We evaluated extended-interval dosing of the investigational echinocandin rezafungin (1, 4, and 16 mg/kg on days 1, 4, and 7 postinoculation) for the treatment of disseminated invasive aspergillosis caused by azole-resistant Aspergillus fumigatus. Survival was significantly improved in mice treated with each dose of rezafungin and supratherapeutic posaconazole (20 mg/kg twice daily).
KEYWORDS: Aspergillus fumigatus, TR34/L98H, azole resistance, invasive aspergillosis, murine model, rezafungin
ABSTRACT
We evaluated extended-interval dosing of the investigational echinocandin rezafungin (1, 4, and 16 mg/kg on days 1, 4, and 7 postinoculation) for the treatment of disseminated invasive aspergillosis caused by azole-resistant Aspergillus fumigatus. Survival was significantly improved in mice treated with each dose of rezafungin and supratherapeutic posaconazole (20 mg/kg twice daily). Kidney fungal burden, as measured by quantitative real-time PCR, was also significantly reduced in mice treated with rezafungin although variability was observed.
TEXT
Growing concern exists for azole-resistant Aspergillus fumigatus due to prolonged exposure to members of this antifungal class in patients with acute invasive or chronic pulmonary aspergillosis, or to environmental exposure of isolates to azoles used in agriculture and various materials to prevent rotting and the growth of molds (1). Azole resistance due to environmental exposure has been associated with mutations within the CYP51A gene along with tandem base pair repeats in the promoter region of this gene (i.e., TR34/L98H and TR46/Y121F/T289A) (2–4). Azole-resistant A. fumigatus isolates with such resistance mechanisms have now been found in numerous countries worldwide (5–7). The rise of azole resistance in A. fumigatus is particularly concerning, since the azoles are the only orally available agents for the treatment of invasive aspergillosis and the duration of therapy is often prolonged. Rezafungin is an investigational echinocandin that demonstrates potent in vitro activity against Aspergillus species, including azole-resistant A. fumigatus isolates (8, 9). Structurally, rezafungin is similar to anidulafungin (10), but with the hemiaminal group replaced with a choline aminal ether, resulting in a more stable compound with a prolonged half-life in multiple animal species and humans (half-life of ∼130 h in humans) (10–13). This long half-life and high plasma exposure of rezafungin translate into sustained drug levels and high tissue penetration with less frequent dosing compared with other echinocandins (14). Our objective was to determine if extended-interval dosing of rezafungin would also be effective against disseminated invasive aspergillosis caused by an azole-resistant isolate.
Male ICR mice were rendered neutropenic with intraperitoneal (i.p.) doses of cyclophosphamide and 5-fluorouracil (200 mg/kg and 5 mg/mouse, respectively, the day prior to inoculation). Mice were inoculated intravenously with a clinical A. fumigatus isolate (UTHSCSA DI15-116) harboring a TR34/L98H mutation (rezafungin minimum effective concentration [MEC], 0.06 μg/ml; posaconazole MIC, 1 μg/ml) (6). Treatment with vehicle control, rezafungin (1, 4, or 16 mg/kg i.p. on days 1, 4, and 7) or supratherapeutic posaconazole (20 mg/kg orally [p.o.] twice a day [BID]) (15) began 24 h postinoculation and continued through day 7. In the fungal burden arm, mice were humanely euthanized on day 8, and kidneys were collected for fungal burden analysis by CFU enumeration (CFU/g) and quantitative real-time PCR (qPCR) as previously described (16). In the survival arm, mice were followed off therapy until day 12. Fungal burden was also measured as mice became moribund in the survival arm. Survival was assessed by Kaplan-Meier analysis and the log rank test, and fungal burden was assessed by analysis of variance (ANOVA) with Tukey’s posttest for multiple comparisons. The animal protocol was approved by the University of Texas Health Science Center Institutional Animal Care and Use Committee.
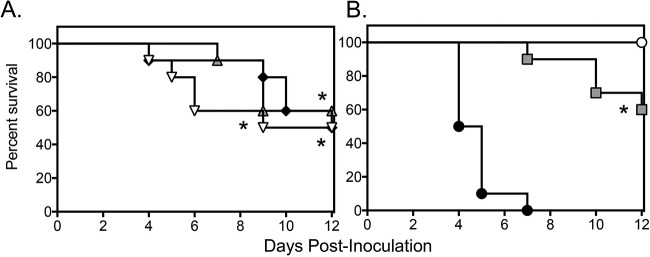
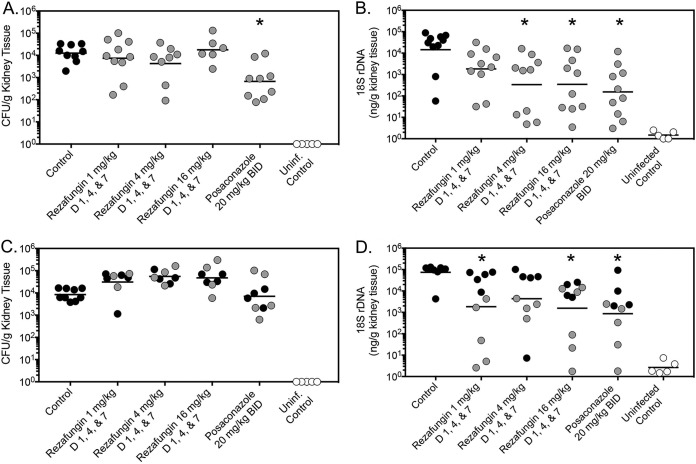
Extended-interval dosing of rezafungin was associated with a survival advantage in this experimental model of disseminated aspergillosis. Both median survival and percent survival were significantly improved at each dosage level of rezafungin (median survival range, 10.5 to 12 days; percent survival, 50% to 60%) compared to vehicle control (4.5 days and 0%; P ≤ 0.0325 for all comparisons) (Fig. 1A). Survival was also improved in mice administered supratherapeutic posaconazole (>12 days and 60%; P < 0.0001 versus vehicle) (Fig. 1B). Changes in fungal burden within kidney tissue were also observed with extended-interval dosing of rezafungin and posaconazole. However, these changes were assay dependent. On day 8 postinoculation, 1 day after therapy stopped, only posaconazole was associated with a significant reduction in CFU (mean, 2.84 log10 CFU/g) compared to vehicle control (4.10 log10 CFU/g; P = 0.0021) (Fig. 2A). In contrast, when measured by qPCR, extended-interval doses of rezafungin of 4 mg/kg and 16 mg/kg (2.53 and 2.54 log10 ng/g DNA, respectively) and posaconazole (2.19 log10 ng/g DNA) significantly lowered fungal burden compared to vehicle control (4.16 log10 ng/g DNA; P ≤ 0.0131 for all comparisons) (Fig. 2B). In the survival arm, no treatment group was associated with significant reductions in CFU (Fig. 2C). In contrast, when measured by qPCR, rezafungin 1 mg/kg and 16 mg/kg (3.26 and 3.19 log10 ng/g DNA, respectively) and posaconazole (2.94 log10 ng/g DNA) reduced fungal burden compared to vehicle control (4.87 log10 ng/g DNA; P ≤ 0.041 for all comparisons) (Fig. 2D). Overall, the fungal burden results observed with rezafungin and posaconazole as measured by qPCR in the survival arm were consistent with the improvements in survival observed in this study, as 94.4% of treated mice whose fungal burden was above the mean value for all treatment groups (3.36 log10 ng/g DNA) succumbed to infection. Similar relationships were not observed when measured by CFU. Others have previously reported the utility of qPCR in assessing changes in fungal burden in animal models of invasive aspergillosis with similar variability (16–18). Interestingly, extended-interval dosing of rezafungin at 4 mg/kg was not associated with a significant reduction in fungal burden in the survival arm, despite improvements in overall survival at this dosage level.
FIG 1.
Survival curves in a neutropenic murine model of invasive aspergillosis caused by an azole-resistant A. fumigatus isolate harboring a TR34/L98H mutations. One day post intravenous inoculation, mice were treated with extended-interval dosing of rezafungin (1, 4, or 16 mg/kg i.p. on days 1, 4, and 7 postinoculation) (A), vehicle control, or posaconazole (20 mg/kg p.o. BID) (B). Treatment continued through day 7, and mice were followed off therapy until day 12. Black circles, vehicle control; gray squares, posaconazole; white circle, uninfected control (to assess for bacterial superinfections); inverted white triangle, rezafungin 1 mg/kg; black diamond, rezafungin 4 mg/kg; gray triangle, rezafungin 16 mg/kg. N = 10 mice per group; lines represent mean values. *, P < 0.05 versus vehicle control.
FIG 2.
Fungal burden in neutropenic mice with invasive aspergillosis caused by an azole-resistant A. fumigatus isolate harboring a TR34/L98H mutations. Fungal burden was measured by CFU enumeration (CFU/g) (A) and quantitative real-time PCR (ng/g DNA) (B), measured on day 8 postinoculation in the fungal burden arm, and on day 12 postinoculation (C) or as the mice became moribund in the survival arm (D). Black circles represent mice that were moribund and humanely euthanized prior to the day 12 time point in the survival arm (C and D), while gray circles represent infected mice that survived to this time point. *, P value < 0.05 versus vehicle control. D, day; rDNA, ribosomal DNA.
The in vivo results of this study are in agreement with the in vitro activity previously reported for rezafungin against azole-resistant A. fumigatus and cryptic Aspergillus species against which the azoles have reduced activity (8, 9). One study also demonstrated good in vivo efficacy of rezafungin with extended-interval dosing in a neutropenic murine model of invasive candidiasis (14). In this previous work, which used the same doses and administration frequency, the rezafungin maximum concentration of drug in serum (Cmax) fell between 2.60 μg/ml to 26.5 μg/ml, and the area under the concentration-time curve from 0 h to infinity (AUC0-inf) was between 93.2 μg · h/ml and 972 μg · h/ml. The extended-interval dosing used in this previous study and in the current work mimicked the pharmacokinetics achieved with once-weekly regimens studied in humans (13, 14, 19). The results of the current study also suggest that extended-interval dosing of rezafungin may be effective in the treatment of invasive aspergillosis, with improved survival and reduction in fungal burden as measured by qPCR, which is also consistent with activity of echinocandins against Aspergillus (17). However, it is unknown if outcomes could be further improved with a different dosing strategy of rezafungin, given the differences observed in echinocandin activity between Candida (fungicidal) and Aspergillus (fungistatic) species (20). Both the AUC/MIC and Cmax/MIC pharmacokinetic/pharmacodynamic parameters have been associated with improved echinocandin treatment outcomes in experimental models of invasive fungal infections, including invasive aspergillosis (18, 21–24). Extended-interval dosing of rezafungin would be advantageous in the treatment of azole-resistant aspergillosis, as daily intravenous administration of the currently approved echinocandins is problematic given the long durations of therapy often needed in patients with these invasive mycoses. However, further studies are warranted to assess the potential benefits of extended-interval dosing of rezafungin, including comparisons with different dosing regimens and the use of additional fungal strains, for the treatment of aspergillosis caused by azole-resistant Aspergillus.
ACKNOWLEDGMENTS
This study was funded by a research grant from Cidara Pharmaceuticals, Inc., to the University of Texas Health Science Center at San Antonio (to T. F. Patterson, principal investigator).
N. P. Wiederhold has received research support to the University of Texas Health Science Center at San Antonio from Astellas, bioMérieux, Cidara, F2G, and Viamet, and has served on advisory boards for Astellas and Mayne Pharma and as a speaker for Gilead. T. F. Patterson has received research grants to UT Health San Antonio from Cidara and has served as a consultant for Astellas, Basilea, Gilead, Merck, Pfizer, Toyama, Viamet, and Scynexis.
REFERENCES
- 1.Wiederhold NP, Patterson TF. 2015. Emergence of azole resistance in Aspergillus. Semin Respir Crit Care Med 36:673–680. doi: 10.1055/s-0035-1562894. [DOI] [PubMed] [Google Scholar]
- 2.Mellado E, Garcia-Effron G, Alcázar-Fuoli L, Melchers WJG, Verweij PE, Cuenca-Estrella M, Rodríguez-Tudela JL. 2007. A new Aspergillus fumigatus resistance mechanism conferring in vitro cross-resistance to azole antifungals involves a combination of cyp51A alterations. Antimicrob Agents Chemother 51:1897–1904. doi: 10.1128/AAC.01092-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snelders E, van der Lee HA, Kuijpers J, Rijs AJ, Varga J, Samson RA, Mellado E, Donders AR, Melchers WJ, Verweij PE. 2008. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med 5:e219. doi: 10.1371/journal.pmed.0050219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howard SJ, Cerar D, Anderson MJ, Albarrag A, Fisher MC, Pasqualotto AC, Laverdiere M, Arendrup MC, Perlin DS, Denning DW. 2009. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg Infect Dis 15:1068–1076. doi: 10.3201/eid1507.090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verweij PE, Chowdhary A, Melchers WJ, Meis JF. 2016. Azole resistance in Aspergillus fumigatus: can we retain the clinical use of mold-active antifungal azoles? Clin Infect Dis 62:362–368. doi: 10.1093/cid/civ885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiederhold NP, Gil VG, Gutierrez F, Lindner JR, Albataineh MT, McCarthy DI, Sanders C, Fan H, Fothergill AW, Sutton DA. 2016. First detection of TR34 L98H and TR46 Y121F T289A Cyp51 mutations in Aspergillus fumigatus isolates in the United States. J Clin Microbiol 54:168–171. doi: 10.1128/JCM.02478-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivero-Menendez O, Alastruey-Izquierdo A, Mellado E, Cuenca-Estrella M. 2016. Triazole resistance in Aspergillus spp.: a worldwide problem? J Fungi (Basel) 2:E21. doi: 10.3390/jof2030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfaller MA, Messer SA, Rhomberg PR, Jones RN, Castanheira M. 2016. Activity of a long-acting echinocandin, CD101, determined using CLSI and EUCAST reference methods, against Candida and Aspergillus spp., including echinocandin- and azole-resistant isolates. J Antimicrob Chemother 71:2868–2873. doi: 10.1093/jac/dkw214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiederhold NP, Locke JB, Daruwala P, Bartizal K. 2018. Rezafungin (CD101) demonstrates potent in vitro activity against Aspergillus, including azole-resistant Aspergillus fumigatus isolates and cryptic species. J Antimicrob Chemother 73:3063–3067. doi: 10.1093/jac/dky280. [DOI] [PubMed] [Google Scholar]
- 10.Krishnan BR, James KD, Polowy K, Bryant BJ, Vaidya A, Smith S, Laudeman CP. 2017. CD101, a novel echinocandin with exceptional stability properties and enhanced aqueous solubility. J Antibiot (Tokyo) 70:130–135. doi: 10.1038/ja.2016.89. [DOI] [PubMed] [Google Scholar]
- 11.James KD, Laudeman CP, Malkar NB, Krishnan R, Polowy K. 2017. Structure-activity relationships of a series of echinocandins and the discovery of CD101, a highly stable and soluble echinocandin with distinctive pharmacokinetic properties. Antimicrob Agents Chemother 61:e01541-16. doi: 10.1128/AAC.01541-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ong V, James KD, Smith S, Krishnan BR. 2017. Pharmacokinetics of the novel echinocandin CD101 in multiple animal species. Antimicrob Agents Chemother 61:e01626-16. doi: 10.1128/AAC.01626-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandison T, Ong V, Lee J, Thye D. 2017. Safety and pharmacokinetics of CD101 IV, a novel echinocandin, in healthy adults. Antimicrob Agents Chemother 61:e01627-16. doi: 10.1128/AAC.01627-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lepak AJ, Zhao M, VanScoy B, Ambrose PG, Andes DR. 2017. Pharmacodynamics of a long-acting echinocandin, CD101, in a neutropenic invasive-candidiasis murine model using an extended-interval dosing design. Antimicrob Agents Chemother 62:e02154-17. doi: 10.1128/AAC.02154-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hope WW, McEntee L, Livermore J, Whalley S, Johnson A, Farrington N, Kolamunnage-Dona R, Schwartz J, Kennedy A, Law D, Birch M, Rex JH. 2017. Pharmacodynamics of the orotomides against Aspergillus fumigatus: new opportunities for treatment of multidrug-resistant fungal disease. mBio 8:e01157-17. doi: 10.1128/mBio.01157-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiederhold NP, Najvar LK, Matsumoto S, Bocanegra RA, Herrera ML, Wickes BL, Kirkpatrick WR, Patterson TF. 2015. Efficacy of the investigational echinocandin ASP9726 in a guinea pig model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother 59:2875–2881. doi: 10.1128/AAC.04857-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowman JC, Abruzzo GK, Anderson JW, Flattery AM, Gill CJ, Pikounis VB, Schmatz DM, Liberator PA, Douglas CM. 2001. Quantitative PCR assay to measure Aspergillus fumigatus burden in a murine model of disseminated aspergillosis: demonstration of efficacy of caspofungin acetate. Antimicrob Agents Chemother 45:3474–3481. doi: 10.1128/AAC.45.12.3474-3481.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiederhold NP, Kontoyiannis DP, Chi J, Prince RA, Tam VH, Lewis RE. 2004. Pharmacodynamics of caspofungin in a murine model of invasive pulmonary aspergillosis: evidence of concentration-dependent activity. J Infect Dis 190:1464–1471. doi: 10.1086/424465. [DOI] [PubMed] [Google Scholar]
- 19.Lepak AJ, Zhao M, Andes DR. 2018. Pharmacodynamic evaluation of rezafungin (CD101) against Candida auris in the neutropenic mouse invasive candidiasis model. Antimicrob Agents Chemother 62:e01572-18. doi: 10.1128/AAC.01572-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowman JC, Hicks PS, Kurtz MB, Rosen H, Schmatz DM, Liberator PA, Douglas CM. 2002. The antifungal echinocandin caspofungin acetate kills growing cells of Aspergillus fumigatus in vitro. Antimicrob Agents Chemother 46:3001–3012. doi: 10.1128/aac.46.9.3001-3012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andes D, Diekema DJ, Pfaller MA, Bohrmuller J, Marchillo K, Lepak A. 2010. In vivo comparison of the pharmacodynamic targets for echinocandin drugs against Candida species. Antimicrob Agents Chemother 54:2497–2506. doi: 10.1128/AAC.01584-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andes D, Diekema DJ, Pfaller MA, Prince RA, Marchillo K, Ashbeck J, Hou J. 2008. In vivo pharmacodynamic characterization of anidulafungin in a neutropenic murine candidiasis model. Antimicrob Agents Chemother 52:539–550. doi: 10.1128/AAC.01061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andes DR, Diekema DJ, Pfaller MA, Marchillo K, Bohrmueller J. 2008. In vivo pharmacodynamic target investigation for micafungin against Candida albicans and C. glabrata in a neutropenic murine candidiasis model. Antimicrob Agents Chemother 52:3497–3503. doi: 10.1128/AAC.00478-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louie A, Deziel M, Liu W, Drusano MF, Gumbo T, Drusano GL. 2005. Pharmacodynamics of caspofungin in a murine model of systemic candidiasis: importance of persistence of caspofungin in tissues to understanding drug activity. Antimicrob Agents Chemother 49:5058–5068. doi: 10.1128/AAC.49.12.5058-5068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]