholine. Canadian Anaesthetists' Society Journal 1986;33(5):556‐62. [PUBMED: 3768764] [DOI] [PubMed] [Google Scholar]
Liu 2006 {published data only}
- Liu Y, Huang C, He M, Zhang L, Cai H, Guo Q. Influences of perioperative metoprolol on hemodynamics and myocardial ischaemia in elderly patients undergoing noncardiac surgery. Journal of Central South University ‐ Medical Sciences 2006;31(2):249‐53. [PUBMED: 16706126] [PubMed] [Google Scholar]
Louizos 2007 {published data only}
- Louizos AA, Hadzilia SJ, Davilis DI, Samanta EG, Georgiou LG. Administration of esmolol in microlaryngeal surgery for blunting the hemodynamic response during laryngoscopy and tracheal intubation in cigarette smokers. Annals of Otology, Rhinology, and Laryngology 2007;116(2):107‐11. [PUBMED: 17388233] [DOI] [PubMed] [Google Scholar]
Magnusson 1986 {published data only}
- Magnusson J, Thulin T, Werner O, Järhult J, Thomson D. Haemodynamic effects of pretreatment with metoprolol in hypertensive patients undergoing surgery. British Journal of Anaesthesiology 1986;58(3):251‐60. [PUBMED: 3511930] [DOI] [PubMed] [Google Scholar]
Mallon 1990 {published data only}
- Mallon JS, Hew E, Wald R, Kapala D. Bolus doses of esmolol for the prevention of postintubation hypertension and tachycardia. Journal of Cardiothoracic Anesthesia 1990;4(5 Suppl 2):27‐30. [20380331] [Google Scholar]
Meftahuzzaman 2014 {published data only}
- Meftahuzzaman SM, Islam MM, Ireen ST, Islam MR, Kabir H, Rashid H, et al. Comparison of efficacy of labetalol and fentanyl for attenuating reflex responses to laryngoscopy and intubation. Mymensingh Medical Journal 2014;23(2):242‐8. [PUBMED: 24858149] [PubMed] [Google Scholar]
Menigaux 2002 {published data only}
- Menigaux C, Guignard B, Adam F, Sessler DI, Joly V, Chauvin M. Esmolol prevents movement and attenuates the BIS response to orotracheal intubation. British Journal of Anaesthesia 2002;89(6):857‐62. [PUBMED: 12453930] [DOI] [PubMed] [Google Scholar]
Mikawa 1991 {published data only}
- Mikawa K, Maekawa N, Goto R, Kaetsu H, Hasegawa M, Yaku H, et al. Effects of pindolol on the cardiovascular response to tracheal intubation. British Journal of Anaesthesia 1991;67(4):416‐20. [PUBMED: 1931398] [DOI] [PubMed] [Google Scholar]
Miller 1990 {published data only}
- Miller DR, Martineau RJ, Hull KA, Hill J. Bolus administration of esmolol for controlling the hemodynamic response to laryngoscopy and intubation: efficacy and effects on myocardial performance. Journal of Cardiothoracic Anesthesia 1990;4(5 Suppl 2):31‐6. [Google Scholar]
Miller 1991 {published data only}
- Miller DR, Martineau RJ, Wynands JE, Hill J. Bolus administration of esmolol for controlling the haemodynamic response to tracheal intubation: the Canadian multicentre trial. Canadian Journal of Anesthesia 1991;38(7):849‐58. [PUBMED: 1683818 ] [DOI] [PubMed] [Google Scholar]
Miyazaki 2009 {published data only}
- Miyazaki M, Kadoi Y, Saito S. Effects of landiolol, a short‐acting beta‐1 blocker, on hemodynamic variables during emergence from anesthesia and tracheal extubation in elderly patients with and without hypertension. Journal of Anesthesia 2009;23(4):483‐8. [PUBMED: 19921354] [DOI] [PubMed] [Google Scholar]
Moon 2011 {published data only}
- Moon YE, Hwang WJ, Koh HJ, Min JY, Lee J. The sparing effect of low‐dose esmolol on sevoflurane during laparoscopic gynaecological surgery. Journal of International Medical Research 2011;39(5):1861‐9. [PUBMED: 22117987 ] [DOI] [PubMed] [Google Scholar]
Neary 2006 {published data only}
- Neary WD, McCrirrick A, Foy C, Heather BP, Earnshaw JJ. Lessons learned from a randomised controlled study of perioperative beta blockade in high risk patients undergoing emergency surgery. Surgeon 2006;4(3):139‐43. [PUBMED: 16764198] [DOI] [PubMed] [Google Scholar]
Ohri 1999 {published data only}
- Ohri AK, Sharma DR, Thakur JR, Sharma GD, Santoshi ID. Effect of esmolol infusion on the duration of action of non‐depolarizing muscle relaxants. Journal of Anaesthesiology, Clinical Pharmacology 1999;15(1):55‐9. [Google Scholar]
Ojima 2017 {published data only}
- Ojima T, Nakamori M, Nakamura M, Katsuda M, Hayata K, Kato T, et al. Randomized clinical trial of landiolol hydrochloride for the prevention of atrial fibrillation and postoperative complications after oesophagectomy for cancer. British Journal of Surgery 2017;104(8):1003‐9. [PUBMED: 28444964] [DOI] [PubMed] [Google Scholar]
Oxorn 1990 {published data only}
- Oxorn D, Know JW, Hill J. Bolus doses of esmolol for the prevention of perioperative hypertension and tachycardia. Canadian Journal of Anaesthesiology 1990;37(2):206‐9. [PUBMED: 1968784] [DOI] [PubMed] [Google Scholar]
Park 2009 {published data only}
- Park SH, Do SH, Shin HY, Jeon YT, Hwang JW, Han SH. Nicardipine is more effective than esmolol at preventing blood pressure increases during emergence from total intravenous anesthesia. Korean Journal of Anesthesiology 2009;57(5):597‐603. [PUBMED: 30625931] [DOI] [PubMed] [Google Scholar]
POBBLE 2005 {published data only}
- Brady AR, Gibbs JS, Greenhalgh RM, Powell JT, Sydes MR, POBBLE trial investigators. Perioperative beta‐blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double‐blind controlled trial. Journal of Vascular Surgery 2005;41(4):602‐9. [PUBMED: 15874923] [DOI] [PubMed] [Google Scholar]
POISE 2008 {published data only}
- Devereaux PJ, Yang H, Yusuf S, Guyatt G, Leslie K, Villar JC, et al. Effects of extended‐release metoprolol succinate in patients undergoing non‐cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008;371(9627):1839‐47. [PUBMED: 18479744] [DOI] [PubMed] [Google Scholar]
PRESAGE 2016 {published data only}
- Cardinale D, Sandri MT, Colombo A, Salvatici M, Tedeschi I, Bacchiani G, et al. Prevention of atrial fibrillation in high‐risk patients undergoing lung cancer surgery: the PRESAGE trial. Annals of Surgery 2016;264(2):244‐51. [PUBMED: 26764872] [DOI] [PubMed] [Google Scholar]
Raby 1999 {published data only}
- Raby KE, Brull SJ, Timimi F, Akhtar S, Rosenbaum S, Naimi C, et al. The effect of heart rate control on myocardial ischemia among high‐risk patients after vascular surgery. Anesthesia and Analgesia 1999;88(3):477‐82. [PUBMED: 10071990] [DOI] [PubMed] [Google Scholar]
Safwat 1984 {published data only}
- Safwat AM, Fung DL, Bilton DC. The use of propranolol in rapid sequence anaesthetic induction: optimal time interval for pretreatment. Canadian Anaesthetists' Society Journal 1984;31(6):638‐41. [PUBMED: 6498579] [DOI] [PubMed] [Google Scholar]
Shailaja 2013 {published data only}
- Shailaja S, Srikantu J. Comparison of effect of esmolol vs. esmolol and fentanyl on hemodynamic response to laryngoscopy and tracheal intubation in controlled hypertensive patients: a randomized controlled double blind study. Anaesthsia, Pain & Intensive Care 2013;17(3):267‐73. [Google Scholar]
Sharma 1996 {published data only}
- Sharma S, Mitra S, Grover VK, Kalra R. Esmolol blunts the haemodynamic responses to tracheal intubation in treated hypertensive patients. Canadian Journal of Anaesthesia 1996;43(8):778‐82. [PUBMED: 8840055] [DOI] [PubMed] [Google Scholar]
Sharma 2018 {published data only}
- Sharma S, Suthar OP, Tak ML, Thanvi A, Paliwal N, Karnawat R. Comparison of esmolol and dexmedetomidine for suppression of hemodynamic response to laryngoscopy and endotracheal intubation in adult patients undergoing elective general surgery: a prospective, randomized controlled double‐blinded study. Anesthesia, Essays and Researches 2018;12(1):262‐6. [PUBMED: 29628593] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shrestha 2011 {published data only}
- Shrestha GS, Marhatta MN, Amatya R. Use of gabapentin, esmolol or their combination to attenuate haemodynamic response to laryngoscopy and intubation. Kathmandu University Medical Journal 2011;9(36):238‐43. [PUBMED: 22710530] [DOI] [PubMed] [Google Scholar]
Shukla 2010 {published data only}
- Shukla S, Gupta K, Gurha P, Sharma M, Sanjay RR, Shukla R, et al. Role of β blockade in anaesthesia and postoperative pain management after major lower abdominal surgery. Internet Journal of Anesthesiology 2010;25(1):pagination not available. [DOI: 10.5580/170f] [DOI] [Google Scholar]
Singh 1995 {published data only}
- Singh H, Vichitvejpaisal P, Gaines GY, White PF. Comparative effects of lidocaine, esmolol, and nitroglycerin in modifying the hemodynamic response to laryngoscopy and intubation. Journal of Clinical Anesthesia 1995;7(1):5‐8. [PUBMED: 7772359] [DOI] [PubMed] [Google Scholar]
Singh 2010 {published data only}
- Singh SP, Quadir A, Malhotra P. Comparison of esmolol and labetalol, in low doses, for attenuation of sympathomimetic response to laryngoscopy and intubation. Saudi Journal of Anaesthesia 2010;4(3):163‐8. [PUBMED: 21189853] [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2012 {published data only}
- Singh S, Laing EF, Owiredu W, Singh A. Attenuation of cardiovascular response by ß‐blocker esmolol during laryngoscopy and intubation. Journal of Medical and Biomedical Sciences 2012;1(4):27‐33. [Google Scholar]
Srivastava 2015 {published data only}
- Srivastava VK, Agrawal S, Gautam SK, Ahmed M, Sharma S, Kumar R. Comparative evaluation of esmolol and dexmedetomidine for attenuation of sympathomimetic response to laryngoscopy and intubation in neurosurgical patients. Journal of Anaesthesiology, Clinical Pharmacology 2015;31(2):186‐90. [PUBMED: 25948898] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stone 1988 {published data only}
- Stone JG, Foex P, Sear JW, Johnson LL, Khambatta HJ, Triner L. Myocardial ischemia in untreated hypertensive patients: effect of a single small oral dose of a beta‐adrenergic blocking agent. Anesthesiology 1988;68(4):495‐500. [PUBMED: 2895596] [PubMed] [Google Scholar]
Sugiura 2007 {published data only}
- Sugiura S, Seki S, Hidaka K, Masuoka M, Tsuchida H. The hemodynamic effects of landiolol, an ultra‐short‐acting beta1‐selective blocker, on endotracheal intubation in patients with and without hypertension. Anesthesia and Analgesia 2007;104(1):124‐9. [PUBMED: 17179256] [DOI] [PubMed] [Google Scholar]
Tendulkar 2017 {published data only}
- Tendulkar MP, Ninave SS. Prospective comparison of pressor and airway responses to IV esmolol and IV dexmedetomidine during emergence from general anaesthesia and extubation. Journal of Krishna Institute of Medical Sciences University (JKIMSU) 2017;6(1):49‐56. [Google Scholar]
Ugur 2007 {published data only}
- Ugur B, Ogurlu M, Gezer E, Nuri Aydin O, Gursoy F. Effects of esmolol, lidocaine and fentanyl on haemodynamic responses to endotracheal intubation: a comparative study. Clinical Drug Investigation 2007;27(4):269‐77. [PUBMED: 17358099] [DOI] [PubMed] [Google Scholar]
Unal 2008 {published data only}
- Unal Y, Ozsoylar O, Sargnüney D, Arslan M, Tardim RS. The efficacy of esmolol to blunt the haemodynamic response to endotracheal extubation in lumbar disc surgery. Research Journal of Medical Sciences 2008;2(2):99‐104. [Google Scholar]
Urias 2016 {published data only}
- Urias E, Ochoa RC, Sandoval A, Arce B, Martinez JI, Chacon E. Perioperative metoprolol effectiveness in patients scheduled for laparoscopic cholecystectomy. Regional Anesthesia and Pain Medicine 2016;41(5 Suppl 1):e161. [Google Scholar]
Van den Berg 1998 {published data only}
- Berg AA. Use of esmolol to attenuate hemodynamic responses during cataract extraction. Middle East Journal of Anesthesiology 1998;14(4):231‐48. [PUBMED: 9557911] [PubMed] [Google Scholar]
Verma 2018 {published data only}
- Verma A, Srivastava D, Paul M, Chatterjee A, Chandra A. Effect of esmolol and diltiazem infusions on hemodynamic response to pneumoperitoneum on laparoscopic simple nephrectomy: a randomized controlled trial. Anesthesia, Essays and Researches 2018;12(1):85‐91. [PUBMED: 29628560] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wajima 2011 {published data only}
- Wajima Z, Tsuchida H, Shiga T, Imanaga K, Inoue T. Intravenous landiolol, a novel β(1)‐adrenergic blocker, reduces the minimum alveolar concentration of sevoflurane in women. Journal of Clinical Anesthesia 2011;23(4):292‐6. [PUBMED: 21663813] [DOI] [PubMed] [Google Scholar]
Wallace 1998 {published data only}
- Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter study of Perioperative Ischemia Research Group. New England Journal of Medicine 1996;335(23):1713‐20. [3145552; PUBMED: 8929262] [DOI] [PubMed] [Google Scholar]
- Wallace A, Layug B, Tateo I, Li J, Hollenberg M, Browner W, et al. Prophylactic atenolol reduces postoperative myocardial ischemia. McSPI Research Group. Anesthesiology 1998;88(1):7‐17. [3145632; PUBMED: 9447850] [DOI] [PubMed] [Google Scholar]
Ward‐Booth 1983 {published data only}
- Ward‐Booth P, Rubin P, Macfarlane P, Hillis S. Metoprolol in the prevention of dysrhythmias during minor dental surgery. American Heart Journal 1983;105(4):689‐90. [PUBMED: 6837424] [DOI] [PubMed] [Google Scholar]
White 2003 {published data only}
- White PF, Wang B, Tang J, Wender RH, Naruse R, Sloninsky A. The effect of intraoperative use of esmolol and nicardipine on recovery after ambulatory surgery. Anesthesia and Analgesia 2003;97(6):1633‐8. [PUBMED: 14633533] [DOI] [PubMed] [Google Scholar]
Whitehead 1980 {published data only}
- Whitehead MH, Whitmarsh VB, Horton JN. Metoprolol in anaesthesia for oral surgery. Anaesthesia 1980;35(8):779‐82. [PUBMED: 7004258] [DOI] [PubMed] [Google Scholar]
Yamazaki 2005 {published data only}
- Yamazaki A, Kinoshita H, Shimogai M, Fujii K, Nakahata K, Hironaka Y, et al. Landiolol attenuates tachycardia in response to endotracheal intubation without affecting blood pressure. Canadian Journal of Anaesthesia 2005;52(3):254‐7. [PUBMED: 15753495] [DOI] [PubMed] [Google Scholar]
Yang 2006 {published data only}
- Yang H, Raymer K, Butler R, Parlow J, Roberts R, Tech M. The effects of perioperative beta‐blockade: results of the metoprolol after vascular surgery (MaVS) study, a randomized controlled trial. American Heart Journal 2006;152(5):983‐90. [PUBMED: 17070177] [DOI] [PubMed] [Google Scholar]
Yang 2008 {published data only}
- Yang X, Wu X, Wang S, Wang Q. Effects of metoprolol on perioperative cardiovascular events in patients with risk or at high risk for coronary artery disease undergoing non‐cardiac surgery. Chinese Medical Journal 2008;88(21):1476‐80. [PUBMED: 18953854] [PubMed] [Google Scholar]
Yoshida 2017 {published data only}
- Yoshida T, Furukita Y, Yamamoto Y, Nishino T, Inoue S, Morimoto M, et al. A randomized, open label study of the efficacy of prophylactic 24‐h low‐dose landiolol for atrial fibrillation in transthoracic esophagectomy. Esophagus 2017;14(1):97‐103. [Google Scholar]
Zaugg 1999 {published data only}
- Zaugg M, Tagliente T, Lucchinetti E, Jacobs E, Drol M, Bodian C, et al. Beneficial effects from beta‐adrenergic blockade in elderly patients undergoing noncardiac surgery. Anesthesiology 1999;91(6):1674‐86. [PUBMED: 10598610] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Chae 1990 {published data only}
- Chae DH, Yoo KY, Park CJ, Ha IH. Effects of verapamil and propranolol on hemodynamic responses to laryngoscopy and tracheal intubation in hypertensive patients. Korean Journal of Anesthesiology 1990;23(3):366‐72. [Google Scholar]
Coleman 1980 {published data only}
- Coleman AJ, Jordan C. Cardiovascular response to anaesthesia. Influence of beta‐adrenergic blockade with metoprolol. Anaesthesia 1980;35(10):972‐8. [PUBMED: 7004251] [DOI] [PubMed] [Google Scholar]
Marwick 2009 {published data only}
- Marwick TH, Branagan H, Venkatesh B, Stewart S. Use of a nurse‐led intervention to optimize beta‐blockade for reducing cardiac events after major noncardiac surgery. American Heart Journal 2009;157(4):784‐90. [PUBMED: 19332211] [DOI] [PubMed] [Google Scholar]
Ryder 1973 {published data only}
- Ryder W, Charlton JE, Gorman PB. Oral atropine and practolol premedication in dental anaesthesia. British Journal of Anaesthesia 1973;45(7):745‐9. [PUBMED: 4147154] [DOI] [PubMed] [Google Scholar]
Sandler 1990 {published data only}
- Sandler AN, Leitch LF, Badner NH, Colmenares M, Kimball B. Esmolol compared with placebo in preventing increases in heart rate and blood pressure during rigid bronchoscopy. Journal of Cardiothoracic Anesthesia 1990;4(5 Suppl 2):44‐50. [Google Scholar]
Sezai 2015 {published data only}
- Sezai A, Osaka S, Yaoita H, Ishii Y, Arimoto M, Hata H, et al. Safety and efficacy of landiolol hydrochloride for prevention of atrial fibrillation after cardiac surgery in patients with left ventricular dysfunction: prevention of atrial fibrillation after cardiac surgery with landiolol hydrochloride for left ventricular dysfunction (PLATON) trial. Journal of Thoracic and Cardiovascular Surgery 2015;150(4):957‐64. [PUBMED: 26254752] [DOI] [PubMed] [Google Scholar]
Taenaka 2013 {published data only}
- Taenaka N, Kikawa S. The effectiveness and safety of landiolol hydrochloride, an ultra‐short‐acting beta1‐blocker, in postoperative patients with supraventricular tachyarrhythmias: a multicenter, randomized, double‐blind, placebo‐controlled study. American Journal of Cardiovascular Drugs 2013;13(5):353‐64. [PUBMED: 23818039] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tan 2002 {published data only}
- Tan PH, Yang LC, Shih HC, Lin CR, Lan KC, Chen CS. Combined use of esmolol and nicardipine to blunt the haemodynamic changes following laryngoscopy and tracheal intubation. Anaesthesia 2002;57(12):1207‐12. [PUBMED: 12479191] [DOI] [PubMed] [Google Scholar]
Vucevic 1992 {published data only}
- Vucevic M, Purdy GM, Ellis FR. Esmolol hydrochloride for management of the cardiovascular stress responses to laryngoscopy and tracheal intubation. British Journal of Anaesthesia 1992;68(5):529‐30. [PUBMED: 1642945] [DOI] [PubMed] [Google Scholar]
Zmora 2016 {published data only}
- Zmora O, Shaashua L, Gutman M, Ben‐Eliyahu S. The perioperative use of a beta‐adrenergic blocker and a COX‐2 inhibitor in colorectal cancer patients for the prevention of cancer recurrence: a preliminary study assessing feasibility and safety. Brain, Behavior, and Immunity 2016;57(Suppl 1):e9. [Google Scholar]
References to studies awaiting assessment
ACTRN12605000639628 {published data only}
- ACTRN12605000639628. Effect of preoperative atenolol on mortality and cardiovascular morbidity after non‐cardiac surgery [A prospective, double‐blind randomised control study comparing the effect of atenolol versus placebo on mortality and cardiovascular morbidity in patients undergoing non‐cardiac surgery]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=828&isReview=true (first received 11 October 2005).
ACTRN12615000889 {published data only}
- ACTRN12615000889550. A phase II randomized study of perioperative beta‐blocker vs placebo on gene expression in newly diagnosed breast cancer [Investigating whether, in patients receiving breast cancer surgery, pre‐operative propranolol compared with placebo, changes the cancer's gene expression]. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12615000889550 (first received 25 August 2015).
Birbicer 2007 {published data only}
- Birbicer H, Yapici D, Camsari A, Doruk, N, Atici S, Bilgin E, et al. Evaluation of esmolol on P‐wave dispersion. Gogus‐Kalp‐Damar Anestezi ve Yogun Bakim Dernegi Dergisi 2007;13(3):105‐8. [Google Scholar]
Boussofara 2001 {published data only}
- Boussofara M, Braco D, Kaddour C, Nafaa MN, Iarbi O, Ravussin P, et al. Control of hemodynamic variations secondary to suspension microlaryngoscopy. Tunisie Medicale 2001;79(10):503‐7. [PUBMED: 11910689 ] [PubMed] [Google Scholar]
Boussofara 2004 {published data only}
- Boussofara M, Chrayti H, Bracco D, Ravussin P. Comparison of three protocols in the control of cardiovascular response to suspended laryngoscopy in ENT surgery. Tunisie Medicale 2004;82(1):19‐24. [PUBMED: 15125352] [PubMed] [Google Scholar]
Gong 1999 {published data only}
- Gong Z, Luo A. Effects of alfentanil and esmolol on hemodynamic and catecholamine response to tracheal intubation. Chinese Medical Sciences Journal 1999;14(3):189‐92. [PubMed] [Google Scholar]
Hornamand 2017 {published data only}
- Honarmand A, Safavi M, Kiani N, Keshavarzi E. Comparative evaluation of the effects of different doses of labetalol on cardiovascular response to tracheal intubation. Journal of Isfahan Medical School 2016;34(393):893‐900. [Google Scholar]
- Honarmand A, Safavi M, Mirjalali K. The effect of labetalol or remifentanil on blood pressure and heart rate after laryngoscopy and intubation. Journal of Isfahan Medical School 2017;34(408):1395‐400. [Google Scholar]
Inada 2002 {published data only}
- Inada Y, Namiki A, Shimizu R, Hanaoka K, Ogawa R, Shimoji K, et al. Clinical evaluation of a short acting beta‐blocker (MR5H3; esmolol hydrochloride) on perioperative tachycardia ‐ an open label, multi‐center clinical trial. Anesthesia and Resuscitation 2002;38(3):131‐44. [Google Scholar]
Itani 2013 {published data only}
- Itani M, Tatara T, Hashimoto K, Shii H, Hirose M. Continuous administration of landiolol during general anesthesia for hepatectomy increases plasma epinephrine concentrations but does not affect the magnitude of tachycardia after tracheal extubation. Anesthesia and Analgesia 2013;116(Suppl 1):no pagination. [Google Scholar]
Joo 2010 {published data only}
- Joo J, Lee J, Kim JE, Park J. Optimal dose of esmolol in combination with nicardipine to stabilize cardiovascular response during anesthetic induction in ambulatory patients. Anesthesia and Pain Medicine 2010;5(4):288‐94. [Google Scholar]
Kajiura 2013 {published data only}
- Kajiura K, Sakiyama S, Takizawa H, Nakagawa Y, Kondo K, Tangoku A. Prophylactic landiolol administration can prevent atrial fibrillation after lobectomy?. Journal of Thoracic Oncology 2013;2:S1124. [71397593] [Google Scholar]
Kawano 2005 {published data only}
- Kawano T, Eguchi S, Iseki A, Oshita S. Effects of landiolol on cardiovascular responses, bispectral index and body movement during endotracheal intubation. Masui. The Japanese Journal of Anesthesiology 2005;54(6):610‐4. [PUBMED: 15966376] [PubMed] [Google Scholar]
NCT02466542 {published data only}
- NCT02466542. Analgesic effect of intraoperative esmolol in mastectomies [Analgesic effect of intraoperative esmolol in mastectomies: single center, prospective, double‐blind, randomized and placebo controlled study]. www.clinicaltrials.gov/ct2/show/record/NCT02466542 (first received 9 June 2015).
Tangoku 2016 {published data only}
- Tangoku A, Yoshida T, Inoue S. Efficacy of prophylactic landiolol for atrial fibrillation in transthoracic esophagectomy. Diseases of the Esophagus 2016;29(Suppl 1):93A. [Google Scholar]
UMIN000024040 {published data only}
- UMIN000024040. Evaluation of perioperative landiolol administration for preventing atrial fibrillation during esophagectomy. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000027684 (first received 13 September 2016).
Wang 1994 {published data only}
- Wang SC, Wu CC, Lin MS, Chang CF. Use of esmolol to prevent hemodynamic changes during intubation in general anesthesia. Acta Anaesthesiologica Sinica 1994;32(3):141‐6. [PUBMED: 7921857] [PubMed] [Google Scholar]
Wang 1999 {published data only}
- Wang L, Luo A, Wu X. Bolus administration of esmolol for preventing the haemodynamic response to tracheal intubation: a multicentre clinical study. Zhonghua Yi Xue Za Zhi 1999;79(11):828‐31. [PUBMED: 11715490] [PubMed] [Google Scholar]
Yuan 1994 {published data only}
- Yuan L, Chia YY, Jan KT, Chen CS, Wang CH, Haung LH, et al. The effect of single bolus dose of esmolol for controlling the tachycardia and hypertension during laryngoscopy and tracheal intubation. Acta Anaesthesiologica Sinica 1994;32(3):147‐52. [PUBMED: 7921858] [PubMed] [Google Scholar]
References to ongoing studies
EUCTR2010‐021844‐17 {published data only}
- EUCTR2010‐021844‐17. Perioperative esmolol infusion for haemodynamic stability during major vascular surgery. www.clinicaltrialsregister.eu/ctr‐search/search?query=2010‐021844‐17 (first received 27 July 2010).
NCT01555554 {published data only}
- NCT01555554. Perioperative propranolol in patients with post traumatic stress disorder (PTSD) [Effect of prophylactic, perioperative propranolol on peri‐ and postoperative complications in patients with post traumatic stress disorder]. www.clinicaltrials.gov/ct2/show/record/NCT01555554 (first received 15 March 2012).
NCT03138603 {published data only}
- NCT03138603. Metoprolol to reduce perioperative myocardial injury. www.clinicaltrials.gov/ct2/show/results/NCT03138603 (first received 3 May 2017).
Additional references
Bangalore 2008
- Bangalore S, Wetterslev J, Pranesh S, Sawhney S, Gluud C, Messerli FH. Perioperative beta blockers in patients having non‐cardiac surgery: a meta‐analysis. Lancet 2008;372(9654):1962‐76. [PUBMED: 19012955] [DOI] [PubMed] [Google Scholar]
Blessberger 2019
- Blessberger H, Lewis SR, Pritchard MW, Fawcett LJ, Domanovits H, Schlager O, et al. Perioperative beta‐blockers for preventing surgery‐related mortality and morbidity in adults undergoing cardiac surgery. Cochrane Database of Systematic Reviews 2019, Issue 9. [DOI: 10.1002/14651858] [DOI] [PMC free article] [PubMed] [Google Scholar]
Borenstein 2010
- Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed‐effect and random‐effects models for meta‐analysis. Research synthesis methods 2010;1(2):97‐111. [PUBMED: 26061376] [DOI] [PubMed] [Google Scholar]
Bouri 2014
- Bouri S, Shun‐Shin MJ, Cole GD, Mayet J, Francis DP. Meta‐analysis of secure randomised controlled trials of β‐blockade to prevent perioperative death in non‐cardiac surgery. Heart 2014;100(6):456‐64. [DOI: 10.1136/heartjnl-2013-304262; PUBMED: 23904357] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chopra 2012
- Chopra V, Eagle KA. Perioperative mischief: the price of academic misconduct. American Journal of Medicine 2012;125(10):953‐5. [PUBMED: 22884175] [DOI] [PubMed] [Google Scholar]
Covidence 2019 [Computer program]
- Veritas Health Innovation. Covidence. Version accessed 2 January 2019. Melbourne, Australia: Veritas Health Innovation.
De Lima 2014
- Lima LG, Saconato H, Atallah ÁN, Silva EM. Beta‐blockers for preventing stroke recurrence. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD007890.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
DECREASE‐IV 2009
- Dunkelgrun M, Boersma E, Schouten O, Koopman‐van Gemert AW, Poorten F, Bax JJ, et al. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate‐risk patients undergoing noncardiovascular surgery: a randomized controlled trial (DECREASE‐IV). Annals of Surgery 2009;249(6):921‐6. [PUBMED: 19474688] [DOI] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG, editor(s) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS editor(s), Cochrane Handbook for Systematic Reviews of Interventions. Version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Devereaux 2015
- Devereaux PJ, Sessler DI. Cardiac complications in patients undergoing major noncardiac surgery. New England Journal of Medicine 2015;373(23):2258‐69. [PUBMED: 26630144] [DOI] [PubMed] [Google Scholar]
Duncan 2018
- Duncan D, Sankar A, Beattie W, Wijeysundera DN. Alpha‐2 adrenergic agonists for the prevention of cardiac complications among adults undergoing surgery. Cochrane Database of Systematic Reviews 2018, Issue 3. [DOI: 10.1002/14651858.CD004126.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Endnote [Computer program]
- Thomson Reuters. Endnote X5. Philadelphia (PA): Thomson Reuters, 2011.
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Hajibandeh 2017
- Hajibandeh S, Hajibandeh S, Antoniou SA, Torella F, Antoniou GA. Effect of beta‐blockers on perioperative outcomes in vascular and endovascular surgery: a systematic review and meta‐analysis. British Journal of Anaesthesia 2017;118(1):11‐21. [PUBMED: 28039238] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Higgins 2017
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration 2017. Available from www.training.cochrane.org/handbook.
Hristovska 2017
- Hristovska AM, Duch P, Allingstrup M, Afshari A. Efficacy and safety of sugammadex versus neostigmine in reversing neuromuscular blockade in adults. Cochrane Database of Systematic Reviews 2017, Issue 8. [DOI: 10.1002/14651858.CD012763] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jørgensen 2018
- Jørgensen ME, Andersson C, Venkatesan S, Sanders RD. Beta‐blockers in noncardiac surgery: did observational studies put us back on safe ground?. British Journal of Anaesthesia 2018;121(1):16‐25. [PUBMED: 29935568] [DOI] [PubMed] [Google Scholar]
Kristensen 2014
- Kristensen SD, Knuuti J, Saraste A, Anker S, Botker HE, DeHert S, et al. 2014 ESC/ESA guidelines on non‐cardiac surgery: cardiovascular assessment and management. European Heart Journal 2014;35(35):2383‐431. [PUBMED: 25086026] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Lewis 2018
- Lewis SR, Pritchard MW, Schofield‐Robinson OJ, Alderson P, Smith AF. Continuation versus discontinuation of antiplatelet therapy for bleeding and ischaemic events in adults undergoing non‐cardiac surgery. Cochrane Database of Systematic Reviews 2018, Issue 7. [DOI: 10.1002/14651858.CD012584.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mangano 1990
- Mangano DT. Perioperative cardiac morbidity. Anesthesiology 1990;72(1):153‐84. [PUBMED: 2404426] [DOI] [PubMed] [Google Scholar]
Myles 2016
- Myles PS, Grocott MP, Boney O, Moonesinghe SR. Standardizing end points in perioperative trials: towards a core and extended outcome set. British Journal of Anaesthesia 2016;116(5):586‐9. [PUBMED: 27106961] [DOI] [PubMed] [Google Scholar]
Oprea 2019
- Oprea AD, Lombard FW, Kertai MD. Perioperative β‐adrenergic blockade in noncardiac and cardiac surgery: a clinical update. Journal of Cardiothoracic and Vascular Anesthesia 2019;33(3):817‐32. [PUBMED: 29934209] [DOI] [PubMed] [Google Scholar]
Poldermans 1999
- Poldermans D, Boersma E, Bax JJ, Thomson IR, Ven LL, Blankensteijn JD, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high‐risk patients undergoing vascular surgery. Dutch echocardiographic cardiac risk evaluation applying stress echocardiography study group. New England Journal of Medicine 1999;341(24):1789‐94. [PUBMED: 10588963] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sellers 2018
- Sellers D, Srinivas C, Djaiani G. Cardiovascular complications after non‐cardiac surgery. Anaesthesia 2018;73 Suppl 1:34‐42. [PUBMED: 29313903] [DOI] [PubMed] [Google Scholar]
VISION 2014
- The vascular events In noncardiac surgery patIents cohort evaluation (VISION) writing group. Myocardial injury after noncardiac surgery. A large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30‐day outcomes. Anesthesiology 2014;120:564‐78. [PUBMED: 24534856] [DOI] [PubMed] [Google Scholar]
Wiysonge 2017
- Wiysonge CS, Bradley HA, Volmink J, Mayosi BM, Opie LH. Beta‐blockers for hypertension. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD002003.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Blessberger 2014
- Blessberger H, Kammler J, Domanovits H, Schlager O, Wildner B, Azar D, et al. Perioperative beta‐blockers for preventing surgery‐related mortality and morbidity. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD004476.pub2] [DOI] [PubMed] [Google Scholar]
Blessberger 2018
- Blessberger H, Kammler J, Domanovits H, Schlager O, Wildner B, Azar D, et al. Perioperative beta‐blockers for preventing surgery‐related mortality and morbidity. Cochrane Database of Systematic Reviews 2018, Issue 3. [DOI: 10.1002/14651858.CD004476.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


