Bacteriophage-derived lysins are being developed as anti-infective agents. In an acute osteomyelitis methicillin-resistant Staphylococcus aureus (MRSA) model, rats receiving no treatment or treatment with daptomycin, exebacase (CF-301), or daptomycin plus exebacase had means of 5.13, 4.09, 4.65, and 3.57 log10 CFU/gram of bone, respectively.
KEYWORDS: CF-301, daptomycin, exebacase, methicillin-resistant Staphylococcus aureus, osteomyelitis
ABSTRACT
Bacteriophage-derived lysins are being developed as anti-infective agents. In an acute osteomyelitis methicillin-resistant Staphylococcus aureus (MRSA) model, rats receiving no treatment or treatment with daptomycin, exebacase (CF-301), or daptomycin plus exebacase had means of 5.13, 4.09, 4.65, and 3.57 log10 CFU/gram of bone, respectively. All treated animals had fewer bacteria than did untreated animals (P ≤ 0.0001), with daptomycin plus exebacase being more active than daptomycin (P = 0.0042) or exebacase (P < 0.001) alone.
INTRODUCTION
Osteomyelitis is a devastating infection that can be challenging to treat and that may be associated with morbidity, including damage to bone tissue and metastatic infection. Staphylococcus aureus is the most common cause of osteomyelitis (1). S. aureus has the ability to form biofilms and to enter and survive within osteoblasts, both of which may allow it to evade the immune system and be resistant to many traditional antimicrobials (2). Current therapeutic options include surgical irrigation and debridement and long-term therapy with antimicrobials such as daptomycin or vancomycin (1, 3, 4). Compounding the challenge of the virulence of S. aureus itself is the increasing antimicrobial resistance of S. aureus. Methicillin-resistant S. aureus (MRSA) osteomyelitis is growing in frequency and has been associated with poorer outcomes than methicillin-susceptible S. aureus infection (5). There is therefore a need for new, more effective antimicrobial strategies for S. aureus, especially MRSA, bone and joint infections.
Bacteriophage-derived lysins offer a novel therapeutic approach using bacterial species-specific enzymes that hydrolyze the peptidoglycan of the bacterial cell wall (6, 7). As direct lytic agents, lysins do not require bacteria to be actively growing to exert their activity and act immediately upon contact with bacterial cells, thus rendering them as promising potential therapeutics for bone and joint infections (8). Exebacase (CF-301) is a recombinantly produced lytic enzyme derived within a Streptococcus suis prophage (6, 7), with a catalytic N-terminal domain linked to a cell wall-binding C-terminal domain with d-alanyl-l-glycyl endopeptidase activity (6, 7). Exebacase may have antibiofilm activity as a result of its causing bacteriolysis within biofilms as a result of its cleavage of peptidoglycan in the structural framework (8). In vitro, exebacase is rapidly bactericidal, shows minimal resistance development, and has synergistic activity with daptomycin and vancomycin against S. aureus (6, 8). In an experimental murine S. aureus bacteremia model, a single intravenous dose of exebacase with and without vancomycin or daptomycin increased survival (6). The results of a phase 2 clinical trial demonstrate a 43% higher clinical response rate with a single dose of exebacase used in addition to standard-of-care antimicrobials versus antimicrobials alone for MRSA bacteremia, including endocarditis (9, 10). We hypothesized that exebacase would be active against MRSA in experimental acute osteomyelitis in rats.
The study strain, MRSA IDRL-6169, had MICs of 0.5 μg/ml for both exebacase and daptomycin, as determined by broth microdilution (11, 12). The minimum biofilm inhibitory concentrations and minimum biofilm bactericidal concentrations were 1 and 4 μg/ml for exebacase and 1 and 2 μg/ml for daptomycin, as determined using previously described methods (14). Exebacase testing was supplemented with 0.5 mM dl-dithiothreitol and 25% horse serum (6).
The studies described were approved by the Institutional Animal Care and Use Committee of the Mayo Clinic. Osteomyelitis was established in 64 male Sprague Dawley rats using a modification of Zak’s model of experimental osteomyelitis designed to mimic human infection (15). Animals were anesthetized with isoflurane and the left knee shaved and disinfected with chlorohexidine. To induce osteomyelitis, the knee joint was bent at a 45° angle to expose the top of the tibial process. A 1-ml syringe with a 21 G needle containing 10 μl arachidonic acid (50 μg/ml) and 50 μl of a 107 CFU suspension of IDRL-6169 in tryptic soy broth was inserted into the tibia. The bacterial suspension was slowly injected into the tibia, the needle removed, the knee joint straightened, and pressure placed on the injection site for 1 min. One week after establishing infection (day 8), rats were randomly assigned to one of four treatment arms, as follows: (i) no treatment, (ii) 60 mg/kg of body weight daptomycin intraperitoneally every 12 h (16) for 4 days, (iii) single-dose 40 mg/kg exebacase in the tail vein, or (iv) single-dose 40 mg/kg exebacase plus 60 mg/kg daptomycin intraperitoneally every 12 h for 4 days. Daptomycin was administered 15 min prior to exebacase injection. Exebacase, formulated for clinical use, was maintained on ice until injection. Rats were sacrificed 4 days after starting therapy (day 12). The left tibia from each animal was collected, weighed, and cryopulverized for quantitative bacterial culture (15). The results of quantitative cultures were compared using SAS software version 9.4 (SAS, Inc., Cary, NC) using the Kruskal-Wallis test as well as Bonferroni correction for multiple comparisons. Means and standard deviations (SD) were reported as log10 CFU/gram of bone. All tests were two sided; P values less than 0.05 were considered statistically significant.
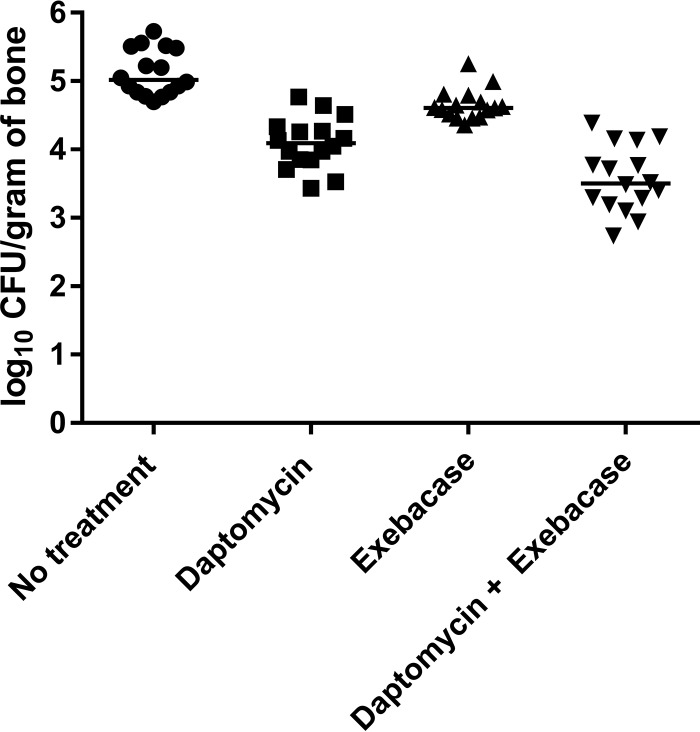
Rats receiving no treatment had a mean (±SD) bacterial density of 5.13 (±0.34) log10 CFU/gram of bone. Rats in the daptomycin, exebacase, and daptomycin plus exebacase groups had mean (±SD) bacterial densities of 4.09 (±0.37), 4.65 (±0.65), and 3.57 (±0.48) log10 CFU/gram of bone, respectively (Fig. 1). The colony counts in all treatment groups were lower than those of the untreated rats (P ≤ 0.0001). Daptomycin combined with exebacase-treated animals had lower colony counts than did those treated with daptomycin (P = 0.0042) or exebacase (P < 0.0001) alone. The conclusions from the study remain unchanged after application of the Bonferroni correction for multiple comparisons.
FIG 1.
Results of quantitative cultures of the tibias after treatment (log10 CFU of MRSA/gram of bone). All treatment groups had lower bacterial loads than those with no treatment (P ≤ 0.0001), with exebacase combined with daptomycin resulting in lower bacterial loads than those with monotherapy with either exebacase (P < 0.0001) or daptomycin (P = 0.0042). The horizontal lines depict median values.
The animal model used here is one of acute MRSA osteomyelitis. S. aureus bone and joint infections are challenging to treat, partly due to the ability of S. aureus to survive in bone tissue (2). Antimicrobials such as vancomycin may not penetrate bone tissue well (17, 18), yet they are often used to treat osteomyelitis (4). It has been suggested that daptomycin has bone penetration (19). We found levels of exebacase in bone to be ∼15% of plasma levels after a single dose of 10 mg/kg. As determined by enzyme-linked immunosorbent assay (ELISA), the maximum concentration in serum (Cmax) and area under the concentration-time curve (AUC) in plasma and bone were 88 μg/ml and 12 μg/g and 28 μg·h/ml and 4 μg·h/g, respectively. Exebacase may therefore offer a strategy to target S. aureus bone and joint infections through its ability to lyse S. aureus at the site of infection. It was previously shown that exebacase is synergistic with daptomycin, possibly by increasing the ability of daptomycin to bind to its target (6). Thus, the use of exebacase plus daptomycin may offer a treatment option for acute osteomyelitis.
In this study, while treatment with daptomycin or exebacase alone showed a reduction in bacterial counts, exebacase in addition to daptomycin showed a more pronounced effect.
ACKNOWLEDGMENTS
This study was sponsored by ContraFect and a grant from the Department of Defense Congressionally Directed Medical Research Program (award W81XWH-16-1-0245).
REFERENCES
- 1.Birt MC, Anderson DW, Toby EB, Wang J. 2017. Osteomyelitis: recent advances in pathophysiology and therapeutic strategies. J Orthop 14:45–52. doi: 10.1016/j.jor.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kavanagh N, Ryan EJ, Widaa A, Sexton G, Fennell J, O’Rourke S, Cahill KC, Kearney CJ, O’Brien FJ, Kerrigan SW. 2018. Staphylococcal osteomyelitis: Disease progression, treatment challenges, and future directions. Clin Microbiol Rev 31:e00084-17. doi: 10.1128/CMR.00084-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu C, Chambers HF, Kaplan SL, Karchmer AW, Levine DP, Rybak MJ, Murray BE, Bayer A, Talan DA, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Infectious Diseases Society of America. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52:e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 4.Fraimow HS. 2009. Systemic antimicrobial therapy in osteomyelitis. Semin Plastic Surg 23:90–99. doi: 10.1055/s-0029-1214161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis WT, Gilbert SR. 2018. Comparison of methicillin-resistant versus susceptible Staphylococcus aureus pediatric osteomyelitis. J Ped Orthop 38:e285–e291. doi: 10.1097/BPO.0000000000001152. [DOI] [PubMed] [Google Scholar]
- 6.Schuch R, Lee HM, Schneider BC, Sauve KL, Law C, Khan BK, Rotolo JA, Horiuchi Y, Couto DE, Raz A, Fischetti V, Huang DB, Nowinski RC, Wittekind M. 2014. Combination therapy with lysin CF-301 and antibiotic is superior to antibiotic alone for treating methicillin-resistant Staphylococcus aureus–induced murine bacteremia. J Infect Dis 209:1469–1478. doi: 10.1093/infdis/jit637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lood R, Molina H, Fischetti VA. 2017. Determining bacteriophage endopeptidase activity using either fluorophore-quencher labeled peptides combined with liquid chromatography-mass spectrometry (LC-MS) or Förster resonance energy transfer (FRET) assays. PLoS One 12:e0173919. doi: 10.1371/journal.pone.0173919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuch R, Khan BK, Raz A, Rotolo JA, Wittekind M. 2017. Bacteriophage lysin CF-301: a potent anti-staphylococcal biofilm agent. Antimicrob Agents Chemother 61:02666-16. doi: 10.1128/AAC.02666-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ClinicalTrials.gov. 2017. Safety, efficacy and pharmacokinetics of CF-301 vs. placebo in addition to antibacterial therapy for treatment of S. aureus bacteremia, NCT 03163446. https://clinicaltrials.gov/ct2/show/NCT03163446. Accessed 18 March 2019.
- 10.Fowler VG, Das A, Lipka J, Schuch R, Cassino C. 2019. Exebacase (lysin CF-301) improved clinical responder rates in methicillin-resistant Staphylococcus aureus bacteremia and endocarditis compared to standard of care antibiotics alone in a first-in-patient phase 2 study. 29th European Congress of Clinical Microbiology and Infectious Diseases Annual Meeting, 13 to 16 April, 2019, Amsterdam, the Netherlands. [Google Scholar]
- 11.CLSI. 2018. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 11th ed. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 12.CLSI. 2019. Performance standards for antimicrobial susceptibility testing, 29th ed. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 13.Reference deleted.
- 14.Schmidt-Malan SM, Greenwood Quaintance KE, Karau MJ, Patel R. 2016. In vitro activity of tedizolid against staphylococci isolated from prosthetic joint infections. Diagn Microbiol Infect Dis 85:77–79. doi: 10.1016/j.diagmicrobio.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 15.O’Reilly T, Mader J. 1999. Rat model of bacterial osteomyelitis of the tibia, p 561–575. In Zak O, Sande M (ed), Handbook of animal models of infection. Academic Press, San Diego, CA. [Google Scholar]
- 16.Rouse MS, Piper KE, Jacobson M, Jacofsky DJ, Steckelberg JM, Patel R. 2006. Daptomycin treatment of Staphylococcus aureus experimental chronic osteomyelitis. J Antimicrob Chemother 57:301–305. doi: 10.1093/jac/dki435. [DOI] [PubMed] [Google Scholar]
- 17.Albayati ZAF, Sunkara M, Schmidt-Malan SM, Karau MJ, Morris AJ, Steckelberg JM, Patel R, Breen PJ, Smeltzer MS, Taylor KG, Merten KE, Pierce WM, Crooks PA. 2016. Novel bone-targeting agent for enhanced delivery of vancomycin to bone. Antimicrob Agents Chemother 60:1865–1868. doi: 10.1128/AAC.01609-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karau MJ, Schmidt-Malan SM, Greenwood Quaintance KE, Mandrekar J, Cai J, Pierce WM, Merten K, Patel R. 2013. Treatment of methicillin-resistant Staphylococcus aureus experimental osteomyelitis with bone-targeted vancomycin. Springerplus 2:329. doi: 10.1186/2193-1801-2-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montange D, Berthier F, Leclerc G, Serre A, Jeunet L, Berard M, Muret P, Vettoretti L, Leroy J, Hoen B, Chirouze C. 2014. Penetration of daptomycin into bone and synovial fluid in joint replacement. Antimicrob Agents Chemother 58:3991–3996. doi: 10.1128/AAC.02344-14. [DOI] [PMC free article] [PubMed] [Google Scholar]