Abstract
Mesenchymal stem cells (MSCs) have been used as a therapy to modulate diverse biological processes. To fulfill the requirements for different MSC therapies, safe and effective gene transfer methods for MSCs are critical. Calcium phosphate transfection is an inexpensive and well-described method without discernible biosafety issues; however, an optimal protocol has not been developed for MSCs. In this report, we optimized the protocol of calcium phosphate transfection for murine MSCs, and compared this protocol with other gene transfer methods in different strains of mice and in human cells. We found that transfection efficiency and cell viability showed an inverse relationship depending on serum concentration during the process of calcium phosphate transfection, in which 2% serum was chosen in the optimized protocol. The optimized protocol of calcium phosphate transfection showed a fine balance between efficiency (about 70–80%) and viability (doubling original cell number) compared to other methods. Human MSCs were more resistant to this protocol (about 30% efficiency) compared with murine MSCs. Moreover, MSC potential for osteogenesis, adipogenesis, and chondrogenesis was not affected by calcium phosphate transfection. Finally, MSCs transfected with the luciferase gene were injected into the murine distal femoral bone marrow cavity to monitor gene expression overtime in vivo. MSCs in the bone marrow environment showed extended expression of the luciferase that was transfected by calcium phosphate. This report provides an optimized protocol for calcium phosphate transfection for murine MSCs and characterizes gene overexpression in MSCs in the in vitro and in vivo environments.
Impact Statement
Mesenchymal stem cells (MSCs) are a promising tool for cell therapy, and gene-modified MSCs further expand their applications. To take full advantage of MSCs as a therapeutic approach, developing effective gene transfer methods is critical. Calcium phosphate transfection is well-established and safe, but the protocols need to be optimized according to different cell types. Currently, there is no optimized protocol for MSCs. This study optimized the protocol of calcium phosphate transfection for MSCs and highlighted the importance of serum during the process of transfection. More interestingly, the behavior of gene overexpression in MSCs in the in vivo environment was verified.
Keywords: mesenchymal stem cells, cell therapy, transfection, serum, calcium phosphate
Introduction
Mesenchymal stem cells (MSCs) are multipotent stromal cells with capabilities of self-renewal and differentiation into mesenchymal lineages such as chondrogenic, osteogenic, and adipogenic cells.1–3 MSCs can be isolated from different sources (e.g., bone marrow, adipose tissue, and dental pulp)4 and are characterized by specific surface markers (CD105, CD73, and CD90) and by the absence of hematopoietic antigens (e.g., CD34 and HLA).5 MSCs can migrate to sites of injury and inflammation to repair damaged tissue and modulate immune responses. Furthermore, the transplanted MSCs from donors do not induce host immunoreactions, which makes MSCs an ideal tool for gene and cell therapy.6,7
Indeed, genetically manipulated MSCs have been used for distinct therapeutic strategies, in which these cells function as immunomodulatory agents, the building blocks for tissue engineering, or drug carriers.2,3 Currently, more than 900 clinical trials relative to MSCs are being carried out in different human diseases, according to ClinicalTrials.gov (accessed in May 2019). Our laboratory has utilized gene-modified IL-4 overexpressing MSCs to alter immune responses, in which M2 macrophages were enhanced in bone healing studies.8,9
Effective methods of gene transfer are critical to take full advantage of MSCs as a therapeutic approach. Presently, gene delivery systems can be divided into viral transduction and nonviral transfection.10 The viral transduction systems, such as lentivirus, are more powerful gene transfer methods for MSCs, which not only reaches high transduction efficiency but also causes limited cell death.11 Lentiviruses infect MSCs efficiently and make cells overexpress genes permanently because of DNA integration, whereas adenoviruses and herpes saimiri viruses express genes in cells transiently.10 Although viral vectors are extensively used in vitro and in basic science studies, their intrinsic biosafety issues (e.g., adverse immune response, uncertainty of DNA integration, and tumorigenesis) limit their applications in the in vivo and clinical arenas.12,13
On the contrary, there are several nonviral transfection methods, such as electroporation, lipofection, calcium phosphate, and others. Electroporation enhances cell permeability to DNA by a short pulse of an intense electric field; liposomes as DNA vehicles can easily merge with the cell membrane and bring DNA into cells; calcium phosphate and DNA form a fine precipitate on cell surface to promote DNA uptake by cells.14,15 Usually the efficiency of nonviral transfection methods is lower compared to viral systems, and nonviral transfection causes more cell death in the condition of high transfection efficiency.15 Therefore, choosing and optimizing the nonviral transfection method according to cell types and the experimental goal are important for successful gene transfer.
Our laboratory is developing new MSC therapies for bone repair for future translational applications. Among the viral and nonviral gene transfer methods, we chose the calcium phosphate method to transfect MSCs because (1) there are fewer biosafety concerns in calcium phosphate transfection compared to the viral systems, (2) calcium phosphate is the cheapest and easiest transfection method to get adequate numbers of transfected MSCs within a limited time frame, and (3) transient gene expression of calcium phosphate transfection is a desired objective for our experiments. Although calcium phosphate is a well-developed transfection method, its efficiency in different cells is variable and affected by many factors, including cell characteristics, cell density, PH of the transfection solution, incubation time, DNA quality and so on.16 Currently there is no published protocol for calcium phosphate transfection that is optimized for MSCs.
In this study, we optimized the calcium phosphate transfection in murine MSCs and showed that the medium serum during the calcium phosphate transfection plays an influential role in the transfection efficiency and cell viability. We also compared different gene transfer methods in MSCs and the accessibility of different MSCs to calcium phosphate transfection. Furthermore, we found gene overexpression, transfected by calcium phosphate, in MSCs was elongated in the in vivo environment. This study provides an improved protocol of calcium phosphate transfection for murine MSCs and will enhance MSC therapy for translational applications.
Materials and Methods
Animals and cells
Eight- to 10-week-old C57BL/6J, C57BL/6J Albino, and BALB/c male mice were purchased from Jackson Laboratory (Bar Harbor, ME). Mice were hosted in Stanford Animal Facility. Stanford's Administrative Panel on Laboratory Animal Care (APLAC) approved all mouse experiments, and institutional guidelines for the care and use of laboratory animals were observed in all aspects of this project.
Murine MSCs were isolated from mouse bone marrow and cultured in alpha-minimal essential medium (α-MEM; Life Technologies, Carlsbad, CA) supplied with 10% MSC certified fetal bovine serum (FBS; Invitrogen, Carlsbad, CA) and antibiotic antimycotic solution (100 U penicillin, 100 μg of streptomycin, and 0.25 μg of Amphotericin B/mL; HyClone; Thermo Fisher Scientific, Waltham, MA) at 37°C in a CO2 (5%) incubator.
The isolation protocol of murine MSCs was described previously.17 In brief, bone marrow was collected from the femurs and tibias of C57BL/6J or BALB/c male mice. Then, the cells were carefully suspended and passed through a 70-μm strainer and cultured in complete growth medium. The culture medium was replaced the next day to remove the unattached cells (passage one). The immunophenotype of isolated MSCs (Sca1+/CD105+/CD44+/CD45−/CD34−/CD11b; BioLegend) was characterized using LSR II flow cytometer (BD Bioscience, Franklin Lakes, NJ) at passage four.8 The murine MSCs between cell passage four and eight were used in the experiments.
Human MSCs (hMSCs), which were purchased from LONZA company (Basel, Switzerland), were isolated from normal (nondiabetic) adult human bone marrow withdrawn from bilateral punctures of the posterior iliac crests of normal volunteers. These hMSCs were cultured in Mesenchymal Stem Cell Growth Medium BulletKit™ (MSCGMTM; LONZA) at 37°C in a CO2 (5%) incubator after thawing of cells according to the protocol provided by LONZA. During the calcium phosphate transfection, hMSCs were temporarily changed to α-MEM with 2% FBS and then recultured in MSCGMTM after transfection. hMSCs between cell passage three and five were used in the experiments.
Human embryonic kidney 293 cells (HEK293) were purchased from the American Type Culture Collection (ATCC, Manassas, VA) and cultured in Dulbecco's modified Eagle's medium (DMEM; Life Technologies) supplied with 10% heat-inactivated FBS (Invitrogen) and antibiotic–antimycotic solution at 37°C in a CO2 (5%) incubator. HEK293T cells were used to produce lentiviral vectors.
MSC transfection
MSCs were transfected with pMaxGFP (LONZA), pCMV-Luc, or pCDH-CMV-mBMP2-EF1α-copGFP to analyze transfection efficiency and the ability of MSC differentiation. pCMV-Luc was a gift from Ralf Kuehn (Addgene plasmid no. 45968; http://n2t.net/addgene:45968; RRID: Addgene_45968). pCDH-CMV-mBMP2-EF1α-copGFP was constructed by insertion of murine bone morphogenetic protein 2 (mBMP2) into pCDH-CMV-MCS-EF1α-copGFP (System Biosciences, Palo Alto, CA). mBMP2 was isolated from cDNA of primary osteoblasts of BALB/c mice by polymerase chain reaction. All plasmid DNA that was used in this study was amplified in the DH5α Escherichia coli strain and isolated by PureLink™ HiPure Plasmid Filter Maxiprep Kit (Invitogen).
For calcium phosphate transfection, MSCs were seeded in 10-cm2 dishes at a density of 5 × 105 cells/dish overnight and then changed to 10 mL mTeSR™1 cell culture medium (STEMCELL Technologies, Vancouver, Canada), α-MEM with 1%, 2%, 5%, or 10% FBS, or α-MEM with 0.5%, 1%, 2%, 5%, or 10% bovine serum albumin (BSA; Alfa Aelar, Haverhill, MA) before transfection.
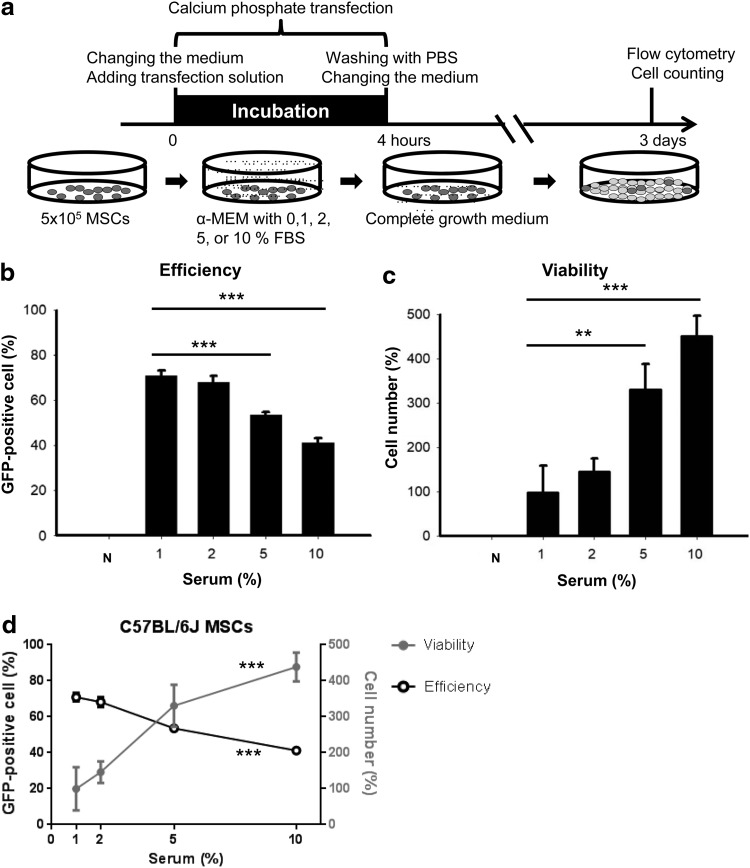
The MSCs were transfected with 40 μg plasmid DNA/dish using CalPhos™ Mammalian Transfection Kit (Clontech, Mountain View, CA) according to the manufacturer's protocol. Forty microgram DNA were mixed with 87μL 2 M calcium solution in 700 μL total volume and then added with an equal volume of 2 × HEPES-buffered saline solution dropwise. The mixture stood for 15 min and was added to the MSCs in 10-cm2 dishes. The cells were incubated in the calcium phosphate-containing medium for 4 h and then washed with phosphate-buffered saline (PBS) three times. After washing, the MSCs were cultured in fresh complete growth medium for 3 days or different time points when indicated for flow cytometry analysis and cell counting. The experimental design is summarized in Figures 1a and 5a.
FIG. 1.
The effect of serum on transfection efficiency and cell viability during the calcium phosphate transfection. (a) Experimental timeline: C57BL/6J MSCs were transfected with 40 μg pMaxGFP by calcium phosphate. The cells were incubated in the calcium phosphate-containing medium with different serum concentrations for 4 h and harvested 3 days after transfection. (b) The transfection efficiency is calculated by percentage of GFP-positive cells (n = 3). (c) The cell viability is analyzed by cell number, which is normalized to the original cell plating number (5 × 105/dish) and shown as a percentage, (n = 3). (d) Combination of the efficiency data (black) and viability data (gray). The data were analyzed by one-way ANOVA (d) followed by the Dunnett's test for multiple comparisons with the 1% serum group (b, c). **p < 0.01 and ***p < 0.001. ANOVA, analysis of variance; GFP, green fluorescent protein; MSC, mesenchymal stem cell.
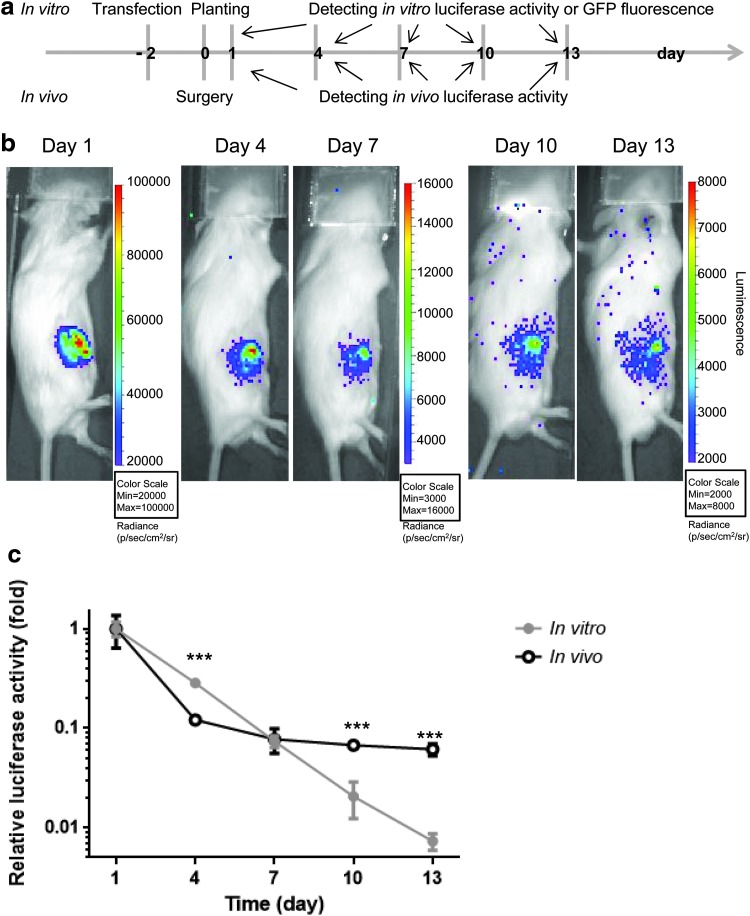
FIG. 5.
Gene overexpression of MSCs transfected by calcium phosphate in the in vivo environment. (a) Experimental design for the in vivo and in vitro luciferase activity detection. C57BL/6J MSCs were transfected with pCMV-Luc using our optimized calcium phosphate protocol and 2 days later, the cells were used for bone marrow implantation (5 × 105 cells/mouse) and seeding in six-well plate (2 × 104 cells/well). The in vivo and in vitro luciferase activities were monitored every 3 days as described in the Materials and Methods section. (b) In Vivo Imaging System intensity images of a representative mouse implanted with luciferase-expressing MSCs. The luciferase signal is seen in the right femur and decreased over time. The images at day 1, 4, 7, 10, and 13 are shown here in three different scales (unit: photon [p]/s/cm2/surface radiance [sr]). (c) Quantification of results of the in vitro (black) and in vivo (gray) luciferase activities. The relative luciferase activity is normalized to the value of the day 1 luciferase activity and shown as fold change (in vitro, n = 3; in vivo, n = 4). The data were analyzed by unpaired Student's t test. ***p < 0.001. Color images are available online.
For the calcium phosphate transfection without specifically described experimental conditions, the transfection was done using our optimal calcium phosphate protocol, in which MSCs are transfected with 40 μg DNA in 2% FBS α-MEM for 4 h during the calcium phosphate transfection process.
For lipofection, MSCs were plated in six-well plates with density of 1 × 105 cells/well overnight and were transfected with 4 μg pMaxGFP/well, which was mixed with 8 μL Lipofectamine™ 2000 (Invitrogen) at a DNA (μg) to Lipofectamine 2000 (μL) ratio of 1:2. The cells were incubated in liposome-containing medium for 4 h and then washed with PBS. After washing, the MSCs were cultured in fresh complete growth medium for 3 days for flow cytometry analysis and cell counting.
For electroporation, mouse MSCs were nucleofected using the Amaxa™ MSC cell Nucleofector™ Kit (LONZA) according to the manufacturer's protocol. In brief, 5 × 105 cells were washed with PBS buffer, mixed with 5 μg of pMaxGFP in 100 μL of MSC nucleofector solution and supplement. Cells were transfected by Nucleofection using program X-001 in AMAXA system. Cells were cultured and harvested in 3 days for flow cytometry analysis and cell counting.
The flow cytometry analysis was used to detect green fluorescent protein (GFP)-positive cells for transfection efficiency. The GFP-positive cells were determined by the untransfected cells, which were incubated in the serum-free medium instead of DNA-calcium phosphate transfection solution during the 4-h incubation time. There was no significant difference between the nontransfection and mock transfection controls (Supplementary Fig. S1). Mean fluorescence intensity was also detected by flow cytometry. Flow cytometry analysis was done in an LSRII flow cytometer (BD Bioscience) at the Stanford Shared FACS Facility. The cell number was counted using a hemocytometer for cell viability.
Preparation and infection of lentiviral vectors
The lentiviral vectors were prepared as previously described.18 HIV1-based (human immunodeficiency virus) lentiviral particles, Lt.pCDH and Lt.BMP2, pseudotyped by VSV-G (vesicular stomatitis virus spike glycoprotein) were generated from the HEK293 cells that were cotransfected with the transfer plasmid (pCDH-CMV-MCS-EF1α-copGFP or pCDH-CMV-mBMP2-EF1α-copGFP), the package plasmid (psPAX2), and the envelop plasmid (pMD2G) by calcium phosphate. The culture supernatant was collected at 48 h after transfection, and the cellular debris was removed by centrifugation.
The virus titer was determined in transduction units by using HEK293 cells. For the MSC infection experiment, MSCs were plated in the six-well plate (5 × 104 cells/well) the day before infection and were infected at different multiplicity of infection (MOI) in the viral supernatant diluted with serum-free α-MEM supplemented with 10 μg/mL of polybrene (Sigma-Aldrich, St. Louis, MO). The transduction efficiency (number of GFP-positive cells) was confirmed by LSRII flow cytometer 3 days postinfection. The cell number was counted using a hemocytometer for cell viability.
MSC differentiation assay
MSCs were transfected with pMaxGFP as previously described and then were cultured in the differentiation media 3 days later. MSCs were differentiated into adipocytes, chondrocytes, and osteoblasts according to published protocols.17 In brief, MSCs were seeded at a density of 1 × 104 per well in a 24-well plate and cultured in 500 μL control medium (α-MEM with 10% FBS) or osteo-differentiation medium (α-MEM with 10% FBS, 10−7 M dexamethasone [Sigma-Aldrich], 10 mMβ-glycerol phosphate [Sigma-Aldrich], and 50 μM ascorbate-2-phosphate [Sigma-Aldrich]) for osteoblast differentiation. The medium was changed twice per week until 28 days when cells were fixed in 4% paraformaldehyde (Sigma-Aldrich) and stained with 40 mM Alizarin Red S (Acros Organics; Thermo Fisher Scientific).
For adipogenesis analysis, MSCs were plated into a 24-well plate at a density of 2 × 104 cells/well and incubated in 500 μL control medium (α-MEM with 10% FBS) or adipo-differentiation medium (α-MEM with 10% FBS, 10−6 M dexamethasone, 0.5 μM IBMX (Sigma-Aldrich), 10 ng/mL insulin (Sigma-Aldrich)) for 2 weeks, and the medium was changed twice per week. The cells were fixed in 4% paraformaldehyde and stained with 0.5% Oil-red-O (Sigma-Aldrich) in isopropanol.
For chondrocyte differentiation, 1 million MSCs were spin down in a 15 mL polypropylene tube and cultured in 500 μL control medium (DMEM with 10% FBS) or chondrocyte-differentiation medium (DMEM with 10% FBS, 10−7 M dexamethasone, 1% insulin-transferrin-sodium selenite media supplement [Sigma-Aldrich], 50 μM ascorbate-2-phosphate, 1 mM sodium pyruvate [Life Technologies], 50 μg/mL Proline [Sigma-Aldrich], and 20 ng/mL TGF-β3 [R&D Systems, Minneapolis, MN]). The medium was changed twice per week until 21 days when cells were fixed in 4% paraformaldehyde and stained with 0.5% toluidine blue (Sigma-Aldrich).
Alkaline phosphatase activity detection
Alkaline phosphatase (ALP) activity was detected by the Alkaline Phosphatase Assay Kit (Colorimetric) (Abcam, Cambridge, UK). The assay was performed according the manufacturer's protocol. In brief, cells were homogenized in 500 μL Assay Buffer, and samples were mixed with p-nitrophenyl phosphate solution for 60 min before measurement at OD405. ALP activity was calculated by B/(▵T × V) (B, amount of p-nitrophenyl determined by standard cure (μmol); ▵T, reaction time (min); V, sample volume [mL]). Total protein was measured by Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific). The ALP activity was normalized by total protein concentration for comparison.
Bone marrow implantation
This implantation model was described previously, in which cells are implanted into the bone marrow of the mouse femur.19 C57BL/6J Albino male mice (10 weeks old) were used as the recipients of MSC implantation. The mice were placed under isoflurane inhalation anesthesia and the right distal femurs were exposed under aseptic conditions via a lateral parapatellar arthrotomy. A 25-gauge needle was used to drill an axial hole to the middle of the intercondylar region to gain access to the medullary cavity. The MSCs, transfected with pCMV-Luc by calcium phosphate, were injected (5 × 105 cells/10 μL PBS) into bone marrow cavity of the right distal femur using a Hamilton microsyringe. The mice were euthanized 3 weeks postoperation. The study design is summarized in Figure 5a.
Quantification of implanted MSCs in vivo
The viability of the injected luciferase reporter MSCs was evaluated by IVIS In Vivo Imaging System (PerkinElmer, Waltham, MA) 1 day after the cell injection and then every 3 days for 2 weeks (Fig. 5a). Luciferase substrate D-Luciferin (Caliper Life Sciences, Waltham MA) was administered by intraperitoneal injection (150 mg/kg) 10 min before imaging. The data were analyzed using Living Image Software (PerkinElmer) and presented as the bioluminescence (recorded as photon [p]/s/cm2/surface radiance [sr]) in the regions of interest drawn over the injected femurs.
In vitro luciferase assay
C57BL/6J MSCs were transfected with pCMV-Luc by calcium phosphate as described in the “MSC transfection” section. Cells were plated in six-well plates (2 × 104 cells/well) 2 days after transfection and were harvested every 3 days (Fig. 5a). Total protein was measured by Pierce BCA Protein Assay Kit, and luciferase activity was analyzed using Luciferase Assay System (Promega, Madison, WI). The manufacturer's protocols were followed carefully. The luciferase activity was normalized by total protein concentration for comparison.
Statistical analysis
One-way analysis of variance (ANOVA) with the Dunnett's test was performed for data with three or more groups, and nonpaired Student's t tests were performed for data with two groups. The statistical analysis was performed using Prism 7 (GraphPad Software). Data are reported as mean ± standard deviations. p < 0.05 was chosen as the threshold of statistical significance.
Results
Serum in the medium reduces transfection efficiency and enhances cell viability during the process of calcium phosphate transfection
The amount of DNA and the incubation time were tested and optimized in our protocol and found to be 40 μg per dish and 4 h of incubation (data not shown). More interestingly, we found that the transfection efficiency and cell viability were also affected by the FBS concentration in the medium during the incubation period of calcium phosphate transfection. Figure 1a shows the experimental design of this in vitro experiment. During the incubation period, MSCs were cultured in media with different serum concentrations (negative control [serum free], 1%, 2%, 5%, and 10%). We found that the medium serum concentration reduced transfection efficiency (Fig. 1b) and protected cells from transfection toxicity (Fig. 1c) in a dose-dependent manner. More GFP-positive cells and fewer cell numbers were counted in the low serum medium, and vice versa.
One exception was the negative control group, in which all the MSCs died (Fig. 1c). Cell death of the negative control group may be due to imbalance of osmotic strength; in other words, the different concentrations of BSA protected the MSCs from death (Supplementary Fig. S2). When we combined both the results of Figure 1b and 1c, we found that the transfection efficiency and cell viability showed an inverse relationship that was dependent on serum concentration (Fig. 1d).
Instead of using serum during the calcium phosphate transfection process, we further tested the serum-free complete cell culture medium, mTeSR1, which is widely used for human embryonic stem cells and induced pluripotent stem cells, and found that the efficiency (40–50%) and cell viability (60–70%) were not as good as when using serum (Supplementary Fig. S3).
Accordingly, we decided to use 2% FBS medium during calcium phosphate transfection in our optimal protocol, in which there were high transfection efficiency (about 70–80%) and high cell viability (doubling of the original cell number). In addition, the transfection efficiency and cell viability of MSCs transfected using this optimal calcium phosphate protocol at different time points (24, 48, and 72 h) are also shown here. We found circular, floating dead cells at 24 h after transfection when the cell number was reduced (Supplementary Fig. S4). Cell number was increased after 24 h, which means that cells recovered from the damage of transfection. The GFP-positive cells increased consistently until day 3 (72 h).
Calcium phosphate transfection displays mild cell toxicity and good efficiency in MSCs compared with other transfection methods
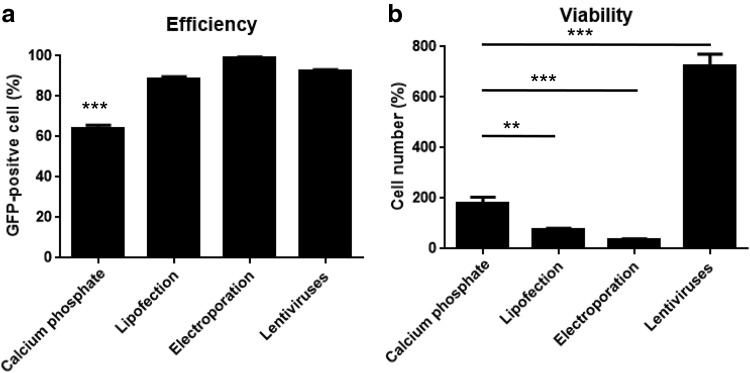
After optimizing the protocol of calcium phosphate transfection in MSCs, we determined the effects and cell viability of different gene transfer methods in MSCs. In this study, we compared our calcium phosphate protocol with electroporation, lipofection, and the lentiviral system. MSCs were transfected or infected and were analyzed 3 days later. Both lipofection and electroporation displayed high transfection efficiency (more than 80% GFP-positive cells) and high cell toxicity, so the cell numbers in both groups was fewer than the original plating cell number (fewer than 100%) after 3 days of culture (Fig. 2).
FIG. 2.
Comparison of different gene transfer methods in MSCs. C57BL/6J MSCs were transfected with pMaxGFP or infected with Lt.pCDH at MOI = 200 as described in the Materials and Methods section at day 0 and then harvested at day 3 for flow cytometry and cell counting. (a) The transfection efficiency is calculated by the percentage of GFP-positive cells (n = 3). (b) The cell viability is analyzed by cell number, which is normalized to the original cell plating number and shown as percentages (n = 3). The data were analyzed by one-way ANOVA followed by the Dunnett's test for multiple comparisons with calcium phosphate group. **p < 0.01 and ***p < 0.001. MOI, multiplicity of infection.
Lentivirus infection showed the best result of more than 90% MSCs expressing GFP without significant cell death, and cell number increased dramatically in this group even when we used high MOI (200) (Fig. 2). The transduction efficiency and cell viability at different MOI are also shown (Supplementary Fig. S5).
MSCs transfected with calcium phosphate using our optimized protocol showed more than 60% cells expressing GFP and good cell viability, and the cell number was twice the original cell number. These data indicated that calcium phosphate transfection using our optimized protocol displayed good efficiency and proliferative ability compared to other nonviral transfection methods (Fig. 2).
Murine MSCs are highly transfectable by calcium phosphate transfection
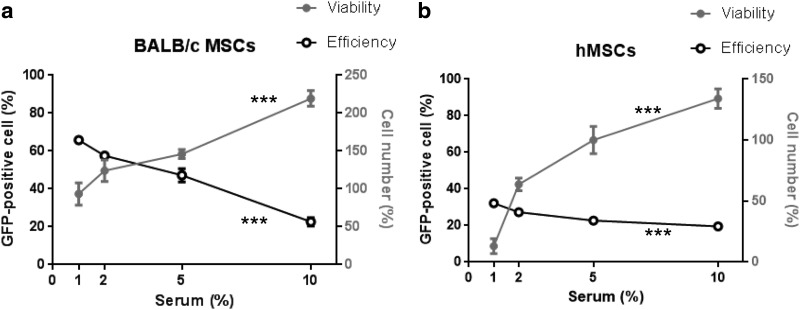
Our previous experiments were performed using MSCs from C57BL/6J mice; here, we examined the effects of calcium phosphate transfection in MSCs from other murine strains. Figure 3a shows that the transfection efficiency and cell viability displayed an inverse relationship with serum concentration when BALB/c MSCs were transfected by calcium phosphate, which was similar to the data using C57BL/6J MSCs (Fig. 1d). These results support the concept that the murine MSCs were highly transfectable by calcium phosphate.
FIG. 3.
Calcium phosphate transfection in murine MSCs and human MSCs. BALB/c MSCs (a) and hMSC (b) were transfected with pMaxGFP by calcium phosphate as described in the Materials and Methods section and were analyzed 3 days after transfection. BALB/c MSCs and hMSCs were transfected at different serum concentrations. The efficiency is calculated by percentage of GFP-positive cells (n = 3), and the viability is shown as the percentage of cell number and normalized to the original cell plating number (5 × 105/dish) (n = 3). The data were analyzed by one-way ANOVA. ***p < 0.001. hMSC, human MSC.
In addition, we transfected hMSCs with pMaxGFP by calcium phosphate in different serum concentration conditions. The result displayed transfection efficiency and cell viability in an inverse relationship with respect to serum concentrations, but lower transfection efficiency and more cell death compared to murine MSCs (Fig. 3b), which suggested that hMSCs were more resistant to calcium phosphate transfection compared to murine MSCs.
MSC multipotency is maintained after calcium phosphate transfection
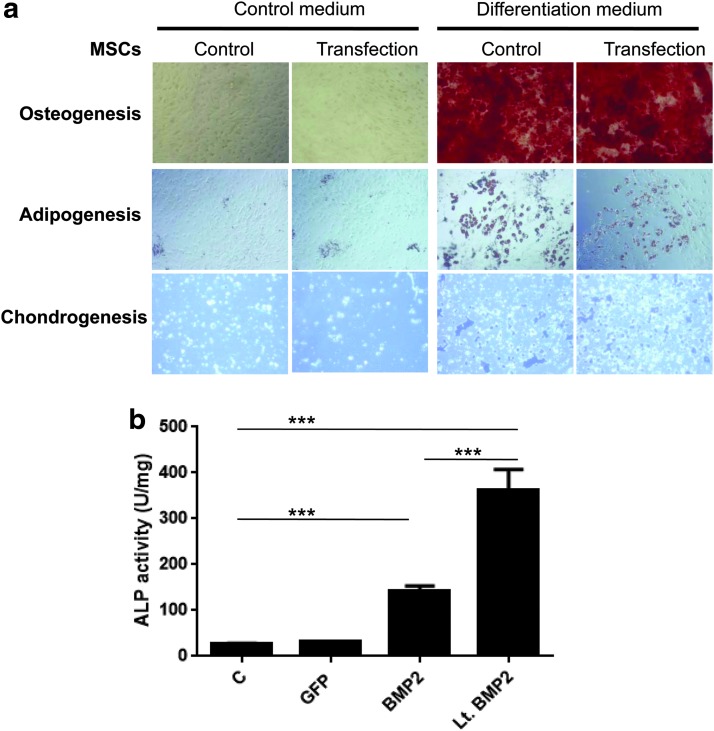
The effect of calcium phosphate transfection on MSC differentiation was investigated. After incubation in different differentiation media, MSCs transfected with pMaxGFP by calcium phosphate and MSCs without transfection, both differentiated into osteoblasts, adipocytes, and chondrocytes (Fig. 4a). We did not find any difference between the two groups (with or without transfection). In addition, ALP activity, which is a marker of osteogenesis, was significantly enhanced by overexpressing murine BMP2 protein in MSCs that transfected by calcium phosphate or infected by the lentiviral vector (Fig. 4b). In summary, calcium phosphate transfection did not affect the potential for MSC differentiation.
FIG. 4.
Osteogenic, adipogenic, and chondrogenic differentiation of the transfected MSCs or control MSCs. (a) C57BL/6J MSCs were transfected with pMaxGFP (transfection) or not (control) at day 0 and, at day 3, incubated in appropriate differentiation media for osteogenesis, adipogenesis, and chondrogenesis as described in the Materials and Methods section. The mature osteoblasts with mineralization were stained with Alizarin Red S and show as red color; the lipidic vacuoles of adipocytes were stained with Oil-red-O and show as red color; the chondrocytes were stained with toluidine blue and show as dark blue. Original magnification, 100 × for osteoblasts and adipocytes; 200 × for chondrocytes. (b) C57BL/6J MSCs were infected with Lt.BMP2 at MOI = 100 (Lt.BMP2), or transfected with pMaxGFP (GFP), pCDH-CMV-mBMP2-EF1α-copGFP (BMP2), or not (control) at day 0. Cells were cultured in the normal growth medium and harvested at day 3 for measurement of ALP activity (U = μmol/min; n = 3) as described in the Materials and Methods section. The cells infected with Lt.BMP2 were used as a positive control. The data were analyzed by one-way ANOVA followed by the Dunnett's test for multiple comparisons. ***p < 0.001. ALP, alkaline phosphatase; BMP2, bone morphogenetic protein 2. Color images are available online.
Gene overexpression in MSCs is extended in the bone marrow environment
Finally, we examined the time frame of gene overexpression in MSCs transfected by calcium phosphate in the in vivo environment. We used an in vivo model in which the luciferase-expressing MSCs were implanted into the bone marrow of the mouse femur. Figure 5a shows the experimental design of this in vivo experiment. In vitro cultured MSCs, which were also transfected with pCMV-Luc or pMaxGFP, functioned as controls.
Figure 5b shows a representative mouse, in which the in vivo luciferase activity decayed overtime. When comparing the in vivo and in vitro data, we found both luciferase signals deceased overtime; the in vivo signal reduced sharply earlier (by day 4) and then slowly (day 7, 10, and 13); the in vitro luciferase signal diminished consistently (Fig. 5c). The values of the in vitro luciferase activity are far greater than background signal, even at day 13 (more than 27 times higher) (Supplementary Fig. S6).
The GFP-positive cells in the in vitro experiment began to decrease after day 4 (day 6 after transfection); the in vitro GFP fluorescence signal dropped quickly at first 7 days and then more slowly when the fluorescence intensity was close to MSC autofluorescence at day 7, which means that the detection limitation of fluorescence signal was reached after 7 days (Supplementary Fig. S7).
The in vivo luciferase signal could be detected until day 20 (data not shown), while our previous data indicated that the in vivo luciferase signal from lentivirus-transduced luciferase-expressing MSCs can be detected in bone marrow until 28 days.8 Thus, our data demonstrated that gene overexpression in MSCs by calcium phosphate transfection was retained for an extended period in the in vivo bone marrow environment.
Discussion
The aim of the present study was to develop and optimize a protocol of calcium phosphate transfection in MSCs for potential translational applications. One previous study showed that calcium phosphate transfection in Chinese hamster ovary cells is more efficient in DMEM with FBS (10%) compared with DMEM alone.20 In this study, we further showed that serum in the medium enhances MSC viability, while serum-free medium causes almost complete cell death during the process of transfection (Fig. 1c); furthermore, serum concentration decreases transfection efficiency in a dose-dependent manner (Fig. 1b).
There are several reasons accounting for the effect of serum. One possibility is that there are numerous growth factors and proteins in serum, which may facilitate cell survival during the transfection process. For example, the major component in the serum is albumin, which has been shown to protect cells from the stimulation of the extracellular environment.21 Our data indicated that BSA enhanced cell survival during calcium phosphate transfection, but did not display a dose-dependent relationship (Supplementary Fig. S2), which means that BSA is just one of the factors protecting cell viability. In addition, BSA cannot enhance MSC transfection efficiency even at a low concentration (0.5%) (Supplementary Fig. S2), which means that BSA is only one of the factors affecting transfection efficiency.
Another possible reason is that serum may affect the precipitation of calcium phosphate and DNA. The reduced precipitation diminishes not only the chance of DNA uptake by cells but also the toxicity of transfection. Indeed, we found that precipitation was decreased in the medium with increased concentration of serum during calcium phosphate transfection (data not shown). The detailed mechanisms, how serum influences cell viability and the precipitation of calcium phosphate during the transfection process, need further investigation.
We developed this protocol of calcium phosphate transfection to obtain sufficient genetically modified MSCs within a reasonable short time for later translational in vivo applications. Although one might surmise that in vitro testing could be performed in small amounts (e.g., six-well plate) and then simply scaled up, the results of scaling-up experiments do not always meet the expectations. Therefore, all of our calcium phosphate transfections, even during the stage of optimizing transfection conditions, were performed in 10-cm2 dishes (5 × 105 MSCs/dish).
Other transfection methods, such as electroporation and lipofectin, can also be accomplished using large numbers of cells, but both methods induce more cell death (Fig. 2b), which may result in inadequate cell numbers for subsequent experiments and applications. Moreover, electroporation and lipofectin are far more expensive than calcium phosphate transfection.
Based on the data (Fig. 3b), hMSCs are less easily transfected by calcium phosphate. Our protocol of calcium phosphate transfection was initiated in C57BL/6J MSCs. Although this protocol is also useful for MSCs from BALB/c mice, the difference between mouse and human MSCs appears to be significant. To improve the effect of calcium phosphate transfection in hMSCs, our current protocol would need to be further optimized.
There are several modified calcium phosphate transfection methods that have been published, in which chemicals were added during the preparation process of the calcium phosphate transfection solution.22,23 These chemicals, such as chitosan and hyaluronic acid, can stabilize and make the formation of calcium phosphate particles more uniform, which enhances cell uptake and reproducibility of transfection efficiency. Our protocol, combined with these modified calcium phosphate transfection methods, may result in improved transfection efficiency in hMSCs. Furthermore, biological agents such as chloroquine and cysteine protease inhibitors may be useful for improving the transfection efficiency.24,25
The therapeutic effect of MSCs are highly dependent on their regenerative and immunomodulatory abilities, which are compromised by MSC senescence.26 Cell manipulation or gene transfection may cause some cell death, which increases the number of cell population doubling (NCPD) of MSCs and results in MSC senescence and aging. For example, transfection by electroporation and lipofectin caused significant MSC death (Fig. 2b). Therefore, measures to prevent MSC death during MSC expansion and manipulation are very important for keeping their therapeutic potential and safety in clinical use.
Our optimized protocol of calcium phosphate transfection significantly reduced the death of MSCs. In addition, our data showed that transfected MSCs, using the optimized protocol of calcium phosphate transfection, can differentiate into osteoblast, adipocytes, and chondrocytes normally compared with the control group (Fig. 4). These results suggest that calcium phosphate transfection does not affect MSC differentiation and that the optimized protocol does not increase NCPD of MSCs and is therefore suitable for MSC manipulation.
When the foreign plasmids do not integrate into the genomic DNA of the cells, the plasmids, transfected by transient transfection, are lost overtime in cells due to DNA degradation, cell division, or other factors. Originally, we thought that the luciferase signal in our bone implantation experiments would disappear quickly because of the transient nature of the transfection and the short survival period of MSCs in the in vivo environment27,28; surprisingly, the transplanted transfected MSCs, which survived in the bone marrow, expressed luciferase longer compared to the cells that were cultured in vitro (Fig. 5).
A previous study showed that the implanted MSCs lined the surface of the endosteum in the distal femur,8 where cells may stay in a quiescent state without proliferation or death, so that they can keep expressing the plasmid and extend the gene expression. In addition, MSCs comprise only 0.01–0.001% of bone marrow cells in human.29 Once MSCs are isolated and cultured in vitro, they present a high capacity of proliferation and expand quickly, which suggests that there are factors holding MSCs quiescent in the environment of bone marrow. Our results suggest that transient gene overexpression in MSCs had a longer effect in the in vivo environment, which could be an advantage of MSCs compared with other methods of cell therapy.
We have optimized the protocol of calcium phosphate transfection in murine MSCs. With this protocol, we can generate enough genetically manipulated MSCs in a reasonable time for subsequent experiments or therapies. Genes that are transiently expressed in MSCs, transfected by the calcium phosphate method, can be used as a short-term enhancer for tissue repair or for temporal immune modulation. For example, our laboratory reported the use of IL-4-expressing MSCs to enhance M2 macrophage polarization for facilitating bone healing.8,9 Transient overexpression of IL-4 by transfected MSCs could potentially mitigate adverse immune responses.
In summary, this study provides an evidence-based protocol for calcium phosphate transfection of murine MSCs and characterized the time-course of these MSCs in an in vivo environment. This information is useful for experiments using genetically engineered MSCs in mice and potentially for future translational applications.
Supplementary Material
Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by a SPARK at Stanford University and NIH grants 1R01AR063717, R01AR073145, R01AR072613, and the Ellenburg Chair in Surgery at Stanford University.
Supplementary Material
References
- 1. Charbord P. Bone marrow mesenchymal stem cells: historical overview and concepts. Hum Gene Ther 21, 1045, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fayaz H.C., Giannoudis P.V., Vrahas M.S., et al. The role of stem cells in fracture healing and nonunion. Int Orthop 35, 1587, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Perez J.R., Kouroupis D., Li D.J., Best T.M., Kaplan L., and Correa D. Tissue engineering and cell-based therapies for fractures and bone defects. Front Bioeng Biotechnol 6, 105, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Millan C., Vivanco J.F., Benjumeda-Wijnhoven I.M., Bjelica S., and Santibanez J.F. Mesenchymal stem cells and calcium phosphate bioceramics: implications in periodontal bone regeneration. Adv Exp Med Biol 1107, 91, 2018 [DOI] [PubMed] [Google Scholar]
- 5. Dominici M., Le Blanc K., Mueller I., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Poggi A., Varesano S., and Zocchi M.R. How to hit mesenchymal stromal cells and make the tumor microenvironment immunostimulant rather than immunosuppressive. Front Immunol 9, 262, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baksh D., Song L., and Tuan R.S. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med 8, 301, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin T., Pajarinen J., Kohno Y., et al. Transplanted interleukin-4—secreting mesenchymal stromal cells show extended survival and increased bone mineral density in the murine femur. Cytotherapy 20, 1028, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin T., Pajarinen J., Nabeshima A., et al. Establishment of NF-kappaB sensing and interleukin-4 secreting mesenchymal stromal cells as an “on-demand” drug delivery system to modulate inflammation. Cytotherapy 19, 1025, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nayerossadat N., Maedeh T., and Ali P.A. Viral and nonviral delivery systems for gene delivery. Adv Biomed Res 1, 27, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van Damme A., Thorrez L., Ma L., et al. Efficient lentiviral transduction and improved engraftment of human bone marrow mesenchymal cells. Stem Cells 24, 896, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Baldo A., van den Akker E., Bergmans H.E., Lim F., and Pauwels K. General considerations on the biosafety of virus-derived vectors used in gene therapy and vaccination. Curr Gene Ther 13, 385, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Collins D.E., Reuter J.D., Rush H.G., and Villano J.S. Viral vector biosafety in laboratory animal research. Comp Med 67, 215, 2017 [PMC free article] [PubMed] [Google Scholar]
- 14. Li S., and Huang L. Nonviral gene therapy: promises and challenges. Gene Ther 7, 31, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Ramamoorth M., and Narvekar A. Non viral vectors in gene therapy—an overview. J Clin Diagn Res 9, Ge01, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kingston R.E., Chen C.A., and Rose J.K. Calcium phosphate transfection. Curr Protoc Mol Biol, 2003; DOI: 10.1002/0471142727.mb0901s63 [DOI] [PubMed] [Google Scholar]
- 17. Zhu H., Guo Z.K., Jiang X.X., et al. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat Protoc 5, 550, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Pajarinen J., Lin T.H., Sato T., et al. Establishment of green fluorescent protein and firefly luciferase expressing mouse primary macrophages for in vivo bioluminescence imaging. PLoS One 10, e0142736, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takada K., Inaba M., Ichioka N., et al. Treatment of senile osteoporosis in SAMP6 mice by intra-bone marrow injection of allogeneic bone marrow cells. Stem Cells 24, 399, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Guo L., Wang L., Yang R., et al. Optimizing conditions for calcium phosphate mediated transient transfection. Saudi J Biol Sci 24, 622, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Francis G.L. Albumin and mammalian cell culture: implications for biotechnology applications. Cytotechnology 62, 1, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee J.E., Yin Y., Lim S.Y., et al. Enhanced transfection of human mesenchymal stem cells using a hyaluronic acid/calcium phosphate hybrid gene delivery system. Polymers (Basel) 11, E798, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Choi B., Cui Z.K., Kim S., Fan J., Wu B.M., and Lee M. Glutamine-chitosan modified calcium phosphate nanoparticles for efficient siRNA delivery and osteogenic differentiation. J Mater Chem B 3, 6448, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luthman H., and Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res 11, 1295, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Coonrod A., Li F.Q., and Horwitz M. On the mechanism of DNA transfection: efficient gene transfer without viruses. Gene Ther 4, 1313, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Yin J.Q., Zhu J., and Ankrum J.A. Manufacturing of primed mesenchymal stromal cells for therapy. Nat Biomed Eng 3, 90, 2019 [DOI] [PubMed] [Google Scholar]
- 27. Li L., Chen X., Wang W.E., and Zeng C. How to improve the survival of transplanted mesenchymal stem cell in ischemic heart? Stem Cells Int 2016, 9682757, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ozeki N., Muneta T., Koga H., et al. Not single but periodic injections of synovial mesenchymal stem cells maintain viable cells in knees and inhibit osteoarthritis progression in rats. Osteoarthritis Cartilage 24, 1061, 2016 [DOI] [PubMed] [Google Scholar]
- 29. Pittenger M.F., Mackay A.M., Beck S.C., et al. Multilineage potential of adult human mesenchymal stem cells. Science 284, 143, 1999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.