Abstract

Spacers are widely used in membrane technologies to reduce fouling and concentration polarization. Fouling can start from the spacer surface and grow, thereby reducing flux, selectivity, and operation lifetime. Fluorescein isothiocyanate labeled bovine serum albumin was used for fouling studies and observed during cross-flow filtration operation for up to 144 h. Here, we mixed carbon nanotubes (CNTs) and polypropylene (PP) to make a spacer with better antifouling than plain PP spacers. The fouling process was observed by scanning electron microscopy and monitored in situ by fluorescence microscopy. Molecular dynamics simulations show that bovine serum albumin has a lower interaction energy with the nanocomposite CNTs/PP spacer than with the plain PP. The findings are relevant for the development of spacers to improve the operation lifetime of membranes in filtration technologies.
Introduction
Membrane technology for water desalination and reclamation is nowadays the most frequently used method in industrial applications. This technology includes reverse osmosis (RO), nano-, ultra-, and microfiltration membranes, usually configured in spiral-wound membrane modules in cross-flow setups. During operation, the service of membrane modules is limited because of several phenomena, fouling being one of the most important. Even though the antifouling properties of the membrane surface are crucial for an efficient long-term operation of the modules, the membrane surface is not the only part of the module prone to fouling. Abundant research has been done toward the optimization of spacers,1−3 which have two main key functions within the membrane modules: separate the membrane active layer sides while inducing a turbulent flow on the surface of the active layer. The latter function is especially important for avoiding the concentration polarization effect, which occurs during the RO operation given the localized high concentration of ions in the interface between the membrane active layer and the solute. Turbulent flow also contributes to avoiding the deposition of organic and inorganic matter on the membrane surface. Both concentration polarization problems and fouling constitute an important problematic phenomenon of membrane modules.
For these reasons, there are potential advantages of a spacer with enhanced antifouling properties. Previous research has explored several alternatives, the most common being the modification of the geometric configuration of the spacer. Such modifications alter the hydrodynamic properties of the whole membrane module by changing the critical spacer characteristics, such as the angle between filaments, filament thickness, and spacing ratio.4−7 Some of these works reported the use of novel manufacturing methods such as three-dimensional printing,5 which are interesting and could open new possibilities for the spacer geometry configuration but are still difficult to implement in large-scale spacer manufacturing. Another route for improving the antifouling properties of spacers is to directly modify their surface chemistry. For instance, coating polypropylene (PP) spacers by sputtering with metallic particles (silver, copper, and gold).3 Alternative approaches are the direct formation of silver nanoparticles,8,9 or a nanostructured zinc oxide10 on the spacer surface, by attaching chelating ligands bound to metal ions,11 or antifouling biomolecules such as polydopamine.12 These examples illustrate the most common routes to improve the biofouling resistance of the spacers in membrane modules, all of them with a promising potential to reduce the biofilm deposition and fouling. Nevertheless, from the manufacturing point of view, additional steps in the preparation of spacers could hinder the practicality of such an approach. Thus, the use of composite materials with intrinsic antifouling properties as spacers would be advantageous.
In recent years, considerable attention has been paid to low-fouling materials that can be used in technologies such as biomedical materials, food technology, and membrane and marine coatings.13 Low-density polyethylene such as vexar has been widely used, but PP has also been used as spacer.14 PP is a low-cost commodity polymer with excellent mechanical properties and good processability. Several studies have shown that both the addition of carbon nanotubes (CNTs) to certain polymers15 or modification of the surface chemistry of spacers, particularly those made of polypropylene,11,16 can improve the antifouling properties. CNTs have been widely used for making nanocomposite materials that exhibit superior properties, such as increased mechanical and chemical resistance and combining light weight and low cost. Furthermore, CNTs have been added to the active layer of polyamide RO membranes with interesting results such as better antifouling properties and increased chlorination resistance.17−21 A couple of years ago, we studied a nanocomposite active layer made of CNTs and aromatic polyamide by conventional interfacial polymerization,17 with further research on its properties for protein fouling and antiscaling resistance. We found through molecular simulations that the initial stages of protein adhesion can be delayed by increasing the molecular stiffness of the membrane molecule by adding nanotubes while improving its hydrophilicity. Even though mechanisms of fouling between spacers materials and membrane materials are different, it is usually considered that improving their hydrophilicity is the easiest way to reduce protein adsorption,16 and this is usually achieved by surface chemical modification. In this work, however, we use a different method to modify the antifouling property by the preparation of a general PP and multiwalled CNTs nanocomposite spacer. The foulant adhesion behavior is compared with that of a conventional PP spacer by examining its fouling resistance and proposing a possible mechanism for the observed improvement in fouling resistance.
Results and Discussion
Composite materials with carbon nanostructures usually present a quenching effect for many fluorescent substances, a property used for their characterization or for novel sensor designs.22 This quenching has been attributed to strong interactions between delocalized electrons in sp2 carbon networks promoting charge transfer between dyes and carbon materials. Since we used fluorescence microscopy, such a phenomenon would make it impossible to monitor protein fouling; therefore, we first confirmed that CNTs did not quench the fluorescein isothiocyanate (FITC)-labeled bovine serum albumin (BSA). Briefly, 0.1 mL of BSA (200 ppm)/FITC solution containing fluorescent dye was poured on the spacers and the intensity of the fluorescence was observed every hour for a maximum of 24 h. The observed images using blue light (λex = 490 nm) are shown in Figure 1. It was confirmed that the fluorescent intensity did not change from 2 h and up to 24 h, both for PP and CNTs/PP nanocomposite feed spacers, with only a slight uniform fluorescence weakening with time, most likely due to photobleaching. Therefore, no quenching effect was observed for the two spacer materials, which validates the usability of the fluorescent dyeing method. We believe that the lack of quenching indicates a good dispersion of CNTs on the PP matrix, resulting in few CNTs being exposed on the nanocomposite surface, preventing direct interactions between the dyes and the surface of CNTs, in turn, avoiding the dye fluorescence quenching. Additional images are shown in the Supporting Information (Figure S1), where a relationship between BSA concentration (100 to 400 ppm) and observed fluorescence intensity can be observed for both pure PP and CNTs/PP-based nanocomposite spacer samples.
Figure 1.
Verification of the absence of quenching of fluorescence in plain PP and 15% CNTs/PP nanocomposite feed spacers (organic foulant: FITC-BSA). Images correspond to optical (a, c) and fluorescence microscopy (b, d) images with time for the droplet on the spacer, respectively (FITC-BSA solution: 8 μL).
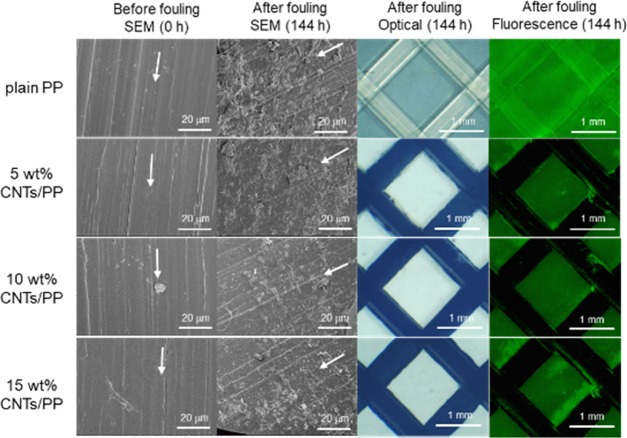
Scanning electron microscopy (SEM) images of PP and various CNTs/PP nanocomposite feed spacers before and 144 h after BSA fouling test are shown in Figure 2, along with the corresponding optical and fluorescence images of the spacers inside the cross-flow experimental cell. The BSA-originated deposits are observed for all the spacers. However, the absence of fluorescence suggests that no protein was deposited on the spacers when CNTs were added to the plain PP, even in small quantity. This result contrasts with the previous findings in polyamide CNTs nanocomposite membranes in which at least 10 wt % CNTs were necessary to improve the antifouling properties.18,19 In the following experiments, we focused mainly on the CNTs/PP nanocomposite spacer containing 15 wt % CNTs (hereafter designated as 15 wt % CNTs/PP) and the BSA-fouling tests.
Figure 2.
SEM, optical, and fluorescence images of the surfaces of PP and different CNTs/PP nanocomposite feed spacers before and after 144 h of BSA fouling test. Arrows indicate the spacer thread main axis.
Figure 3 shows the fluorescence microscopy images taken every 24 h of the spacers placed on commercial RO membranes after immersing in the foulant solution for up to 144 h. A weak fluorescence signal was observed for the PP and CNTs/PP nanocomposite feed spacers at the beginning of the experiment. The photographs show that the deposition of foulant on PP increased with time. On the contrary, the deposition amount of foulant on CNTs/PP is significantly less than that for PP regardless of the content of CNTs. Furthermore, the deposition does not increase with time. The central area of the RO membrane surrounded by the CNTs/PP nanocomposite feed spacer has the deposition of the foulant, in contrast to the CNTs/PP filament. These images show that the CNTs/PP nanocomposite spacer has an outstanding antifouling functionality against BSA.
Figure 3.
(a) Fluorescence microscopy images of PP and CNTs/PP nanocomposite spacers observed in the cross-flow fouling test using organic foulant (BSA) stained by FITC with time. (b) Intensity of the fluorescence from the spacer surface are plotted for each spacer as a function of fouling time.
As reported before,23 the foulant first deposit in the gap between the spacer and the membrane or accumulates in the vicinity and upper surface of the spacer, and then those protein deposits extend to the remaining free membrane surface. However, in the case of the CNTs/PP nanocomposite feed spacer, the extension of the foulant accumulated in the vicinity of the filament toward the membrane surface occurred far less than that in the case of the PP spacer, and the deposition of the foulant on the spacer filament was rare. The observation suggests that no deposition of the foulant occurs on the surface of the CNTs/PP filament. Therefore, it can be considered that the deposition of the foulant develops from the closing portion of the water flow. The accumulation of the foulant on the CNTs/PP spacer correlates with the water flow in the narrow portion between the membrane and the feed spacer, where the water flow is expected to be much lower.
To confirm the influence of the RO membrane on the fouling of the spacers, optical and fluorescence images of the surfaces of the PP and 15 wt % CNTs/PP nanocomposite feed spacers without the membrane were observed and are shown in Figure S2. The result is the same as the fluorescence images in Figure 3, confirming that RO membranes do not promote the fouling behavior of the spacers, although some material is deposited in the gap between the spacer and the membrane in all cases.
Figure 4 shows the observed ζ-potential (two measurements for each sample) of the feed spacers (plain PP, 5, 10, and 15 wt % CNTs/PP); all the specimens are negatively charged at pH above 4. There is a trend of slight increase in the ζ-potential of the spacer with the content of CNTs. It is well-known that the ζ-potential can influence the absorption of foulants electrostatic interactions dominate. BSA has a isoelectric point between 4.6 and 5.1, thus it is negatively charged under desalination conditions, i.e., pH between 7 and 8.5. Negatively charged substrates thus have a larger electrostatic repulsion toward BSA, which increases with their negative charge. However, in the presence of salts, the adhesion of BSA toward polymeric substrates is dominated by hydrophobic interactions because the ions screen the charge interactions.24
Figure 4.

ζ-Potential of various feed spacers (plain PP, 5, 10, and 15 wt % CNTs/PP) measured (each 2 points) as a function of pH.
We also carried out molecular dynamics simulations to study the fouling phenomena on PP. Typically, a BSA molecule was equilibrated on the surface of a plain PP or CNTs/PP model. After 0.5 ns of simulation, the contact surface between PP and BSA (Figure 5a) is considerably larger than that between CNTs/PP-BSA (Figure 5a); this trend increases over time, as shown in Figure 5c. This difference in adsorption originates from the difference in the interaction energy between CNTs/PP-BSA (Figure 5d, black dotted lines) and PP-BSA (Figure 5d, red dotted lines), which is slightly lower in the former. In addition, it is well-known that surface-bound water also plays a role in preventing protein adsorption. The same plot shows that the interaction energy between CNTs/PP and water (Figure 5d, solid black line) is stronger than that between PP and water (Figure 5d, solid red line), while the interaction energy between PP and BSA is lower than that between PP and water. Indeed, Figure 5e shows that plain PP has a slightly thinner surface-bound water layer on the surface compared with CNTs/PP (Figure 5f).
Figure 5.
Molecular dynamics snapshots of (a) BSA-on-PP and (b) BSA-on-CNTs/PP models. (c) Contact surface area evolution between BSA and the two spacer models. (d) Interaction energies between PP and CNTs/PP and water (solid lines) and PP and CNTs/PP and BSA (dotted lines). Visualization of the surface-bound water layer on (e) plain PP and (f) CNTs/PP model.
In addition to the effect of interaction energy, the surface morphology should be considered as the reason why the present CNTs/PP nanocomposite feed spacer shows a higher resistance for fouling deposition in comparison with the typical PP-based spacer. Figure S3 shows the laser scanning microscopy images of the surfaces of (a) PP and (b) 15 wt % CNTs/PP nanocomposite feed spacers. The latter has a smoother surface on which BSA molecules are harder to be anchored. The relatively low degree of interactions between BSA and PP might be a reason why BSA is not attached to the CNTs/PP surface despite a small increase in the contact angle, as shown in Figure S4. Indeed, usually polyolefins with a larger contact angle show a higher propensity for BSA fouling; however, in this study, the CNTs/PA mixture does not follow that trend. We believe the mechanism is thus dominated by a relatively inert substrate with a reduced roughness that prevents protein adsorption along with a lower interaction of the CNTs/PP matrix with the BSA molecule.
Conclusions
We have successively developed a feed spacer using a CNTs/PP nanocomposite with organic antifouling properties. The present CNTs/PP nanocomposite feed spacer shows a high resistance for fouling deposition in comparison with the typical PP-based spacer currently used, which is evaluated by the fouling test for organic matter: BSA foulant under cross-flow experimental setup with RO membrane. This excellent antifouling property of CNTs/PP nanocomposite feed spacer against the commercially used and laboratory made PP-based spacers can be attributed to the smooth morphology and weak negatively charged properties compared to the typical PP feed spacers. The fouling resistance of the CNTs/PP nanocomposite feed spacer is expected to reduce the pressure loss by foulant deposition, improving the overall membrane module performance. Our proposed nanocomposite spacer exhibits promising characteristics, which make it feasible for efficient membrane modules in various water treatment operations ranging from seawater desalination to groundwater, industrial water, and sewage, which contain considerable amounts of foulants and impurities. In addition, this feed spacer could be attractive for applications in the field of membrane technology related to biological, pharmaceutical, and food processing.
Experimental Section
CNTs/PP Nanocomposite Spacer Fabrication
Composite feed spacers are usually made by rotary extrusion; however, we studied laboratory-made samples fabricated by injection molding (Sodick Co., Ltd., TR60EH) using a batch of PP (Japan Polypropylene Corporation, J137M, melt flow rate 30 g/10 min, density 0.9 g/cm3, Rockwell hardness 115, heat deflection temperature 125 °C, room temperature charpy impact strength 2.5 kJ/m2, yield stress 42 MPa, modulus of elasticity 2.3 MPa) with CNTs (CNano Technology, FloTube9000) in ratios between 5.0 and 15.0 wt %. Details of the CNTs/PP blend preparation are provided in the Supporting Information (Figure S5). The nanocomposites for the spacers were prepared using a CNTs/PP masterbatch (CNano Technology, CP360-20), and injection-molded into circular samples (33 mm in diameter) with and without CNTs (black and white samples shown in Figure 6a, respectively). The optical microscopy images of the dimensions for the spacer are shown in Figure 6b,c and those of the fracture surfaces are shown in Figure 6d,e, while the optical images and profilometries of the surface are shown in Figure 6f–i.
Figure 6.
(a) Samples of the PP and CNTs/PP nanocomposite feed spacers. Optical microscopy images of (b) PP and (c) 15 wt % CNTs/PP with cross-sectional images. SEM images of the cross sections of (d) PP and (e) 15 wt % CNTs/PP. (f) Optical microscopy image of the PP surface. (g) Topography obtained by laser confocal microscopy. (h) Optical microscopy image of the 15 wt % CNTs/PP spacer surface. (i) Topography obtained by laser confocal microscopy. Wide-angle X-ray scattering (WAXS) patterns of the (j) plain PP spacer and (k) 15 wt % CNTs/PP spacer. The insets show the two-dimensional (2D) scattering pattern, where a clear biaxial orientation can be seen.
Figure 6j,k shows the wide-angle X-ray scattering (WAXS) patterns obtained for the plain polypropylene material and the carbon nanotube nanocomposite containing 15 wt % CNTs. We observed a higher scattering in the nanocomposite due to the highly ordered CNTs material embedded in the nanocomposite and an apparent increment in crystallinity. The peak-to-peak ratio of the crystalline peaks to the amorphous phase halo area increases after adding the CNTs, suggesting a nucleating effect. The (001) peak of graphitic carbon is absent due to the perpendicular orientation of the nanotubes to the beam, so it might be very weak and/or overlapped by the polymer peaks. However, the small-angle X-ray scattering (SAXS) patterns (Supporting Information, Figure S6) evidence a dramatic reduction of the spherulite size upon addition of the CNTs. The WAXS patterns before integrations show a clear biaxial orientation due to the orientation of the polymer during the injection flow. This orientation effect is markedly higher in the CNTs/PP composites. Indeed, the CNTs have been shown to induce polymer orientation during injection due to shear stress,21 improving the mechanical properties of the nanocomposite. Molecular dynamics simulations in Figure S7 show how PP is well-attached to the walls of CNTs (Figure S7a) and has a lower mobility due to the good interaction and charge transfer with the nanotubes, thus the mobility map shows as a gradient from the bottom to the top, with the PP being closer to the CNTs showing a lower diffusion. Bulk PP on the other hand has a relatively homogeneous mobility (Figure S7b). These results show that carbon nanotubes are well-dispersed and well-aligned within the polypropylene matrix and support a possible improvement in the mechanical properties.
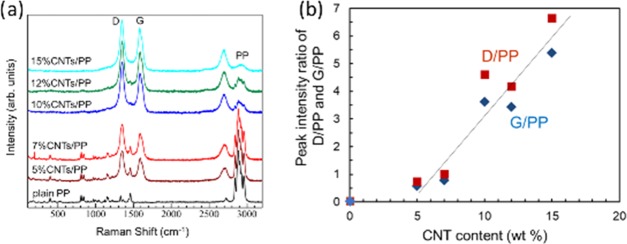
Figure 7a shows the Raman spectra of PP and various CNTs/PP nanocomposite feed spacers, and the typical carbon signature peaks denoted as D and G peaks are clearly observed. The relationship between the CNT content and peak intensity ratios against PP original peak at 2900 cm–1 (denoted as D/PP and G/PP) shows a linear characteristic with the threshold at about 4 wt % CNT content (Figure 7b). It is shown that the CNTs are well-dispersed in the nanocomposite, and the Raman spectroscopy is useful for the determination of the CNT content in the CNTs/PP nanocomposite feed spacer by using the D or G peaks from carbon divided by the signal of PP at 2800 cm–1 as the calibration curve.
Figure 7.
(a) Raman spectra of the PP and various CNTs/PP nanocomposite feed spacers. (b) Relationship between CNT content and peak intensity ratio of D/PP and G/PP.
Cross-Flow Tests of Organic and Inorganic Fouling of the Feed Spacers
Figure 8a shows the optical microscopy image of the prepared CNTs/PP nanocomposite feed spacer. The PP and CNTs/PP feed spacers and permeate spacer (Figure 8b) samples were placed in transparent cross-flow cells as shown in Figure 8c. The solutes were a mixture of 0.06 wt % NaCl (Kanto Chemical Co., Inc., 37144-01) aqueous solution plus bovine serum albumin (BSA) (Jackson Immuno Research Laboratories, IgG-Free and protease-free, 001-000-162). Fluorescein isothiocyanate (FITC) (Dojindo Molecular Technologies, Inc., 349-03661) was used to label the BSA as a fluorescent organic foulant. The schematic illustration of the experimental system is shown in Figure 8d. A cross-flow filtration system (Membrane Solutions Technology, Tokyo, FTU-1) was operated at 0.7 MPa with a flow rate of 500 mL/min through the cross-flow cell. The 1 mm thick feed spacers were placed on the membrane’s surface to replicate a typical large-scale RO module. The water flow rate was 500 mL/min along the surface of the membrane. The water source temperature was kept at 21 ± 1 °C. A 0.06 wt % NaCl aqueous solution was used for the initial compaction process for 72 h, until reaching a stable permeate flux. The NaCl rejection, permeate flux, and salt permeability were measured on the commercial RO membrane during compaction, whereas during the steady state of the fouling experiments, the permeate flux was recorded.
Figure 8.

Optical microscopy image of (a) CNTs/PP nanocomposite feed spacer, (b) permeate collector spacer, (c) acrylic transparent cell, and (d) cross-flow fouling tests system of the spacers using fluorescence microscope for in situ observation.
At first, 100 ppm of the FITC-BSA solution as an organic foulant was dissolved in 0.06 wt % NaCl aqueous solution after the membrane compaction was completed in the cross-flow test at pH 7. The fouling tests were conducted at pH 10. The BSA deposition on the membrane surface and spacers was monitored by blue light (λex = 490 nm) fluorescent optical microscopy. The camera used was a CMOS microscope digital eyepiece camera (MC500, Ostec, Guanzhou, China) with an exposure time of 196 ms and equipped with an epifluorescence mode using a stereomicroscope (SMZ18, Nikon, Tokyo) with a Nikon P2-EFLC green filter. The surface of the membrane was monitored through the CNTs/PP feed spacer at the center of the acrylic cross-flow cell every 24 h over the 144 h fouling period.
Spacer Characterization
Samples surface and cross sections were observed by scanning electron microscopy (SEM, Hitachi, Ltd., SU-8000) before and after cross-flow fouling tests. The samples were coated with Au to avoid charging during the SEM observation. Biofouling evaluations were also carried out by means of the fluorescent dyeing of BSA by FITC, as described above. Additional structural characterization was done by melting–mixing PP and CNTs in different mass fractions. Then, net-like samples with a shape typical of a membrane spacer were injected. Samples of about 1 mm of radius were cut from this material and analyzed by WAXS and SAXS, at the Aichi Synchrotron facility, using the BL8 line. The resulting diffraction patterns were analyzed using Fit2D software.25
Molecular Dynamics
Computer simulations were done by running classical molecular dynamics using LAMMPS simulation package.26 For the CNTs/PP nanocomposite structure modeling, we used three layers of graphene and PP, which is a good approach for evaluating experimental interactions between PP and CNTs walls. One hundred chains of PP were relaxed to conform a block representing the bulk PP. To avoid coalescing into spherical aggregates, spring-like points were added to selected PP atoms. For both PP and grafted polypropylene (GPP) models, atom charges were set by using a charge equilibration method.27,28 The molecular structure of BSA was extracted from the PDB file downloaded from the Protein Data Bank (4F5S). We used the General Amber Force Field29 for PP and CNTs/PP, and CHARMM force field30 for BSA. The SPC/E model was adopted for water molecules.31 The interaction between molecules was calculated by Lennard-Jones and Coulombic interactions with a particle–particle mesh solver.32 The interaction of water layer with PP was calculated considering the water molecules within 4 Å from the PP surface. All calculations were performed by using NPT ensemble at 1 atm and at 300 K with the Nosé–Hoover method33 under periodic boundary conditions with a time step of 1 fs.
Acknowledgments
This research was supported by the Center of Innovation Program “Global Aqua Innovation Center for Improving Living Standards and Water-sustainability” (Grant Number JPMJCE1316) from Japan Science and Technology Agency, JST. This work was also partly supported (for M.E.) by JSPS KAKENHI Grant Number JP17H03401(Simulation part). The authors would like to thank the Aichi Synchrotron Radiation Center and the kind assistance of Dr. Hiroko Yamamoto, Dr. Azuma Hirozumi, and Dr. Tetsuo Nagami during WAXS and SAXS data acquisition.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b01757.
Fluorescence optical microscopy images of BSA solutions on PP and CNTs/PP plates; optical and fluorescence images for the surfaces of spacers without the membrane at the beginning and after cross-flow filtration; laser scanning microscopy images of the surface of the spacers; contact angle data of the surface of the spacers; scheme and details of the spacers prepared by injection; small-angle X-ray scattering patterns of the spacers; molecular dynamics simulation of the mobility of the spacer materials (PDF)
Author Contributions
K.T. and H.K. contributed equally to this work. M.E., K.T., and R.C.-S. proposed and directed the project and wrote the manuscript. H.K. and K.T. synthesized the samples. M.F. and H.K. performed antifouling experiments, fluorescence microscope observation, and ζ-potential measurement. M.O. performed the SEM observation. A.M.-G. performed Raman spectroscopy. A.Y. and S.T. performed molecular dynamics simulations and analysis. J.O.-M., A.M-G., R.C.-S., N.A., and A.Y. advised and discussed the results during the project. All authors reviewed and approved the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Abid H. S.; Johnson D. J.; Hashaikeh R.; Hilal N. A review of efforts to reduce membrane fouling by control of feed spacer characteristics. Desalination 2017, 420, 384–402. 10.1016/j.desal.2017.07.019. [DOI] [Google Scholar]
- Linares R. V.; Bucs S. S.; AbuGhdeeb Z.; Li M.; Amy G.; Vrouwenvelder J. S. Impact of spacer thickness on biofouling in forward osmosis. Water Res. 2014, 57, 223–233. 10.1016/j.watres.2014.03.046. [DOI] [PubMed] [Google Scholar]
- Araújo P. A.; Kruithof J. C.; Van Loosdrecht M. C. M.; Vrouwenvelder J. S. The potential of standard and modified feed spacers for biofouling control. J. Membr. Sci. 2012, 403-404, 58–70. 10.1016/j.memsci.2012.02.015. [DOI] [Google Scholar]
- Bucs S. S.; Farhat N.; Kruithof J. C.; Picioreanu C.; van Loosdrecht M. C. M.; Vrouwenvelder J. S. Review on strategies for biofouling mitigation in spiral wound membrane systems. Desalination 2018, 434, 189–197. 10.1016/j.desal.2018.01.023. [DOI] [Google Scholar]
- Siddiqui A.; Farhat N.; Bucs S. S.; Linares R. V.; Picioreanu C.; Kruithof J. C.; van Loosdrecht M. C. M.; Kidwell J.; Vrouwenvelder J. S. Development and characterization of 3D-printed feed spacers for spiral wound membrane systems. Water Res. 2016, 91, 55–67. 10.1016/j.watres.2015.12.052. [DOI] [PubMed] [Google Scholar]
- Gu B.; Adjiman C. S.; Xu X. Y. The effect of feed spacer geometry on membrane performance and concentration polarisation based on 3D CFD simulations. J. Membr. Sci. 2017, 527, 78–91. 10.1016/j.memsci.2016.12.058. [DOI] [Google Scholar]
- Koutsou C. P.; Karabelas A. J. Towards optimization of spacer geometrical characteristics for spiral wound membrane modules. Desalin. Water Treat. 2010, 18, 139–150. 10.5004/dwt.2010.1382. [DOI] [Google Scholar]
- Yang H.-L.; Lin J. C.-T.; Huang C. Application of nanosilver surface modification to RO membrane and spacer for mitigating biofouling in seawater desalination. Water Res. 2009, 43, 3777–3786. 10.1016/j.watres.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Ronen A.; Lerman S.; Ramon G. Z.; Dosoretz C. G. Experimental characterization and numerical simulation of the anti-biofuling activity of nanosilver-modified feed spacers in membrane filtration. J. Membr. Sci. 2015, 475, 320–329. 10.1016/j.memsci.2014.10.042. [DOI] [Google Scholar]
- Ronen A.; Semiat R.; Dosoretz C. G. Impact of ZnO embedded feed spacer on biofilm development in membrane systems. Water Res. 2013, 47, 6628–6638. 10.1016/j.watres.2013.08.036. [DOI] [PubMed] [Google Scholar]
- Hausman R.; Gullinkala T.; Escobar I. C. Development of low-biofouling polypropylene feedspacers for reverse osmosis. J. Appl. Polym. Sci. 2009, 114, 3068–3073. 10.1002/app.30755. [DOI] [Google Scholar]
- Miller D. J.; Araújo P. A.; Correia P. B.; Ramsey M. M.; Kruithof J. C.; van Loosdrecht M. C. M.; Freeman B. D.; Paul D. R.; Whiteley M.; Vrouwenvelder J. S. Short-term adhesion and long-term biofouling testing of polydopamine and poly(ethylene glycol) surface modifications of membranes and feed spacers for biofouling control. Water Res. 2012, 46, 3737–3753. 10.1016/j.watres.2012.03.058. [DOI] [PubMed] [Google Scholar]
- Fritzmann C.; Löwenberg J.; Wintgens T.; Melin T. State-of-the-art of reverse osmosis desalination. Desalination 2007, 216, 1–76. 10.1016/j.desal.2006.12.009. [DOI] [Google Scholar]
- Araújo P. A.; Miller D. J.; Correia P. B.; van Loosdrecht M. C. M.; Kruithof J. C.; Freeman B. D.; Paul D. R.; Vrouwenvelder J. S. Impact of feed spacer and membrane modification by hydrophilic, bactericidal and biocidal coating on biofouling control. Desalination 2012, 295, 1–10. 10.1016/j.desal.2012.02.026. [DOI] [Google Scholar]
- Madaeni S. S.; Zinadini S.; Vatanpour V. Preparation of superhydrophobic nanofiltration membrane by embedding multiwalled carbon nanotube and polydimethylsiloxane in pores of microfiltration membrane. Sep. Purif. Technol. 2013, 111, 98–107. 10.1016/j.seppur.2013.03.033. [DOI] [Google Scholar]
- Yang Q.; Xu Z.-K.; Dai Z.-W.; Wang J.-L.; Ulbricht M. Surface Modification of Polypropylene Microporous Membranes with a Novel Glycopolymer. Chem. Mater. 2005, 17, 3050–3058. 10.1021/cm048012x. [DOI] [Google Scholar]
- Inukai S.; Cruz-Silva R.; Ortiz-Medina J.; Morelos-Gomez A.; Takeuchi K.; Hayashi T.; Tanioka A.; Araki T.; Tejima S.; Noguchi T.; Terrones M.; Endo M. High-performance multi-functional reverse osmosis membranes obtained by carbon nanotube·polyamide nanocomposite. Sci. Rep. 2015, 5, 13562 10.1038/srep13562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa Y.; Inukai S.; Araki T.; Cruz-Silva R.; Uemura N.; Morelos-Gomez A.; Ortiz-Medina J.; Tejima S.; Takeuchi K.; Kawaguchi T.; Noguchi T.; Hayashi T.; Terrones M.; Endo M. Antiorganic Fouling and Low-Protein Adhesion on Reverse-Osmosis Membranes Made of Carbon Nanotubes and Polyamide Nanocomposite. ACS Appl. Mater. Interfaces 2017, 9, 32192–32201. 10.1021/acsami.7b06420. [DOI] [PubMed] [Google Scholar]
- Takizawa Y.; Inukai S.; Araki T.; Cruz-Silva R.; Ortiz-Medina J.; Morelos-Gomez A.; Tejima S.; Yamanaka A.; Obata M.; Nakaruk A.; Takeuchi K.; Hayashi T.; Terrones M.; Endo M. Effective Antiscaling Performance of Reverse-Osmosis Membranes Made of Carbon Nanotubes and Polyamide Nanocomposites. ACS Omega 2018, 3, 6047–6055. 10.1021/acsomega.8b00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Medina J.; Inukai S.; Araki T.; Morelos-Gomez A.; Cruz-Silva R.; Takeuchi K.; Noguchi T.; Kawaguchi T.; Terrones M.; Endo M. Robust water desalination membranes against degradation using high loads of carbon nanotubes. Sci. Rep. 2018, 8, 2748 10.1038/s41598-018-21192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y.; Zhang X.; Cao W.; Wang K.; Tan H.; Zhang Q.; Du R.; Fu Q. Dispersion and mechanical properties of polypropylene/multiwall carbon nanotubes composites obtained via dynamic packing injection molding. J. Appl. Polym. Sci. 2007, 104, 1880–1886. 10.1002/app.25852. [DOI] [Google Scholar]
- Zhang H.; Zhang H.; Aldalbahi A.; Zuo X.; Fan C.; Mi X. Fluorescent biosensors enabled by graphene and graphene oxide. Biosens. Bioelectron. 2017, 89, 96–106. 10.1016/j.bios.2016.07.030. [DOI] [PubMed] [Google Scholar]
- Baker J.; Stephenson T.; Dard P.; Côté S. Characterisation of Fouling of Nanofiltration Membranes used to Treat Surface Waters. Environ. Technol. 1995, 16, 977–985. 10.1080/09593331608616335. [DOI] [Google Scholar]
- Shaikh A. R.; Karkhanechi H.; Yoshioka T.; Matsuyama H.; Takaba H.; Wang D.-M. Adsorption of Bovine Serum Albumin on Poly(vinylidene fluoride) Surfaces in the Presence of Ions: A Molecular Dynamics Simulation. J. Phys. Chem. B 2018, 122, 1919–1928. 10.1021/acs.jpcb.7b10221. [DOI] [PubMed] [Google Scholar]
- Hammersley A. P. FIT2D: a multi-purpose data reduction, analysis and visualization program. J. Appl. Crystallogr. 2016, 49, 646–652. 10.1107/S1600576716000455. [DOI] [Google Scholar]
- Plimpton S. Fast parallel algorithms for short-range molecular-dynamics. J. Comput. Phys. 1995, 117, 1–19. 10.1006/jcph.1995.1039. [DOI] [Google Scholar]
- Rappe A. K.; Goddard W. A. Charge equilibration for molecular dynamics simulations. J. Phys. Chem. A. 1991, 95, 3358–3363. 10.1021/j100161a070. [DOI] [Google Scholar]
- Aktulga H. M.; Fogarty J. C.; Pandit S. A.; Grama A. Y. Parallel reactive molecular dynamics: Numerical methods and algorithmic techniques. Parallel Comput. 2012, 38, 245–259. 10.1016/j.parco.2011.08.005. [DOI] [Google Scholar]
- Cornell W. D.; Cieplak P.; Bayly C. I.; Gould I. R.; Merz K. M.; Ferguson D. M.; Spellmeyer D. C.; Fox T.; Caldwell J. W.; Kollman P. A. A Second Generation Force Field for the Simulation of Proteins, Nucleic Acids, and Organic Molecules. J. Am. Chem. Soc. 1995, 117, 5179–5197. 10.1021/ja00124a002. [DOI] [Google Scholar]
- MacKerell A. D.; Banavali N.; Foloppe N. Development and Current Status of the CHARMM Force Field for Nucleic Acids. Biopolymers 2000, 56, 257–265. . [DOI] [PubMed] [Google Scholar]
- Berendsen H. J. C.; Grigera J. R.; Straatsma T. P. The missing term in effective pair potentials. J. Phys. Chem. A. 1987, 91, 6269–6271. 10.1021/j100308a038. [DOI] [Google Scholar]
- Pollock E. L.; Glosli J. Comments on P(3)M, FMM, and the Ewald method for large periodic Coulombic systems. Comput. Phys. Commun. 1996, 95, 93–110. 10.1016/0010-4655(96)00043-4. [DOI] [Google Scholar]
- Hoover W. G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695–1697. 10.1103/PhysRevA.31.1695. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.