Delafloxacin is a broad-spectrum anionic fluoroquinolone that has completed a phase 3 study for community-acquired bacterial pneumonia. We investigated the pharmacodynamic target for delafloxacin against 12 Klebsiella pneumoniae and 5 Pseudomonas aeruginosa strains in the neutropenic murine lung infection model. The median 24-h free-drug area under the curve (fAUC)/MIC values associated with net stasis and 1-log kill were 28.6 and 64.1 for K. pneumoniae, respectively.
KEYWORDS: Klebsiella, Pseudomonas aeruginosa, delafloxacin, pharmacodynamics
ABSTRACT
Delafloxacin is a broad-spectrum anionic fluoroquinolone that has completed a phase 3 study for community-acquired bacterial pneumonia. We investigated the pharmacodynamic target for delafloxacin against 12 Klebsiella pneumoniae and 5 Pseudomonas aeruginosa strains in the neutropenic murine lung infection model. The median 24-h free-drug area under the curve (fAUC)/MIC values associated with net stasis and 1-log kill were 28.6 and 64.1 for K. pneumoniae, respectively. The 24-h fAUC/MIC values associated with net stasis and 1-log kill for P. aeruginosa were 5.66 and 14.3, respectively.
INTRODUCTION
Delafloxacin is a novel fluoroquinolone antibiotic indicated in adults for the treatment of acute bacterial skin and skin structure infections, and a phase 3 study for community-acquired bacterial pneumonia has been completed (1–3). Delafloxacin has a broad spectrum of activity that includes Gram-positive and Gram-negative bacteria (4–7). We previously characterized the pharmacokinetic and pharmacodynamic (PK/PD) activity of delafloxacin against Staphylococcus aureus and Streptococcus pneumoniae using a neutropenic murine pneumonia infection model (8). In the current studies, we explored the in vivo activity of delafloxacin against multiple strains of K. pneumoniae and P. aeruginosa to delineate target PK/PD exposures for stasis and 1-log reduction in the neutropenic murine pneumonia infection model.
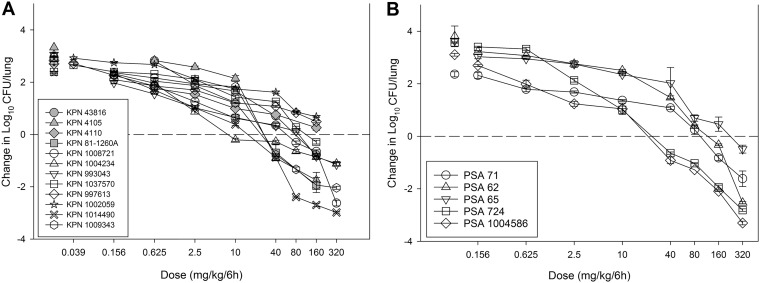
Eight K. pneumoniae strains and five P. aeruginosa strains were utilized in the current in vivo study. Analysis of previous data in this model (our laboratory) with four K. pneumoniae strains was integrated into the present data set. The strains, MIC phenotypes, and genotypes (when available) are presented in Table 1. MICs were determined in triplicate according to CLSI guidelines (9). The MICs ranged widely from 0.03 to 4 mg/liter for K. pneumoniae and from 0.12 to 4 mg/liter for P. aeruginosa, which are similar to the MIC ranges for delafloxacin identified in an in vitro surveillance study (4). The neutropenic murine lung infection model was used for in vivo study of delafloxacin. Animals were maintained in accordance with American Association for Accreditation of Laboratory Animal Care criteria. All animal studies were approved by the Animal Research Committee of the William S. Middleton Memorial VA Hospital and the University of Wisconsin. Mice were infected with 6.7 ± 0.3 log10 CFU of each strain via nasal inhalation. The in vivo fitness of each strain was assessed, and study demonstrated robust growth over 24 h. Two hours after lung infection, delafloxacin was administered to mice subcutaneously every 6 h for the duration of the 24-h experiment. Treatment included a dose range of 0.0156 to 1,280 mg/kg in 24 h. The highest doses of delafloxacin reduced the lung organism burden by up to 3 log10 compared with the burden at the start of therapy (Fig. 1). A 1-log10 kill was achieved against 7 of 12 K. pneumoniae strains, and the dose-response curves correlated well with our previous studies on K. pneumoniae (8). A 1-log10 kill was achieved for all but the highest MIC strain of P. aeruginosa.
TABLE 1.
Susceptibility results of delafloxacin and comparators against K. pneumoniae and P. aeruginosa strains
| Organism and strain | MIC (mg/liter) |
Strain description | ||
|---|---|---|---|---|
| Delafloxacin | Levofloxacin | Ciprofloxacin | ||
| K. pneumoniae | ||||
| 43816a | 0.06 | 0.06 | Wild type | |
| 4105a | 1 | 1 | TEM26, SHV4 | |
| 4110a | 0.5 | 1 | TEM1, SHV1 | |
| 81-1260Aa | 0.06 | 0.06 | CTX-M, AmpC | |
| 1037570 | 0.5 | 1 | 2 | |
| 997613 | 1 | 0.5 | 0.25 | |
| 1002059 | 4 | 1 | 0.5 | |
| 993043 | 0.03 | 0.03 | 0.03 | |
| 1004234 | 0.06 | 0.06 | 0.03 | |
| 1008721 | 0.12 | 0.12 | 0.12 | |
| 1009343 | 0.25 | 0.12 | 0.25 | |
| 1014490 | 0.25 | 0.12 | 0.06 | |
| P. aeruginosa | ||||
| 71 | 1 | 2 | Wild type | |
| 62 | 2 | 4 | Wild type | |
| 65 | 4 | >8 | parC S87L, gyrA T83I | |
| 724 | 0.12 | ≤0.06 | Wild type | |
| 1004586 | 0.5 | 0.5 | 0.12 | |
Results for these strains were retrieved from our previous study (8).
FIG 1.
Dose-response curves for delafloxacin against 12 K. pneumoniae strains (A) and 5 P. aeruginosa strains (B) in the neutropenic murine lung infection model. Symbols represents means and standard deviations from three lung infection replicates. Gray symbols, strains retrieved from our previous study (8). Eight different dose levels were administered by subcutaneous route every 6 h. The burden of organisms was enumerated at the start and end of therapy over a 24-h study period. Horizontal dashed line at 0, burden of organisms at the start of therapy. Data points above the line, net growth (i.e., increase) in burden; data points below the line, reduction in bacterial burden.
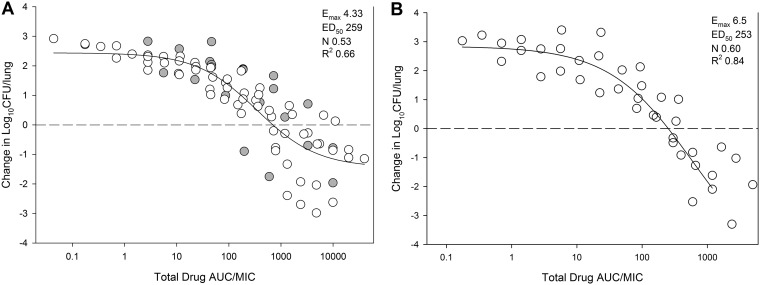
We utilized the PK of delafloxacin from this model recently reported from our lab to estimate the plasma area under the curve (AUC) over the current dose range (8). Murine protein binding of 97.6% (Melinta Therapeutics, Inc; data on file) was used to determine free-drug concentrations. The resultant AUC/MIC exposures are shown in Fig. 2. The PK/PD index AUC/MIC correlated well with the therapeutic effect (R2 = 0.66 for K. pneumoniae and 0.84 for P. aeruginosa) when modeled using the sigmoid maximum effect (Emax) model.
FIG 2.
In vivo exposure-response relationship between the PK parameter 24-h AUC/MIC and treatment effect for 12 K. pneumoniae strains (A) and 5 P. aeruginosa strains (B) in the neutropenic murine lung infection model. Each symbol is the mean of three lung infection replicates. Eight total drug dosing regimens were fractionated into an every-6-h regimen. The delafloxacin exposure is represented on the x axis as plasma 24-h AUC/MIC. The burden of organisms was measured at the start and end of therapy over a 24-h period. Horizontal dashed line at 0, burden of organisms at the start of therapy. Data points above the line, net growth (i.e., increase) in burden; data points below the line, net reduction in bacterial burden. Line drawn through the data, best-fit line based on the sigmoid Emax model (Hill equation). Additional PD paremeters shown are Emax, 50% maximal effect point (ED50), slope of the line (N), and R2 (coefficient of determination).
The AUC/MIC exposures associated with net stasis and 1-log10 kill for each strain are shown in Table 2. The median PD target 24-h free-drug plasma AUC/MIC associated with net stasis and 1-log10 kill for K. pneumoniae were 28.6 and 64.1, respectively. The median PD target 24-h free-drug plasma AUC/MIC associated with net stasis and 1-log10 kill for P. aeruginosa were 5.66 and 14.3, respectively.
TABLE 2.
Delafloxacin pharmacodynamic target exposures for each K. pneumoniae and P. aeruginosa strain in the murine lung infection model
| Organism and strain | Bacterial burden at start of therapy (log10 CFU/lung) | Growth in control at 24 h (Δlog10CFU/lung) | Stasis |
1-Log kill |
||||
|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg/24 h) | 24-h AUC/MIC |
Dose (mg/kg/24 h) | 24-h AUC/MIC |
|||||
| Total drug | Free drug | Total drug | Free drug | |||||
| K. pneumoniae | ||||||||
| 43816a | 6.30 | 2.86 | 304 | 5,287 | 127 | |||
| 4105a | 6.30 | 3.33 | 106 | 128 | 3.08 | 196 | 228 | 5.47 |
| 4110a | 6.32 | 2.82 | NAb | |||||
| 81-1260Aa | 6.28 | 2.83 | 84.8 | 1,681 | 40.3 | 238 | 4,369 | 105 |
| 1037570 | 6.53 | 2.95 | NA | |||||
| 997613 | 6.56 | 3.04 | NA | |||||
| 1002059 | 6.64 | 3.05 | 639 | 1,192 | 28.6 | |||
| 993043 | 6.99 | 2.39 | 134 | 1,365 | 32.8 | 545 | 4,312 | 103 |
| 1004234 | 6.64 | 2.70 | 50.7 | 952 | 22.8 | 862 | 13,394 | 321 |
| 1008721 | 6.71 | 2.37 | 157 | 6,467 | 155 | |||
| 1009343 | 6.92 | 2.76 | 109 | 528 | 12.7 | 233 | 1,032 | 24.8 |
| 1014490 | 7.26 | 2.72 | 37.2 | 161 | 3.87 | 98.5 | 474 | 11.4 |
| Median | 109 | 1,192 | 28.6 | 235 | 2,672 | 64.1 | ||
| SE | 63.0 | 764 | 18.3 | 117 | 2,033 | 48.8 | ||
| P. aeruginosa | ||||||||
| 71 | 6.23 | 2.36 | 294 | 309 | 7.41 | 830 | 774 | 18.6 |
| 62 | 6.92 | 3.81 | 354 | 179 | 4.30 | 903 | 421 | 10.1 |
| 65 | 6.93 | 3.64 | 1,057 | 236 | 5.66 | NA | ||
| 724 | 6.31 | 3.57 | 107 | 1,035 | 24.8 | 246 | 2,152 | 51.6 |
| 1004586 | 6.52 | 3.11 | 61.5 | 142 | 3.41 | 169 | 409 | 9.82 |
| Median | 294 | 236 | 5.66 | 538 | 598 | 14.3 | ||
| SE | 179 | 166 | 3.99 | 192 | 413 | 9.91 | ||
The results for these strains were retrieved from our previous study (8).
NA, not achieved.
Previous studies have demonstrated that PD targets of fluoroquinolones associated with clinical and microbiological responses vary for different bacterial species (10–14). These studies with older fluoroquinolones demonstrated that the AUC/MIC associated with clinical success was ≥100 for S. aureus isolates and Gram-negative bacteria, whereas an AUC/MIC of <40 appeared to be linked to treatment efficacy for S. pneumoniae isolates. This divergence in species targets holds true for delafloxacin as demonstrated by integrating the PK/PD targets identified in the current study with those of previous PK/PD studies with delafloxacin. The previous study with a murine pneumonia model demonstrated that PK/PD targets for S. aureus and S. pneumoniae were ∼50- to 100-fold lower than those of comparative fluoroquinolones (8, 15). The delafloxacin PK/PD targets were somewhat lower than those described for other fluoroquinolones in this model (10). The mechanistic basis for this numeric difference is not clear.
As expected, free-drug AUC/MIC was strongly correlated with in vivo efficacy of delafloxacin, which is consistent with other fluoroquinolones (8, 10, 12–14). Previous preclinical and clinical evaluations have demonstrated the predictive value of stasis endpoints in the murine model with clinical outcome for patients with community-acquired respiratory tract infections (14). The 24-h free-drug AUC/MICs required for stasis for K. pneumoniae and P. aeruginosa infections were 28.6 and 5.66, respectively. The human steady-state PK of delafloxacin in healthy subjects using 450-mg oral (16) and 300-mg intravenous (17) clinical dosing regimens demonstrated free AUC from 0 to 24 h (AUC0–24) of 9.9 and 7.5 mg · h/liter. The current PK/PD stasis targets indicate that human PK would predict efficacy against K. pneumoniae infection with MICs up to 0.25 mg/liter and against P. aeruginosa infection with MICs of ≤1 mg/liter. These MIC values included 78.2% of K. pneumoniae strains and 75% of P. aeruginosa strains from large surveillance studies with delafloxacin (4).
In conclusion, these results suggest that delafloxacin is a promising agent against K. pneumoniae and P. aeruginosa infections. The PK targets identified in the murine pneumonia model for net stasis are achievable in most cases when examining the targets in the context of human PK of approved dosing regimens and epidemiological MIC distribution. These animal model PK/PD targets should be useful for future designs of delafloxacin dosing regimens and the development of susceptibility breakpoints.
ACKNOWLEDGMENT
This study was funded by Melinta Therapeutics.
REFERENCES
- 1.Shiu J, Ting G, Kiang TK. 2019. Clinical pharmacokinetics and pharmacodynamics of delafloxacin. Eur J Drug Metab Pharmacokinet 44:305–317. doi: 10.1007/s13318-018-0520-8. [DOI] [PubMed] [Google Scholar]
- 2.Tulkens PM, Van Bambeke F, Zinner SH. 2019. Profile of a novel anionic fluoroquinolone-delafloxacin. Clin Infect Dis 68:S213–S222. doi: 10.1093/cid/ciy1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho JC, Crotty MP, White BP, Worley MV. 2018. What is old is new again: delafloxacin, a modern fluoroquinolone. Pharmacotherapy 38:108–121. doi: 10.1002/phar.2050. [DOI] [PubMed] [Google Scholar]
- 4.Pfaller MA, Sader HS, Rhomberg PR, Flamm RK. 2017. In vitro activity of delafloxacin against contemporary bacterial pathogens from the United States and Europe, 2014. Antimicrob Agents Chemother 61:e02609-16. doi: 10.1128/AAC.02609-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCurdy S, Lawrence L, Quintas M, Woosley L, Flamm R, Tseng C, Cammarata S. 2017. In vitro activity of delafloxacin and microbiological response against fluoroquinolone-susceptible and nonsusceptible Staphylococcus aureus isolates from two phase 3 studies of acute bacterial skin and skin structure infections. Antimicrob Agents Chemother 61:e00772-17. doi: 10.1128/AAC.00772-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soge OO, Salipante SJ, No D, Duffy E, Roberts MC. 2016. In vitro activity of delafloxacin against clinical Neisseria gonorrhoeae isolates and selection of gonococcal delafloxacin resistance. Antimicrob Agents Chemother 60:3106–3111. doi: 10.1128/AAC.02798-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flamm RK, Rhomberg PR, Huband MD, Farrell DJ. 2016. In vitro activity of delafloxacin tested against isolates of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Antimicrob Agents Chemother 60:6381–6385. doi: 10.1128/AAC.00941-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lepak AJ, Andes DR. 2016. In vivo pharmacodynamic target assessment of delafloxacin against Staphylococcus aureus, Streptococcus pneumoniae, and Klebsiella pneumoniae in a murine lung infection model. Antimicrob Agents Chemother 60:4764–4769. doi: 10.1128/AAC.00647-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically—9th ed. CLSI document M07-A9. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 10.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–10; quiz 11–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 11.Dudley MN. 1991. Pharmacodynamics and pharmacokinetics of antibiotics with special reference to the fluoroquinolones. Am J Med 91:45S–50S. doi: 10.1016/0002-9343(91)90311-k. [DOI] [PubMed] [Google Scholar]
- 12.Andes D, Craig WA. 2002. Pharmacodynamics of the new fluoroquinolone gatifloxacin in murine thigh and lung infection models. Antimicrob Agents Chemother 46:1665–1670. doi: 10.1128/aac.46.6.1665-1670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andes D, Craig WA. 2003. Pharmacodynamics of the new des-f(6)-quinolone garenoxacin in a murine thigh infection model. Antimicrob Agents Chemother 47:3935–3941. doi: 10.1128/AAC.47.12.3935-3941.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambrose PG, Grasela DM, Grasela TH, Passarell J, Mayer HB, Pierce PF. 2001. Pharmacodynamics of fluoroquinolones against Streptococcus pneumoniae in patients with community-acquired respiratory tract infections. Antimicrob Agents Chemother 45:2793–2797. doi: 10.1128/AAC.45.10.2793-2797.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thabit AK, Crandon JL, Nicolau DP. 2016. Pharmacodynamic and pharmacokinetic profiling of delafloxacin in a murine lung model against community-acquired respiratory tract pathogens. Int J Antimicrob Agents 48:535–541. doi: 10.1016/j.ijantimicag.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Hoover R, Hunt T, Benedict M, Paulson SK, Lawrence L, Cammarata S, Sun E. 2016. Single and multiple ascending-dose studies of oral delafloxacin: effects of food, sex, and age. Clin Ther 38:39–52. doi: 10.1016/j.clinthera.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Hoover R, Hunt T, Benedict M, Paulson SK, Lawrence L, Cammarata S, Sun E. 2016. Safety, tolerability, and pharmacokinetic properties of intravenous delafloxacin after single and multiple doses in healthy volunteers. Clin Ther 38:53–65. doi: 10.1016/j.clinthera.2015.11.019. [DOI] [PubMed] [Google Scholar]