The use of antifungal agents in clinical settings is limited by the appearance of drug resistance and adverse side effects. There is, therefore, an urgent need to develop new drugs to strengthen the treatment of invasive fungal diseases. The aim of this study is to describe the potential repurposing of ribavirin as an adjunct therapy against Candida spp. Primary screening of a Prestwick Chemical library against Candida albicans ATCC 90028 and fluconazole-resistant Candida albicans strains was performed.
KEYWORDS: Candida species, antifungals, in vitro activity, ribavirin
ABSTRACT
The use of antifungal agents in clinical settings is limited by the appearance of drug resistance and adverse side effects. There is, therefore, an urgent need to develop new drugs to strengthen the treatment of invasive fungal diseases. The aim of this study is to describe the potential repurposing of ribavirin as an adjunct therapy against Candida spp. Primary screening of a Prestwick Chemical library against Candida albicans ATCC 90028 and fluconazole-resistant Candida albicans strains was performed. Subsequently, we evaluated the responses of 100 Candida sp. strains to ribavirin, an antiviral agent, using the broth microdilution method as recommended by CLSI. We checked the involvement of efflux pump activity in the development of ribavirin resistance. We studied time-kill curves and performed a checkerboard assay for a ribavirin-antifungal combination study. Twenty-one nonstandard antifungal compounds were identified, including ribavirin. Ribavirin had antifungal activity in vitro against 63 Candida strains, including strains of C. albicans, C. parapsilosis, and C. tropicalis, with MICs ranging from 0.37 to 3.02 μg/ml, while MICs for C. krusei, C. glabrata, C. lusitaniae, and some C. albicans strains remained high (≥24.16 μg/ml). No relation was observed between efflux pump activity and ribavirin resistance. Ribavirin exhibited fungistatic activity against multidrug-resistant (MDR) C. albicans and fungicidal activity against a C. parapsilosis strain. In addition, ribavirin acted synergistically with azoles against Candida strains for which ribavirin MICs were <24.4 μg/ml. This study highlights the potential clinical application of ribavirin, alone or in association with other antifungal agents, as an adjunct anti-Candida drug.
INTRODUCTION
Fungi are considered to be among the main causes of human infections, particularly for immunocompromised individuals and hospitalized patients with serious immunosuppressive conditions, such as HIV and organ transplantation (1). Infections due to Candida spp. are among the most common invasive fungal diseases (2). In a recent study, Candida spp. were identified as the most frequent cause of bloodstream infection in hospitalized patients (3). Only three classes of antifungal agents are currently used to treat invasive Candida species infections: azoles, polyenes, and echinocandins (4). Although these classes of antifungal agents are usually active against many fungal infections, their applicability and efficacy might be compromised due to their high toxicity, their narrow spectrum of activity, and the development of drug resistance (5). Hence, there is a constant need for other compounds that possess antifungal properties. An interesting approach in this field is drug repurposing, whereby FDA-approved drugs may be tested and used in another therapeutic class (6). Applying this approach by screening 1,920 compounds belonging to three FDA-approved drug libraries against Candida albicans strains, Tournu et al. recently identified ribavirin, a purine nucleoside analog, as a potential C. albicans vacuole-disrupting agent (7). Ribavirin is a guanosine analog that produces broad-spectrum activity against many RNA and DNA viruses. However, it is used mainly to treat hepatitis C virus (HCV) in combination with alpha interferon (8). In this study, we investigated the in vitro activities of ribavirin against different clinical Candida species, including emerging fluconazole-resistant Candida albicans strains. We also evaluated the synergy between ribavirin and common antifungal agents.
RESULTS AND DISCUSSION
By testing of an FDA-approved library of 1,280 drugs against C. albicans ATCC 90028 (quality control) and a C. albicans strain (Q181103513) that has high resistance to fluconazole and all echinocandin antifungal agents, 21 nonstandard antifungal hits showed fungal growth inhibition greater than 90%. These primary compounds include 7 antibacterial drugs, 3 anthelmintic agents, and 11 compounds belonging to different therapeutic classes (Table 1).
TABLE 1.
Hits obtained by the primary screening of 1,280 FDA-approved drugs of the Prestwick Chemical library at 10 μM against both C. albicans ATCC 90028 and fluconazole-resistant C. albicans strain Q181103513
| Drug | Therapeutic class | Fungal growth inhibition (%) | Reference |
|---|---|---|---|
| Auranofin | Analgesic | 99 | 25 |
| Disulfiram | Antabuse effect | 98 | 26 |
| Pinaverium bromide | Antispastic | 98 | Unknown |
| Avermectin B1 | Anthelmintic | 98 | Unknown |
| Pyrvinium pamoate | Anthelmintic | 97 | 27 |
| Triclabendazole | Anthelmintic | 96 | Unknown |
| Pentamidine isethionate | Antiprotozoal | 96 | 28 |
| Tetraethylenepentamine pentahydrochloride | Antilipemic | 94 | Unknown |
| Ribavirin | Antiviral | 96 | 7 |
| Pentetic acid | Chelating/radioprotectant | 98 | 28 |
| Thioguanine | Antineoplastic | 96 | Unknown |
| Anthralin | Antipsoriatic | 96 | Unknown |
| Thonzonium bromide | Antiseptic | 98 | 29 |
| Clioquinol | Antiamebic/antiseptic | 98 | 7 |
| Clotrimazole | Antibacterial | 98 | 30 |
| Chloroxine | Antibacterial | 98 | 31 |
| Chlorhexidine | Antibacterial/antiseptic | 98 | 31 |
| Dequalinium dichloride | Antibacterial/antiseptic | 98 | 32 |
| Methylbenzethonium chloride | Antibacterial | 98 | 33 |
| Benzethonium chloride | Antibacterial/antiseptic | 98 | 33 |
| Ciclopirox ethanolamine | Antibacterial | 97 | 34 |
The principal aim of our study is to identify new molecules whose antifungal action is not known and/or well investigated and which can be used in clinical practice without any constraints for patients. Among the 21 hits obtained, 14 have already been identified and well studied in previous work (Table 1), confirming the validity and reproducibility of our results and our screening technique. However, to our knowledge, the fungal growth inhibition induced by six of the remaining seven compounds—including pinaverium bromide, avermectin B1, triclabendazole, tetraethylenepentamine pentahydrochloride, thioguanine, and anthralin—has never been reported previously, and that induced by ribavirin has not been well studied (Table 1).
Among the aforementioned antifungal hits, we focused our study on the drug ribavirin for several reasons. (i) It presents a 96% reduction in fungal growth. (ii) It has been shown to disrupt vacuolar function in the pathogen C. albicans (7); however, no report describes its antifungal efficacy directly (i.e., MICs, fungicidal or fungistatic activity, synergy with antifungals, etc.). (iii) Most of these seven hits have been designed for a specific noninfectious indication (antispastic, antilipemic, antineoplastic, antipsoriatic) (Table 1), whereas ribavirin was approved to treat infectious disease of the liver (9), so its repurposing as an antifungal agent could be admissible. (iv) Reversible hemolytic anemia is the unique adverse effect observed with ribavirin treatment, in contrast to the cytotoxic effects that other molecules, such as thioguanine and avermectin B1, may have on human cells due to their nonselective activities (10, 11). (v) Ribavirin can be administered orally (as a capsule or tablet), intravenously, or through inhalation (9), while only topical application is possible for some molecules, such as anthralin (12).
That is why, in this study, a large panel of 100 Candida sp. strains was tested for susceptibility to ribavirin, and ribavirin MICs were determined. Consequently, we report here the efficacy of this compound against a collection of 60 Candida sp. strains, including 6 Candida parapsilosis, 5 C. tropicalis, and 49 C. albicans strains, with MICs ranging from 1.56 to 12.5 μM (i.e., 0.37 to 3.02 μg/ml). Among these 60 clinical strains tested, C. parapsilosis and C. tropicalis strains showed the greatest susceptibility to ribavirin; MICs were mainly ≤3.02 μM (i.e., ≤0.75 μg/ml). Interestingly, ribavirin was effective against the clinical strain that was resistant to both fluconazole and echinocandin agents (C. albicans Q181103513), with a low MIC of 6.25 μM (i.e., 1.51 μg/ml). On the other hand, the ribavirin MICs obtained for C. krusei, C. lusitaniae, C. auris, and C. glabrata were mainly ≥100 μM (i.e., ≥24.16 μg/ml) (Fig. 1).
FIG 1.

Distribution of ribavirin MICs for all clinical Candida sp. strains tested in this study. Data are color coded by strain as follows: brown, C. parapsilosis; green, C. glabrata; yellow, C. auris; red, C. albicans; blue, C. krusei; black, C. lusitaniae; gray, C. tropicalis.
In order to study the fungicidal and fungistatic activities of the ribavirin compound, time-kill curves were performed with C. albicans and C. parapsilosis. Ribavirin exhibited fungistatic activity against the multidrug-resistant (MDR) C. albicans strain tested and C. albicans ATCC 90028. At ribavirin concentrations equal to 0.5× MIC, no inhibitory effect was observed, and the curve was nearly identical to those for the control. At concentrations of ≥1× MIC, a concentration-independent fungistatic effect was observed. In contrast, fungicidal activity of ribavirin against C. parapsilosis was noted, with a minimal fungicidal concentration (MFC) of ≥2-fold the ribavirin MIC (MFC, 3.12 μM [i.e., 1.5 μg/ml]) (Fig. 2).
FIG 2.
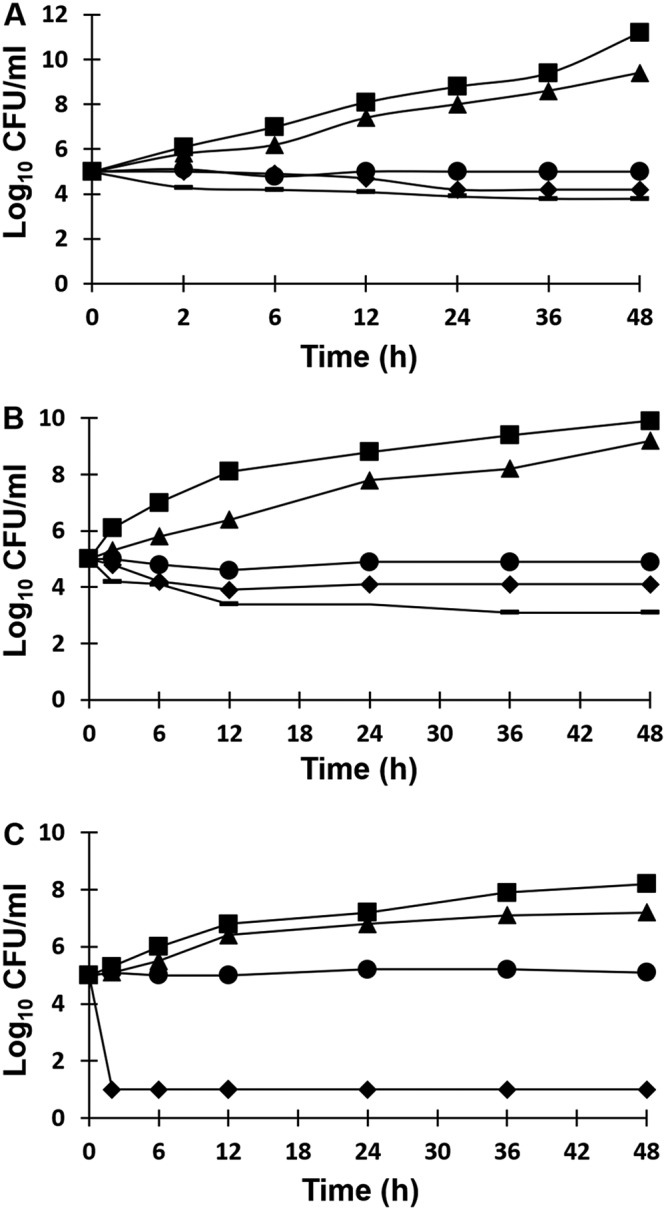
CFU counts (expressed as log10 CFU per milliliter) versus time for Candida strains tested against ribavirin at the following concentrations: control (squares), 0.5× MIC (triangles), 1× MIC (circles), 2× MIC (diamonds), 4× MIC (heavy lines). (A) C. albicans Q181103513; (B) C. albicans ATCC 90028; (C) C. parapsilosis Q181208447.
Ribavirin is on the WHO Model List of Essential Medicines as an effective and safe drug used in a health system. Ribavirin is administered orally at a dose of 1,200 mg (600 mg twice a day) over 4 weeks to achieve a stable concentration of 2.2 μg/ml in plasma (9). A retrospective study performed by J. F. Jen et al. on patients infected with hepatitis C virus (HCV) showed that 49% of the sustained virological response was achieved at week 4 via plasma ribavirin concentrations ranging from 3.5 to 4 μg/ml. In addition, the authors achieved higher plasma ribavirin concentrations in the treatment of a patient infected with HCV genotype 1 (13). Moreover, a prospective open-label study of patients infected with HCV genotype 1 demonstrated that an increase in the ribavirin treatment dose (a maximum daily dose of 3,600 mg) resulted in plasma ribavirin concentrations of 5.37 μg/ml. In this case, HCV RNA was rendered undetectable in 9/10 patients during the fourth week, but this result was associated with more-frequent side effects, such as anemia (14). Thus, concentrations in plasma equal to the MICs obtained from most of the Candida sp. strains tested in this study can be easily achieved. The main side effect of ribavirin treatment is reversible hemolytic anemia, which may be observed in patients with chronic hepatitis C (15). The use of this drug for short-term therapy, along with the use of hematopoietic growth factors, can manage the risk of severe anemia by maintaining an effective concentration.
Nonetheless, some Candida strains were resistant to ribavirin, including some C. albicans strains and all the C. glabrata, C. krusei, and C. lusitaniae strains tested. Among the many drug resistance mechanisms that have been described in yeasts, one specific or nonspecific mechanism is the overexpression of membrane-associated carriers acting as drug efflux pumps (16). Thus, we supposed that reduced permeability of the membrane to the ribavirin drug and the potential role of efflux pumps in the extrusion of the ribavirin molecule may be the mechanisms that explain ribavirin resistance. That is why we checked the efflux pump activity using the nonspecific efflux pump inhibitor (EPI) carbonyl cyanide 3-chlorophenylhydrazone (CCCP) and the specific calcium channel blocker verapamil for ribavirin-resistant strains. The activity of ribavirin did not change with the addition of CCCP or verapamil. No changes were reported in the ribavirin MICs for any of the strains tested after EPI treatment, which could indicate the potentially normal transport of ribavirin in the isolates tested, although we did not quantify the intracellular level of ribavirin. This result leads us to the possibility of the existence of a specific pathway that could induce the development of ribavirin resistance in yeasts.
The mutation frequencies of C. albicans ATCC 90028 on RPMI medium containing 1.52, 3.04, and 6.08 μg/ml of ribavirin were 1.5 × 10−4, 1.1 × 10−4, and 9.2 × 10−4, respectively. The mutation frequencies of C. albicans Q181103513 on RPMI agar plates with 3.04, 6.08, and 12.16 μg/ml of ribavirin were 1.6 × 10−4, 1.1 × 10−4, and 9.8 × 10−4, respectively. Thus, the overall mutation frequency was in the range of 10−3 to 10−4 per strain, relatively higher than that reported for classical antifungals (17). This result indicates that it is preferable to use ribavirin for a short time or in association with other antimicrobial drugs. Therefore, in addition to the discovery of new agents effective against fungi, a pragmatic approach would be to improve the activities of old antifungal agents by using a combination of drugs. Thus, testing ribavirin-antifungal combinations in vitro could be very useful for increasing the antimicrobial spectra of the usual antifungals used and for minimizing the development of drug resistance and side effects by reducing the drug concentration.
Investigations for potential in vitro synergy between ribavirin and antifungal agents were carried out with Candida strains resistant to an azole (fluconazole [6 strains], itraconazole [9 strains], posaconazole [19 strains], and/or flucytosine [11 strains]) (Table 2). The combination of ribavirin and fluconazole was evaluated against six fluconazole-resistant Candida sp. strains (five C. glabrata strains and one MDR C. albicans strain) (Table 2). Interestingly, significant synergism (fractional inhibitory concentration index [FICI], 0.12) was demonstrated against the MDR C. albicans strain (Q181103513), since this combination lowered the MICs of fluconazole and ribavirin from 256 μg/ml and 1.5 μg/ml to 1 μg/ml and 0.18 μg/ml, respectively. However, this combination (i.e., ribavirin with fluconazole) was synergistic for only one C. glabrata strain and was indifferent for the remaining four C. glabrata strains tested. It is noteworthy that the latter strains had initially high ribavirin MICs (≥24.4 μg/ml) (Table 2). When ribavirin was combined with itraconazole and tested against nine itraconazole-resistant strains, synergistic activities were observed for the two C. albicans strains tested, while indifferent activities were noted for most C. glabrata strains (5/7) analyzed in this study (Table 2). Furthermore, in ribavirin-posaconazole combinations, the highest rates of synergy were observed for C. albicans (7/9 [78%]) and C. tropicalis (2/2 [100%]) strains (Table 2) but not for C. glabrata (1/7) and C. krusei (0/1) strains. It is worth mentioning again that the ribavirin-azole association was synergistic mainly against Candida strains for which ribavirin MICs were <24.4 μg/ml. Synergy was not seen with ribavirin in combination with flucytosine against any of the Candida sp. strains tested in this study (11 strains) (Table 2). This could be explained by the fact that both molecules (i.e., ribavirin and flucytosine) belong to the same therapeutic class of nucleoside analogs, which can disturb the DNA synthesis of a given microorganism. Finally, none of the combinations tested was found to be antagonistic against Candida strains.
TABLE 2.
MICs and fractional inhibitory concentration indexes for the combinations of ribavirin with antifungalsa
| Candida species and strain | MIC |
FICI |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| FLU | ITRA | POSA | 5-FC | RBV | RBV-FLU | RBV-ITRA | RBV-POSA | RBV–5-FC | |
| C. glabrata | |||||||||
| Q181201614 | 8 | 8 | 1 | <0.06 | >24.4 | ND | 0.7 (I) | ND | ND |
| Q181198565 | 64 | 1 | 8 | <0.06 | >24.4 | 0.7 (I) | 1 (I) | 0.7 (I) | ND |
| Q181203672 | 128 | 1 | >8 | <0.06 | >24.4 | 0.6 (I) | 0.6 (I) | 0.4 (S) | ND |
| Q181202903 | 16 | 4 | 8 | 0.06 | >24.4 | ND | 0.7 (I) | 1 (I) | ND |
| Q181255715 | 64 | 16 | 4 | <0.06 | >24.4 | 0.6 (I) | 0.6 (I) | 0.8 (I) | ND |
| Q181280604 | 4 | 0.25 | 0.5 | <0.06 | 24.4 | ND | ND | 0.7 (I) | ND |
| 8070855959 | 128 | 16 | 8 | <0.06 | >24.4 | 0.6 (I) | 0.1 (S) | 2 (I) | ND |
| Q181208658 | 128 | 16 | 8 | <0.06 | 24.4 | 0.5 (S) | 0.5 (S) | 1 (I) | ND |
| C. albicans | |||||||||
| Q181103513 | 256 | 0.25 | 0.25 | <0.06 | 1.52 | 0.12 (S) | ND | 0.1 (S) | ND |
| Q181198103 | 4 | 0.25 | 1 | <0.06 | 3.05 | ND | ND | 0.2 (S) | ND |
| Q181201380 | 0.5 | 0.06 | 0.03 | >64 | 1.52 | ND | ND | ND | 1.5 (I) |
| Q181203339 | 1 | 0.06 | 0.12 | <0.06 | >24.4 | ND | ND | 0.7 (I) | ND |
| 490890568 | 1 | 0.5 | 0.25 | 0.5 | 3.05 | ND | ND | 0.4 (S) | ND |
| Q181264539 | 0.25 | 0.12 | 0.06 | >64 | 0.76 | ND | ND | ND | 1.2 (I) |
| Q181252599 | 1 | 0.12 | 0.12 | 0.12 | 6.1 | ND | ND | 0.3 (S) | ND |
| Q181253570 | <0.012 | 0.03 | 0.15 | 0.12 | >24.4 | ND | ND | 0.5 (S) | ND |
| Q181211559 | 2 | 1 | 0.5 | 1 | 12.2 | ND | 0.4 (S) | 0.6 (I) | ND |
| Q181226987 | 0.25 | 0.06 | 0.03 | 64 | 6.1 | ND | ND | ND | 2 (I) |
| Q181260978 | 2 | 1 | 0.5 | 0.5 | 12.2 | ND | 0.5 (S) | 0.5 (S) | ND |
| Q181108884 | 16 | 0.5 | 0.5 | 1 | 1.52 | ND | ND | 0.3 (S) | ND |
| C. krusei | |||||||||
| ATCC 6258 | 32 | 0.12 | 0.25 | 8 | >24.4 | ND | ND | 0.6 (I) | ND |
| Q181262141 | 32 | 0.12 | 0.12 | 4 | 24.4 | ND | ND | ND | 0.8 (I) |
| Q181263662 | 16 | 0.12 | 0.12 | 2 | 12.2 | ND | ND | ND | 0.6 (I) |
| Q181208438 | 32 | 0.25 | 0.25 | 8 | 24.4 | ND | ND | ND | 0.6 (I) |
| C. lusitaniae Q181206420 | 0.5 | 0.12 | 0.03 | 32 | >24.4 | ND | ND | ND | 0.7 (I) |
| C. tropicalis | |||||||||
| Q181257439 | 1 | 0.12 | 0.12 | 64 | 0.76 | ND | ND | 0.4 (S) | 0.7 (I) |
| Q181250041 | 1 | 0.12 | 0.06 | 64 | 1.52 | ND | ND | ND | 1 (I) |
| 8070845333 | 2 | 0.12 | 0.12 | >64 | 1.52 | ND | ND | 0.4 (S) | 1.1 (I) |
| Q181203338 | 1 | 0.12 | 0.06 | 64 | 0.76 | ND | ND | ND | 1 (I) |
Abbreviations: FLU, fluconazole; ITRA, itraconazole; POSA, posaconazole; 5-FC, flucytosine; RBV, ribavirin; S, synergy; I, indifference; ND, not determined.
The mechanisms of action of ribavirin on HCV have been correctly elucidated, and four pathways have been described: (i) inhibition of HCV replication (RNA polymerase), (ii) inhibition of the IMP dehydrogenase (IMPDH) enzyme, (iii) induction of RNA mutagenesis, and (iv) adaptation of the immune response to HCV (9). In contrast, the mechanism of ribavirin action on Candida species remains unclear. Thus, the most important aim of further studies will be the characterization of the mode of action of ribavirin against Candida species.
Our findings demonstrate that ribavirin exhibits antifungal activities against Candida species, especially C. albicans, C. parapsilosis, and C. tropicalis. Its efficacy against MDR C. albicans could offer a promising alternative strategy for the treatment of invasive fungal infections. Nonetheless, the ribavirin MICs for some Candida strains (especially strains of C. glabrata, C. krusei, and C. lusitaniae) remain higher than the concentration achievable in human plasma. This phenomenon of high MICs is not associated with the overexpression of efflux pump activity. Finally, ribavirin has synergistic activities with fluconazole, itraconazole, or posaconazole, but not with flucytosine, particularly against Candida strains for which ribavirin MICs are <24.4 μg/ml. Consequently, the in vivo activities of ribavirin need further investigation in order to introduce ribavirin as an antifungal agent, alone or in association with other antifungals, in clinical mycology practice.
MATERIALS AND METHODS
Screening of 1,280 FDA-approved drugs against C. albicans.
Using the concept of drug repurposing, an initial screen of an FDA-approved library of 1,280 drugs (Prestwick, Illkirch-Graffenstaden, France) (18) at a fixed concentration of 10 μM was performed against Candida albicans ATCC 90028 (quality control) and a clinical Candida albicans strain that is resistant to fluconazole and all echinocandin agents. This concentration was chosen according to previous screening studies in order to select “hits” that present antifungal activities at low concentrations (thus avoiding the toxicity and adverse effects of drugs tested at high concentrations). The fungal inoculum was prepared using RPMI 1640 medium (Sigma-Aldrich, St. Louis, MO) according to the Clinical and Laboratory Standards Institute protocol (19). Sixteen 96-well plates were used. Each plate contained 80 compounds; the first column served for the positive control with untreated fungi, and the last column contained the medium as a negative control. The plates were incubated for 24 h at 37°C. Then the optical density (OD) was determined using a spectrophotometer, and the percentages of fungal growth inhibition were calculated in relation to the growth in the untreated wells.
Fungal strains.
A collection of 96 Candida sp. strains was tested, including 72 C. albicans, 5 Candida parapsilosis, 8 Candida glabrata, 5 Candida tropicalis, 3 Candida krusei, and 3 Candida lusitaniae strains with different antifungal susceptibilities (see Table S1 in the supplemental material). The species of all isolates were identified using matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (20). All strains were recovered from La Timone University Hospital in Marseille, France, and were isolated from different clinical samples, mainly from blood cultures, urine, and vaginal swabs (Table S1). C. albicans ATCC 90028, C. parapsilosis ATCC 22019, C. auris DSMZ 21092, and C. krusei ATCC 6258 were used as quality controls (Table S1).
Antifungal susceptibility testing.
Antifungal susceptibility testing was performed using commercial broth microdilution plates (Sensititre YeastOne; Thermo Fisher Scientific, Schwerte, Germany) containing nine antifungals belonging to the four therapeutic classes: polyenes (amphotericin B), azoles (fluconazole, posaconazole, voriconazole, and itraconazole), echinocandins (anidulafungin, caspofungin, and micafungin), and nucleoside analogs (5-flucytosine). The MICs obtained for the Candida sp. strains tested (Table S1 in the supplemental material) were compared to the breakpoints provided by the manufacturers, or to the CLSI cutoffs (19), so as to assess the susceptibilities of each strain to the different antifungal agents tested.
Ribavirin susceptibility testing.
Using the broth microdilution method as outlined by the CLSI (19), ribavirin MICs were determined. First, ribavirin powder (Sigma-Aldrich, St. Louis, MO) was dissolved in water to obtain a stock solution at a concentration of 10 mg/ml. Then the ribavirin stock solution was diluted in RPMI 1640 broth medium (Sigma-Aldrich, St. Louis, MO) to yield a concentration of 200 μM (i.e., 48.32 μg/ml), 2-fold more concentrated than the starting concentration. Fungal suspensions at a 0.5 McFarland standard were prepared, and working yeast suspensions were obtained by a 1:100 dilution followed by a 1:20 dilution of the initial suspension in RPMI broth medium. Further, serial ribavirin dilutions (2-fold dilutions) ranging from 100 to 0.2 μM (i.e., 24.16 to 0.047 μg/ml) were tested by inoculating a well containing 100 μl of ribavirin at the appropriate concentration with 100 μl of the final fungal suspension. The ribavirin concentration achievable in human plasma without toxic effects (≈6 μg/ml) was considered in order to establish the concentration ranges used in the 2-fold dilution testing. The MIC of ribavirin was defined as the concentration that resulted in complete growth inhibition relative to the growth in untreated control wells. This MIC was later determined visually after 24 h of incubation at 37°C by using the OD obtained by spectrophotometric measurement.
Time-kill assay.
In order to assess the fungicidal or fungistatic activity of the ribavirin compound against three fungal strains (2 C. albicans strains and 1 C. parapsilosis strain), ribavirin time-kill analysis was performed as described previously (21); C. albicans ATCC 90028 was used as the quality control. The action of ribavirin was investigated at four different concentrations (0.5× MIC, 1× MIC, 2× MIC, 4× MIC), and fungal growth was determined by the CFU count (expressed as CFU per milliliter) after 2, 6, 12, 24, 36, or 48 h of incubation at 37°C. The results were analyzed using GraphPad Prism software (version 5.3; GraphPad Inc., San Diego, CA, USA).
Testing of efflux pump activity.
The involvement of efflux pumps in the ribavirin resistance mechanism in some ribavirin-resistant Candida sp. strains (two C. glabrata, one C. krusei, one C. lusitaniae, and one C. albicans strain) was verified using the inhibitors CCCP (carbonyl cyanide 3-chlorophenylhydrazone) and verapamil, which decrease the MICs when resistance is correlated to this mechanism (22). The CCCP and verapamil inhibitors were tested at two final concentrations (0.5 μg/ml and 10 μg/ml). To ensure that these concentrations did not affect yeast growth, control growth wells containing efflux pump inhibitors (EPIs) alone in RPMI medium were added. We determined ribavirin MICs for all strains before and after adding EPIs to the RPMI medium.
Mutation frequency.
The frequencies of spontaneous mutations were determined on C. albicans ATCC 90028 and the multidrug-resistant (MDR) C. albicans strain Q181103513 by plating 100 μl of the growing yeast at an appropriate dilution (103 CFU) on ribavirin-free plates and without dilution (105 CFU) on ribavirin-containing plates (17). RPMI 1640 agar plates with different ribavirin concentrations (1× MIC, 2× MIC, 4× MIC) were prepared as described previously (23). Spontaneous mutation frequencies were calculated, after 24 h of incubation at 37°C, by dividing the number of resistant colonies grown on a given plate by the initial inoculum pipetted. Mutant resistance phenotypes were confirmed by determining the ribavirin MICs for mutant colonies.
In vitro interaction studies of combinations of ribavirin with antifungal drugs.
In order to exploit the potential for improved efficacy, reduced toxicity, and reduced risk of drug resistance development (24), ribavirin–antifungal agent combinations were tested against 29 Candida sp. strains, including 12 C. albicans strains, 8 C. glabrata strains, 4 C. tropicalis strains, 1 C. lusitaniae strain, and 4 C. krusei strains. Based on the checkerboard association assay, combinations of ribavirin with each of the following antifungals were tested: fluconazole, itraconazole, posaconazole, and flucytosine (Sigma-Aldrich, St. Louis, MO). The effects of different combinations on different strains were analyzed using the fractional inhibitory concentration index (FICI), calculated as described previously (24), and interpreted as follows: an FICI of ≤0.5 indicates synergy between the antimicrobials tested; an FICI of >0.5 but ≤4 indicates indifference or additivity; and an FICI of >4 indicates antagonism between the antimicrobials tested.
Supplementary Material
ACKNOWLEDGMENTS
We thank CookieTrad for English reviewing.
This work was supported by the French Government under the Investments for the Future (Investissements d’avenir) program managed by the National Agency for Research (Agence Nationale de la Recherche [ANR]) (reference no. Méditerranée Infection 10-IAHU-03) and by the Région Provence-Alpes-Côte d’Azur and European funding (FEDER [Fonds européen de développement régional] PRIMMI [Plateformes de Recherche et d'Innovation Mutualisées Méditerranée Infection]).
We declare that there is no conflict of interest and there is no need for financial disclosure.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00263-19.
REFERENCES
- 1.Geddes-McAlister J, Shapiro RS. 2019. New pathogens, new tricks: emerging, drug-resistant fungal pathogens and future prospects for antifungal therapeutics. Ann N Y Acad Sci 1435:57–78. doi: 10.1111/nyas.13739. [DOI] [PubMed] [Google Scholar]
- 2.Singh R, Parija SC. 2012. Candida parapsilosis: an emerging fungal pathogen. Indian J Med Res 136:671–673. [PMC free article] [PubMed] [Google Scholar]
- 3.Spivak ES, Hanson KE. 2017. Candida auris: an emerging fungal pathogen. J Clin Microbiol 56:e01588-17. doi: 10.1128/JCM.01588-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassetti M, Peghin M, Timsit JF. 2016. The current treatment landscape: candidiasis. J Antimicrob Chemother 71(Suppl 2):ii13–ii22. doi: 10.1093/jac/dkw392. [DOI] [PubMed] [Google Scholar]
- 5.Pfaller MA. 2012. Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am J Med 125:S3–S13. doi: 10.1016/j.amjmed.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Ogundeji AO, Pohl CH, Sebolai OM. 2016. Repurposing of aspirin and ibuprofen as candidate anti-Cryptococcus drugs. Antimicrob Agents Chemother 60:4799–4808. doi: 10.1128/AAC.02810-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tournu H, Carroll J, Latimer B, Dragoi AM, Dykes S, Cardelli J, Peters TL, Eberle KE, Palmer GE. 2017. Identification of small molecules that disrupt vacuolar function in the pathogen Candida albicans. PLoS One 12:e0171145. doi: 10.1371/journal.pone.0171145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Te HS, Randall G, Jensen DM. 2007. Mechanism of action of ribavirin in the treatment of chronic hepatitis C. Gastroenterol Hepatol (N Y) 3:218–225. [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas E, Ghany MG, Liang TJ. 2012. The application and mechanism of action of ribavirin in therapy of hepatitis C. Antivir Chem Chemother 23:1–12. doi: 10.3851/IMP2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Truong M, Monahan LG, Carter DA, Charles IG. 2018. Repurposing drugs to fast-track therapeutic agents for the treatment of cryptococcosis. PeerJ 6:e4761. doi: 10.7717/peerj.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soyuncu S, Oktay C, Berk Y, Eken C. 2007. Abamectin intoxication with coma and hypotension. Clin Toxicol (Phila) 45:299–300. doi: 10.1080/15563650601072225. [DOI] [PubMed] [Google Scholar]
- 12.Wu SZ, Wang S, Ratnaparkhi R. 2018. Treatment of pediatric alopecia areata with anthralin: a retrospective study of 37 patients. Pediatr Dermatol 6:817–820. doi: 10.1111/pde.13703. [DOI] [PubMed] [Google Scholar]
- 13.Jen JF, Glue P, Gupta S, Zambas D, Hajian G. 2000. Population pharmacokinetic and pharmacodynamic analysis of ribavirin in patients with chronic hepatitis C. Ther Drug Monit 22:555–565. doi: 10.1097/00007691-200010000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Lindahl K, Stahle L, Bruchfeld A, Schvarcz R. 2005. High-dose ribavirin in combination with standard dose peginterferon for treatment of patients with chronic hepatitis C. Hepatology 41:275–279. doi: 10.1002/hep.20563. [DOI] [PubMed] [Google Scholar]
- 15.Bodenheimer HC, Lindsay KL, Davis GL, Lewis JH, Thung SN, Seeff LB. 1997. Tolerance and efficacy of oral ribavirin treatment of chronic hepatitis C: a multicenter trial. Hepatology 26:473–477. doi: 10.1002/hep.510260231. [DOI] [PubMed] [Google Scholar]
- 16.Prasad R, Rawal MK. 2014. Efflux pump proteins in antifungal resistance. Front Pharmacol 5:202. doi: 10.3389/fphar.2014.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Locke JB, Almaguer AL, Zuill DE, Bartizal K. 2016. Characterization of in vitro resistance development to the novel echinocandin CD101 in Candida species. Antimicrob Agents Chemother 60:6100–6107. doi: 10.1128/AAC.00620-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peyclit L, Baron SA, Yousfi H, Rolain JM. 2018. Zidovudine: a salvage therapy for mcr-1 plasmid-mediated colistin-resistant bacterial infections? Int J Antimicrob Agents 52:11–13. doi: 10.1016/j.ijantimicag.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 19.NCCLS. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard—2nd ed NCCLS document M27-A2, vol 22, no 15 NCCLS, Wayne, PA. [Google Scholar]
- 20.Cassagne C, Normand A-C, L'Ollivier C, Ranque S, Piarroux R. 2016. Performance of MALDI-TOF MS platforms for fungal identification. Mycoses 59:678–690. doi: 10.1111/myc.12506. [DOI] [PubMed] [Google Scholar]
- 21.Klepser ME, Wolfe EJ, Jones RN, Nightingale CH, Pfaller MA. 1997. Antifungal pharmacodynamic characteristics of fluconazole and amphotericin B tested against Candida albicans. Antimicrob Agents Chemother 41:1392–1395. doi: 10.1128/AAC.41.6.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guinea J, Sánchez-Somolinos M, Cuevas O, Peláez T, Bouza E. 2006. Fluconazole resistance mechanisms in Candida krusei: the contribution of efflux-pumps. Med Mycol 44:575–578. doi: 10.1080/13693780600561544. [DOI] [PubMed] [Google Scholar]
- 23.Jorgensen JH, Pfaller MA, Carroll KC, Funke G, Landry ML, Richter SS, Warnock DW (ed). 2015. Manual of clinical microbiology, 11th ed ASM Press, Washington, DC. [Google Scholar]
- 24.Foucquier J, Guedj M. 2015. Analysis of drug combinations: current methodological landscape. Pharmacol Res Perspect 3:e00149. doi: 10.1002/prp2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiederhold NP, Patterson TF, Srinivasan A, Chaturvedi AK, Fothergill AW, Wormley FL, Ramasubramanian AK, Lopez-Ribot JL. 2017. Repurposing auranofin as an antifungal: in vitro activity against a variety of medically important fungi. Virulence 8:138–142. doi: 10.1080/21505594.2016.1196301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan S, Singhal S, Mathur T, Upadhyay DJ, Rattan A. 2007. Antifungal potential of disulfiram. Nippon Ishinkin Gakkai Zasshi 48:109–113. doi: 10.3314/jjmm.48.109. [DOI] [PubMed] [Google Scholar]
- 27.De Cremer K, Lanckacker E, Cools TL, Bax M, De Brucker K, Cos P, Cammue BPA, Thévissen K. 2015. Artemisinins, new miconazole potentiators resulting in increased activity against Candida albicans biofilms. Antimicrob Agents Chemother 59:421–426. doi: 10.1128/AAC.04229-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miletti KE, Leibowitz MJ. 2000. Pentamidine inhibition of group I intron splicing in Candida albicans correlates with growth inhibition. Antimicrob Agents Chemother 44:958–966. doi: 10.1128/aac.44.4.958-966.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olsen I. 2014. Attenuation of Candida albicans virulence with focus on disruption of its vacuole functions. J Oral Microbiol 6. doi: 10.3402/jom.v6.23898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kasper L, Miramón P, Jablonowski N, Wisgott S, Wilson D, Brunke S, Hube B. 2015. Antifungal activity of clotrimazole against Candida albicans depends on carbon sources, growth phase and morphology. J Med Microbiol 64:714–723. doi: 10.1099/jmm.0.000082. [DOI] [PubMed] [Google Scholar]
- 31.Calamari SE, Bojanich MA, Barembaum SR, Berdicevski N, Azcurra AI. 2011. Antifungal and post-antifungal effects of chlorhexidine, fluconazole, chitosan and its combinations on Candida albicans. Med Oral Patol Oral Cir Bucal 16:e23–e28. doi: 10.4317/medoral.16.e23. [DOI] [PubMed] [Google Scholar]
- 32.Mendling W, Weissenbacher ER, Gerber S, Prasauskas V, Grob P. 2016. Use of locally delivered dequalinium chloride in the treatment of vaginal infections: a review. Arch Gynecol Obstet 293:469–484. doi: 10.1007/s00404-015-3914-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim K, Zilbermintz L, Martchenko M. 2015. Repurposing FDA approved drugs against the human fungal pathogen, Candida albicans. Ann Clin Microbiol Antimicrob 14:32. doi: 10.1186/s12941-015-0090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niewerth M, Kunze D, Seibold M, Schaller M, Korting HC, Hube B. 2003. Ciclopirox olamine treatment affects the expression pattern of Candida albicans genes encoding virulence factors, iron metabolism proteins, and drug resistance factors. Antimicrob Agents Chemother 47:1805–1817. doi: 10.1128/aac.47.6.1805-1817.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


