The quorum-sensing (QS) system is an intercellular cell-cell communication mechanism that controls the expression of genes involved in a variety of cellular processes and that plays critical roles in the adaption and survival of bacteria in their environment. The LuxS/AI-2 QS system, which uses AI-2 (autoinducer-2) as a signal molecule, has been identified in both Gram-negative and Gram-positive bacteria.
KEYWORDS: LuxS/AI-2 system, bacterial resistance, biofilm formation, efflux pump
ABSTRACT
The quorum-sensing (QS) system is an intercellular cell-cell communication mechanism that controls the expression of genes involved in a variety of cellular processes and that plays critical roles in the adaption and survival of bacteria in their environment. The LuxS/AI-2 QS system, which uses AI-2 (autoinducer-2) as a signal molecule, has been identified in both Gram-negative and Gram-positive bacteria. As one of the important global regulatory networks in bacteria, it responds to fluctuations in the numbers of bacteria and regulates the expression of a number of genes, thus affecting cell behavior. We summarize here the known relationships between the LuxS/AI-2 system and drug resistance, discuss the inhibition of LuxS/AI-2 system as an approach to prevent bacterial resistance, and present new strategies for the treatment of drug-resistant pathogens.
INTRODUCTION
The quorum-sensing (QS) system is an intercellular cell-cell communication mechanism that controls the expression of genes involved in a variety of cellular processes and that plays critical roles in the adaption and survival of bacteria in their environment (1). For intra- and interspecific communication, bacteria use chemical signals and their corresponding receptors (2). When an extracellular threshold concentration is reached, these molecules bind to their receptors, thereby activating the QS system. With the discovery of autoinducer-2 (AI-2) and its corresponding synthase, LuxS, the first possible interspecies QS system was found, as the synthase is widespread among the bacterial kingdom in both Gram-positive and Gram-negative bacteria (3). The LuxS/AI-2 QS system modulates various cellular processes involved mainly in the regulation of virulence factors, bacterial luminescence, sporulation, motility, toxin production, biofilm formation, and drug resistance (4, 5).
The emergence of antibiotic-resistant bacterial pathogens was reported in a major health challenge worldwide (6). Recently, some studies have shown that the QS system may be involved in bacterial resistance (7–9). Consequently, inhibition of bacterial QS has become a new and promising antibacterial strategy, which not only prevents the development of bacterial resistance but also eliminates the virulence factor gene expression related to population density (10–12).
LUXS/AI-2 QS SYSTEM
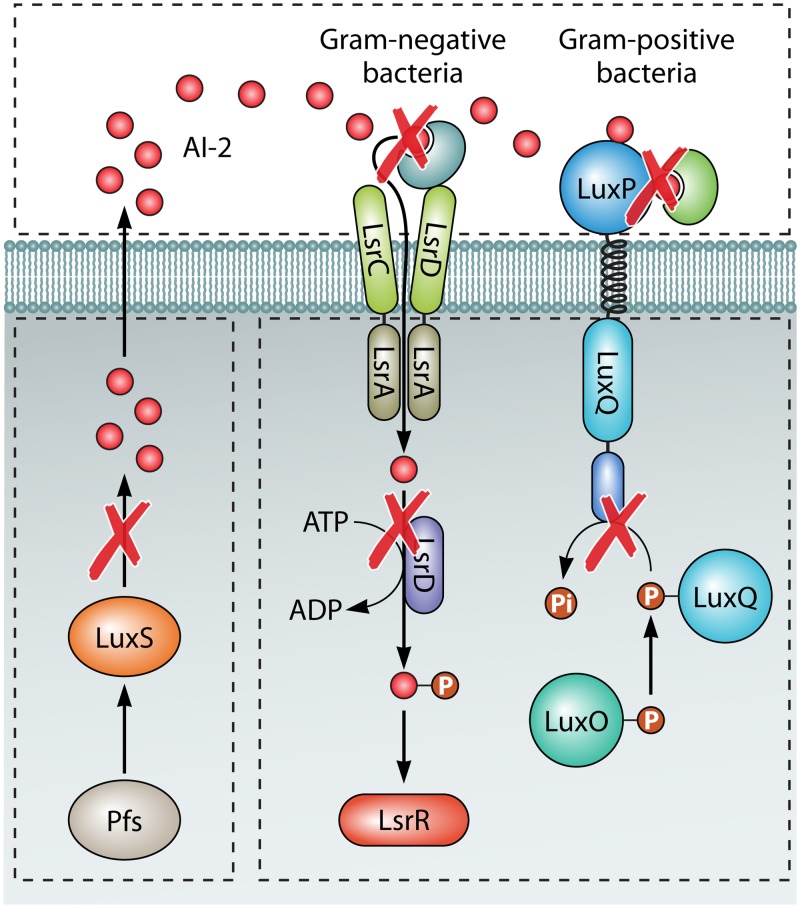
The LuxS/AI-2 system, coexisting in both Gram-negative and Gram-positive bacteria, is a QS regulatory mechanism that enables bacteria to make collective decisions with respect to the expression of a specific set of genes by secreting and detecting the signal molecule AI-2, a furanosyl-borate-diester as it has been identified in Vibrio harveyi, as a furan molecule existing in Escherichia coli (13, 14). The synthesis of AI-2 involves the conversion of S-adenosylhomocysteine (SAH) to homocysteine either by a one-step reaction using the enzyme SAH hydrolase (SahH) or a two-step reaction that requires the SAH nucleosidase (Pfs) and LuxS, which catalyzes the cleavage of the thioether linkage of SRH to produce 4,5 dihydroxy-2,3-pentanedione (DPD), which can rearrange to R- or S-2-methyl-2,3,3,4-tetrahydroxytetrahydrofuran (R- or S-THMF), both better known as AI-2 (15–19). The discovery that the AI-2 signal molecule produced by one bacterial species can be sensed by bacteria of different species led to the proposition that AI-2 is probably a universal signaling molecule that functions in interspecies communication (20). LuxS, the AI-2 synthase, has been identified in a wide range of Gram-negative and Gram-positive bacteria (1). LuxS not only participates in the production of AI-2 signaling molecules but also plays an important role in activating the methyl cycle as part of the bacterial central metabolism (18, 19). LuxS is mainly responsible for the hydrolysis of S-adenosyl homocysteine to S-adenosylmethionine (SAMe) as a methyl donor, which is an important pathway for bacteria to recover methyl groups and plays an important role in bacterial vitamin synthesis and polyamine formation (21). Challan Belval et al. showed that the luxS mutation can also cause changes in the extracellular concentration of S-ribosyl homocysteine (SAMe with LuxS function) (18). The biological importance of the LuxS/AI-2 QS system has been demonstrated by numerous experimental evidences, which showed that LuxS/AI-2 is involved in physiological processes such as biofilm formation, conjugation, virulence, and antibiotic resistance (22–24).
LUXS/AI-2 QS SYSTEM CONTRIBUTES TO ANTIBIOTIC RESISTANCE
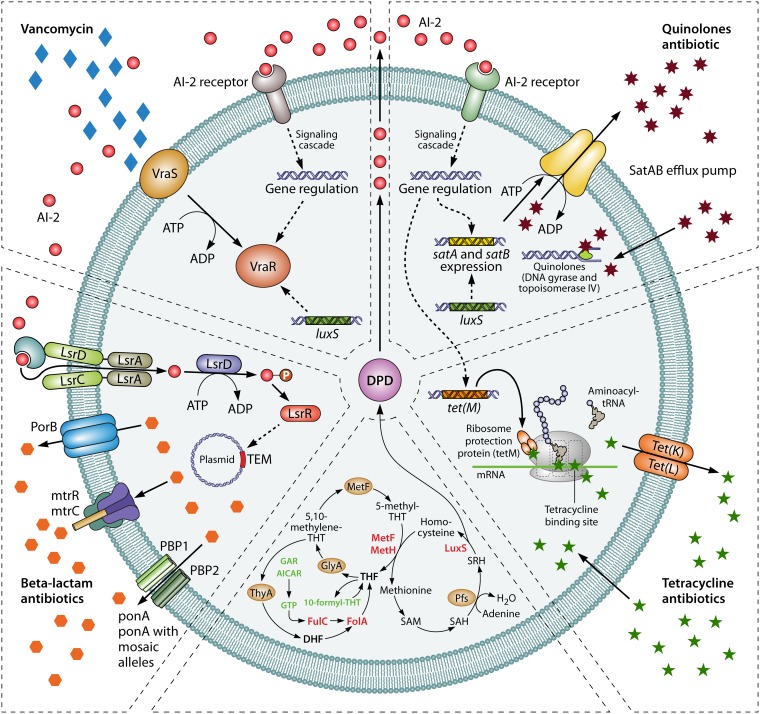
The modulation of antibiotic bacterial resistance by the LuxS/AI-2 system is complex (25–27). As summarized in Fig. 1, the LuxS/AI-2 system contributes to antibiotic resistance through effects on efflux pumps, mobile genetic elements, the VraSR two-component system, and the folate synthesis pathway. The fact that the LuxS/AI-2 system coordinates bacterial biofilm formation further contributes to bacterial resistance. Each of these aspects will be discussed separately. Table 1 lists the antimicrobials mentioned in this review, as well as their major mechanisms of action.
FIG 1.
Regulation mechanism between LuxS/AI-2 and bacterial resistance. Abbreviations: AI-2 autoinducer-2; LuxS, AI-2 synthase; LrsC, response regulator; MetF, 5,10-methylenetetrahydrofolate reductase; MetE, methionine synthase; MetH, B12-dependent homocysteine-5-methyltetrahydrofolate methyltransferase; THF, tetrahydrofolate; GlyA, serine hydroxymethyltransferase; ThyA, thymidylate synthase; AICAR, 5-aminoimidazole-4-carboxamine ribonucleotide; DHF, dihydrofolate; FolC, dihydrofolate synthase; FolA, dihydrofolate reductase; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine; Pfs, S-adenosylhomocysteine nucleosidase; SRH, S-ribosylhomocysteine; LuxS, S-ribosylhomocysteinase; DPD, 4,5-dihydroxy-2,3-pentanedione (the precursor of AI-2).
TABLE 1.
Antibiotics involved in the regulation of LuxS/AI-2 QS system and their resistance mechanisms
| Antibiotic class | Example(s) | Major mechanism of action | Major mechanism of resistance |
|---|---|---|---|
| Nucleic acid synthesis inhibitors | |||
| Quinolones | Norfloxacin, enrofloxacin | Inhibit DNA replication by complexing with DNA and DNA gyrase or topoisomerase IV | Target enzymes (DNA gyrase and topoisomerase IV) of changes and efflux pump protein expression |
| Sulfonamides | Trimethoprim-sulfamethoxazole | Ultimately prevent THFa synthesis by inhibiting dihydrofolate reductase (DHFR) and dihydropteroate synthetase (DHPS) | Acquired resistance genes located on mobile genetic elements; and mutations on the chromosomal DHFR and DHPS gene |
| Protein synthesis inhibitors | |||
| Tetracyclines | Tetracycline, chlorotetracycline | Bind to the 16S rRNA of the 30S ribosomal subunit, prevent-ing aminoacyl-tRNA from binding to the ribosomal A site | Acquisition of mobile genetic elements with resistance genes, efflux, ribosomal protection, and enzymatic inactivation |
| Cell wall synthesis inhibitors | |||
| β-Lactams | Penicillin, cephalosporin, aztreonam | Prevent transpeptidation of peptidoglycan by inhibiting transpeptidases (penicillin-binding proteins [PBPs]) | Reduced access to the PBPs, reduced PBPs binding affinity, expression of enzymes that bind and hydrolyze β-lactams. |
| Glycopeptides | Vancomycin | Bind to the terminal D-Ala–D-Ala residues of peptidoglycan subunits, preventing transpeptidation | Alteration of the peptidoglycan synthesis pathway, specifically D-Ala–D-Ala to either D-Ala–D-Lac or D-Ala–D-Ser |
Tetrahydrofolate (THF) is a cofactor required for the de novo biosynthesis of purines and of thymidine.
LuxS/AI-2 affects drug resistance through efflux pumps.
Overexpression of multidrug (MDR) efflux pumps is considered one of the main mechanisms of bacterial resistance (28–30). The expression of the efflux system is regulated in multiple levels, involving local and global transcriptional regulation, as well as posttranscriptional and posttranslational regulation (31). Studies have shown that overexpression of the QS regulator SdiA leads to an increased expression of the AcrAB efflux pump, in addition to participate in the MDR efflux pump system in E. coli (32). We recently showed that the LuxS/AI-2 QS system affects the expression of the efflux pump SatAB, further affecting the resistance to quinolone antibiotics in Streptococcus suis (33). This study also showed that the reduced resistance of the luxS gene deletion mutant strain to the quinolone antibiotics norfloxacin and enrofloxacin was achieved through the luxS gene affecting the expression levels of the efflux pump genes SatA and SatB. Mou et al. (34) showed no significant difference in cmeB efflux gene expression levels in the luxS mutant compared to the wild type in Campylobacter jejuni. However, there is a decrease in cmeR efflux gene expression in the luxS mutant, resulting in fewer CmeR proteins, thereby reducing CmeABC inhibition. This may in turn lead to an increase in efflux expression and function. Despite the lack of changes in cmeB expression, the luxS deletion may trigger expression of other efflux systems associated with CmeR regulatory factors (34). In addition, bacterial signaling molecules need to be exported and released outside the cell to be recognized by other bacteria. In Gram-negative bacteria, the signaling molecule AHL is actively transported across the cell membrane by the MexAB-OprM efflux pump (35). Our previous studies have brought evidence that the signal molecule AI-2 is involved in the resistance to quinolone antibiotics (33). Once the AI-2 produced by the luxS gene is excreted from the cells, it binds to the corresponding receptors and regulates the overexpression of efflux pump SatAB involved in bacterial resistance in S. suis. Further research in this area will reveal in depth the role of the LuxS/AI-2 system in controlling the effects of these efflux pumps on bacterial resistance (33). The ability of the QS system to regulate MDR efflux pumps represents a potential target for antibiotic resistance (36).
LuxS/AI-2 affects drug resistance through mobile genetic elements.
Mobile genetic elements such as plasmids, integron gene cassettes and transposable elements play an important role in bacterial resistance (37). Plasmid-mediated resistance can cause horizontal transfers among different bacteria, leading to the spread of bacterial resistance, which can cause serious public health problems (38). Extended-spectrum beta-lactamase (ESBL) is a type of lactamase encoded in plasmids that hydrolyzes most of the beta-lactam antibiotics such as penicillin, cephalosporin, and aztreonam (39, 40). TEM-type ESBL includes TEM-1 and TEM-2 (41). Xue et al. (42) showed that exogenous AI-2 increased the antibiotic resistance of clinical E. coli strains isolated from cow’s papillitis by upregulating the expression of TEM-type enzyme in an LsrR-dependent manner. Transposons are a group of mobile genetic elements that are defined as a DNA sequence (43). Because of its ability to move between bacterial chromosomes, plasmids, and phages, resistance on the transposon is more easily transmitted and disseminated horizontally (44, 45). The antibiotic resistance gene tet(M) is located on the Tn916 family of junctional transposons (46). Our previous studies have shown that exogenously added AI-2 affects the resistance of S. suis to tetracycline through an upregulation of tet(M) gene expression. Although the specific signaling mechanism needs to be further studied, LuxS/AI-2 appears to be the main target for preventing the spread of bacterial resistance.
LuxS/AI-2 affects drug resistance through the VraSR two-component regulatory system.
The two-component signal transduction system is widely distributed in bacteria. The VraSR two-component system is an important regulatory system in Staphylococcus aureus, allowing bacteria to sense changes in the external environment and to adjust their response to maintain its homeostasis (47, 48). The VraSR two-component system consists of a histidine kinase sensor protein (VraS) and an effector regulatory protein (VraR) (49, 50). VraS autophosphorylates in vitro and rapidly transfers phosphate groups to VraR, which selectively dephosphorylates VlaS-mediated signaling pathways (51). Mutation or increased expression of the VraSR two-component system is one of the mechanisms of resistance to vancomycin in Staphylococcus aureus (48). Xue et al. (52) have shown that the loss of S. aureus luxS gene leads to a decrease in susceptibility to cell wall synthesis inhibitor antibiotics accompanied by upregulation of the VraSR two-component system. This revealed that the luxS gene may regulate bacterial resistance through a VraSR two-component regulatory system (52). In the presence of exogenous AI-2, the susceptibility of the luxS deletion mutant to cell wall synthesis inhibitors was restored, demonstrating that LuxS is involved in the antibiotic susceptibility of S. aureus, which may be primarily due to AI-2 signaling (52). In addition, as a two-component regulatory system, VraSR is able to detect conditions that may disrupt bacterial cell wall synthesis and regulate cell wall biosynthesis pathways (51, 53). Higher VraSR levels in the luxS deletion strain indicate that cells can respond to cell wall structure damage more rapidly than the wild type when exposed to cell wall synthesis inhibitor antibiotics (52). Therefore, the LuxS/AI-2 system affects the resistance of S. aureus to cell wall synthesis inhibitors through a VraSR two-component regulatory system.
LuxS/AI-2 affects drug resistance by inhibiting the folate synthesis pathway.
Folic acid refers to substances such as tetrahydrofolate and its derivatives, which are important cofactors for mediating carbon transfer and participate in many important reactions in organisms (54). Studies have shown that specific target binding-like interaction with LuxR may contribute to transcriptional activation and that sulfonamides compete with dihydropterylic acid synthetase for binding, which inhibits the biosynthesis of folate and causes toxicity (55). Yu et al. (56) showed that the presence of exogenous AI-2 increased the sensitivity of avian pathogenic Escherichia coli strain to trimethoprim-sulfamethoxazole (SXT) in the folate synthesis-dependent pathway, but does not rely on the LsrR-dependent pathway. The addition of the exogenous AI-2 precursor molecule DPD triggers product feedback inhibition and then reduces the expression of luxS and a number of other products of LuxS, such as homocysteine (56). Homocysteine is a substrate for methionine synthase E (MetE) and methionine synthase H (MetH), which are important enzymes in the folate synthesis pathway (57). Substrate inhibition caused by a decrease in homocysteine downregulates the expression of metE and metH, which in turn leads to a decrease in the intermediate metabolite tetrahydrofolate (THF), an important substrate for the synthesis of purines and pyrimidines (58). In THF metabolism, purines and pyrimidines are two important intermediate metabolites for the resynthesis of THF, and folA and folC are two important folate synthase-encoding genes (59). In the absence of SXT, AI-2 downregulates the transcriptional levels of the folate synthase-encoding genes folA and folC only by the folate pathway (56). However, in the presence of SXT, exogenous AI-2 enhances the growth inhibition of the APEC strain by SXT by downregulating the transcriptional level of the folate-related gene (56). Further information is provided on the potential drug targets for prophylactic and adjuvant antibiotic treatment.
LuxS/AI-2 affects drug resistance through biofilm formation.
Bacterial biofilms, which are surface-attached communities of bacterial cells composed of polymers produced by the microorganisms themselves embedded in an extracellular polymeric matrix, are a cause of multidrug resistance (60). The ability of S. suis to form biofilm was significantly increased when a small amount of AI-2 was added during growth, whereas deleting the luxS gene leads to a decreased ability to form a biofilm (4, 61–63). These observations suggest that the LuxS/AI-2 QS system modulates the formation of bacterial biofilms. Biofilm formation by Helicobacter pylori decreases its susceptibility to antibiotics, and antibiotic resistance mutations in H. pylori are more frequently generated in biofilms than in planktonic cells (64). The luxS gene is the only known QS gene found in the genome sequence of H. pylori (65). Some reports indicated that H. pylori produces extracellular signaling molecules associated with AI-2 and that AI-2 production is dependent on luxS function (66). Bacterial biofilm is associated with increased antibiotic resistance and is involved in many persistent diseases (67). The main mechanisms of bacterial biofilm resistance are QS, activation of efflux pumps, the formation of biofilms, and the production of inactive enzymes and antibiotic-modifying enzymes (68). Alone, each of these mechanisms only partially accounts for the increased antimicrobial recalcitrance observed in biofilms. However, the influence of LuxS/AI-2 on biofilm formation is likely a combination of various mechanisms and environmental changes.
TARGET THE LUXS/Al-2 SYSTEM: NEW ANTIBACTERIAL STRATEGY FOR BACTERIAL RESISTANCE
Inhibition of bacterial QS system represents a novel antibacterial strategy, which not only prevents the development of bacterial resistance but also eliminates the density-induced control of bacterial virulence factors that contributes to serious infections (69). Several strategies developed to prevent bacterial resistance by inhibiting QS systems are summarized in Fig. 2, while an overview of LuxS/AI-2 QS system inhibition strategies is summarized in Table 2.
FIG 2.
Strategies for QS interception. (1) Signal-generating enzymes (LuxS and Pfs). (2) Signal sequestration and degradation outside the cell. (3) Receptors and transducers in the signal transduction cascade.
TABLE 2.
Overview of LuxS/AI-2 QS inhibition strategies
| Inhibitor | Action mode | Biological effect | Reference |
|---|---|---|---|
| Inhibition target: signal generators | |||
| MT-DADMe-ImmA (transition state analog) | Pfs inhibitor | Inhibit AI-2 production | 73 |
| Hydroxylated pyrrolidines derivatives |
Inhibitors of SAH/MTA nucleosidase. |
Inhibit AI-2 production | 76 |
| TNRHNPHHLHHV (peptide) | Interact with the LuxS enzyme | Inhibit enzyme activity | 86 |
| Inhibition target: signal molecule | |||
| Ex vivo addition of LsrK and ATP | Phosphorylation and degradation of AI-2 | Inhibits bioluminescence in V. harveyi and lsr expression in E. coli and S.Typhimurium | 90 |
| Imidazole | A furan carbocyclic analog of AI-2 | Inhibiting AI-2 function | 93 |
| Inhibition target: signal receptor/transduction | |||
| d-Galactose | Inhibitor AI-2 activity | Targeting AI-2 activity for prevention biofilm formation. | 99 |
| Small peptide, 5906 | Prevents LuxS homodimer formation | Inhibits LuxS activity by binding specifically to LuxS | 100 |
Inhibition of signal molecule synthesis.
In QS systems, the synthesis of signal molecules plays an essential role in the communication between cells (70). Among them, the signal molecule AI-2 is an important molecule for signal exchange between different bacterial species (71). The precursor S-ribosyl homocysteine (SRH) of AI-2 is formed by the action of 5′-methylthioadenosine/S-adenosylhomocysteine nucleosidase (MTAN) on SAH (72). The inhibition of MTAN results in an accumulation of 5′-methylthioadenosine (MTA) and SAH and consequently the inhibition of AI-2 production (73). The production of AI-2 is significantly reduced in MTAN knockout strains or in the presence of tight-binding inhibitors of MTAN (74). Since MTAN is not expressed in humans, it provides a potential target for antibacterial drug design for QS signaling. 5′-Methylthioadenosine phosphorylase (MTAP) plays a role in the polyamine pathway by circulating MTA and maintaining SAM (75). Its transition state structure is used to direct the synthesis of MT-DADMe-ImmA, a picomolar inhibitor that blocks QS in Vibrio cholerae without altering the rate of bacterial growth (73). Transition state analog inhibitors have shown promise as anticancer agents and antibacterial agents (73). These results indicate that MTAN inhibition is a possible drug strategy since it provides a “single injection” target for LuxS/AI-2-controlled bacteria. Guillermo et al. first showed that hydroxylated pyrrolidine represents a SAH/MTA inhibitor and speculated that these compounds might be transitional analogs (76). However, inhibition of Pfs is fatal to cells because the accumulation of MTA and SAH is toxic to cells (77, 78). Excess MTA levels in cells inhibit growth processes and DNA synthesis by indirectly preventing the synthesis of polyamines involved in these important processes (79, 80). Few studies have focused on the inhibition of S-ribosylhomocysteinase (LuxS). LuxS inhibition should not affect the processes essential for growth and survival (81–83). This enzyme is a DPD synthetase and is involved in the detoxification of SAH; it plays a minor role in the sulfur cycle pathway (84, 85). Han and Lu (86) used phage display technology to screen for a phage-encoded peptide that specifically interacts with the LuxS enzyme in S. suis. This LuxS peptide inhibitor (TNRHNPHHLHHV) showed partial inhibition of enzyme activity (86).
Inhibition of signaling molecules.
Inactivation or denaturation of the signal molecule itself, which can be achieved by various mechanism, is the most basic way to use the QS system to prevent bacterial resistance and to study new antibacterial strategies (87). Some microorganisms have the ability to metabolize AI-2 and consequently to inhibit QS function. Signal molecule degradation can be achieved by adding LsrK (AI-2 kinase) and ATP into the bacterial culture, in which AI-2 is phosphorylated outside the cell; the bacterial cross talk controlled by AI-2 is then significantly reduced (88, 89). In vitro-phosphorylated AI-2 quenched the QS response in E. coli, Salmonella Typhimurium, and Haber’s bacillus (90). Phosphorylated AI-2 is more hydrophilic and is thought to fail to cross the cell membrane and act as a QS signal (89). This strategy might be particularly effective in mixed infections because LsrK can phosphorylate DPD (the precursor molecule of AI-2) (91). Moreover, it may be effective regardless of the AI-2 structure and transport/sensor mechanism used by different bacterial QS systems (92). Yu et al. (93) and others reported that exogenous imidazole, a furan carbocyclic analogue of AI-2, reduced the antibiotic resistance of clinical E. coli strains to β-lactam antibiotics by inhibiting the function of AI-2.
Inhibit signal molecule conduction or binding to receptors.
In the activated methyl cycle, S-adenosylmethionine acts as a methyl donor, resulting in the accumulation of the toxic intermediate S-adenosylhomocysteine (SAH) in bacterial cells (94). The LuxS enzyme plays a role in the detoxification of SAH with homocysteine and DPD (19, 95). DPD is a highly active pre-AI-2 molecule. Destruction of the activated methyl cycle by inactivation of luxS may result in a series of chemical reactions (96). Therefore, further complementary studies were performed using synthetic DPD. Recently study showed a correlation between threshold DPD concentration and antibiotic susceptibility in Streptococcus anginosus (97, 98). These results are consistent with other studies on DPD and AI-2 and show the importance of achieving appropriate AI-2 threshold levels in bacterial populations (97). AI-2 does not have a specific structure; rather, it represents a class of molecules. The precursor DPD of AI-2 is cyclized in solution to form various isomers. The most significant inhibitory effect of propyl and butyl-DPD relates to Salmonella Typhimurium QS (99). Ryu et al. showed that d-galactose, as an inhibitor of AI-2 activity, inhibits biofilm formation by periodontal pathogens (99). The d-galactose-binding protein shows high sequence similarity to ribose-binding protein (RbsB), a known AI-2 receptor of Actinobacillus sp. (99). Sun et al. (100) identified the small peptide 5906 that inhibits Edwardsiella tarda LuxS activity by specifically binding LuxS in a manner that may prevent the formation of a functional LuxS homodimer. Furthermore, the AI-2 activity of Aeromonas hydrophila and Vibrio harveyi can be inhibited, and fish supplemented with DH5α/p5906 exhibit enhanced resistance to both bacteria. The results indicate that 5906 or an analog/derivative thereof can be used to develop a broad-spectrum antimicrobial agent for the prevention and control of bacterial diseases in fish (100). Bacterial QS responses are not necessarily triggered by AI-2 produced by organisms of the same species, genus, or even classes (20). AI-2-mediated QS typically occurs in bacterial communities composed of many different types of microorganisms. Several studies have demonstrated the QS phenotype in multimicrobial communities, including LuxS/AI-2, mediates activity between normal microflora and pathogens (101). Since LuxS/AI-2 regulates pathogen virulence in multimicrobial communication networks, disrupting signaling in these networks provides another goal for QS quenchers (102).
CONCLUSION
The LuxS/AI-2 QS system plays a key role in antibiotic resistance in bacteria. The LuxS/AI-2 system controls the expression of a variety of genes and then regulates the cellular activities of bacteria to adapt to different environments (97, 103–105). Since QS controls the expression of many virulence factors and drug-resistant genes in bacteria, any process that blocks QS signaling molecules or receptor-recognizing signaling molecules can attenuate the virulence and resistance gene expression of bacterial QS-dependent genes (106–109). The concerns regarding the rising in antibiotic resistance require an alternative approach to antibacterial therapy. Extensive AI-2 communication between bacteria makes it a possible therapeutic target. Therefore, understanding the role of AI-2 QS in antibiotic susceptibility is of great interest.
ACKNOWLEDGMENTS
We acknowledge the support of Henan University of Science and Technology and all study participants.
This study was funded by the National Natural Science Foundation of China (grants 31772761 and 31540095) and the Natural Science Foundation of Henan Province (grant 182300410047).
REFERENCES
- 1.Waters CM, Bassler BL. 2005. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol 21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 2.Bassler BL, Losick R. 2006. Bacterially speaking. Cell 125:237–246. doi: 10.1016/j.cell.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Orchard SS, Goodrich-Blair H. 2004. Identification and functional characterization of a Xenorhabdus nematophila oligopeptide permease. Appl Environ Microbiol 70:5621–5627. doi: 10.1128/AEM.70.9.5621-5627.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Yi L, Zhang Z, Fan H, Cheng X, Lu C. 2013. Overexpression of luxS cannot increase autoinducer-2 production, only affect the growth and biofilm formation in Streptococcus suis. Sci World J 2013:1–6. doi: 10.1155/2013/924276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Wang Y, Sun L, Grenier D, Yi L. 2018. The LuxS/AI-2 system of Streptococcus suis. Appl Microbiol Biotechnol 102:7231–7238. doi: 10.1007/s00253-018-9170-7. [DOI] [PubMed] [Google Scholar]
- 6.Bidault P, Chandad F, Grenier D. 2007. Risk of bacterial resistance associated with systemic antibiotic therapy in periodontology. J Can Dent Assoc 73:721–725. [PubMed] [Google Scholar]
- 7.Xu GM. 2016. Relationships between the regulatory systems of quorum sensing and multidrug resistance. Front Microbiol 7:958. doi: 10.3389/fmicb.2016.00958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tavío MM, Aquili VD, Poveda JB, Antunes NT, Sánchez-Céspedes J, Vila J. 2010. Quorum-sensing regulator sdiA and marA overexpression is involved in in vitro-selected multidrug resistance of Escherichia coli. J Antimicrob Chemother 65:1178–1186. doi: 10.1093/jac/dkq112. [DOI] [PubMed] [Google Scholar]
- 9.Tavio MM, Aquili VD, Fabrega A, Vila J, Poveda JB. 2012. Overexpression of the quorum-sensing regulator sdiA and soxS is involved in low-level multidrug resistance induced in Escherichia coli AG100 by haloperidol, diazepam and NaCl. Int J Antimicrob Agents 39:91–93. doi: 10.1016/j.ijantimicag.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Kalia VC, Wood TK, Kumar P. 2014. Evolution of resistance to quorum-sensing inhibitors. Microb Ecol 68:13–23. doi: 10.1007/s00248-013-0316-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krasovec R, Belavkin RV, Aston JA, Channon A, Aston E, Rash BM, Kadirvel M, Forbes S, Knight CG. 2014. Where antibiotic resistance mutations meet quorum-sensing. Microb Cell 1:250–252. doi: 10.15698/mic2014.07.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Contreras R, Maeda T, Wood TK. 2016. Can resistance against quorum-sensing interference be selected? ISME J 10:4–10. doi: 10.1038/ismej.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Surette MG, Miller MB, Bassler BL. 1999. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc Natl Acad Sci U S A 96:1639–1644. doi: 10.1073/pnas.96.4.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Wang L, Hashimoto Y, Tsao CY, Wood TK, Valdes JJ, Zafiriou E, Bentley WE. 2006. A stochastic model of Escherichia coli AI-2 quorum signal circuit reveals alternative synthesis pathways. Mol Syst Biol 2:67. doi: 10.1038/msb4100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung V, Levesque CM. 2012. A stress-inducible quorum-sensing peptide mediates the formation of persister cells with noninherited multidrug tolerance. J Bacteriol 194:2265–2274. doi: 10.1128/JB.06707-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.García-Contreras R, Nuñez-López L, Jasso-Chávez R, Kwan BW, Belmont JA, Rangel-Vega A, Maeda T, Wood TK. 2015. Quorum sensing enhancement of the stress response promotes resistance to quorum quenching and prevents social cheating. ISME J 9:115–125. doi: 10.1038/ismej.2014.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandes R, Bentley WE. 2009. AI-2 biosynthesis module in a magnetic nanofactory alters bacterial response via localized synthesis and delivery. Biotechnol Bioeng 102:390–399. doi: 10.1002/bit.22078. [DOI] [PubMed] [Google Scholar]
- 18.Challan Belval S, Gal L, Margiewes S, Garmyn D, Piveteau P, Guzzo J. 2006. Assessment of the roles of LuxS, S-ribosyl homocysteine, and autoinducer 2 in cell attachment during biofilm formation by Listeria monocytogenes EGD-e. Appl Environ Microbiol 72:2644–2250. doi: 10.1128/AEM.72.4.2644-2650.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu X, Wang Y, Gao L, Jiang W, Lin W, Niu C, Yuan K, Ma R, Huang Z. 2018. The impairment of methyl metabolism from luxS mutation of Streptococcus mutans. Front Microbiol 9:404. doi: 10.3389/fmicb.2018.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson JA, Oliveira RA, Djukovic A, Ubeda C, Xavier KB. 2015. Manipulation of the quorum sensing signal AI-2 affects the antibiotic-treated gut microbiota. Cell Rep 10:1861–1871. doi: 10.1016/j.celrep.2015.02.049. [DOI] [PubMed] [Google Scholar]
- 21.Bonhoure N, Byrnes A, Moir RD, Hodroj W, Preitner F, Praz V, Marcelin G, Chua SC Jr, Martinez-Lopez N, Singh R, Moullan N, Auwerx J, Willemin G, Shah H, Hartil K, Vaitheesvaran B, Kurland I, Hernandez N, Willis IM. 2015. Loss of the RNA polymerase III repressor MAF1 confers obesity resistance. Genes Dev 29:934–947. doi: 10.1101/gad.258350.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma Y, Hao L, Ke H, Liang Z, Ma J, Liu Z, Li Y. 2017. LuxS/AI-2 in Streptococcus agalactiae reveals a key role in acid tolerance and virulence. Res Vet Sci 115:501–507. doi: 10.1016/j.rvsc.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 23.Ma R, Qiu S, Jiang Q, Sun H, Xue T, Cai G, Sun B. 2017. AI-2 quorum sensing negatively regulates rbf expression and biofilm formation in Staphylococcus aureus. Int J Med Microbiol 307:257–267. doi: 10.1016/j.ijmm.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Wu X, Jacobs NT, Bozio C, Palm P, Lattar SM, Hanke CR, Watson DM, Sakai F, Levin BR, Klugman KP, Vidal JE. 2017. Competitive dominance within biofilm consortia regulates the relative distribution of pneumococcal nasopharyngeal density. Appl Environ Microbiol 83:e00953-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang M, Li N, Yan M, Chang W, Hao J, Pang X, Wang X. 2015. Expression of Edwardsiella tarda luxS gene at different growth stage. Wei Sheng Wu Xue Bao 55:1201–1207. (In Chinese.) [PubMed] [Google Scholar]
- 26.Ju X, Li J, Zhu M, Lu Z, Lv F, Zhu X, Bie X. 2018. Effect of the luxS gene on biofilm formation and antibiotic resistance by Salmonella serovar Dublin. Food Res Int 107:385–393. doi: 10.1016/j.foodres.2018.02.039. [DOI] [PubMed] [Google Scholar]
- 27.Meng L, Du Y, Liu P, Li X, Liu Y. 2017. Involvement of LuxS in Aeromonas salmonicida metabolism, virulence, and infection in Atlantic salmon (Salmo salar L). Fish Shellfish Immunol 64:260–269. doi: 10.1016/j.fsi.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Yoon EJ, Balloy V, Fiette L, Chignard M, Courvalin P, Grillot-Courvalin C. 2016. Contribution of the Ade resistance-nodulation-cell division-type efflux pumps to fitness and pathogenesis of Acinetobacter baumannii. mBio 7:e00697–16. doi: 10.1128/mBio.00697-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horiyama T, Nikaido E, Yamaguchi A, Nishino K. 2011. Roles of Salmonella multidrug efflux pumps in tigecycline resistance. J Antimicrob Chemother 66:105–110. doi: 10.1093/jac/dkq421. [DOI] [PubMed] [Google Scholar]
- 30.Bruchmann S, Dotsch A, Nouri B, Chaberny IF, Haussler S. 2013. Quantitative contributions of target alteration and decreased drug accumulation to Pseudomonas aeruginosa fluoroquinolone resistance. Antimicrob Agents Chemother 57:1361–1368. doi: 10.1128/AAC.01581-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weston N, Sharma P, Ricci V, Piddock L. 2018. Regulation of the AcrAB-TolC efflux pump in Enterobacteriaceae. Res Microbiol 169:425–431. doi: 10.1016/j.resmic.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Yang S, Lopez CR, Zechiedrich EL. 2006. Quorum sensing and multidrug transporters in Escherichia coli. Proc Natl Acad Sci U S A 103:2386–2391. doi: 10.1073/pnas.0502890102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Liu B, Li J, Gong S, Dong X, Mao C, Yi L. 2019. LuxS/AI-2 system is involved in fluoroquinolones susceptibility in Streptococcus suis through overexpression of efflux pump SatAB. Vet Microbiol 233:154–158. doi: 10.1016/j.vetmic.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Mou KT, Plummer PJ. 2016. The impact of the LuxS mutation on phenotypic expression of factors critical for Campylobacter jejuni colonization. Vet Microbiol 192:43–51. doi: 10.1016/j.vetmic.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 35.Moore JD, Gerdt JP, Eibergen NR, Blackwell HE. 2014. Active efflux influences the potency of quorum sensing inhibitors in Pseudomonas aeruginosa. Chembiochem 15:435–442. doi: 10.1002/cbic.201300701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pawar S, Ashraf MI, Mujawar S, Mishra R, Lahiri C. 2018. In silico identification of the indispensable quorum sensing proteins of multidrug-resistant Proteus mirabilis. Front Cell Infect Microbiol 8:269. doi: 10.3389/fcimb.2018.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma L, Li B, Zhang T. 2014. Abundant rifampin resistance genes and significant correlations of antibiotic resistance genes and plasmids in various environments revealed by metagenomic analysis. Appl Microbiol Biotechnol 98:5195–5204. doi: 10.1007/s00253-014-5511-3. [DOI] [PubMed] [Google Scholar]
- 38.Gama JA, Zilhao R, Dionisio F. 2018. Impact of plasmid interactions with the chromosome and other plasmids on the spread of antibiotic resistance. Plasmid 99:82–88. doi: 10.1016/j.plasmid.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 39.Brolund A, Rajer F, Giske CG, Melefors O, Titelman E, Sandegren L. 2019. Dynamics of resistance plasmids in extended-spectrum-β-lactamase-producing Enterobacteriaceae during postinfection colonization. Antimicrob Agents Chemother 63:e02201–18. doi: 10.1128/AAC.02201-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castanheira M, Farrell SE, Krause KM, Jones RN, Sader HS. 2014. Contemporary diversity of beta-lactamases among Enterobacteriaceae in the nine U.S. census regions and ceftazidime-avibactam activity tested against isolates producing the most prevalent beta-lactamase groups. Antimicrob Agents Chemother 58:833–838. doi: 10.1128/AAC.01896-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonnet R, De Champs C, Sirot D, Chanal C, Labia R, Sirot J. 1999. Diversity of TEM mutants in Proteus mirabilis. Antimicrob Agents Chemother 43:2671–2677. doi: 10.1128/AAC.43.11.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xue T, Yu L, Shang F, Li W, Zhang M, Ni J, Chen X. 2016. Short communication: the role of autoinducer 2 (AI-2) on antibiotic resistance regulation in an Escherichia coli strain isolated from a dairy cow with mastitis. J Dairy Sci 99:4693–4698. doi: 10.3168/jds.2015-10543. [DOI] [PubMed] [Google Scholar]
- 43.Babakhani S, Oloomi M. 2018. Transposons: the agents of antibiotic resistance in bacteria. J Basic Microbiol 58:905–917. doi: 10.1002/jobm.201800204. [DOI] [PubMed] [Google Scholar]
- 44.Florez AB, Reimundo P, Delgado S, Fernandez E, Alegria A, Guijarro JA, Mayo B. 2012. Genome sequence of Lactococcus garvieae IPLA 31405, a bacteriocin-producing, tetracycline-resistant strain isolated from a raw-milk cheese. J Bacteriol 194:5118–5119. doi: 10.1128/JB.00975-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toleman MA, Walsh TR. 2011. Combinatorial events of insertion sequences and ICE in Gram-negative bacteria. FEMS Microbiol Rev 35:912–935. doi: 10.1111/j.1574-6976.2011.00294.x. [DOI] [PubMed] [Google Scholar]
- 46.Roberts AP, Mullany P. 2009. A modular master on the move: the Tn916 family of mobile genetic elements. Trends Microbiol 17:251–258. doi: 10.1016/j.tim.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 47.Taglialegna A, Varela MC, Rosato RR, Rosato AE. 2019. VraSR and virulence trait modulation during daptomycin resistance in methicillin-resistant Staphylococcus aureus infection. mSphere 4:e00557-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mehta S, Cuirolo AX, Plata KB, Riosa S, Silverman JA, Rubio A, Rosato RR, Rosato AE. 2012. VraSR two-component regulatory system contributes to mprF-mediated decreased susceptibility to daptomycin in in vivo-selected clinical strains of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 56:92–102. doi: 10.1128/AAC.00432-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kato Y, Suzuki T, Ida T, Maebashi K. 2010. Genetic changes associated with glycopeptide resistance in Staphylococcus aureus: predominance of amino acid substitutions in YvqF/VraSR. J Antimicrob Chemother 65:37–45. doi: 10.1093/jac/dkp394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gardete S, Wu SW, Gill S, Tomasz A. 2006. Role of VraSR in antibiotic resistance and antibiotic-induced stress response in Staphylococcus aureus. Antimicrob Agents Chemother 50:3424–3434. doi: 10.1128/AAC.00356-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen H, Xiong Z, Liu K, Li S, Wang R, Wang X, Zhang Y, Wang H. 2016. Transcriptional profiling of the two-component regulatory system VraSR in Staphylococcus aureus with low-level vancomycin resistance. Int J Antimicrob Agents 47:362–367. doi: 10.1016/j.ijantimicag.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 52.Xue T, Zhao L, Sun B. 2013. LuxS/AI-2 system is involved in antibiotic susceptibility and autolysis in Staphylococcus aureus NCTC 8325. Int J Antimicrob Agents 41:85–89. doi: 10.1016/j.ijantimicag.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 53.Belcheva A, Golemi-Kotra D. 2008. A close-up view of the VraSR two-component system. A mediator of Staphylococcus aureus response to cell wall damage. J Biol Chem 283:12354–12364. doi: 10.1074/jbc.M710010200. [DOI] [PubMed] [Google Scholar]
- 54.Chen C, Ke J, Zhou XE, Yi W, Brunzelle JS, Li J, Yong EL, Xu HE, Melcher K. 2013. Structural basis for molecular recognition of folic acid by folate receptors. Nature 500:486–489. doi: 10.1038/nature12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deng Z, Lin Z, Zou X, Yao Z, Tian D, Wang D, Yin D. 2012. Model of hormesis and its toxicity mechanism based on quorum sensing: a case study on the toxicity of sulfonamides to Photobacterium phosphoreum. Environ Sci Technol 46:7746–7754. doi: 10.1021/es203490f. [DOI] [PubMed] [Google Scholar]
- 56.Yu L, Li W, Zhang M, Cui Y, Chen X, Ni J, Yu L, Shang F, Xue T. 2018. Autoinducer-2 affects trimethoprim-sulfamethoxazole susceptibility in avian pathogenic Escherichia coli dependent on the folate synthesis-associate pathway. Microbiologyopen 7:e00582. doi: 10.1002/mbo3.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pejchal R, Ludwig ML. 2005. Cobalamin-independent methionine synthase (MetE): a face-to-face double barrel that evolved by gene duplication. PLoS Biol 3:e31. doi: 10.1371/journal.pbio.0030031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh R, Fouladi-Nashta AA, Li D, Halliday N, Barrett DA, Sinclair KD. 2006. Methotrexate induced differentiation in colon cancer cells is primarily due to purine deprivation. J Cell Biochem 99:146–155. doi: 10.1002/jcb.20908. [DOI] [PubMed] [Google Scholar]
- 59.Sybesma W, Starrenburg M, Kleerebezem M, Mierau I, de Vos WM, Hugenholtz J. 2003. Increased production of folate by metabolic engineering of Lactococcus lactis. Appl Environ Microbiol 69:3069–3076. doi: 10.1128/aem.69.6.3069-3076.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dufour D, Leung V, Lévesque CM. 2010. Bacterial biofilm: structure, function, and antimicrobial resistance. Endod Topics 22:2–16. doi: 10.1111/j.1601-1546.2012.00277.x. [DOI] [Google Scholar]
- 61.Wang Y, Yi L, Zhang Z, Fan H, Cheng X, Lu C. 2014. Biofilm formation, host-cell adherence, and virulence genes regulation of Streptococcus suis in response to autoinducer-2 signaling. Curr Microbiol 68:575–580. doi: 10.1007/s00284-013-0509-0. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y, Zhang W, Wu Z, Lu C. 2011. Reduced virulence is an important characteristic of biofilm infection of Streptococcus suis. FEMS Microbiol Lett 316:36–43. doi: 10.1111/j.1574-6968.2010.02189.x. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y, Zhang W, Wu Z, Zhu X, Lu C. 2011. Functional analysis of luxS in Streptococcus suis reveals a key role in biofilm formation and virulence. Vet Microbiol 152:151–160. doi: 10.1016/j.vetmic.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 64.Yonezawa H, Osaki T, Kamiya S. 2015. Biofilm formation by Helicobacter pylori and its involvement for antibiotic resistance. Biomed Res Int 2015:1. doi: 10.1155/2015/914791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee WK, Ogura K, Loh JT, Cover TL, Berg DE. 2006. Quantitative effect of luxS gene inactivation on the fitness of Helicobacter pylori. Appl Environ Microbiol 72:6615–6622. doi: 10.1128/AEM.01291-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen F, Hobley L, Doherty N, Loh JT, Cover TL, Sockett RE, Hardie KR, Atherton JC. 2010. In Helicobacter pylori autoinducer-2, but not LuxS/MccAB catalysed reverse transsulphuration, regulates motility through modulation of flagellar gene transcription. BMC Microbiol 10:210. doi: 10.1186/1471-2180-10-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bonifait L, Grignon L, Grenier D. 2008. Fibrinogen induces biofilm formation by Streptococcus suis and enhances its antibiotic resistance. Appl Environ Microbiol 74:4969–4972. doi: 10.1128/AEM.00558-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y, Wang Y, Sun L, Grenier D, Yi L. 2018. Streptococcus suis biofilm: regulation, drug-resistance mechanisms, and disinfection strategies. Appl Microbiol Biotechnol 102:9121–9129. doi: 10.1007/s00253-018-9356-z. [DOI] [PubMed] [Google Scholar]
- 69.Bhardwaj AK, Vinothkumar K, Rajpara N. 2013. Bacterial quorum sensing inhibitors: attractive alternatives for control of infectious pathogens showing multiple drug resistance. Recent Pat Antiinfect Drug Discov 8:68–83. doi: 10.2174/1574891X11308010012. [DOI] [PubMed] [Google Scholar]
- 70.Abbamondi GR, De Rosa S, Iodice C, Tommonaro G. 2014. Cyclic dipeptides produced by marine sponge-associated bacteria as quorum sensing signals. Nat Prod Commun 9:229–232. [PubMed] [Google Scholar]
- 71.Zaitseva IV, Popova AA. , Khmel IA. 2014. Quorum sensing regulation in bacteria of the family Enterobacteriaceae Genetika 50:373–391. (In Russian.) [PubMed] [Google Scholar]
- 72.Singh V, Evans GB, Lenz DH, Mason JM, Clinch K, Mee S, Painter GF, Tyler PC, Furneaux RH, Lee JE, Howell PL, Schramm VL. 2005. Femtomolar transition state analogue inhibitors of 5′-methylthioadenosine/S-adenosylhomocysteine nucleosidase from Escherichia coli. J Biol Chem 280:18265–18273. doi: 10.1074/jbc.M414472200. [DOI] [PubMed] [Google Scholar]
- 73.Schramm VL, Gutierrez JA, Cordovano G, Basu I, Guha C, Belbin TJ, Evans GB, Tyler PC, Furneaux RH. 2008. Transition state analogues in quorum sensing and SAM recycling. Nucleic Acids Symp Ser 52:75–76. doi: 10.1093/nass/nrn038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang S, Thomas K, Schramm VL. 2014. Catalytic site cooperativity in dimeric methylthioadenosine nucleosidase. Biochemistry 53:1527–1535. doi: 10.1021/bi401589n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Namanja-Magliano HA, Stratton CF, Schramm VL. 2016. Transition state structure and inhibition of Rv0091, a 5′-deoxyadenosine/5′-methylthioadenosine nucleosidase from Mycobacterium tuberculosis. ACS Chem Biol 11:1669–1676. doi: 10.1021/acschembio.6b00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guillerm G, Varkados M, Auvin S, Goffic FL. 1987. Synthesis of hydroxylated pyrrolidines derivatives as potential inhibitors of SAH/MTA nucleosidase. Tetrahedron Lett 28:535–538. doi: 10.1016/S0040-4039(00)95775-7. [DOI] [Google Scholar]
- 77.Heurlier K, Vendeville A, Halliday N, Green A, Winzer K, Tang CM, Hardie KR. 2009. Growth deficiencies of Neisseria meningitidis pfs and luxS mutants are not due to inactivation of quorum sensing. J Bacteriol 191:1293–1302. doi: 10.1128/JB.01170-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang J, He J, Yu M, Li T, Luo L, Liu P. 2016. The efficacy and safety of platinum plus gemcitabine (PG) chemotherapy with or without molecular targeted agent (MTA) in first-line treatment of non-small cell lung cancer (NSCLC). Medicine (Baltimore, MD) 95:e5599. doi: 10.1097/MD.0000000000005599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kumar R, Wang RA. 2016. Structure, expression and functions of MTA genes. Gene 582:112–121. doi: 10.1016/j.gene.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kothari A, Hittelman WN, Chambers TC. 2016. Cell cycle-dependent mechanisms underlie vincristine-induced death of primary acute lymphoblastic leukemia cells. Cancer Res 76:3553–3561. doi: 10.1158/0008-5472.CAN-15-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yun B, Oh S, Song M, Hong YS, Park S, Park DJ, Griffiths MW, Oh S. 2015. Inhibitory effect of epigallocatechin gallate on the virulence of Clostridium difficile PCR ribotype 027. J Food Sci 80:M2925–M2931. doi: 10.1111/1750-3841.13145. [DOI] [PubMed] [Google Scholar]
- 82.Li L, Sun L, Song Y, Wu X, Zhou X, Liu Z, Zhou R. 2013. Screening of Actinobacillus pleuropneumoniae LuxS inhibitors. Curr Microbiol 67:564–571. doi: 10.1007/s00284-013-0403-9. [DOI] [PubMed] [Google Scholar]
- 83.Park H, Lee K, Yeo S, Shin H, Holzapfel WH. 2017. Autoinducer-2 quorum sensing influences viability of Escherichia coli O157:H7 under osmotic and in vitro gastrointestinal stress conditions. Front Microbiol 8:1077. doi: 10.3389/fmicb.2017.01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wnuk SF, Robert J, Sobczak AJ, Meyers BP, Malladi VL, Zhu J, Gopishetty B, Pei D. 2009. Inhibition of S-ribosylhomocysteinase (LuxS) by substrate analogues modified at the ribosyl C-3 position. Bioorg Med Chem 17:6699–6706. doi: 10.1016/j.bmc.2009.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Malladi VL, Sobczak AJ, Meyer TM, Pei D, Wnuk SF. 2011. Inhibition of LuxS by S-ribosylhomocysteine analogues containing a [4-aza]ribose ring. Bioorg Med Chem 19:5507–5519. doi: 10.1016/j.bmc.2011.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Han X, Lu C. 2009. Biological activity and identification of a peptide inhibitor of LuxS from Streptococcus suis serotype 2. FEMS Microbiol Lett 294:16–23. doi: 10.1111/j.1574-6968.2009.01534.x. [DOI] [PubMed] [Google Scholar]
- 87.Metz B, Jiskoot W, Hennink WE, Crommelin DJ, Kersten GF. 2003. Physicochemical and immunochemical techniques predict the quality of diphtheria toxoid vaccines. Vaccine 22:156–167. doi: 10.1016/j.vaccine.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 88.Quan Y, Meng F, Ma X, Song X, Liu X, Gao W, Dang Y, Meng Y, Cao M, Song C. 2017. Regulation of bacteria population behaviors by AI-2 “consumer cells” and “supplier cells. BMC Microbiol 17:198. doi: 10.1186/s12866-017-1107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhu J, Hixon MS, Globisch D, Kaufmann GF, Janda KD. 2013. Mechanistic insights into the LsrK kinase required for autoinducer-2 quorum sensing activation. J Am Chem Soc 135:7827–7830. doi: 10.1021/ja4024989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roy V, Fernandes R, Tsao C, Bentley W. 2010. Cross species quorum quenching using a native AI-2 processing enzyme. ACS Chem Biol 5:223–232. doi: 10.1021/cb9002738. [DOI] [PubMed] [Google Scholar]
- 91.Xavier KB, Miller ST, Lu W, Kim JH, Rabinowitz J, Pelczer I, Semmelhack MF, Bassler BL. 2007. Phosphorylation and processing of the quorum-sensing molecule autoinducer-2 in enteric bacteria. ACS Chem Biol 2:128–136. doi: 10.1021/cb600444h. [DOI] [PubMed] [Google Scholar]
- 92.Stotani S, Gatta V, Medarametla P, Padmanaban M, Karawajczyk A, Giordanetto F, Tammela P, Laitinen T, Poso A, Tzalis D, Collina S. 2019. DPD-inspired discovery of novel LsrK kinase inhibitors: an opportunity to fight antimicrobial resistance. J Med Chem 62:2720–2737. doi: 10.1021/acs.jmedchem.9b00025. [DOI] [PubMed] [Google Scholar]
- 93.Yu L, Li W, Zhang M, Cui Y, Chen X, Ni J, Yu L, Shang F, Xue T. 2018. Imidazole decreases the ampicillin resistance of an Escherichia coli strain isolated from a cow with mastitis by inhibiting the function of autoinducer 2. J Dairy Sci 101:3356–3362. doi: 10.3168/jds.2017-13761. [DOI] [PubMed] [Google Scholar]
- 94.Weickhmann AK, Keller H, Wurm JP, Strebitzer E, Juen MA, Kremser J, Weinberg Z, Kreutz C, Duchardt-Ferner E, Wöhnert J. 2019. The structure of the SAM/SAH-binding riboswitch. Nucleic Acids Res 47:2654–2665. doi: 10.1093/nar/gky1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu D, Zuo J, Chen Z, Lv X, Hu J, Wu X, Qi K, Mi R, Huang Y, Miao J, Jiang W, Wang S, Wang C, Han X. 2017. Different activated methyl cycle pathways affect the pathogenicity of avian pathogenic Escherichia coli. Vet Microbiol 211:160–168. doi: 10.1016/j.vetmic.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 96.Kaur A, Capalash N, Sharma P. 2018. Quorum sensing in thermophiles: prevalence of autoinducer-2 system. BMC Microbiol 18:62. doi: 10.1186/s12866-018-1204-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ahmed NA, Petersen FC, Scheie AA. 2007. AI-2 quorum sensing affects antibiotic susceptibility in Streptococcus anginosus. J Antimicrob Chemother 60:49–53. doi: 10.1093/jac/dkm124. [DOI] [PubMed] [Google Scholar]
- 98.Ahmed NA, Petersen FC, Scheie AA. 2009. AI-2/LuxS is involved in increased biofilm formation by Streptococcus intermedius in the presence of antibiotics. Antimicrob Agents Chemother 53:4258–4263. doi: 10.1128/AAC.00546-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ryu EJ, Sim J, Sim J, Lee J, Choi BK. 2016. D-Galactose as an autoinducer 2 inhibitor to control the biofilm formation of periodontopathogens. J Microbiol 54:632–637. doi: 10.1007/s12275-016-6345-8. [DOI] [PubMed] [Google Scholar]
- 100.Sun B, Zhang M. 2016. Analysis of the antibacterial effect of an Edwardsiella tarda LuxS inhibitor. Springerplus 5:92. doi: 10.1186/s40064-016-1733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Neiditch MB, Federle MJ, Pompeani AJ, Kelly RC, Swem DL, Jeffrey PD, Bassler BL, Hughson FM. 2006. Ligand-induced asymmetry in histidine sensor kinase complex regulates quorum sensing. Cell 126:1095–1108. doi: 10.1016/j.cell.2006.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shao C, Shang W, Yang Z, Sun Z, Li Y, Guo J, Wang X, Zou D, Wang S, Lei H, Cui Q, Yin Z, Li X, Wei X, Liu W, He X, Jiang Z, Du S, Liao X, Huang L, Wang Y, Yuan J. 2012. LuxS-dependent AI-2 regulates versatile functions in Enterococcus faecalis V583. J Proteome Res 11:4465–4475. doi: 10.1021/pr3002244. [DOI] [PubMed] [Google Scholar]
- 103.Armbruster CE, Hong W, Pang B, Weimer KE, Juneau RA, Turner J, Swords WE. 2010. Indirect pathogenicity of Haemophilus influenzae and Moraxella catarrhalis in polymicrobial otitis media occurs via interspecies quorum signaling. mBio 1:e00102–10. doi: 10.1128/mBio.00102-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ramos S, Silva N, Hebraud M, Santos HM, Nunes-Miranda JD, Pinto L, Pereira JE, Capelo JL, Poeta P, Igrejas G. 2016. Proteomics for drug resistance on the food chain? Multidrug-resistant Escherichia coli proteomes from slaughtered pigs. OMICS 20:362–374. doi: 10.1089/omi.2016.0044. [DOI] [PubMed] [Google Scholar]
- 105.Zhou JW, Chen TT, Tan XJ, Sheng JY, Jia AQ. 2018. Can resveratrol, a quorum-sensing inhibitor, function as an aminoglycoside antibiotic-accelerant against Pseudomonas aeruginosa? Int J Antimicrob Agents. doi: 10.1016/j.ijantimicag.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 106.Jones MB, Peterson SN, Benn R, Braisted JC, Jarrahi B, Shatzkes K, Ren D, Wood TK, Blaser MJ. 2010. Role of luxS in Bacillus anthracis growth and virulence factor expression. Virulence 1:72–83. doi: 10.4161/viru.1.2.10752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yoon Y, Sofos JN. 2010. Absence of association of autoinducer-2-based quorum sensing with heat and acid resistance of Salmonella. J Food Sci 75:M444–M448. doi: 10.1111/j.1750-3841.2010.01744.x. [DOI] [PubMed] [Google Scholar]
- 108.Ðapa T, Dapa T, Leuzzi R, Ng YK, Baban ST, Adamo R, Kuehne SA, Scarselli M, Minton NP, Serruto D, Unnikrishnan M. 2013. Multiple factors modulate biofilm formation by the anaerobic pathogen Clostridium difficile. J Bacteriol 195:545–555. doi: 10.1128/JB.01980-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thompson JA, Oliveira RA, Xavier KB. 2014. Can chatter between microbes prevent cholera? Trends Microbiol 22:660–662. doi: 10.1016/j.tim.2014.10.006. [DOI] [PubMed] [Google Scholar]