Abstract
Development of resistance to antibiotics is one of the major reasons of difficulties in treatments of diseases caused by antibiotic-resistant bacteria, and this resistance makes the investigation of alternative antimicrobials a key priority. Phenolic acids are plant- and fungi-originating natural antimicrobial products, and there is no known bacterial resistance after exposure to them. The purpose of this study was to investigate the resistance ability of bacteria against phenolic acids. Therefore, the ability of methicillin-resistant Staphylococcus aureus and methicillin-susceptible S. aureus to gain resistance against two phenolic acids and an antibiotic upon exposure to subinhibitory concentrations was tested. Herein, we evaluated the minimum inhibitory concentrations (MICs) of vanillic acid (VA), 2-hydroxycinnamic acid (2-HCA), and vancomycin in the beginning of the experiment and the MICs were found to be 2.5 mg/mL VA, 1.6 mg/mL 2-HCA, and 0.01 mg/mL vancomycin for both bacteria. Following continuous treatments with increasing subinhibitory concentrations, MICs were evaluated once more. Exposure to subinhibitory concentrations of vancomycin induced the development of resistance immediately; however, resistance to both phenolic acids could not be induced. These data indicated the potential of phenolic acids to be used as effective antimicrobials in the inhibition of antibiotic-resistant pathogenic bacteria.
1. Introduction
One of the major health and economic problems of all countries is the spreading of resistance against antibiotics.1−3 Extreme misuse of antibiotics in humans and animals has lead to the development of antibiotic-resistant bacteria. Although there is a possibility that bacteria are intrinsically resistant to some of the antibiotics, they can overcome the probable susceptibility by several mechanisms.4 Antibiotics mostly inhibit the bacterial growth by acting on the synthesis of the cell wall, the cell membrane, proteins, DNA, and RNA.2,3 Apart from these classical targets, another action mechanism of antibiotics is resulting in reactive oxygen species formation which then affects the structure and stability of lipids, proteins, and DNA of the bacteria.2 The ability of bacteria to develop resistance against antibiotics can be due to mechanisms such as horizontal gene transfer, selection of previously resistant strains among the population, and mutagenesis.2,5−7 Studies reported that the exposure to subinhibitory antibiotic concentrations not only induces resistance but also affects the virulence factors of the human pathogens which may lead to a rise in illnesses and deaths.8,9 The accelerating pace of resistance to antibiotics poses crucial problems worldwide from the points of human health and increased costs of treatments caused by infections.2,4,10 In 2013, about two million infections with antibiotic-resistant bacterial strains which then directly resulted in the death of about 23 000 people were reported in the USA.3,4 Besides, it is estimated that about 1.5 billion Euros are disbursed in Europe.4
Staphylococcus aureus is a Gram-positive bacterium that naturally can live on the human skin and in the nasopharynx.11−13 It is a global major source of infections caused by bacteria. Staphylococcal infections can vary from skin infections, such as boils and abscesses to toxic shock syndrome and mortal pneumonia.12−15 Although about 30% of all people asymptomatically carry S. aureus, its presence is related with the infections occur subsequently.5,13,14S. aureus is able to develop resistance against multiple antibiotics.11−14,16 and these resistant strains are responsible for many epidemics and pandemics. Among others, methicillin-resistant S. aureus (MRSA) is known as the most common pathogen that has resistance against multiple antibiotics.4 The presence of MRSA both as hospital-associated and community-associated strains carries these bacteria to the top of the list of healthcare problems.17 According to the literature, methicillin resistance is observed in more than 60% of S. aureus isolates and the development of resistance to more than 20 of antimicrobial drugs has been achieved in many strains.16 The ability to gain resistance against almost all the antibiotics in use is one of the main reasons that make the treatment of S. aureus infections more difficult. This resistance then leads to an increase in the cost of the treatments and the loss of labor.17,18 Therefore, the investigation and development of effective antimicrobial agents against antibiotic-resistant bacteria has vital importance worldwide.13 According to the literature, although about 80% of the pharmaceuticals were produced from plants, not much is used for their possessed antimicrobial properties. This situation prompts the screening of plant-based materials to find the novel antimicrobial agents.1
Phenolic compounds are produced by the plants and fungi as secondary metabolites. They are consumed in human diet and beneficial to human health due to their antioxidant,19,20 anticarcinogen,21 anti-inflammatory,21,22 and antiviral23 properties. Recent studies indicated their neuroprotective24 and radiation-mitigating25 properties. They also have antibacterial activities which were commonly shown in many studies.10,13,26−29 Vanillic acid (VA) and 2-hydroxycinnamic acid (2-HCA) used in this study are phenolic acids made of an aromatic ring that is attached with hydroxyl groups. Antimicrobial and antioxidant properties of phenolic acids are determined by the hydroxyl groups’ position, number, and also by the presence of other groups attached to the ring.26 Plant-based simple phenolic acids are classified in two major groups: hydroxybenzoic acid and hydroxycinnamic acids. While VA is a derivative of hydroxybenzoic acids;22,30 2-HCA is a derivative of hydroxycinnamic acids.26
The aim of our study was to test the resistance development ability of multidrug resistant bacteria against VA and 2-HCA and to determine the possible difference against phenolic acids among two S. aureus strains for the first time. Herein, we report that MRSA and methicillin-susceptible S. aureus (MSSA) did not develop resistance to both VA and 2-HCA whereas the two strains developed resistance to vancomycin.
2. Results and Discussion
2.1. Determination of Subinhibitory and Minimum Inhibitory Concentrations
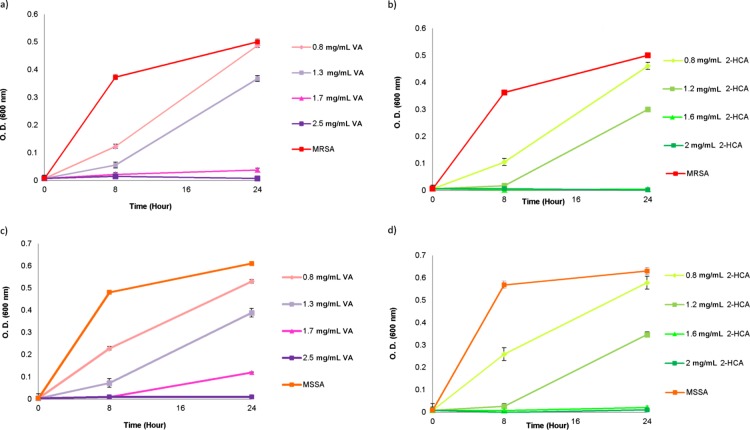
Antimicrobial properties of VA, 2-HCA, and vancomycin on MRSA and MSSA were determined via a macro dilution method. The effect of different concentrations of phenolic acids against MRSA and MSSA were presented in Figure 1.
Figure 1.
Growth of MRSA and MSSA in the presence of different concentrations of VA (a,c) and 2-HCA (b,d). The absorbance measurement was taken at 600 nm at 0th, 8th and 24th time points during incubation at 37 °C.
The minimum inhibitory concentrations (MICs) and subinhibitory concentrations were given in Table 1. MICs of tested antimicrobial agents were found as 2.5 mg/mL for VA; 1.6 mg/mL for 2-HCA, and 0.01 mg/mL for vancomycin for both organisms. Subinhibitory concentrations for phenolic acids were same for MRSA and MSSA: 1.3 mg/mL for VA and 1.2 mg/mL for 2-HCA. However, vancomycin concentrations showed differences, while 0.005 mg/mL vancomycin resulted in the inhibition of about half of MRSA; it was 0.0075 mg/mL vancomycin in the case of MSSA. These concentrations were used as starter concentrations to induce the resistance ability of bacteria.
Table 1. List of Used Concentrations of Phenolic Acids and Vancomycin to Induce Resistance and Incubation Times.
| incubation
time |
||||||
|---|---|---|---|---|---|---|
| 24 h | 24 h | 48 h | 72 h | 24 h | ||
| antimicrobial compound (mg/mL) | initial MIC | subinhibitory concentration | 1st increment | 2nd increment | final MIC | |
| MRSA | VA | 2.5 | 1.3 | 1.4 | 1.5 | 2.5 |
| 2-HCA | 1.6 | 1.2 | 1.3 | 1.4 | 1.6 | |
| vancomycin | 0.01 | 0.005 | 0.0055 | 0.006 | 0.015 | |
| MSSA | VA | 2.5 | 1.3 | 1.4 | 1.5 | 2.5 |
| 2-HCA | 1.6 | 1.2 | 1.3 | 1.4 | 1.6 | |
| vancomycin | 0.01 | 0.0075 | 0.008 | 0.0085 | 0.025 | |
2.2. Effect of Exposure to Subinhibitory Concentrations of Phenolic Acids and Vancomycin
The antibiotic resistance not only results in serious health problems but also loss of labor due to the hospitalization of infected people and increased costs of treatments. When compared with the infections caused by antibiotic susceptible strains, the infections caused by resistant ones lead to about a twofold increase in deaths.10 Vancomycin is an intravenous antibiotic used for the treatment of infections caused by the community acquired or hospital acquired MRSA.11,14 However, it does not provide a complete solution due to the occurrence of bacteremia and nephrotoxicity during antibiotic treatment, high incidence of treatment failures, and emergence of resistant strains.14 Several studies showed the increased resistance to vancomycin upon exposure of bacteria to inhibitory concentrations.31−33 In our study, after determination of the antibacterial effect of VA, 2-HCA, and vancomycin on MRSA and MSSA, the induction of resistance was promoted with the incubation of bacteria within the subinhibitory concentrations of tested antimicrobial agents for 24 h. At the same time, MRSA and MSSA were exposed to previously determined MIC concentrations of these antimicrobials to confirm the obtained MICs shown in Table 1. Transfer of bacteria from media containing subinhibitory concentrations of antimicrobials to freshly prepared media containing an appropriately incremented concentration of antimicrobials resulting in a difference in percent inhibitions of same concentrations at the end of the 48 h incubation. While there was no difference in the MIC concentrations of VA and 2-HCA after the first increment, both MRSA and MSSA rapidly developed resistance to vancomycin (Figure 2).
Figure 2.
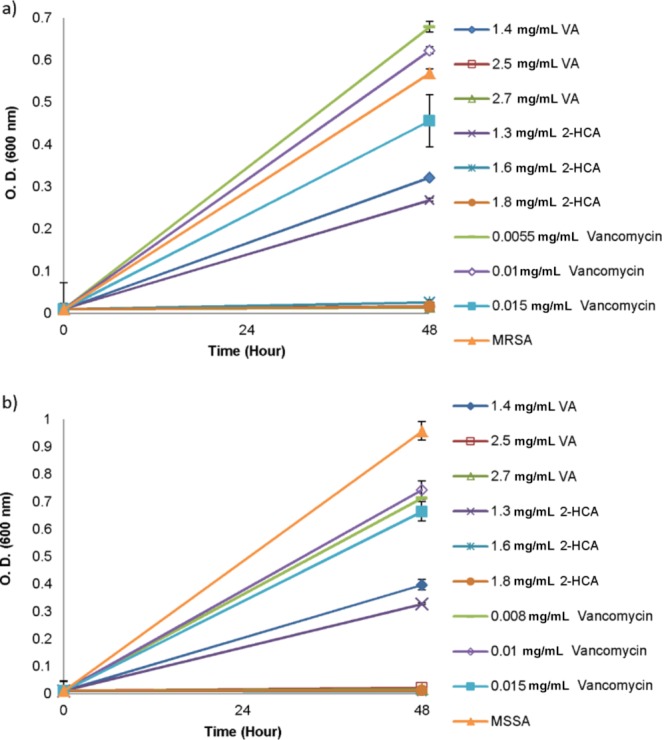
Growth of MRSA (a) and MSSA (b) in the presence of VA, 2-HCA and vancomycin after the first increment in concentrations of antimicrobials. Data represent the mean (±SD) of two independent experiments.
Consistently with the literature,31 one of our findings was that vancomycin resistance developed not only in MRSA but also in MSSA, indicating that a difference in resistance to one type of antibiotic did not confine bacteria to develop resistance to other one. According to Figure 2, treatment of both bacteria with vancomycin elevated the MIC values. Even though both MRSA and MSSA were treated with 0.015 mg/mL vancomycin, it was not enough to inhibit the bacterial growth after 48 h incubation period. To assess whether incubation time affects the resistance profile of bacteria on antibiotic and phenolic acids, another increment in the concentrations was done. This time, transferred bacteria were grown in the presence of slightly increased subinhibitory concentrations of antimicrobials for 72 h (Figure 3).
Figure 3.
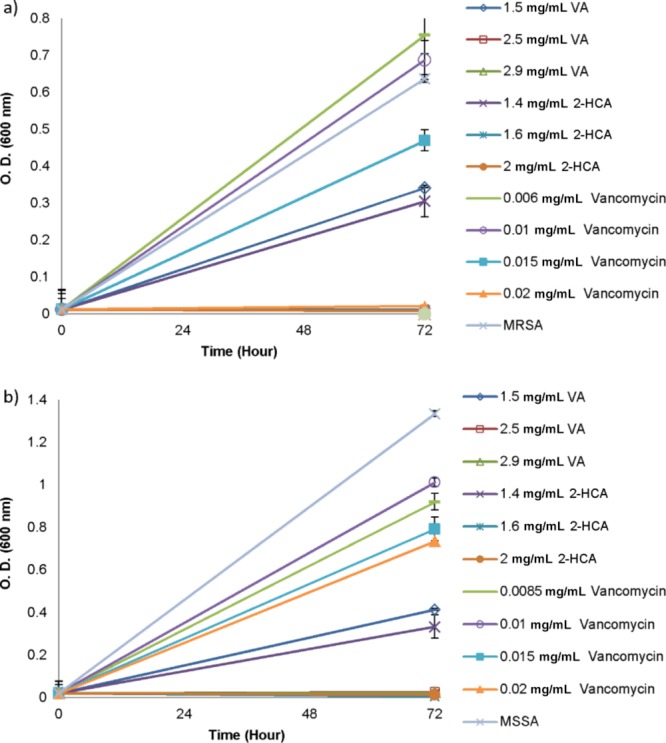
Growth of MRSA (a) and MSSA (b) in the presence of VA, 2-HCA and vancomycin after the second increment in concentrations of antimicrobials. Data represent the mean (±SD) of two independent experiments.
As expected, the development of resistance to vancomycin was observed for both bacteria but not the subinhibitory concentrations of phenolic acids. After second incrementation in subinhibitory concentrations, bacteria were able to grow in the presence of vancomycin concentrations and reached to 0.015 mg/mL as shown in Figure 3. While 0.02 mg/mL vancomycin was required to inhibit total growth of MRSA, it was not enough to inhibit total growth of MSSA when the incubation period was protracted to 72 h.
The determination of MICs for VA, 2-HCA and vancomycin on MRSA and MSSA before, during and after exposure to subinhibitory concentrations allowed the investigation of the ability of bacteria to develop resistance against increasing subinhibitory concentrations. In contrast to antibiotic treatment, challenge of both bacteria with VA and 2-HCA did not result in acquisition of resistance. Moreover, although vancomycin susceptibility of MRSA and MSSA changed following exposure of subinhibitory concentrations, no difference between MRSA and MSSA was observed in the case of their phenolic acid susceptibility as shown in Table 2.
Table 2. Viable Counts of the Bacteria Able to Grown in the Initial MIC Values That Obtained Prior to Exposure and Final MIC Values That Obtained after the Exposure of the Antimicrobials.
| MIC (mg/mL) |
cfu/mL |
||||
|---|---|---|---|---|---|
| initial | final | initial | final | ||
| MRSA | control | 3 × 108 | 7 × 108 | ||
| VA | 2.5 | 2.5 | 1.7 × 105 | 6 × 105 | |
| 2-HCA | 1.6 | 1.6 | 1.2 × 105 | 3 × 105 | |
| van | 0.01 | 0.015 | 7 × 104 | 3 × 108a | |
| MSSA | control | 7 × 108 | 1 × 109 | ||
| VA | 2.5 | 2.5 | 5.7 × 105 | 1.3 × 105 | |
| 2-HCA | 1.6 | 1.6 | 6.7 × 105 | 2.5 × 106 | |
| van | 0.01 | 0.025 | 4 × 106 | 1 × 109a | |
This count does belong to 0.01 mg/mL for vancomycin which was obtained as initial MIC.
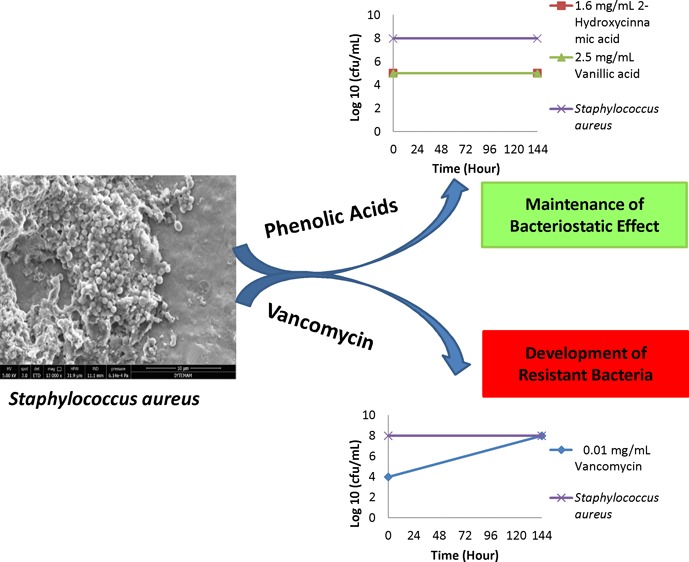
Transfer of bacteria that had been grown in media containing subinhibitory concentrations of antimicrobials for 6 days (144 h continuous exposure) to antimicrobial-free TSB (tryptic soy broth) media provided the information whether the increased MIC values for vancomycin were stable or not.6 All MRSA and MSSA cultures transferred to the antimicrobial-free environment were able to grow in the TSB which were consistent with the information that none of the obtained MIC value was bactericidal against MRSA or MSSA (Table 2).
The determination of final MIC values displayed that the MICs which were elevated for vancomycin following the continuous exposure to subinhibitory concentrations were remained elevated. Additional 48 h incubation in the absence of the antimicrobials did not change the response of bacteria in terms of the acquired resistance to vancomycin. Because there was no increase in the MICs for phenolic acids at the end of continuous exposure, no difference in the MICs was observed neither for MRSA nor MSSA after additional 48 h growth in TSB.
Treatment of bacteria with subinhibitory concentrations of vancomycin resulted in different elevated levels of antibiotic resistance for MRSA and MSSA after additional incubation in an antimicrobial-free environment. While 0.015 mg/mL vancomycin inhibited the total growth of MRSA, the same concentration resulted in an incomplete inhibition of MSSA. MIC of vancomycin was found as 0.025 mg/mL for MSSA (Figure 4).
Figure 4.
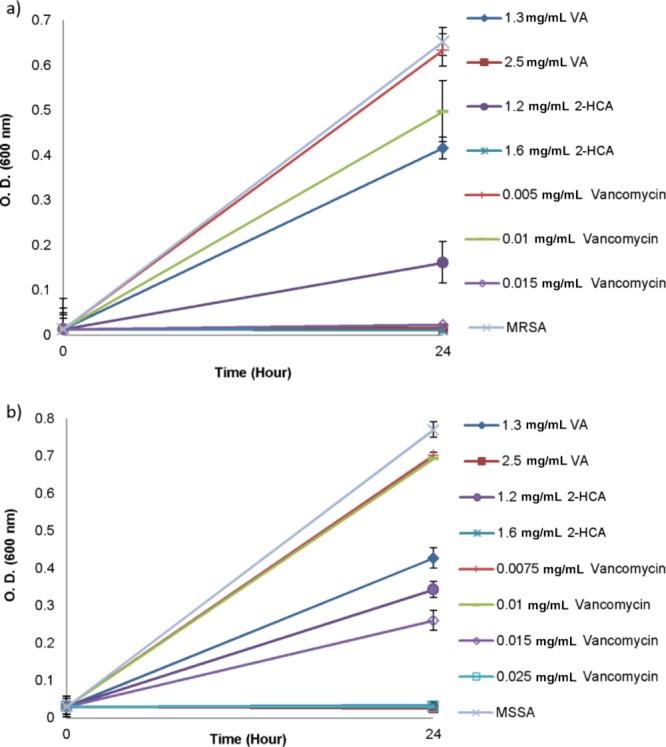
Growth of MRSA (a) and MSSA (b) in the presence of VA, 2-HCA, and vancomycin after exposure to subinhibitory concentrations. Data represent the mean (±SD) of two independent experiments.
The second significant finding of this study was that even bacterial cultures were transferred to an antimicrobial-free environment they maintained their resistance and susceptibility profiles to vancomycin and phenolic acids. Interestingly, there was difference for increased MIC values for bacteria following continuous exposure to subinhibitory concentrations (Figure 3) and after additional growth in an antibiotic-free environment (Figure 4). This difference might be due to the effect of the increased incubation period in the presence of antibiotic. Longer time might be required to generate more resistant strains in the population.
The percent inhibitions of treatment concentrations of all antimicrobial agents and the confirmation of the MIC values at the beginning and at the end of the transfer series, and after each increment are listed in Table 3. While the percent growth inhibitions of the bacteria in the presence of subinhibitory phenolic acid concentrations remained similar, the subinhibitory vancomycin concentration lose its effectiveness on both bacteria after the first transfer. Moreover, our results showed that the prolonged exposure to both phenolic acids increased the inhibitory effect on both bacteria (Table 3).
Table 3. Percent Growth Inhibitions of MRSA and MSSA in the Presence of Subinhibitory Concentrations of VA, 2-HCA, and Vancomycin (Van) and the Confirmation of Minimum Inhibitory Concentrations at Each Transfera,b.
| % growth inhibitions
of bacteria |
||||
|---|---|---|---|---|
| initial | 1st increment | 2nd increment | final | |
| MRSA | ||||
| subinhibitory [VA] | 35 ± 4.5 | 43 ± 1.4 | 46 ± 1.6 | 36 ± 11.6 |
| MIC (2.5 mg/mL) VA | NC | NC | NC | NC |
| subinhibitory [2-HCA] | 46 ± 3.1 | 53 ± 2.6 | 52 ± 14 | 75 ± 15 |
| MIC (1.6 mg/mL) 2-HCA | NC | NC | NC | NC |
| subinhibitory [van] | 72 ± 6.5 | –19 ± 8.1 | –18 ± 19 | 2 ± 17 |
| MIC (0.01 mg/mL) van | NC | >1.5 fold MIC | =2 fold MIC | =1.5 fold MIC |
| MSSA | ||||
| subinhibitory [VA] | 31 ± 6.0 | 59 ± 5.3 | 69 ± 0.1 | 45 ± 9.5 |
| MIC (2.5 mg/mL) VA | NC | NC | NC | NC |
| Subinhibitory [2-HCA] | 46 ± 14.8 | 66 ± 7.5 | 75 ± 8.5 | 55 ± 7.3 |
| MIC (1.6 mg/mL) 2-HCA | NC | NC | NC | NC |
| subinhibitory [van] | 57 ± 4.2 | 25 ± 14.2 | 31 ± 4.4 | 8 ± 4.3 |
| MIC (0.01 mg/mL) van | NC | >1.5 fold MIC | >2 fold MIC | =2.5 fold MIC |
Data represents the mean (±SD) of two independent experiments.
NC: not changed.
Interestingly, continuous exposure of bacteria with 2-HCA resulted in the increased susceptibility (from 46% growth inhibition to 75% growth inhibition) to that antimicrobial at the end of the transfer process, while the bacterial growth percentage was similar to a previous inhibitory effect for VA. This might be related with the chemical differences in the structures of phenolic acids. The observed difference in our results showing elevated MIC values for MRSA (0.015 mg/mL) and MSSA (0.025 mg/mL) might be due to the presence of a subpopulation that are already resistant to vancomycin. Also an enhanced resistance might be explained with the presence of the internal plasmid that carries the transposon responsible for vancomycin resistance.14
Our results showed that the method used in this study was convenient for studying the induction of resistance because the experimental procedure did not result in the adaptation of bacteria to the presence of antibiotics and allowed the development of resistance. Any differences among the determined MICs for used antimicrobials reported in previous studies could be due to working with different strains, difference in the initial concentration of bacteria, use of different methodological approaches, or preparation of solutions. Use of different solvents for the complete solution of phenolic compounds in different concentrations might have an effect on MIC determination.10,34,35
The third and the most significant finding of our study were for antibacterial effects of phenolic acids. Inhibitory effects of phenolic compounds on pathogenic bacteria have been shown in the literature.13,19,20,29,36 The action mechanisms of different phenolic compounds were also studied and their interaction with RNA and protein synthesis,29 their interaction with genomic DNA,40 and membrane structure and function13,26 were determined. However, the previous studies showing a similar phenomenon in which the bacteria develop no resistance against the tested agent are limited with a few antimicrobial compounds. Along these lines, the study performed by Blair (2009) that includes the experimental steps our study is based on showed that pathogenic bacteria were not able not develop resistance against subinhibitory concentrations of Leptospermum honey, while they rapidly acquire resistance to antibiotics.37 Apolonio (2014) and his colleagues evaluated the antimicrobial resistance induction in S. aureus, MRSA and Listeria monocytogenes strains against continuous exposure to eugenol and citral. They did not observe resistance development for these two essential oils upon continuous exposure for the testes strains.38 A more similar example was shown by Takahashi (2015) in which an important food-borne pathogen L. monocytogenes acquired no resistance to ferulic acid upon treatment with subinhibitory concentrations.39
This study has focused on not only the antibacterial activity of VA and 2-HCA on MRSA and MSSA but also the maintenance of the antibacterial activity in terms of induction of resistance. Here, we have shown for the first time that the antibacterial activity of both VA and 2-HCA were maintained upon continuous exposure to subinhibitory concentrations regardless of the type of phenolic acid, duration of incubation period, and antibiotic resistance profile of S. aureus. Because the development of the resistance against antibiotics commonly gained by the acquisition of one responsible gene among bacteria, it seems unlikely to develop resistance against phenolic acids due to complex action mechanisms. According to the literature, phenolic acids inhibit bacterial growth by several action mechanisms such as changing membrane permeability, changing potassium efflux from the cell, causing nucleotide leakage,28 changing bacterial surface charge,26 binding to proteins,10 and binding to DNA.40 Although there are phenolic compounds degrading bacteria in the environment, especially in the soils,41−43 studies showed that different enzymes and activation of several metabolic pathways were needed to metabolize phenolic acids. Considering what is known about phenolic acid action mechanism and phenolic acid-metabolizing mechanisms of bacteria and their native niches, it seems that there is minimal risk for MRSA and MSSA to gain resistance against phenolic acids in the near future.
3. Conclusions
This study highlighted the importance of phenolic acids as alternative antimicrobial compounds by showing that even antibiotic-resistant bacteria, namely, MRSA and MSSA, were not able to develop resistance to them. Therefore, the findings obtained from our study supported the idea of the usage of phenolic acids to fight against pathogenic bacteria. However, further data are required to determine the exact potential of phenolic acids to be used in treatments of illnesses caused by antibiotic-resistant bacteria.
4. Experimental Section
4.1. Micro-organism and Culture Conditions
The following strains were used in this study: methicillin-resistant S. aureus (MRSA) (39) N315 type II SCCmecA and methicillin-susceptible S. aureus (MSSA) (28) ATCC 29213. Bacteria were maintained on tryptic soy agar (TSA) and broth (TSB) (Sigma-Aldrich 22092). Both strains were grown by the inoculation of a single colony of bacteria on TSA or in 4 mL TSB and overnight incubation at 37 °C without shaking (NuVe). The observation of the desired concentrations of overnight bacterial cultures was done via spectrophotometric measurements and viable cell count methods.
4.2. Preparation of Phenolic Acids and Vancomycin
For the determination of the antimicrobial effect of phenolic acids, VA (abbreviated as VA) (Sigma-Aldrich 94770) (synonym: 4-hydroxy-3-methoxybenzoic acid), and 2-HCA (abbreviated as 2-HCA) (Sigma-Aldrich H22809) (synonym: o-coumaric acid) were used. Solubility of phenolic acids in TSB media was increased by the addition of 0.6% of dimethyl sulfoxide (DMSO) (Sigma-Aldrich D5879) as a final concentration into the phenolic acid solutions. Bacterial growth was not affected by the presence of 0.6% DMSO final concentration in the medium (data not shown). The tested concentrations for all antimicrobial agents were freshly prepared prior to experiments. The stock concentrations of VA and 2-HCA were prepared as two-fold of the intended highest concentration. Although the concentrations of the compounds seem higher when compared with antibiotics, the ODs of the antimicrobials alone did not get effected by the concentrations (the spectrophotometric absorbance measurements of 2.5 mg/mL VA, 1.6 mg/mL 2-HCA, and 0.01 mg/mL vancomycin were 0.1022, 0.1034, and 0.1010, respectively; while TSB alone was measured as 0.1013 at 600 nm at the end of the 24 h incubation period).
Vancomycin (Vancotek, Koçak Farma) was dissolved in double distilled water (ddH2O) (as 10 mg/mL stock concentration) and sterilized by filter sterilization using 0.2 μm pore-sized filter and stored at −20 °C.
4.3. Determination of Minimum Inhibitory and Subinhibitory Concentrations
The MIC and subinhibitory concentrations of vancomycin, VA, and 2-HCA, were determined via the broth macro-dilution method with some modifications.44 Briefly, the concentrations of all antimicrobial agents were freshly prepared in TSB media. Final test concentrations were ranged between: 0.001 and 0.05 mg/mL for vancomycin, 0.8–2.5 mg/mL for VA, and 0.8–2 mg/mL for 2-HCA. Addition of inoculum from the overnight culture as 2:100 into the tubes resulted in final bacterial concentrations of 5 × 106 cfu/mL for MRSA and 1 × 107 cfu/mL for MSSA. The time of inoculation of bacteria into the media containing different antimicrobial concentrations was accepted as the initial time point for the spectrophotometric measurement and optical density (OD) was taken at 600 nm (Spectrophotometer, Thermo). After 24 h incubation at 37 °C, the OD measurement was repeated and MICs were determined for each antimicrobial agent. MIC tests were repeated at least two times. Subinhibitory concentrations were accepted as the concentration that inhibits about half of the bacteria after 24 h growth. Percent inhibitions in the growth rates of micro-organisms were calculated according to the following formula: 100 × 1 – (test group OD/control group OD), where the test group OD corresponded to bacteria treated with antimicrobial compound concentrations and control group corresponded to the untreated bacteria grown under normal conditions. The determined initial MIC and subinhibitory concentrations for both organisms (prior to exposure to subinhibitory antimicrobial concentrations) were listed in Table 1.
4.4. Enumeration of Bacteria
The determination of viability in the test tubes that contain antimicrobial agent or not was done by spreading 0.1 mL of serially diluted bacteria from each tube onto TSA plates and incubated at 37 °C for 24 h. A viable cell count allowed the determination of bacterial number in the test tubes at the beginning and at the end of the process of exposure to antimicrobials. To be able to compare the effect of the exposure to subinhibitory antimicrobial concentrations, first, bacteria grown under normal conditions were tested with the mentioned (subinhibitory and MIC) concentrations of all antimicrobials. Their inhibitory effect on bacterial growth as well as the viable number of cells was recorded. Then, the bacterial culture grown in the subinhibitory concentrations of the antimicrobials were transferred into the freshly prepared antimicrobial compounds which have slightly increased concentrations (1st and 2nd increments) and were allowed to grow for an indicated incubation period (Table 1). Following these transfers, bacteria were incubated in antimicrobial-free TSB for 48 h. Then, these bacteria were transferred into the TSB containing initially determined concentrations (MIC and subinhibitory concentrations) of antimicrobial compounds for 24 h. The inhibitory effect of the antimicrobials and the number of viable cells were noted once more. The comparison of viable cells subjected to the same antimicrobial concentration prior to continuous exposure and following the exposure was shown in Table 2.
4.5. Exposure to Subinhibitory Concentrations of Phenolic Acids and Vancomycin
The inducement of resistance against VA, 2-HCA, and vancomycin was based on the study of Blair37 with some modifications. The determination of resistance development to VA, 2-HCA, and vancomycin was started with the addition of 2% inoculum from an overnight culture of bacteria into the media containing both subinhibitory and inhibitory concentrations of all tested antimicrobial agents (Table 1). The control groups were MRSA and MSSA grown in TSB media without the addition of any antimicrobial agent. Following 24 h incubation at 37 °C, 2% inoculums from the cultures containing a subinhibitory concentration of antimicrobials were taken and subcultured into freshly prepared phenolic acid and vancomycin solutions (4 mL total volume) that had 0.1 mg/mL increment for phenolic acids and 0.0005 mg/mL increment for vancomycin in the concentration (Table 1). To test the development of resistance to the antimicrobial agents, MICs were retested with each transfer by using previously determined and increased concentrations of the corresponding antimicrobial agent. MICs were increased with increments of 0.2 mg/mL for phenolic acids and 0.005 mg/mL for vancomycin. The cultures were incubated for 48 h at 37 °C and the same process was repeated with second increments in the concentrations for 72 h incubation. After 72 h incubation, 2% inoculums from the cultures containing increased subinhibitory concentrations, MIC and increased MIC of antimicrobial agents were transferred into TSB media. 48 h incubation in this antimicrobial-free media provided a demonstration of whether increased MIC values were stable or not. After the 48 h incubation period in TSB, MICs for MRSA and MSSA were retested for resistance to phenolic acids and vancomycin. The experiments conducted for testing resistance development were performed with two biological repeats.
Acknowledgments
We thank Dr. Zafer Atbasi for kindly providing the bacteria. This work was funded by the Izmir Institute of Technology Research Fund (Project # 2017IYTE25) to F.S., and a PhD grant to D.K. by the Izmir Institute of Technology.
Author Present Address
† Current address of the author is different from the research affiliation.
Author Contributions
Both the authors contributed to the design of the study, to the analysis and interpretation of data and to the writing of the manuscript. D.K. performed the experiments.
The authors declare no competing financial interest.
References
- Deliorman Orhan D.; Özçelik B.; Hoşbaş S.; Vural M. Assessment of antioxidant, antibacterial, antimycobacterial, and antifungal activities of some plants used as folk remedies in Turkey against dermatophytes and yeast-like fungi. Turk. J. Biol. 2012, 36, 672–686. 10.3906/biy-1203-33. [DOI] [Google Scholar]
- Laureti L.; Matic I.; Gutierrez A. Bacterial Responses and Genome Instability Induced by Subinhibitory Concentrations of Antibiotics. Antibiotics 2013, 2, 100–114. 10.3390/antibiotics2010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maria-Neto S.; de Almeida K. C.; Macedo M. L. R.; Franco O. L. Understanding bacterial resistance to antimicrobial peptides: From the surface to deep inside. Biochim. Biophys. Acta, Biomembr. 2015, 1848, 3078–3088. 10.1016/j.bbamem.2015.02.017. [DOI] [PubMed] [Google Scholar]
- Pasberg-Gauhl C. A need for new generation antibiotics against MRSA resistant bacteria. Drug Discovery Today: Technol. 2014, 11, 109–116. 10.1016/j.ddtec.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Chambers H. F.; DeLeo F. R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009, 7, 629–641. 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohanski M. A.; DePristo M. A.; Collins J. J. Sublethal Antibiotic Treatment Leads to Multidrug Resistance via Radical-Induced Mutagenesis. Mol. Cell 2010, 37, 311–320. 10.1016/j.molcel.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T.; Okuma K.; Ma X. X.; Yuzawa H.; Hiramatsu K. Insights on antibiotic resistance of from its whole genome: genomic island SCC. Drug Resist. Updates 2003, 6, 41–52. 10.1016/s1368-7646(03)00003-7. [DOI] [PubMed] [Google Scholar]
- Ohlsen K.; Ziebuhr W.; Koller K.-P.; Hell W.; Wichelhaus T. A.; Hacker J. Effects of Subinhibitory Concentrations of Antibiotics on Alpha-Toxin (hla) Gene Expression of Methicillin-Sensitive and Methicillin-Resistant Staphylococcus aureusIsolates. Antimicrob. Agents Chemother. 1998, 42, 2817–2823. 10.1128/aac.42.11.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M. P.; Martin E.; Badiou C.; Lebrun S.; Bes M.; Vandenesch F.; Etienne J.; Lina G.; Dumitrescu O. Effects of subinhibitory concentrations of antibiotics on virulence factor expression by community-acquired methicillin-resistant. J. Antimicrob. Chemother. 2013, 68, 1524–1532. 10.1093/jac/dkt073. [DOI] [PubMed] [Google Scholar]
- Alves M. J.; Ferreira I. C. F. R.; Froufe H. J. C.; Abreu R. M. V.; Martins A.; Pintado M. Antimicrobial Activity of Phenolic Compounds Identified in Wild Mushrooms, SAR Analysis and Docking Studies. J. Appl. Microbiol. 2013, 115, 346–357. 10.1111/jam.12196. [DOI] [PubMed] [Google Scholar]
- Harris L. G.; Foster S. J.; Richards R. G. An Introduction to and Techniques for Identifying and Quantifying Adhesins in Relation to Adhesion to Biomaterials: Review. Eur. Cell Mater. 2002, 4, 39–60. 10.22203/ecm.v004a04. [DOI] [PubMed] [Google Scholar]
- Parekh J.; Chanda S. V. Antibacterial Activity of Aqueous and Alcoholic Extracts of 34 Indian Medicinal Plants against Some. Turk. J. Biol. 2008, 32, 63–71. [Google Scholar]
- Kumar P.; Kandi S. K.; Manohar S.; Mukhopadhyay K.; Rawat D. S. Monocarbonyl Curcuminoids with Improved Stability as Antibacterial Agents against and Their Mechanistic Studies. ACS Omega 2019, 4, 675–687. 10.1021/acsomega.8b02625. [DOI] [Google Scholar]
- McGuinness W. A.; Malachowa N.; DeLeo F. R. Vancomycin Resistance in Staphylococcus aureus. Yale J. Biol. Med. 2017, 90, 269–281. [PMC free article] [PubMed] [Google Scholar]
- DeLeo F. R.; Otto M.; Kreiswirth B. N.; Chambers H. F. Community-associated meticillin-resistant Staphylococcus aureus. Lancet 2010, 375, 1557–1568. 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D.; Yu L.; Xiang H.; Fan J.; He L.; Guo N.; Feng H.; Deng X. Global transcriptional profiles ofStaphylococcus aureustreated with berberine chloride. FEMS Microbiol. Lett. 2008, 279, 217–225. 10.1111/j.1574-6968.2007.01031.x. [DOI] [PubMed] [Google Scholar]
- Redwan E. M.; El-Baky N. A.; Al-Hejin A. M.; Baeshen M. N.; Almehdar H. A.; Elsaway A.; Gomaa A.-B. M.; Al-Masaudi S. B.; Al-Fassi F. A.; Abuzeid I. E.; Uversky V. N. Significant antibacterial activity and synergistic effects of camel lactoferrin with antibiotics against methicillin-resistant (MRSA). Res. Microbiol. 2016, 167, 480–491. 10.1016/j.resmic.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Yao W.; Xu G.; Bai B.; Wang H.; Deng M.; Zheng J.; Li D.; Deng X.; Liu X.; Lin Z.; Chen Z.; Li G.; Deng Q.; Yu Z. In vitro-induced erythromycin resistance facilitates cross-resistance to the novel fluoroketolide, solithromycin, in. FEMS Microbiol. Lett. 2018, 365, 1–6. 10.1093/femsle/fny116. [DOI] [PubMed] [Google Scholar]
- Karaosmanoglu H.; Soyer F.; Ozen B.; Tokatli F. Antimicrobial and Antioxidant Activities of Turkish Extra Virgin Olive Oils. J. Agric. Food Chem. 2010, 58, 8238–8245. 10.1021/jf1012105. [DOI] [PubMed] [Google Scholar]
- Bridi R.; Atala E.; Pizarro P. N.; Montenegro G. Honeybee Pollen Load: Phenolic Composition and Antimicrobial Activity and Antioxidant Capacity. J. Nat. Prod. 2019, 82, 559–565. 10.1021/acs.jnatprod.8b00945. [DOI] [PubMed] [Google Scholar]
- Heleno S. A.; Martins A.; Queiroz M. J. R. P.; Ferreira I. C. F. R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A Review. Food Chem. 2015, 173, 501–513. 10.1016/j.foodchem.2014.10.057. [DOI] [PubMed] [Google Scholar]
- Calixto-Campos C.; Carvalho T. T.; Hohmann M. S. N.; Pinho-Ribeiro F. A.; Fattori V.; Manchope M. F.; Zarpelon A. C.; Baracat M. M.; Georgetti S. R.; Casagrande R.; Verri W. A. Jr. Vanillic Acid Inhibits Inflammatory Pain by Inhibiting Neutrophil Recruitment, Oxidative Stress, Cytokine Production, and NFκB Activation in Mice. J. Nat. Prod. 2015, 78, 1799–1808. 10.1021/acs.jnatprod.5b00246. [DOI] [PubMed] [Google Scholar]
- Cho H.-M.; Ha T.-K. -Q.; Dang L.-H.; Pham H.-T. -T.; Tran V.-O.; Huh J.; An J.-P.; Oh W.-K. Prenylated Phenolic Compounds from the Leaves of Sabia limoniacea and Their Antiviral Activities against Porcine Epidemic Diarrhea Virus. J. Nat. Prod. 2019, 82, 702–713. 10.1021/acs.jnatprod.8b00435. [DOI] [PubMed] [Google Scholar]
- Liu X.; Fu J.; Yao X.-J.; Yang J.; Liu L.; Xie T.-G.; Jiang P.-C.; Jiang Z.-H.; Zhu G.-Y. Phenolic Constituents Isolated from the Twigs of Cinnamomum cassia and Their Potential Neuroprotective Effects. J. Nat. Prod. 2018, 81, 1333–1342. 10.1021/acs.jnatprod.7b00924. [DOI] [PubMed] [Google Scholar]
- God̵evac D.; Stanković J.; Novaković M.; And̵elković B.; Dajić-Stevanović Z.; Petrović M.; Stanković M. Phenolic Compounds from and Their Radiation-Mitigating Activity. J. Nat. Prod. 2015, 78, 2198–2204. 10.1021/acs.jnatprod.5b00273. [DOI] [PubMed] [Google Scholar]
- Borges A.; Ferreira C.; Saavedra M. J.; Simões M. Antibacterial Activity and Mode of Action of Ferulic and Gallic Acids against Pathogenic Bacteria. Microb. Drug Resist. 2013, 19, 256–265. 10.1089/mdr.2012.0244. [DOI] [PubMed] [Google Scholar]
- Cueva C.; Moreno-Arribas M. V.; Martín-Álvarez P. J.; Bills G.; Vicente M. F.; Basilio A.; Rivas C. L.; Requena T.; Rodríguez J. M.; Bartolomé B. Antimicrobial Activity of Phenolic Acids against Commensal, Probiotic and Pathogenic Bacteria. Res. Microbiol. 2010, 161, 372–382. 10.1016/j.resmic.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Lou Z.; Wang H.; Zhu S.; Ma C.; Wang Z. Antibacterial Activity and Mechanism of Action of Chlorogenic Acid. J. Food Sci. 2011, 76, M398–M403. 10.1111/j.1750-3841.2011.02213.x. [DOI] [PubMed] [Google Scholar]
- Lucchini J. J.; Corre J.; Cremieux A. Antibacterial Activity of Phenolic Compounds and Aromatic Alcohols. Res. Microbiol. 1990, 141, 499–510. 10.1016/0923-2508(90)90075-2. [DOI] [PubMed] [Google Scholar]
- Khadem S.; Marles R. J. Monocyclic Phenolic Acids; Hydroxy- and Polyhydroxybenzoic acids: Occurrence and Recent Bioactivity Studies. Molecules 2010, 15, 7985–8005. 10.3390/molecules15117985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu K. Vancomycin-resistant Staphylococcus aureus : a new model of antibiotic resistance. Lancet Infect. Dis. 2001, 1, 147–155. 10.1016/s1473-3099(01)00091-3. [DOI] [PubMed] [Google Scholar]
- Mongodin E.; Finan J.; Climo M. W.; Rosato A.; Gill S.; Archer G. L. Microarray Transcription Analysis of Clinical Isolates Resistant to Vancomycin. J. Bacteriol. 2003, 185, 4638–4643. 10.1128/jb.185.15.4638-4643.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L.; Ma X.; Sato K.; Okuma K.; Tenover F. C.; Mamizuka E. M.; Gemmell C. G.; Kim M.-N.; Ploy M.-C.; El Solh N.; Ferraz V.; Hiramatsu K. Cell Wall Thickening is a Common Feature of Vancomycin Resistance in. J. Clin. Microbiol. 2003, 41, 5–14. 10.1128/jcm.41.1.5-14.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuete V.; Nana F.; Ngameni B.; Mbaveng A. T.; Keumedjio F.; Ngadjui B. T. Antimicrobial activity of the crude extract, fractions and compounds from stem bark of (Moraceae). J. Ethnopharmacol. 2009, 124, 556–561. 10.1016/j.jep.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Kuete V.; Ango P. Y.; Fotso G. W.; Kapche G. D.; Dzoyem J. P.; Wouking A. G.; Ngadjui B. T.; Abegaz B. M. Antimicrobial activities of the methanol extract and compounds from Artocarpus communis (Moraceae). BMC Complement Altern. Med. 2011, 11, 42. 10.1186/1472-6882-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belofsky G.; Aronica M.; Foss E.; Diamond J.; Santana F.; Darley J.; Dowd P. F.; Coleman C. M.; Ferreira D. Antimicrobial and Antiinsectan Phenolic Metabolites of Dalea searlsiae. J. Nat. Prod. 2014, 77, 1140–1149. 10.1021/np401083g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair S. E.; Cokcetin N. N.; Harry E. J.; Carter D. A. The unusual antibacterial activity of medical-grade Leptospermum honey: antibacterial spectrum, resistance and transcriptome analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1199–1208. 10.1007/s10096-009-0763-z. [DOI] [PubMed] [Google Scholar]
- Apolónio J.; Faleiro M. L.; Miguel M. G.; Neto L. No induction of antimicrobial resistance inStaphylococcus aureusandListeria monocytogenesduring continuous exposure to eugenol and citral. FEMS Microbiol. Lett. 2014, 354, 92–101. 10.1111/1574-6968.12440. [DOI] [PubMed] [Google Scholar]
- Takahashi H.; Takada K.; Tsuchiya T.; Miya S.; Kuda T.; Kimura B. Listeria monocytogenes develops no resistance to ferulic acid after exposure to low concentrations. Food Control 2015, 47, 560–563. 10.1016/j.foodcont.2014.07.062. [DOI] [Google Scholar]
- Lou Z.; Wang H.; Rao S.; Sun J.; Ma C.; Li J. p-coumaric acid kills bacteria through dual damage mechanisms. Food Control 2012, 25, 550–554. 10.1016/j.foodcont.2011.11.022. [DOI] [Google Scholar]
- Narbad A.; Gasson M. J. Metabolism of ferulic acid via vanillin using a novel CoA-dependent pathway in a newly-isolated strain of Pseudomonas fluorescens. Microbiology 1998, 144, 1397–1405. 10.1099/00221287-144-5-1397. [DOI] [PubMed] [Google Scholar]
- Fritsch C.; Heinrich V.; Vogel R. F.; Toelstede S. Phenolic acid degradation potential and growth behavior of lactic acid bacteria in sunflower substrates. Food Microbiol. 2016, 57, 178–186. 10.1016/j.fm.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Wang J.; Liang J.; Gao S. Biodegradation of Lignin Monomers Vanillic, p-Coumaric, and Syringic Acid by the Bacterial Strain, Sphingobacterium sp. HY-H. Curr. Microbiol. 2018, 75, 1156–1164. 10.1007/s00284-018-1504-2. [DOI] [PubMed] [Google Scholar]
- Hacek D. M.; Dressel D. C.; Peterson L. R. Highly Reproducible Bactericidal Activity Test Results by Using a Modified National Committee for Clinical Laboratory Standards Broth Macrodilution Technique. J. Clin. Microbiol. 1999, 37, 1881–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]