We report the cases of a 39-year-old woman with chronic lymphocytic leukemia and a 21-year-old man with chronic granulomatous disease treated for cerebral aspergillosis. The patients required radical surgery for infection progression despite adequate isavuconazole plasma concentration or neurological complication. We thus decided to measure the brain isavuconazole concentration.
KEYWORDS: cerebral aspergillosis, isavuconazole, pharmacokinetic, therapeutic drug monitoring
ABSTRACT
We report the cases of a 39-year-old woman with chronic lymphocytic leukemia and a 21-year-old man with chronic granulomatous disease treated for cerebral aspergillosis. The patients required radical surgery for infection progression despite adequate isavuconazole plasma concentration or neurological complication. We thus decided to measure the brain isavuconazole concentration. These results suggest that the concentrations of isavuconazole obtained in the infected brain tissue clearly differ from those obtained in the normal brain tissue and the cerebrospinal fluid.
TEXT
First, we report the case of a 39-year-old persistently neutropenic woman with chronic lymphocytic leukemia (CLL) admitted to hospital because of headache and fever. Magnetic resonance imaging (MRI) showed several cerebral abscesses. A cerebral biopsy was performed and showed acute septate thin hyaline filamentous fungi. Fungal culture grew Aspergillus fumigatus susceptible to triazoles with MICs at 0.5 mg/liter for voriconazole (VCZ), at 0.19 mg/liter for posaconazole, and at 0.38 mg/liter for itraconazole. She initially received 2 weeks of liposomal amphotericin B (L-AmB) in combination with VCZ followed by VCZ monotherapy. Three months after the diagnosis of aspergillosis, she received bendamustin for CLL progression, leading to a more profound neutropenia for 2 weeks. She experienced clinical deterioration with seizures associated with MRI worsening and low VCZ plasma residual concentration (0.790 mg/liter). At that time, we added a treatment with high-dose caspofungin (150 mg/day). She improved clinically, but 2 months later she developed severe cytolytic hepatitis ascribed to voriconazole, which was switched for isavuconazole (ISZ), 200 mg/day after the loading dose. ISZ plasma residual concentrations were 2.5 mg/liter, and 1 month thereafter, the patient reported frontal headache again. A new MRI showed a large edema surrounding the frontal lesions, radical surgery was performed, and the ISZ dosage was increased to 300 mg daily to reach higher plasma residual concentrations (4.3 mg/liter). Fungal culture remained positive. Four months after surgery, while she was still on ISZ and caspofungin, the frontal lesion increased again, and she underwent another neurosurgical intervention, and this opportunity was taken to measure ISZ local concentrations (Fig. 1). In the same period, the ISZ concentration in cerebrospinal fluid (CSF) was undetectable. Fungal culture remained positive. Plasma and tissue ISZ concentrations were measured using a method previously published by our group (1). Tissue samples were first diluted 1/5 with water and homogenized as previously described (2), considering a tissue density of 1.04 (3). The following results were obtained: 0.405 mg/liter in normal brain, 2.62 mg/liter in inflamed dura mater, and 5.11 mg/liter and 5.09 mg/liter within the fungal abscess from the first and second surgical procedures, respectively. The corresponding plasma concentrations were 4.3 mg/liter and 3.2 mg/liter, respectively, with a 300 mg daily ISZ dosage. We also verified that the most recent Aspergillus fumigatus isolate obtained remained susceptible, with MICs at 1 mg/liter for VCZ and ISZ (EUCAST methodology).
FIG 1.
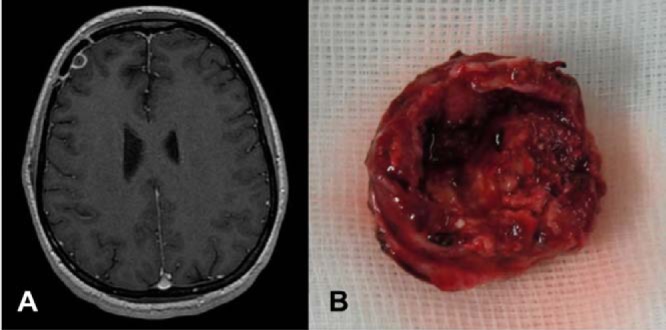
(A) T1-gadolinium cerebral magnetic resonance imaging showing a frontal abscess due to Aspergillus fumigatus infection before the second surgical procedure and (B) the resection specimen.
More recently, we managed the case of a 21-year-old man with chronic granulomatous disease treated for probable pulmonary aspergillosis and cerebral aspergillosis since 2013. The diagnosis relied on microscopic morphological findings compatible with Aspergillus. In addition to two initial radical surgeries, two interventions were necessary for excision of cystic lesions responsible for brainstem compression and blocking of CSF flow. The patient received different antifungal treatments (VCZ and caspofungin, then posaconazole, then VCZ and caspofungin, then liposomal amphotericin B, then VCZ, then VCZ and caspofungin) and interferon gamma immunotherapy. Finally, an allogeneic bone marrow transplant procedure was performed 2 years after the aspergillosis diagnosis because of refractory infection. The VCZ treatment had to be changed to ISZ (oral treatment, 200 mg/day once daily after the loading dose) due to phototoxicity. One and then two years thereafter, two neurosurgical procedures were needed to remove cystic cerebellar lesions. We proved mycological failure during the first one with a positive culture with Aspergillus fumigatus sensitive to all azoles. Thus, measurement of the ISZ concentration in the central nervous system was performed during the last surgical intervention. The concentration in the noninfected cerebellar tissue was below the limit of detection (i.e., 0.025 mg/liter) and was 0. 7949 mg/liter in the cystic wall. Pathological examination of the cystic wall showed a gliotic and fibro-inflammatory tissue, while microbiological tools, including a molecular approach, failed to reveal aspergillosis locally. At the same time, the ISZ plasma concentration was 5.606 mg/liter.
Until now, there were scarce data available on ISZ diffusion in human brain (4). In our first case, we found a high ISZ concentration (more than 5 mg/liter) in the brain abscess, even higher than those expected in plasma (i.e., 2 to 5 mg/liter) (5). We also found encouraging ISZ concentrations in inflamed meninges, contrasting with much lower concentrations in normal brain. With the second case, we confirmed that the concentrations of isavuconazole obtained in the brain markedly differed between inflammatory and normal tissues. Of note, although evidenced in only the two patients studied, our data suggest that the daily isavuconazole dosage might be increased to 300 mg/day during cerebral aspergillosis.
Our pharmacokinetic data obtained in a patient with cerebral aspergillosis are reminiscent of those obtained preclinically and are concordant with an animal model (6) and the efficacy of ISZ in experimental cryptococcal meningitis (7), cerebral mucormycosis (8), or endophthalmitis caused by Aspergillus fumigatus (9). In humans, data regarding the efficacy of ISZ during cerebral fungal infections are scarce (4, 10), considering that results of the noninferiority SECURE trial comparing ISZ to VCZ for the treatment of invasive aspergillosis or those of the VITAL trial involving patients with mucormycosis did not report specifically the efficacy of ISZ in those with cerebral mold infections (11, 12). Even if the examination of the relationship between brain antifungal pharmacokinetics and efficacy were limited, we can note that a high isavuconazole concentration in infected tissue is obtained.
Alternative hypotheses for treatment failure might have been the patient’s sustained immunosuppression, as the efficacy of isavuconazole is reduced during neutropenia (13) more than ISZ, based on the MIC values obtained here, as only those >16 μg/ml were associated with reduced efficacy (14).
ACKNOWLEDGMENTS
We thank all the people who took part in the care of these patients, especially Felipe Suarez, Stéphane Blanche, Ambroise Marçais, Claire Aguilar, David Lebeaux, Sylvain Poirée, Christophe Delavaud, and Giula Disnan.
REFERENCES
- 1.Toussaint B, Lanternier F, Woloch C, Fournier D, Launay M, Billaud E, Dannaoui E, Lortholary O, Jullien V. 2017. An ultra performance liquid chromatography-tandem mass spectrometry method for the therapeutic drug monitoring of isavuconazole and seven other antifungal compounds in plasma samples. J Chromatogr B Analyt Technol Biomed Life Sci 1046:26–33. doi: 10.1016/j.jchromb.2017.01.036. [DOI] [PubMed] [Google Scholar]
- 2.Schmitt-Hoffmann A-H, Kato K, Townsend R, Potchoiba MJ, Hope WW, Andes D, Spickermann J, Schneidkraut MJ. 2017. Tissue distribution and elimination of isavuconazole following single and repeat oral-dose administration of isavuconazonium sulfate to rats. Antimicrob Agents Chemother 61:e01292-17. doi: 10.1128/AAC.01292-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barber TW, Brockway JA, Higgins LS. 1970. The density of tissues in and about the head. Acta Neurol Scand 46:85–92. doi: 10.1111/j.1600-0404.1970.tb05606.x. [DOI] [PubMed] [Google Scholar]
- 4.Lamoth F, Mercier T, André P, Pagani JL, Pantet O, Maduri R, Guery B, Decosterd LA. 2019. Isavuconazole brain penetration in cerebral aspergillosis. J Antimicrob Chemother 74:1751–1753. doi: 10.1093/jac/dkz050. [DOI] [PubMed] [Google Scholar]
- 5.Desai A, Schmitt-Hoffmann A-H, Mujais S, Townsend R. 2016. Population pharmacokinetics of isavuconazole in subjects with mild or moderate hepatic impairment. Antimicrob Agents Chemother 60:3025–3031. doi: 10.1128/AAC.02942-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee A, Prideaux B, Lee MH, Zimmerman M, Dolgov E, Perlin DS, Zhao Y. 2019. Tissue distribution and penetration of isavuconazole at the site of infection in experimental invasive aspergillosis in mice with underlying chronic granulomatous disease. Antimicrob Agents Chemother 63:e00524-19. doi: 10.1128/AAC.00524-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiederhold NP, Kovanda L, Najvar LK, Bocanegra R, Olivo M, Kirkpatrick WR, Patterson TF. 2016. Isavuconazole is effective for the treatment of experimental cryptococcal meningitis. Antimicrob Agents Chemother 60:5600–5603. doi: 10.1128/AAC.00229-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo G, Gebremariam T, Lee H, Edwards JE, Kovanda L, Ibrahim AS. 2014. Isavuconazole therapy protects immunosuppressed mice from mucormycosis. Antimicrob Agents Chemother 58:2450–2453. doi: 10.1128/AAC.02301-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guest JM, Singh PK, Revankar SG, Chandrasekar PH, Kumar A. 2018. Isavuconazole for treatment of experimental fungal endophthalmitis caused by Aspergillus fumigatus. Antimicrob Agents Chemother 62:e01537-18. doi: 10.1128/AAC.01537-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peixoto D, Gagne LS, Hammond SP, Gilmore ET, Joyce AC, Soiffer RJ, Marty FM. 2014. Isavuconazole treatment of a patient with disseminated mucormycosis. J Clin Microbiol 52:1016–1019. doi: 10.1128/JCM.03176-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maertens JA, Raad II, Marr KA, Patterson TF, Kontoyiannis DP, Cornely OA, Bow EJ, Rahav G, Neofytos D, Aoun M, Baddley JW, Giladi M, Heinz WJ, Herbrecht R, Hope W, Karthaus M, Lee D-G, Lortholary O, Morrison VA, Oren I, Selleslag D, Shoham S, Thompson GR, Lee M, Maher RM, Schmitt-Hoffmann A-H, Zeiher B, Ullmann AJ. 2016. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet Lond Engl 387:760–769. doi: 10.1016/S0140-6736(15)01159-9. [DOI] [PubMed] [Google Scholar]
- 12.Marty FM, Ostrosky-Zeichner L, Cornely OA, Mullane KM, Perfect JR, Thompson GR, Alangaden GJ, Brown JM, Fredricks DN, Heinz WJ, Herbrecht R, Klimko N, Klyasova G, Maertens JA, Melinkeri SR, Oren I, Pappas PG, Ráčil Z, Rahav G, Santos R, Schwartz S, Vehreschild JJ, Young J-AH, Chetchotisakd P, Jaruratanasirikul S, Kanj SS, Engelhardt M, Kaufhold A, Ito M, Lee M, Sasse C, Maher RM, Zeiher B, Vehreschild MJGT, VITAL and FungiScope Mucormycosis Investigators. 2016. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis 16:828–837. doi: 10.1016/S1473-3099(16)00071-2. [DOI] [PubMed] [Google Scholar]
- 13.Kontoyiannis DP, Selleslag D, Mullane K, Cornely OA, Hope W, Lortholary O, Croos-Dabrera R, Lademacher C, Engelhardt M, Patterson TF. 2017. Impact of unresolved neutropenia in patients with neutropenia and invasive aspergillosis: a post hoc analysis of the SECURE trial. J Antimicrob Chemother 73:757–763. doi: 10.1093/jac/dkx423. [DOI] [PubMed] [Google Scholar]
- 14.Andes DR, Ghannoum MA, Mukherjee PK, Kovanda LL, Lu Q, Jones ME, Santerre Henriksen A, Lademacher C, Hope WW. 2018. Outcomes by minimum inhibitory concentrations for patients treated with isavuconazole or voriconazole for invasive aspergillosis in the phase 3 SECURE and VITAL trials. Antimicrob Agents Chemother 63:e01634-18. doi: 10.1128/AAC.01634-18. [DOI] [PMC free article] [PubMed] [Google Scholar]


