The mortality rate associated with Vibrio vulnificus sepsis remains high. An in vitro time-kill assay revealed synergism between tigecycline and ciprofloxacin. The survival rate was significantly higher in mice treated with tigecycline plus ciprofloxacin than in mice treated with cefotaxime plus minocycline. Thus, combination treatment with tigecycline-ciprofloxacin may be an effective novel antibiotic regimen for V. vulnificus sepsis.
KEYWORDS: sepsis, tigecycline, V. vulnificus
ABSTRACT
The mortality rate associated with Vibrio vulnificus sepsis remains high. An in vitro time-kill assay revealed synergism between tigecycline and ciprofloxacin. The survival rate was significantly higher in mice treated with tigecycline plus ciprofloxacin than in mice treated with cefotaxime plus minocycline. Thus, combination treatment with tigecycline-ciprofloxacin may be an effective novel antibiotic regimen for V. vulnificus sepsis.
TEXT
Vibrio vulnificus is an opportunistic human pathogen that causes skin and soft tissue infections and septicemia. It is introduced by ingestion of contaminated seafood or contact of a wound with seawater (1, 2). Antibiotics used to treat this infection include combination therapy consisting of minocycline plus third-generation cephalosporin and quinolone monotherapy, based on the results of in vitro (3) and in vivo (4, 5) studies. However, the mortality rate for V. vulnificus sepsis remains high (≥50%) despite application of these regimens.
(Presented in part at IDWeek 2017, San Diego, CA, 4 to 8 October 2017.)
Tigecycline is the first member of the glycylcycline class of antibiotics and was approved for the treatment of complicated skin and soft tissue infections and intra-abdominal infections, based on noninferiority studies (7, 8). Moreover, tigecycline is active against Vibrio species in vitro, with a low resistance rate (9). Therefore, tigecycline may be an effective therapeutic agent for V. vulnificus infections of skin and soft tissues, including necrotizing fasciitis. However, few in vivo studies are available on the therapeutic use of tigecycline for V. vulnificus infection (10, 11). In this study, we evaluated the activity of tigecycline-based therapy against V. vulnificus infection in vitro and in vivo.
Two strains clinically obtained from patients with Vibrio sepsis, V. vulnificus CMCP6 and V. vulnificus MO6-24/O, were used in the time-kill study, and V. vulnificus CMCP6 was used in animal experiments. The MICs of cefotaxime, minocycline, ciprofloxacin, and tigecycline were measured using the microdilution method (12). To evaluate synergy in vitro, a time-kill assay was performed as described previously (13). Cefotaxime (Chong Kun Dang Pharmaceutical, Seoul, Republic of Korea), ciprofloxacin (Ildong Pharmaceutical, Seoul, Republic of Korea), minocycline (Sigma-Aldrich, St. Louis, MO), and tigecycline (Pfizer Korea, Seoul, Republic of Korea) were used in the in vitro and in vivo animal studies. Antibiotic synergy was defined as a ≥2-log10-CFU/ml decrease at 24 h by combination treatment compared with the most active single antibiotic agent and a ≥2-log10-CFU/ml decrease at 24 h compared with the starting inoculum (14, 15).
V. vulnificus CMCP6 isolates were incubated overnight at 37°C in a shaking incubator in cation-adjusted Mueller-Hinton broth. A total of 100 μl of the bacterial suspension was transferred to 10 ml of the same fresh broth and incubated for 3 h at 37°C. Bacteria grown were collected by centrifugation, and the pellet was resuspended in 0.85% saline, as described previously (5). For in vivo mice experiments, 8-week-old female BALB/c mice (Samtako, Osan, Republic of Korea) with an average body weight of 20 g were used in the study. To establish iron-overload status, 1,000 μg of ferric ammonium citrate was administered intraperitoneally (i.p.) 30 min before V. vulnificus inoculation (16). Next, 1 × 108 CFU V. vulnificus were inoculated subcutaneously (s.c.) on the right thigh, as described previously (5, 17). The mice were randomly assigned to the treatment groups. All antibiotics were initially given 2 h after the animals were infected. Control mice were treated with 0.1 ml sterile saline i.p. every 6 h, and antibiotics were given for 42 h. The animals were monitored every 6 h over a 48-h period and then every 24 h for another 48 h.
Tigecycline 6.25 mg/kg was administered s.c. every 12 h, in accordance with a recent V. vulnificus study in septic mice (10) and another study that measured serum and tissue concentrations and pharmacokinetic parameters of tigecycline (18). The tigecycline dose with the most similar pharmacokinetic effects to the human dose was determined (19). Cefotaxime 180 mg/kg/day i.p. and minocycline 4 to 6 mg/kg/day i.p. were used in most previous studies (4, 5, 17, 20). In this study, to ensure that we did not underestimate the activity of cefotaxime and minocycline, higher doses of cefotaxime (150 mg/kg every 6 h [600 mg/kg/day]) and minocycline (50 mg/kg every 24 h) were given i.p. in accordance with recent mouse V. vulnificus infection models (10); these doses corresponded to the maximum human doses in recent pharmacokinetic mouse studies (21, 22). Ciprofloxacin 8 mg/kg was administered i.p. every 12 h as in previous mouse V. vulnificus infection models (4, 17, 23, 24), in which similar pharmacokinetics in mice and humans were seen (25, 26). In the survival study, animals were euthanized when they exhibited combined clinical criteria totaling ≥8 points. The clinical criteria were defined according to the Korean Food and Drug Administration (KFDA) guidelines as changes in body weight (0 to 3 points), hair coat (0 to 2 points), eye opening (0 to 2 points), activity (0 to 2 points), and posture (0 to 3 points) (27); the higher the score, the worse the condition of the mouse. All experimental mice were housed in a semi-specific pathogen-free (SPF) facility. All animal experiments were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Chonnam National University and KFDA (27). The study protocol was approved by the IACUC of Chonnam National University Hwasun Hospital. The Kaplan-Meier method and log-rank test were used for survival analyses. P values of <0.05 indicated statistical significance. Statistical analyses were performed using SPSS (v.24.0; SPSS Inc., Chicago, IL) and GraphPad Prism (v. 7.0; GraphPad Software, La Jolla, CA) software.
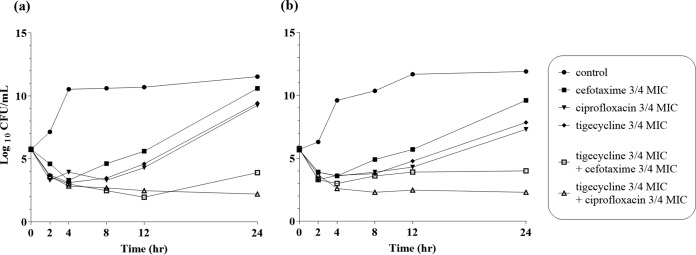
The MICs of cefotaxime, minocycline, ciprofloxacin, and tigecycline for V. vulnificus CMCP6 and MO6-24/O isolates were 0.0625, 0.0625, 0.03, and 0.0625 mg/liter, respectively. Antibiotic synergy was observed in vitro in 3/4 MIC time-kill assays for tigecycline-ciprofloxacin after 24 h in V. vulnificus strains CMCP6 (Fig. 1a) and MO6-24/O (Fig. 1b). The tigecycline-cefotaxime combination showed a ≥2-log10-CFU/ml decrease at 24 h compared with each antibiotic agent used alone but did not meet the criteria for synergy because the bacterial count after treatment decreased by <2 log10 CFU/ml compared with the starting inoculum (CMCP6, 5.7 × 105 to 8.0 × 103 CFU/ml; MO6-24/O, 5.7 × 105 to 1.0 × 104 CFU/ml).
FIG 1.
Time-kill curves for V. vulnificus CMCP6 (a) and MO6-24/O (b) isolates after incubation with 3/4 MICs of single and multiple antibiotics.
Figure 2 presents the survival rates in each treatment group after inoculation with 1 × 108 CFU of V. vulnificus CMCP6. The 96-h survival rate was significantly higher in mice treated with tigecycline-ciprofloxacin (71% [17/24]) than in mice treated with cefotaxime-minocycline (42% [10/24]; log-rank test, P = 0.04). The 96-h survival rate of the tigecycline-cefotaxime group (67% [16/24]) was higher than that of the cefotaxime-minocycline group, but the difference was not statistically significant (log-rank test, P = 0.09). The survival rate of the tigecycline-ciprofloxacin group was also higher than that of the ciprofloxacin (56% [14/25]) and tigecycline (54% [13/24]) monotherapy groups; however, the differences were not significant (log-rank test, P = 0.20 and 0.29, respectively).
FIG 2.
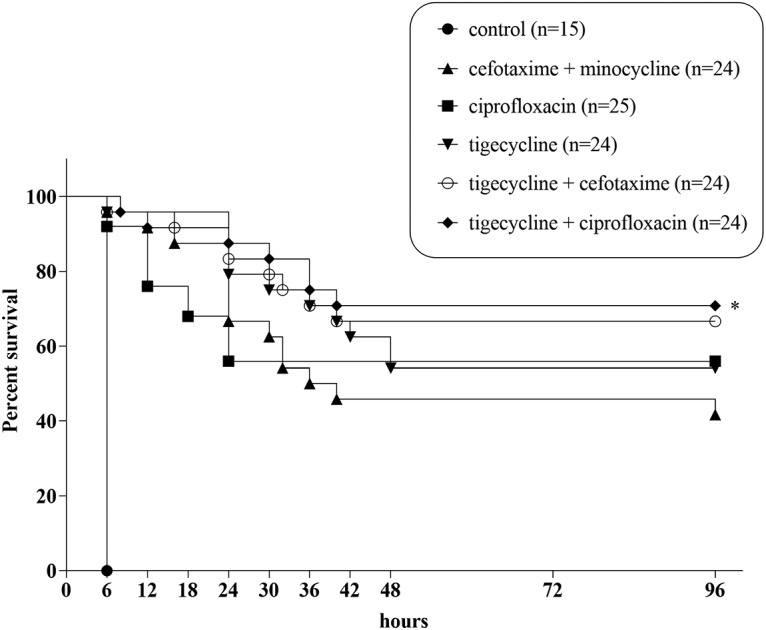
Survival curve of mice infected with 1 × 108 CFU V. vulnificus CMCP6. *, P < 0.05.
Tigecycline monotherapy may not be appropriate for V. vulnificus sepsis because V. vulnificus sepsis commonly accompanies bacteremia and tigecycline enters tissues rapidly after administration, resulting in low serum levels. Moreover, a recent study suggested that combination therapy has a better treatment outcome than monotherapy in V. vulnificus sepsis (23). Few studies have explored the in vivo therapeutic activity of tigecycline-cephalosporin against V. vulnificus infection (10). Lin et al. (11) reported the case of a 12-year-old boy administered tigecycline-cefpirome salvage therapy, but the therapeutic activity of this combination cannot be determined by a single case report. Tang et al. (10) showed that a tigecycline-cefotaxime regimen was associated with superior survival compared with cefotaxime-minocycline and tigecycline monotherapy in mice infected with a 1.25 × 106 inoculum of a V. vulnificus Vv14-3 clinical isolate. However, in vitro synergy of cefotaxime and tigecycline was not seen in 25% (2/8) of the tested isolates. In addition, the therapeutic activity of tigecycline-quinolone remains unknown. In our study, tigecycline-cefotaxime combination failed to show in vitro synergy in two reference strains against V. vulnificus infection, but the in vivo activity was comparable to that of combination treatment with tigecycline-ciprofloxacin. We consistently found that tigecycline-ciprofloxacin showed in vitro synergy and was associated with better survival than cefotaxime-minocycline in mice infected with V. vulnificus.
In conclusion, our in vitro and in vivo studies suggest that tigecycline in combination with ciprofloxacin is a potent option for the treatment of invasive V. vulnificus infection. Further studies are required to evaluate the activity of tigecycline-ciprofloxacin combination therapy in a clinical setting.
ACKNOWLEDGMENTS
This work was supported financially by the Chonnam National University Hospital Biomedical Research Institute (grant no. CRI17021-1).
We have no conflicts of interest to declare.
REFERENCES
- 1.Oliver JD. 2015. The biology of Vibrio vulnificus. Microbiol Spectr 3. doi: 10.1128/microbiolspec.VE-0001-2014. [DOI] [PubMed] [Google Scholar]
- 2.Hlady WG, Klontz KC. 1996. The epidemiology of Vibrio infections in Florida, 1981-1993. J Infect Dis 173:1176–1183. doi: 10.1093/infdis/173.5.1176. [DOI] [PubMed] [Google Scholar]
- 3.Chuang YC, Liu JW, Ko WC, Lin KY, Wu JJ, Huang KY. 1997. In vitro synergism between cefotaxime and minocycline against Vibrio vulnificus. Antimicrob Agents Chemother 41:2214–2217. doi: 10.1128/AAC.41.10.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang HJ, Chang MC, Ko WC, Huang KY, Lee CL, Chuang YC. 2002. In vitro and in vivo activities of newer fluoroquinolones against Vibrio vulnificus. Antimicrob Agents Chemother 46:3580–3584. doi: 10.1128/aac.46.11.3580-3584.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chuang YC, Ko WC, Wang ST, Liu JW, Kuo CF, Wu JJ, Huang KY. 1998. Minocycline and cefotaxime in the treatment of experimental murine Vibrio vulnificus infection. Antimicrob Agents Chemother 42:1319–1322. doi: 10.1128/AAC.42.6.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reference deleted.
- 7.Ellis-Grosse EJ, Babinchak T, Dartois N, Rose G, Loh E, Tigecycline 300 cSSSI Study Group, Tigecycline 305 cSSSI Study Group. 2005. The efficacy and safety of tigecycline in the treatment of skin and skin-structure infections: results of 2 double-blind phase 3 comparison studies with vancomycin-aztreonam. Clin Infect Dis 41(Suppl 5):S341–S353. doi: 10.1086/431675. [DOI] [PubMed] [Google Scholar]
- 8.Babinchak T, Ellis-Grosse E, Dartois N, Rose GM, Loh E, Tigecycline 301 Study Group, Tigecycline 306 Study Group. 2005. The efficacy and safety of tigecycline for the treatment of complicated intra-abdominal infections: analysis of pooled clinical trial data. Clin Infect Dis 41(Suppl 5):S354–S367. doi: 10.1086/431676. [DOI] [PubMed] [Google Scholar]
- 9.Liu CY, Huang YT, Liao CH, Hsueh PR. 2008. In vitro activities of tigecycline against clinical isolates of Aeromonas, Vibrio, and Salmonella species in Taiwan. Antimicrob Agents Chemother 52:2677–2679. doi: 10.1128/AAC.00002-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang HJ, Chen CC, Lai CC, Zhang CC, Weng TC, Chiu YH, Toh HS, Chiang SR, Yu WL, Ko WC, Chuang YC. 2018. In vitro and in vivo antibacterial activity of tigecycline against Vibrio vulnificus. J Microbiol Immunol Infect 51:76–81. doi: 10.1016/j.jmii.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Lin YS, Hung MH, Chen CC, Huang KF, Ko WC, Tang HJ. 2016. Tigecycline salvage therapy for necrotizing fasciitis caused by Vibrio vulnificus: case report in a child. J Microbiol Immunol Infect 49:138–141. doi: 10.1016/j.jmii.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically—11th ed. CLSI document M07-A10. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 13.Kim DM, Lym Y, Jang SJ, Han H, Kim YG, Chung CH, Hong SP. 2005. In vitro efficacy of the combination of ciprofloxacin and cefotaxime against Vibrio vulnificus. Antimicrob Agents Chemother 49:3489–3491. doi: 10.1128/AAC.49.8.3489-3491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.George M, Eliopoulos RC, Moellering JR, Lorian V (ed). 1996. Antibiotics in laboratory medicine, 4th ed, Chapter 9, p 338–342. Williams and Wilkins, Baltimore, MD. [Google Scholar]
- 15.George M, Eliopoulos RC, Moellering JR, Lorian V (ed). 1996. Antibiotics in laboratory medicine, 4th ed, p 338–342. Williams and Wilkins, Baltimore, MD. [Google Scholar]
- 16.Lee SE, Kim SY, Kim CM, Kim MK, Kim YR, Jeong K, Ryu HJ, Lee YS, Chung SS, Choy HE, Rhee JH. 2007. The pyrH gene of Vibrio vulnificus is an essential in vivo survival factor. Infect Immun 75:2795–2801. doi: 10.1128/IAI.01499-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang HC, Choi SM, Kim HK, Kim SE, Kang SJ, Park KH, Ryu PY, Lee TH, Kim YR, Rhee JH, Jung SI, Choy HE. 2014. In vivo efficacy of the combination of ciprofloxacin and cefotaxime against Vibrio vulnificus sepsis. PLoS One 9:e101118. doi: 10.1371/journal.pone.0101118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koomanachai P, Kim A, Nicolau DP. 2009. Pharmacodynamic evaluation of tigecycline against Acinetobacter baumannii in a murine pneumonia model. J Antimicrob Chemother 63:982–987. doi: 10.1093/jac/dkp056. [DOI] [PubMed] [Google Scholar]
- 19.Muralidharan G, Micalizzi M, Speth J, Raible D, Troy S. 2005. Pharmacokinetics of tigecycline after single and multiple doses in healthy subjects. Antimicrob Agents Chemother 49:220–229. doi: 10.1128/AAC.49.1.220-229.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang HJ, Ko WC, Chuang YC, Chen CC, Chiang SR. 2010. Cefazolin plus minocycline against a clinical isolate of Vibrio vulnificus: in vitro and animal studies. Jpn J Infect Dis 63:16–18. [PubMed] [Google Scholar]
- 21.Ko WC, Lee HC, Chuang YC, Ten SH, Su CY, Wu JJ. 2001. In vitro and in vivo combinations of cefotaxime and minocycline against Aeromonas hydrophila. Antimicrob Agents Chemother 45:1281–1283. doi: 10.1128/AAC.45.4.1281-1283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou J, Ledesma KR, Chang KT, Abodakpi H, Gao S, Tam VH. 2017. Pharmacokinetics and pharmacodynamics of minocycline against Acinetobacter baumannii in a neutropenic murine pneumonia model. Antimicrob Agents Chemother 61:e02371-16. doi: 10.1128/AAC.02371-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trinh SA, Gavin HE, Satchell KJF. 2017. Efficacy of ceftriaxone, cefepime, doxycycline, ciprofloxacin, and combination therapy for Vibrio vulnificus foodborne septicemia. Antimicrob Agents Chemother 61:e01106-17. doi: 10.1128/AAC.01106-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neupane GP, Kim DM, Yun NR, Shin SH, Lim SC, Choi CH. 2012. Quantitative PCR and in vivo efficacy of antibiotics in the treatment of Vibrio vulnificus infection in a mouse model. Eur J Clin Microbiol Infect Dis 31:2461–2467. doi: 10.1007/s10096-012-1592-z. [DOI] [PubMed] [Google Scholar]
- 25.Guillard T, Cambau E, Chau F, Massias L, de Champs C, Fantin B. 2013. Ciprofloxacin treatment failure in a murine model of pyelonephritis due to an AAC(6')-Ib-cr-producing Escherichia coli strain susceptible to ciprofloxacin in vitro. Antimicrob Agents Chemother 57:5830–5835. doi: 10.1128/AAC.01489-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.FDA US. 2016. Medication guide (highlights of prescribing information of ciprofloxacin). https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/019537s086lbl.pdf. Accessed 7 June 2019.
- 27.FDA Korea. 2016. Guidelines for animal experiments and IACUC standard operation. http://www.mfds.go.kr/brd/m_218/view.do?seq=24563&srchFr=&srchTo=&srchWord=%EB%8F%99%EB%AC%BC%EC%8B%A4%ED%97%98&srchTp=0&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=1#. Accessed 7 June 2019.