The delayed-release tablet formulation of posaconazole (POS-tab) results in higher plasma POS trough concentrations (Cmin) than the oral suspension (POS-susp), which raises the question of the utility of therapeutic drug monitoring (TDM). We aimed to compare the variability of the POS Cmin for the two formulations and identify determinants of the POS-tab Cmin and its variability.
KEYWORDS: hematopoietic stem cell transplantation, posaconazole, therapeutic drug monitoring, trough concentration, variability
ABSTRACT
The delayed-release tablet formulation of posaconazole (POS-tab) results in higher plasma POS trough concentrations (Cmin) than the oral suspension (POS-susp), which raises the question of the utility of therapeutic drug monitoring (TDM). We aimed to compare the variability of the POS Cmin for the two formulations and identify determinants of the POS-tab Cmin and its variability. Demographic, biological, and clinical data from 77 allogeneic hematopoietic stem cell transplant patients (874 Cmin) treated with POS-tab (n = 41), POS-susp (n = 29), or both (n = 7) from January 2015 to December 2016 were collected retrospectively. Interpatient and within-subject coefficients of variation (CVs) of the Cmin adjusted to dose (D) were calculated for each formulation. Between-group comparisons were performed using a linear mixed effects model. The POS Cmin was higher for the tablet than for the suspension (median [25th–75th percentile]: 1.8 [1.2–2.4] mg/liter versus 1.2 [0.7–1.6] mg/liter, P < 0.0001). Interpatient CVs for the tablet and suspension were 60.8 versus 63.5% (P = 0.7), whereas within-subject CVs were 39.7 and 44.9%, respectively (P = 0.3). Univariate analysis showed that age and treatment by POS-tab were significantly and positively associated with the POS Cmin, whereas diarrhea was associated with a diminished POS Cmin. Multivariate analysis identified treatment with POS-tab and diarrhea as independent factors of the POS Cmin, with a trend toward a lower impact of diarrhea during treatment with POS-tab (P = 0.07). Despite increased POS exposure with the tablet formulation, the variability of the POS Cmin was not significantly lower than that of the suspension. This suggests that TDM may still be useful to optimize tablet POS therapy.
INTRODUCTION
Posaconazole (POS) is an antifungal agent widely used for prophylaxis of invasive fungal infections in patients with hematological malignancies at high risk, especially allogeneic hematopoietic stem-cell transplant (AHSCT) patients with graft-versus-host disease. Since 2015, POS has been commercially available in Europe as a new formulation, i.e., delayed-release tablets (POS-tab), allowing a single 300-mg dose per day (1). This new formulation improves POS bioavailability relative to the former oral suspension formulation (POS-susp) (2–4). Hence, the proportion of patients achieving the recommended target goal of >0.7 mg/liter (5) while taking the tablet has increased over that of patients taking the oral suspension formulation (3, 6), which raises the question of the utility of POS therapeutic drug monitoring (TDM).
Increased POS exposure of patients using the tablet formulation over that of patients using the oral suspension is now well established (2–4). However, although the variability of the POS-susp trough concentrations (Cmin) is known to be significant, with interindividual and within-subject coefficients of variation (CV) as high as 64% and 49%, respectively (7), little is known about the variability of the POS-tab Cmin. A recent crossover study showed lower within-subject CV for the POS-tab Cmin in lung-transplant patients treated with POS-susp and then with POS-tab (8). However, the number of patients was quite low (n = 24), and interindividual CVs were not studied. Moreover, determinants involved in such variability are yet to be clarified. Indeed, the impact of diarrhea (7), mucositis, and proton pump inhibitors (PPIs) on the POS Cmin has been well described for POS-susp (9). However, the influence of these factors on the POS Cmin during POS-tab therapy is not clear, as recent results are conflicting (3, 4, 10–14). For example, several studies reported a significant impact of diarrhea on the POS-tab Cmin (11, 12), whereas others did not (4, 13–15).
The primary aim of this study was to compare the POS Cmin, as well as its intra- and interindividual variability between the two galenic formulations (tablet versus suspension) in AHSCT patients. We further aimed to identify factors that influence the POS Cmin and its variability.
RESULTS
Baseline characteristics of patients.
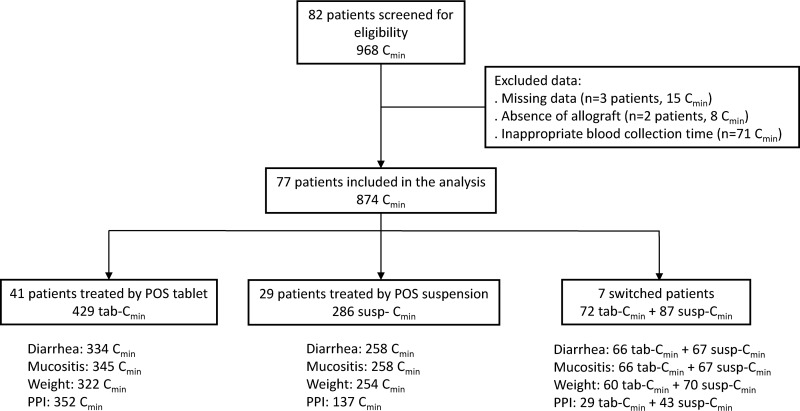
A total of 874 POS Cmin, determined in 79 patients, were included in this study; 41 patients received POS-tab only, 31 received POS-susp only, and 7 switched from the suspension to the tablet formulation (see Fig. 1). The baseline characteristics of patients are shown in Table 1.
FIG 1.
Flow chart. For each group of patients (tablet, suspension, and switched patients), the number of posaconazole trough concentrations (POS Cmin) for which potential factors that influence the POS Cmin were known are specified at the bottom.
TABLE 1.
Baseline characteristics of patientsa
| Characteristic | All patients (n = 77) |
|---|---|
| Demographics | |
| Age (yrs) | 53.0 (22.0–64.7) |
| Male (%) | 41 (53.2) |
| Weight (kg) | 64.0 (48.9–84.3) |
| Underlying hematological diseases | |
| Acute myeloid leukemia | 35 |
| Acute lymphoblastic leukemia | 16 |
| Myelodysplastic syndrome | 11 |
| Lymphoma | 8 |
| Chronic myeloid leukemia | 1 |
| Chronic lymphoblastic leukemia | 1 |
| Othersb | 5 |
| Longitudinal follow-up of POS treatment | |
| Duration of follow-up (days) | 84 (41–376) |
| No. of dose adjustments/patient | 1 (0–2) |
| No. of POS Cmin/patient | 9 (4–18) |
Data are indicated as numbers (%) or median (25th–75th percentiles).
Others include one idiopathic medullary aplasia, two nondifferentiated acute leukemia, and two acute biclonal leukemia.
Posaconazole trough concentrations and their determinants.
POS Cmin were significantly higher during treatment with POS-tab than with POS-susp (Table 2; Fig. S1 in the supplemental material), resulting in a higher proportion of therapeutic POS Cmin with POS-tab without reducing numbers of POS dose adjustment (Table 2). The results of univariate and multivariate analyses performed for all 874 POS-Cmin are shown in Table 3. Treatment with POS-tab was associated with a higher POS Cmin, whereas the presence of diarrhea was significantly associated with a lower POS Cmin (Table 3). Age was positively associated with the POS Cmin in the univariate analysis, but did not reach statistical significance in the multivariate analysis. Conversely, mucositis, cotreatment with a PPI, and body mass index (BMI) were not associated with the POS Cmin. In addition, there was a trend toward an interaction (P = 0.07) between the presence of diarrhea and galenic formulation, suggesting a lower POS Cmin in the presence of diarrhea with the suspension versus the tablet formulation (Fig. S2).
TABLE 2.
Description of plasma POS Cmin according to galenic formulation
| Characteristic | POS-susp Cmin | POS-tab Cmin | P value |
|---|---|---|---|
| No. of POS Cmin | 373 | 501 | |
| POS Cmin (mg/l) | |||
| Mediana (25th–75th percentiles) | 1.2 (0.7–1.6) | 1.8 (1.2–2.4) | <0.0001 |
| Minimum POS-Cmin | <0.1 | <0.1 | |
| Maximum POS-Cmin | 4.7 | 8.2 | |
| No. of therapeutic POS Cmin (%) | 294 (79) | 462 (92) | <0.0001 |
| Median POS dosea (mg/day) | 600 (600–800) | 300 (200–300) | <0.0001 |
| No. of POS dose adjustments (%) | 39 (10.4) | 48 (9.6) | 0.7 |
| Coefficient of variation of POS Cmin/dose | |||
| Interindividual CV (%) | 63.5 | 60.8 | 0.7 |
| Within-subject CV (%) | 44.9 | 39.7 | 0.3 |
| No. (%) of POS Cminb associated with: | |||
| Mucositis | |||
| Yes | 18 (5.5) | 73 (17.7) | |
| No | 307 (94.5) | 338 (82.3) | |
| Diarrhea | |||
| Yes | 42 (12.9) | 51 (12.7) | |
| No | 283 (87.1) | 349 (87.3) | |
| Proton pump inhibitor | |||
| Yes | 141 (78.3) | 352 (92.4) | |
| No | 39 (21.7) | 29 (7.6) |
Data are indicated as median (25th–75th percentiles).
As indicated in Fig. 1, some data are missing. Thus, the percentage of POS-Cmin with these potential determinants could vary.
TABLE 3.
Mixed-effects model of potential determinants of POS Cmin
| Variable | Nonmissing data (%) | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|---|
| Estimate ± SE | P value | Estimate ± SE | P value | ||
| POS-tablet | 100 | 0.92 ± 0.11 | <0.001 | 1.13 ± 0.18 | <0.001 |
| Age | 100 | 0.33 ± 0.07 | <0.001 | −0.19 ± 0.11 | 0.07 |
| Body mass index | 76 | 0.11 ± 0.03 | 0.72 | ||
| Proton pump inhibitor | 76 | 0.14 ± 0.19 | 0.47 | ||
| Mucositis | 82 | 0.11 ± 0.08 | 0.17 | ||
| Diarrhea | 80 | −0.37 ± 0.08 | <0.001 | −0.30 ± 0.07 | <0.001 |
Variability of posaconazole plasma trough concentrations and its determinants.
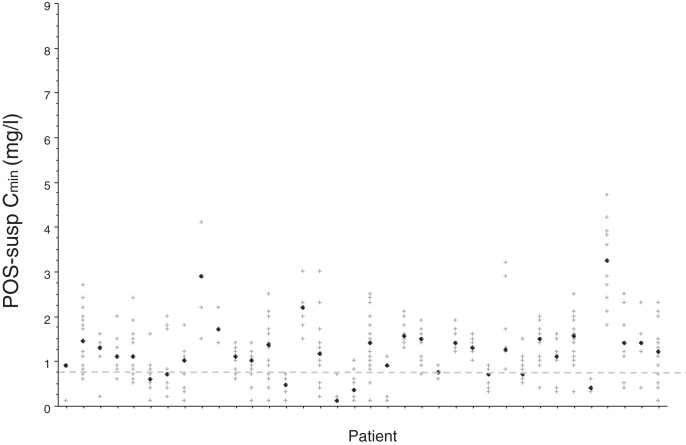
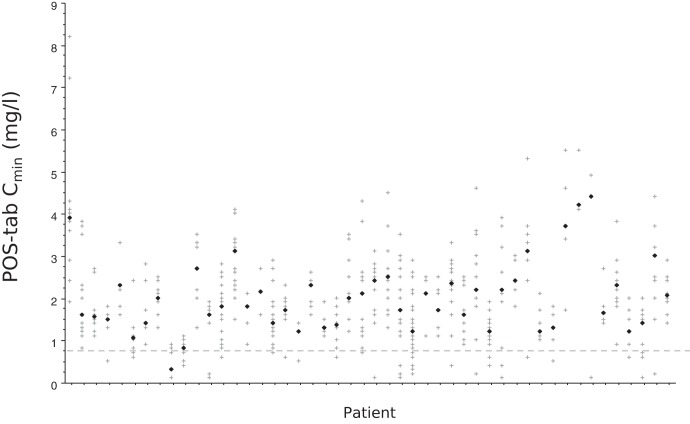
There was a large range of POS Cmin among patients (Fig. 2 and 3), which translated into high intra- and interindividual variability of POS Cmin/dose (D), regardless of the galenic formulation (Table 2). CVs and within-subject CVs of POS-tab Cmin/D were slightly but not significantly lower than those of POS-susp (Table 2). Intra- and interindividual variability was not significantly associated with age, BMI, mucositis, diarrhea, or treatment with a PPI (Tables S1 and S2).
FIG 2.
Variability of plasma posaconazole (POS) trough concentration (Cmin) in 36 patients treated with POS suspension. Each vertical series of crosses corresponds to repetitive POS trough concentrations in one patient, with the black diamond indicating the median POS Cmin per patient. The dotted line indicates the prophylactic threshold of POS Cmin (5).
FIG 3.
Variability of plasma posaconazole (POS) trough concentration (Cmin) in 48 patients treated with POS tablet. Each vertical series of crosses corresponds to repetitive POS trough concentrations in one patient, with the black diamond indicating the median POS Cmin per patient. The dotted line indicates the prophylactic threshold of POS Cmin (5).
Onset of invasive aspergillosis.
Invasive aspergillosis (IA) occurred in three patients treated with POS-tab (3/48) and four treated with POS-susp (4/36). The characteristics of these patients and their POS Cmin are shown in the supplemental material (Table S3). The mean POS Cmin per patient before IA diagnosis was 2.3 mg/liter for patients treated with POS-tab and 0.65 mg/liter for those treated with POS-susp.
DISCUSSION
Retrospective analysis of 874 POS Cmin measured in 77 AHSCT patients demonstrates that the POS tablet formulation results in higher POS exposure than the POS oral suspension, without reducing either inter- or intraindividual variability of the POS Cmin.
Enhanced exposure with posaconazole tablets.
POS Cmin determined during longitudinal TDM of AHSCT patients were significantly higher during POS-tab treatment than those obtained with POS-susp, in accordance with the results of a phase III study of POS-tab (4) and several other retrospective studies (2, 3, 6, 11, 13, 16). Logically, this increased POS exposure resulted in a higher percentage of therapeutic POS Cmin with POS-tab but did not reduce the number of POS dose adjustments. The magnitude of the increase in POS Cmin that we observed (mean increase of 0.6 mg/liter, which represents a 1.5-fold increase) for POS-tab is close to that previously reported by Pham et al. (1.4-fold increase) (6) and Jung et al. (2.5-fold increase) (2).
Determinants of posaconazole trough concentrations.
Among the potential determinants of POS Cmin, we explored diarrhea, mucositis, age, BMI, and concomitant treatment with a PPI. We found a significant impact of diarrhea, which decreased the POS Cmin in both univariate and multivariate analyses. This finding is in accordance with the previously described negative impact of diarrhea on POS-susp Cmin (7, 9) but also with several retrospective studies conducted in hematological patients treated with POS-tab (11, 12, 15). However, the impact of diarrhea appeared to be lower during POS-tab therapy than POS-susp treatment (Figure S2), even if the interaction did not reach statistical significance. The lower magnitude of the effect of diarrhea with the tablet might explain why other studies did not find an influence of diarrhea on POS-tab Cmin (13, 16). However, other factors could be involved, such as heterogeneous study populations, i.e., lung-transplant recipients and hematological patients (16) or hematological patients without a distinction between allografted or nonallografted patients (13).
In addition, we found a positive association between age and POS Cmin in univariate analyses, which is in accordance with previous studies performed in hematological patients treated with POS-susp (7, 17) or POS-tab (18). However, this association did not reach significance in multivariate analyses, suggesting that the influence of age on POS Cmin was dependent on another variable in our cohort. Conversely, we found no effect of BMI, mucositis, or PPI cotreatment on the POS Cmin, whereas several studies did (3, 11, 19). However, these studies were not all comparable in terms of sample size and patient inclusion criteria, which could explain, at least in part, these discrepancies.
Variability of posaconazole trough concentrations.
The originality of our study consists of the comparison of intra- and interindividual variability of POS Cmin/D for both galenic formulations. As dose adjustments occurred during longitudinal TDM (Table 1 and 2), we adjusted the POS Cmin for the POS dose to overcome this factor of variability. Although the interindividual CV and within-subject CV of the POS Cmin/D were lower for the POS-tab than for the POS-susp (Table 2 and Fig. 2 and 3), the difference was small and neither clinically relevant nor statistically significantly different, suggesting that the variability of POS-tab Cmin is still as large as that of POS-susp Cmin (20). Aside from one study, which reported lower POS Cmin variability with the tablet regimen in 24 lung-transplant patients (8), such a comparison of the intra- and interpatient variability of POS Cmin between the two oral galenic formulations of POS has never been performed. Several studies reported CVs for the POS-tab Cmin, but comparing CV values is problematic since calculation methods of CV differ. Here, we used the method described by Bland (21) to calculate within-subject CV, whereas a different approach was used in other studies, i.e., the ratio between standard error and the mean POS Cmin in each patient (18, 22). Finally, as explained above, we adjusted the POS Cmin for the POS dose in our cohort, which was not done in the other studies (18, 22). Moreover, differences in study populations may also account for the differences observed between the studies. For example, the within-subject CV of the POS-tab Cmin was 39.7% in AHSCT patients in our study, whereas it was estimated to be 16% in 24 lung transplant recipients and 48% in 15 patients with hematological malignancies (18). Despite the significant variability of POS Cmin, we did not identify any variable associated with such variability in our cohort, suggesting that factors other than those studied here, such as genetic variants of UGT1A4 (23) or reduced hepatic function, still need to be investigated.
Efficacy of posaconazole.
Finally, the central question is probably the efficacy of POS-tab relative to that of POS-susp. In our study, we could not perform any statistical analysis to compare the number of treatment failures between groups, because episodes of IA were very rare during POS therapy, regardless of the galenic formulation used. In addition, the retrospective design of our study and heterogeneity in follow-up enhance the risk of bias. To date, only one study has demonstrated a lower number of IA episodes during treatment with POS-tab than POS-susp (15), whereas others have reported similar rates of invasive fungal infections between the two galenic formulations (3, 13, 24, 25). In our cohort, we reported treatment failure in 6.3% of patients receiving POS-tab (3/48), although all these patients had a therapeutic POS Cmin. Such findings have already been described for POS-susp (13, 18), which suggests that some therapeutic failures may not be related to insufficient POS exposure.
Limitations.
Our study had several limitations. First, this study was retrospective and performed in a single center. Thus, some data were missing, especially for potential factors influencing the POS-tab Cmin (allograft-versus-host disease, PPI, weight, and gradation of diarrhea and mucositis). In the same vein, POS safety was not evaluated, while high POS Cmin could be associated with occurrence of adverse events as recently described in several case reports (14, 18, 26, 27). Despite these limitations, our study also had several strengths, since it is notably the first study to compare the variability of the POS Cmin between POS-susp and POS-tab. In addition, our study was conducted in a homogeneous and large population of recipients of allograft stem-cell transplantation (77 patients and 874 POS Cmin), which is not true of several previous studies (3, 11, 12, 16).
Conclusion.
Although the tablet formulation increased POS exposure, variability of the POS Cmin was still high and not significantly lower than that observed with the oral suspension. Therefore, these results suggest that TDM may still be useful for AHSCT patients treated with POS-tab.
MATERIALS AND METHODS
Study design.
This retrospective study was conducted at the Grenoble University Hospital, France. Adult (>18 years old) AHSCT patients with graft-versus-host disease, treated with POS according to the European Conference on Infections in Leukemia 6 guidelines (5), with longitudinal TDM (defined by at least three POS Cmin determinations per patient and per galenic form) from January 2014 to December 2016 were eligible (see Fig. 1 for details). Demographic (age, sex) and clinical data (diarrhea, mucositis, and weight), as well as records concerning POS therapy (daily dose, galenic form, and POS Cmin) and concomitant PPI treatment were collected retrospectively. Concerning the factors that could potentially affect the POS Cmin, we considered only those that are concomitant to the determination of the POS Cmin. For clinical events (diarrhea, mucositis), we considered only those that occurred within 7 days before the POS Cmin determination. Similarly, only PPI therapy initiated at least 7 days before measuring the POS Cmin was considered. Due to the retrospective design of our study, some data are missing, especially for potential determinants of the POS-tab Cmin (see Fig. 1 for details). Concerning comedications, only PPIs were considered to be a risk factor in our model, but the absence of any other drug that could interact with POS was carefully checked. All patients gave written consent for collection and use of their data.
Posaconazole therapeutic drug monitoring.
All patients received POS for prophylaxis of invasive fungal infection. Patients treated with POS-susp were asked to take their POS with an acidic beverage to improve POS absorption. All patients treated with POS received regular POS TDM. Blood samples were drawn just before drug intake or at least 20 h after intake for the POS-tab. After treatment initiation or dose adjustment, a 7-day period was considered necessary to obtain a pharmacokinetic steady state. POS Cmin were excluded in case of inappropriate blood collection time (sample handled before pharmacokinetic steady state or less than 20 h after POS-tab intake) (see Fig. 1 for details). Plasma POS Cmin were measured by validated liquid chromatography tandem-mass spectrometry, as previously described (28). The plasma drug standard curve ranged from 0.1 to 20 mg/liter with adequate between-day and within-day variabilities (CV < 15%) and accuracy. A therapeutic threshold of 0.7 mg/liter defined for prophylaxis was considered (5).
Evaluation of efficacy.
The efficacy of POS was assessed by the absence of invasive fungal infections (especially infections due to Aspergillus spp.) during the follow-up period for each patient. The follow-up period was defined as the time elapsed between the initiation of POS therapy and the last determination of the POS Cmin. All cases were prospectively reviewed monthly by a multidisciplinary team including chest physicians, a microbiologist, a hygienist, and a pharmacologist (29). Cases were classified according to guidelines from the European Organization for Research and Treatment of Cancer/European Invasive Infections Cooperative Group and the criteria of the National Institute of Allergy and Infectious Diseases-Mycoses Study Group (EORTC/MSG) (30).
Statistical analysis.
Categorical variables are expressed as frequencies (and percentages) and quantitative variables, as medians (with the 25th to 75th percentiles). The analysis of POS exposure, depending on galenic formulation or potential determinants, was performed for the POS Cmin, whereas the study of variability was performed on the POS Cmin adjusted for dose (Cmin/D) to overcome the influence of dosage adjustments occurring during longitudinal follow-up. CVs and within-subject CVs were calculated as previously described (21) for each POS formulation to assess inter- and intraindividual variability, respectively. They were subsequently compared using a method adapted from Levene’s test. As some patients received both formulations, we used mixed effects models, in which the formulation was a fixed factor and the patient a random factor. We performed a log transformation of the Cmin/D ratio because the data were not normally distributed. A P value of 0.05 was considered statistically significant. Statistical analyses were performed with SPSS Statistics version 21 (IBM, Armonk, NY, USA).
Supplementary Material
ACKNOWLEDGMENTS
We thank Karine Scalabrino, Cécile Pistone-Girard, and Mélanie Davallet-Pin for their excellent technical assistance.
This study was financed by a grant from MSD.
We declare no conflict of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00484-19.
REFERENCES
- 1.Merck Sharp & Dohme Limited. 2012. Noxafil (posaconazole) oral suspension, summary of product characteristics. Merck Sharp & Dohme Limited, Hertfordshire, United Kingdom: https://www.ema.europa.eu/en/documents/product-information/noxafil-epar-product-information_en.pdf. [Google Scholar]
- 2.Jung DS, Tverdek FP, Kontoyiannis DP. 2014. Switching from posaconazole suspension to tablets increases serum drug levels in leukemia patients without clinically relevant hepatotoxicity. Antimicrob Agents Chemother 58:6993–6995. doi: 10.1128/AAC.04035-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cumpston A, Caddell R, Shillingburg A, Lu X, Wen S, Hamadani M, Craig M, Kanate AS. 2015. Superior serum concentrations with posaconazole delayed-release tablets compared to suspension formulation in hematological malignancies. Antimicrob Agents Chemother 59:4424–4428. doi: 10.1128/AAC.00581-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornely OA, Duarte RF, Haider S, Chandrasekar P, Helfgott D, Jiménez JL, Candoni A, Raad I, Laverdiere M, Langston A, Kartsonis N, Van Iersel M, Connelly N, Waskin H. 2016. Phase 3 pharmacokinetics and safety study of a posaconazole tablet formulation in patients at risk for invasive fungal disease. J Antimicrob Chemother 71:718–726. doi: 10.1093/jac/dkv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tissot F, Agrawal S, Pagano L, Petrikkos G, Groll AH, Skiada A, Lass-Flörl C, Calandra T, Viscoli C, Herbrecht R. 2017. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica 102:433–444. doi: 10.3324/haematol.2016.152900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pham AN, Bubalo JS, Lewis JS. 2016. Comparison of posaconazole serum concentrations from haematological cancer patients on posaconazole tablet and oral suspension for treatment and prevention of invasive fungal infections. Mycoses 59:226–233. doi: 10.1111/myc.12452. [DOI] [PubMed] [Google Scholar]
- 7.Tonini J, Thiébaut A, Jourdil JF, Berruyer AS, Bulabois CE, Cahn JY, Stanke-Labesque F. 2012. Therapeutic drug monitoring of posaconazole in allogeneic hematopoietic stem cell transplantation patients who develop gastrointestinal graft-versus-host disease. Antimicrob Agents Chemother 56:5247–5252. doi: 10.1128/AAC.00815-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stelzer D, Weber A, Ihle F, Matthes S, Ceelen F, Zimmermann G, Kneidinger N, Schramm R, Winter H, Zoller M, Vogeser M, Behr J, Neurohr C. 2018. Posaconazole liquid vs tablet formulation in lung transplant recipients. Mycoses 61:186–194. doi: 10.1111/myc.12724. [DOI] [PubMed] [Google Scholar]
- 9.Dolton MJ, Ray JE, Chen SCA, Ng K, Pont L, McLachlan AJ. 2012. Multicenter study of posaconazole therapeutic drug monitoring: exposure-response relationship and factors affecting concentration. Antimicrob Agents Chemother 56:5503–5510. doi: 10.1128/AAC.00802-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kraft WK, Chang PS, van Iersel LPS, Waskin H, Krishna G, Kersemaekers WM. 2014. Posaconazole tablet pharmacokinetics: lack of effect of concomitant medications altering gastric pH and gastric motility in healthy subjects. Antimicrob Agents Chemother 58:4020–4025. doi: 10.1128/AAC.02448-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miceli MH, Perissinotti AJ, Kauffman CA, Couriel DR. 2015. Serum posaconazole levels among haematological cancer patients taking extended release tablets is affected by body weight and diarrhoea: single centre retrospective analysis. Mycoses 58:432–436. doi: 10.1111/myc.12339. [DOI] [PubMed] [Google Scholar]
- 12.Tang LA, Marini BL, Benitez L, Nagel JL, Miceli M, Berglund C, Perissinotti AJ. 2017. Risk factors for subtherapeutic levels of posaconazole tablet. J Antimicrob Chemother 72:2902–2905. doi: 10.1093/jac/dkx228. [DOI] [PubMed] [Google Scholar]
- 13.Lenczuk D, Zinke-Cerwenka W, Greinix H, Wölfler A, Prattes J, Zollner-Schwetz I, Valentin T, Lin TC, Meinitzer A, Hoenigl M, Krause R. 2018. Antifungal prophylaxis with posaconazole delayed-release tablet and oral suspension in a real-life setting: plasma levels, efficacy, and tolerability. Antimicrob Agents Chemother 62:e02655-17. doi: 10.1128/AAC.02655-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeong W, Snell GI, Levvey BJ, Westall GP, Orla Morrissey C, Wolfe R, Ivulich S, Neoh CF, Slavin MA, Kong DCM. 2018. Single-centre study of therapeutic drug monitoring of posaconazole in lung transplant recipients: factors affecting trough plasma concentrations. J Antimicrob Chemother 73:748–756. doi: 10.1093/jac/dkx440. [DOI] [PubMed] [Google Scholar]
- 15.Leclerc E, Combarel D, Uzunov M, Leblond V, Funck-Brentano C, Zahr N. 2018. Prevention of invasive Aspergillus fungal infections with the suspension and delayed-release tablet formulations of posaconazole in patients with haematologic malignancies. Sci Rep 8:1681. doi: 10.1038/s41598-018-20136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chin A, Pergam SA, Fredricks DN, Hoofnagle AN, Baker KK, Jain R. 2017. Evaluation of posaconazole serum concentrations from delayed-release tablets in patients at high risk for fungal infections. Antimicrob Agents Chemother 61:e00569-17. doi: 10.1128/AAC.00569-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohl V, Müller C, Cornely OA, Abduljalil K, Fuhr U, Vehreschild JJ, Scheid C, Hallek M, Rüping MJGT. 2010. Factors influencing pharmacokinetics of prophylactic posaconazole in patients undergoing allogeneic stem cell transplantation. Antimicrob Agents Chemother 54:207–212. doi: 10.1128/AAC.01027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tverdek FP, Heo ST, Aitken SL, Granwehr B, Kontoyiannis DP. 2017. Real-life assessment of the safety and effectiveness of the new tablet and intravenous formulations of posaconazole in the prophylaxis of invasive fungal infections via analysis of 343 courses. Antimicrob Agents Chemother 61:e00188-17. doi: 10.1128/aac.00188-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vanstraelen K, Prattes J, Maertens J, Lagrou K, Schoemans H, Peersman N, Vermeersch P, Theunissen K, Mols R, Augustijns P, Annaert P, Hoenigl M, Spriet I. 2016. Posaconazole plasma exposure correlated to intestinal mucositis in allogeneic stem cell transplant patients. Eur J Clin Pharmacol 72:953–963. doi: 10.1007/s00228-016-2057-6. [DOI] [PubMed] [Google Scholar]
- 20.Dolton MJ, Brüggemann RJM, Burger DM, McLachlan AJ. 2014. Understanding variability in posaconazole exposure using an integrated population pharmacokinetic analysis. Antimicrob Agents Chemother 58:6879–6885. doi: 10.1128/AAC.03777-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bland M. 2000. An introduction to medical statistics, p 269–272. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 22.Stelzer D, Weber A, Ihle F, Matthes S, Ceelen F, Zimmermann G, Kneidinger N, Schramm R, Winter H, Zoller M, Vogeser M, Behr J, Neurohr C. 2017. Comparing azole plasma trough levels in lung transplant recipients: percentage of therapeutic levels and intrapatient variability. Ther Drug Monit 39:93–101. doi: 10.1097/FTD.0000000000000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suh HJ, Yoon SH, Yu KS, Cho JY, Park SI, Lee E, Lee JO, Koh Y, Song KH, Choe PG, Kim ES, Bang SM, Kim HB, Kim I, Kim NJ, Song SH, Park WB, Oh M. d. 2018. The genetic polymorphism UGT1A4*3 is associated with low posaconazole plasma concentrations in hematological malignancy patients receiving the oral suspension. Antimicrob Agents Chemother 62:e02230-17. doi: 10.1128/AAC.02230-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belling M, Kanate AS, Shillingburg A, Lu X, Wen S, Shah N, Craig M, Cumpston A. 2017. Evaluation of serum posaconazole concentrations in patients with hematological malignancies receiving posaconazole suspension compared to the delayed-release tablet formulation. Leuk Res Treatment 2017:1–6. doi: 10.1155/2017/3460892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furuno JP, Tallman GB, Noble BN, Bubalo JS, Forrest GN, Lewis JS, Bienvenida AF, Holmes CA, Weber BR, McGregor JC. 2018. Clinical outcomes of oral suspension versus delayed-release tablet formulations of posaconazole for prophylaxis of invasive fungal infections. Antimicrob Agents Chemother 62:e00893-18. doi: 10.1128/AAC.00893-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foolad F, Kontoyiannis DP. 2018. Persistent CNS toxicity in a patient receiving posaconazole tablets after discontinuation of voriconazole due to supratherapeutic serum levels. J Antimicrob Chemother 73:256–258. doi: 10.1093/jac/dkx362. [DOI] [PubMed] [Google Scholar]
- 27.Parkes LO, Cheng MP, Sheppard DC. 2016. Visual hallucinations associated with high posaconazole concentrations in serum. Antimicrob Agents Chemother 60:1170–1171. doi: 10.1128/AAC.02739-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jourdil J-F, Tonini J, Stanke-Labesque F. 2013. Simultaneous quantitation of azole antifungals, antibiotics, imatinib, and raltegravir in human plasma by two-dimensional high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 919–920:1–9. doi: 10.1016/j.jchromb.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 29.Garnaud C, Brenier-Pinchart MP, Thiebaut-Bertrand A, Hamidfar R, Quesada JL, Bosseray A, Lebeau B, Mallaret MR, Maubon D, Saint-Raymond C, Pinel C, Hincky V, Plantaz D, Cornet M, Pelloux H. 2012. Seven-year surveillance of nosocomial invasive aspergillosis in a French University Hospital. J Infect 65:559–567. doi: 10.1016/j.jinf.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 30.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Muñoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG). Clin Infect Dis 46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.