Abstract
Genetically encoded fluorescent and luminescent indicators have revolutionized our ability to monitor physiology in real time, but the separate development of new sensors for each of these imaging modalities involves substantial effort and resources. Methods to rapidly engineer multimodal sensors would, therefore, significantly accelerate the diversification of sensors for simultaneous use in different systems and applications. We hypothesized that the enhanced Nano-lanterns could be incorporated into modular ratiometric sensors as an efficient approach to creating dual-mode fluorescent-luminescent sensors. As a proof-of-concept, we engineered an Epac1-based sensor that responds to cyclic adenosine monophosphate binding with a greater than 80% change in both Förster Resonance Energy Transfer and bioluminescent resonance energy transfer (BRET) modes. We also demonstrate that our new sensor reports cellular changes in G-protein-coupled signaling, and that the ratiometric BRET mode is bright enough for subcutaneous measurements in mice.
Introduction
Genetically encoded indicators (GEIs) have been engineered to sense a diverse array of cellular signals by converting a change in the target molecule concentration to a change in the fluorescent or bioluminescent properties of the GEI.1,2 These sensors confer a large spatiotemporal advantage over previous techniques for interrogating molecular signaling events since they can read out dynamics in real time in genetically targeted cell types and subcellular compartments. GEIs, thus, enable the study of these molecules from specific contexts in vivo to subcellular signaling microdomains in vitro.1,2
A common GEI design strategy is to fuse a pair of resonance energy transfer (RET)-compatible proteins across the termini of a protein that naturally binds the molecule of interest.1,3 Upon binding the target molecule, the protein undergoes a conformational change that alters either the distance or orientation of the RET pair with respect to each other, changing the RET efficiency, and, therefore, the light output of the RET pair. This approach has the advantage that the ratio of the donor and acceptor emissions provides a metric for the target molecule concentration that is independent of the sensor expression level.
Given the significant effort required to engineer new GEIs, we sought a method for rapidly improving existing sensors and extending their use to new systems. For example, fluorescence-based sensors have found extensive application in examining subcellular signaling mechanisms in vitro, but in vivo their application is limited to use in surface tissues due to the requirement for multiphoton excitation.1,2 Similarly, bioluminescence-based sensors have been successfully applied toward in vitro drug screening for their low background,4,5 but their application in vivo is limited by the low light emission from traditional luciferases.6 NanoLuc, a new, exceptionally bright shrimp luciferase, has the potential to overcome this limitation for luminescent sensors in vivo.6,7 However, despite having spectral properties similar to other luciferases, its incorporation into existing sensors is not always straightforward.8
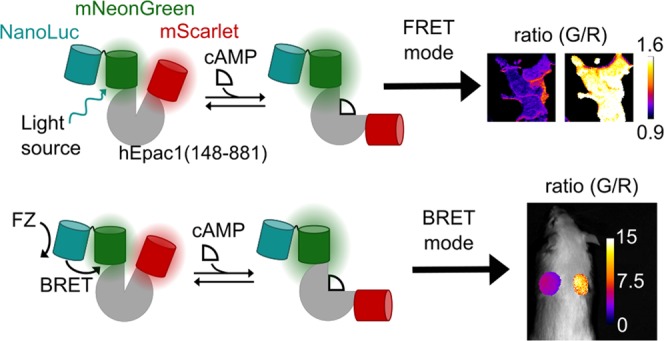
One advancement that may aid the incorporation of NanoLuc into sensors is the recent development of “Enhanced Nano-lanterns” (eNLs).9 eNLs are engineered protein fusions between NanoLuc and one of several fluorescent proteins (FPs) in such a way that NanoLuc undergoes constitutive bioluminescent RET (BRET) with the FP.9 These constructs, therefore, red-shift NanoLuc emission from the autoluminescence of its substrate, further reducing the background signal, and several eNLs have a brightness greater than NanoLuc alone. Since the eNL maintains the fluorescent capabilities of the original FP, we reasoned that incorporating eNLs into existing Förster resonance energy transfer (FRET) sensor designs could be an effective method for rapidly generating dual-mode sensors capable of FRET- and BRET-based reporting. These sensors would, therefore, overcome the brightness limitations of in vivo bioluminescence studies while retaining FRET capabilities for use in subcellular studies on traditional fluorescence microscopes.
As a proof-of-concept, we chose to engineer an eNL-based FRET–BRET cyclic adenosine monophosphate (cAMP) sensor because of the central importance of cAMP as a second messenger of G-protein signaling following G-protein-coupled receptor (GPCR) activation.10 The relevance of cAMP dynamics is underscored by previous efforts to develop fluorescence- and bioluminescence-based sensors for measuring its dynamics.11−13 For example, cAMP levels are a preferred metric for G-protein-dependent signaling in opioid receptors (ORs),14 and the balance of G-protein dependent to independent signaling at these receptors has behavioral effects that are guiding drug development efforts.15 Thus, creating a bright dual-mode FRET- and BRET-capable sensor for detecting OR G-protein signaling that is scalable from in vitro screening to in vivo mechanism validation could accelerate these efforts.
In this work, we demonstrate that eNLs are capable of modular inclusion into a previously designed ratiometric cAMP sensor architecture, with the similar dynamic range as the original sensor. In doing so, we give a proof-of-concept that eNLs can be used to make dual-mode sensors capable of operating in either FRET or BRET modes. We show that our sensor responds to cAMP changes in both modes in intact cells, and that in the BRET mode, the ratio remains constant even as overall luminescence decays from the NanoLuc (NL) substrate turnover. Finally, we show that our sensor is bright enough to ratiometrically report cAMP through tissue and fur in a mouse.
Results
Design and Screen for a Ratiometric, Dual-Mode cAMP Sensor
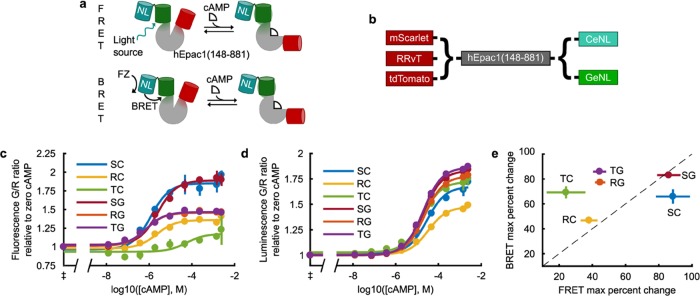
To generate a dual-mode cAMP sensor, we chose to re-engineer the FRET-based cAMP sensor, Indicator of cAMP using Epac 3 (ICUE3).11 ICUE3 is a fusion protein of a fragment of the cAMP-binding protein, exchange protein activated by cAMP 1 (Epac1), with CFP and a circularly permuted mVenus at its N- and C-termini, respectively. Since the FP in each eNL is at its N-terminus, we reasoned that an efficient approach would be to replace the C-terminal FP in ICUE3 with an eNL and the N-terminal FP with a RET-compatible red FP (Figure 1a). The molecular “reporting” should, therefore, still primarily occur from RET between the two FPs on either side of Epac1. Thus, only one interaction needs to be optimized, whether we supply excitation energy through the NanoLuc substrate in the BRET mode or we excite the FP in the eNL directly to operate in the FRET mode. We designed six constructs (Figure 1b), testing three alternative bright red fluorescent proteins at the N-terminus of Epac1 and two RET-compatible eNL variants at its C-terminus. To directly assess the maximal cAMP response of each construct, we first measured their cAMP dose–response curves (Figure 1c,d). Epac1-based sensors are difficult to purify from bacteria, as they are readily cleaved in this expression system.13 Though Jiang et al.12 report that purifying their sensor with a 6xHis tag, we could not replicate their results due to cleavage at multiple sites between the fluorescent domains, observable via sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel, even when using their buffers, protease inhibitors, or a low-temperature prep. Other groups have successfully measured Epac1-based sensor responses in the cell lysate and found the resulting EC50 values to be similar to that of purified protein.13,16,17 Therefore, we chose to characterize our sensors in the diluted HEK293 cell lysate. We observed that the switch architecture is very robust to the choice of protein, and nearly every construct responds in at least one optical detection mode. Interestingly, the maximum percent change for each construct is not identical for both modes, with many performing better in the BRET mode than in the FRET mode (Figure 1e). Since RRvT and tdTomato are dimers,18 it may be that the second FP is able to accept RET in the unbound state in the FRET mode, but that in the BRET mode, direct RET from NanoLuc to the red FP compensates for this effect.
Figure 1.
Screen for a dual-mode FRET and BRET cAMP sensor. (a) Diagram showing how a dual-mode FRET and BRET sensor using eNL could function. Excitation can be supplied from direct excitation of FP in the eNL to operate via FRET or through the NanoLuc (NL) bioluminescence in the presence of furimazine (FZ) to operate via BRET. (b) Constructs screened for the construction of the sensor. Cyan (CeNL) and green (GeNL) eNLs incorporate an mTurqoise2 or mNeonGreen, respectively. (c, d) Dose–response curves of sensors in the HEK cell lysate in FRET (c) and BRET (d) modes (mean ± σ, n = 3). ‡Measurements at 0 cAMP put at 10–10 M cAMP for fitting and plotting. (e) Maximum percent change in ratio of each construct of the fits in (c, d) plotted against each other. The dashed line is y = x and data is given as mean ± 95% c.i., n = 3. Construct abbreviations (see (b) also): S, mScarlet; R, RRvT; T, tdTomato; C, CeNL (mTurquoise2-NL); G, GeNL (mNeonGreen-NL).9
We also observed that although the EC50 values for both modes are near the 8.8–12.5 μM published range for Epac1-based sensors,12,17 the constructs each had higher EC50 values in the BRET mode (Supporting Information (SI) Table 1). For example, mScarlet-Epac-GeNL has a mean (95% c.i.) EC50 of 2.5 (1.64, 3.92) μM in FRET but 19.8 (17.2, 22.8) μM in BRET (SI Table 1). In the BRET mode, the NanoLuc must turn over the substrate, setting up a linked equilibrium with the cAMP-binding reaction that can be affected by intramolecular interactions within the protein in the cAMP-bound and -unbound states. The differences in filters and instrumentation between the measurements may also contribute to the range in EC50 values. Regardless of these differences, the affinities in either mode are still well-tuned to detect fluctuations in physiological levels of cAMP. To evaluate this, we continued the characterization of our highest performing sensor in cells.
Overall, the mScarlet-Epac-GeNL construct performed best in both BRET and FRET modes, achieving ∼80% maximum signal change in each. This is comparable to previous cAMP sensors and is only a 20% difference from the original ICUE3,11 confirming that our approach is an effective method for adding functionality to FRET-based sensors. Evaluating the fluorescence and bioluminescence spectra for mScarlet-Epac-GeNL, we observed the expected changes upon cAMP binding, namely, a decrease in emission from mScarlet at 594 nm and an increase in emission from mNeonGreen at 517 nm (SI, Figure 1). These data together demonstrate that mScarlet-Epac-GeNL is a dual-mode, ratiometric cAMP sensor.
Live-Cell Validation of mScarlet-Epac-GeNL
We next sought to validate the function of both modes of our sensor with live-cell imaging. In HEK cells stably expressing the κ-opioid receptor (κOR), the fluorescence emission ratio faithfully increases in response to forskolin (FSK), a drug that stimulates cAMP production by directly activating adenylyl cyclase (Figure 2). This change is reversed upon addition of the endogenous κOR agonist dynorphin, which stimulates Gαi through κOR to inhibit adenylyl cyclase. The maximum change in cAMP observed using the mScarlet-Epac-GeNL sensor is approximately 44%, comparable to that for ICUE3.11 This suggests that inserting the eNL into the ICUE3 architecture largely preserves the sensor’s FRET capabilities and confirms that the FP in the eNL is a viable donor for FRET-based reporting of cAMP.
Figure 2.
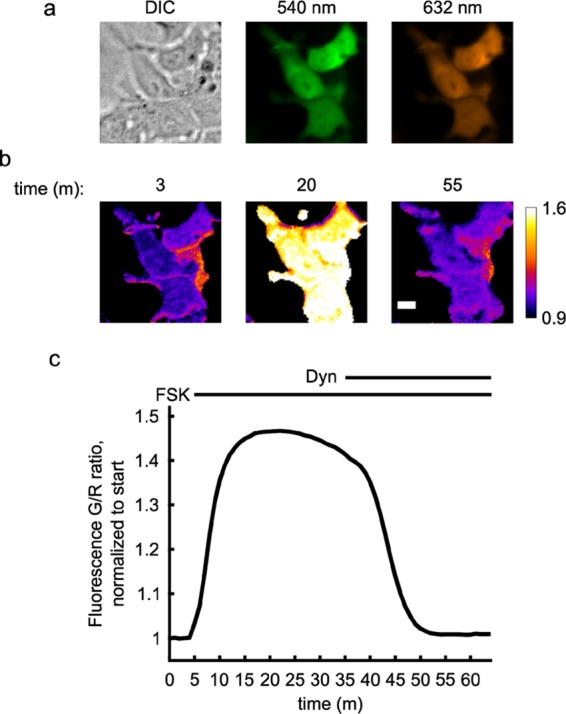
mScarlet-Epac-GeNL responds to changes in cellular cAMP in the FRET mode. HEK293 cells stably expressing κ-opioid receptor (κOR) were transiently transfected with mScarlet-Epac-GeNL and imaged in the FRET mode. (a) Images of a representative group of cells showing that cells are healthy and have an even distribution of the sensor in the cytosol. (b, c) After 5 min, cells were treated with 50 μM forskolin (FSK), which activates adenylyl cyclases and stimulates cAMP production. After 30 min with FSK, 9 nM dynorphin (dyn), the endogenous agonist to κOR, was added, leading to the inhibition of cellular adenylyl cyclase and cellular cAMP returns to previous levels. (b) Ratio images of HEK293 cells in (a) undergoing treatment at different time points (scale bar = 10 μm). (c) Quantification of data (mean ± σ, n = 3) of three independent wells with 20 cells each.
We similarly sought to validate the BRET functionality of mScarlet-Epac-GeNL in living cells. We seeded HEK293A cells in a 96-well plate and imaged the well population in the presence of the NanoLuc substrate furimazine using a Spectral Instruments Ami HT animal imager designed for bioluminescence quantification. We find that the cell population shows a ratiometric change of approximately 29% in response to adenylyl cyclase stimulation by FSK (Figure 3a). The BRET response is similar to the change seen in the FRET mode (Figure 2), validating that mScarlet-Epac-GeNL can be used as a dual-mode sensor in cells. Importantly, the ratio of the green-to-red emission holds constant even as the luminescence signal from both channels decays as furimazine is turned over by NanoLuc (Figure 3b). This result underscores the consistency of our ratiometric design and suggests that this feature will allow for better comparisons between samples and trials.
Figure 3.
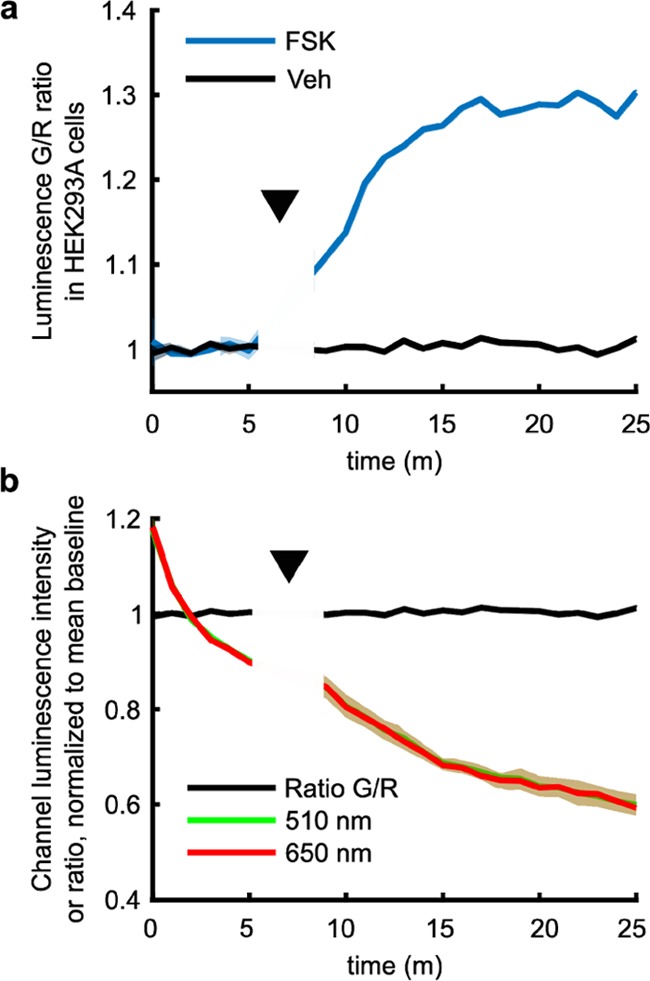
mScarlet-Epac-GeNL reports cAMP changes in cells in the BRET mode. (a) HEK293A cells seeded into a 96-well plate and transiently transfected with mScarlet-Epac-GeNL respond to 50 μM forskolin (FSK) addition. (b) Vehicle (Veh) data in (a) is plotted with the channel intensity values to show that the ratio remains constant even as intensity drops dramatically due to consumption of FZ. Traces in both plots normalized to mean baseline and shaded area for both plots are mean ± σ, n = 3.
Ratiometric cAMP Response of mScarlet-Epac-GeNL through Mouse Tissue
After determining that our sensor functions in both modes in cells, we sought to evaluate the brightness of our sensor in animal tissues. We injected HEK cell lysate containing mScarlet-Epac-GeNL corresponding to ∼1200 transfected cells subcutaneously into the deceased mice (Figure 4). We find that the protein exhibits a higher response to cAMP when injected into animals, an approximate 147% change, compared to an 83% change in the dose–response curves (compare Figures 4b to 1c,d). The raw ratio values are lower in the tissue, as expected from the protein luminescence spectra and the higher scattering and absorption of 510 nm light over 650 nm light in tissues (SI, Figures 1 and 2). However, the high cAMP ratio did not scale to the same degree as the 0 cAMP condition, resulting in the higher observed dynamic range (SI, Figure 2).19 Although the underlying reason for this difference is unclear, the increase in the dynamic range suggests that our sensor will maintain or perhaps exceed the 30% dynamic range it showed in the cell culture when expressed in vivo. This supports the application of our sensor to measuring cAMP changes in multiple systems.
Figure 4.
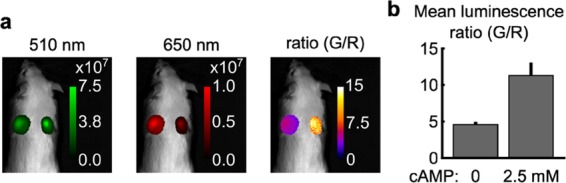
mScarlet-Epac-GeNL is sufficiently bright to report cAMP through the tissue and fur in a mouse. (a) HEK293 lysate containing mScarlet-Epac-GeNL corresponding to ∼1200 transfected cells was injected subcutaneously into a mouse having either 0 mM (left) or 2.5 mM (right) cAMP. Shown are the images for the 510 and 650 channels (radiance, photons·s–1·cm–2·sr–1) and the computed ratio image for a representative experiment in a mouse. (b) Quantification of data for three independent experiments performed as in (a), mean ± σ, n = 3.
Importantly, the radiance values for both the 510 and 650 nm channels are 1–2 orders of magnitude greater than the background (Figure 4a, mean background is ∼1.5 × 104 photons·s–1·cm–2·sr–1 with an upper 2σ value of ∼6.4 × 105 photons·s–1·cm–2·sr–1 for either channel), supporting the potential use of mScarlet-Epac-GeNL in tissue studies even in the unshaved mice.
Discussion
In this study, we established mScarlet-Epac-GeNL as a dual-mode FRET and BRET sensor. Previous efforts have made single-mode ratiometric FRET or BRET sensor for cAMP11,12 and other molecules.1,2 Using a split NanoLuc approach, intensiometric luminescent sensors have been developed using Nano-lanterns as a basis, including a sensor for cAMP. However, these sensors significantly reduce the brightness of NanoLuc, reducing the benefits of using the eNL.9,20 In contrast, our approach demonstrates that eNLs can be incorporated into existing ratiometric sensors with rapid optimization and no disruption of the NanoLuc structure, taking full advantage of its brightness and the ability of eNL to red-shift NanoLuc emission from autoluminescence caused by background oxidation of the substrate. Thus, the advantages of the eNL are maximized.
The BRET-based approach we built on here9 is a part of a wider effort to red-shift luciferase emission.2,21,22 NanoLuc, although bright, has a peak emission at 460 nm, which is highly scattered and absorbed by tissues.7,19 Although other efforts have successfully red-shifted NanoLuc emission through direct mutation or through chemical modifications to its substrate furimazine,23−25 our approach employs the red-shifted eNLs to modularly add bioluminescence capabilities to existing FRET sensors. It would be interesting if these red-shifted mutant NanoLuc enzymes could be fused with still longer wavelength-emitting fluorescent proteins to employ a similar approach to ours to generate ratiometric sensors that were truly in the “optical window” for in vivo imaging.
We were able to generate a dual-mode FRET and BRET sensor with eNL using a screen of only six constructs, suggesting that other dual-mode ratiometric sensors could be rapidly engineered using our approach. However, the conformational change in Epac is particularly robust to the addition of different fluorescent proteins on its termini.26 Thus, it may not be expected that eNL incorporation into other sensors would be as facile. Fortunately, our approach aligns closely with that of Komatsu et al.,8 who used the Renilla luciferase-containing Nano-Lanterns to make hybrid FRET–BRET kinase sensors using AKAR sensors as a scaffold, and Aper et al., who generated a zinc FRET–BRET sensor.27 Our study improves upon their results by using the brighter eNLs and extends the strategy to the family of Epac-based cAMP sensors. Together, our results suggest that this approach may be generalizable to other ratiometric sensors.
One significant application of BRET-based cAMP sensors is in cell-based drug screening assays.4,28 Ideally, a single sensor could function in high-throughput screening assays and then be used for the mechanism of action and animal studies. Our sensor functions well in cells in vitro in the BRET mode and maintains the FRET mode functionality for subcellular assessment of the drug function. In mice, mScarlet-Epac-GeNL from approximately 1200 cells is bright over noise by 1–2 orders of magnitude and faithfully reports cAMP changes in a ratiometric manner, with a lower reporter limit <300 cells in our assay (SI, Figures 3 and 4). However, even after accounting for the equivalent number of cells represented by the amount of injected protein, direct injection of lysate into a deceased mouse is a crude method of estimating the signal strength. The overall brightness of our sensor in the future animal work will depend on the cell number and protein expression levels in the cell population being examined. Future extensions of this work into animals will need to optimize their assay conditions according to their observed brightness, as is standard with any sensor.29 Nonetheless, we have demonstrated that differentials in the tissue scattering of the 510 vs 650 nm light in the mice did not disrupt the ability of our sensor to ratiometrically report cAMP differences in deceased mice. Furthermore, from the signal to noise in the BRET cell-based assay (Figure 3), we estimate that in an ideal case, our sensor could statistically identify changes in cAMP leading to a 9% change in the ratio, corresponding to cAMP deviations of ∼3 μM. This is well within the sensitivity needed to detect fluctuations commonly seen in neurons responding to natural and synthetic agonists acting at the membrane in cultured cells, including neurons.12,30 Furthermore, the large signal-to-noise ratio observed through the tissue with the lysate derived from a small number of cells, together with its apparent resolution in cells, suggests that mScarlet-Epac-GeNL could, in principle, be used in vivo for initial drug validation. Furthermore, by applying our approach to sensors that monitor different aspects of GPCR signaling,1 a suite of sensors could be developed to screen and characterize novel drugs from subcellular compartments to in vivo animals, minimizing time spent on unproductive compounds and mechanism validation.31
In conclusion, we have generated a cross-platform FRET and BRET cAMP ratiometric sensor that may find applications in monitoring cAMP dynamics in vitro and in vivo and may contribute to screening for new therapeutic targets at GPCRs. Our use of GeNL should reduce interference from shorter wavelength background autoluminescence, and our use of mScarlet facilitates filter-based imaging because of the large spectral difference between the donor and acceptor. Furthermore, our method of adding bright BRET functionality to existing FRET sensors through replacement of its C-terminal FP with an eNL may be extendable to other GEIs, promoting rapid diversification of sensors and their applications.
Materials and Methods
Molecular Biology
ICUE3 (Addgene #61622) was a generous gift from Zhang.11 mScarlet-N1 (Addgene #85066) was a generous gift from Gadella.32 HisB-RRvT (Addgene # 87364) was a generous gift from Campbell.18 tdTomato (Addgene #54856) and mTurquoise2 (Addgene # 54844) were generous gifts from Davidson.33,34 mNeonGreen-N1 was a generous gift from Day.35 pT7-CalfluxVTN (Addgene #83926) containing NanoLuc was a generous gift from Johnson.36
Red fluorescent proteins, hEpac1 (148-881), mNeonGreen (ΔC10, res. 1-226), mTurqoise2 (ΔC10, res. 1-229), and NanoLuc fragments, were amplified using PCR and ligated into the pGW1 vector at the XbaI/EcoRI restriction sites in a single reaction using Gibson cloning (NEB HiFi kit). For GeNL constructs, a GF linker was inserted between mNeonGreen (ΔC10) and NanoLuc (ΔN5, res. 6-171); for CeNL constructs, an LH linker was inserted between mTurquoise2ΔC10 and NanoLuc (ΔN3, res. 4-171).9 An AGT linker was placed between the RFP and hEpac1 (148-881), and an EQ linker was used between hEpac1 (148-881) and GeNL/CeNL, similar to the FRET-based sensor ICUE3.11
Cell Culture and Transfection
Transient transfections of the sensor constructs in the pGW1 vector were carried out in HEK293A cells or a stable HEK293 κ-opioid receptor line (HEKKOR), as indicated using the calcium phosphate method from Jordan et al.37 Salmon sperm carrier DNA (Invitrogen 15632011) was used to dilute sensor DNA for cell viability according to the needs of the experiment ranging from 1:1 to 7:1 carrier/sensor DNA mass ratios. HEK lines were maintained in a 37 °C incubator at 10% CO2 in Dulbecco’s modified Eagle’s medium (Fisher #31600034) pH 7.08, with 10% HyClone CCS (Fisher #SH3008703) and passaged every 3–4 days. Media for HEKKOR cells were supplemented with 0.35 mg mL–1 of G418 (Sigma G8168).
Dose–Response Curves and Protein Spectra with HEK Lysate
HEKKOR cells were transfected with sensor constructs in a six-well plate and allowed to express protein for 36 h before being lysed with a hypotonic lysis solution (5 mM Tris·HCl, 2 mM ethylenediaminetetraacetic acid, pH = 7.3).16 Briefly, cells were transferred to imaging media (15 mM N-(2-hydroxyethyl)piperazine-N′-ethanesulfonic acid, 120 mM NaCl, 3 mM KCl, 3 mM NaHCO3, 1.25 mM NaH2PO4, 10 mM glucose, 2 mM CaCl2, 1 mM MgCl2) and imaged (see “Determination of FRET cAMP response in cells”) to evaluate transfection efficiency. Cells were then rinsed 1× with 1× Dulbecco’s phosphate-buffered saline (DPBS) (Fisher #14-200-075) and nonenzymatically dissociated (Fisher #13 150-016) at 37 °C for 8–9 min. Cells were then resuspended in 1 mL of imaging media and pelleted at 200g for 5 min. Cells were rinsed in 0.5 mL of ice-cold DPBS and repelleted before being resuspended in 0.44 mL of ice-cold lysis buffer. Cells were left in the lysis buffer for 10 min on ice and pipetted 5× three times during incubation to ensure protein escape from membranes. Cell debris was then pelleted at 17 000g for 10 min, and protein-containing supernatant was removed for analysis.
cAMP stocks (25 mM) were made by dissolving cAMP (Millipore-Sigma, #A6885) in the lysis buffer. Serial dilutions of this stock were added stepwise to wells containing the sensor lysate. FRET-based measurements were performed on a Synergy H4 plate reader (Biotek) at a gain setting of 60 and the following filters/mirrors: for CeNL constructs, 420/50 ex, 485/20 & 620/15 em; for GeNL constructs, 485/20 ex, 528/20 & 620/15 em; and a 50% 200–850 nm ex/em dichroic mirror for all channels. To maximize the signal, the protein was left at a full concentration in the lysate.
Protein for BRET-based dose–response curves was diluted to a common effective concentration based on the direct fluorescence of the RFP that was determined to slow furimazine decay while allowing high signal:noise. The protein was diluted in 100 μL of the lysis buffer in a 96-well plate and 25 μL of the Nano-Glo substrate (Promega N2011), prepared with furimazine at 1× concentration according to the manufacturer’s guidelines, was added just prior to starting the experiment. The 96-well plate was imaged in a Spectral Ami animal imager using a group acquisition with 2 × 2 binning, exposure time 2 s, FOV 20, Fstop 1.2, object height 10 cm for both luminescence channels 510/20 and 650/20. During the 510/20 acquisition, the Ami took a photo using the default settings. Protein received the same cAMP dosing as when determining the FRET dose–response curves.
For fluorescence and luminescence spectra of mScarlet-Epac-GeNL, the lysate was prepared as above and quantified in a Synergy H4 plate reader (Biotek) using a 9 nm bandwidth and read height of 8.0 mm. For fluorescence spectra, 480/9 excitation was applied and spectra were read from 500 to 700 nm with a 5 nm step size. For luminescence spectra, the scanning speed seemed to affect the observed differences in spectra. After optimizing the protein concentration, the best balance for signal:noise and speed was to use a 0.1 s integration time and a 10 nm step size from 450 to 700 nm. An autogain function was used to determine the optimum gain for each set of curves and was set at the first read and held constant through the rest of the repetition.
For all dose–response curves and spectra, a single experiment/repetition constitutes the lysate prepped from an independently transfected well in a 6-well plate. Three background wells containing the lysis buffer (and the Nano-Glo substrate, where applicable) were included with each read and received cAMP. The average background was subtracted from each data point prior to normalization or analysis. The lysate from untransfected cells did not differ from the lysis buffer alone. During analysis, dose–response curve repetitions are normalized to their zero cAMP value prior to averaging. Normalized values were then fitted to
| 1 |
using the nonlinear least squares method in custom MATLAB scripts. Spectra are presented with each repetition normalized to its own area 100×. Figures were plotted in MATLAB and arranged in Inkscape.
Determination of FRET cAMP Response in Cells
mScarlet-Epac-GeNL was transiently transfected into HEKKOR cells and allowed to express for 36 h. Media were exchanged for imaging media (as above) and cells were imaged on an Olympus IX83 microscope with an Andor Zyla4.2 sCMOS camera, Lumencor LED light source, and prior motorized stage controlled by the Andor iQ3 software. mTurquoise2 fluorescence and FRET from mTurquoise2 were observed with 438/29x (Chroma), 470/24m ET (Chroma), and 632/60m ET (Chroma) filters with a 69008bs dichroic mirror (Chroma). mNeonGreen fluorescence and FRET from mNeonGreen were observed using 475/34x (Chroma), 525/50m ET (Chroma), and 632/60m ET (Chroma) filters with a 59 022bs dichroic mirror (Chroma). A plan Apo VC 20x objective (0.75 NA) was used for capture with exposure time 100 ms and 2 × 2 binning each channel.
For each repetition, a baseline was acquired for six frames before imaging media was removed and replaced with media containing the indicated drugs, either 50 μM forskolin (FSK, Tocris #1099) or 50 μM FSK + 9 nM Dynorphin A (1–17) (dyn, Genscript custom peptide). Frames were collected at 1 min intervals, and media changes performed between frames. Images were background-subtracted using the rolling ball algorithm (radius = 50) in ImageJ prior to computing the ratio image.38 ROIs of cells were selected based on thresholded images in either the green or red channel and applied to the ratio image to get each cell’s average ratio in each frame. For Figure 2b, a mask was applied to eliminate noncell pixels from the image. This is because many background pixels in the ratio image have nonreal or saturated values from dividing by zero or near-zero values after background subtraction.
For FRET cAMP responses in cells, data from 20 cells imaged in an independently transfected well were averaged to form a single experimental repetition/trial. The G/R ratio time course of each cell was normalized to the average G/R ratio of its own 6 frame baseline prior to averaging. Images and measurements were processed in ImageJ, data was analyzed, and plotted in MATLAB, and Figure 2 was arranged in Inkscape.
Demonstration of BRET cAMP Response in Cells
HEK293A cells were seeded and transfected in a 96-well plate and imaged 36–48 h later in the Spectral Ami animal imager using the filter setup above. Cell growth media was changed to Opti-MEM media (Invitrogen #22600-050) upon removing cells from the incubator. Just prior to imaging, 25 μL of the Nano-Glo substrate having 0.5× furimazine was added to each well. At the indicated time, 80 μL of the well solution was drawn out and mixed with FSK or vehicle and then returned to the well. This was necessary due to the low solubility of FSK and the desire to not alter the furimazine state in the well. The parameters used for imaging were 2 s exposure time, FOV = 20, FStop = 1.2, object height = 10 cm. This imager is not setup to do fast imaging but a delay of 27 s approximated an imaging time step of 1 min for the 2 s exposure time setting. The plate was taped down to a 10 cm pipette tip box to reduce well-to-well luminescence bleed through. Thresholded images in either the 510 or 650 nm channel were used to generate ROIs for each well, and the ratio of the mean ROI intensities was calculated for Figure 3a. Images were processed using ImageJ, and the data was plotted in MATLAB. An independently transfected well of cells served as a repetition/trial, and three independent repetitions were used for each condition.
Animal Work
Fvb mice p111–112 were sacrificed using CO2 euthanasia. For each repetition/trial, a pool of lysate was further diluted into conditions with either 0 or 2.5 mM cAMP and the Nano-Glo substrate having 1× furimazine. Each mixture (25 μL), corresponding to ∼1200 transfected cells, was injected subcutaneously into the dorsal caudal surface of a mouse, with 0 cAMP lysate on the left side and 2.5 mM cAMP on the right. Mice were imaged on Spectral Ami animal imager with exposure time of 5 s, FOV = 20, Fstop = 1.2, object height 2 cm. No background subtraction was needed for these images. The raw images were masked with an arbitrary threshold to eliminate pixels with nonreal values and overlaid with the photoimage of the mouse using ImageJ. Pixel values were converted to radiance using the conversion factor calculated from the Spectral Aura software. Values quantified in Figure 4b are calculated from the unmasked areas in the ratio image of each repetition. Data was processed and analyzed in MATLAB.
All animal works were carried out with the approval of the Purdue Animal Care and Use Committee and performed in accordance with their guidelines.
Acknowledgments
This work was supported by NIH grants R21EY026425 to M.T. and F32MH115432 to A.R.F. Additional support was provided from the Indiana Clinical and Translational Sciences Institute funded, in part by Grant Number UL1TR002529 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors acknowledge the use of the facilities of the Bindley Bioscience Center, a core facility of the NIH-funded Indiana Clinical and Translational Sciences Institute.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b01770.
Fitted parameters of the FRET and BRET cAMP dose–response curves (Table 1); fluorescence and bioluminescence spectra of mScarlet-Epac-GeNL (Figure 1); comparing raw ratio values of mScarlet-Epac-GeNL at zero and saturating cAMP in the dose–response curves and animal experiment (Figure 2); showing cAMP reporting of the diluted sensor in the tissue (Figure 3); sensor signal stability in the tissue (Figure 4) (PDF)
The authors declare no competing financial interest.
Notes
All animal works were carried out with the approval of the Purdue Animal Care and Use Committee and performed in accordance with their guidelines. DNA encoding each sensor construct will be made available on Addgene.org.
Supplementary Material
References
- Greenwald E. C.; Mehta S.; Zhang J. Genetically Encoded Fluorescent Biosensors Illuminate the Spatiotemporal Regulation of Signaling Networks. Chem. Rev. 2018, 118, 11707–11794. 10.1021/acs.chemrev.8b00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh H.-W.; Ai H.-W.. Development and Applications of Bioluminescent and Chemiluminescent Reporters and Biosensors Annu. Rev. Anal. Chem. 2019, 12, 129–150. 10.1146/annurev-anchem-061318-115027. [DOI] [PMC free article] [PubMed]
- Sanford L.; Palmer A.. Recent Advances in Development of Genetically Encoded Fluorescent Sensors; 1st ed; Elsevier Inc., 2017; Vol. 589. [DOI] [PubMed] [Google Scholar]
- Manglik A.; Lin H.; Aryal D. K.; McCorvy J. D.; Dengler D.; Corder G.; Levit A.; Kling R. C.; Bernat V.; Hübner H.; et al. Structure-Based Discovery of Opioid Analgesics with Reduced Side Effects. Nature 2016, 537, 185–190. 10.1038/nature19112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masri B.; Salahpour A.; Didriksen M.; Ghisi V.; Beaulieu J.-M.; Gainetdinov R. R.; Caron M. G. Antagonism of Dopamine D2 Receptor/ β-Arrestin 2 Interaction Is a Common Property of Clinically Effective Antipsychotics. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 13656–13661. 10.1073/pnas.0803522105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzanotte L.; van ‘t Root M.; Karatas H.; Goun E. A.; Löwik C. W. G. M. In Vivo Molecular Bioluminescence Imaging: New Tools and Applications. Trends Biotechnol. 2017, 35, 640–652. 10.1016/j.tibtech.2017.03.012. [DOI] [PubMed] [Google Scholar]
- Hall M. P.; Unch J.; Binkowski B. F.; Valley M. P.; Butler B. L.; Wood M. G.; Otto P.; Zimmerman K.; Vidugiris G.; MacHleidt T.; et al. Engineered Luciferase Reporter from a Deep Sea Shrimp Utilizing a Novel Imidazopyrazinone Substrate. ACS Chem. Biol. 2012, 7, 1848–1857. 10.1021/cb3002478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu N.; Terai K.; Imanishi A.; Kamioka Y.; Sumiyama K.; Jin T.; Okada Y.; Nagai T.; Matsuda M. A Platform of BRET-FRET Hybrid Biosensors for Optogenetics, Chemical Screening, and in vivo Imaging. Sci. Rep. 2018, 8, 8984 10.1038/s41598-018-27174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K.; Kimura T.; Shinoda H.; Bai G.; Daniels M. J.; Arai Y.; Nakano M.; Nagai T. Five Colour Variants of Bright Luminescent Protein for Real-Time Multicolour Bioimaging. Nat. Commun. 2016, 7, 13718 10.1038/ncomms13718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone-Corsi P. The Cyclic AMP Pathway. Cold Spring Harbor Perspect. Biol. 2012, 4, a011148 10.1101/cshperspect.a011148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPilato L. M.; Zhang J. The Role of Membrane Microdomains in Shaping β2-Adrenergic Receptor-Mediated cAMP Dynamics. Mol. Biosyst. 2009, 5, 832. 10.1039/b823243a. [DOI] [PubMed] [Google Scholar]
- Jiang L. I.; Collins J.; Davis R.; Lin K.-M.; DeCamp D.; Roach T.; Hsueh R.; Rebres R. A.; Ross E. M.; Taussig R.; et al. Use of a cAMP BRET Sensor to Characterize a Novel Regulation of cAMP by the Sphingosine 1-Phosphate/G 13 Pathway. J. Biol. Chem. 2007, 282, 10576–10584. 10.1074/jbc.M609695200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarenbeek J.; Goedhart J.; van Batenburg A.; Groenewald D.; Jalink K. Fourth-Generation Epac-Based FRET Sensors for cAMP Feature Exceptional Brightness, Photostability and Dynamic Range: Characterization of Dedicated Sensors for FLIM, for Ratiometry and with High Affinity. PLoS One 2015, 10, e0122513 10.1371/journal.pone.0122513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avidor-Reiss T.; Zippel R.; Levy R.; Saya D.; Ezra V.; Barg J.; Matus-Leibovitch N.; Vogel Z. Kappa-Opioid Receptor-Transfected Cell Lines: Modulation of Adenylyl Cyclase Activity Following Acute and Chronic Opioid Treatments. FEBS Lett. 1995, 361, 70–74. 10.1016/0014-5793(95)00154-2. [DOI] [PubMed] [Google Scholar]
- Valentino R. J.; Volkow N. D. Untangling the Complexity of Opioid Receptor Function. Neuropsychopharmacology 2018, 43, 2514–2520. 10.1038/s41386-018-0225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev V. O.; Bünemann M.; Hein L.; Hannawacker A.; Lohse M. J. Novel Single Chain cAMP Sensors for Receptor-Induced Signal Propagation. J. Biol. Chem. 2004, 279, 37215–37218. 10.1074/jbc.C400302200. [DOI] [PubMed] [Google Scholar]
- Violin J. D.; Elston T. C.; Zhang J.; Yildirim N.; Lefkowitz R. J.; DiPilato L. M. β2 -Adrenergic Receptor Signaling and Desensitization Elucidated by Quantitative Modeling of Real Time CAMP Dynamics. J. Biol. Chem. 2008, 283, 2949–2961. 10.1074/jbc.M707009200. [DOI] [PubMed] [Google Scholar]
- Wiens M. D.; Shen Y.; Li X.; Salem M. A.; Smisdom N.; Zhang W.; Brown A.; Campbell R. E. A Tandem Green–Red Heterodimeric Fluorescent Protein with High FRET Efficiency. ChemBioChem 2016, 17, 2361–2367. 10.1002/cbic.201600492. [DOI] [PubMed] [Google Scholar]
- Jacques S. L. Optical Properties of Biological Tissues: A Review. Phys. Med. Biol. 2013, 58, R37–61. 10.1088/0031-9155/58/11/R37. [DOI] [PubMed] [Google Scholar]
- Saito K.; Chang Y.-F.; Horikawa K.; Hatsugai N.; Higuchi Y.; Hashida M.; Yoshida Y.; Matsuda T.; Arai Y.; Nagai T. Luminescent Proteins for High-Speed Single-Cell and Whole-Body Imaging. Nat. Commun. 2012, 3, 1262 10.1038/ncomms2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J.; Oh Y.; Sens A.; Ataie N.; Dana H.; Macklin J. J.; Laviv T.; Welf E. S.; Dean K. M.; Zhang F.; Kim B. B.; Tang C. T.; Hu M.; Baird M. A.; Davidson M. W.; Kay M. A.; Fiolka R.; Yasuda R.; Kim D. S.; Ng H. L.; Lin M. Z. A Bright Cyan-Excitable Orange Fluorescent Protein Facilitiates Dual-Emission Microscopy and Enhances Bioluminescence Imaging In Vivo. Nat. Biotechnol. 2016, 34, 760–767. 10.1038/nbt.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh Y.; Park Y.; Cho J. H.; Wu H.; Paulk N. K.; Liu L. X.; Kim N.; Kay M. A.; Wu J. C.; Lin M. Z. An Orange Calcium-Modulated Bioluminescent Indicator for Non-Invasive Activity Imaging. Nat. Chem. Biol. 2019, 15, 433–436. 10.1038/s41589-019-0256-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh H.-W.; Karmach O.; Ji A.; Carter D.; Martins-Green M. M.; Ai H. Red-Shifted Luciferase–Luciferin Pairs for Enhanced Bioluminescence Imaging. Nat. Methods 2017, 14, 971–974. 10.1038/nmeth.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakhmin A.; Hall M. P.; Machleidt T.; Walker J. R.; Wood K. V.; Kirkland T. A. Coelenterazine Analogues Emit Red-Shifted Bioluminescence with NanoLuc. Org. Biomol. Chem. 2017, 15, 8559–8567. 10.1039/C7OB01985H. [DOI] [PubMed] [Google Scholar]
- Yeh H. W.; Xiong Y.; Wu T.; Chen M.; Ji A.; Li X.; Ai H. W. ATP-Independent Bioluminescent Reporter Variants to Improve in Vivo Imaging. ACS Chem. Biol. 2019, 14, 959–965. 10.1021/acschembio.9b00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Krogt G. N. M.; Ogink J.; Ponsioen B.; Jalink K. A Comparison of Donor-Acceptor Pairs for Genetically Encoded FRET Sensors: Application to the Epac cAMP Sensor as an Example. PLoS One 2008, 3, e1916 10.1371/journal.pone.0001916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aper S. J. A.; Dierickx P.; Merkx M. Dual Readout BRET/FRET Sensors for Measuring Intracellular Zinc. ACS Chem. Biol. 2016, 11, 2854–2864. 10.1021/acschembio.6b00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkowski B. F.; Butler B. L.; Stecha P. F.; Eggers C. T.; Otto P.; Zimmerman K.; Vidugiris G.; Wood M. G.; Encell L. P.; Fan F.; Wood K. V. A luminescent biosensor with increased dynamic range for intracellular cAMP. ACS Chem. Biol. 2011, 6, 1193–1197. 10.1021/cb200248h. [DOI] [PubMed] [Google Scholar]
- Yeh H.-W.; Wu T.; Chen M.; Ai H. Identification of Factors Complicating Bioluminescence Imaging. Biochemistry 2019, 58, 1689–1697. 10.1021/acs.biochem.8b01303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntean B. S.; Zucca S.; MacMullen C. M.; Dao M. T.; Johnston C.; Iwamoto H.; Blakely R. D.; Davis R. L.; Martemyanov K. A. Interrogating the Spatiotemporal Landscape of Neuromodulatory GPCR Signaling by Real-Time Imaging of cAMP in Intact Neurons and Circuits. Cell Rep. 2018, 22, 255–268. 10.1016/j.celrep.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich A. T.; Semache M.; Gross F.; Da Fonte D. F.; Runtz L.; Colley C.; Mezni A.; LeGouill C.; Lukashova V.; Hogue M.; et al. Biased Signaling of the Mu Opioid Receptor Revealed in Native Neurons. iScience 2019, 14, 47–57. 10.1016/j.isci.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindels D. S.; Haarbosch L.; Van Weeren L.; Postma M.; Wiese K. E.; Mastop M.; Aumonier S.; Gotthard G.; Royant A.; Hink M. A.; et al. MScarlet: A Bright Monomeric Red Fluorescent Protein for Cellular Imaging. Nat. Methods 2017, 14, 53–56. 10.1038/nmeth.4074. [DOI] [PubMed] [Google Scholar]
- Shaner N. C.; Campbell R. E.; Steinbach P. A.; Giepmans B. N. G.; Palmer A. E.; Tsien R. Y. Improved Monomeric Red, Orange and Yellow Fluorescent Proteins Derived from Discosoma Sp. Red Fluorescent Protein. Nat. Biotechnol. 2004, 22, 1567–1572. 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- Goedhart J.; Von Stetten D.; Noirclerc-Savoye M.; Lelimousin M.; Joosen L.; Hink M. A.; Van Weeren L.; Gadella T. W. J.; Royant A. Structure-Guided Evolution of Cyan Fluorescent Proteins towards a Quantum Yield of 93%. Nat. Commun. 2012, 3, 751 10.1038/ncomms1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner N. C.; Lambert G. G.; Chammas A.; Ni Y.; Cranfill P. J.; Baird M. A.; Sell B. R.; Allen J. R.; Day R. N.; Israelsson M.; et al. A Bright Monomeric Green Fluorescent Protein Derived from Branchiostoma Lanceolatum. Nat. Methods 2013, 10, 407–409. 10.1038/nmeth.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.; Cumberbatch D.; Centanni S.; Shi S. Q.; Winder D.; Webb D.; Johnson C. H. Coupling Optogenetic Stimulation with NanoLuc-Based Luminescence (BRET) Ca++ Sensing. Nat. Commun. 2016, 7, 13268 10.1038/ncomms13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan M.; Schallhorn A.; Wurm F. M. Transfecting Mammalian Cells: Optimization of Critical Parameters Affecting Calcium-Phosphate Precipitate Formation. Nucleic Acids Res. 1996, 24, 596–601. 10.1093/nar/24.4.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C. A.; Rasband W. S.; Eliceiri K. W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.