In recent years, cases involving terbinafine-resistant Trichophyton isolates have been reported increasingly, particularly in India. We present 14 cases of terbinafine treatment failure in Trichophyton-infected Danish patients due to acquired resistance. Patients infected with Trichophyton rubrum (n = 12) or Trichophyton interdigitale (n = 2) with elevated terbinafine MICs during 2013–2018 were included.
KEYWORDS: Trichophyton, antifungal resistance, dermatophytes, squalene epoxidase mutations, terbinafine
ABSTRACT
In recent years, cases involving terbinafine-resistant Trichophyton isolates have been reported increasingly, particularly in India. We present 14 cases of terbinafine treatment failure in Trichophyton-infected Danish patients due to acquired resistance. Patients infected with Trichophyton rubrum (n = 12) or Trichophyton interdigitale (n = 2) with elevated terbinafine MICs during 2013–2018 were included. Antifungal susceptibility testing (AFST) was performed following a modified EUCAST E.Def 9.3.1 method (5 days of incubation) with or without cycloheximide and chloramphenicol (CC) supplementation of the growth medium. The squalene epoxidase (SE) target gene was sequenced, and 3-dimensional enzyme homology modeling was performed. Most patients (12/14 [86%]) were male. The mean age was 53.5 years (range, 11 to 77 years). The mean duration of infections was 4.8 years at the time of resistance detection. Prior systemic terbinafine treatment was documented for all patients, and topical therapy for 62% (information was missing in one case). Overall, nine isolates (64%) displayed high terbinafine resistance (MICs, 4 to >8 mg/liter), while two (14%) displayed moderate (MICs, 1 to 2 mg/liter) and three (21%) displayed low (MICs, 0.125 to 0.25 mg/liter) terbinafine resistance compared with control isolates. MICs generated with or without CC supplementation were similar, but CC prevented contamination. Known and novel SE amino acid substitutions (F397L, L393F, L393S, F415S, H440Y F484Y, and I121M V237I) were detected in resistant but not control isolates. Three-dimensional homology modeling suggested a role of the novel I121M and V237I alterations. Terbinafine resistance has been detected in Denmark using a modified EUCAST method, which facilitated susceptibility testing of dermatophytes. Action is needed for this emerging public health problem.
INTRODUCTION
Topical antifungals are available in many countries as over-the-counter medications, whereas oral antifungals require a prescription. Oral antifungal treatment is reserved for moderate to severe and/or recurrent cases where topical treatment is ineffective. Terbinafine (TRB), an allylamine antifungal drug, is considered to be the first-line drug for tinea caused by Trichophyton infections, since it is fungicidal (1). In recent years, an increasing number of terbinafine-resistant strains have been reported, especially in India (2–4). Terbinafine inhibits the enzyme squalene epoxidase (SE), thereby interfering with the biosynthesis of ergosterol, an essential cell membrane component (5, 6). Mutations in the squalene epoxidase gene have been documented in association with clinical terbinafine resistance (2, 4, 6–9). In particular, a recent in vitro study has verified that alterations in the specific amino acid (aa) hot spots L393, F397, F415, and H440 in squalene epoxidase raise terbinafine MICs for both Trichophyton rubrum and Trichophyton interdigitale (2). Most dominant are the two variants L393F and F397L, leading to high resistance, while other variants have been associated with lower MIC elevations but still potentially pose a risk of treatment failure (2, 4, 7).
An increasing number of Trichophyton infections with clinical resistance since 2013 have led to increased awareness of this emerging problem in Denmark (10, 11). One major concern is that susceptibility testing of dermatophytes is not common practice, and thus, resistance may be overlooked. Furthermore, confirmation of terbinafine resistance is challenging when one is using the microdilution mold susceptibility testing methods (5, 6). This is due to the required extended incubation period for dermatophytes, which entails a risk of bacterial or mold contamination, complicating or even precluding interpretation of terbinafine MICs.
This paper presents 14 cases of clinical terbinafine treatment failure in Trichophyton-infected patients. Furthermore, SE-associated resistance mechanisms are evaluated, and finally, a proposal for a EUCAST protocol for antifungal susceptibility testing (AFST) of dermatophytes is presented.
RESULTS
Fourteen Trichophyton isolates with reduced terbinafine susceptibility were obtained from 14 patients seen at departments of dermatology (Aarhus [n = 2], Gentofte [n = 2], Roskilde [n = 4], and Bispebjerg [n = 1] hospitals) and private dermatologic clinics in Birkerød (n = 2), Horsens (n = 1), Viborg (n = 1), and Skive (n = 1), Denmark.
Patients.
Most of the patients were male (n = 12 [86%]), and the mean age was 53.5 years (range, 11 to 77 years). Five (36%) of the patients suffered from skin diseases: atopic dermatitis (n = 3 [21%]), hand eczema (n = 1 [7%]), or Darier disease (n = 1 [7%]). One patient (7%) had diabetes. The patients had, on average, suffered from dermatophyte infections for 4.8 years at the time of resistance detection (median, 7 months; range, 3 months to >54 years; information not available for one patient). The anatomical regions involved were the lower extremities inclusive of toes, plantar areas, feet, legs, and groin (n = 12), nails (n = 8), trunk (n = 6), face (n = 1), neck (n = 1), and hands (n = 1); the majority (n = 11) had more than one region involved (Table 1). The origins of the isolates are specified in Table 2.
TABLE 1.
Summary of patient and clinical characteristics
| Case ID | Gender | Age (yr) | Area involved | Tinea duration (mo)a | Skin disease | Other diseasesb |
|---|---|---|---|---|---|---|
| 1 | Male | 54 | Body | 24 | No | No |
| 2 | Male | 65 | Body, groin, nails | 44 | Darier disease | No |
| 3 | Male | 76 | Face, nails | 60 | No | CLL, hypertension, nephrectomy, PST |
| 4 | Male | 77 | Feet/toes, nails | 180 | No | No |
| 5 | Male | 47 | Feet/toes | 96 | Atopic dermatitis | No |
| 6 | Male | 11 | Body, legs, feet/toes | 28 | Atopic dermatitis, congenital ichthyosiform erythroderma | ALOXE3 mutation |
| 7 | Male | 44 | Feet/toes, nails | 216 | Hand eczema | No |
| 8 | Female | 64 | Feet/toes, leg, nails | 84 | No | No |
| 9 | Male | 52 | Nails | NA | No | No |
| 10 | Male | 42 | Feet/toes | >300 | Atopic dermatitis | Urticaria (terbinafine related) |
| 11 | Male | 51 | Body, groin, hands, neck, feet/toes | 156 | No | No |
| 12 | Male | 71 | Feet, groin, nails, body (nates) | 648 | No | No |
| 13 | Male | 43 | Feet/toes | 36 | No | No |
| 14 | Female | 25 | Body, groin, hands, neck, legs, feet/toes | 3 | No | Diabetes |
NA, not available.
CLL, chronic lymphocytic leukemia; PST, paroxysmal supraventricular tachycardia.
TABLE 2.
Origins and susceptibility patterns of patient isolates and control strains
| Case or control ID | Isolate no. | Origin of isolate | Specimen | Species | Terbinafine MIC (mg/liter)a
|
SE sequencing resultb | |
|---|---|---|---|---|---|---|---|
| Without CC | With CC | ||||||
| Cases | |||||||
| 1 | SSI-8807 | Abdomen | Skin | T. rubrum | 0.125 | 0.125 | H440Y, F484Y |
| 2 | SSI-8138 | Back | Skin | T. rubrum | >8 | >8 | F397L |
| 3 | SSI-7885 | Toenail | Nail | T. rubrum | >8 | 8 | F397L |
| 4 | SSI-7942 | Toenail | Nail | T. rubrum | 2 | 1 | L393S |
| 5 | SSI-7359 | Foot (sole) | Skin | T. rubrum | >8 | >8 | F397L |
| 6 | SSI-7387 | Leg | Skin | T. rubrum | 4 | NP | F397L |
| 7 | SSI-5313 | Foot | Skin | T. rubrum | 8 | NP | F397L |
| 8 | SSI-9111 | Leg | Skin | T. rubrum | 8 | >8 | F397L |
| 9 | SSI-7906 | Toenail | Nail | T. rubrum | >8 | >8 | L393F |
| 10 | SSI-10382 | Foot (sole, heel, nail) | Skin | T. rubrum | NP | 0.25 | F415S |
| 11 | SSI-9866 | Abdomen | Skin | T. rubrum | NP | 1 | L393S |
| 12 | SSI-7549 | Buttocks/nates | Skin | T. rubrum | 0.125 | 0.125 | I121M, V237I |
| 13 | SSI-8865 | Foot (sole) | Skin | T. interdigitale | >8 | >8 | L393F |
| 14 | SSI-9723 | Leg | Skin | T. interdigitale | NP | >4 | F397L |
| Controls | |||||||
| C-1 | SSI-8153 | Chest/thorax | Skin | T. rubrum | 0.016 | 0.016 | WT |
| C-2 | SSI-8004 | Toenail | Nail | T. rubrum | 0.016 | 0.03 | WT |
| C-3 | SSI-6926 | Armpit/axilla | Skin | T. rubrum | 0.03 | 0.03 | WT |
| C-4 | CBS 289.86 | Buttocks/nates | Skin | T. rubrum | 0.03 | 0.03 | WT |
| C-5 | SSI-7583 | Armpit/axilla | Skin | T. rubrum | 0.03 | 0.03 | WT |
| C-6 | SSI-7936 | Hand | Skin | T. rubrum | 0.03 | 0.03 | WT |
| C-7 | SSI-8042 | Toenail | Nail | T. rubrum | 0.03 | 0.03 | WT |
| C-8 | SSI-7914 | Erythema, unspecified | Skin | T. rubrum | 0.06 | 0.06 | WT |
| C-9 | SSI-2047 | Foot | Skin | T. interdigitale | 0.016 | 0.03 | WT |
| C-10 | SSI-2048 | Foot (sole) | Skin | T. interdigitale | 0.008 | 0.016 | WT |
| C-11 | SSI-2123 | Toenail | Nail | T. interdigitale | 0.016 | 0.016 | WT |
| C-12 | SSI-3215 | Foot | Skin | T. interdigitale | 0.016 | 0.016 | WT |
| C-13 | SSI-3398 | Toenail | Nail | T. interdigitale | 0.008 | 0.016 | WT |
| C-14 | SSI-3399 | Foot | Skin | T. interdigitale | 0.016 | 0.016 | WT |
| C-15 | SSI-3455 | Foot | Skin | T. interdigitale | 0.008 | 0.016 | ND |
| C-16 | SSI-7933 | Toenail | Nail | T. interdigitale | 0.016 | 0.016 | ND |
CC, medium supplemented with chloramphenicol (50 mg/liter) and cycloheximide (300 mg/liter); NP, not possible to culture the strain (from frozen stocks) for retesting with or without CC. Testing with CC was carried out by the EUCAST microdilution method (23).
Given as the aa substitution(s) or “WT.” ND, not done.
Drug exposure.
All of the patients were exposed to terbinafine previously; of these, 62% had received topical treatment and all had received systemic treatment. Terbinafine hydrochloride for one application only (Lamisil Once) was used by one patient. Information on previous use of antifungals other than terbinafine was available for 71% of the patients (Table 3).
TABLE 3.
Prior exposure to antifungal therapy for patients with non-wild-type Trichophyton isolates
| Case ID | Antifungal(s) to which patient was exposeda
|
Time of first terbinafine exposure (yr)b
|
No. of treatment courses with terbinafine (wks of treatment) |
|||
|---|---|---|---|---|---|---|
| Topical | Systemic | Topical | Systemic | Topical | Systemic | |
| 1 | Terbinafine | Terbinafine | NA | 2017 | 0 (0) | 1 (NA) |
| 2 | None | Terbinafine | — | 2014 | 0 | 1 (2) |
| 3 | Terbinafine, amorolfine, ketoconazole | Terbinafine, itraconazole | NA | 2013 | 6c (8) | 2 (24) |
| 4 | Terbinafine, amorolfine, ciclopirox | Terbinafine, itraconazole, fluconazole | 2004 | 2010 | 3 (7) | 3 (53) |
| 5 | Terbinafine, ketoconazole | Terbinafine, itraconazole | 2010 | 2015 | 9c (20) | 2 (6) |
| 6 | Terbinafine | Terbinafine, itraconazole | 2014 | 2015 | 3 (8) | 2 (17) |
| 7 | Amorolfine | Terbinafine, fluconazole, itraconazole, griseofulvin | NA | 2000 | NA (NA) | 3 (36) |
| 8 | Terbinafine, amorolfine, ketoconazole | Terbinafine, itraconazole | 2012 | 2005 | 2 (24) | 3 (28) |
| 9 | None | Terbinafine, itraconazole | — | 2015 | 0 (0) | 1 (NA) |
| 10 | NA | Terbinafine, itraconazole | NA | Before 1990 | NA (NA) | NA (>104) |
| 11 | Terbinafine, miconazole-hydrocortisone combination | Terbinafine, itraconazole | 2017 | 2017 | 2 (10) | 3 (28) |
| 12 | Terbinafine, amorolfine, ketoconazole | Terbinafine, itraconazole, griseofulvin | 2009 | 2008 | NA (NA) | 2 (24) |
| 13 | Terbinafine | Terbinafine | 2010 | 2014 | NA (NAd ) | NA (NA) |
| 14 | Terbinafine | Terbinafine, itraconazole | 2018 | 2018 | 1 (16) | 1 |
NA, not available.
—, no first-exposure year, since topical terbinafine was not used.
Two of the treatment courses consisted of one application only of terbinafine hydrochloride.
Several treatment courses (2 to 4 weeks) in a 2-year period.
Fungal identification and susceptibility.
All 14 dermatophyte infections were confirmed by culturing. The internal transcribed spacer (ITS) sequences of 12 isolates (86%) were identical, with a 100% match (579/579 bp), to the sequence of T. rubrum strain CBS 392.58, and for 2 isolates (14%), a 100% match (657/657 bp) was found to the reference sequence of T. interdigitale strain CBS 558.66 (and 507/507 and 506/507 bp matched neotype T. interdigitale [CBS 428.63NT] and neotype Trichophyton mentagrophytes [IHEM 4268NT], respectively). Susceptibility testing of the Trichophyton isolates was performed with and without the addition of chloramphenicol and cycloheximide (CC) to the inoculum. The terbinafine MICs against the eight T. rubrum comparator isolates were 0.016 to 0.06 mg/liter (geometric mean, 0.03 mg/liter) and 0.016 to 0.06 mg/liter (geometric mean, 0.028 mg/liter) with and without CC supplementation, respectively (Table 2). Terbinafine MICs against eight T. interdigitale comparator isolates were identical for four out of eight and one twofold dilution step different for the remaining four isolates, with and without CC, ranging from 0.008 to 0.03 mg/liter (geometric mean, 0.017 and 0.012 mg/liter, respectively). Consequently, T. rubrum and T. interdigitale study isolates with MICs of >0.06 mg/liter and >0.03 mg/liter, respectively, were regarded as non-wild-type (non-WT) susceptible to terbinafine. Overall, seven T. rubrum patient isolates and both T. interdigitale patient isolates (64%) displayed high terbinafine resistance (MICs, 4 to >8 mg/liter), two T. rubrum isolates (14%) showed moderate resistance (MICs, 1 to 2 mg/liter), and three T. rubrum isolates (21%) showed low resistance (MICs, 0.125 to 0.25 mg/liter). Among 30 susceptibility-tested isolates, 25 were successfully tested with and without CC (Table 2). For three isolates. MICs were available only for the susceptibility test with CC (due to contamination without CC), and for two, MICs were available only for the test without CC, because the isolates could not be revived from stock for additional susceptibility testing with CC. Perfect agreement was observed between MICs determined with and without CC: MICs were identical for 17/25 (68%) isolates and within one 2-fold dilution for 8/25 (32%).
Squalene epoxidase analysis.
Well-known aa substitutions in squalene epoxidase were found in all nine isolates displaying high-level resistance (F397L [n = 7] and L393F [n = 2]) (Table 2). Similarly, another well-described alteration was found in two moderately resistant isolates (aa substitution L393S), while both known and novel missense mutations, leading to aa substitutions F415S, H440Y F484Y, and I121M V237I, were detected in three low-resistant isolates (Table 2). All terbinafine-susceptible comparator isolates had wild-type squalene epoxidase.
Squalene epoxidase protein modeling.
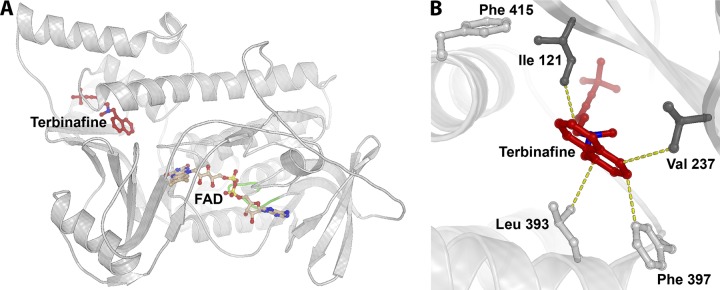
The I121M V237I and F484Y alterations have not been described previously. The isoleucine (I121) and valine (V237) are situated in conserved aa regions across diverse fungal species (Trichophyton species, Aspergillus fumigatus, Candida albicans, and Saccharomyces cerevisiae [data not shown]). Hence, protein modeling was performed to analyze both positions in relation to the proposed binding of terbinafine (Fig. 1). As visualized, I121 and V237 are both in close proximity to the terbinafine binding site, as are F397 and L393. Both substitutions are to similar aa (methionine and isoleucine, respectively) with nonpolar aliphatic side chains. F484Y may be insignificant for terbinafine MICs, since this aa position is not conserved across other species and this alteration is combined with H440Y, which has already been shown to confer elevated terbinafine MICs similar to those in our study (2).
FIG 1.
Trichophyton rubrum squalene epoxidase model. (A) Overall view of protein fold with the cofactor FAD (light brown) superimposed from the structurally homologous flavoprotein/dehydrogenase from Cytophaga hutchinsonii (PDB entry 3NIX). The coordinates for terbinafine (red) are superimposed from the model of Saccharomyces cerevisiae SE (28), followed by refinement of the binding site positioning using AutoDock Vina. The loop comprising the conserved 45GXGXXG50 motif of the Rossmann fold is marked in green. (B) Close-up of the binding site of terbinafine. Amino acid positions where substitutions have been shown to be crucial for terbinafine resistance are depicted as sticks. The dark gray sticks represent the positions of newly discovered resistance mutations (I121 and V237), while the light gray sticks represent those identified previously (L393, F397, and F415) (2). Terbinafine is shown in red, and the yellow dotted lines represent distances in the range of 3 to 4 Å between terbinafine and surrounding residues.
DISCUSSION
Fourteen cases of terbinafine-resistant Trichophyton infection diagnosed over the past 5 years are documented. All resistant isolates harbored target gene alterations, including one not previously reported (the I121M V237I double substitution), the potential importance of which was suggested by protein-modeling analysis. Taken together, these observations suggest that susceptibility testing is important in treatment failure cases and that terbinafine resistance may be emerging in T. rubrum and T. interdigitale strains in Denmark.
Clinical resistance due to, e.g., lack of absorption of the antifungal agent or low penetration, as seen in nail dermatophytomas, is a well-recognized challenge (12). In contrast, clinical failure due to acquired terbinafine resistance has been described only sporadically in Denmark and only with regard to T. rubrum infections (10, 11). Susceptibility testing of dermatophytes is not routine practice, and therefore, it is very likely that terbinafine resistance is underestimated. In our experience, dermatophyte susceptibility testing is often complicated by contamination of the susceptibility plate despite primary isolation on selective agars, probably due in part to the necessary prolonged incubation. The proposed EUCAST microdilution protocol for dermatophytes presented here may contribute to making terbinafine susceptibility testing more accessible by preventing the overgrowth of nondermatophyte molds and bacteria. Importantly, MICs were not affected by CC supplementation: 100% MIC agreement within one 2-fold dilution was observed.
In vitro resistance is classified as primary (intrinsic) drug resistance or secondary (acquired) resistance after exposure to an antifungal. Both types of terbinafine resistance have been reported in dermatophyte infections (13, 14). Cutoff values for defining terbinafine resistance in T. rubrum and T. interdigitale have been suggested to be ≥1 mg/liter (15, 16). In a recent study by Khurana et al., wild-type T. interdigitale strains isolated from patients with clinical treatment failure had higher MIC values (≥0.25 mg/liter), supporting the assumption that in vitro resistance correlates to clinical treatment failure (16).
There are no established epidemiological cutoff values (ECOFFs) for either T. rubrum or T. interdigitale. In our study, the modal MIC for T. rubrum isolates with wild-type SE was 0.03 mg/liter, and all but one had terbinafine MIC values of ≤0.03 mg/liter, suggesting an epidemiological cutoff value of 0.06 or 0.125 mg/liter. The majority of wild-type T. interdigitale isolates had a MIC of 0.016 mg/liter (range, 0.008 to 0.03 mg/liter). Variable Clinical and Laboratory Standards Institute (CLSI) MIC ranges have been reported in the literature for both T. rubrum and T. interdigitale isolates. For both species, ranges stretch from 0.004 mg/liter to >32 mg/liter, mainly due to the inclusion of isolates with acquired resistance. Overall, however, most isolates display CLSI terbinafine MIC ranges from 0.004 to 0.125 mg/liter, with MIC50 values of 0.03 to 0.06 mg/liter (6, 7, 17–21), which we regard as equivalent to our findings. Nonetheless, two Danish cases of treatment failure involved T. rubrum isolates displaying EUCAST terbinafine MICs of 0.125 mg/liter, indicating a risk of misclassification of nonsusceptible isolates unless a restrictive clinical breakpoint of ≤0.06 mg/liter for susceptibility is selected.
The MIC ranges for wild-type T. rubrum and T. interdigitale isolates in this study were observed to be lower than those described in India (4, 7). Indeed, a review of the literature revealed notable differences in the MIC ranges and MIC50 values across various studies overall, and for two studies from India, MIC ranges (MIC50 values) were 0.015 to 8 mg/liter (0.03 mg/liter) (18) and 0.25 to ≥32 mg/liter (1 mg/liter) (16). Such differences suggest that interlaboratory variation and method-specific differences between EUCAST and CLSI are important factors causing this variation and that further method standardization is warranted before formal ECOFFs/estimated components of variation (ECVs) and clinical breakpoints can be established.
Recently, an epidemic of terbinafine-resistant infections was described in India. The combination of topical steroids and antifungals has been suggested as an explanation, since topical steroid use may activate fungal metabolism and cell membrane-protective activity (16). It was not within the scope of this study to investigate if the patients had previously used topical steroids, but it was noted as earlier treatment in five of the cases.
This paper is limited by the retrospective review of patient data, the limited number of isolates, and the single-center format with respect to the evaluation of the proposed EUCAST method. Therefore, epidemiological surveys and multicenter validation of the proposed method with more isolates are warranted.
In conclusion, terbinafine resistance has been detected in Denmark using a modified EUCAST AFST method, which facilitated dermatophyte susceptibility testing. All patients whose medical histories were known had been previously exposed to topical and systemic terbinafine, which probably contributed to the selection/development of terbinafine resistance. Over-the-counter self-medication may contribute to this. It is therefore suggested that antifungal medication be accessible only through a doctor’s prescription and that AFST be performed for nonresponders. Action is needed for this emerging public health problem.
MATERIALS AND METHODS
Patients.
Patients with confirmed Trichophyton infections with reduced terbinafine susceptibilities who were seen routinely at hospital dermatology clinics or private dermatologic clinics during the years 2013 to 2018 were included retrospectively.
Clinical information.
Information on age, sex, concomitant diseases, and previous and current antifungal treatment was obtained through medical records. Case numbers 2 and 7 have been published previously (10, 11).
Clinical isolates and species identification.
Clinical samples from 14 patients with dermatophyte infections who did not respond to terbinafine treatment were sent to the mycology reference laboratory, Statens Serum Institut, Copenhagen, Denmark, for species identification and AFST. Primary detection was performed by direct microscopy of the clinical specimen using Blankophor in 10% potassium hydroxide supplemented with 10% glycerol (SSI Diagnostica, Hillerød, Denmark). Culturing was performed on Sabouraud glucose agar supplemented with chloramphenicol and cycloheximide (SSI Diagnostica, Hillerød, Denmark), and cultures were incubated at 25°C for as long as 6 weeks. Identification to genus and species levels was performed by micro- and macromorphology and was confirmed by DNA sequencing (ITS1 and ITS2) (22).
Eight T. rubrum and eight T. interdigitale clinical isolates susceptible to terbinafine were included as comparators, and the upper limit of the MIC distribution for these isolates was used as the cutoff between wild-type and non-wild-type organisms.
AFST.
Stock solutions of antifungal compounds were prepared in dimethyl sulfoxide (DMSO; 5,000 mg/liter; Sigma-Aldrich). Microtiter plates (cell culture-treated Nunc MicroWell 96-well microplates, catalog no. 167008; Thermo Fisher Scientific) were prepared using 2-fold serial dilutions in double-concentrated medium according to the EUCAST E.Def 9.3.1 methodology (23), with pipette tips changed twice during the titration. The microtiter plates were frozen at −80°C for at least 24 h prior to use, which is part of routine practice in most laboratories. The antimycotics (all purchased from Sigma-Aldrich) (concentration ranges) applied were as follows: terbinafine and voriconazole (range, 0.008 to 8 mg/liter) and fluconazole and itraconazole (range, 0.03 to 32 mg/liter). Aspergillus flavus ATCC 204304 and A. flavus CNM-CM-1813 were used as quality controls for susceptibility testing and were read after 2 days of incubation at 37°C.
AFST of dermatophytes was performed by following a modified EUCAST E.Def 9.3.1 protocol (23) using a standardized inoculum by filtration (11-nm pore size; Merck Millipore, Sigma-Aldrich) and endpoint reading after 5 to 7 days of incubation at 25°C. Challenges with bacterial growth were prevented by supplementing with chloramphenicol and cycloheximide (CC) (final concentrations in the inoculated susceptibility plate, 50 mg/liter and 300 mg/liter, respectively), and the Trichophyton isolates were thus tested with and without the CC supplementation. Endpoint reading was performed visually (full inhibition) and spectrophotometrically (90% inhibition) for all isolates. An elevation in the optical density at 490 nm (OD490) beyond the background level of ≥0.200 for the positive controls was used as the criterion for sufficient growth for both the Trichophyton isolates and the controls.
Molecular analysis of the gene encoding squalene epoxidase.
Genomic DNA was extracted by obtaining fungal specimens directly from cultured plates. Fungal cell material was subjected to bead beating with 1.4-mm molecular-biology-grade zirconium beads (OPS Diagnostics, Lebanon, NJ, USA) and was subsequently extracted on the automated NucliSENS easyMag platform (bioMérieux Nordic, Gothenburg, Sweden). The entire gene encoding squalene epoxidase was amplified by PCR using primers TRUB SE-F0 (TTACCCCATCAATAAGTTACTAC) and TRUB SE-R0 (GAGTTAGAGATAAGCCTATCTGC) for T. rubrum and primers Tricho SE-F0 (TGACAGCGACAAGTGCCA) and TINT SE-R0 (AAAGAGCTAGAGATAAGCCTATCTG) for T. interdigitale. PCR products were Sanger sequenced (Macrogen, Netherlands) using additional sequencing primers: TRUB SE-F2 (AATATCTCCCCATACAACCAG) and TRUB SE-R2 (AACCCTCCCTTCTCCAACGCA) for T. rubrum and TRI SE-F3 (GGAATATCTCCCCATACAACCAG) and TRI SE-R3 (CCTCCCTTCTCCAACGCAG) for T. interdigitale. Sequences were aligned and compared to wild-type reference sequences in GenBank for T. rubrum SE (accession no. AY282411) and T. interdigitale SE (accession no. EZF33561) (4, 8).
3D homology model.
The 3-dimensional (3D) homology structure of squalene epoxidase from Trichophyton rubrum was predicted using I-TASSER (24). The model with the highest confidence score was chosen, and the atomic geometry was further refined by ModRefiner (25). The chosen model had high structural resemblance to the flavoprotein/dehydrogenase from Cytophaga hutchinsonii in complex with flavin adenine dinucleotide (FAD) (PDB entry 3NIX) as well as the modeled structure of the SE-terbinafine complex from S. cerevisiae (26). Therefore, the FAD from the 3NIX complex could be superimposed on the corresponding binding site of the T. rubrum SE model without significant steric hindrance. Terbinafine from the S. cerevisiae model was also superimposed on the model of T. rubrum SE, and its position was refined by a docking procedure carried out by AutoDock Vina software (27). Docking was performed with a grid box narrowing the accepted space to the terbinafine binding pocket suggested in the SE model from S. cerevisiae.
Ethical consideration.
The study investigates antifungal resistance in dermatophytes and not the human host biology, and thus, ethical approval as described in paragraph 2 in the Danish Ethical Committee Law is not required. Since May 2018, a local register of activities has been established according to paragraph 30 in the General Data Protection Regulation (GDPR). The local journal number at Statens Serum Institut covering activities related to this project is 18/04265. Informed consent to use patient data was obtained in writing from the dermatological departments and clinics involved.
ACKNOWLEDGMENTS
We thank Eva Due (Dermatologic Clinic, Birkerød, Denmark) and Małgorzata Wanat-Krzak (Dermatologic Clinic, Skive, Denmark) for their case contribution. We also thank Birgit Brandt and Nissrine Abou-Chakra (Unit of Mycology, Department for Bacteria, Parasites and Fungi, Statens Serum Institut, Copenhagen, Denmark) for excellent laboratory assistance, for technical support for species identification, and for performing the susceptibility testing.
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship. D.M.L.S. participated in planning, collected data, wrote, and contributed to the critical review of the manuscript. R.K.H. participated in planning, did laboratory work, wrote, and contributed to the critical review of the manuscript. K.M.J. did laboratory work, wrote, and contributed to the critical review of the manuscript. R.J. carried out protein modeling, wrote, and contributed to the critical review of the manuscript. M.D., C.O.Z., S.F.T., L.B.-H., and K.K. collected data and contributed to the critical review of the manuscript. M.C.A. participated in planning, did laboratory work, wrote, and contributed to the critical review of the manuscript.
D.M.L.S. was paid as a consultant for advisory board meetings by AbbVie, Janssen, and Sanofi and received speaker’s honoraria and/or grants from the following companies during the past 5 years: AbbVie, Desitin, Pfizer, Galderma, Astellas, Novartis, and LEO Pharma. R.K.H. has received a research grant from Gilead and travel grants from Gilead, Astellas, MSD, and Pfizer. K.M.J. has received travel grants from Amplyx and F2G and a meeting grant from MSD. M.C.A. has received personal speaker’s honoraria from Astellas, Basilea, Gilead, MSD, Pfizer, T2 Biosystems, and Novartis. She has received research grants and obtained contract work (for which the Statens Serum Institute was paid) from Amplyx, Astellas, Basilea, Cidara, F2G, Gilead, MSD, Novabiotics, Pfizer, Scynexis, and T2 Biosystems over the past 5 years. C.O.Z. has served as a scientific consultant for AbbVie, Pfizer, Janssen-Cilag, Merck & Co., Inc., Eli Lilly, Takeda, CSL, and Novartis and as a clinical study investigator for AbbVie, Amgen, Eli Lilly, Merck & Co., Inc., Takeda, LEO Pharma, and Novartis. S.F.T. has been a paid speaker for AbbVie, LEO Pharma, Eli Lilly, Novartis, Pierre Fabre, and Sanofi, has served on advisory boards for AbbVie, Celgene, Eli Lilly, Janssen, LEO Pharma, Novartis, Roche, and Sanofi, and has received research grants from AbbVie, Novartis, Sanofi, UCB, and LEO Pharma. M.D. has been a paid advisor, speaker, and/or investigator for AbbVie, LEO Pharma, Eli Lilly, Pfizer, Pierre Fabre, Meda, Galapagos, Sanofi Genzyme, and Regeneron. K.K. has been a paid speaker for AbbVie, LEO Pharma, Eli Lilly, Meda, Takeda, Merck, Bristol-Myers Squibb, and Galderma, has served on advisory boards for AbbVie, Celgene, LEO Pharma, and Novartis, and has received research grants from Novartis. L.B.-H. and R.J. have nothing to disclose.
All expenses were covered by the investigators. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Ghannoum M, Isham N, Herbert J, Henry W, Yurdakul S. 2011. Activity of TDT 067 (terbinafine in Transfersome) against agents of onychomycosis, as determined by minimum inhibitory and fungicidal concentrations. J Clin Microbiol 49:1716–1720. doi: 10.1128/JCM.00083-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamada T, Maeda M, Alshahni MM, Tanaka R, Yaguchi T, Bontems O, Salamin K, Fratti M, Monod M. 2017. Terbinafine resistance of Trichophyton clinical isolates caused by specific point mutations in the squalene epoxidase gene. Antimicrob Agents Chemother 61:e00115-17. doi: 10.1128/AAC.00115-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santos HL, Lang EAS, Segato F, Rossi A, Martinez-Rossi NM. 2018. Terbinafine resistance conferred by multiple copies of the salicylate 1-monooxygenase gene in Trichophyton rubrum. Med Mycol 56:378–381. doi: 10.1093/mmy/myx044. [DOI] [PubMed] [Google Scholar]
- 4.Singh A, Masih A, Khurana A, Singh PK, Gupta M, Hagen F, Meis JF, Chowdhary A. 2018. High terbinafine resistance in Trichophyton interdigitale isolates in Delhi, India harbouring mutations in the squalene epoxidase (SQLE) gene. Mycoses 61:477–484. doi: 10.1111/myc.12772. [DOI] [PubMed] [Google Scholar]
- 5.Gupta AK, Foley KA, Versteeg SG. 2017. New antifungal agents and new formulations against dermatophytes. Mycopathologia 182:127–141. doi: 10.1007/s11046-016-0045-0. [DOI] [PubMed] [Google Scholar]
- 6.Salehi Z, Shams-Ghahfarokhi M, Razzaghi-Abyaneh M. 2018. Antifungal drug susceptibility profile of clinically important dermatophytes and determination of point mutations in terbinafine-resistant isolates. Eur J Clin Microbiol Infect Dis 37:1841–1846. doi: 10.1007/s10096-018-3317-4. [DOI] [PubMed] [Google Scholar]
- 7.Rudramurthy SM, Shankarnarayan SA, Dogra S, Shaw D, Mushtaq K, Paul RA, Narang T, Chakrabarti A. 2018. Mutation in the squalene epoxidase gene of Trichophyton interdigitale and Trichophyton rubrum associated with allylamine resistance. Antimicrob Agents Chemother 62:e02522-17. doi: 10.1128/AAC.02522-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osborne CS, Leitner I, Favre B, Ryder NS. 2005. Amino acid substitution in Trichophyton rubrum squalene epoxidase associated with resistance to terbinafine. Antimicrob Agents Chemother 49:2840–2844. doi: 10.1128/AAC.49.7.2840-2844.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osborne CS, Leitner I, Hofbauer B, Fielding CA, Favre B, Ryder NS. 2006. Biological, biochemical, and molecular characterization of a new clinical Trichophyton rubrum isolate resistant to terbinafine. Antimicrob Agents Chemother 50:2234–2236. doi: 10.1128/AAC.01600-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Digby SS, Hald M, Arendrup MC, Hjort SV, Kofoed K. 2017. Darier disease complicated by terbinafine-resistant Trichophyton rubrum: a case report. Acta Derm Venereol 97:139–140. doi: 10.2340/00015555-2455. [DOI] [PubMed] [Google Scholar]
- 11.Schøsler L, Andersen LK, Arendrup MC, Sommerlund M. 2018. Recurrent terbinafine resistant Trichophyton rubrum infection in a child with congenital ichthyosis. Pediatr Dermatol 35:259–260. doi: 10.1111/pde.13411. [DOI] [PubMed] [Google Scholar]
- 12.Roberts DT, Evans EG. 1998. Subungual dermatophytoma complicating dermatophyte onychomycosis. Br J Dermatol 138:189–190. doi: 10.1046/j.1365-2133.1998.02050.x. [DOI] [PubMed] [Google Scholar]
- 13.Pai V, Ganavalli A, Kikkeri N. 2018. Antifungal resistance in dermatology. Indian J Dermatol 63:361–368. doi: 10.4103/ijd.IJD_131_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukherjee PK, Leidich SD, Isham N, Leitner I, Ryder NS, Ghannoum MA. 2003. Clinical Trichophyton rubrum strain exhibiting primary resistance to terbinafine. Antimicrob Agents Chemother 47:82–86. doi: 10.1128/aac.47.1.82-86.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Favre B, Ghannoum MA, Ryder NS. 2004. Biochemical characterization of terbinafine-resistant Trichophyton rubrum isolates. Med Mycol 42:525–529. doi: 10.1080/13693780410001661482. [DOI] [PubMed] [Google Scholar]
- 16.Khurana A, Masih A, Chowdhary A, Sardana K, Borker S, Gupta A, Gautam RK, Sharma PK, Jain D. 2018. Correlation of in vitro susceptibility based on MICs and squalene epoxidase mutations with clinical response to terbinafine in patients with tinea corporis/cruris. Antimicrob Agents Chemother 62:e01038-18. doi: 10.1128/AAC.01038-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiruma J, Kitagawa H, Noguchi H, Kano R, Hiruma M, Kamata H, Harada K. 2019. Terbinafine-resistant strain of Trichophyton interdigitale strain isolated from a tinea pedis patient. J Dermatol 46:351–353. doi: 10.1111/1346-8138.14809. [DOI] [PubMed] [Google Scholar]
- 18.Pathania S, Rudramurthy SM, Narang T, Saikia UN, Dogra S. 2018. A prospective study of the epidemiological and clinical patterns of recurrent dermatophytosis at a tertiary care hospital in India. Indian J Dermatol Venereol Leprol 84:678–684. doi: 10.4103/ijdvl.IJDVL_645_17. [DOI] [PubMed] [Google Scholar]
- 19.Badali H, Mohammadi R, Mashedi O, de Hoog GS, Meis JF. 2015. In vitro susceptibility patterns of clinically important Trichophyton and Epidermophyton species against nine antifungal drugs. Mycoses 58:303–307. doi: 10.1111/myc.12315. [DOI] [PubMed] [Google Scholar]
- 20.Yenişehirli G, Tunçoğlu E, Yenişehirli A, Bulut Y. 2013. In vitro activities of antifungal drugs against dermatophytes isolated in Tokat, Turkey. Int J Dermatol 52:1557–1560. doi: 10.1111/ijd.12100. [DOI] [PubMed] [Google Scholar]
- 21.Altınbaş R, Özakkaş F, Barış A, Turan D, Şen S. 2018. In vitro susceptibility of seven antifungal agents against dermatophytes isolated in İstanbul. Turk J Med Sci 48:615–619. doi: 10.3906/sag-1709-157. [DOI] [PubMed] [Google Scholar]
- 22.Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, Chen W, Bolchacova E, Voigt K, Crous PW, Miller AN, Wingfield MJ, Aime MC, An K-D, Bai F-Y, Barreto RW, Begerow D, Bergeron M-J, Blackwell M, Boekhout T, Bogale M, Boonyuen N, Burgaz AR, Buyck B, Cai L, Cai Q, Cardinali G, Chaverri P, Coppins BJ, Crespo A, Cubas P, Cummings C, Damm U, de Beer ZW, de Hoog GS, Del-Prado R, Dentinger B, Dieguez-Uribeondo J, Divakar PK, Douglas B, Duenas M, Duong TA, Eberhardt U, Edwards JE, Elshahed MS, Fliegerova K, Furtado M, Garcia MA, Ge Z-W, Griffith GW, Griffiths K, Groenewald JZ, Groenewald M, Grube M, Gryzenhout M, Guo L-D, Hagen F, Hambleton S, et al. 2012. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Natl Acad Sci 109:6241–6246. doi: 10.1073/pnas.1117018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arendrup MC, Meletiadis J, Mouton JW, Lagrou K, Hamal P, Guinea J, Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). January 2017. EUCAST Definitive Document E.Def 9.3.1. Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia forming moulds. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E_Def_9_3_1_Mould_testing__definitive.pdf.
- 24.Zhang Y. 2008. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics 9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu D, Zhang Y. 2011. Improving the physical realism and structural accuracy of protein models by a two-step atomic-level energy minimization. Biophys J 101:2525–2534. doi: 10.1016/j.bpj.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nowosielski M, Hoffmann M, Wyrwicz LS, Stepniak P, Plewczynski DM, Lazniewski M, Ginalski K, Rychlewski L. 2011. Detailed mechanism of squalene epoxidase inhibition by terbinafine. J Chem Inf Model 51:455–462. doi: 10.1021/ci100403b. [DOI] [PubMed] [Google Scholar]
- 27.Trott O, Olson AJ. 2010. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruckenstuhl C, Lang S, Poschenel A, Eidenberger A, Baral PK, Kohút P, Hapala I, Gruber K, Turnowsky F. 2007. Characterization of squalene epoxidase of Saccharomyces cerevisiae by applying terbinafine-sensitive variants. Antimicrob Agents Chemother 51:275–284. doi: 10.1128/AAC.00988-06. [DOI] [PMC free article] [PubMed] [Google Scholar]