The carbapenem-hydrolyzing class D β-lactamases (CHDLs) are the main mechanism of carbapenem resistance in Acinetobacter baumannii. CHDLs are not effectively inactivated by clinically available β-lactam-type inhibitors. We have previously described the in vitro efficacy of the inhibitor LN-1-255 in combination with carbapenems.
KEYWORDS: Acinetobacter baumannii, animal models, antimicrobial agents, beta-lactamase inhibitor, class D carbapenemases, preclinical drug studies
ABSTRACT
The carbapenem-hydrolyzing class D β-lactamases (CHDLs) are the main mechanism of carbapenem resistance in Acinetobacter baumannii. CHDLs are not effectively inactivated by clinically available β-lactam-type inhibitors. We have previously described the in vitro efficacy of the inhibitor LN-1-255 in combination with carbapenems. The aim of this study was to compare the efficacy of LN-1-255 with that of imipenem in murine pneumonia using A. baumannii strains carrying their most extended carbapenemases, OXA-23 and OXA-24/40. The blaOXA-23 and blaOXA-24/40 genes were cloned into the carbapenem-susceptible A. baumannii ATCC 17978 strain. Clinical isolates Ab1 and JC12/04, producing the enzymes OXA-23 and OXA-24/40, respectively, were used in the study. Pharmacokinetic (PK) parameters were determined. An experimental pneumonia model was used to evaluate the efficacy of the combined imipenem–LN-1-255 therapy. MICs of imipenem decreased between 32- and 128-fold in the presence of LN-1-255. Intramuscular treatment with imipenem–LN-1-255 (30/50 mg/kg) decreased the bacterial burden by (i) 4 and 1.7 log10 CFU/g lung in the infection with the ATCC 17978-OXA-23 and Ab1 strains, respectively, and by (ii) 2.5 and 4.5 log10 CFU/g lung in the infection produced by the ATCC 17978-OXA-24/40 and the JC12/04 strains, respectively. In all assays, combined therapy offered higher protection against pneumonia than that provided by monotherapy. No toxicity was observed in treated mice. Imipenem treatment combined with LN-1-255 treatment significantly reduced the severity of infection by carbapenem-resistant A. baumannii strains carrying CHDLs. Preclinical assays demonstrated the potential of LN-1-255 and imipenem therapy as a new antibacterial treatment.
INTRODUCTION
Acinetobacter baumannii is a nosocomial pathogen that frequently acquires and easily develops mechanisms of resistance to multiple antibiotics, which are associated with an increased risk of clinical failure, prolonging the lengths of hospital stays and therefore increasing health care costs (1). Drug-resistant infections are currently responsible for more than half a million deaths worldwide each year. It is expected that, by 2050, antimicrobial resistance will have caused the death of more than ten million people, and it is projected that related health care costs worldwide will be greater than $300 million per year (2). A. baumannii is considered one of the most threatening opportunistic pathogens for global health, and it has been included in a “critical priority” category by the World Health Organization (3).
The β-lactams are the most relevant family of antibiotics. In particular, carbapenems are extensively used for multidrug-resistant (MDR) pathogens such as species in the genus Acinetobacter, particularly A. baumannii (4, 5). Consequently, the production of β-lactamase enzymes is the most relevant mechanism of antibiotic inactivation through the cleavage of the β-lactam ring. The carbapenem-hydrolyzing class D β-lactamases (CHDLs), such as OXA-23 or OXA-24/40, are the most important carbapenemases produced by A. baumannii (6–10). The spread of OXA carbapenemases in the last decade is seriously compromising the use of these antibiotics, threatening the sustainability of carbapenem efficacy against A. baumannii. Few real alternatives against this pathogen have been commercialized in the last two decades.
Drug discovery has led to the development of β-lactamase inhibitors and to the rescue of failing β-lactams, combating the clinical challenge given by antimicrobial resistance. Over the last years, an increasing number of inhibitors have been developed, including avibactam, relebactam, and several boronic acid β-lactamase inhibitors (11). These new inhibitors present excellent activity against class A and class C β-lactamases and against many carbapenemases. It has been demonstrated that OXA-48 from Enterobacteriaceae can be inactivated by avibactam, which, in combination with ceftazidime, ceftaroline or aztreonam, is a very interesting option to treat infections caused by bacteria carrying this enzyme (12). However, the CHDLs of A. baumannii are not effectively inactivated by the clinically available β-lactam-type inhibitors and remain nonsusceptible to the new inhibitor combinations (11, 13). There is a shortage of relevant literature that describes candidates for new inhibitors of the OXA carbapenemases of A. baumannii. Thus, the treatment of Acinetobacter sp. isolates expressing CHDLs is an increasingly relevant clinical problem (1, 14), and the development of efficient inhibitors is needed to preserve the efficacy of carbapenems (15).
Our group has previously demonstrated that a series of β-lactam derivatives, substituted penicillin sulfones, present an exceptional potency against the Acinetobacter sp. CHDL OXA-24/40. Within a series of developed compounds, the compound LN-1-255 presented affinity in the nM range for all of the A. baumannii CHDLs tested, including the relevant OXA-23, OXA-24/40, OXA-58, and OXA-143 CHDLs (10, 16–18). The combination of carbapenems with LN-1-255 led to an important decrease in the MICs of these antibiotics (10). Similarly, this inhibitor also demonstrated good inhibitory activity against the CHDL OXA-48, as assessed by both kinetic and microbiological studies (10, 17). Other OXA or class A noncarbapenemase β-lactamases are also inhibited by this compound (19).
Since the inhibition potency of LN-1-255 against the major representative enzymes of all CHDL families described to date in A. baumannii has been previously demostrated by our group (10), in the present study we propose a new antimicrobial strategy. Therefore, the objective of this study is the evaluation of a new therapy combining imipenem and LN-1-255 for the treatment of an experimental murine pneumonia model caused by carbapenem-resistant A. baumannii transformants and clinical isolates carrying CHDLs.
RESULTS
Antimicrobial susceptibility assays.
In vitro susceptibility to a combined therapy of imipenem and LN-1-255 was compared to susceptibility to imipenem alone. All transformants studied were imipenem resistant. As previously described, the inhibitor was able to reduce imipenem MICs by between 8- and 32-fold at 4 mg/liter and between 32- and 128-fold at 16 mg/liter (Table 1). Data indicated that a clear synergy occurred when imipenem was combined with LN-1-255. No antimicrobial activity was observed with LN-1-255 alone (MIC > 512 mg/liter).
TABLE 1.
Imipenem susceptibility profile
| Laboratory strain or clinical isolate | MIC (mg/liter) fora
: |
||
|---|---|---|---|
| Imipenem | Imipenem + LN-1-255 (4 mg/liter) | Imipenem + LN-1-255 (16 mg/liter) | |
| A. baumannii ATCC 17978 | 0.5 | 0.5 | 0.5 |
| A. baumannii ATCC 17978 + pET-RA/OXA-23 | 16 | 2 | 0.5 |
| A. baumannii Ab1(OXA-23) | 32 | 4 | 1 |
| A. baumannii ATCC 17978 + pET-RA/OXA-24/40 | 64 | 2 | 0.5 |
| A. baumannii JC12/04 (OXA-24/40) | 32 | 2 | 0.5 |
MICs for LN-1-255 were >512 mg/liter for all tested A. baumannii strains.
blaOXA-51-like expression analysis.
Reverse transcription-quantitative PCR (qRT-PCR) analyses were performed in order to investigate the effect of a possible overexpression of the chromosomal OXA-51-like β-lactamase of the A. baumannii strains, which could sequester the inhibitor during treatments. Data revealed that blaOXA-51-like was not overexpressed in any of the tested strains. Taking the relative expression of the blaOXA-51-like gene in the ATCC 17978 strain as 1, the expression of this gene in ATCC 17978+pET-RA/OXA-23, ATCC 17978+pET-RA/OXA-24/40, Ab1, and JC12/04 strains was 0.86, 0.91, 0.41, and 0.57, respectively. Therefore, a similar expression pattern of the blaOXA-51-like gene was observed in the studied strains, and no overexpression was detected in any case.
Efficacy of the treatment.
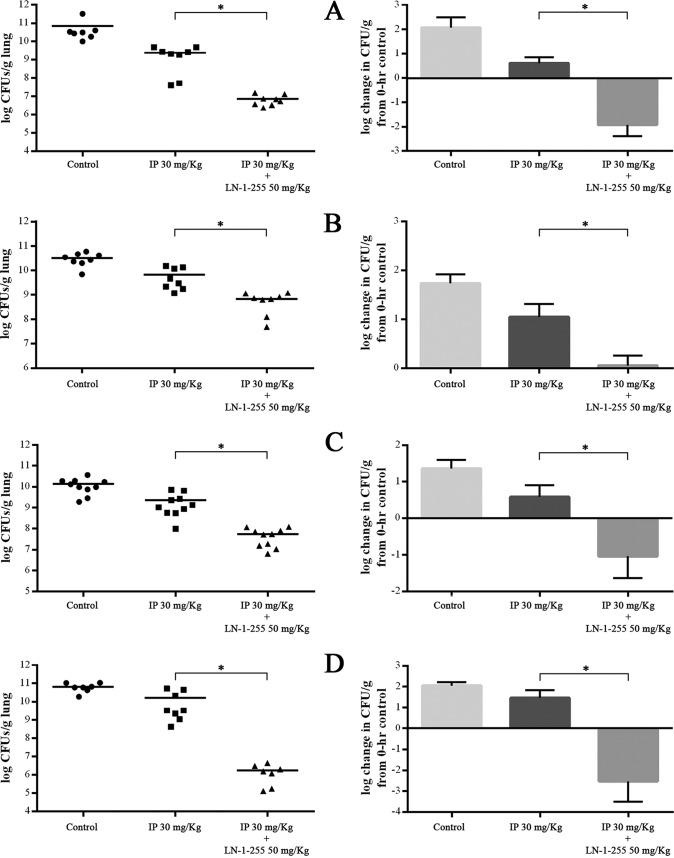
The 100% lethal dose (LD100) used in the pneumonia models was 2.1 × 107 CFU/mouse for ATCC 17978 carrying pET-RA/OXA-24/40, 0.7 × 107 CFU/mouse for JC12/04, 2.9 × 107 CFU/mouse for ATCC 17978 carrying pET-RA/OXA-23, and 3.2 × 107 CFU/mouse for Ab1. After treatment, bacterial concentration in the lungs was consistently reduced when the inhibitor LN-1-255 was used intramuscularly at 50 mg/kg (Fig. 1, Table 2). Although all strains used that harbored CHDLs were imipenem resistant, the treatment with imipenem led to a slight decrease of the bacterial load in the infected lungs compared to that in the untreated control groups (0.5 to 1.5 log10 CFU/g lung). However, more interestingly, the treatment with imipenem and LN-1-255 decreased the bacterial load by 4 log10 CFU/g lung and 1.7 log10 CFU/g lung in the infections caused by the ATCC 17978 strain carrying pET-RA/OXA-23 and by the clinical isolate Ab1, respectively, showing statistical differences from the results obtained in imipenem monotherapy (P < 0.02). Conversely, the combined treatment with imipenem and LN-1-255 decreased the bacterial load by 2.5 log10 CFU/g lung and 4.5 log10 CFU/g lung in the infections caused by the ATCC 17978 strain carrying pET-RA/OXA-24/40 and by the clinical isolate JC12/04, respectively, and it also showed statistical differences from the imipenem treatment (P < 0.01).
FIG 1.
Effect of the combined therapy with imipenem and LN-1-255 on the clearance of A. baumannii strains in mouse lung. Left, each value and means of total counts in lungs are reflected. Right, values are means, and bars indicate standard deviation of total counts related to the bacterial load of the infected control mice at 0 h. (A) A. baumannii ATCC 17978 + pET-RA/OXA-23. (B) A. baumannii Ab1. (C) A. baumannii ATCC 17978 + pET-RA/OXA-24/40. (D) A. baumannii JC12/04. Student’s t test was used to validate the experimental data (*, P < 0.05).
TABLE 2.
Effects of monotherapy and combined therapy on the clearance of strains from mouse lungs
| A. baumannii strain | Bacterial load (mean log10CFU/g lung ± SEM) |
Decrease in bacterial load compared with that of nontreated mice (log10CFU/g lung) |
|||
|---|---|---|---|---|---|
| Nontreated | Imipenem | Imipenem + LN-1-255 | Imipenem | Imipenem + LN-1-255 | |
| ATCC 17978 + pET-RA/OXA-23 | 10.6 ± 4.5 | 9.1 ± 2.4 | 6.6 ± 1.4 | 1.5 | 4.0 |
| Ab1 (OXA-23) | 10.3 ± 1.8 | 9.6 ± 2.6 | 8.6 ± 2.1 | 0.7 | 1.7 |
| ATCC 17978 + pET-RA/OXA-24/40 | 10.0 ± 2.3 | 9.2 ± 3.1 | 7.5 ± 1.8 | 0.8 | 2.5 |
| JC12/04 (OXA-24/40) | 10.7 ± 1.9 | 10.2 ± 3.6 | 6.2 ± 1.7 | 0.5 | 4.5 |
Other intramuscular dosages of LN-1-255 (20 and 150 mg/kg) in combination with imipenem also reduced the bacterial load of lungs infected by clinical isolates and transformants carrying OXA-23 and OXA-24/40 compared to that when treated with imipenem alone. However, none of these dosages presented a higher bacterial clearance than the dosage of 50 mg/kg. Similarly, neither the intraperitoneal nor subcutaneous routes improved on the results obtained using the intramuscular route (data not shown).
No antimicrobial activity was observed when LN-1-255 was used alone; this inhibitor in treatments by itself did not decrease the bacterial load compared to that of the untreated groups. When imipenem combined with LN-1-255 was used to treat the infection caused by the imipenem-susceptible parental strain ATCC 17978 A. baumannii, no increment of efficacy was observed; the use of imipenem–LN-1-255 did not decrease the bacterial load compared with that in the control treatment (imipenem alone; data not shown).
Pharmacokinetic parameters in mice.
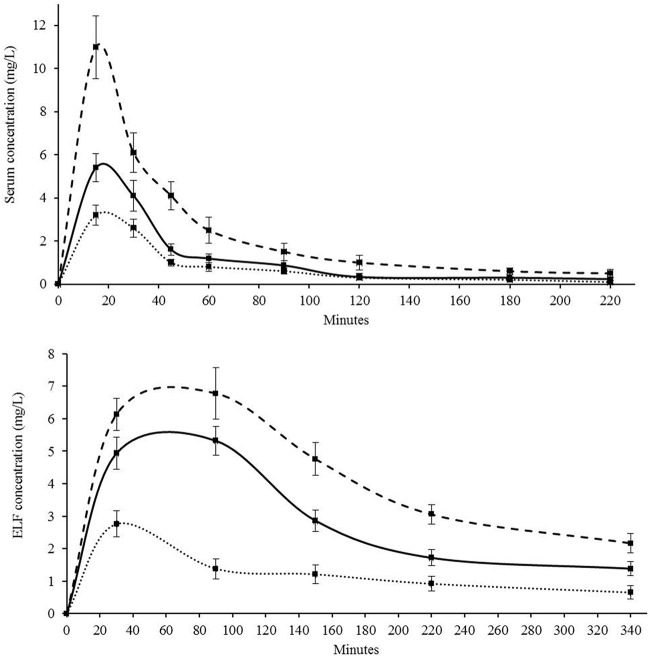
Dosages of 20, 50, and 150 mg/kg of LN-1-255 inoculated intramuscularly were tested for pharmacokinetic activity. Pharmacokinetic curves of the inhibitor serum and epithelial lining fluid (ELF) concentration are shown in Fig. 2 and Table 3. The inhibitor maximum concentration of drug in serum (Cmax) obtained in serum and ELF at 50 mg/kg were 5.4 and 5.6 mg/liter, showing a half-life (t1/2) of 22 and 98 min, respectively. For this dosage, the area under the concentration-time curve (AUC) was also better in ELF (4.1 mg · h/liter) than in blood (2.8 mg · h/liter). Pharmacokinetic (PK) parameters were lower at 20 mg/kg than at 50 mg/kg. However, PK results were slightly more favorable at 150 mg/kg, showing a Cmax value 20% higher in ELF than that for 50 mg/kg.
FIG 2.
Pharmacokinetics of LN-1-255 in mice after intramuscular administration measured in (a) serum and (b) ELF. Dotted line, 20 mg/kg; solid line, 50 mg/kg; dashed line, 150 mg/kg. Three replicates were carried out for each time point.
TABLE 3.
Pharmacokinetic parameters of LN-1-255 in blood and epithelial lining fluid
| Dosage (mg/kg) | Pharmacokinetic parameters fora: |
|||||
|---|---|---|---|---|---|---|
| Serum |
Lung |
|||||
| Cmax | t1/2 (min) | AUC (mg · h/liter) | Cmax | t1/2 (min) | AUC (mg · h/liter) | |
| 20 | 3.3 ± 0.45 | 21 ± 2 | 1.8 | 2.8 ± 0.43 | 58 ± 7 | 1.8 |
| 50 | 5.4 ± 0.65 | 22 ± 2 | 2.8 | 5.6 ± 0.48 | 98 ± 12 | 4.1 |
| 150 | 11.3 ± 1.45 | 16 ± 1 | 5.4 | 7.0 ± 0.81 | 135 ± 13 | 5.1 |
Cmax, maximum concentration of drug in serum; t1/2, half-life.
Using as reference an imipenem-susceptible strain of A. baumannii such as ATCC 17978 (MIC for imipenem, 0.12 mg/liter), the literature shows that the time above the MIC (T>MIC) parameter of imipenem in murine plasma using 30 mg/kg is ca. 2 h. Thus, if dosages were administered every 3 h in our study, about 66% of time during the treatment imipenem concentration in plasma would be over the MIC. For therapeutic success with carbapenems, it is necessary to reach a concentration over the MIC at least 40% of the time (20, 21). The T>MIC parameter for imipenem against the imipenem-resistant strains used in this study was minimal or nonexistent (MICs of 16 to 64 mg/liter). Thus, based on the pharmacokinetic/pharmacodynamic (PK/PD) data of LN-1-255, it was necessary to establish a pattern between doses that would allow reaching a compound concentration during the infection capable of inhibiting the carbapenemases and to decrease the MICs to imipenem as long as possible to obtain an appropriate T>MIC parameter. Using the intramuscular dosage for LN-1-55 of 50 mg/kg every 3 h and taking as reference an effective inhibitor concentration of 4 mg/liter (minimal effective concentration [MEC]), we can see in Fig. 2 that the time above the MEC (T>MEC) parameter of LN-1-255 is 1.81 h, ca. 60% of the treatment duration. This allowed us to match the T>MIC parameter of imipenem, previously described, with the T>MEC of LN-1-255 during the treatments, in order to maximize the synergy between both compounds.
Toxicity.
LN-1-255 was well tolerated in mice. No mortality was observed in the groups of uninfected mice treated with a single intramuscular dose of 350 mg/kg of LN-1-255 or in those groups which received six doses of 150 mg/kg every 3 h. Adverse effects such as muscle spasms, weight loss, or transitory movement disorders were not observed at the administered doses over 7 days.
DISCUSSION
The carbapenems remain the most-used antibiotics to treat A. baumannii infections (22). However, the isolation of carbapenem-resistant strains is increasing globally (23). Relevant efforts have been made to develop new β-lactamase inhibitors to recover the activity of β-lactams in species such as Pseudomonas aeruginosa or A. baumannii. The greatest advances have taken place in the development of non-β-lactam inhibitors, especially the diazabicyclooctanones (DBOs), such as avibactam (approved for clinical use) or relebactam (in the late stage of development), which are able to inhibit class A and class C β-lactamases (24). Avibactam also presents activity against the relevant carbapenemase OXA-48 but does not present any activity against CHDLs of A. baumannii (17, 25).
A quality leap forward was made on the development of DBOs when compounds with activity against class A, C, and D β-lactamases (including carbapenemases) were described. Recently, the new combination of sulbactam and ETX2514 has been able to restore the activity of sulbactam. The authors demonstrated its activity in a collection of A. baumannii clinical isolates and in a murine thigh model and a pneumonia model similar to the one presented in this work (26, 27). Other β-lactamases inhibitors include the boronates, such as vaborbactam (RPX7009), in combination with meropenem (phase IV). However, this inhibitor lacks activity against A. baumannii or P. aeruginosa (28, 29).
LN-1-255, a new β-lactamase inhibitor, is being developed to address growing carbapenemase resistance due to CHDLs. It has been previously shown to preserve imipenem activity against OXA-48-producing strains (17) and against the most relevant CHDLs of A. baumannii (10). Porin loss seems not to affect this inhibitor, probably due to its structural similarity with siderophores (17). The significant activity of LN-1-255 against CHDLs is mainly due to its excellent kinetics; it shows a very high affinity for these β-lactamases (inhibits the OXA carbapenemases with low Ki values in the nM range), and the turnover number (the time-dependent partitioning of the initial enzyme/inhibitor complex between hydrolysis and enzyme inactivation) was remarkable and similar for all of the CHDLs tested, 2-6 (10). The LN-1-255 inhibitor also demonstrated an excellent microbiological synergy and a significantly higher activity than tazobactam and avibactam against all tested CHDLs (OXA-23, OXA-24/40, OXA-58, OXA-143, OXA-235, and the chromosomally encoded OXA-51) (10). Analyzing the good results obtained in vitro and these new in vivo results, it is possible to hypothesize that this inhibitor could be used to treat the lung infections caused by all of the CHDL-carrying A. baumannii strains. Moreover, in vitro synergy was also observed between meropenem and LN-1-255, which could be another alternative to treat infections caused by carbapenem-resistant A. baumannii strains.
The pneumonia model was chosen in this study because the majority of A. baumannii strains are isolated from the respiratory tract of hospitalized patients, this type of nosocomial infection being the most predominant caused by this pathogen (1). Usually the dose regimen for treatment is calculated to obtain serum concentrations of imipenem above the MIC (T>MIC) during 35 to 40% of the interval between doses (30, 31), which means approximately 3 h against imipenem-susceptible strains. In this study, we have used imipenem-resistant strains, which do not meet the criteria when a “standard” imipenem dosage is used, and thus the regimen of imipenem 30 mg/kg in monotherapy will result in a therapeutic failure. Imipenem dosage at 30 mg/kg reaches a Cmax in serum of 16.9 to 26.7 mg/liter (20, 21), minimally decreasing the bacterial load in lungs of the imipenem-resistant strains used in this study (MICs, 16 to 64 mg/liter). Previous literature has defined the Cmax value in plasma only, so there is no information for murine lungs. The plasma Cmax of imipenem is similar or slightly lower than the MICs of used strains (1 to 2 dilutions), which may produce the slight observed decrease in bacterial load (0.5 to 1.5 log10 CFU/g lung), along with the immune response of nonimmunosuppressed mice. With LN-1-255 in combined therapy with imipenem, we have tried to recover the effectiveness of the standard regimen, and thus, finally, the interval between drug doses was 3 h and an intraperitoneal dosage of 50 mg/kg of LN-1-255 was used to avoid therapeutic failure. The other concentrations and routes tested were less effectiveness at decreasing the bacterial load of infected lungs. The Cmax of LN-1-255 at 50 mg/kg obtained in mice lungs was 5.6 mg/liter, which was enough to decrease by 1.7 to 4.5 log10 the bacterial load in pneumonia when the inhibitor was used in combination with imipenem. In vitro assays showed a clear synergy when the inhibitor was used at 4 mg/liter against class D carbapenemase-producing A. baumannii strains in this and previously published studies (10), a concentration that was maintained for 60% of the time between doses in this study.
The intramuscular administration of 50 mg/kg of LN-1-255 reached in ELF a Cmax and T>MEC about 20% and 30% lower, respectively, than those obtained using 150 mg/kg. However, these apparently better PK/PD parameters obtained with the dosage of 150 mg/kg did not increase the clearance in lungs during the infection. We hypothesized that, although LN-1-255 is not toxic at these doses in healthy mice, very high doses used repeatedly may have some deleterious effect in unhealthy (infected) mice. Last, β-lactam dosages used in treatments are usually lower than 50 to 100 mg/kg, and thus 50 mg/kg of the compound LN-1-255 (a penicillin sulfone) was the selected dosage to study in combination with imipenem for experimental treatments. It is also necessary to highlight the important role of the immune system (neutrophils) in combating bacterial infections. The depletion of neutrophils results in deficiency in bacterial clearance, and severe neutropenia or immunosuppression has been associated with higher mortality (32). In this study, immunocompetent mice were used, and the immune system certainly has a relevant role in decreasing the infection by carbapenem-resistant A. baumannii strains treated with the studied imipenem–LN-1-255 combination.
To our knowledge to date, no inhibitors have presented efficacy in preclinical studies against CHDL-produced A. baumannii strains except for the recently published DBO ETX2514 and the β-lactam-based inhibitor LN-1-255. The ETX2514 inhibitor was tested in thigh and pneumonia murine infection models (26). Using different sulbactam-ETX2514 concentrations, the authors observed a 1- to 5-log10 decrease of bacterial load in pneumonia caused by a multidrug-resistant strain expressing an OXA-24/40 variant. Also, when sulbactam-EXT2514 was used at 15/50 mg/kg (similar to our dosage) in a thigh murine model, the bacterial load decreased by approximately 3.5 log. In the present study, the combination imipenem–LN-1-255 at 30/50 mg/kg in a pneumonia model infection caused by the clinical isolate with OXA-24/40 decreased the bacterial load by 3.8 log. Thus, the results obtained here showed similar in vivo efficacy of both inhibitors against CHDL-producing carbapenem-resistant A. baumannii strains.
Infections caused by antibiotic-resistant bacteria have increased rapidly in the last decades, and new therapeutic targets and antimicrobial agents are urgently needed. In antimicrobial development, PK/PD evaluation in experimental infection models are essential to design optimal dosing regimens and planning clinical trials, which are extremely costly. In this work, we have probed the in vivo efficacy of the LN-1-255 inhibitor, using a murine model of infection caused by CHDL-carrying A. baumannii transformant strains and clinical isolates. We have also described the pharmacokinetic parameters and demonstrated the lack of toxicity of this compound. The scarce arsenal against multidrug-resistant (MDR) A. baumannii infection supports the potential clinical use of the LN-1-255 inhibitor in combination with β-lactams.
MATERIALS AND METHODS
Bacterial strains, culture media, cloning, and susceptibility testing.
The recipient strain for the expression of OXA-23 and OXA-24/40 enzymes was the antibiotic-susceptible A. baumannii ATCC 17978 strain. The A. baumannii clinical isolates used were Ab1 (carrying OXA-23) and JC12/04 (carrying OXA-24/40), both previously isolated in our hospital (Table 4). All strains were grown in Luria-Bertani (LB) broth or agar at 37°C. MICs and checkerboard assays were performed by microdilution in Mueller-Hinton II broth (Becton, Dickinson and Company, Sparks, MD, USA) (33, 34). Bacterial strains were frozen in LB broth with 15% glycerol and were maintained at –80°C until analysis. All chemicals were purchased from Sigma-Aldrich (Madrid, Spain) unless otherwise mentioned. LN-1-255 was synthetized by our group, as previously reported (Fig. 3) (35).
TABLE 4.
Strains and plasmids used in this worka
| Strain or plasmid | blaOXA gene | Features | Reference or source |
|---|---|---|---|
| Strains | |||
| A. baumannii ATCC 17978 | NA | A. baumannii type strain | ATCC |
| A. baumannii ATCC 17978 + pET-RA/OXA-23 | OXA-23 | A. baumannii ATCC 17978 harboring pET-TRA-KM plasmid overexpressing OXA-23 | 10 |
| A. baumannii Ab1 | OXA-23 | A. baumannii clinical isolate from A Coruña (Spain) | 42 |
| A. baumannii ATCC 17978 + pET-RA/OXA-24/40 | OXA-24/40 | A. baumannii ATCC 17978 harboring pET-TRA-KM plasmid overexpressing OXA-24/40 | 10 |
| A. baumannii JC12/04 | OXA-24/40 | A. baumannii clinical isolate from A Coruña (Spain) | This study |
| Escherichia coli BL21 | OXA-24/40 | E. coli type strain | GE Healthcare (Chicago, IL, USA) |
| Plasmids | |||
| pET-RA-KM | Cloning vector harboring kanamycin and rifampicin resistance markers | 10 | |
| pGEX-6P-1 | Cloning vector for expressing GST fusion proteins with a PreScission protease site | GE Healthcare (Chicago, IL, USA) |
NA, not applicable; ATTC, American Type Culture Collection; GST, glutathione S-transferase.
FIG 3.
β-Lactam compounds used in this study.
The cloning procedures for blaOXA genes using the pET-RA-Km vector and the susceptibility testing of imipenem and LN-1-255 have been previously described by Vázquez-Ucha et al. (10).
RNA extraction and qRT-PCR analysis.
RNA extraction from strains grown at an optical density at 600 nm (OD600) of 1.0 was performed using the High Pure RNA isolation kit (Roche, Basel, Switzerland). Real-time PCR was done using rpoB as the housekeeping gene (36), the LightCycler 480 RNA Master hydrolysis probe kit (Roche), and the LightCycler 480 RNA instrument (Roche). The following primers were used: OXA51.FW-3′-GTCGATAATTTTTGGCTGGTG-5′ and OXA51.REV-3′-TTAGCCTAGCTTGTAAGCAAACTGTG-5′. The relative expression of blaOXA-51-like from the different A. baumannii strains was compared. All assays were done in triplicate.
Animal infection.
Immunocompetent BALB/c male mice from 9 to 11 weeks old and weighing 20 to 25 g were housed in regulation cages with food and water ad libitum. Mice were bred in our colony and used for all experiments. All mice were maintained in the specific-pathogen-free facility at the Centro Tecnológico de Formación de la Xerencia de Xestión Integrada A Coruña (CTF-XXIAC). The study was approved by the Ethics and Clinical Research Committee (CHUAC, project code P2015/82; Spain), in accordance with the Helsinki Declaration of 1975. This committee follows the recommendations of the National Committee for the Replacement, Refinement and Reduction of Animal Research (NC3Rs).
An experimental pneumonia model was used to evaluate the efficacy of LN-1-255 and imipenem against carbapenem-resistant A. baumannii during the course of the infection caused by transformant and clinical strains.
For bacterial inoculation, the strains were cultured at 37°C until they reached an OD600 of 0.7. The cultures were centrifuged, and pellets were washed and adjusted in sterile NaCl 0.9% to the required cellular suspension. The CFU of bacterial inoculums were quantified by colony counting in LB plates.
Briefly, mice anesthetized by inhalation of Sevoflurane (Zoetis, Madrid, Spain) were suspended by their incisors on a board in a semivertical position. An endoscope was used on the oral cavity to confirm the intratracheal inoculation. The trachea was accessed using a blunt-tipped needle and a 100-μl Hamilton syringe for the inoculation of a 40-μl bacterial suspension made in sterile saline solution and 10% porcine mucin (wt/vol) mixed at a 1:1 ratio. Immediately after the inoculation, a solution of ketamine (500 μg/mouse; Pfizer, Madrid, Spain) and medetomidine (15 μg/mouse; Ecuphar, Barcelona, Spain) was intraperitoneally injected in order to keep the mice over 10 min in a vertical position and then 20 min in a 30° inclined position. Antisedan (15 μg/mouse; Ecuphar, Barcelona, Spain) was injected, also intraperitoneally, for restoring the state of consciousness and normalizing the physiological variables of mice. Mice were finally euthanized with an overdose of thiopental sodium (Sandoz, Holzkirchen, Germany) after 22 h of treatment.
Infections were performed using the LD100 of each strain. In order to calculate the exact LD100, previous to the treatment, lung inoculations in groups of 4 mice infected with each strain were performed, using the following inocula: 0.1 × 107, 0.4 × 107, 1 × 107, 4 × 107, and 20 × 107 CFU/mouse.
Treatments.
In order to obtain the best results by decreasing the bacterial load in synergy with imipenem, a screening was performed using different concentrations of LN-1-255 (20, 50, and 150 mg/kg) and three inoculation routes (intraperitoneal, intramuscular, and subcutaneous). The screening of the best LN-1-255 dosage was performed using reduced (n = 4 to 6) groups of mice in assays by duplicate. The optimum imipenem dose was 30 mg/kg, intramuscularly, a dosage previously described as effective against A. baumannii in experimental pneumonia models (20, 21, 37).
For establishing the best treatment model, groups of 9 to 11 mice were infected following the optimum dosage and inoculation route. Mice were randomly located in each group, and treatments were begun 90 min after bacterial inoculation in lungs, where the initial infection took place. For each assay, the first group (control) did not receive antimicrobial treatment (only sterile phosphate-buffered saline [PBS]), the second group was treated only with imipenem (30 mg/kg intramuscularly), and the third group was treated with imipenem (30 mg/kg, intramuscularly) and LN-1-255 at the best dosage and inoculation route observed (50 mg/kg, intramuscularly). For final established treatments, the interval between drug doses was 3 h. Mice were monitored for 22 h, euthanized with an overdose of thiopental sodium, and analyzed immediately after death. After the treatments, lungs were extracted in aseptic conditions, homogenized in sterile PBS, and CFU were enumerated by plating 10−2-fold serial dilutions in LB plates. Results were expressed as means ± standard deviations of the log10 CFU per gram of lung.
Two series of assays were used as control to discard the possible specific antimicrobial activity of the inhibitor, as follows: (i) similarly to that described above, pneumonia infections caused by both carbapenem-resistant derivatives of ATCC 17978 carrying OXA-23 or OXA-24/40 CHDLs were treated with only LN-1-255 (50 mg/kg, intramuscularly) and compared with untreated infections, and (ii) pneumonia infections caused by the susceptible parental strain ATCC 17978 were treated intramuscularly with imipenem (30 mg/kg) and compared with those treated by the combination of imipenem (30 mg/kg) and LN-1-255 (50 mg/kg).
LN-1-255 pharmacokinetics.
Pharmacokinetics of LN-1-255 were determined after intramuscular administration of a single dose of 20, 50, or 150 mg/kg of body weight. To obtain pharmacokinetic parameters in serum, blood samples were collected from submandibular vein of anesthetized mice, or the animals were euthanized and blood was extracted from the periorbital plexuses. Three mice per time point (0, 30, 45, 60, 90, 120, and 180 min) were used. Sera were separated from the blood cells by centrifugation (2,000 × g, 20 min) and stored at −80°C until subsequent analysis. To obtain the pharmacokinetic parameters in lung, the concentrations of LN-1-255 in epithelial lining fluid (ELF) were measured following the protocol described by Gotfried et al. (38). Bronchoalveolar lavage was performed with 2 ml of 0.9% NaCl in three anesthetized mice per time point (0, 30, 90, 150, 220, and 340 min). The supernatant and cells were separated by centrifugation (400 × g, 5 min) and frozen at −80°C. To calculate the ELF obtained, curves of urea concentration in plasma and bronchoalveolar lavage (BAL) fluid were determined using the urea nitrogen colorimetric detection kit (Thermo Fisher, Waltham, MA, USA) and an Epoch 2.0 spectrophotometer (BioTek, Winooski, VT, USA).
The serum and ELF drug concentrations were calculated using a kinetic assay, which detected the inhibition of hydrolysis of the reporter substrate nitrocefin (Oxoid, Hampshire, UK) by the purified β-lactamase OXA-24/40. The enzyme OXA-24/40 was expressed in Escherichia coli BL21 and purified using the previously described procedures. All assays were performed at 25°C using the proteins under steady-state conditions on an Epoch 2 spectrophotometer (39). Briefly, 40 ng/ml of carbapenemase was added to the reaction mixture, which included 200 μM nitrocefin and mice serum diluted to 10% vol/vol. Final concentrations of inhibitor in serum were estimated by extrapolation from the regression line of known LN-1-255 concentrations (0.01 to 25 mg/liter). The lower limits of detection were 0.007 mg/liter.
In vivo toxicity.
Once the optimum therapeutic dose of LN-1-255 was established, toxicity studies were performed. The in vivo toxicity was evaluated using the previously described methods (40, 41). Groups of 4 mice received a single intramuscular dose of LN-1-255 at 350 mg/kg (7-fold normal treatment). Also, two groups of 4 mice received 6 intramuscular or intraperitoneal doses of 150 mg/kg (3-fold normal treatment), respectively. Over 7 days, survival and signs of toxicity in mice were monitored.
Statistics.
Differences between groups in the presence of imipenem and the inhibitor LN-1-255 were established using the Student’s t test, carried out using Prism (GraphPad Software, San Diego, CA, USA). Differences were considered significant at P ≤ 0.05.
ACKNOWLEDGMENTS
This work has been funded by projects PI15/00860 to G.B., CP13/00226 to A.B., and P14/00059 and PI17/01482 to M.P. and A.B., all of which were integrated into the National Plan for Scientific Research, Development and Technological Innovation 2013–2016 and funded by the ISCIII–General Subdirection of Assessment and Promotion of the Research-European Regional Development Fund (FEDER) “A way of making Europe,” The study was also funded by project IN607A 2016/22 (Consellería de Cultura, Educación e Ordenación Universitaria) to G.B. This study was also funded by the Spanish Ministry of Economy and Competiveness (SAF2016-75638-R), Xunta de Galicia (Centro Singular de Investigación de Galicia accreditation 2016 to 2019, ED431G/09), and by the European Regional Development Fund (ERDF) to C.G.-B. This work was also supported by Planes Nacionales de I+D+i 2008-2011/2013-2016 and by Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía y Competitividad, Spanish Network for Research in Infectious Diseases (REIPI RD16/0016/006), cofinanced by the European Development Regional Fund “A way to achieve Europe” and operative program Intelligent Growth 2014–2020. Also, this study was supported in part by funds from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (USA) under award numbers R01AI063517, R01AI072219, and R01AI100560, by funds and/or facilities provided by the Cleveland Department of Veterans Affairs, the Veterans Affairs Merit Review Program award 1I01BX001974, and by the Geriatric Research Education and Clinical Center VISN 10 to R.A.B. J.D.B. acknowledges support from the National Institutes of Allergy and Infectious Diseases under award number R15AI142699. J.C.V.-U. was financially supported by the Miguel Servet Programme (CP13/00226; ISCIII, Spain) and by Consellería de Cultura, Educación e Ordenación Universitaria (IN607A 2016/22), and M.M.-G. was financially supported by a Clara Roy grant (Spanish Society of Clinical Microbiology and Infectious Diseases [SEIMC]).
The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health or the Department of Veterans Affairs.
We have no conflicts of interest to declare.
REFERENCES
- 1.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Review on Antimicrobial Resistance. 2014. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Review on Antimicrobial Resistance, United Kingdom. [Google Scholar]
- 3.World Health Organization. 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery and development of new antibiotics. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 4.Toth M, Antunes NT, Stewart NK, Frase H, Bhattacharya M, Smith CA, Vakulenko SB. 2016. Class D β-lactamases do exist in Gram-positive bacteria. Nat Chem Biol 12:9–14. doi: 10.1038/nchembio.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poole K. 2004. Resistance to beta-lactam antibiotics. Cell Mol Life Sci 61:2200–2223. doi: 10.1007/s00018-004-4060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaitany KC, Klinger NV, June CM, Ramey ME, Bonomo RA, Powers RA, Leonard DA. 2013. Structures of the class D carbapenemases OXA-23 and OXA-146: mechanistic basis of activity against carbapenems, extended-spectrum cephalosporins, and aztreonam. Antimicrob Agents Chemother 57:4848–4855. doi: 10.1128/AAC.00762-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Queenan AM, Bush K. 2007. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev 20:440–458. doi: 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walther-Rasmussen J, Høiby N. 2006. OXA-type carbapenemases. J Antimicrob Chemother 57:373–383. doi: 10.1093/jac/dki482. [DOI] [PubMed] [Google Scholar]
- 9.Smith CA, Antunes NT, Stewart NK, Toth M, Kumarasiri M, Chang M, Mobashery S, Vakulenko SB. 2013. Structural basis for carbapenemase activity of the OXA-23 β-lactamase from Acinetobacter baumannii. Chem Biol 20:1107–1115. doi: 10.1016/j.chembiol.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vázquez-Ucha JC, Maneiro M, Martínez-Guitián M, Buynak J, Bethel CR, Bonomo RA, Bou G, Poza M, González-Bello C, Beceiro A. 2017. Activity of the β-lactamase inhibitor LN-1-255 against carbapenem-hydrolyzing class D β-lactamases from Acinetobacter baumannii. Antimicrob Agents Chemother 61:e01172-17. doi: 10.1128/AAC.01172-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drawz SM, Papp-Wallace KM, Bonomo RA. 2014. New beta-lactamase inhibitors: a therapeutic renaissance in an MDR world. Antimicrob Agents Chemother 58:1835–1846. doi: 10.1128/AAC.00826-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Livermore DM, Mushtaq S, Warner M, Zhang J, Maharjan S, Doumith M, Woodford N. 2011. Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 55:390–394. doi: 10.1128/AAC.00756-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naas T, Nordmann P. 1999. OXA-type beta-lactamases. Curr Pharm Des 5:865–879. [PubMed] [Google Scholar]
- 14.Shlaes DM. 2013. New β-lactam-β-lactamase inhibitor combinations in clinical development. Ann N Y Acad Sci 1277:105–114. doi: 10.1111/nyas.12010. [DOI] [PubMed] [Google Scholar]
- 15.Perez-Llarena FJ, Bou G. 2009. Beta-lactamase inhibitors: the story so far. Curr Med Chem 16:3740–3765. doi: 10.2174/092986709789104957. [DOI] [PubMed] [Google Scholar]
- 16.Bou G, Santillana E, Sheri A, Beceiro A, Sampson JM, Kalp M, Bethel CR, Distler AM, Drawz SM, Pagadala SR, van den Akker F, Bonomo RA, Romero A, Buynak JD. 2010. Design, synthesis, and crystal structures of 6-alkylidene-2′-substituted penicillanic acid sulfones as potent inhibitors of Acinetobacter baumannii OXA-24 carbapenemase. J Am Chem Soc 132:13320–13331. doi: 10.1021/ja104092z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vallejo JA, Martínez-Guitián M, Vázquez-Ucha JC, González-Bello C, Poza M, Buynak JD, Bethel CR, Bonomo RA, Bou G, Beceiro A. 2016. LN-1-255, a penicillanic acid sulfone able to inhibit the class D carbapenemase OXA-48. J Antimicrob Chemother 71:2171–2180. doi: 10.1093/jac/dkw105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pattanaik P, Bethel CR, Hujer AM, Hujer KM, Distler AM, Taracila M, Anderson VE, Fritsche TR, Jones RN, Pagadala SR, van den Akker F, Buynak JD, Bonomo RA. 2009. Strategic design of an effective beta-lactamase inhibitor: LN-1-255, a 6-alkylidene-2′-substituted penicillin sulfone. J Biol Chem 284:945–953. doi: 10.1074/jbc.M806833200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drawz SM, Bethel CR, Doppalapudi VR, Sheri A, Pagadala SR, Hujer AM, Skalweit MJ, Anderson VE, Chen SG, Buynak JD, Bonomo RA. 2010. Penicillin sulfone inhibitors of class D beta-lactamases. Antimicrob Agents Chemother 54:1414–1424. doi: 10.1128/AAC.00743-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beceiro A, López-Rojas R, Domínguez-Herrera J, Docobo-Pérez F, Bou G, Pachón J, Spanish Network for Research in Infectious Diseases (REIPI). 2009. In vitro activity and in vivo efficacy of clavulanic acid against Acinetobacter baumannii. Antimicrob Agents Chemother 53:4298–4304. doi: 10.1128/AAC.00320-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodríguez-Hernández MJ, Pachón J, Pichardo C, Cuberos L, Ibáñez-Martínez J, García-Curiel A, Caballero FJ, Moreno I, Jiménez-Mejías ME. 2000. Imipenem, doxycycline and amikacin in monotherapy and in combination in Acinetobacter baumannii experimental pneumonia. J Antimicrob Chemother 45:493–501. doi: 10.1093/jac/45.4.493. [DOI] [PubMed] [Google Scholar]
- 22.Ruiz J, Núñez ML, Pérez J, Simarro E, Martínez-Campos L, Gómez J. 1999. Evolution of resistance among clinical isolates of Acinetobacter over a 6-year period. Eur J Clin Microbiol Infect Dis 18:292–295. doi: 10.1007/s100960050280. [DOI] [PubMed] [Google Scholar]
- 23.Poirel L, Nordmann P. 2002. Acquired carbapenem-hydrolyzing beta-lactamases and their genetic support. Curr Pharm Biotechnol 3:117–127. doi: 10.2174/1389201023378427. [DOI] [PubMed] [Google Scholar]
- 24.Coleman K. 2011. Diazabicyclooctanes (DBOs): a potent new class of non-β-lactam β-lactamase inhibitors. Curr Opin Microbiol 14:550–555. doi: 10.1016/j.mib.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 25.Lahiri SD, Mangani S, Jahic H, Benvenuti M, Durand-Reville TF, De Luca F, Ehmann DE, Rossolini GM, Alm RA, Docquier JD. 2015. Molecular basis of selective inhibition and slow reversibility of avibactam against class D carbapenemases: a structure-guided study of OXA-24 and OXA-48. ACS Chem Biol 10:591–600. doi: 10.1021/cb500703p. [DOI] [PubMed] [Google Scholar]
- 26.Durand-Réville TF, Guler S, Comita-Prevoir J, Chen B, Bifulco N, Huynh H, Lahiri S, Shapiro AB, McLeod SM, Carter NM, Moussa SH, Velez-Vega C, Olivier NB, McLaughlin R, Gao N, Thresher J, Palmer T, Andrews B, Giacobbe RA, Newman JV, Ehmann DE, de Jonge B, O'Donnell J, Mueller JP, Tommasi RA, Miller AA. 2017. ETX2514 is a broad-spectrum β-lactamase inhibitor for the treatment of drug-resistant Gram-negative bacteria including Acinetobacter baumannii. Nat Microbiol 2:17104. doi: 10.1038/nmicrobiol.2017.104. [DOI] [PubMed] [Google Scholar]
- 27.Barnes MD, Bethel CR, Rutter JD, Akker FVD, Papp-Wallace KM, Bonomo RA. 2017. The novel β-lactamase inhibitor, ETX-2514, in combination with sulbactam effectively inhibits Acinetobacter baumannii. Open Forum Infect Dis 4:S368. doi: 10.1093/ofid/ofx163.900. [DOI] [Google Scholar]
- 28.Cho JC, Zmarlicka MT, Shaeer KM, Pardo J. 2018. Meropenem/vaborbactam, the first carbapenem/β-lactamase inhibitor combination. Ann Pharmacother 52:769–779. doi: 10.1177/1060028018763288. [DOI] [PubMed] [Google Scholar]
- 29.Lapuebla A, Abdallah M, Olafisoye O, Cortes C, Urban C, Quale J, Landman D. 2015. Activity of meropenem combined with RPX7009, a novel β-lactamase inhibitor, against Gram-negative clinical isolates in New York City. Antimicrob Agents Chemother 59:4856–4860. doi: 10.1128/AAC.00843-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nielsen EI, Cars O, Friberg LE. 2011. Pharmacokinetic/pharmacodynamic (PK/PD) indices of antibiotics predicted by a semimechanistic PKPD model: a step toward model-based dose optimization. Antimicrob Agents Chemother 55:4619–4630. doi: 10.1128/AAC.00182-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–10. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 32.Guo B, Abdelraouf K, Ledesma KR, Chang KT, Nikolaou M, Tam VH. 2011. Quantitative impact of neutrophils on bacterial clearance in a murine pneumonia model. Antimicrob Agents Chemother 55:4601–4605. doi: 10.1128/AAC.00508-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsieh MH, Yu CM, Yu VL, Chow JW. 1993. Synergy assessed by checkerboard. A critical analysis. Diagn Microbiol Infect Dis 16:343–349. doi: 10.1016/0732-8893(93)90087-N. [DOI] [PubMed] [Google Scholar]
- 34.Clinical and Laboratory Standards Institute. 2012. Performance standards for antimicrobial susceptibility testing. 17th informational supplement, M07-A9 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 35.Buynak JD, Rao AS, Doppalapudi VR, Adam G, Petersen PJ, Nidamarthy SD. 1999. The synthesis and evaluation of 6-alkylidene-2′beta-substituted penam sulfones as beta-lactamase inhibitors. Bioorg Med Chem Lett 9:1997–2002. doi: 10.1016/S0960-894X(99)00325-X. [DOI] [PubMed] [Google Scholar]
- 36.Álvarez-Fraga L, Vázquez-Ucha JC, Martínez-Guitián M, Vallejo JA, Bou G, Beceiro A, Poza M. 2018. Pneumonia infection in mice reveals the involvement of the feoA gene in the pathogenesis of Acinetobacter baumannii. Virulence 9:496–509. doi: 10.1080/21505594.2017.1420451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parra Millán R, Jiménez Mejías ME, Sánchez Encinales V, Ayerbe Algaba R, Gutiérrez Valencia A, Pachón Ibáñez ME, Díaz C, Pérez Del Palacio J, López Cortés LF, Pachón J, Smani Y. 2016. Efficacy of lysophosphatidylcholine in combination with antimicrobial agents against Acinetobacter baumannii in experimental murine peritoneal sepsis and pneumonia models. Antimicrob Agents Chemother 60:4464–4470. doi: 10.1128/AAC.02708-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gotfried MH, Danziger LH, Rodvold KA. 2001. Steady-state plasma and intrapulmonary concentrations of levofloxacin and ciprofloxacin in healthy adult subjects. Chest 119:1114–1122. doi: 10.1378/chest.119.4.1114. [DOI] [PubMed] [Google Scholar]
- 39.Santillana E, Beceiro A, Bou G, Romero A. 2007. Crystal structure of the carbapenemase OXA-24 reveals insights into the mechanism of carbapenem hydrolysis. Proc Natl Acad Sci U S A 104:5354–5359. doi: 10.1073/pnas.0607557104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.López-Rojas R, Sánchez-Céspedes J, Docobo-Pérez F, Domínguez-Herrera J, Vila J, Pachón J. 2011. Pre-clinical studies of a new quinolone (UB-8902) against Acinetobacter baumannii resistant to ciprofloxacin. Int J Antimicrob Agents 38:355–359. doi: 10.1016/j.ijantimicag.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 41.O’Reilly T, Andes DA, Ostergaard C, Frimodt-Moller N. 2005. Evaluation of antimicrobials in experimental animal infections In Lorian V. (ed), Antibiotics in laboratory medicine, 5th ed Lippincott Williams and Wilkins, Philadelphia, PA. [Google Scholar]
- 42.Higgins PG, Pérez-Llarena FJ, Zander E, Fernández A, Bou G, Seifert H. 2013. OXA-235, a novel class D β-lactamase involved in resistance to carbapenems in Acinetobacter baumannii. Antimicrob Agents Chemother 57:2121–2126. doi: 10.1128/AAC.02413-12. [DOI] [PMC free article] [PubMed] [Google Scholar]