Plazomicin is a new FDA-approved aminoglycoside antibiotic for complicated urinary tract infections (cUTI). In the product labeling, trough-based therapeutic drug management (TDM) is recommended for cUTI patients with renal impairment to prevent elevated trough concentrations associated with serum creatinine increases of ≥0.5 mg/dl above baseline.
KEYWORDS: aminoglycoside, dosing, nephrotoxicity, pharmacokinetics
ABSTRACT
Plazomicin is a new FDA-approved aminoglycoside antibiotic for complicated urinary tract infections (cUTI). In the product labeling, trough-based therapeutic drug management (TDM) is recommended for cUTI patients with renal impairment to prevent elevated trough concentrations associated with serum creatinine increases of ≥0.5 mg/dl above baseline. Herein, the utility of the Hartford nomogram to prevent plazomicin trough concentrations exceeding the TDM trough of 3 μg/ml and optimize the area under the curve (AUC) was assessed. The AUC reference range was defined as the 5th to 95th percentile AUC observed in the phase 3 cUTI trial (EPIC) (121 to 368 μg · h/ml). Observed 10-h plazomicin concentrations from patients in EPIC (n = 281) were plotted on the nomogram to determine an eligible dosing interval (every 24 h [q24h], q36h, q48h). Based on creatinine clearance (CLcr), a 15- or 10-mg/kg of body weight dose was simulated with the nomogram-derived interval. The nomogram recommended an extended interval (q36h and q48h) in 31% of patients. Compared with the 15 mg/kg q24h regimen received by patients with CLcr of ≥60 ml/min in EPIC, the nomogram-derived interval reduced the proportion of patients with troughs of ≥3 μg/ml (q36h, 27% versus 0%, P = 0.021; q48h, 57% versus 0%, P = 0.002) while significantly increasing the number of patients within the AUC range. Compared with the 8 to 12 mg/kg q24h regimen (received by patients with CLcr of >30 to 59 ml/min in EPIC), the nomogram-derived interval significantly reduced the proportion of troughs of ≥3μg/ml in the q48h cohort (72% versus 0%, P < 0.001) while maintaining a similar proportion of patients in the AUC range. Simulated application of the Hartford nomogram optimized plazomicin exposures in patients with cUTI while reducing troughs to <3 μg/ml.
INTRODUCTION
Aminoglycoside antibiotics have long held a therapeutic role in the treatment of Gram-negative infections in the urinary tract (1, 2). The increasing rate of β-lactam and fluoroquinolone resistance has prompted a renewed interest in aminoglycoside use (3, 4). In an effort to optimize aminoglycoside exposures for clinical efficacy and minimize dose-related toxicities, therapeutic drug monitoring (TDM) for the currently available aminoglycoside antibiotics has become routine (5, 6). The Hartford Hospital extended-interval aminoglycoside dosing nomogram (referred to as the Hartford nomogram) was developed in the mid-1990s as a simple and reliable tool for determining a daily (every 24 [q24h]) or extended (q36h or q48h) dosing interval for gentamicin, tobramycin, and later, amikacin when using a higher than conventional mg/kg dose (7). Plotting of a single random aminoglycoside concentration collected 6 to 14 h after administration of a high dose (i.e., gentamicin or tobramycin at 7 mg/kg of body weight) proposed a dosing interval that resulted in trough concentrations less than or equal to 1 μg/ml. Accordingly, reductions in aminoglycoside-induced nephrotoxicity have been observed with the application of nomogram-derived dosing intervals (5, 7, 8).
Plazomicin is a recently FDA-approved aminoglycoside antibiotic for the treatment of complicated urinary tract infections (cUTI), including pyelonephritis, in patients 18 years of age or older (9). Plazomicin is structurally modified to avoid degradation by clinically relevant aminoglycoside-modifying enzymes that would otherwise result in resistance to other aminoglycosides (10–12). Plazomicin inhibits bacterial protein synthesis and has rapid bactericidal activity against many extended-spectrum β-lactamase (ESBL)-producing, aminoglycoside-resistant, and carbapenem-resistant isolates (13–16).
In the ACHN-490-009 phase 3 clinical trial (ClinicalTrials.gov identifier NCT02486627; EPIC trial) in patients with cUTI or acute pyelonephritis, plazomicin dosing was guided by daily creatinine clearance (CLcr) without the use of therapeutic drug monitoring (TDM) (17). Plazomicin achieved the primary objective of noninferiority against meropenem. Serum creatinine increases of ≥0.5 mg/dl above baseline at any time during EPIC, were observed in approximately 4% and 7% of the meropenem-treated and plazomicin-treated patients, respectively. These changes in serum creatinine primarily occurred in patients with creatinine clearance (CLcr) of ≤90 ml/min and were generally associated with a plazomicin trough concentration of ≥3 μg/ml. Most serum creatinine increases were ≤1 mg/dl above baseline and returned to <0.5 mg/dl from the baseline value after completion of plazomicin therapy (17).
Given a similar pharmacokinetic profile of plazomicin to other aminoglycosides (9, 11), we hypothesized that plazomicin concentrations, with application of the appropriate dose ratio, could be plotted on the Hartford nomogram in order to select a dosing interval that would reduce the proportion of patients with a simulated trough concentration of ≥3 μg/ml while maintaining a high proportion of patients with simulated area under the curve (AUC) exposures within the AUC reference range from EPIC. Similar to amikacin, a dose ratio is used to correct for differences in the mg/kg clinical dose for plazomicin compared to gentamicin and tobramycin, for which the nomogram was developed. Application of the Hartford nomogram, which relies on a single measurement 6 to 14 h after dosing, could allow an interval adjustment 10 to 18 h prior to the next dose for patients on a q24h dosing interval. Plazomicin-treated patients enrolled in EPIC had plasma collected after the second, third, or fourth dose for plazomicin concentration determination and pharmacokinetic analyses. At least one of the collected plasma samples was within the 6- to 14-h time window, permitting application of the Hartford nomogram to select a dosing interval, followed by simulation of that regimen to evaluate theoretical trough concentrations and AUC exposures.
RESULTS
Patient characteristics.
A total of 609 patients were enrolled in the EPIC trial, with 303 receiving at least one dose of plazomicin. Of the 303 plazomicin-treated patients, 22 were excluded from these analyses, as pharmacokinetic parameters could not be estimated based on the collected pharmacokinetic (PK) data, leaving 281 patients available for application of the Hartford nomogram and simulation. Table 1 highlights the clinical characteristics for these evaluable patients. Males and females were equally represented, and the majority of patients (189/281, 67%) had a CLcr of ≥60 ml/min.
TABLE 1.
Characteristics of evaluable plazomicin-treated patientsa
| Participant characteristics (n = 281) | Value |
|---|---|
| Female sex, n (%) | 157 (55.9) |
| Infection type | |
| cUTI, n (%) | 169 (60.1) |
| AP, n (%) | 112 (39.9) |
| Age (years), mean (SD) | 58 (18) |
| Total body weight (kg), mean (SD) | 76 (16) |
| CLcr (ml/min)b , median (IQR) | 77.4 (55.4–95.5) |
| CLcr, n (%) | |
| ≥60 ml/min | 189 (67.3) |
| 59–31 ml/min | 92 (32.7) |
| Plazomicin dose (mg), median (IQR) | 960 (770–1,120) |
| Any rise in Scr ≥0.5 mg/dl, n (%) | 17 (6.0) |
CLcr obtained on day of plazomicin concentration sampling.
AP, acute pyelonephritis; SD, standard deviation; IQR, interquartile range; Scr, serum creatinine.
Hartford nomogram application.
The observed mean (standard deviation [SD]) plazomicin concentration at 10 h was 7.7 (5.9) μg/ml for the 281 patients. Mean (SD) 10-h concentrations were higher for patients with lower CLcr (CLcr of >30 to 59 ml/min, 9.0 [5.6] μg/ml versus CLcr of ≥60 ml/min, 7.1 [5.9] μg/ml). When corrected by the dose ratio (i.e., the ratio of administered dose to 7 mg/kg [the original gentamicin/tobramycin dose used in the Hartford nomogram]), 194 (69%) and 87 (31%) patients were eligible for a q24h dosing interval and an extended interval (q36h or q48h), respectively. A significantly larger proportion of patients with CLcr of >30 to 59 ml/min were eligible for an extended dosing interval than patients with CLcr of ≥60 ml/min (55.4% [51/92] versus 19% [36/189], P < 0.001). For patients with a CLcr of ≥60 ml/min (n = 189), 12% were eligible for the q36h regimen and 7% were eligible for the q48h regimen. For patients with CLcr of >30 to 59 ml/min (n = 92), the nomogram recommended q36h and q48h intervals in 24% (n = 22) and 32% (n = 29) of patients, respectively.
Serum creatinine increases of ≥0.5 mg/dl above baseline.
Serum creatinine increases of ≥0.5 mg/dl above baseline at any time during the trial were observed in 17/281 (6.0%) plazomicin-treated patients for whom PK parameters could be estimated. Compared with patients with trough concentrations of <3 μg/ml, the incidence of serum creatinine increases of ≥0.5 mg/dl above baseline was significantly higher in patients with observed troughs of ≥3 μg/ml (6/231 [2.6%] versus 11/50 [22.0%], P < 0.001). Of note, 39/87 (44.8%) of patients eligible for an extended interval had observed trough concentrations of ≥3 μg/ml compared with 11/194 (5.8%) of patients eligible for a q24h interval (P < 0.001). Similarly, patients eligible for an extended interval had a higher incidence of serum creatinine increases of ≥0.5 mg/dl above baseline compared with patients eligible for a q24h interval (12.6% versus 3.1%, P = 0.002).
Simulated plazomicin exposures.
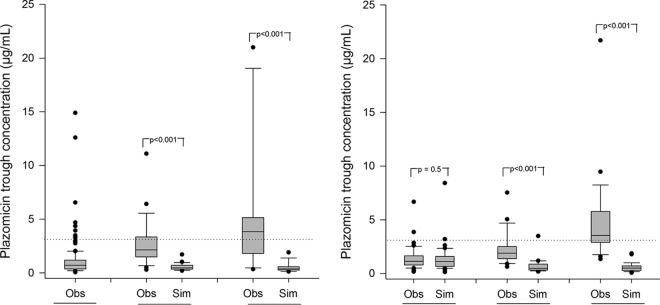
Plazomicin trough concentrations and interval-normalized AUC0–24h (nAUC) values were simulated for patients eligible for an extended dosing interval and compared with the troughs and AUC0–24h values observed in EPIC (Table 2). The nAUC for the simulated patients was defined as the AUC0–24h for q24h-eligible patients, AUC over the 36 h divided by 1.5 for q36h-eligible patients, and AUC over the 48 h divided by 2 for q48h-eligible patients. Fig. 1 illustrates the median trough concentrations for the observed versus simulated patients in each dosing interval cohort (i.e., patients eligible for a q24h, q36h, and q48h interval).
TABLE 2.
Proportion of patients with trough of ≥3 μg/ml and mean nAUC (μg/ml) after simulation with Hartford nomogram-recommended extended interval
| Dosing interval | CLcr ≥ 60 ml/min (n = 189)b
|
CLcr > 30 to 59 mL/min (n = 92)b
|
||||
|---|---|---|---|---|---|---|
| Trough ≥3 μg/ml (n [%]) | nAUC,d mean (SD) | Trough <3 μg/ml and nAUC within reference rangee (n [%]) | Trough ≥3 μg/ml (n [%]) | nAUC,d mean (SD) | Trough <3 μg/ml and nAUC within reference rangee (n [%]) | |
| Eligible for q24h interval | ||||||
| Observeda | 9/153 (5.9%) | 234 (83) | 128/153 (83.7%) | 2/41 (4.9%) | 182 (54) | 32/41 (78%) |
| Simulated | –c | – | – | 2/41 (4.9%) | 166 (47) | – |
| P value | NDf | ND | ND | 1.000 | 0.156 | ND |
| Eligible for q36h interval | ||||||
| Observed | 6/22 (27.3%) | 312 (71) | 12/22 (54.5%) | 4/22 (18.2%) | 253 (73) | 16/22 (72.7%) |
| Simulated | 0/22 (0%) | 203 (36) | 22/22 (100%) | 1/22 (4.5%) | 153 (37) | 16/22 (72.7%) |
| P value | 0.021 | <0.001 | <0.001 | 0.345 | <0.001 | 1.000 |
| Eligible for q48h interval | ||||||
| Observed | 8/14 (57.1%) | 407 (102) | 5/14 (35.7%) | 21/29 (72.4%) | 323 (127) | 8/29 (27.6%) |
| Simulated | 0/14 (0%) | 205 (53) | 14/14 (100%) | 0/29 (0%) | 153 (56) | 23/29 (79.3%) |
| P value | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Observed AUC0–24h (μg · h/ml) and trough values based on q24h regimen received during the EPIC trial.
CLcr determined per EPIC trial protocol using the Cockcroft-Gault formula.
Simulation of nAUC and trough concentrations not performed, as patients were Hartford nomogram eligible for an interval that was utilized in EPIC (i.e., plazomicin 15 mg/kg q24h).
Interval-normalized AUC0–24h (nAUC, μg · h/ml) is AUC0–24h for q24h patients, AUC0–36h divided by 1.5 for q36h patients, and AUC0–48h divided by 2 for q48h patients.
The AUC reference range was defined as the 5th to 95th percentile AUC observed in the phase 3 cUTI trial (EPIC) (121 to 368 μg · h/ml).
ND, not done.
FIG 1.
Median observed (obs) versus simulated (sim) plazomicin trough concentrations according to Hartford nomogram dosing eligibility among patients with (left) CLcr of ≥60 ml/min and (right) CLcr of >30 to 59 ml/min. Boxes represent the median and 25th and 75th percentiles. Whiskers represent the 5th and 95th percentiles. Data points (dots) represent individual concentration outliers. The dashed line represents the toxicity threshold (3 μg/ml).
For patients with CLcr of ≥60 ml/min, simulation of a 15-mg/kg dose with a nomogram-derived extended interval (q36h or q48h) in eligible patients decreased the proportion of patients with AUC above the reference AUC range (121 to 368 μg · h/ml), resulting in a mean nAUC (± standard deviation) (q36h, 203 ± 36 μg · h/ml; q48h, 205 ± 53 μg · h/ml) comparable to the mean AUC of patients eligible to continue a q24h dosing interval (q24h, 234 ± 83 μg · h/ml) (Table 2). Similarly, for patients with CLcr of >30 to 59 ml/min, simulation of a 10-mg/kg dose with a nomogram-derived extended interval in eligible patients resulted in mean nAUC (q36h, 153 ± 37 μg · h/ml; q48h, 153 ± 56 μg · h/ml) comparable to the mean AUC of patients eligible to continue a q24h dosing interval (q24h, 182 ± 54 μgml).
Most importantly, at the plazomicin doses (mg/kg) recommended for patients in both renal function cohorts, application of the Hartford nomogram resulted in marked improvement in the proportion of patients that achieved plazomicin exposures (nAUC) within the reference range and concurrently had a trough of <3 μg/ml. Overall, 73% to 100% of patients met this safety and efficacy metric (Table 2).
DISCUSSION
Optimizing antimicrobial therapy is critical to improving clinical outcomes among patients with serious and life-threatening bacterial infections (18–20). Conventionally, aminoglycosides have been administered as multiple daily doses with serial determinations of plasma concentrations to evaluate efficacy and monitor toxicity. Early understanding of the pharmacodynamic and pharmacokinetic principles underlying aminoglycosides led to the development of alternative dosing strategies in an effort to maximize the peak concentration (Cpeak)/MIC ratio and reduce the risk of toxicity (8, 21). Plazomicin, administered as a once daily aminoglycoside, takes advantage of this class-wide pharmacodynamic strategy. Of note, the plazomicin AUC/MIC ratio been shown to correlate best with efficacy in animal and in vitro models of infection (9, 22).
Per the plazomicin FDA product label, TDM is recommended to maintain plasma trough concentrations below 3 μg/ml for cUTI patients with CLcr of ≥15 ml/min and <90 ml/min (9). Unfortunately, TDM protocols incorporating safety and efficacy for clinicians and pharmacists do not exist—hence the urgent need to provide a simple and reliable tool for TDM and dosage selection. With this study, we show that the validated and widely adopted Hartford nomogram for traditional aminoglycoside dosing can be used for plazomicin TDM. Our results suggest that use of the Hartford nomogram will successfully reduce trough concentrations and increase the proportion of AUC values in the desired range in patients with cUTI. Among all patients evaluated in the current analyses, the reported incidence of serum creatinine increases ≥0.5 mg/dl above baseline was 6%. The incidence of serum creatinine increases of ≥0.5 mg/dl above baseline was substantially higher among patients with a trough of ≥3 μg/ml than among patients with a trough of <3 μg/ml. Similarly, elevated troughs and corresponding serum creatinine increases of ≥0.5 mg/dl above baseline were more common in patients with CLcr of >30 to 59 ml/min versus CLcr of ≥60 ml/min. This reaffirms the well-described association between declining renal function and an increased risk of aminoglycoside-induced nephrotoxicity (23–25). Irrespective of baseline renal function, however, patients eligible for extended intervals based on their 10-h concentrations were more likely to have higher troughs and observed serum creatinine increases of ≥0.5 mg/dl above baseline compared with patients who would continue to require q24h dosing.
One in three patients (31%) with cUTI included in this analysis was eligible for an extended-interval regimen after application of the Hartford nomogram, indicating a substantial role for the utilization of the nomogram in clinical practice. The primary finding in this study was the reduction in the proportion of simulated patients with a trough of ≥3 μg/ml relative to observed troughs in the EPIC trial, in which trough-based TDM was not implemented per the protocol. Indeed, in patients eligible for extended-interval dosing regimens, simulated plazomicin trough concentrations were on average 5-fold lower than trough concentrations observed following the q24h regimen administered in the EPIC trial (Fig. 1). This is not surprising given the linear, dose-proportional pharmacokinetics of aminoglycosides, including plazomicin.
Given the favorable clinical and microbiological outcomes observed in the pivotal phase 3 trial (17), a surrogate for maintaining comparable plazomicin efficacy, the 5th to 95th percentile AUC0–24h values observed in EPIC (121 to 368 mgliter) were selected as the benchmark AUC range. For all patients with CLcr of ≥60 ml/min and a 10-h concentration necessitating an extended interval, the simulated mean nAUC fell within this reference range. By extending the interval from q24h to q36h or q48h, the mean nAUC was lower, and a corresponding shift in the number of patients with nAUC above range to within range was observed. Among patients with moderate renal impairment (CLcr, >30 to 59 ml/min), the simulated mean nAUC was significantly lower than the q24h regimen received; however, no significant difference in the proportion of patients achieving an nAUC within the reference range was observed. Overall, application of the Hartford nomogram resulted in marked improvement in the number of patients that concurrently maintained AUC exposures within the reference range and achieved trough concentrations of <3 μg/ml. This combined metric provides clinical relevance by taking into account both the safety and efficacy of the Hartford nomogram for plazomicin dosing.
For cUTI patients with CLcr of ≥15 ml/min and <90 ml/min, TDM-based adjustment in the current plazomicin product label involves extending the dosing interval by 1.5-fold (i.e., from q24h to q36h or from q48h to q72h) for plasma trough concentrations of ≥3 μg/ml (9). This strategy utilizes the trough, drawn approximately 30 minutes prior to the subsequent dose, as the monitoring parameter to minimize dose-related toxicities. Consequently, a second plazomicin dose can be administered before the results of TDM samples are available for the dosage interval decision. On the other hand, obtaining a plazomicin concentration earlier (i.e., drawn between 6 and 14 h for plotting on the Hartford nomogram) is highly attractive, as it could lead clinicians to identify patients at risk for elevated trough concentrations sooner and permit the selection of a new dosing interval before a second dose is administered. However, since trough-based TDM was not utilized within the EPIC trial, no conclusions can be made with regard to the performance of the Hartford nomogram compared with trough-based TDM recommended within the product label.
The following study limitations should be considered when interpreting results. Patients with a baseline CLcr of ≤30 ml/min were excluded from the EPIC trial. As such, these analyses are not applicable to this patient population and warrant further studies. Furthermore, clinicians should ensure that individual patients match the population for which the original nomogram was developed (i.e., exclude pediatric, pregnant, burn, ascite, and dialysis patients) until otherwise proven applicable. Individualization of patient therapy remains critical to obtaining optimal outcomes. As with original application of the nomogram for gentamicin and tobramycin, if the single plazomicin concentration falls near the q36h or q48h recommendation line, the longer interval should be chosen to avoid drug accumulation and associated dose-related toxicities (7).
We acknowledge that a TDM strategy for plazomicin (and all aminoglycosides) that utilizes serial plasma concentrations at exact time points from a patient during a dosing interval will allow one to better characterize the pharmacokinetic drug profile of that individual. However, while this is feasible in research studies, in the clinical setting, where resources and sampling time are limited, the availability of once-daily nomograms such as the Hartford nomogram (which requires a single blood draw) provides convenience, cost saving, and equivalent patient outcomes (6). Of note, this current study utilized CLcr values available from the various study sites enrolled in the EPIC trial, in contrast to the central study laboratory CLcr values. This was used for nomogram application in order to incorporate a level of variability in CLcr estimates that can be observed with different clinical laboratories, thus adding robustness to the study findings. Our study design involved trough and AUC exposure simulations in the patients who participated in the EPIC trial based on their individual pharmacokinetic parameters derived from a previously conducted population pharmacokinetic analysis (26). The application of patient-specific pharmacokinetics and the product labeling dosing recommendations provided a robust framework for the utility of the Hartford nomogram in patients receiving plazomicin for cUTI. As a result of this methodology, a prospective trial utilizing the Hartford nomogram should be conducted to clinically validate our findings and the real-world incidence of plazomicin nephrotoxicity.
In conclusion, the application of the Hartford nomogram allowed for identification of daily and extended plazomicin dosing intervals in patients with cUTI while decreasing the exposure associated with nephrotoxicity in this population. Due to increasing resistance to other classes of antimicrobials, plazomicin has the potential for significant adoption in patients, and a lack of individual dosing guidance could lead to underdosing or overdosing with toxicity. The positive results of this study should therefore have a valuable impact on plazomicin TDM.
MATERIALS AND METHODS
Study design.
This was a retrospective analysis of patient data collected during the ACHN-490-009 phase 3 cUTI/AP trial (NCT02486627; EPIC). In brief, patients who participated in the EPIC trial and who had pharmacokinetic parameter estimates available had plazomicin concentrations simulated using a 15- or 10-mg/kg dose with the dosing interval based on their observed 10-h concentration and the Hartford nomogram. The study was reviewed and approved by the Hartford Hospital Institutional Review Board. Informed consent was waived because all patient data were available and collected for the purposes of the EPIC trial.
Study population.
Patients enrolled in the EPIC trial who received at least one dose of plazomicin were considered for inclusion in this study. Patients were excluded from these analyses if pharmacokinetic parameter estimates were unavailable. During the phase 3 EPIC trial, plazomicin was administered as a daily intravenous infusion over 30 minutes without the use of trough-based TDM; daily doses were adjusted based on body weight and estimated CLcr as follows: CLcr of >60 ml/min, 15 mg/kg q24h; CLcr of >50 to 60 ml/min, 12 mg/kg q24h; CLcr of >40 to 50 ml/min, 10 mg/kg q24h; and CLcr of >30 to 40 ml/min, 8 mg/kg q24h. Plazomicin concentrations for each patient were available at 0.5 h prior to the subsequent dose (trough) and at 1.5 h, 4 h, and 10 h after the start of infusion on study day 3 (±1 day). The following patient characteristics were extracted from the EPIC data set and provided by Achaogen, Inc., to the Center for Anti-Infective Research and Development for utilization during the analyses: age, sex, height, total body weight (TBW), ideal body weight (IBW), date and time plazomicin doses were administered, CLcr on day of plazomicin sample collection, and individual pharmacokinetic parameter estimates (i.e., clearance [CL], volume of distribution [V], distribution CL of peripheral compartment 1 and 2 [CLD1, CLD2], volume of peripheral compartment 1 and 2 [VP1 and VP2], and observed AUC0–24h. CLcr, available as the reported values from individual study sites from the EPIC trial, was estimated with the Cockcroft-Gault formula using TBW or IBW for patients with TBW greater than IBW by 25% or more. Additionally, nephrotoxicity (defined in the EPIC trial as any rise in serum creatinine of ≥0.5 mg/dl above the baseline value at any time during the study, including on and/or post-IV drug therapy) was also recorded.
Hartford nomogram application.
A stepwise approach was utilized to identify a dose and dosing interval for each individual simulation. As a first step, individuals were assigned a dose based on renal function. In line with product labeling recommendations, a 15-mg/kg dose was selected for patients with a baseline CLcr of ≥60 ml/min, and a 10-mg/kg dose was selected for patients with a CLcr of <60 ml/min. The dose (mg) was calculated based on actual body weight unless the patient was obese (i.e., 25% over IBW). If the patient was obese, adjusted body weight was utilized. As a second step, a daily or extended dosing interval (i.e., q36h or q48h) was determined by application of the Hartford nomogram. Clinical application of the Hartford nomogram is performed by obtaining a single random blood sample between 6 and 14 h after the start of an aminoglycoside infusion (7). The observed 10-h postdose plazomicin concentration was plotted on the nomogram after application of a ratio based on the mg/kg dose received (Equation 1):
| (1) |
where Plot C denotes concentration plotted on nomogram, ObsC is the observed plazomicin concentration, and dose is the plazomicin dose received. The dosing intervals evaluated in the simulation, q36h and q48h, were based on application of a patient’s observed 10-h concentration on the Hartford nomogram. Concentration thresholds for extending the dose were at a corrected 10-h concentration as follows: <4.8 μg/ml, eligible for q24h interval; 4.8 to 7 μg/ml, eligible for q36h interval; >7 μg/ml, eligible for q48h interval.
Pharmacokinetic analyses.
A three-compartment pharmacokinetic simulation was performed using Crystal Ball (Oracle, Inc., Redwood Shores, CA), in which plazomicin concentrations were predicted for all individual patients eligible for an extended dosing interval (q36h or q48h). Simulations were based on (i) individual pharmacokinetic parameter estimates, (ii) a 15-mg/kg or 10-mg/kg dose (determined by CLcr), and (iii) a nomogram-recommended dosing interval (based on the 10-h sampling concentration). From these simulations, a trough (0.5 h prior to the subsequent dose) and interval-normalized AUC0–24h (nAUC) based on the dosing interval were determined. All AUC values were determined using the trapezoidal rule. The nAUC for the simulated patients was defined as the AUC0–24h for q24h-eligible patients, AUC over the initial 36 h divided by 1.5 for q36h-eligible patients, and AUC over the initial 48 h divided by 2 for q48h-eligible patients. As a measure of plazomicin exposure after Hartford nomogram application, the proportion of patients within the AUC range was assessed by comparing nAUC with the 5th to 95th percentile AUC0–24h values observed in the EPIC trial (121 to 368 μgml). To evaluate safety, the proportion of patients with an observed or simulated trough of ≥3 μg/ml was evaluated, as this threshold was associated with serum creatinine increases of ≥0.5 mg/dl in the EPIC trial.
Statistical analyses.
Descriptive statistics were performed using Sigma Plot 14 (Systat Software, Inc., San Jose, CA). For categorical variables including the proportion of patients with a trough of <3 or ≥3 μg/ml, analyses were performed using the χ2 test or Fisher’s exact test. For continuous variables, including nAUC and median trough concentrations, Student’s t test, analysis of variance (ANOVA), or Mann Whitney Rank Sum test were utilized when appropriate. In each instance, a two-tailed test was carried out, and a prespecified alpha level of 0.05 was used.
ACKNOWLEDGMENTS
This study was funded by Achaogen, Inc. (South San Francisco, CA). At the time of the study, A.S.K. and J.D.S. were employees of Achaogen, Inc. The other authors have no conflicts to disclose. We acknowledge Kristie Kooken and Ellie Hershberger from Achaogen for assistance with facilitation of data transfer and for thoughtful discussion, respectively.
REFERENCES
- 1.Colgan R, Hooton T, Gupta K, Gomolin I, Childs S, Gould M. 2000. Urinary tract infections: current approaches, future directions. Postgrad Med 108:007–015. doi: 10.3810/pgm.12.2000.suppl11.57. [DOI] [PubMed] [Google Scholar]
- 2.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE, Infectious Diseases Society of America, European Society for Microbiology and Infectious Diseases. 2011. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 52:e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 3.Gupta N, Limbago BM, Patel JB, Kallen AJ. 2011. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis 53:60–67. doi: 10.1093/cid/cir202. [DOI] [PubMed] [Google Scholar]
- 4.Redgrave LS, Sutton SB, Webber MA, Piddock LJV. 2014. Fluoroquinolone resistance: mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol 22:438–445. doi: 10.1016/j.tim.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Chuck SK, Raber SR, Rodvold KA, Areff D. 2000. National survey of extended-interval aminoglycoside dosing. Clin Infect Dis 30:433–439. doi: 10.1086/313692. [DOI] [PubMed] [Google Scholar]
- 6.Stankowicz MS, Ibrahim J, Brown DL. 2015. Once-daily aminoglycoside dosing: an update on current literature. Am J Heal Pharm 72:1357–1364. doi: 10.2146/ajhp140564. [DOI] [PubMed] [Google Scholar]
- 7.Nicolau DP, Freeman CD, Belliveau PP, Nightingale CH, Ross JW, Quintiliani R. 1995. Experience with a once-daily aminoglycoside program administered to 2,184 adult patients. Antimicrob Agents Chemother 39:650–655. doi: 10.1128/aac.39.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman CD, Nicolau DP, Belliveau PP, Nightingale CH. 1997. Once-daily dosing of aminoglycosides: review and recommendations for clinical practice. J Antimicrob Chemother 39:677–686. doi: 10.1093/jac/39.6.677. [DOI] [PubMed] [Google Scholar]
- 9.Achaogen, Inc. 2018. Zemdri (plazomicin) injection package insert. Achaogen, Inc, South San Francisco, CA: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210303Orig1s000lbl.pdf. [Google Scholar]
- 10.Aggen JB, Armstrong ES, Goldblum AA, Dozzo P, Linsell MS, Gliedt MJ, Hildebrandt DJ, Feeney LA, Kubo A, Matias RD, Lopez S, Gomez M, Wlasichuk KB, Diokno R, Miller GH, Moser HE. 2010. Synthesis and spectrum of the neoglycoside ACHN-490. Antimicrob Agents Chemother 54:4636–4642. doi: 10.1128/AAC.00572-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhanel GG, Lawson CD, Zelenitsky S, Findlay B, Schweizer F, Adam H, Walkty A, Rubinstein E, Gin AS, Hoban DJ, Lynch JP, Karlowsky JA. 2012. Comparison of the next-generation aminoglycoside plazomicin to gentamicin, tobramycin and amikacin. Expert Rev Anti Infect Ther 10:459–473. doi: 10.1586/eri.12.25. [DOI] [PubMed] [Google Scholar]
- 12.Cox G, Ejim L, Stogios PJ, Koteva K, Bordeleau E, Evdokimova E, Sieron AO, Savchenko A, Serio AW, Krause KM, Wright GD. 2018. Plazomicin retains antibiotic activity against most aminoglycoside modifying enzymes. ACS Infect Dis 4:980–987. doi: 10.1021/acsinfecdis.8b00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walkty A, Adam H, Baxter M, Denisuik A, Lagacé-Wiens P, Karlowsky JA, Hoban DJ, Zhanel GG. 2014. In vitro activity of plazomicin against 5,015 Gram-negative and Gram-positive clinical isolates obtained from patients in Canadian hospitals as part of the CANWARD study, 2011-2012. Antimicrob Agents Chemother 58:2554–2563. doi: 10.1128/AAC.02744-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castanheira M, Davis AP, Mendes RE, Serio AW, Krause KM, Flamm RK. 2018. In vitro activity of plazomicin against Gram-negative and Gram-positive isolates collected from U.S. hospitals and comparative activities of aminoglycosides against carbapenem-resistant Enterobacteriaceae and isolates carrying carbapenemase genes. Antimicrob Agents Chemother 62:e00313-18. doi: 10.1128/AAC.00313-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thwaites M, Hall D, Shinabarger D, Serio AW, Krause KM, Marra A, Pillar C. 2018. Evaluation of the bactericidal activity of plazomicin and comparators against multidrug-resistant Enterobacteriaceae. Antimicrob Agents Chemother 62:e00236-18. doi: 10.1128/AAC.00236-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdelraouf K, Kim A, Krause KM, Nicolau DP. 2018. In vivo efficacy of plazomicin alone or in combination with meropenem or tigecycline against Enterobacteriaceae isolates exhibiting various resistance mechanisms in an immunocompetent murine septicemia model. Antimicrob Agents Chemother 62:e01074-18. doi: 10.1128/AAC.01074-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagenlehner FME, Cloutier DJ, Komirenko AS, Cebrik DS, Krause KM, Keepers TR, Connolly LE, Miller LG, Friedland I, Dwyer JP. 2019. Once-daily plazomicin for complicated urinary tract infections. N Engl J Med 380:729–740. doi: 10.1056/NEJMoa1801467. [DOI] [PubMed] [Google Scholar]
- 18.Gillespie EL, Kuti JL, Nicolau DP. 2005. Pharmacodynamics of antimicrobials: treatment optimisation. Expert Opin Drug Metab Toxicol 1:351–361. doi: 10.1517/17425255.1.3.351. [DOI] [PubMed] [Google Scholar]
- 19.Rea RS, Capitano B. 2007. Optimizing use of aminoglycosides in the critically ill. Semin Respir Crit Care Med 28:596–603. doi: 10.1055/s-2007-996406. [DOI] [PubMed] [Google Scholar]
- 20.Craig WA. 2011. Optimizing aminoglycoside use. Crit Care Clin 27:107–121. doi: 10.1016/j.ccc.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Drusano GL, Louie A. 2011. Optimization of aminoglycoside therapy. Antimicrob Agents Chemother 55:2528–2531. doi: 10.1128/AAC.01314-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trang M, Seroogy JD, Van Wart SA, Bhavnani SM, Kim A, Gibbons JA, Ambrose PG, Rubino CM. 2019. Population pharmacokinetic analyses for plazomicin using pooled data from phase 1, 2, and 3 clinical studies. Antimicrob Agents Chemother 63. doi: 10.1128/AAC.02329-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith CR, Moore RD, Lietman PS. 1986. Studies of risk factors for aminoglycoside nephrotoxicity. Am J Kidney Dis 8:308–313. doi: 10.1016/S0272-6386(86)80103-2. [DOI] [PubMed] [Google Scholar]
- 24.Paterson DL, Robson JMB, Wagener MM. 1998. Risk factors for toxicity in elderly patients given aminoglycosides once daily. J Gen Intern Med 13:735–739. doi: 10.1046/j.1525-1497.1998.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beaucaire G. 2000. Does once-daily dosing prevent nephrotoxicity in all aminoglycosides equally? Clin Microbiol Infect 6:357–362. [DOI] [PubMed] [Google Scholar]
- 26.Bhavnani SM, Hammel JP, Trang M, Kim A, Krause KM, Castanheira M, Rubino CM, Ambrose PG, Drusano GL. 2018. Pharmacokinetic-pharmacodynamic target attainment analyses to support plazomicin dose selection and recommendations for interpretive criteria for in vitro susceptibility testing for Enterobacteriaceae, poster #518. American Society for Microbiology Microbe (ASM), Atlanta, GA. [Google Scholar]