WCK 4282 is a combination product of cefepime (FEP) and tazobactam (TAZ) in a 1:1 ratio currently under development for the treatment of multidrug-resistant Gram-negative bacterial infections. We investigated the effect of renal impairment on the pharmacokinetics (PK) and safety of WCK 4282 in 48 subjects with various degrees of renal function. Subjects were categorized on the basis of their Cockcroft-Gault equation-estimated creatinine clearance (CLCR).
KEYWORDS: Gram-negative bacterial infections, antibacterial agents, bacterial, β-lactamases, multidrug resistance
ABSTRACT
WCK 4282 is a combination product of cefepime (FEP) and tazobactam (TAZ) in a 1:1 ratio currently under development for the treatment of multidrug-resistant Gram-negative bacterial infections. We investigated the effect of renal impairment on the pharmacokinetics (PK) and safety of WCK 4282 in 48 subjects with various degrees of renal function. Subjects were categorized on the basis of their Cockcroft-Gault equation-estimated creatinine clearance (CLCR). We enrolled 6 subjects each into those with mild (CLCR, 60 to <90 ml/min), moderate (CLCR, 30 to <60 ml/min), or severe (CLCR, <30 ml/min) renal impairment and those with end-stage renal disease (ESRD) requiring hemodialysis and 24 healthy control subjects (CLCR, ≥90 ml/min). Healthy subjects and subjects with mild and moderate renal impairment received a single 90-min infusion of 4 g of WCK 4282 (2 g FEP and 2 g TAZ). Subjects with severe renal impairment and ESRD received 2 g of WCK 4282 (1 g FEP and 1 g TAZ) over 90 min. The plasma exposure of FEP-TAZ increased as renal function decreased. In subjects with mild, moderate, and severe renal impairment and ESRD, the mean exposure (area under the plasma concentration versus time curve from time zero extrapolated to infinity) of FEP and TAZ increased by 1.3- and 1.2-fold, 2.3- and 2.3-fold, 4.7- and 4.0-fold, and 8.5- and 11.6-fold, respectively. The urinary recovery of FEP and TAZ decreased with increasing renal impairment. There were no adverse events reported during the study. The findings suggest that dose adjustments for WCK 4282 will be required according to the degree of renal impairment. A single infusion of WCK 4282 was found to be safe and well tolerated in subjects with normal and impaired renal function. (This study has been registered at ClinicalTrials.gov under identifier NCT02709382.)
INTRODUCTION
WCK 4282 is an injectable antibacterial combination product consisting of cefepime (FEP) and tazobactam (TAZ) in a 2 g:2 g ratio currently under development for the treatment of multidrug-resistant Gram-negative bacterial infections. Cefepime-tazobactam (FEP-TAZ) consists of 2 g cefepime plus 2 g tazobactam that is administered every 8 h via a 90-min infusion as a therapeutic option for the treatment of Gram-negative bacterial infections prevalent in hospital settings, such as complicated urinary tract infections, nosocomial pneumonia, and complicated intra-abdominal infections (1–7). Both cefepime and tazobactam have been extensively evaluated. The dosing of cefepime at 1 g every 12 h (q12h) is considered a low dose, and the dosing of cefepime at 2 g every 8 h (q8h) is considered a high dose (7). Cefepime-tazobactam combines an AmpC-stable cephalosporin with an established inhibitor which is active against extended-spectrum β-lactamases (ESBLs) (1–7). Cefepime-tazobactam is active against Pseudomonas aeruginosa and Enterobacteriaceae strains, including ESBL screening-positive-phenotype Escherichia coli strains and ceftazidime-nonsusceptible Enterobacter spp. (1). Cefepime-tazobactam provided coverage of 96% to 100% of Enterobacteriaceae with penicillinases, AmpC, ESBLs, or K1 or OXA-48 β-lactamases (2).
Tazobactam, a triazolyl-substituted penicillanic acid sulfone, is a β-lactamase inhibitor that has been successfully combined with piperacillin to protect this antibiotic from class A ESBLs which mediate hydrolysis (8). U.S. FDA-approved indications include appendicitis/peritonitis, uncomplicated and complicated skin and soft tissue infections, postpartum endometritis, community-acquired pneumonia, and nosocomial pneumonia (8–11).
Peak plasma concentrations of TAZ are attained immediately after completion of an intravenous (i.v.) infusion. Tazobactam is 39% bound to plasma proteins. The plasma elimination half-life (t1/2) of TAZ was found to range from 0.7 to 1.2 h and was unaffected by the dose or the duration of infusion (8). Tazobactam is metabolized to a single metabolite that lacks pharmacological and antibacterial activities. Tazobactam and its metabolite are eliminated primarily by renal excretion, with 80% of the administered dose being excreted as unchanged drug and the remainder being excreted as the single metabolite. Tazobactam is also secreted into the bile (8).
The average plasma concentrations of cefepime observed in healthy adult male volunteers (n = 9) at various times following single infusions of cefepime at 500 mg, 1 g, and 2 g were found to produce a maximum observed plasma concentration (Cmax) of 39.1 to 163.91 μg/ml and an area under the plasma concentration-versus-time curve (AUC) of 70.8 to 284.8 μg·h/ml. Cefepime is 16 to 19% bound to proteins, and elimination is primarily via renal excretion. The mean t1/2 and total body clearance (CL) of FEP are approximately 2 h and 120 ml/min, respectively (11–14). Cefepime exhibits linear pharmacokinetics (PK) across the dosage range of 250 mg to 2 g. The renal excretion of unchanged FEP accounts for approximately 85% of the total dose. Cefepime clearance is reduced and t1/2 and the area under the plasma concentration-versus-time curve from time zero extrapolated to infinity (AUC0–∞) are increased with increasing degrees of renal impairment (13–15).
It is expected that the elimination of both FEP and TAZ is prolonged in patients with reduced renal function. Accordingly, this study was conducted to evaluate the effect of renal impairment on the PK and safety of FEP and TAZ.
RESULTS
Forty-eight subjects were enrolled into this study. Each subject received a single 90-min i.v. infusion of WCK 4282. The demographic and baseline characteristics for all enrolled subjects are summarized in Table 1. Forty subjects were male, and 8 subjects were female. The mean (standard deviation [SD]) age and body mass index (BMI) of the study population were 60.2 (8.2) years and 27.6 (4.4) kg/m2, respectively. Thirty-nine subjects were white (Hispanic or Latino), and 9 subjects were black or African American. All the renal impairment groups were closely matched to their corresponding control groups with regard to age, BMI, and the male/female proportion. Baseline Cockcroft and Gault equation-estimated creatinine clearance (CLCR) met impairment group criteria and were consistent within treatment groups.
TABLE 1.
Demographic and baseline characteristicsa
| Characteristic | Value |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mild |
Moderate |
Severe |
ESRD on HD |
Total (n = 48) | |||||
| Impairment (n = 6) | Control (n = 6) | Impairment (n = 6) | Control (n = 6) | Impairment (n = 6) | Control (n = 6) | Impairment (n = 6) | Control (n = 6) | ||
| Mean (SD) age (yr) | 62.7 (7.0) | 59.5 (6.9) | 65.7 (7.7) | 60.2 (7.9) | 62.0 (12.8) | 58.2 (10.5) | 57.3 (5.7) | 55.8 (4.5) | 60.2 (8.2) |
| No. of subjects by gender | |||||||||
| Male | 6 | 6 | 5 | 5 | 4 | 4 | 5 | 5 | 40 |
| Female | 0 | 0 | 1 | 1 | 2 | 2 | 1 | 1 | 8 |
| No. of subjects by ethnicity | |||||||||
| Hispanic or Latino | 5 | 5 | 6 | 6 | 5 | 6 | 0 | 6 | 39 |
| Not Hispanic or Latino | 1 | 1 | 0 | 0 | 1 | 0 | 6 | 0 | 9 |
| No. of subjects by race | |||||||||
| Black | 2 | 0 | 0 | 0 | 1 | 0 | 6 | 0 | 9 |
| White | 4 | 6 | 6 | 6 | 5 | 6 | 0 | 6 | 39 |
| Mean (SD) ht (cm) | 171.0 (5.9) | 175.3 (6.3) | 169.8 (7.8) | 171.8 (7.4) | 166.8 (4.4) | 170.3 (10.5) | 170.2 (6.0) | 173.3 (3.5) | 171.1 (6.7) |
| Mean (SD) wt (kg) | 77.6 (12.6) | 84.2 (15.8) | 83.3 (15.2) | 85.5 (9.1) | 85.0 (19.8) | 84.2 (9.1) | 72.0 (13.3) | 74.0 (7.2) | 80.7 (13.3) |
| Mean (SD) BMI (kg/m2) | 26.5 (3.9) | 27.3 (4.2) | 28.9 (5.7) | 29.1 (3.6) | 30.4 (6.1) | 29.2 (3.6) | 24.7 (3.1) | 24.6 (2.1) | 27.6 (4.4) |
| Mean (SD) CLCR (ml/min) | 80.3 (6.5) | 120.8 (20.8) | 45.6 (6.4) | 134.0 (35.4) | 19.3 (3.7) | 144.0 (33.7) | 10.4 (2.3) | 126.8 (29.2) | 85.1 (54.8) |
BMI, body mass index; ESRD, end-stage renal disease; HD, hemodialysis.
Effect of renal impairment on FEP and TAZ PK parameters.
Forty-seven of 48 subjects (97.9%) were included in the plasma PK analysis, and 42 subjects (87.5%) were included in the urine PK analysis. None of the 6 subjects undergoing hemodialysis (HD) were able to produce urine, and these subjects were therefore excluded from the urine PK analysis. Plasma PK parameters were evaluated for subjects with mild, moderate, and severe renal impairment and subjects with end-stage renal disease (ESRD) on HD. Both FEP and TAZ demonstrated a graded reduction in clearance and corresponding increases in t1/2, the AUC from time zero to the time (t) of the last measured concentration (AUC0–t), and AUC0–∞ with decreasing renal function. The plasma concentration-versus-time curves for FEP and TAZ are displayed in Fig. 1A and B, respectively. Log-linear plots of the plasma concentration versus time for FEP and TAZ are displayed in Fig. 2A and B, respectively. The effects of renal impairment on FEP and TAZ PK parameters are summarized in Tables 2 and 3.
FIG 1.
(A) Plasma FEP concentration versus time. (B) Plasma TAZ concentration versus time.
FIG 2.
(A) Natural log plasma FEP concentration versus time. (B) Natural log plasma TAZ concentration versus time.
TABLE 2.
Summary of PK parameters of FEP and TAZ
| Drug and parameter | Mild |
Moderate |
Severe |
ESRD on HD |
||||
|---|---|---|---|---|---|---|---|---|
| Impairment (n = 6) | Control (n = 6) | Impairment (n = 6) | Control (n = 6) | Impairment (n = 6) | Control (n = 6) | Impairment (n = 5) | Control (n = 6) | |
| FEP | ||||||||
| AUC0–t (μg·h/ml) | ||||||||
| Mean | 418.5 | 326.2 | 719.5 | 307.3 | 818.2 | 348.0 | 1,426.0 | 333.2 |
| SD | 51.8 | 59.7 | 204.3 | 46.0 | 219.8 | 33.3 | 335.8 | 66.5 |
| AUC0–∞ (μg·h/ml) | ||||||||
| Mean | 420.7 | 328.5 | 728.7 | 308.9 | 882.7 | 349.9 | 1,820.5 | 335.2 |
| SD | 51.7 | 59.6 | 211.3 | 46.5 | 237.4 | 32.8 | 353.6 | 66.0 |
| Cmax (μg/ml) | ||||||||
| Mean | 102.7 | 99.9 | 96.9 | 96.7 | 66.7 | 108.6 | 291.7 | 101.7 |
| SD | 20.1 | 11.8 | 35.0 | 10.4 | 19.7 | 19.8 | 273.9 | 21.5 |
| Tmax (h) | ||||||||
| Mean | 1.5 | 1.4 | 1.9 | 1.4 | 1.4 | 1.3 | 1.2 | 1.3 |
| SD | 0.1 | 0.1 | 1.1 | 0.1 | 0.2 | 0.1 | 0.5 | 0.1 |
| t1/2 (h) | ||||||||
| Mean | 3.5 | 2.8 | 6.3 | 2.7 | 12.9 | 3.3 | 23.0 | 2.9 |
| SD | 0.5 | 0.3 | 1.8 | 0.5 | 2.2 | 1.0 | 4.6 | 0.6 |
| λz (1/h) | ||||||||
| Mean | 0.2 | 0.2 | 0.1 | 0.3 | 0.1 | 0.2 | 0.03 | 0.3 |
| SD | 0.03 | 0.02 | 0.04 | 0.05 | 0.01 | 0.1 | 0.01 | 0.1 |
| CL total (ml/min) | ||||||||
| Mean | 80.2 | 104.2 | 50.3 | 109.9 | 20.9 | 96.0 | 9.1 | 103.4 |
| SD | 9.2 | 18.2 | 20.0 | 15.7 | 9.2 | 9.5 | 1.8 | 24.3 |
| CLR (ml/min) | ||||||||
| Mean | 79.2 | 106 | 39.1 | 105 | 9.51 | 93.5 | NAa | 111 |
| SD | 23.5 | 22.1 | 10.6 | 14.6 | 3.22 | 10.9 | NA | 26.5 |
| VZ (liters) | ||||||||
| Mean | 24.1 | 25.4 | 27.2 | 25.1 | 23.4 | 27.3 | 18.2 | 25.0 |
| SD | 3.3 | 4.6 | 13.0 | 2.7 | 11.2 | 9.2 | 5.6 | 3.0 |
| Vss (liters) | ||||||||
| Mean | 19.1 | 17.6 | 24.2 | 17.5 | 20.6 | 17.0 | 16.8 | 17.1 |
| SD | 4.7 | 2.3 | 11.8 | 2.0 | 7.7 | 4.2 | 5.7 | 2.6 |
| Mean residence time (h) | ||||||||
| Mean | 4.0 | 2.8 | 8.3 | 2.7 | 16.8 | 3.0 | 30.5 | 2.8 |
| SD | 1.0 | 0.3 | 3.3 | 0.3 | 3.2 | 0.7 | 6.6 | 0.7 |
| TAZ | ||||||||
| AUC0–t (μg·h/ml) | ||||||||
| Mean | 129.2 | 110.9 | 224.8 | 98.3 | 229.5 | 115.6 | 624.5 | 107.4 |
| SD | 27.4 | 24.4 | 60.0 | 18.8 | 57.6 | 11.5 | 257.2 | 23.7 |
| AUC0–∞ (μg·h/ml) | ||||||||
| Mean | 130.8 | 111.7 | 227.2 | 99.4 | 231.4 | 116.3 | 632.1 | 108.3 |
| SD | 27.6 | 24.5 | 59.7 | 18.2 | 57.5 | 11.4 | 257.8 | 23.8 |
| Cmax (μg/ml) | ||||||||
| Mean | 58.0 | 53.0 | 64.7 | 48.3 | 53.3 | 59.0 | 300.3 | 51.7 |
| SD | 6.9 | 8.1 | 22.0 | 6.5 | 13.5 | 11.6 | 288.8 | 12.2 |
| Tmax (h) | ||||||||
| Mean | 1.4 | 1.3 | 1.6 | 1.3 | 1.4 | 1.3 | 1.1 | 1.3 |
| SD | 0.2 | 0.1 | 0.5 | 0.2 | 0.2 | 0.1 | 0.4 | 0.2 |
| t1/2 (h) | ||||||||
| Mean | 1.5 | 1.4 | 2.2 | 1.3 | 3.7 | 1.5 | 7.2 | 1.4 |
| SD | 0.4 | 0.2 | 0.4 | 0.4 | 1.0 | 0.5 | 1.6 | 0.6 |
| λz (1/h) | ||||||||
| Mean | 0.5 | 0.5 | 0.3 | 0.6 | 0.2 | 0.5 | 0.1 | 0.5 |
| SD | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.02 | 0.2 |
| CL total (ml/min) | ||||||||
| Mean | 264.0 | 309.0 | 160.8 | 344.5 | 76.2 | 289.2 | 29.7 | 322.3 |
| SD | 52.3 | 59.4 | 65.4 | 59.5 | 20.7 | 30.3 | 14.1 | 80.2 |
| CLR (ml/min) | ||||||||
| Mean | 250 | 276 | 110 | 308 | 23.1 | 268 | NA | 340 |
| SD | 96.6 | 57 | 29 | 60.5 | 8.85 | 33.5 | NA | 88.6 |
| Vz (liters) | ||||||||
| Mean | 34.1 | 38.6 | 30.5 | 39.0 | 23.8 | 38.7 | 17.3 | 36.9 |
| SD | 3.8 | 10.7 | 14.5 | 16.3 | 7.7 | 14.5 | 5.5 | 7.4 |
| Vss (liters) | ||||||||
| Mean | 24.2 | 22.3 | 26.7 | 24.3 | 19.8 | 20.5 | 13.4 | 22.3 |
| SD | 5.8 | 3.3 | 11.9 | 8.2 | 6.1 | 4.8 | 7.5 | 3.4 |
| Mean residence time (h) | ||||||||
| Mean | 1.6 | 1.2 | 2.9 | 1.2 | 4.5 | 1.2 | 7.4 | 1.2 |
| SD | 0.5 | 0.2 | 1.2 | 0.3 | 1.2 | 0.2 | 2.6 | 0.3 |
NA, not applicable.
TABLE 3.
Effect of renal impairment on FEP and TAZ PK parameters
| Drug and PK parameter | Renal impairment category | Impairment group geometric mean | Control group geometric mean | Geometric mean ratio impairment/control | 95% CI for geometric mean ratio | Correlation coefficient |
|---|---|---|---|---|---|---|
| FEP | ||||||
| AUC0–t (μg·h/ml) | Mild | 415.9 | 321.8 | 1.3 | 0.9319–1.7925 | −0.8988 |
| Moderate | 690.3 | 304.5 | 2.3 | 1.6346–3.1439 | ||
| Severe | 1,569.4 | 346.6 | 4.5 | 3.2649–6.2798 | ||
| ESRD on HD | 2,791.0 | 327.1 | 8.8 | 6.1170–12.5234 | ||
| AUC0–∞ (μg·h/ml) | Mild | 418.1 | 324.1 | 1.3 | 0.9287–1.7923 | −0.8967 |
| Moderate | 698.1 | 306.1 | 2.3 | 1.6416–3.1680 | ||
| Severe | 1,691.5 | 348.5 | 4.9 | 3.4937–6.7421 | ||
| ESRD on HD | 3,587.7 | 329.2 | 11.2 | 7.7947–16.0163 | ||
| Cmax (μg/ml) | Mild | 100.8 | 99.3 | 1.0 | 0.5500–1.8713 | −0.4743 |
| Moderate | 90.6 | 96.3 | 0.9 | 0.5104–1.7367 | ||
| Severe | 128.5 | 107.2 | 1.2 | 0.6496–2.2103 | ||
| ESRD on HD | 377.2 | 99.9 | 3.8 | 1.9344–7.3978 | ||
| TAZ | ||||||
| AUC0–t (μg·h/ml) | Mild | 126.9 | 108.8 | 1.2 | 0.8243–1.6495 | −0.8668 |
| Moderate | 215.9 | 96.9 | 2.2 | 1.5757–3.1531 | ||
| Severe | 446.3 | 115.1 | 3.9 | 2.7407–5.4844 | ||
| ESRD on HD | 1,161.4 | 105.0 | 11.2 | 7.6524–16.3617 | ||
| AUC0–∞ (μg·h/ml) | Mild | 128.4 | 109.7 | 1.2 | 0.8343–1.6430 | −0.8673 |
| Moderate | 218.5 | 98.0 | 2.2 | 1.5881–3.1272 | ||
| Severe | 450.3 | 115.8 | 3.9 | 2.7715–5.4576 | ||
| ESRD on HD | 1,176.7 | 105.9 | 11.2 | 7.7485–16.2779 | ||
| Cmax (μg/ml) | Mild | 57.7 | 52.5 | 1.1 | 0.5837–2.0637 | −0.6086 |
| Moderate | 60.9 | 47.9 | 1.3 | 0.6767–2.3924 | ||
| Severe | 103.6 | 58.1 | 1.8 | 0.9479–3.3511 | ||
| ESRD on HD | 371.8 | 50.6 | 7.5 | 3.7324–14.8851 |
Effect of renal impairment on FEP PK parameters.
Mean peak FEP concentrations were consistent among the control groups and reached a maximum of 96.7 to 108.6 μg/ml shortly before or just after the end of the 90-min infusion. Clearances were consistent (96.0 to 109.9 ml/min) across the control groups. The concentrations in the control groups declined in a roughly monophasic manner, with the mean t1/2 being 2.7 to 3.3 h. Drug concentrations in subjects with mild, moderate, and severe renal insufficiency also reached a maximum shortly before or just after the end of the 90-min infusion. A decrease in clearance was observed beginning with the subjects with mild renal dysfunction and became more apparent with an increase in renal dysfunction, with the concentration-versus-time plots becoming flatter as the severity of renal disease increased. The key PK parameters analyzed and utilized for comparisons across renal impairment groups were AUC0–∞, Cmax, t1/2, and plasma clearance (CL). The variability of AUC0–∞, Cmax, t1/2, and CL increased with an increasing degree of renal insufficiency.
(i) Mild renal impairment. A single 4-g dose of WCK 4282 (2 g FEP + 2 g TAZ) in mild renal impairment resulted in a mean (SD) FEP serum Cmax value of 102.7 (20.1) μg/ml, whereas the mean (SD) FEP serum Cmax was 99.9 (11.8) μg/ml in healthy control subjects. Cmax was reached within the 90-min infusion time frame. The mean (SD) plasma clearance decreased to 80.2 (9.2) ml/min in subjects with mild renal impairment and was 104.2 (18.2) ml/min in control subjects, and the mean (SD) AUC0–∞ was 420.7 (51.7) μg·h/ml in subjects with mild impairment and 328.5 (59.6) μg·h/ml in control subjects.
(ii) Moderate renal impairment. A single 4-g dose of WCK 4282 (2 g FEP + 2 g TAZ) in moderate renal impairment produced a mean (SD) FEP serum Cmax of 96.9 (35.0) μg/ml, and the mean (SD) FEP serum Cmax was 96.7 (10.4) μg/ml in control subjects. The mean Cmax was reached shortly after the 90-min infusion time. The mean (SD) plasma clearance was reduced to 50.3 (20.0) ml/min in subjects with moderate renal impairment and was 109.9 (15.7) ml/min in control subjects, and the mean (SD) AUC0–∞ was markedly increased to 728.7 (211.3) μg·h/ml in subjects with moderate renal impairment and was 308.9 (46.5) μg·h/ml in control subjects.
(iii) Severe renal impairment. Following a single 2-g dose of WCK 4282 (1 g FEP + 1 g TAZ), the mean FEP serum Cmax was reached within the 90-min infusion period, similar to the findings for control subjects, who received a 4-g dose of WCK 4282 (2 g FEP + 2 g TAZ). The mean (SD) Cmax was 66.7 (19.7) μg/ml, approximately half the Cmax of 108.6 (19.8) μg/ml reached in healthy control subjects. This finding was consistent with that for the 2-g dose in the severe renal impairment group versus the 4-g dose in healthy control subjects. The mean (SD) plasma clearance was reduced to 20.9 (9.2) ml/min in subjects with severe renal impairment, roughly one-fifth of the plasma clearance of 96.0 (9.5) ml/min in healthy control subjects. The mean (SD) AUC0–∞ was increased to 882.7 (237.4) μg·h/ml in subjects with severe renal impairment and was 349.9 (32.8) μg·h/ml in healthy control subjects.
(iv) ESRD requiring HD. In subjects with ESRD requiring HD, the mean (SD) FEP plasma clearance was markedly reduced to 9.1 (1.8) ml/min; in contrast, it was 103.4 (24.3) in the control subjects. Cmax values for ESRD subjects were quite erratic and were not consistent with those for the other renal impairment groups. Mean Cmax values were 291.7 μg/ml (among the five evaluable subjects) and highly variable (273.9 μg/ml). One subject with ESRD had an exceedingly high Cmax during infusion of 4,148.4 μg/ml. This concentration was considered erroneous, and this subject was excluded from the PK analysis.
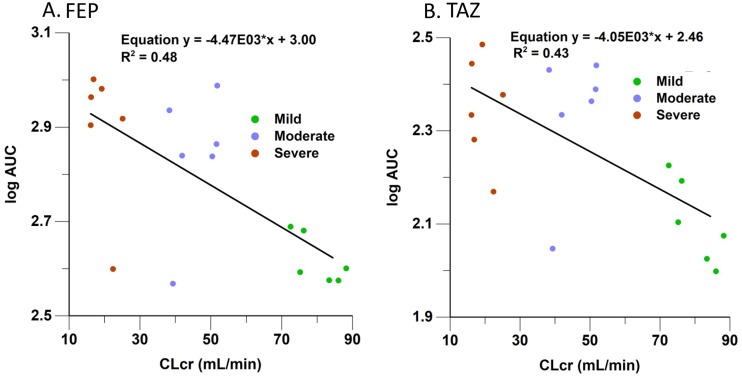
(v) Summary of FEP PK parameters across renal impairment groups. As expected, Cmax was consistent across the renal impairment groups, allowing for modest variability in patient size and the volume of distribution (V) (Table 2 and Fig. 3A). Consistent with FEP renal elimination, we observed graded decreases in plasma clearance and increases in t1/2 and AUC0–∞ with declining renal function. Figure 3A demonstrates the increase in the FEP log AUC0–∞ with declining creatinine clearance.
FIG 3.
Correlation of FEP and TAZ log AUC versus CLCR.
Effect of renal impairment on TAZ PK parameters.
Mean peak TAZ concentrations were consistent across the control groups. A Cmax of 48.3 to 59.0 μg/ml was achieved shortly before or just after the end of the 90-min infusion. The plasma clearances were consistent (289.2 to 344.5 ml/min). The concentrations in the control groups declined in a roughly monophasic manner, with the t1/2 being 1.28 to 1.53 h. Cmax reached a maximum shortly before or just after the end of the 90-min infusion in subjects with mild to moderate renal insufficiency, and the Cmax values in subjects with renal insufficiency were roughly equivalent to those in control subjects, except for those subjects requiring HD. The departure from the control profiles was not as abrupt as that seen with FEP. There was an overlap of mild renal dysfunction profiles with control subjects. The difference between the renal impairment and control profiles was more evident in the moderate impairment group. The variability of the AUC0–∞, Cmax, t1/2, and CL parameters increased with the degree of renal insufficiency.
(i) Mild renal impairment. A 4-g dose of WCK 4282 resulted in a mean (SD) Cmax of 58.0 (6.9) μg/ml, roughly equivalent to that observed in control subjects 53.0 (8.1) μg/ml. Cmax was reached within the 90-min infusion time. The mean (SD) plasma clearance was reduced to 264.0 (52.3) ml/min in subjects with mild renal impairment and was 309.0 (59.4) ml/min in controls subjects, and the mean (SD) AUC0–∞ was 130.8 (27.6) μg·h/ml in subjects with mild renal impairment and 111.7 (24.5) μg·h/ml in control subjects.
(ii) Moderate renal impairment. A single 4-g dose of WCK 4282 produced a mean (SD) Cmax of 64.7 (22.0) μg/ml; in contrast, it was 48.3 (6.5) μg/ml in the control subjects. The mean Cmax was achieved shortly after the 90-min infusion time. The mean (SD) plasma clearance was markedly reduced to 160.8 (65.4) ml/min in subjects with moderate renal impairment and was 344.5 (59.5) ml/min in control subjects, and the mean (SD) AUC0–∞ was increased to 227.2 (59.7) μg·h/ml in subjects with moderate renal impairment and was 99.4 (18.2) μg·h/ml in control subjects.
(iii) Severe renal impairment. With a single 2-g dose of WCK 4282 (1 g FEP + 1 g TAZ), the mean Cmax was reached within the 90-min infusion time. The mean (SD) Cmax was 53.3 (13.5) μg/ml in subjects with sever renal impairment, whereas it was 59.0 (11.6 μg/ml) in healthy control subjects.
The mean (SD) plasma clearance was reduced to 76.2 (20.7) ml/min in subjects with severe renal impairment and was 289.2 (30.3) ml/min in healthy control subjects. The mean (SD) AUC0–∞ was increased to 231.4 (57.5) μg·h/ml in subjects with severe renal impairment and was 116.3 (11.4) μg·h/ml in healthy control subjects.
(iv) ESRD requiring HD. The TAZ Cmax values for ESRD subjects on HD were as erratic as the values for FEP. The same subject who was excluded from the FEP PK analysis was also excluded from TAZ PK analysis because of a markedly elevated Cmax (4,146.3 μg/ml). The mean (SD) plasma clearance was markedly reduced to 29.7 (14.1) ml/min in ESRD patients, whereas it was 322.3 (80.2) μg·h/ml in healthy controls. The mean (SD) AUC0–∞ was 632.1 (257.8) μg·h/ml in ESRD patients and 108.3 (23.8) μg·h/ml in the healthy control subjects.
(v) Summary of TAZ PK parameters across renal impairment groups. As expected, Cmax was consistent across the renal impairment groups, allowing for modest variability in patient size and the volume of distribution (Table 2 and Fig. 3B). Consistent with TAZ renal elimination, we observed graded decreases in plasma clearance and increases in t1/2 and AUC0–∞ with declining renal function. Figure 3B demonstrates the increase in the TAZ log AUC0–∞ with declining creatinine clearance.
Urine excretion and renal clearance for FEP and TAZ.
Urine PK parameters for FEP and TAZ are displayed in Table 4. The amounts of FEP and TAZ excreted from time zero to the end of each collection time are summarized in Table 5. Renal elimination accounted for almost all of the intact drug in healthy subjects. Approximately 90% of the intact drug was excreted in the urine after 8 h.
TABLE 4.
FEP and TAZ urine PK parameters
| Drug, parameter (units), and statistic | Mild |
Moderate |
Severe |
ESRD on HD control (n = 6) | |||
|---|---|---|---|---|---|---|---|
| Impairment (n = 6) | Control (n = 6) | Impairment (n = 5) | Control (n = 6) | Impairment (n = 6) | Control (n = 6) | ||
| FEP | |||||||
| Ae0–48 (mg) | |||||||
| Mean | 1,981.1 | 2,030.4 | 1,562.8 | 1,914.8 | 438.7 | 1,950.2 | 2,158.1 |
| SD | 563.0 | 330.4 | 563.3 | 308.3 | 102.6 | 276.1 | 374.7 |
| CLR (ml/min) | |||||||
| Mean | 79.2 | 105.9 | 39.1 | 104.5 | 9.5 | 93.5 | 111.2 |
| SD | 23.5 | 22.1 | 10.6 | 14.6 | 3.2 | 10.8 | 26.5 |
| TAZ | |||||||
| Ae0–48 (mg) | |||||||
| Mean | 1,907.2 | 1,791.0 | 1,365.1 | 1,776.6 | 296.1 | 1,842.9 | 2,088.2 |
| SD | 639.3 | 291.0 | 412.9 | 269.3 | 53.2 | 174.8 | 241.4 |
| CLR (ml/min) | |||||||
| Mean | 249.7 | 275.9 | 109.8 | 308.0 | 23.1 | 267.6 | 339.7 |
| SD | 96.6 | 57.0 | 29.0 | 60.5 | 8.9 | 33.5 | 88.6 |
TABLE 5.
Urine recovery of FEP and TAZ
| Drug, period,a and statistic | Mild |
Moderate |
Severe |
|||
|---|---|---|---|---|---|---|
| Impairment (n = 6) | Control (n = 6) | Impairment (n = 5) | Control (n = 6) | Impairment (n = 6) | Control (n = 6) | |
| FEP | ||||||
| 0–2 h | ||||||
| Mean | 620.8 | 730.5 | 308.4 | 762.9 | ||
| SD | 191.0 | 164.4 | 252.0 | 159.4 | ||
| 0–4 h | ||||||
| Mean | 1,198.4 | 1,354.1 | 659.2 | 1,446.0 | ||
| SD | 471.1 | 243.9 | 362.3 | 183.6 | ||
| 0–8 h | ||||||
| Mean | 1,666.5 | 1,816.9 | 1,047.5 | 1,773.9 | ||
| SD | 539.0 | 262.0 | 419.3 | 272.4 | ||
| 0–12 h | ||||||
| Mean | 1,848.8 | 1,962.4 | 1,295.5 | 1,865.7 | ||
| SD | 576.4 | 301.3 | 541.3 | 301.2 | ||
| 0–24 h | ||||||
| Mean | 1,977.6 | 2,030.4 | 1,524.5 | 1,914.8 | 359.1 | 1,950.2 |
| SD | 560.4 | 330.4 | 559.7 | 308.3 | 99.3 | 276.1 |
| 0–48 h | ||||||
| Mean | 1,981.1 | 2,030.4 | 1,562.8 | 1,914.8 | 438.7 | 1,950.2 |
| SD | 563.0 | 330.4 | 563.3 | 308.3 | 102.6 | 276.1 |
| TAZ | ||||||
| 0–2 h | ||||||
| Mean | 1,053.1 | 1,086.6 | 557.8 | 1,168.9 | ||
| SD | 279.0 | 255.1 | 321.9 | 221.3 | ||
| 0–4 h | ||||||
| Mean | 1,656.7 | 1,558.3 | 1,021.2 | 1,683.7 | ||
| SD | 575.0 | 219.7 | 403.0 | 258.6 | ||
| 0–8 h | ||||||
| Mean | 1,874.6 | 1,778.2 | 1,293.0 | 1,776.6 | ||
| SD | 622.3 | 269.6 | 408.0 | 269.3 | ||
| 0–12 h | ||||||
| Mean | 1,907.2 | 1,791.0 | 1,365.1 | 1,776.6 | ||
| SD | 639.3 | 291.0 | 412.9 | 269.3 | ||
| 0–24 h | ||||||
| Mean | 1,907.2 | 1,791.0 | 1,365.1 | 1,776.6 | 296.1 | 1,842.9 |
| SD | 639.3 | 291.0 | 412.9 | 269.3 | 53.2 | 174.8 |
| 0–48 h | ||||||
| Mean | 1,907.2 | 1,791.0 | 1,365.1 | 1,776.6 | 296.1 | 1,842.9 |
| SD | 639.3 | 291.0 | 412.9 | 269.3 | 53.2 | 174.8 |
Postdose times are indicated.
Eighty-four percent of intact FEP was eliminated within 8 h in the mild renal impairment group. In the moderate and severe renal impairment groups, the mean recoveries of FEP were 74 and 43%, respectively, up to 48 h. As anticipated, renal clearances (CLR) declined in a graded relationship with worsening renal impairment. Renal clearance was reduced from 93.5 to 105.9 ml/min for control subjects to a mean (SD) of 9.5 (3.2) ml/min in severely impaired subjects.
The amounts of TAZ excreted from time zero to the end of each collection time are summarized in Table 5. Ninety percent of intact TAZ was excreted by 4 h in control subjects. The urinary recoveries of TAZ in the mild, moderate, and severe renal impairment groups were 95, 63, and 30%, respectively, at 48 h.
Renal clearances declined with increasing level of impairment, falling from 268 to 308 ml/min for control subjects to a mean (SD) of 23.1 (8.9) ml/min for severely impaired subjects. Dialysis subjects were anuric and were excluded from the urine PK analysis for both compounds (Table 5).
Parallel changes in FEP and TAZ clearances in renal impairment subjects.
To assess the relative match of CLR and systemic clearance (CLsys) for FEP and TAZ, we employed the methods developed by Derendorf and colleagues (16, 17). The plots are displayed in Fig. 4. The results of regression analysis applied to determine the mean slope and confidence intervals (CI) for impaired subject data are displayed in Table 6. The CLR- and CLsys-versus-CLCR plots show that CLR and CLsys were about 3-fold higher for TAZ than for FEP, with the CLR- and CLsys-versus-CLCR regression slopes being 1.1 and 0.93, respectively, for FEP and 3.62 and 3.0, respectively, for TAZ. These results indicate that TAZ clearances are not closely matched to FEP clearances but that the FEP/TAZ concentration ratios should be relatively independent of the degree of renal impairment, with the ratio range being approximately 3 to 3.5. This may be due to the renal secretion of TAZ, as the renal clearance for TAZ was approximately 3- to 3.5-fold higher than the CLCR and equal to the systemic clearance. This is supported by the data in Table 6.
FIG 4.
Renal clearance (CLR) and systemic clearance (CLsys) versus CLCR for FEP and TAZ.
TABLE 6.
Linear mixed-effects analysis of CLR and CLsys versus CLCR for renal impairment subjects
| Compound | Parameter | Mixed-effect model | Mean slope | Lower CI | Upper CI | P value |
|---|---|---|---|---|---|---|
| FEP | CLR | Intercept + CLCR | 1.10 | 0.78 | 1.42 | 2.16E−06 |
| TAZ | 3.62 | 2.42 | 4.83 | 9.41E−06 | ||
| FEP | CLsys | Intercept + CLCR | 0.93 | 0.64 | 1.21 | 3.55E−06 |
| TAZ | 3.00 | 2.03 | 3.96 | 6.28E−06 |
Safety evaluation.
All 48 subjects were included in the safety evaluation of the single intravenous dose of WCK 4282. No treatment-emergent adverse events (TEAEs) or severe adverse events were observed in any treatment group. No increasing or decreasing trends from the baseline were observed in clinical laboratory test parameters, vital signs, electrocardiogram (ECG) results, or physical examination findings. There were no clinically significant abnormal findings noted for any of these safety parameters.
DISCUSSION
A phase 1, open-label, single-dose study was conducted to evaluate the PK and safety of FEP and TAZ following a single 90-min i.v. infusion of WCK 4282 in male and female subjects with normal and impaired renal function. The primary route of elimination for both FEP and TAZ is via renal excretion. Because of the potential for safety concerns, a reduced dose of FEP-TAZ was employed in the groups with severe renal impairment and ESRD. A reduction in clearance and corresponding increases in t1/2 and AUC0–∞ were observed beginning with the mild renal impairment group and became more pronounced in the moderate and severe impairment groups and the group with ESRD on HD. In addition, the variability of the PK parameters increased with increasing renal impairment. The recovery of intact FEP and TAZ was high for control subjects and subjects with mild renal impairment. On the other hand, the renal recovery of intact drug was markedly reduced in subjects with moderate and severe renal impairment.
Our results for FEP PK are consistent with those of previous investigations (13–15) in subjects with renal impairment. Our group observed a mean (SD) t1/2 of 2.56 (0.612) h and a mean (SD) CL of 102 (21) ml/min in matched healthy subjects and a t1/2 of 10.4 (4.77) h and a mean (SD) CL of 25.7 (10.6) ml/min in subjects with severe renal impairment with a mean (SD) creatinine clearance of 17.98 (6.46) ml/min. As anticipated, TAZ elimination was prolonged in subjects with renal impairment. The mean (SD) plasma clearance was reduced from 289.2 (30.3) ml/min in control subjects to 76.2 (20.7) ml/min in subjects with severe renal impairment. The mean (SD) half-life increased from 1.53 (0.49) h in control subjects to 3.70 (1.00) h in subjects with severe renal impairment. Our findings indicate that modification of the dose and/or the duration of infusion of WCK 4282 is indicated for subjects with moderate and severe renal impairment and subjects on HD.
The dosing of cefepime at 1 g every 12 h (q12h) is considered a low dose, and the dosing of cefepime at 2 g q8h is considered a high dose (7). Cefepime at the higher dose of 2 g every 8 h has an incidence of probably related adverse events, consisting of rash (4%), diarrhea (3%), nausea (2%), vomiting (1%), pruritus (1%), fever (1%), and headache (1%). During postmarketing surveillance, significant neurotoxicity reactions have been reported, including encephalopathy, myoclonus, and seizures, mostly in subjects with renal impairment who did not receive an appropriate dosage adjustment. The doses of FEP and TAZ used in this study are approved and used in other pharmaceutical products in humans. Significant accumulation is not expected with either FEP or TAZ because the plasma elimination half-life is short. Nevertheless, repeated dosing in patients with significant renal impairment has been associated with the possibility for neurotoxicity. Accordingly, dose adjustment to the lower dose of 1 g is recommended for the severe impairment and ESRD groups.
Spuriously elevated drug concentrations were observed for both FEP and TAZ in the ESRD population. In one subject, the concentrations measured during infusion were manyfold higher for both FEP and TAZ than the values for the other subjects with ESRD. We consider that the erratic FEP and TAZ concentrations were produced by the infusion of drug and the drawing of PK samples from the same nonaccess arm, combined with a distorted venous anatomy and a consequent shunting from the site of infusion to the site of sampling. Each of the dialysis subjects had a vascular access for HD in one arm, and therefore, blood drawing or i.v. catheter placement could not be performed in that arm. Consequently, both i.v. WCK 4282 infusion and blood PK sampling were conducted in the same nonaccess arm. To prevent shunting from the infusion site to the sampling site, the i.v. dose was infused proximally into a large antecubital vein and PK sampling was from a catheter placed in a large distal hand vein.
Subjects on HD may have a rearrangement of the venous anatomy due to the damage to veins that occurs over time with multiple phlebotomies, i.v. catheters, and previous access surgeries. Distortion of the venous anatomy may have resulted in some runoff from the infusion site directly into the sampling site, resulting in such high PK concentrations. Consistent with this explanation is that both FEP and TAZ plasma concentrations were reduced to the expected range rapidly after the i.v. infusion was complete. Further, the concentrations dropped immediately when the infusion finished.
A small runoff of a 1-g dose could produce a substantial spurious increase in Cmax. In addition, the erroneous Cmax values were observed for both FEP and TAZ in the same patient. Given the large number of samples collected and the fact that both FEP and TAZ concentrations were elevated in parallel, we consider that phlebotomist error or contamination was unlikely. The PK samples obtained from this subject were excluded from PK summary analysis.
The entire study cohort of 48 subjects was included in the safety evaluation. Following a single i.v. dose of WCK 4282, we observed no treatment-emergent adverse events, serious adverse events, or deaths in this single-dose study. In addition, we observed no clinically significant findings or increasing or decreasing trends from the baseline in any safety parameter, including clinical assessment, laboratory evaluation, or ECG.
Our investigation determined that the clearances of both FEP and TAZ, administered as a 90-min i.v. infusion, were reduced and the corresponding t1/2 and AUC0–∞ were increased in a graded relationship with worsening renal impairment. These alterations were markedly amplified in the severe impairment and ESRD cohorts. Our results are consistent with the fact that a large percentage of both drugs is eliminated by renal excretion and indicate that adjustments to the dose and/or the duration of infusion will be required depending on the degree of renal impairment. A single i.v. infusion of WCK 4282 was generally well tolerated in subjects with renal impairment and subjects with normal renal function.
MATERIALS AND METHODS
The primary objective of the study was to assess the effect of renal impairment on the PK of WCK 4282. The secondary objective of the study was to assess the safety and tolerability of WCK 4282 following a single intravenous dose in subjects with normal and impaired renal function. This investigation (ClinicalTrials.gov identifier NCT02709382) was approved by the Western Institutional Review Board and the Human Subjects Research Office of the University of Miami and was performed in accordance with U.S. FDA regulations, ICH E6 (R1) good clinical practice, and the Declaration of Helsinki. Written informed consent was obtained directly from all participants prior to entry into the study and prior to any study procedures.
Overall study design.
This investigation was a phase 1, open-label, parallel-group study conducted at a single academic phase 1 research center to evaluate the PK, safety, and tolerability of WCK 4282 in subjects with renal impairment and healthy control subjects. The U.S. FDA recommends conducting a pharmacokinetic study in subjects with impaired renal function when the drug is likely to be used in such patients and when renal impairment is likely to alter the pharmacokinetics of the drug and/or its active metabolites (18, 20, 21). The design and conduct of this study follow recommendations described in an U.S. FDA guidance document dated March 2010 (18).
The study cohorts consisted of 48 male and female subjects 18 to 80 years of age with mild renal impairment (n = 6), moderate renal impairment (n = 6), severe renal impairment (n = 6), and stable end-stage renal disease (ESRD) requiring hemodialysis (HD; n = 6) and 24 healthy control subjects matched for sex, age (±10 years), and BMI (±15%) enrolled in a 1:1 ratio. The enrollment of all 48 study participants was conducted over a period of 3 to 4 months in 2016.
A 90-min infusion of 4 g WCK 4282 (2 g FEP + 2 g TAZ) was administered to mild and moderate renal impairment subjects and matched control subjects, and a 90-min infusion of 2 g WCK 4282 (1 g FEP + 1 g TAZ) was administered to severe renal impairment subjects and patients with ESRD requiring HD (Table 7). Subjects with renal impairment were stratified into impairment and dosage groups on the basis of the Cockcroft-Gault-estimated creatinine clearance (in milliliters per minute) (22).
TABLE 7.
Renal impairment group and WCK 4282 dosage
| Group | Impairment category | CLCR (ml/min) | No. of subjects | WCK 4282 dose (g) |
|---|---|---|---|---|
| 1 | Healthy | ≥90 | 24 | 4 (2 FEP and 2 TAZ) |
| 2 | Mild | 60–89 | 6 | 4 (2 FEP and 2 TAZ) |
| 3 | Moderate | 30–59 | 6 | 4 (2 FEP and 2 TAZ) |
| 4 | Severe | <30a | 6 | 2 (1 FEP and 1 TAZ) |
| 5 | ESRD on HD | 6 | 2 (1 FEP and 1 TAZ) |
The subjects were not on dialysis.
The study consisted of a screening period (days −28 to −1), check-in (day −1), 4 total days of confinement (days −1 to 3), and a follow-up visit within 7 to 14 days after discharge. Subjects remained confined in the Clinical Pharmacology Research Unit from day −1 (1 day prior to study drug administration) through the completion of the scheduled study procedures on the end-of-confinement day (day 3). Subjects received a standardized menu during confinement days. On day 1, each enrolled participant received a single i.v. infusion of WCK 4282 over 90 min. Blood and urine samples for determination of FEP and TAZ concentrations were obtained predose and at time points up to 48 h postinitiation of WCK 4282 infusion. Subjects requiring HD were admitted to the Clinical Pharmacology Research Unit after their routine dialysis and administered the drug on a nondialysis day.
Safety was assessed throughout the study by evaluation of local tolerability at the injection site, adverse event (AE) monitoring, clinical laboratory tests, electrocardiogram (ECG), physical examination, and monitoring of vital signs.
Study participants.
To be eligible for study participation, men or nonpregnant women were required to be 18 to 80 years of age and have a body mass index of between 18 and 40 kg/m2. Subjects with renal impairment were required to have had a stable renal function for at least 1 month prior to screening, as determined by the investigator. Subjects receiving hemodialysis were required to have a stable dialysis prescription, a stable clinical status (no significant changes in symptomatology, physical findings, or medical regimen), and a stable laboratory profile for 2 months, in the estimation of the investigator.
Healthy control subjects were required to have no evidence of any disease or condition that could affect the PK of FEP or TAZ and normal or nonclinically significant findings at physical examination, by clinical laboratory evaluations, and on the electrocardiogram. Subjects with renal impairment were required to have a resting blood pressure of 90 to 155/50 to 100 mm Hg, whereas healthy volunteers were required to have a resting blood pressure of 90 to 145/60 to 95 mmHg, a resting heart rate of 40 to 100/min, and a 12-lead ECG with no clinically significant findings. The QT interval corrected by Fredericia (QTcF) was required to be <500 ms in subjects with renal impairment and <470 ms in healthy volunteers. Males agreed to not donate sperm for 6 weeks after the dose of study drug. Female subjects had to have been of nonchildbearing potential with at least 12 months of spontaneous amenorrhea, had to have undergone sterilization at least 6 months prior to screening or had to practice 2 highly effective methods of birth control (determined by the investigator; one of the methods was required to be a barrier technique) until 6 weeks following administration of the study drug.
Potential subjects were excluded if they were participating in another investigational drug or device study or had been treated with an investigational drug within 30 days or 5 half-lives, whichever was longer, before dosing. Potential subjects were excluded if there was evidence of an uncontrolled cardiovascular, central nervous, respiratory, endocrine, immune, or hematologic system disorder, a malignancy, or any other disorder that, in the opinion of the investigator, would confound the subject’s participation and follow-up in the clinical trial. Other exclusion criteria were a history of clinically significant drug allergy to the study drug or related drugs, a history of significant drug abuse during the past 1 year, or any positive test for drug abuse at screening or day −1, unless the positive drug screen was due to documented prescription drug use. Medications essential for the management of renal impairment and concomitant stable medical conditions for the renal impairment subjects were allowed per the discretion of the investigator. Potential subjects were excluded if they had received FEP or TAZ within 2 months of study drug administration or were allergic to or intolerant of either FEP or TAZ.
Healthy volunteers were excluded for use of any concomitant medication or supplement within 7 days from screening, except for those deemed safe for the study by the investigator and medical monitor. Healthy volunteers and participants with renal impairment were excluded if there was a history of any clinically significant chronic and/or active hepatic disease or elevations of aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels to more than 1.5 times the upper limit of normal (ULN), hepatitis (hepatitis A, B, or C), or biliary tract disease or a history of any significant gastrointestinal surgery.
Blood collection for pharmacokinetic analysis.
Six-milliliter blood samples for PK analysis for FEP and TAZ were collected predose and at 0.25, 0.5, 0.75, 1.0, 1.25, 1.5, 1.75, 2.0, 2.5, 3.0, 4, 6, 8, 10, 12, 18, 24, 30, 36, and 48 h postinitiation of WCK 4282 infusion for all cohorts. Urine was collected predose and at intervals of 0 to 2, 2 to 4, 4 to 8, 8 to 12, 12 to 24, and 24 to 48 h in subjects with mild and moderate renal impairment and healthy controls and at intervals of 0 to 24 and 24 to 48 h in subjects with severe renal impairment. Subjects with ESRD on HD were unable to produce urine and were not included in the urine drug recovery analysis. Both plasma and urine samples were shipped to Keystone Bioanalytical Inc., North Wales, PA.
Bioanalytical methods.
The concentrations of FEP and TAZ were analyzed using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method. Chromatographic separation was carried out on two Imtakt Unison UK-C18 columns (the first column, 30 mm by 2 mm [inside diameter {i.d.}], 3 μm; the second column, 50 by 2 mm [i.d.], 3 μm) with a column switch. Mass spectrometric detection was performed on a triple quadrupole tandem mass spectrometer (API 5500; Applied Biosystems/MDS Sciex, Canada) equipped with a turbo ion spray source operated in the positive ionization mode. Cefepime-d3 was used as an internal standard (IS) for determination of FEP and TAZ. Plasma samples were treated with 70:30 acetonitrile-water for protein precipitation, and urine samples were diluted with 70:30 acetonitrile-water. After vortexing and centrifugation, the supernatants were transferred into injection vials containing 0.05% acetic acid and 10 mM ammonium acetate in water, and the aliquots were injected into the LC-MS/MS system for FEP and TAZ concentration analysis.
Cefepime, TAZ, and cefepime-d3 were detected in the positive mode. The ion spray voltage was set at 5.5 kV, the source temperature was maintained at 600°C, and the collision energy was set at 19 for FEP and 21 for TAZ. Quantification was obtained by using the multiple-reaction monitoring (MRM) mode of the transitions at m/z 481.1 → 396.1 for FEP, at m/z 301.1 → 168.1 for TAZ, and at m/z 484.1 → 396.1 for cefepime-d3.
Data acquisition and integration of the MRM chromatograms were performed using the software package Analyst (version 1.5.2; AB Sciex, Framingham, MA). For further data processing, including the calculation of concentration data, LIMS (version 7.4.1) software (Thermo Scientific) was used. A linear regression (weighted 1/×2) was applied to a plot of the peak area ratio versus concentration for the standards to obtain the calibration curve. The lower limit of quantitation (LLOQ) was 0.25 μg/ml for FEP and TAZ in human plasma and 15 μg/ml for FEP and 45 μg/ml TAZ in urine samples. The quantitation range was 0.25 to 150 μg/ml for FEP and TAZ in human plasma and 15 to 1,000 μg/ml for FEP and 45 to 3,000 μg/ml for TAZ in human urine.
Quality control (QC) samples were used to demonstrate adequate precision (percent coefficient of variation) and accuracy (percent relative error) of the assay. Acceptable sample stability and sensitivity were also demonstrated. For plasma QC samples, precision for intraday variability ranged from 3.12% to 8.92% for FEP and 1.89% to 13.91% for TAZ, and the accuracy for intraday variability ranged from −5.82% to 2.63% for FEP and −5.98% to −8.67% for TAZ. Precision for interday variability ranged from 3.28% to 8.38% for FEP and 3.91% to 10.09% for TAZ, and accuracy for interday variability ranged from −5.04% to 0.66% for FEP and −8.13% to 5.67% for TAZ. Recovery was 98.39% for FEP, 99.35% for TAZ, and 104.12% for cefepime-d3. Reinjection stability was confirmed for up to 67.2 h at 5°C. Postpreparative stability was confirmed for up to 53.9 h with refrigeration at 2 to 8°C. Benchtop stability was confirmed for up to 8 h in ice-water. Freeze-thaw stability was confirmed for up to 4 cycles at −70°C. Long-term stability was confirmed for up to 234 days at −70°C and 15 days at −20°C. No hemolysis effects were observed in 0.75- and 125-μg/ml FEP-TAZ QC samples prepared in hemolyzed human sodium heparin plasma. No hyperlipidemic impact was observed in 0.75- and 125-μg/ml FEP-TAZ hyperlipidemic QC samples. No matrix effect impact was observed in 6 different lots of human plasma.
For urine QC samples, precision for intraday variability ranged from 1.74% to 8.29% for FEP and 0.96% to 4.05% for TAZ, and accuracy for intraday variability ranged from −8.41% to 3.69% for FEP and −9.14% to 5.76% for TAZ. Precision for interday variability ranged from 2.58% to 6.45% for FEP and 4.03% to 8.83% for TAZ, and accuracy for interday variability ranged from 0.25% to 4.72% for FEP and 0.65% to 7.59% for TAZ. Recovery in urine was 100.01% for FEP, 101.39% for TAZ, and 96.52% for cefepime-d3. Reinjection stability was confirmed for up to 59.8 h at 5°C. Postpreparative stability was confirmed for up to 64.6 h with refrigeration at 2 to 8°C. Benchtop stability was confirmed for up to 7 h in ice-water. Freeze-thaw stability was confirmed for up to 4 cycles at −70°C. Long-term stability was confirmed for up to 192 days at −70°C. No matrix effect impact was observed in 6 different lots of human urine.
Statistical methods and pharmacokinetic analysis.
The sample size of 6 for each renal impairment group (mild, moderate, and severe impairment and ESRD) and 6 healthy matching subjects for each renal impairment group was based upon a U.S. FDA guidance document dated March 2010 (18). This guidance provides direction for the design and conduct of studies conducted to determine the effects of renal impairment on drug PK/safety. The key PK parameters analyzed were Cmax, systemic clearance (CL; in milliliters per minute), t1/2, and AUC0–∞. The primary endpoint was to compare the PK of FEP and TAZ after a single i.v. dose of WCK 4282 in subjects with renal impairment versus those in healthy volunteers with normal renal function. Noncompartmental analysis (NCA) was used to calculate the PK parameters for FEP and TAZ using WinNonlin (version 6.4) software (Pharsight Corporation, Mountain View, CA, USA). The terminal elimination rate constant (λz) evaluations necessary for calculating t1/2 were done using the default criteria in WinNonlin. Statistical evaluations and the generation of tables and figures were conducted using SAS (version 9.3; SAS Institute, Cary, NC, USA) or WinNonlin software.
The area under the concentration-time curve from time zero to the time of the last measured concentration (AUC0–t) and to infinity (AUC0–∞) for both FEP and TAZ were determined for the comparison of systemic exposure between subjects with renal impairment and healthy volunteers. The plasma PK parameters maximum observed plasma concentration (Cmax), time to the maximum observed plasma concentration (Tmax), terminal elimination rate constant (λz), elimination half-life (t1/2), systemic clearance (CL), apparent volume of distribution (Vz), and volume of distribution at steady state (Vss), based on plasma FEP and TAZ concentrations, were also determined and compared between subjects with renal impairment and healthy volunteers. Cumulative urinary excretion to the end of each postdose collection interval (Ae0–48) was determined along with the renal clearance (CLR) for comparison between the renal impairment and healthy subjects.
Plasma concentrations and PK parameters are listed and summarized by renal impairment group using descriptive statistics. The PK parameters from each group of renal impairment subjects were compared to those from their matched controls by analysis of variance (ANOVA) using the general linear models (GLM) procedure in SAS (version 9.3) software. The Cmax, AUC0–t, and AUC0–∞ values were natural log transformed prior to statistical analysis. The ratios of the geometric mean PK parameters for each renal impairment group and their matched control group were determined. Simultaneous 95% confidence intervals on the ratios of the impaired-to-control geometric means were calculated.
Because both FEP and TAZ are primarily eliminated by the kidney, a question arises as to whether subjects with mild, moderate, and severe renal impairment will have the same relative ratios of FEP/TAZ concentrations. An imbalance of the FEP/TAZ ratio could happen if renal clearance (CLR) and systemic clearance (CLsys) do not change in approximately the same direction and magnitude for both drugs. Derendorf and colleagues (16, 17) have previously approached this potential problem for the combination piperacillin-tazobactam by regression of the values of CLR, and CLsys with CLCR for piperacillin and for tazobactam and plotted the results for both drugs. The plots were essentially parallel, indicating that the drugs were appropriately matched for a combination product. We employed a similar regression approach to the combination FEP-TAZ.
Safety monitoring and adverse events.
Adverse events (AEs) were recorded from the time that the informed consent form was signed until the completion of the follow-up visit. The safety of a single intravenous dose of WCK 4282 was assessed by the incidence of treatment-emergent adverse events (TEAEs), clinical laboratory tests, physical examinations, vital signs, and 12-lead ECGs. Subjects were asked nonleading questions to determine the occurrence of AEs at regular intervals during the study. Severity, seriousness, and expectedness were assessed for each AE observed. Adverse events were collected throughout the study. All AEs were assessed and treated, if necessary, by the study principal investigator. The period of observation for the collection of AEs extended from the time when the subject signed the informed consent form until and including the date of the last visit or contact. An AE was defined as treatment emergent (TEAE) if its time of onset was on or after the time of study drug administration. The relationship to FEP-TAZ was rated as certainly related, probably/likely related, possibly related, unlikely related, not related, or nonassessable. An AE was defined as treatment related if its relationship to the study drug was recorded as certain, probable/likely, or possible.
ACKNOWLEDGMENTS
We acknowledge Rolando Rodco and Alberto B. Alonso for study coordination, Olga Acosta for regulatory and institutional review board coordination, Michelle Monteagudo for administrative and finance support, and Ana Meza for data management. We thank our colleagues Cynthia Gates, Kenia Viamonte, Evelyne Bital, Joseph Datko, Stephanie Venero, and Karla Fongyee and the University of Miami Human Subjects Research Office for their efficient and thorough review of our project. We also acknowledge Katuska Barbery, Marjorie Paterson, and the JHS Office of Research for their careful review of our research study. Finally, we thank Barbara Cole, D. Stewart MacIntyre III, Jill Frazier Tincher, Karen Hurdle, Tatyana Vikhlyantseva, and the University of Miami Office of Research Administration for their valuable contract evaluation and execution.
This work was supported by a clinical research grant from Wockhardt Limited, Mumbai, India.
Richard A. Preston has received a stipend for manuscript preparation from Wockhardt Limited. This relationship has been reported to and approved by the Office of Disclosures & Relationship Management of the University of Miami. Grigor Mamikonyan is an employee of Clinartis LLC, and Clinartis LLC received a clinical research grant for conduct of parts of this clinical research work. Christopher J. Kemper and Allan Xu were contracted directly by Clinartis for biostatistical and bioanalytical support, respectively. Mushtaque Mastim, Ravindra Yeole, Rajesh Chavan, and Ashima Bhatia are employees of Wockhardt Limited, Mumbai, India. H. David Friedland is an employee of Wockhardt's Morton Grove Pharmaceutical Inc., Morton Grove, IL, USA. Dyal Garg is an employee of Clinical Research Services Inc.
REFERENCES
- 1.Sader HS, Castanheira M, Mendes RE, Flamm RK, Jones RN. 2017. Antimicrobial activity of high-proportion cefepime-tazobactam (WCK 4282) against a large number of Gram-negative isolates collected worldwide in 2014. Antimicrob Agents Chemother 61:e02409-16. doi: 10.1128/AAC.02409-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Livermore DM, Mushtaq S, Warner M, Turner SJ, Woodford N. 2018. Potential of high-dose cefepime/tazobactam against multiresistant Gram-negative pathogens. J Antimicrob Chemother 73:126–133. doi: 10.1093/jac/dkx360. [DOI] [PubMed] [Google Scholar]
- 3.Castanheira M, Duncan LR, Rhomberg PR, Sader HS. 2017. Enhanced activity of cefepime-tazobactam (WCK 4282) against KPC-producing Enterobacteriaceae when tested in media supplemented with human serum or sodium chloride. Diagn Microbiol Infect Dis 89:305–309. doi: 10.1016/j.diagmicrobio.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Riedel S, Huband MD, Sader HS, Flamm RK, Jones RN. 2017. Determination of disk diffusion and MIC quality control guidelines for high-dose cefepime-tazobactam (WCK 4282), a novel antibacterial combination consisting of a β-lactamase inhibitor and a fourth-generation cephalosporin. J Clin Microbiol 55:3130–3134. doi: 10.1128/JCM.00788-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghafur A, Tayade A, Kannaian P. 2012. Clinical profile of patients treated with cefepime/tazobactam: a new beta-lactam/beta-lactamase inhibitor combination. J Microbiol Infect Dis 2:79–86. doi: 10.5799/ahinjs.02.2012.03.0049. [DOI] [Google Scholar]
- 6.Kaur R, Gautam V, Singhal L, Ray P. 2014. Antimicrobial activity of cefepime-tazobactam combination tested against clinical isolates of Enterobacteriaceae. J Antibiot 67:603–604. doi: 10.1038/ja.2014.45. [DOI] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. 2016. M100-526 Performance standards for antimicrobial susceptibility testing: 24th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 8.Pfizer. 2017. Zosyn (piperacillin and tazobactam) for injection package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/050684s88s89s90_050750s37s38s39lbl.pdf.
- 9.Wooley M, Miller B, Krishna G, Hershberger E, Chandorkar G. 2014. Impact of renal function on the pharmacokinetics and safety of ceftolozane-tazobactam. Antimicrob Agents Chemother 58:2249–2255. doi: 10.1128/AAC.02151-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wise R, Logan M, Cooper M, Andrews JM. 1991. Pharmacokinetics and tissue penetration of tazobactam administered alone and with piperacillin. Antimicrob Agents Chemother 35:1081–1084. doi: 10.1128/aac.35.6.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drugs.com. 2019. Cefepime hydrochloride (USP) for injection. FDA prescribing information, side effects and uses. http://www.drugs.com/pro/maxipime.html.
- 12.Endimiani A, Perez F, Bonomo RA. 2008. Cefepime: a reappraisal in an era of increasing antimicrobial resistance. Expert Rev Anti Infect Ther 6:805–824. doi: 10.1586/14787210.6.6.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbhaiya RH, Knupp CA, Forgue ST, Matzke GR, Guay DRP, Pittman KA. 1990. Pharmacokinetics of cefepime in subjects with renal insufficiency. Clin Pharmacol Ther 48:268–276. doi: 10.1038/clpt.1990.149. [DOI] [PubMed] [Google Scholar]
- 14.Tam VH, McKinnon PS, Akins RL, Drusano GL, Rybak MJ. 2003. Pharmacokinetics and pharmacodynamics of cefepime in patients with various degrees of renal function. Antimicrob Agents Chemother 47:1853–1861. doi: 10.1128/aac.47.6.1853-1861.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Preston RA, Mamikonyan G, DeGraff S, Chiou J, Kemper CJ, Xu A, Mastim M, Yeole R, Chavan R, Patel A, Friedland HD, Bhatia A. 2018. Single-center evaluation of the pharmacokinetics of WCK 5222 (cefepime-zidebactam combination) in subjects with renal impairment. Antimicrob Agents Chemother 63:e01484-18. doi: 10.1128/AAC.01484-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De la Pena A, Derendorf H. 1999. Pharmacokinetic properties of beta-lactamase inhibitors. Int J Clin Pharmacol Ther 37:63–75. [PubMed] [Google Scholar]
- 17.Derendorf H, Costa TD. 1996. Pharmacokinetics of piperacillin, tazobactam and its metabolite in renal impairment. Int J Clin Pharmacol Ther 34:482–488. [PubMed] [Google Scholar]
- 18.Center for Drug Evaluation and Research. 2010. Pharmacokinetics in patients with impaired renal function—study design, data analysis, and impact on dosing and labeling (draft guidance). Guidance document www.fda.gov/downloads/drugs/guidances/ucm204959.pdf.
- 19.Reference deleted.
- 20.Huang SM, Temple R, Xiao S, Zhang L, Lesko LJ. 2009. When to conduct a renal impairment study during drug development: US Food and Drug Administration perspective. Clin Pharmacol Ther 86:475–479. doi: 10.1038/clpt.2009.190. [DOI] [PubMed] [Google Scholar]
- 21.Lalonde RL, Wagner JA. 2009. Drug development perspective on pharmacokinetic studies of new drugs in patients with renal impairment. Clin Pharmacol Ther 86:557–561. doi: 10.1038/clpt.2009.182. [DOI] [PubMed] [Google Scholar]
- 22.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]