This single-arm trial (n = 104) in western Cambodia showed high efficacy for 3-day treatment with pyronaridine-artesunate plus single-dose primaquine in Plasmodium falciparum malaria.
KEYWORDS: Cambodia, Plasmodium falciparum, Plasmodium vivax, antimalarial agents, artemisinin, drug resistance, malaria, pyronaridine-artesunate
ABSTRACT
This single-arm trial (n = 104) in western Cambodia showed high efficacy for 3-day treatment with pyronaridine-artesunate plus single-dose primaquine in Plasmodium falciparum malaria. Day 42 PCR-adjusted adequate clinical and parasitological response (ACPR) was 98.3% (58/59) (95% confidence interval [CI], 90.9 to 100.0) in Trapeng Chau in Kampong Speu and 100% (41/41) (95% CI, 91.4 to 100) in Veal Veng in Pursat; 80.6% (83/103) of the patients had P. falciparum with drug resistance molecular markers. For Plasmodium vivax malaria, pyronaridine-artesunate day 28 ACPR was 98.3% (59/60) (95% CI, 91.1 to 100) and 100% (60/60) (95% CI, 94.0 to 100), respectively. (This study is registered in the Australian New Zealand Clinical Trials Registry [ANZCTR] under reference no. ACTRN12618001999224.)
TEXT
Multidrug-resistant Plasmodium falciparum malaria is highly prevalent in western Cambodia, and therapeutic options for the treatment of uncomplicated malaria are increasingly limited (1–5). New therapeutic options are needed to support malaria elimination in the region (6). Pyronaridine-artesunate is an artemisinin-based combination therapy for the treatment of uncomplicated P. falciparum and Plasmodium vivax malaria. Previous studies with pyronaridine-artesunate in P. falciparum malaria indicated >95% efficacy in eastern Cambodia (7), while efficacy was lower in western Cambodia (Pursat/Pailin) (87.2%; 95% confidence interval [CI], 79.7 to 92.6) (8). The current study evaluated the efficacy and safety of pyronaridine-artesunate plus single-dose primaquine in uncomplicated P. falciparum malaria in two areas of known P. falciparum multidrug resistance in western Cambodia. This study follows the same methodology as a previous study in eastern Cambodia (7), except that pyronaridine-artesunate efficacy and safety in P. vivax malaria was also investigated.
Patients with microscopically confirmed malaria were recruited between September and December 2018 from health centers in western Cambodia (Trapeng Chau in Kampong Speu and Veal Veng in Pursat). Eligible patients were ≥7 years old, weighed ≥20 kg, had fever/history of fever, and had P. falciparum or P. vivax monoinfection (500 to 250,000 sexual parasites μl−1). Exclusion criteria were pregnancy, severe or complicated malaria, malnutrition, concomitant disease, known kidney or liver disease, hypersensitivity, and contraindication to the study drugs. All patients or their guardians provided written informed consent plus assent from patients aged <18 years. Ethical approval was granted by the National Ethics Committee at the National Institute of Public Health, Phnom Penh, and the Ethics Review Committee of the World Health Organization Regional Office for the Western Pacific.
The study was conducted using the standard World Health Organization protocol for the assessment of antimalarial efficacy (9). Study procedures, evaluations, and analyses were consistent with a previously reported study conducted in eastern Cambodia (7). Patients received oral pyronaridine-artesunate (Shin Poong Pharmaceutical Co., Ansan, South Korea) once daily for 3 days, dosed according to body weight, plus single-dose primaquine 15 mg on day 0 for P. falciparum cases (7, 10). The primary endpoint was adequate clinical and parasitological response (ACPR), defined as no parasitemia without previous treatment failure (9). The primary outcome was evaluated for P. falciparum isolates at day 42 and for P. vivax isolates at day 28. For P. falciparum isolates, ACPR was adjusted for reinfection with PCR genotyping using published methods (11). Data were analyzed by using a WHO Excel spreadsheet. To determine the prevalence of P. falciparum molecular markers to artemisinin, piperaquine, and mefloquine, the Kelch13 (K13) gene was sequenced, and gene copy numbers for plasmepsin 2 (Pfpm2) and multidrug resistance 1 (Pfmdr1) were determined using published methods (12, 13). The threshold for genic amplification was defined as >1.5 copies.
The study population included 104 patients with P. falciparum and 120 with P. vivax malaria; baseline data were similar across the two sites, although P. falciparum parasitemia was higher in Trapeng Chau (Table 1). For P. falciparum isolates, day 42 PCR-adjusted ACPR was 98.3% (95% CI, 88.8 to 99.8) in Trapeng Chau and 100% in Veal Veng (Kaplan-Meier analysis). In a per-protocol analysis, the proportion of patients with day 42 PCR-adjusted ACPR was 98.3% (58/59) (95% CI, 90.9 to 100.0) in Trapeng Chau and 100% (41/41) (95% CI, 91.4 to 100) in Veal Veng.
TABLE 1.
Baseline characteristics and population disposition
| Characteristic | Results for: |
|||
|---|---|---|---|---|
|
P. falciparum from: |
P. vivax from: |
|||
| Trapeng Chau | Veal Veng | Trapeng Chau | Veal Veng | |
| Male/female (n) | 60/2 | 36/6 | 58/2 | 59/1 |
| Mean age (SD) (yr [range]) | 26.5 (10.4) (7–56) | 30.7 (13.7) (9–60) | 21.9 (7.9) (6–53) | 25.2 (10.8) (8–55) |
| Mean weight (SD) (kg [range]) | 53.2 (10.8) (20–75) | 51.8 (10.7) (20–73) | 51.5 (11.0) (20–77) | 52.9 (12.5) (22–82) |
| Geometric mean parasitemia (μl−1 blood [range]) | 20,363 (1,530–251,752) | 12,875 (892–107,936) | 6,801 (1,164–42,435) | 6,829 (1,794–21,367) |
| Safety population | 62 | 42 | 60 | 60 |
| PCR-adjusted per-protocol population | 59 | 41 | 60 | 60 |
| Withdrawn | 0 | 0 | 0 | 0 |
| Lost to follow-up | 2 | 0 | 0 | 0 |
| PCR adjustment | 1 | 1 | Not applicable | Not applicable |
Previous data from Pursat indicated a day 42 ACPR of 89.8% (95% CI, 78.8 to 95.3%) (Kaplan-Meier analysis) (8). Efficacy in the current study was higher in Veal Veng (Pursat) (100%), but this difference was not statistically significant (P = 0.4). The single recrudescence at Trapeng Chau occurred in a 7-year-old child at day 35 consecutively with an infection by an artemisinin- and piperaquine-resistant strain (K13 C580Y, Pfpm2 amplification). Only two patients (both from Trapeng Chau) had P. falciparum gametocytes at day 0, which is an unusually low baseline gametocyte prevalence (1.9% [2/104]), with rates of ∼10% previously reported from Cambodia (14, 15). However, stringent quality control for microscopy at these experienced sentinel sites supports these findings. No further gametocytes were detected throughout the study in any patient, indicating the high efficacy of primaquine in suppressing transmission.
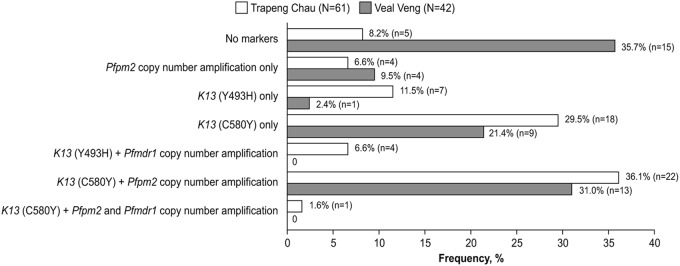
The day 3 parasite positivity rate was 41.7% (25/60) (95% CI, 29.1 to 55.1) in Trapeng Chau and 28.6% (12/42) (95% CI, 15.7 to 44.6) in Veal Veng. This is consistent with a higher prevalence of K13 molecular markers associated with artemisinin resistance in Trapeng Chau (85.2% [52/61]) versus Veal Veng (54.8% [23/42]) (Fig. 1). The prevalence of multiresistant strains, with K13 mutations and Pfpm2 and/or Pfmdr1 copy number amplification, was similar in Trapeng Chau (44.3% [27/61]) and Veal Veng (40.5% [17/42]) (Fig. 1). A triple mutant, with molecular markers associated with artemisinin, piperaquine, and mefloquine resistance, was identified in Trapeng Chau, as was previously noted in northeastern Cambodia (Preah Vihear province) (16).
FIG 1.
Prevalence of molecular markers associated with resistance to artemisinin (K13), piperaquine (Pfpm2), and mefloquine (Pfmdr1). Note that data were incomplete for one patient from Trapeng Chau.
For P. vivax malaria, day 28 ACPR was 98.3% (59/60) (95% CI, 91.1 to 100) in Trapeng Chau and 100% (60/60) (95% CI, 94.0 to 100) in Veal Veng. The day 3 parasite positivity rate was 0% (0/60) in Trapeng Chau and 1.7% (1/60) in Veal Veng.
Adverse events were consistent with symptoms of malaria and generally declined in frequency during treatment (Table 2). Notably, there were no cases of dark urine in P. falciparum cases, consistent with recent data indicating the safety of single-dose primaquine administration (17). There were no serious adverse events or deaths.
TABLE 2.
Frequency of adverse events of any cause by study day
| Adverse event | Frequency (%) in: |
|||||
|---|---|---|---|---|---|---|
|
P. falciparum (n = 104) on: |
P. vivax (n = 120) on: |
|||||
| Day 0 | Day 1 | Day 2 | Day 0 | Day 1 | Day 2 | |
| Fever | 99.0 | 56.3 | 8.8 | 100 | 49.2 | 0.8 |
| Chills | 80.8 | 28.2 | 2.9 | 85.8 | 37.5 | 0.8 |
| Headache | 94.2 | 58.3 | 44.1 | 90.8 | 53.3 | 29.2 |
| Nausea | 18.3 | 9.7 | 3.9 | 14.2 | 7.5 | 0.8 |
| Vomiting | 15.4 | 9.7 | 1.0 | 9.2 | 2.5 | 0.8 |
| Body pain | 84.6 | 51.5 | 29.4 | 75.0 | 40 | 10 |
| Fatigue | 71.2 | 34.0 | 15.7 | 67.5 | 30 | 10.8 |
| Vertigo | 31.7 | 23.3 | 6.9 | 23.3 | 15.8 | 1.7 |
| Confusion | 7.7 | 4.9 | 0 | 1.7 | 0 | 0 |
| Insomnia | 25.0 | 6.8 | 2.0 | 20.8 | 3.3 | 1.7 |
| Deafness | 15.4 | 5.8 | 0 | 0 | 0.8 | 0 |
| Diarrhea | 10.6 | 7.8 | 2.0 | 3.3 | 1.7 | 0 |
| Abdominal pain | 19.2 | 13.6 | 2.9 | 13.3 | 11.7 | 0 |
| Rash | 0 | 0 | 0 | 0 | 0.8 | 0 |
| Palpitations | 1.9 | 3.9 | 1.0 | 0.8 | 0.8 | 0 |
| Tachycardia | 1.0 | 0 | 0 | 0 | 0 | 0 |
Pyronaridine-artesunate had >98% efficacy in two sentinel sites in western Cambodia in both P. falciparum and P. vivax malaria with no adverse events of concern. This is in the context of a high prevalence of molecular markers for artemisinin resistance (72.8% [75/103]) in P. falciparum isolates, which was associated with markers for piperaquine and/or mefloquine resistance in 42.7% (44/103) of the isolates. Pyronaridine-artesunate is a suitable option for the treatment of malaria in Kampong Speu and Pursat, with continuing efficacy surveillance at these sites.
ACKNOWLEDGMENTS
This work was supported by the Bill & Melinda Gates Foundation and USAID-PMI through the World Health Organization. The funding sources were not involved in the design and conduct of the study, the interpretation of the results, or the development of this publication.
Naomi Richardson of Magenta Communications Ltd. developed a first draft of this article from the statistical output, collated author contributions, and provided graphic services and was funded by the World Health Organization. We acknowledge the contributions of the local health staff.
R.L., H.C., and R.H. designed the study. D.M.B. and M.D.B. entered the data and validated microscopy. R.L., M.D.B., P.R., and B.W. analyzed and interpreted the data. M.M.K., N.K., and B.W. conducted the laboratory work (therapeutic efficacy, PCR adjustment, and analysis of molecular markers). All authors critically reviewed the paper and approved the final version of the paper for submission.
D.M.B., M.D.B., and P.R. are staff members of the World Health Organization.
The authors alone are responsible for the views expressed in this publication, and they do not necessarily represent the decisions, policy, or views of the World Health Organization.
We have no conflicts of interest to report.
REFERENCES
- 1.Leang R, Taylor WR, Bouth DM, Song L, Tarning J, Char MC, Kim S, Witkowski B, Duru V, Domergue A, Khim N, Ringwald P, Menard D. 2015. Evidence of Plasmodium falciparum malaria multidrug resistance to artemisinin and piperaquine in western Cambodia: dihydroartemisinin-piperaquine open-label multicenter clinical assessment. Antimicrob Agents Chemother 59:4719–4726. doi: 10.1128/AAC.00835-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amaratunga C, Lim P, Suon S, Sreng S, Mao S, Sopha C, Sam B, Dek D, Try V, Amato R, Blessborn D, Song L, Tullo GS, Fay MP, Anderson JM, Tarning J, Fairhurst RM. 2016. Dihydroartemisinin-piperaquine resistance in Plasmodium falciparum malaria in Cambodia: a multisite prospective cohort study. Lancet Infect Dis 16:357–365. doi: 10.1016/S1473-3099(15)00487-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denis MB, Tsuyuoka R, Poravuth Y, Narann TS, Seila S, Lim C, Incardona S, Lim P, Sem R, Socheat D, Christophel EM, Ringwald P. 2006. Surveillance of the efficacy of artesunate and mefloquine combination for the treatment of uncomplicated falciparum malaria in Cambodia. Trop Med Int Health 11:1360–1366. doi: 10.1111/j.1365-3156.2006.01690.x. [DOI] [PubMed] [Google Scholar]
- 4.Miotto O, Almagro-Garcia J, Manske M, Macinnis B, Campino S, Rockett KA, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Duong S, Nguon C, Chuor CM, Saunders D, Se Y, Lon C, Fukuda MM, Amenga-Etego L, Hodgson AVO, Asoala V, Imwong M, Takala-Harrison S, Nosten F, Su X-Z, Ringwald P, Ariey F, Dolecek C, Hien TT, Boni MF, Thai CQ, Amambua-Ngwa A, Conway DJ, Djimdé AA, Doumbo OK, Zongo I, Ouedraogo J-B, Alcock D, Drury E, Auburn S, Koch O, Sanders M, Hubbart C, Maslen G, Ruano-Rubio V, Jyothi D, Miles A, O'Brien J, Gamble C, Oyola SO, Rayner JC, Newbold CI, Berriman M, Spencer CCA, McVean G, Day NP, White NJ, Bethell D, Dondorp AM, Plowe CV, Fairhurst RM, Kwiatkowski DP. 2013. Multiple populations of artemisinin-resistant Plasmodium falciparum in Cambodia. Nat Genet 45:648–655. doi: 10.1038/ng.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM, Artemisinin R, In Cambodia 1, Study C . 2008. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med 359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. 2015. Strategy for malaria elimination in the Greater Mekong subregion (2015-2030). World Health Organization, Geneva, Switzerland: http://iris.wpro.who.int/bitstream/handle/10665.1/10945/9789290617181_eng.pdf;jsessionid=3204C3285C2E6290730A3453BFFD279D?sequence=1. [Google Scholar]
- 7.Leang R, Mairet-Khedim M, Chea H, Huy R, Khim N, Mey Bouth D, Dorina Bustos M, Ringwald P, Witkowski B. 2019. Efficacy and safety of pyronaridine-artesunate plus single-dose primaquine for the treatment of uncomplicated Plasmodium falciparum malaria in eastern Cambodia. Antimicrob Agents Chemother 63:e02242-18. doi: 10.1128/AAC.02242-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leang R, Canavati SE, Khim N, Vestergaard LS, Borghini Fuhrer I, Kim S, Denis MB, Heng P, Tol B, Huy R, Duparc S, Dondorp AM, Menard D, Ringwald P. 2016. Efficacy and safety of pyronaridine-artesunate for treatment of uncomplicated Plasmodium falciparum malaria in western Cambodia. Antimicrob Agents Chemother 60:3884–3890. doi: 10.1128/AAC.00039-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. 2009. Methods for surveillance of antimalarial drug efficacy. World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/publications/atoz/9789241597531/en/. [Google Scholar]
- 10.World Health Organization. 2015. Policy brief on single-dose primaquine as a gametocytocide in Plasmodium falciparum malaria. World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/publications/atoz/policy-brief-single-dose-primaquine-pf/en/. [Google Scholar]
- 11.World Health Organization. 2008. Methods and techniques for clinical trials on antimalarial drug efficacy: genotyping to identify parasite populations. World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/publications/atoz/9789241596305/en/. [Google Scholar]
- 12.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois A-C, Khim N, Kim S, Duru V, Bouchier C, Ma L, Lim P, Leang R, Duong S, Sreng S, Suon S, Chuor CM, Bout DM, Ménard S, Rogers WO, Genton B, Fandeur T, Miotto O, Ringwald P, Le Bras J, Berry A, Barale J-C, Fairhurst RM, Benoit-Vical F, Mercereau-Puijalon O, Ménard D. 2014. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witkowski B, Duru V, Khim N, Ross LS, Saintpierre B, Beghain J, Chy S, Kim S, Ke S, Kloeung N, Eam R, Khean C, Ken M, Loch K, Bouillon A, Domergue A, Ma L, Bouchier C, Leang R, Huy R, Nuel G, Barale J-C, Legrand E, Ringwald P, Fidock DA, Mercereau-Puijalon O, Ariey F, Ménard D. 2017. A surrogate marker of piperaquine-resistant Plasmodium falciparum malaria: a phenotype-genotype association study. Lancet Infect Dis 17:174–183. doi: 10.1016/S1473-3099(16)30415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin JT, Lon C, Spring MD, Sok S, Chann S, Ittiverakul M, Kuntawunginn W, My M, Thay K, Rahman R, Balasubramanian S, Char M, Lanteri CA, Gosi P, Ubalee R, Meshnick SR, Saunders DL. 2017. Single dose primaquine to reduce gametocyte carriage and Plasmodium falciparum transmission in Cambodia: an open-label randomized trial. PLoS One 12:e0168702. doi: 10.1371/journal.pone.0168702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin JT, Patel JC, Levitz L, Wojnarski M, Chaorattanakawee S, Gosi P, Buathong N, Chann S, Huy R, Thay K, Sea D, Samon N, Takala-Harrison S, Fukuda M, Smith P, Spring M, Saunders D, Lon C. 2018. Gametocyte carriage, antimalarial use, and drug resistance in Cambodia, 2008–2014. Am J Trop Med Hyg 99:1145–1149. doi: 10.4269/ajtmh.18-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossi G, De Smet M, Khim N, Kindermans JM, Menard D. 2017. Emergence of Plasmodium falciparum triple mutant in Cambodia. Lancet Infect Dis 17:1233. doi: 10.1016/S1473-3099(17)30635-7. [DOI] [PubMed] [Google Scholar]
- 17.Dysoley L, Kim S, Lopes S, Khim N, Bjorges S, Top S, Huch C, Rekol H, Westercamp N, Fukuda MM, Hwang J, Roca-Feltrer A, Mukaka M, Menard D, Taylor WR. 2019. The tolerability of single low dose primaquine in glucose-6-phosphate deficient and normal falciparum-infected Cambodians. BMC Infect Dis 19:250. doi: 10.1186/s12879-019-3862-1. [DOI] [PMC free article] [PubMed] [Google Scholar]