Antibiotic tolerance contributes to the inability of standard antimicrobial therapies to clear the chronic Pseudomonas aeruginosa lung infections that often afflict patients with cystic fibrosis (CF). Metabolic potentiation of bactericidal antibiotics with carbon sources has emerged as a promising strategy to resensitize tolerant bacteria to antibiotic killing.
KEYWORDS: Pseudomonas aeruginosa, antibiotic tolerance, fumarate, metabolic potentiation, rpoN, tobramycin
ABSTRACT
Antibiotic tolerance contributes to the inability of standard antimicrobial therapies to clear the chronic Pseudomonas aeruginosa lung infections that often afflict patients with cystic fibrosis (CF). Metabolic potentiation of bactericidal antibiotics with carbon sources has emerged as a promising strategy to resensitize tolerant bacteria to antibiotic killing. Fumarate (FUM), a C4-dicarboxylate, has been recently shown to resensitize tolerant P. aeruginosa to killing by tobramycin (TOB), an aminoglycoside antibiotic, when used in combination (TOB+FUM). Fumarate and other C4-dicarboxylates are taken up intracellularly by transporters regulated by the alternative sigma factor RpoN. Once in the cell, FUM is metabolized, leading to enhanced electron transport chain activity, regeneration of the proton motive force, and increased TOB uptake. In this work, we demonstrate that a ΔrpoN mutant displays impaired FUM uptake and, consequently, nonsusceptibility to TOB+FUM treatment. RpoN was also found to be essential for susceptibility to other aminoglycoside and C4-dicarboxylate combinations. Importantly, RpoN loss-of-function mutations have been documented to evolve in the CF lung, and these loss-of-function alleles can also result in TOB+FUM nonsusceptibility. In a mixed-genotype population of wild-type and ΔrpoN cells, TOB+FUM specifically killed cells with RpoN function and spared the cells that lacked RpoN function. Unlike C4-dicarboylates, both d-glucose and l-arginine were able to potentiate TOB killing of ΔrpoN stationary-phase cells. Our findings raise the question of whether TOB+FUM will be a suitable treatment option in the future for CF patients infected with P. aeruginosa isolates that lack RpoN function.
INTRODUCTION
Pseudomonas aeruginosa is an important pathogen in patients with cystic fibrosis (CF), and chronic P. aeruginosa lung infection is a significant contributor to morbidity and mortality (1, 2). Treatment with antibiotics such as the aminoglycoside tobramycin (TOB) can control, but not eradicate, chronic P. aeruginosa infections (3, 4). There is therefore an urgent need to identify novel treatment strategies that would improve antibiotic efficacy in treating chronic P. aeruginosa lung infections.
The failure of antibiotics like TOB to clear P. aeruginosa can be attributed to a combination of genetic determinants and environmental factors (5–7). Environmental conditions, such as nutrient limitation, can trigger changes in bacterial physiology that confer phenotypic antibiotic tolerance, which is defined as the survival of bacteria in the presence of a concentration of a bactericidal antibiotic that would otherwise be lethal (8). Antibiotic tolerance is reversible once the environmental conditions that promoted the tolerant phenotype have changed. For instance, growth arrest and metabolic dormancy triggered by high bacterial density and low nutrient availability (conditions experienced by cells in stationary-phase cultures, biofilms, and the multicellular aggregates observed in the CF lung) contribute to tolerance to TOB and many other antibiotics (9–18).
Nutrient limitation and the ensuing starvation response can promote tolerance to TOB by reducing TOB entry into the cell. Aminoglycoside uptake into bacteria is energy dependent and requires a threshold membrane potential (19–21). Cells that are metabolically quiescent have a decreased proton motive force (PMF) compared to actively growing cells, and therefore, TOB entry is significantly reduced in cells that are starved (22). Thus, nutrient supplementation combined with TOB is a logical strategy to regenerate PMF, promote TOB uptake, and resensitize the cells to TOB. Indeed, the addition of various metabolites to starved, tolerant cells in order to promote aminoglycoside uptake and killing has been successfully employed by other groups (22–30). For instance, Meylan et al. showed that fumarate (FUM), a C4-dicarboxylate, can sensitize starved, phenotypically tolerant P. aeruginosa cells to TOB killing (22).
P. aeruginosa uses two transporters, DctA and DctPQM, to take up C4-dicarboxylates into the cell (31). Control over dctA and dctPQM transcription involves multiple players, including the alternative sigma factor RpoN (31, 32). Consequently, RpoN is required for growth on C4-dicarboxylates (31). Although RpoN is an important regulator of genes involved in metabolism and virulence (33, 34), several studies have reported that the loss of RpoN function is, somewhat paradoxically, a relatively common mechanism of pathoadaptation to the CF lung (35–41). For instance, Smith et al. found that almost 20% of patients in their study had isolates with nonsynonymous or frameshift mutations in rpoN (36).
Given the critical role of RpoN in promoting the expression of FUM transporters, we hypothesized that P. aeruginosa lacking RpoN function would be nonsusceptible to TOB and FUM combination treatment (TOB+FUM). We found that loss of RpoN function, through either gene deletion or clinically observed loss-of-function point mutations, renders P. aeruginosa nonsusceptible to TOB+FUM due to a lack of FUM uptake. This finding raises concerns about whether TOB+FUM will be effective as a future treatment modality for CF patients infected with P. aeruginosa RpoN loss-of-function mutants.
(Portions of this work were presented by C. W. Hall at the 2019 AMMI Canada-CACMID Annual Conference [Ottawa, ON].)
RESULTS
FUM-mediated potentiation of TOB lethality is RpoN dependent.
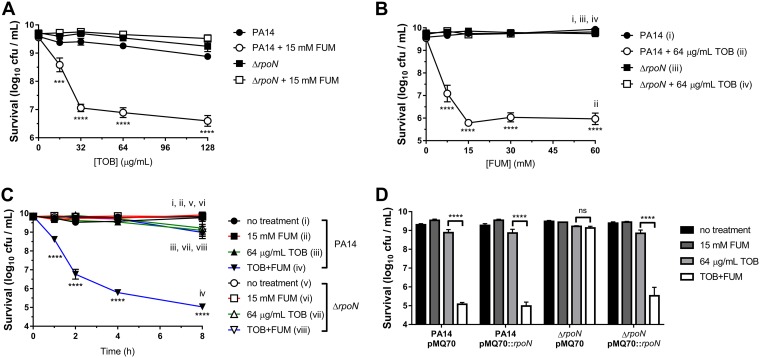
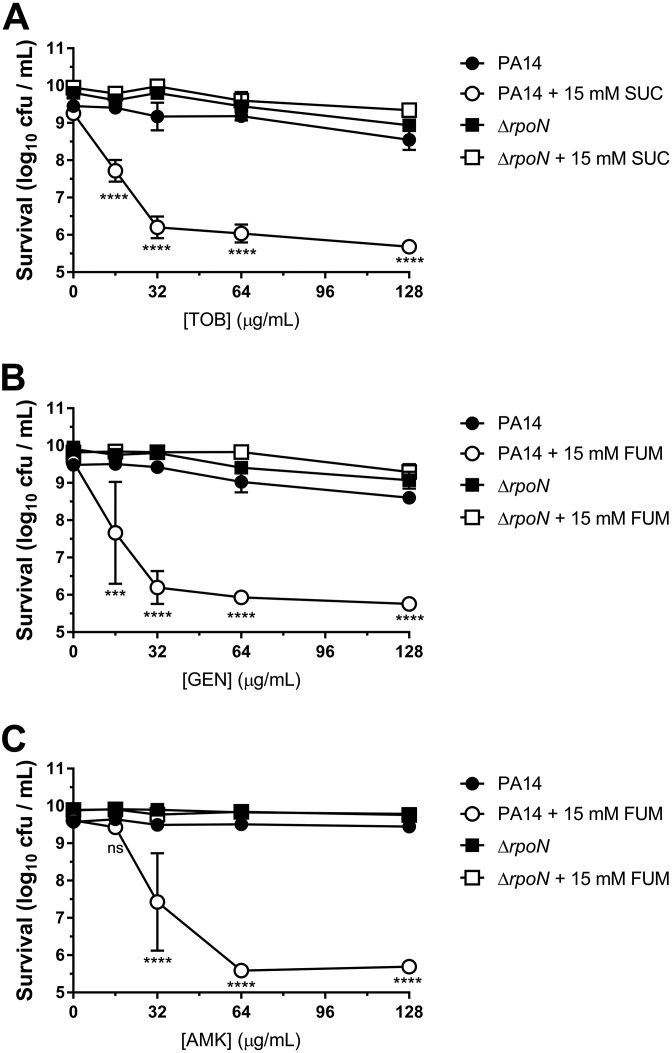
Survival of PA14 wild-type and ΔrpoN stationary-phase cells was determined following incubation for 4 h with or without various concentrations of TOB and/or 15 mM FUM (Fig. 1A), for 4 h with or without 64 μg/ml TOB and various concentrations of FUM (Fig. 1B), and for various amounts of time with or without 64 μg/ml TOB and/or 15 mM FUM (Fig. 1C). In the TOB concentration ([TOB])-dependent, FUM concentration ([FUM])-dependent, and time-dependent killing assays, both wild-type and ΔrpoN cells were tolerant to TOB in the absence of FUM. Moreover, FUM alone did not cause cell death or significant growth. Wild-type cells were significantly more susceptible to TOB+FUM than TOB alone, as previously described (22, 28); however, ΔrpoN cells remained tolerant to TOB even in the presence of FUM under all [TOB], [FUM], and incubation times tested. Introduction of the complementation vector pMQ70::rpoN restored susceptibility of ΔrpoN stationary-phase cells to TOB+FUM (64 μg/ml TOB and 15 mM FUM for 4 h) (Fig. 1D). RpoN was also required for stationary-phase susceptibility to TOB with succinate (SUC) (Fig. 2A), another C4-dicarboxylate, and to FUM combined with the aminoglycoside antibiotic gentamicin (GEN) (Fig. 2B) or amikacin (AMK) (Fig. 2C). Therefore, the results suggest that RpoN is generally important for potentiation of aminoglycoside lethality by C4-dicarboxylates in antibiotic-tolerant P. aeruginosa stationary-phase cells. To ensure that RpoN was important for TOB+FUM susceptibility in another model of antibiotic tolerance, we assessed TOB+FUM susceptibility of wild-type and ΔrpoN colony biofilms. While TOB+FUM killed wild-type biofilms to a much greater extent than TOB alone, there was no significant difference in the survival of ΔrpoN biofilms following TOB monotherapy or TOB+FUM combination treatment (see Fig. S1 in the supplemental material).
FIG 1.
Stationary-phase ΔrpoN cells are not susceptible to TOB+FUM treatment. (A) Survival of PA14 wild-type and ΔrpoN stationary-phase cells that were incubated for 4 h with or without various concentrations of TOB and/or 15 mM FUM. (B) Survival of PA14 wild-type and ΔrpoN stationary-phase cells that were incubated for 4 h with or without 64 μg/ml TOB and/or various concentrations of FUM. (C) Survival of PA14 wild-type and ΔrpoN stationary-phase cells that were incubated for various periods of time with or without 64 μg/ml TOB and/or 15 mM FUM. (D) Survival of PA14 wild-type and ΔrpoN stationary-phase cells carrying pMQ70 or pMQ70::rpoN following incubation for 4 h with or without 64 μg/ml TOB and/or 15 mM FUM. Data in panels A to D are presented as mean log10 CFU per milliliter ± standard errors of the means (SEM) for at least three biological replicates.
FIG 2.
RpoN is required for stationary-phase susceptibility to aminoglycosides combined with C4-dicarboxylates. Shown are survival data for PA14 wild-type and ΔrpoN stationary-phase cells that were incubated for 4 h with no treatment, various concentrations of an aminoglycoside, 15 mM a C4-dicarboxylate, or an aminoglycoside with a C4-dicarboxylate. The tested aminoglycoside and C4-dicarboxylate combinations were TOB+SUC (A), GEN+FUM (B), and AMK+FUM (C). Data are presented as mean log10 CFU per milliliter ± SEM for three biological replicates.
Koeva et al. reported that TOB-resistant strains are not susceptible to TOB+FUM treatment (28). We confirmed that the aminoglycoside MICs for the ΔrpoN mutant were identical to those for the wild type (Table S1), indicating that increased resistance could not explain the nonsusceptibility of the ΔrpoN mutant to TOB+FUM. The addition of 2 mM l-glutamine during the killing assay with TOB+FUM also did not render the ΔrpoN mutant susceptible to TOB+FUM (Fig. S2), demonstrating that the dependence of PA14 ΔrpoN on exogenous l-glutamine as a nitrogen source (42) did not influence the lack of ΔrpoN TOB+FUM susceptibility.
Overall, the data presented above support our hypothesis that RpoN plays an important role in mediating TOB+FUM susceptibility in the PA14 laboratory strain.
FUM is not taken up intracellularly and does not promote respiration or TOB uptake in the ΔrpoN mutant.
Meylan et al. found that the extracellular FUM concentration decreases when stationary-phase cells are treated with FUM (22), suggesting that FUM is taken up into the cell, where it is subsequently metabolized. Koeva et al. made the interesting observation that strains that do not grow with FUM as the sole carbon source are not susceptible to TOB+FUM treatment (28). FUM uptake through the DctA and DctPQM transporters is transcriptionally regulated by RpoN (31). Thus, ΔrpoN mutants, including our PA14 ΔrpoN strain (Fig. S3), fail to grow in minimal medium with FUM as the sole carbon source (31). We therefore hypothesized that regulation of FUM uptake by RpoN is an essential step in FUM-mediated potentiation of TOB lethality.
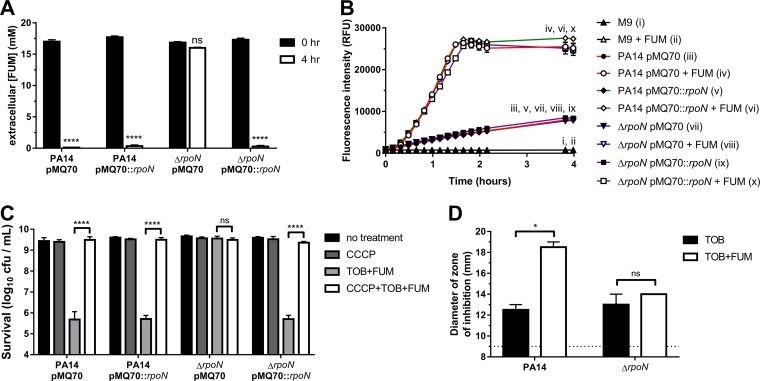
To assess the extent of FUM uptake by PA14 and ΔrpoN cells, we measured the concentration of extracellular FUM immediately after the addition of FUM to the cultures (0 h) and following a 4-h incubation (Fig. 3A). In contrast to PA14/pMQ70, extracellular [FUM] did not appreciably decrease after 4 h in FUM-treated ΔrpoN/pMQ70 cells, which is consistent with the inability of the ΔrpoN mutant to take up FUM. Complementation of the ΔrpoN mutant with pMQ70::rpoN led to a significant reduction in extracellular [FUM] after 4 h.
FIG 3.
FUM fails to promote respiration and TOB uptake in ΔrpoN cells. (A) Loss of rpoN impairs FUM uptake. Extracellular [FUM] in cultures of PA14 or ΔrpoN stationary-phase cells carrying pMQ70 or pMQ70::rpoN was measured after 0 or 4 h. Data are presented as mean extracellular [FUM] and SEM for three biological replicates. (B) FUM promotes respiration in wild-type but not ΔrpoN stationary-phase cells. Reduction of resazurin to resorufin was measured over time in PA14 or ΔrpoN stationary-phase cells carrying pMQ70 or pMQ70::rpoN exposed to 0 or 15 mM FUM. Data are shown as mean fluorescence intensity ± SEM for two independent experiments with 3 technical replicates per experiment. Since some data sets were obscured by others on the graph, roman numerals are used to show the locations of the data sets. RFU, relative fluorescence units. (C) PMF is required for TOB+FUM killing. Shown are survival data for stationary-phase cells following 4 h of treatment with or without CCCP and/or TOB+FUM. Data are presented as mean log10 CFU per milliliter ± SEM for three biological replicates. (D) FUM increases intracellular TOB accumulation in wild-type but not ΔrpoN stationary-phase cells. Zones of E. coli DH5α growth inhibition caused by lysates of PA14 or ΔrpoN cells treated with TOB or TOB+FUM were used to approximate relative amounts of intracellular TOB. Data are shown as mean diameters and SEM of the growth inhibition zones for two independent experiments. The dashed line indicates the limit of detection (the diameter of the wells).
FUM potentiates TOB killing by promoting electron transport chain (ETC) activity, which regenerates the PMF of stationary-phase cells and enables subsequent TOB uptake across the cytoplasmic membrane (22). We assessed the ability of FUM to promote respiration using a resazurin assay (Fig. 3B). Resazurin can be reduced to fluorescent resorufin by components of the ETC. A small, basal increase in fluorescence over time was observed for all strains in the absence of FUM. When PA14/pMQ70, PA14/pMQ70::rpoN, and ΔrpoN/pMQ70::rpoN strains were treated with FUM, there was a rapid increase in resazurin reduction to resorufin (Fig. 3B), which is suggestive of increased ETC activity and metabolic reduction. In contrast, the addition of FUM to the ΔrpoN/pMQ70 strain did not lead to an increase in fluorescence relative to the basal fluorescence in the absence of FUM (Fig. 3B), suggesting that FUM does not promote respiration in the ΔrpoN mutant. To confirm that the FUM-dependent increase in resazurin reduction was due to ETC activity, we treated cells with the cytochrome c oxidase inhibitor sodium azide (NaN3) in the presence or absence of FUM. NaN3 impaired FUM-dependent resazurin reduction in the wild type (Fig. S4).
To probe the role of the PMF generated by the ETC in TOB+FUM killing, we treated cells with TOB+FUM in the presence of carbonyl cyanide m-chlorophenyl hydrazone (CCCP), a proton ionophore that dissipates the PMF (43). CCCP completely abolished the ability of pMQ70::rpoN to restore ΔrpoN TOB+FUM susceptibility (Fig. 3C), which is consistent with a role for the PMF in TOB+FUM killing.
Given that FUM was unable to promote increased ETC activity in the ΔrpoN mutant (Fig. 3B), we anticipated that FUM treatment would also not lead to increased TOB uptake in the ΔrpoN mutant. TOB accumulation was determined using a modified version of a previously described microbiological assay (44). Stationary-phase cells treated with TOB or TOB+FUM were lysed, and the lysate was added to plates that had been inoculated with a lawn of TOB-sensitive Escherichia coli cells. Following incubation, the diameters of the zones of growth inhibition were measured. Larger zones of inhibition were interpreted as being due to the presence of a relatively higher concentration of TOB in the lysate and, thus, a larger amount of intracellular TOB in P. aeruginosa cells. We observed a larger zone of growth inhibition with lysates from PA14 cells treated with TOB+FUM than with those from PA14 cells treated with TOB alone, suggesting that wild-type cells accumulated more TOB in the presence of FUM (Fig. 3D). As predicted, no difference in the diameter of the zone of inhibition and, therefore, in TOB accumulation was observed in TOB- versus TOB+FUM-treated ΔrpoN cells (Fig. 3D). A picture of a representative experiment is shown in Fig. S5. Importantly, lysates from untreated cells or cells treated with FUM alone did not produce zones of growth inhibition on the lawn of E. coli cells (data not shown).
RpoN-independent expression of the DctA transporter is sufficient to promote ΔrpoN TOB+FUM susceptibility.
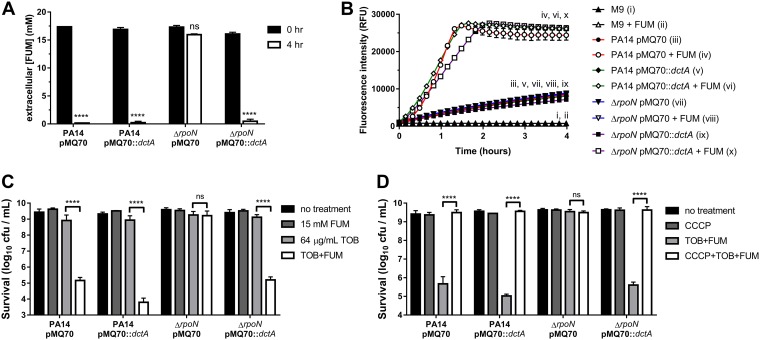
Given that RpoN is required for expression of the dctA and dctPQM FUM transporter genes (31), we hypothesized that the ΔrpoN strain was not susceptible to TOB+FUM because FUM transporters were not expressed and FUM was not being taken up by the cell. Thus, restoring FUM uptake by overexpressing a FUM transporter should restore TOB+FUM susceptibility in the ΔrpoN mutant. We constructed pMQ70::dctA, which carries the dctA C4-dicarboxylate transporter gene under the control of PBAD, and introduced this plasmid into the wild-type and ΔrpoN strains. DctA is a more efficient transporter than DctPQM at the millimolar FUM concentrations used in the assay (31). The pMQ70::dctA plasmid restored FUM uptake (indirectly measured as extracellular FUM depletion) in the ΔrpoN strain (Fig. 4A). Consistent with this finding, pMQ70::dctA also promoted FUM-dependent respiration (Fig. 4B). The pMQ70::dctA plasmid restored the susceptibility of ΔrpoN stationary-phase cells to TOB+FUM (Fig. 4C), suggesting that a lack of FUM uptake can explain the nonsusceptibility of the ΔrpoN mutant to TOB+FUM. CCCP treatment abolished the ability of the pMQ70::dctA plasmid to promote TOB+FUM susceptibility in the ΔrpoN mutant (Fig. 4D), indicating that TOB+FUM susceptibility of the ΔrpoN/pMQ70::dctA strain was PMF dependent.
FIG 4.
RpoN-independent DctA expression restores TOB+FUM susceptibility in ΔrpoN stationary-phase cells. (A) Extracellular [FUM] in cultures of PA14 or ΔrpoN stationary-phase cells carrying pMQ70 or pMQ70::dctA was measured after 0 or 4 h. Data are presented as mean extracellular [FUM] and SEM for three biological replicates. (B) Reduction of resazurin to resorufin was measured over time in PA14 or ΔrpoN stationary-phase cells carrying pMQ70 or pMQ70::dctA exposed to 0 or 15 mM FUM. Data are shown as mean fluorescence intensities ± SEM for two independent experiments with 3 technical replicates per experiment. Since some data sets were obscured by others on the graph, roman numerals are used to indicate the locations of the data sets. (C) Survival of PA14 wild-type and ΔrpoN stationary-phase cells carrying pMQ70 or pMQ70::dctA following incubation for 4 h with or without 64 μg/ml TOB and/or 15 mM FUM. Data are presented as mean log10 CFU per milliliter ± SEM for three biological replicates.
RpoN alleles from clinical isolates with predicted loss-of-function mutations cannot rescue TOB+FUM susceptibility in the PA14 ΔrpoN mutant.
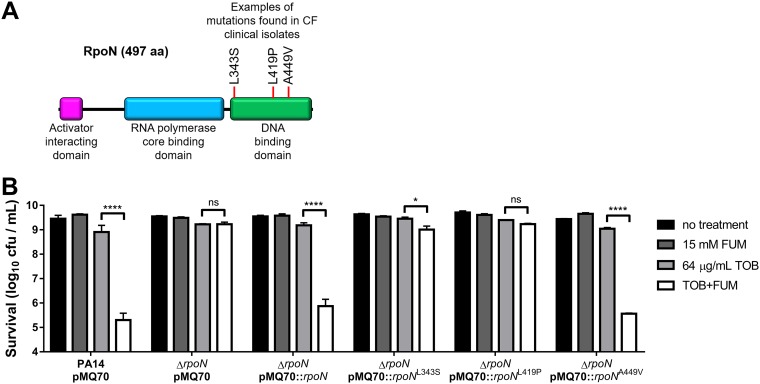
Loss of RpoN function is a recognized means of P. aeruginosa pathoadaptation to the CF lung; however, only some reports have documented specific rpoN mutations that are observed clinically in CF isolates. The AAJ010 isolates used by Huus et al. (45) carried the rpoN nonsynonymous amino acid change L343S arising from a T1028C mutation (Alex Wong, personal communication). The Danish transmissible DK2 lineage carried a T1256C missense, loss-of-function mutation leading to an L419P amino acid change (37, 46). Smith et al. found rpoN missense, nonsense, and frameshift mutations in isolates from several CF patients, including a C1346T mutation leading to A449V that was predicted to negatively impact RpoN function (36). The L343S, L419P, and A449V mutations all map to the RpoN DNA binding domain (Fig. 5A).
FIG 5.
Loss-of-function RpoN alleles from CF clinical isolates impair TOB+FUM killing. (A) Schematic representation of RpoN showing some mutations in the DNA binding domain that have been previously observed in CF clinical isolates. aa, amino acids. (B) Survival of PA14 wild-type and ΔrpoN stationary-phase cells carrying pMQ70, pMQ70::rpoN, or pMQ70::rpoN with clinically relevant point mutations following incubation for 4 h with or without 64 μg/ml TOB and/or 15 mM FUM. Data are presented as mean log10 CFU per milliliter ± SEM for three biological replicates.
We constructed pMQ70::rpoNL343S, pMQ70::rpoNL419P, and pMQ70::rpoNA449V and assessed the ability of these plasmids to complement the ΔrpoN mutant in TOB+FUM killing assays (Fig. 5B). The plasmids carrying the L343S and the L419P rpoN alleles were unable to restore TOB+FUM susceptibility to an appreciable extent in ΔrpoN stationary-phase cells, indicating that these were loss-of-function mutations. The A449V mutation did not lead to a loss of RpoN function in the killing assay given that pMQ70::rpoNA449V was able to restore TOB+FUM killing of the ΔrpoN strain to wild-type levels (Fig. 5B). This result was not entirely unexpected given the conservative nature of the A449V amino acid change. Overall, these data suggest that some rpoN missense mutations observed in P. aeruginosa CF clinical isolates can lead to TOB+FUM nonsusceptibility.
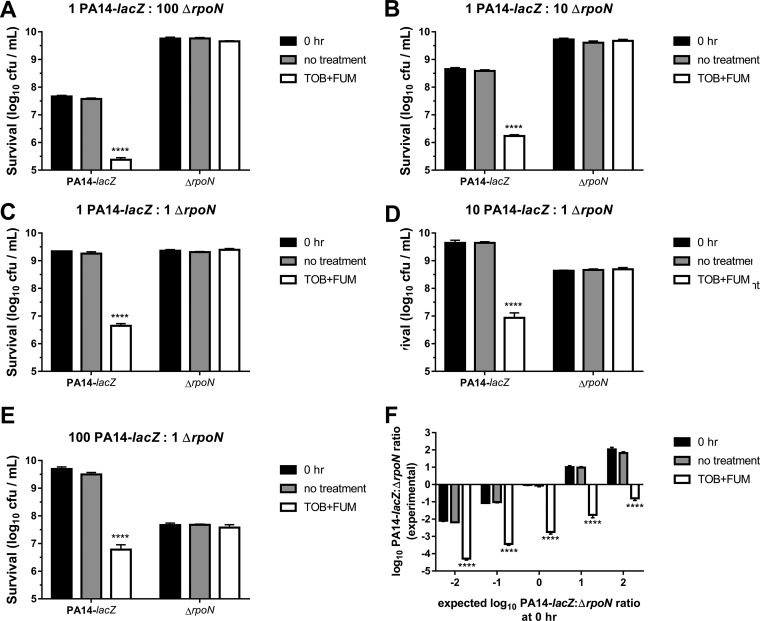
TOB+FUM kills wild-type cells and spares ΔrpoN cells in mixed-genotype cultures.
P. aeruginosa within the CF lung is genetically and phenotypically heterogeneous. It is therefore likely that bacteria retaining RpoN function (RpoN+) and bacteria with pathoadaptive loss of RpoN function (RpoN−) coexist in the lungs of a given patient. To model a heterogeneous population, we mixed stationary-phase PA14-lacZ (RpoN+) and ΔrpoN (RpoN−) cells at various PA14-lacZ/ΔrpoN starting ratios (1:100, 1:10, 1:1, 10:1, and 100:1) and incubated the populations for 4 h without treatment or with TOB+FUM treatment (64 μg/ml TOB and 15 mM FUM). Survival of each genotype was assessed by colony counting on plates supplemented with 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-gal) or both X-gal and tetracycline (TET). PA14-lacZ cells were selectively killed, while ΔrpoN cells were spared upon treatment of the mixed-genotype populations with TOB+FUM (Fig. 6A to E). The colony counts of the PA14-lacZ and ΔrpoN strains were also used to calculate log10 PA14-lacZ/ΔrpoN ratios under each condition (Fig. 6F). The PA14-lacZ/ΔrpoN ratios of the inocula were similar to the PA14-lacZ/ΔrpoN ratios of the untreated bacteria, indicating that the relative genotype proportions in the population remained stable over 4 h in the absence of TOB+FUM. In the presence of TOB+FUM, however, the PA14-lacZ/ΔrpoN ratios decreased. Overall, these data support the conclusion that in mixed-genotype stationary-phase populations, cells with intact RpoN function are killed by TOB+FUM, while cells lacking RpoN function survive treatment with TOB+FUM. The consequence is enrichment of RpoN− cells within the population relative to RpoN+ cells. While the experimental system is simplistic, these data suggest that TOB+FUM treatment may select for RpoN− cell survival in more complex settings, such as in the CF lung, where RpoN loss of function is known to evolve. The clinical consequences of a high proportion of RpoN− isolates in the CF lung are not known and require further study if TOB+FUM is to be used as a future treatment option for chronic P. aeruginosa lung infections.
FIG 6.
TOB+FUM specifically kills cells with RpoN function in mixed-genotype populations. (A to E) PA14-lacZ and ΔrpoN stationary-phase cells were mixed at 1:100 (A), 1:10 (B), 1:1 (C), 10:1 (D), and 100:1 (E) ratios, and survival of individual genotypes was determined prior to treatment (0 h) or following 4 h of incubation with either no treatment or TOB+FUM (64 μg/ml TOB and 15 mM FUM). Data are presented as mean log10 CFU per milliliter ± SEM for three biological replicates. (F) Data in panels A to E were used to calculate log10 PA14-lacZ/ΔrpoN ratios of the populations. TOB+FUM treatment consistently reduced the log10 PA14-lacZ/ΔrpoN ratios, indicating a decrease in PA14-lacZ cells relative to ΔrpoN cells in the population.
l-Arginine or d-glucose can promote TOB killing of ΔrpoN stationary-phase cells.
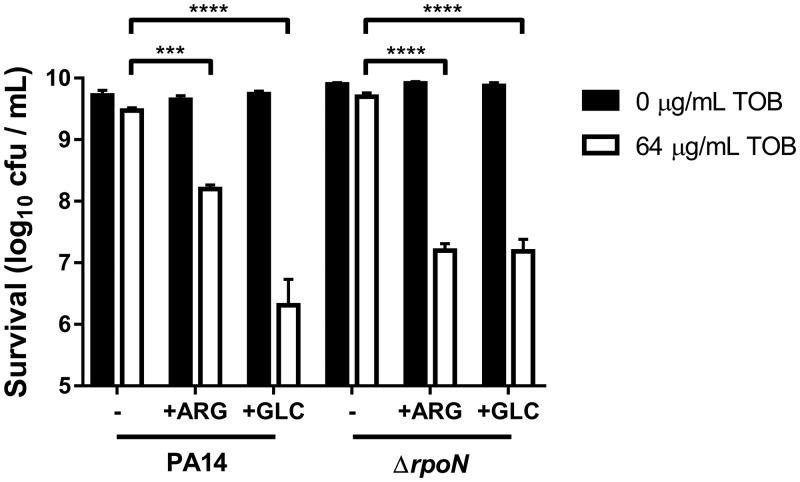
Faced with the unsuitability of TOB+FUM and of other aminoglycosides with C4-dicarboxylate combinations for killing of ΔrpoN cells, we wondered if metabolites other than C4-dicarboxylates would be able to potentiate TOB killing of the ΔrpoN mutant. Unbuffered l-arginine (ARG) was previously shown to promote TOB killing of wild-type P. aeruginosa cells (23, 27). We found that TOB+ARG treatment (64 μg/ml TOB and 10 mM ARG) was also effective at killing ΔrpoN cells (Fig. 7).
FIG 7.
l-Arginine or d-glucose can potentiate TOB killing of ΔrpoN cells. Shown are survival data for PA14 wild-type and ΔrpoN stationary-phase cells following incubation for 4 h with or without 64 μg/ml TOB in the presence or absence of 10 mM l-arginine (ARG) or 10 mM d-glucose (GLC). Data are presented as mean log10 CFU per milliliter ± SEM for three biological replicates.
Other than FUM and SUC, Meylan et al. found other carbon sources, such as acetate, gluconate, and d-glucose (GLC), that potentiated TOB lethality (22). We did not observe significant killing with 30 mM acetate and 64 μg/ml TOB in either PA14 or ΔrpoN cells (data not shown). Moreover, we did not test the ability of gluconate to potentiate TOB killing of ΔrpoN cells because ΔrpoN cultures have been shown to accumulate gluconate, suggesting a defect in gluconate utilization (47). Intriguingly, GLC was able to potentiate TOB killing of PA14 and, to a slightly lesser extent, the ΔrpoN mutant (Fig. 7). Determining whether ARG and GLC also promote TOB killing by enhancing respiration and TOB uptake will be the topic of future study. TOB combined with either ARG or GLC may prove to be a more attractive combination therapy than TOB+FUM in the setting of a high burden of RpoN loss-of-function mutants.
DISCUSSION
Chronic bacterial infections that are recalcitrant to standard antimicrobial treatment regimens contribute to patient morbidity and mortality, promote the emergence of antibiotic resistance, and pose a significant cost to the health care system (48–50). It is important to acknowledge that chronic infections involve a complex combination of antibiotic tolerance, antibiotic resistance, and host factors (7, 51). Therefore, different treatment strategies, likely used in combination, will be needed in order to address these mechanistically different phenomena. Novel therapeutic strategies, such as metabolic potentiation of existing antibiotics, have been proposed in order to specifically combat antibiotic tolerance (29, 52). TOB+FUM was recently identified as a combination treatment that can kill antibiotic-tolerant P. aeruginosa (22, 28), which contributes to the chronic lung infections that afflict patients with CF. Genetic requirements for TOB+FUM susceptibility have not been previously explored.
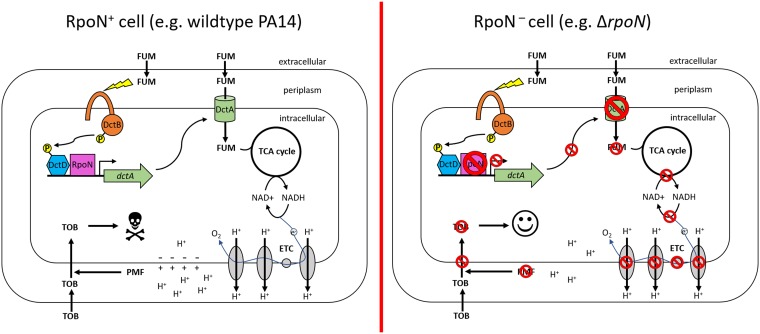
In this work, we have shown that the sigma factor RpoN is required for susceptibility of antibiotic-tolerant P. aeruginosa stationary-phase and biofilm cells to TOB+FUM as well as to other aminoglycoside and C4-dicarboxylate combinations. RpoN is an activator of C4-dicarboxylate transporter gene expression (31), and we have shown that the nonsusceptibility of ΔrpoN cells to TOB+FUM is due to a loss of FUM uptake. The proposed mechanism is presented schematically in Fig. 8. Importantly, RpoN loss-of-function mutations are often seen in P. aeruginosa strains isolated from the CF lung (38), and we observed that clinical RpoN loss-of-function mutations result in TOB+FUM nonsusceptibility. Moreover, TOB+FUM selectively killed cells retaining RpoN function and spared cells with loss of RpoN function in a heterogenous population, effectively leading to selection for rpoN mutant survival. Whether TOB+FUM also selects for the evolution of rpoN mutations will be addressed in the future by experimentally evolving wild-type populations exposed in vitro to TOB+FUM. Overall, our data suggest that TOB+FUM might not be an effective strategy to eradicate P. aeruginosa from the lungs of CF patients with a high burden of RpoN loss-of-function mutants. However, further in vitro experiments with clinical isolates and clinical data from animal and human trials will be needed to further validate this concern. In light of our work, we suggest that clinical trials assessing TOB+FUM effectiveness should consider stratifying patients based on the relative frequency of isolates with rpoN mutations to determine if this parameter influences treatment response. Allelic frequency could be determined by FREQ-Seq (frequency sequencing) (53), which has been previously used to determine the frequency of lasR mutants in CF sputum samples (54).
FIG 8.
Proposed model for RpoN-dependent susceptibility to TOB+FUM. (Left) In the wild-type RpoN+ strain, FUM enters the cell through C4-dicarboxylate transporters (e.g., DctA) that are transcriptionally regulated by RpoN. Once in the cell, FUM is metabolized in the TCA cycle to produce reducing equivalents that promote ETC activity. Increased respiration leads to regeneration of the membrane potential, TOB uptake, and subsequent TOB-dependent cell death. (Right) On the other hand, in the ΔrpoN RpoN− strain, the lack of functional RpoN means that C4-dicarboxylate transporters are not expressed, and FUM cannot enter the cell. As a consequence, TOB uptake is not increased, and the cell remains tolerant to TOB.
Recently, exciting strategies have been proposed to prevent the expression of RpoN-regulated virulence factors such as flagella in an effort to impair P. aeruginosa pathogenicity (55–57). While these strategies are likely to be relevant in the context of acute infectious processes that rely on RpoN-dependent virulence factors, they are unlikely to be helpful in the context of chronic P. aeruginosa CF lung infections where RpoN loss of function seems to provide a fitness benefit. Several theories have been proposed to explain why rpoN mutants evolve in the CF lung. These include immune evasion (35) and improved anaerobic survival (58). Perhaps rpoN mutation is also beneficial because it renders bacteria relatively insensitive to antibiotic potentiation by certain metabolites, like C4-dicarboxylates. Some support for this conjecture can be found in the recent observation that, unlike for RpoN+ strains, TOB susceptibility of the RpoN− DK2 strain is not increased by host-derived metabolites (30). Indeed, nutritional conditions in the CF lung have been previously shown to play a role in selection for antibiotic-tolerant lasR mutants (59). Whatever the underlying reason(s) may be for pathoadaptive loss of RpoN function in the context of the CF lung, what this means for patient outcomes has not been investigated and would be an interesting avenue for future study.
While our data suggest that aminoglycosides combined with C4-dicarboxylates might not be effective in killing P. aeruginosa isolates with RpoN loss of function, we have shown that two other carbon sources, ARG and GLC, can potentiate TOB killing of both wild-type and ΔrpoN antibiotic-tolerant cells. In P. aeruginosa, GLC diffuses across the outer membrane through the OprB porin into the periplasm (60, 61). From there, GLC either is directly transported across the inner membrane via the Glt transporter or is converted to gluconate or 2-ketogluconate, which is transported into the cell through GntP or KguT, respectively (60, 61). In the cytoplasm, GLC, gluconate, and 2-ketogluconate are then converted to 6-phosphogluconate, which is metabolized via the Entner-Doudoroff pathway to produce pyruvate and, ultimately, acetyl-CoA that can enter the tricarboxylic acid (TCA) cycle (60, 61). Transcription of the genes involved in GLC uptake and metabolism is highly regulated by several transcription factors, including the repressors HexR, PtxS, GntR, and GltR as well as the activator PtxR (60, 61). Additionally, some genes involved in glucose metabolism are under catabolite repression control (60, 61). GLC (and gluconate) metabolism is dysregulated in ΔrpoN cells, likely due to the importance of RpoN in alleviating catabolite repression control imposed by Crc through the two-component system CbrAB and the small RNA (sRNA) crcZ (47). ARG can be metabolized as a carbon and nitrogen source via four catabolic pathways in P. aeruginosa (62). Genes involved in ARG uptake and metabolism are regulated by the ArgR transcriptional regulator and, under anaerobic conditions, by Anr (63–65). Catabolite repression control also plays an important role in regulating ARG utilization (66). In spite of the importance of RpoN in indirectly regulating efficient utilization of GLC and ARG through fine-tuning of catabolite repression control, ΔrpoN mutants can still use these substrates as carbon sources, unlike the case for C4-dicarboxylates (67, 68). If TOB+ARG and TOB+GLC proceed down the developmental pipeline as potential therapeutics for CF patients infected with P. aeruginosa, it will be important to understand mechanisms of nonsusceptibility to these treatments.
Metabolic potentiation of antibiotic activity has tremendous potential to help patients who are battling chronic bacterial infections that are tolerant to antibiotics. Taking the case of TOB+FUM and P. aeruginosa as an example, we propose that when developing antibiotic and metabolite combinations for a given infection, it will be important to consider whether genetic mutations impairing sensing, uptake, and/or metabolism of the metabolite can evolve or are already present in the bacterial population. Ultimately, we hope that the work presented here will help guide efforts to personalize TOB+FUM treatment to CF patients who will benefit the most from this combination therapy.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains and plasmids are listed in Table S2 in the supplemental material. Primers used in this study are listed in Table S3. Our laboratory’s wild-type UCBPP-PA14 (PA14) strain (69) and the isogenic ΔrpoN mutant were routinely cultured aerobically in LB-Miller broth (Bio Basic) supplemented with 2 mM l-glutamine (Bio Basic). M9 medium was prepared as a 5× stock (33.9 g/liter Na2HPO4, 15 g/liter KH2PO4, 2.5 g/liter NaCl, 5 g/liter NH4Cl). 1× M9 medium was sterilized by autoclaving and supplemented with 2 mM MgSO4 and 0.1 mM CaCl2. When needed, media were solidified with 1.5% agar (Bio Basic). Plasmids were constructed using standard molecular cloning techniques and mobilized into E. coli DH5α or S17-1 (70) by CaCl2 chemical transformation. Plasmids were introduced into P. aeruginosa strains by conjugation with E. coli S17-1. When required for selection or plasmid maintenance, antibiotics were used at the following concentrations: 100 μg/ml ampicillin (AMP), 20 μg/ml GEN, and 20 μg/ml nalidixic acid (NAL) for E. coli and 150 to 200 μg/ml carbenicillin (CAR), 50 μg/ml GEN, and 100 μg/ml TET for P. aeruginosa.
Construction of the ΔrpoN mutant.
Deletion of rpoN in PA14 was achieved using two-step allelic exchange (71). Briefly, regions upstream and downstream of the rpoN coding region were amplified by PCR using the rpoNdelUF/rpoNdelUR and rpoNdelDF/rpoNdelDR primer pairs. Fragments were cloned into pEX18Gm (72) using standard molecular cloning techniques to create pEX18Gm::ΔrpoN. pEX18Gm::ΔrpoN was mobilized into P. aeruginosa by conjugation with E. coli S17-1 and plating onto LB agar supplemented with 2 mM l-glutamine, 50 μg/ml GEN, and 20 μg/ml NAL. GEN-resistant colonies were counterselected on no-salt LB agar plates supplemented with 10% sucrose (Wisent) and 2 mM l-glutamine. Sucrose-resistant, GEN-susceptible colonies were screened by PCR for the ΔrpoN allele with the rpoNDxF/rpoNDxR primer pair. We further confirmed that our ΔrpoN mutant had decreased pyocyanin production compared to wild-type PA14 and was unable to swim and to twitch (data not shown), since loss of rpoN has been previously reported to lead to these phenotypes (68, 73).
Construction of pMQ70 derivatives.
The coding regions of rpoN and dctA were amplified by PCR from PA14 genomic DNA using primers pMQrpoNF/pMQrpoNR and pMQdctAF/pMQdctAR, respectively. An engineered ribosomal binding site was included in the forward primers upstream of the start codon. Point mutations were introduced into rpoN by splicing by overlap extension PCR with complementary primers carrying the desired mutation. Briefly, upstream fragments were amplified using pMQrpoNF with L343S_R, L419P_R, or A449V_R, and downstream fragments were amplified using pMQrpoNR with L343S_F, L419P_F, or A449V_F. Upstream and downstream fragments were then used as the templates for a second PCR with the pMQrpoNF/pMQrpoNR primer set in order to splice the fragments. Amplified genes were cloned downstream of the arabinose-inducible PBAD promoter in SacI/HindIII-digested pMQ70 (74). Plasmids were sequenced using pMQseqF and pMQseqR (StemCore Laboratories, Ottawa Hospital Research Institute). Sequence-verified plasmids were mobilized into P. aeruginosa strains by conjugation with E. coli S17-1, and transconjugants were selected on LB agar supplemented with 2 mM l-glutamine, 200 μg/ml CAR, and 20 μg/ml NAL.
MIC assays.
MIC assays were performed in LB broth with 2 mM l-glutamine using broth macrodilution or broth microdilution methods as outlined in guideline M07-A10 from the Clinical and Laboratory Standards Institute (CLSI) (Wayne, PA, USA) (75). Assay mixtures were incubated statically at 37°C for 16 to 18 h. The MIC was interpreted as the lowest concentration of antibiotic that prevented visible growth.
Stationary-phase killing assay.
The killing assay for stationary-phase cells was adapted from previously reported protocols (22, 28). Briefly, 50 ml of LB broth supplemented with 2 mM l-glutamine in a 250-ml Erlenmeyer flask was inoculated at a 1:10,000 dilution with stationary-phase cultures grown overnight. A total of 150 μg/ml CAR was added as necessary for maintenance of pMQ70 derivatives. Cultures were grown aerobically to stationary phase at 37°C at 225 rpm for 16 to 18 h. If bacteria carried pMQ70 derivatives, PBAD activity was induced in the stationary-phase cultures by adding l-(+)-arabinose (Sigma) to a final concentration of 0.2% (from a 10% [wt/vol] stock in water) and incubating the cultures at 37°C at 225 rpm for an additional 2 h. Stationary-phase cells were harvested by centrifugation, washed in 10 ml phosphate-buffered saline (PBS) (pH 7.4), and then resuspended in 1 volume of M9 medium. Cells were aliquoted into separate glass culture tubes. When indicated, 50 μM CCCP (Sigma) was added to the cells 15 min prior to the addition of antibiotics and carbon sources. CCCP was prepared as a 10 mM stock in dimethyl sulfoxide. Aminoglycoside antibiotics and carbon sources (concentration normalized to 60 mM total carbon unless indicated otherwise) were added to the cells, and the cells were incubated at 37°C at 225 rpm. Tobramycin sulfate (Research Products International), gentamicin sulfate salt (Sigma), and amikacin hydrate (Sigma) were prepared as 10-mg/ml stocks in sterile water. Sodium fumarate dibasic (Sigma), sodium succinate dibasic (Sigma), l-arginine monohydrochloride (Sigma), and d-glucose (Fisher Scientific) were prepared as sterile 1 M stocks in water and used at the indicated concentrations. Following treatment (4 h unless otherwise indicated), aliquots of cells were washed in PBS. Cell survival was assessed by serial microdilution in PBS and drop plating triplicate 10-μl spots for each dilution onto LB agar plates supplemented with 2 mM l-glutamine. A total of 100 μg/ml TET and/or 40 μg/ml X-gal was added to plates when necessary to differentiate PA14-lacZ (76) and ΔrpoN colonies. Plates were incubated for 18 h at 37°C prior to colony counting, and survival for each strain under the different treatment conditions was expressed as log10 CFU per milliliter.
Colony biofilm killing assay.
The colony biofilm assay was performed as described previously (22, 28). Briefly, UV-sterilized 0.2-μm polycarbonate membrane discs (GVS Life Sciences) were placed on LB agar plates supplemented with 2 mM l-glutamine and inoculated with 5 × 105 CFU from PA14 or ΔrpoN cultures grown overnight. Biofilms were incubated at 37°C for 4 h prior to being transferred to M9 plates with or without 16 μg/ml TOB and/or 15 mM FUM for an additional 20 h. Biofilms were homogenized in PBS by vortexing. Survival was assessed by serial microdilution and drop plating of the dilutions onto LB agar plates supplemented with 2 mM l-glutamine. Plates were incubated for 18 h at 37°C prior to colony counting, and survival for each strain under the different treatment conditions was expressed as log10 CFU per biofilm.
Extracellular FUM quantification.
For extracellular FUM quantification, stationary-phase cells were prepared in M9 medium as described above for the killing assay. Cells were treated with 15 mM FUM for 0 h or 4 h, after which supernatants were collected and filter sterilized. The concentration of FUM in the supernatants was determined using the colorimetric fumarate assay kit (Sigma) exactly as described by the manufacturer.
Respiration assay.
For the respiration assay, stationary-phase cells were prepared in M9 medium as described above for the killing assay. Resazurin sodium salt (Sigma) was prepared as a sterile 1-mg/ml stock in water and added to a final concentration of 0.1 mg/ml, followed by 0 or 15 mM FUM. When indicated, 0.1% NaN3 was also added. Cells were aliquoted into a 96-well plate, and the plate was incubated with shaking at 37°C in a Synergy HT multimode plate reader (BioTek). Resazurin reduction to resorufin was measured by fluorescence (excitation wavelength [Ex]/emission wavelength [Em] of 550/590 nm, with a gain of 55).
TOB accumulation assay.
Intracellular TOB accumulation was indirectly assayed using a modified version of a previously described qualitative microbiological assay (44). Stationary-phase cells were prepared as described above for the TOB+FUM killing assay in M9 medium and subsequently treated for 1 h with 64 μg/ml TOB or 64 μg/ml TOB and 15 mM FUM. After treatment, 1 ml of cells was washed once in PBS to remove extracellular antibiotic, and cells were subsequently lysed for 1 h at room temperature in 100 μl of 0.1 M glycine (pH 3.0). The lysate was dried using a nitrogen evaporator (Organomation). The dried lysate was resuspended in 50 μl of sterile water. LB agar plates were freshly inoculated with a lawn of E. coli DH5α cells, and wells were punched into the agar. The lysate was added to wells. The plates were incubated at 37°C for 16 h, after which the diameter of the zone of E. coli DH5α growth inhibition was measured. A larger zone of growth inhibition was interpreted as being due to an increased amount of tobramycin present in the P. aeruginosa cell lysate.
Statistical analysis.
GraphPad Prism 7 software was used to prepare all graphs and to perform all statistical analyses. Unless otherwise stated, two-way analysis of variance (ANOVA) was performed with either Tukey’s or Sidak’s multiple-comparison test. The level of statistical significance is presented for relevant comparisons in the figures (ns, not significant [P > 0.05]; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001).
Supplementary Material
ACKNOWLEDGMENTS
We thank Rees Kassen (University of Ottawa) and Aaron Hinz (University of Ottawa and Carleton University) for providing the PA14-lacZ strain, Morgan Fullerton’s laboratory (University of Ottawa) for use of their plate reader, and Conor O’Dwyer (University of Ottawa) for technical assistance with the nitrogen evaporator. We thank Xian-Zhi Li (Health Canada) for critical review of the manuscript prior to submission for peer review.
C.W.H. was supported by a Vanier Canada graduate scholarship (Canadian Institutes of Health Research) and an Audrey J. Boyce M.D./Ph.D. fellowship (University of Ottawa). This work was supported by the Lung Association-Ontario grant-in-aid program and the Natural Sciences and Engineering Research Council of Canada.
C.W.H. was responsible for project conceptualization and experimental design. C.W.H., E.F., and L.Z. performed experiments. C.W.H. performed data analysis. C.W.H. wrote the original draft. C.W.H. and T.-F.M. reviewed and edited the manuscript. All authors reviewed and approved the final manuscript prior to submission. T.-F.M. obtained funding and provided reagents/materials/equipment.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01313-19.
REFERENCES
- 1.Ciofu O, Hansen CR, Høiby N. 2013. Respiratory bacterial infections in cystic fibrosis. Curr Opin Pulm Med 19:251–258. doi: 10.1097/MCP.0b013e32835f1afc. [DOI] [PubMed] [Google Scholar]
- 2.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. 2002. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol 34:91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 3.Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams-Warren J, Vasiljev-K M, Borowitz D, Bowman CM, Marshall BC, Marshall S, Smith AL. 1999. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. N Engl J Med 340:23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 4.Smith WD, Bardin E, Cameron L, Edmondson CL, Farrant KV, Martin I, Murphy RA, Soren O, Turnbull AR, Wierre-Gore N, Alton EW, Bundy JG, Bush A, Connett GJ, Faust SN, Filloux A, Freemont PS, Jones AL, Takats Z, Webb JS, Williams HD, Davies JC. 2017. Current and future therapies for Pseudomonas aeruginosa infection in patients with cystic fibrosis. FEMS Microbiol Lett 364:fnx121. doi: 10.1093/femsle/fnx121. [DOI] [PubMed] [Google Scholar]
- 5.Poole K. 2011. Pseudomonas aeruginosa: resistance to the max. Front Microbiol 2:65. doi: 10.3389/fmicb.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis K. 2008. Multidrug tolerance of biofilms and persister cells. Curr Top Microbiol Immunol 322:107–131. [DOI] [PubMed] [Google Scholar]
- 7.Hall CW, Mah T-F. 2017. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol Rev 41:276–301. doi: 10.1093/femsre/fux010. [DOI] [PubMed] [Google Scholar]
- 8.Brauner A, Fridman O, Gefen O, Balaban NQ. 2016. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat Rev Microbiol 14:320–330. doi: 10.1038/nrmicro.2016.34. [DOI] [PubMed] [Google Scholar]
- 9.Eng RH, Padberg FT, Smith SM, Tan EN, Cherubin CE. 1991. Bactericidal effects of antibiotics on slowly growing and nongrowing bacteria. Antimicrob Agents Chemother 35:1824–1828. doi: 10.1128/AAC.35.9.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans DJ, Allison DG, Brown MR, Gilbert P. 1991. Susceptibility of Pseudomonas aeruginosa and Escherichia coli biofilms towards ciprofloxacin: effect of specific growth rate. J Antimicrob Chemother 27:177–184. doi: 10.1093/jac/27.2.177. [DOI] [PubMed] [Google Scholar]
- 11.Walters MC, Roe F, Bugnicourt A, Franklin MJ, Stewart PS. 2003. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob Agents Chemother 47:317–323. doi: 10.1128/AAC.47.1.317-323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borriello G, Werner E, Roe F, Kim AM, Ehrlich GD, Stewart PS. 2004. Oxygen limitation contributes to antibiotic tolerance of Pseudomonas aeruginosa in biofilms. Antimicrob Agents Chemother 48:2659–2664. doi: 10.1128/AAC.48.7.2659-2664.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen D, Joshi-Datar A, Lepine F, Bauerle E, Olakanmi O, Beer K, McKay G, Siehnel R, Schafhauser J, Wang Y, Britigan BE, Singh PK. 2011. Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science 334:982–986. doi: 10.1126/science.1211037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verstraeten N, Knapen WJ, Kint CI, Liebens V, Van den Bergh B, Dewachter L, Michiels JE, Fu Q, David CC, Fierro AC, Marchal K, Beirlant J, Versées W, Hofkens J, Jansen M, Fauvart M, Michiels J. 2015. Obg and membrane depolarization are part of a microbial bet-hedging strategy that leads to antibiotic tolerance. Mol Cell 59:9–21. doi: 10.1016/j.molcel.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Stewart PS, Franklin MJ, Williamson KS, Folsom JP, Boegli L, James GA. 2015. Contribution of stress responses to antibiotic tolerance in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 59:3838–3847. doi: 10.1128/AAC.00433-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alhede M, Kragh KN, Qvortrup K, Allesen-Holm M, van Gennip M, Christensen LD, Jensen PØ, Nielsen AK, Parsek M, Wozniak D, Molin S, Tolker-Nielsen T, Høiby N, Givskov M, Bjarnsholt T. 2011. Phenotypes of non-attached Pseudomonas aeruginosa aggregates resemble surface attached biofilm. PLoS One 6:e27943. doi: 10.1371/journal.pone.0027943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutierrez A, Jain S, Bhargava P, Hamblin M, Lobritz MA, Collins JJ. 2017. Understanding and sensitizing density-dependent persistence to quinolone antibiotics. Mol Cell 68:1147.e3–1154.e3. doi: 10.1016/j.molcel.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Martins D, McKay G, Sampathkumar G, Khakimova M, English AM, Nguyen D. 2018. Superoxide dismutase activity confers (p)ppGpp-mediated antibiotic tolerance to stationary-phase Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 115:9797–9802. doi: 10.1073/pnas.1804525115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bryan LE, Kwan S. 1983. Roles of ribosomal binding, membrane potential, and electron transport in bacterial uptake of streptomycin and gentamicin. Antimicrob Agents Chemother 23:835–845. doi: 10.1128/aac.23.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serio AW, Keepers T, Andrews L, Krause KM. 16 November 2018, posting date Aminoglycoside revival: review of a historically important class of antimicrobials undergoing rejuvenation. EcoSal Plus 2018. doi: 10.1128/ecosalplus.ESP-0002-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taber HW, Mueller JP, Miller PF, Arrow AS. 1987. Bacterial uptake of aminoglycoside antibiotics. Microbiol Rev 51:439–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meylan S, Porter CBM, Yang JH, Belenky P, Gutierrez A, Lobritz MA, Park J, Kim SH, Moskowitz SM, Collins JJ. 2017. Carbon sources tune antibiotic susceptibility in Pseudomonas aeruginosa via tricarboxylic acid cycle control. Cell Chem Biol 24:195–206. doi: 10.1016/j.chembiol.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borriello G, Richards L, Ehrlich GD, Stewart PS. 2006. Arginine or nitrate enhances antibiotic susceptibility of Pseudomonas aeruginosa in biofilms. Antimicrob Agents Chemother 50:382–384. doi: 10.1128/AAC.50.1.382-384.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allison KR, Brynildsen MP, Collins JJ. 2011. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature 473:216–220. doi: 10.1038/nature10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barraud N, Buson A, Jarolimek W, Rice SA. 2013. Mannitol enhances antibiotic sensitivity of persister bacteria in Pseudomonas aeruginosa biofilms. PLoS One 8:e84220. doi: 10.1371/journal.pone.0084220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price KE, Orazi G, Ruoff KL, Hebert WP, O’Toole GA, Mastoridis P. 2015. Mannitol does not enhance tobramycin killing of Pseudomonas aeruginosa in a cystic fibrosis model system of biofilm formation. PLoS One 10:e0141192. doi: 10.1371/journal.pone.0141192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lebeaux D, Chauhan A, Létoffé S, Fischer F, de Reuse H, Beloin C, Ghigo J-M. 2014. pH-mediated potentiation of aminoglycosides kills bacterial persisters and eradicates in vivo biofilms. J Infect Dis 210:1357–1366. doi: 10.1093/infdis/jiu286. [DOI] [PubMed] [Google Scholar]
- 28.Koeva M, Gutu AD, Hebert W, Wager JD, Yonker LM, O’Toole GA, Ausubel FM, Moskowitz SM, Joseph-McCarthy D. 2017. An antipersister strategy for treatment of chronic Pseudomonas aeruginosa infections. Antimicrob Agents Chemother 61:e00987-17. doi: 10.1128/AAC.00987-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meylan S, Andrews IW, Collins JJ. 2018. Targeting antibiotic tolerance, pathogen by pathogen. Cell 172:1228–1238. doi: 10.1016/j.cell.2018.01.037. [DOI] [PubMed] [Google Scholar]
- 30.Crabbé A, Ostyn L, Staelens S, Rigauts C, Risseeuw M, Dhaenens M, Daled S, Van Acker H, Deforce D, Van Calenbergh S, Coenye T. 2019. Host metabolites stimulate the bacterial proton motive force to enhance the activity of aminoglycoside antibiotics. PLoS Pathog 15:e1007697. doi: 10.1371/journal.ppat.1007697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valentini M, Storelli N, Lapouge K. 2011. Identification of C4-dicarboxylate transport systems in Pseudomonas aeruginosa PAO1. J Bacteriol 193:4307–4316. doi: 10.1128/JB.05074-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valentini M, Lapouge K. 2013. Catabolite repression in Pseudomonas aeruginosa PAO1 regulates the uptake of C4-dicarboxylates depending on succinate concentration. Environ Microbiol 15:1707–1716. doi: 10.1111/1462-2920.12056. [DOI] [PubMed] [Google Scholar]
- 33.Potvin E, Sanschagrin F, Levesque RC. 2008. Sigma factors in Pseudomonas aeruginosa. FEMS Microbiol Rev 32:38–55. doi: 10.1111/j.1574-6976.2007.00092.x. [DOI] [PubMed] [Google Scholar]
- 34.Schulz S, Eckweiler D, Bielecka A, Nicolai T, Franke R, Dötsch A, Hornischer K, Bruchmann S, Düvel J, Häussler S. 2015. Elucidation of sigma factor-associated networks in Pseudomonas aeruginosa reveals a modular architecture with limited and function-specific crosstalk. PLoS Pathog 11:e1004744. doi: 10.1371/journal.ppat.1004744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahenthiralingam E, Campbell ME, Speert DP. 1994. Nonmotility and phagocytic resistance of Pseudomonas aeruginosa isolates from chronically colonized patients with cystic fibrosis. Infect Immun 62:596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith EE, Buckley DG, Wu Z, Saenphimmachak C, Hoffman LR, D’Argenio DA, Miller SI, Ramsey BW, Speert DP, Moskowitz SM, Burns JL, Kaul R, Olson MV. 2006. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci U S A 103:8487–8492. doi: 10.1073/pnas.0602138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang L, Jelsbak L, Marvig RL, Damkiær S, Workman CT, Rau MH, Hansen SK, Folkesson A, Johansen HK, Ciofu O, Høiby N, Sommer MOA, Molin S. 2011. Evolutionary dynamics of bacteria in a human host environment. Proc Natl Acad Sci U S A 108:7481–7486. doi: 10.1073/pnas.1018249108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winstanley C, O’Brien S, Brockhurst MA. 2016. Pseudomonas aeruginosa evolutionary adaptation and diversification in cystic fibrosis chronic lung infections. Trends Microbiol 24:327–337. doi: 10.1016/j.tim.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faure E, Kwong K, Nguyen D. 2018. Pseudomonas aeruginosa in chronic lung infections: how to adapt within the host? Front Immunol 9:2416. doi: 10.3389/fimmu.2018.02416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeukens J, Boyle B, Kukavica-Ibrulj I, Ouellet MM, Aaron SD, Charette SJ, Fothergill JL, Tucker NP, Winstanley C, Levesque RC. 2014. Comparative genomics of isolates of a Pseudomonas aeruginosa epidemic strain associated with chronic lung infections of cystic fibrosis patients. PLoS One 9:e87611. doi: 10.1371/journal.pone.0087611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marvig RL, Dolce D, Sommer LM, Petersen B, Ciofu O, Campana S, Molin S, Taccetti G, Johansen HK. 2015. Within-host microevolution of Pseudomonas aeruginosa in Italian cystic fibrosis patients. BMC Microbiol 15:218. doi: 10.1186/s12866-015-0563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hendrickson EL, Plotnikova J, Mahajan-Miklos S, Rahme LG, Ausubel FM. 2001. Differential roles of the Pseudomonas aeruginosa PA14 rpoN gene in pathogenicity in plants, nematodes, insects, and mice. J Bacteriol 183:7126–7134. doi: 10.1128/JB.183.24.7126-7134.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hopfer U, Lehninger AL, Thompson TE. 1968. Protonic conductance across phospholipid bilayer membranes induced by uncoupling agents for oxidative phosphorylation. Proc Natl Acad Sci U S A 59:484–490. doi: 10.1073/pnas.59.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang L, Mah T-F. 2008. Involvement of a novel efflux system in biofilm-specific resistance to antibiotics. J Bacteriol 190:4447–4452. doi: 10.1128/JB.01655-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huus KE, Joseph J, Zhang L, Wong A, Aaron SD, Mah T-F, Sad S. 2016. Clinical isolates of Pseudomonas aeruginosa from chronically infected cystic fibrosis patients fail to activate the inflammasome during both stable infection and pulmonary exacerbation. J Immunol 196:3097–3108. doi: 10.4049/jimmunol.1501642. [DOI] [PubMed] [Google Scholar]
- 46.Damkiær S, Yang L, Molin S, Jelsbak L. 2013. Evolutionary remodeling of global regulatory networks during long-term bacterial adaptation to human hosts. Proc Natl Acad Sci U S A 110:7766–7771. doi: 10.1073/pnas.1221466110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Behrends V, Bell TJ, Liebeke M, Cordes-Blauert A, Ashraf SN, Nair C, Zlosnik JEA, Williams HD, Bundy JG. 2013. Metabolite profiling to characterize disease-related bacteria: gluconate excretion by Pseudomonas aeruginosa mutants and clinical isolates from cystic fibrosis patients. J Biol Chem 288:15098–15109. doi: 10.1074/jbc.M112.442814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lewis K. 2007. Persister cells, dormancy and infectious disease. Nat Rev Microbiol 5:48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- 49.Levin-Reisman I, Ronin I, Gefen O, Braniss I, Shoresh N, Balaban NQ. 2017. Antibiotic tolerance facilitates the evolution of resistance. Science 355:826–830. doi: 10.1126/science.aaj2191. [DOI] [PubMed] [Google Scholar]
- 50.Levin BR, Rozen DE. 2006. Non-inherited antibiotic resistance. Nat Rev Microbiol 4:556–562. doi: 10.1038/nrmicro1445. [DOI] [PubMed] [Google Scholar]
- 51.Lebeaux D, Ghigo J-M, Beloin C. 2014. Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol Mol Biol Rev 78:510–543. doi: 10.1128/MMBR.00013-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crabbé A, Jensen PØ, Bjarnsholt T, Coenye T. 6 June 2019. Antimicrobial tolerance and metabolic adaptations in microbial biofilms. Trends Microbiol. doi: 10.1016/j.tim.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 53.Chubiz LM, Lee M-C, Delaney NF, Marx CJ. 2012. FREQ-Seq: a rapid, cost-effective, sequencing-based method to determine allele frequencies directly from mixed populations. PLoS One 7:e47959. doi: 10.1371/journal.pone.0047959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.LaFayette SL, Houle D, Beaudoin T, Wojewodka G, Radzioch D, Hoffman LR, Burns JL, Dandekar AA, Smalley NE, Chandler JR, Zlosnik JE, Speert DP, Bernier J, Matouk E, Brochiero E, Rousseau S, Nguyen D. 2015. Cystic fibrosis-adapted Pseudomonas aeruginosa quorum sensing lasR mutants cause hyperinflammatory responses. Sci Adv 1:e1500199. doi: 10.1126/sciadv.1500199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lloyd MG, Lundgren BR, Hall CW, Gagnon LB-P, Mah T-F, Moffat JF, Nomura CT. 2017. Targeting the alternative sigma factor RpoN to combat virulence in Pseudomonas aeruginosa. Sci Rep 7:12615. doi: 10.1038/s41598-017-12667-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Payne SR, Pau DI, Whiting AL, Kim YJ, Pharoah BM, Moi C, Boddy CN, Bernal F. 2018. Inhibition of bacterial gene transcription with an RpoN-based stapled peptide. Cell Chem Biol 25:1059.e4–1066.e4. doi: 10.1016/j.chembiol.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lloyd MG, Vossler JL, Nomura CT, Moffat JF. 2019. Blocking RpoN reduces virulence of Pseudomonas aeruginosa isolated from cystic fibrosis patients and increases antibiotic sensitivity in a laboratory strain. Sci Rep 9:6677. doi: 10.1038/s41598-019-43060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoon SS, Hennigan RF, Hilliard GM, Ochsner UA, Parvatiyar K, Kamani MC, Allen HL, DeKievit TR, Gardner PR, Schwab U, Rowe JJ, Iglewski BH, McDermott TR, Mason RP, Wozniak DJ, Hancock REW, Parsek MR, Noah TL, Boucher RC, Hassett DJ. 2002. Pseudomonas aeruginosa anaerobic respiration in biofilms: relationships to cystic fibrosis pathogenesis. Dev Cell 3:593–603. doi: 10.1016/S1534-5807(02)00295-2. [DOI] [PubMed] [Google Scholar]
- 59.Hoffman LR, Richardson AR, Houston LS, Kulasekara HD, Martens-Habbena W, Klausen M, Burns JL, Stahl DA, Hassett DJ, Fang FC, Miller SI. 2010. Nutrient availability as a mechanism for selection of antibiotic tolerant Pseudomonas aeruginosa within the CF airway. PLoS Pathog 6:e1000712. doi: 10.1371/journal.ppat.1000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rojo F. 2010. Carbon catabolite repression in Pseudomonas: optimizing metabolic versatility and interactions with the environment. FEMS Microbiol Rev 34:658–684. doi: 10.1111/j.1574-6976.2010.00218.x. [DOI] [PubMed] [Google Scholar]
- 61.Udaondo Z, Ramos J-L, Segura A, Krell T, Daddaoua A. 2018. Regulation of carbohydrate degradation pathways in Pseudomonas involves a versatile set of transcriptional regulators. Microb Biotechnol 11:442–454. doi: 10.1111/1751-7915.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jann A, Matsumoto H, Haas D. 1988. The fourth arginine catabolic pathway of Pseudomonas aeruginosa. Microbiology 134:2633. doi: 10.1099/00221287-134-9-2633. [DOI] [PubMed] [Google Scholar]
- 63.Galimand M, Gamper M, Zimmermann A, Haas D. 1991. Positive FNR-like control of anaerobic arginine degradation and nitrate respiration in Pseudomonas aeruginosa. J Bacteriol 173:1598–1606. doi: 10.1128/jb.173.5.1598-1606.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lu CD, Winteler H, Abdelal A, Haas D. 1999. The ArgR regulatory protein, a helper to the anaerobic regulator ANR during transcriptional activation of the arcD promoter in Pseudomonas aeruginosa. J Bacteriol 181:2459–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu C-D, Yang Z, Li W. 2004. Transcriptome analysis of the ArgR regulon in Pseudomonas aeruginosa. J Bacteriol 186:3855–3861. doi: 10.1128/JB.186.12.3855-3861.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li W, Lu C-D. 2007. Regulation of carbon and nitrogen utilization by CbrAB and NtrBC two-component systems in Pseudomonas aeruginosa. J Bacteriol 189:5413–5420. doi: 10.1128/JB.00432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caiazza NC, O’Toole GA. 2004. SadB is required for the transition from reversible to irreversible attachment during biofilm formation by Pseudomonas aeruginosa PA14. J Bacteriol 186:4476–4485. doi: 10.1128/JB.186.14.4476-4485.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Totten PA, Lara JC, Lory S. 1990. The rpoN gene product of Pseudomonas aeruginosa is required for expression of diverse genes, including the flagellin gene. J Bacteriol 172:389–396. doi: 10.1128/jb.172.1.389-396.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rahme L, Stevens E, Wolfort S, Shao J, Tompkins R, Ausubel F. 1995. Common virulence factors for bacterial pathogenicity in plants and animals. Science 268:1899–1902. doi: 10.1126/science.7604262. [DOI] [PubMed] [Google Scholar]
- 70.Simon R, Priefer U, Pühler A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Nat Biotechnol 1:784–791. doi: 10.1038/nbt1183-784. [DOI] [Google Scholar]
- 71.Hmelo LR, Borlee BR, Almblad H, Love ME, Randall TE, Tseng BS, Lin C, Irie Y, Storek KM, Yang JJ, Siehnel RJ, Howell PL, Singh PK, Tolker-Nielsen T, Parsek MR, Schweizer HP, Harrison JJ. 2015. Precision-engineering the Pseudomonas aeruginosa genome with two-step allelic exchange. Nat Protoc 10:1820–1841. doi: 10.1038/nprot.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77–86. doi: 10.1016/S0378-1119(98)00130-9. [DOI] [PubMed] [Google Scholar]
- 73.Ishimoto KS, Lory S. 1989. Formation of pilin in Pseudomonas aeruginosa requires the alternative sigma factor (RpoN) of RNA polymerase. Proc Natl Acad Sci U S A 86:1954–1957. doi: 10.1073/pnas.86.6.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shanks RMQ, Caiazza NC, Hinsa SM, Toutain CM, O’Toole GA. 2006. Saccharomyces cerevisiae-based molecular tool kit for manipulation of genes from Gram-negative bacteria. Appl Environ Microbiol 72:5027–5036. doi: 10.1128/AEM.00682-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—10th ed. CLSI document M07-A10. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 76.Wong A, Rodrigue N, Kassen R. 2012. Genomics of adaptation during experimental evolution of the opportunistic pathogen Pseudomonas aeruginosa. PLoS Genet 8:e1002928. doi: 10.1371/journal.pgen.1002928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.