Abstract
Background
We aimed to assess the prevalence and degree of overlap of potential embolic sources (PES) in patients with embolic stroke of undetermined source (ESUS).
Methods and Results
In a pooled data set derived from 3 prospective stroke registries, patients were categorized in ≥1 groups according to the PES that was/were identified. We categorized PES as follows: atrial cardiopathy, atrial fibrillation diagnosed during follow‐up, arterial disease, left ventricular disease, cardiac valvular disease, patent foramen ovale, and cancer. In 800 patients with ESUS (43.1% women; median age, 67.0 years), 3 most prevalent PES were left ventricular disease, arterial disease, and atrial cardiopathy, which were present in 54.4%, 48.5%, and 45.0% of patients, respectively. Most patients (65.5%) had >1 PES, whereas only 29.7% and 4.8% of patients had a single or no PES, respectively. In 31.1% of patients, there were ≥3 PES present. On average, each patient had 2 PES (median, 2). During a median follow‐up of 3.7 years, stroke recurrence occurred in 101 (12.6%) of patients (23.3 recurrences per 100 patient‐years). In multivariate analysis, the risk of stroke recurrence was higher in the atrial fibrillation group compared with other PES, but not statistically different between patients with 0 to 1, 2, or ≥3 PES.
Conclusions
There is major overlap of PES in patients with ESUS. This may possibly explain the negative results of the recent large randomized controlled trials of secondary prevention in patients with ESUS and offer a rationale for a randomized controlled trial of combination of anticoagulation and aspirin for the prevention of stroke recurrence in patients with ESUS.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT02766205.
Keywords: atrial fibrillation, embolic stroke, embolic stroke of undetermined source, embolism
Subject Categories: Cerebrovascular Disease/Stroke, Ischemic Stroke
Clinical Perspective
What Is New?
In this large multicenter data set of 800 consecutive patients with embolic stroke of undetermined source (ESUS), there was major overlap of potential embolic sources: two thirds of patients with ESUS had at least 2, whereas one third of patients had at least 3, with the most prevalent being left ventricular disease, arterial disease, and atrial cardiopathy (each one of which was present in nearly half of the study population).
What Are the Clinical Implications?
These results may possibly explain the negative results of the NAVIGATE ESUS (Rivaroxaban Versus Aspirin in Secondary Prevention of Stroke and Prevention of Systemic Embolism in Patients With Recent Embolic Stroke of Undetermined Source) and RE‐SPECT ESUS (Dabigatran Etexilate for Secondary Stroke Prevention in Patients With ESUS) trials, which showed that anticoagulation is not superior to aspirin for prevention of stroke recurrence in patients with ESUS and offer a rationale for a randomized controlled trial of combination of anticoagulation and aspirin for the prevention of stroke recurrence in patients with ESUS.
Introduction
The term embolic stroke of undetermined source (ESUS) was introduced by the Cryptogenic Stroke/ESUS International Working Group to describe patients with stroke of undetermined cause despite adequate diagnostic workup (ie, nonlacunar strokes without proximal arterial stenosis or a major cardiac source of embolism, like intracardiac thrombus, atrial fibrillation [AF], mechanical heart valves, and others).1, 2 Approximately 17% of all patients with ischemic stroke are classified as having ESUS, which is associated with a considerable rate of stroke recurrence (≈5% per year).1, 3, 4
Embolism in patients with ESUS may be causally related to a large number of conditions, like covert AF, other non‐AF supraventricular arrhythmias, structural abnormalities of the left atrium or the left ventricle (LV), aortic atherosclerotic plaques, carotid atherosclerotic plaques causing low‐degree stenosis (ie, <50%), paradoxical embolism through patent foramen ovale (PFO), atrial septal defect or pulmonary arteriovenous fistula, cardiac valvular pathological conditions, cancer, and others.1, 2 As it may be the case also for the non‐ESUS ischemic stroke population, >1 potential embolic source (PES) may be present in a single patient, rendering it challenging to conclude on the actual cause.5 There is scarce information about the prevalence and, in particular, the degree of PES overlap in the ESUS population. This information may be important as it could assist in the interpretation of the recent trials of secondary prevention in patients with ESUS and generate hypotheses that could be tested in future research.
The objective of the study was to assess the prevalence and the degree of overlap of PES in patients with ESUS. In addition, we aimed to provide information about the rates of stroke recurrence associated with each PES, as well as with the number of PES per patient.
Methods
We will make the data, methods used in the analysis, and materials used to conduct the research available to any researcher for purposes of reproducing the results or replicating the procedure on reasonable request.
We pooled the data of all consecutive patients with ESUS registered in 3 prospective stroke registries: the ASTRAL (Acute Stroke Registry and Analysis of Lausanne), the Athens Stroke Registry, and the Larissa Stroke Registry.6, 7, 8 We used a standardized form with prespecified parameters to collect data. The use of these registry data for research was approved by the local Institutional Review Boards. Study participants provided written or verbal informed consent for the research use of their deidentified data. The study is registered at Clinicaltrials.gov (NCT02766205), and its methods were previously described.9
Definition of ESUS
ESUS was defined according to the criteria proposed by the Cryptogenic Stroke/ESUS International Working Group as a nonlacunar brain infarct in the absence of the following: (1) extracranial or intracranial atherosclerosis causing ≥50% luminal stenosis in arteries supplying the area of ischemia; (2) major‐risk cardioembolic source; and (3) any other specific cause of stroke (eg, arteritis, dissection, migraine/vasospasm, or drug misuse).2 For pragmatic reasons, imaging of the intracranial arteries was not required for the definition of ESUS, similar to the approach followed in the NAVIGATE ESUS (Rivaroxaban Versus Aspirin in Secondary Prevention of Stroke and Prevention of Systemic Embolism in Patients With Recent Embolic Stroke of Undetermined Source) trial.10
Classification of potential embolic sources
Patients were categorized in ≥1 groups according to the PES that was/were identified. We categorized PES as follows: atrial cardiopathy (AC), AF diagnosed during follow‐up, arterial disease, LV disease, cardiac valvular disease, PFO, and cancer. When >1 PES was identified in a single patient, the patient was categorized in all applicable PES groups. Hence, the overall sum of the number of patients (calculated by adding the number of patients in each PES group) is higher than the total number of patients in our population.
Definitions of potential embolic sources
On the basis of previously published associations with the risk of stroke, AC was diagnosed if the echocardiogram reported left atrial dilatation or increased left atrial diameter (>38 mm for women and >40 mm for men)11 or if supraventricular extrasystoles were present at the 12‐lead ECGs performed during hospitalization.12 We diagnosed arterial disease in case of presence of any ipsilateral atherosclerotic carotid plaque causing luminal stenosis of <50%13 or aortic arch atherosclerosis14, 15, 16 based on the imaging reports. We did not review the images. We did not include contralateral carotid atherosclerosis in this PES. LV disease was diagnosed if low LV ejection fraction (<35%), LV hypertrophy, or left‐sided heart failure was reported at the echocardiogram, or if LV hypertrophy was identified at the ECG (Sokolow index ≥35 mm). We diagnosed cardiac valvular disease if moderate‐to‐severe stenosis or regurgitation of the mitral or aortic valve was reported at the echocardiogram. AF was assessed during on‐site patient visits at the outpatient clinic and/or by contact with the patient and/or the next of kin or the patient's primary physician; it was considered present if confirmed by an ECG performed for any reason, including palpitations, irregular pulse on clinical examination, in‐hospital surveillance, or portable outpatient monitoring.
Assessment of outcome
The assessment of stroke recurrence was performed by on‐site patient visits at the outpatient clinic and/or by contact with the patient and/or the next of kin or the patient's primary physician and was ascertained by reviewing the patient's medical chart and imaging, whenever possible.
Statistical Analysis
Continuous covariates are summarized as median and interquartile range. All comparisons were performed using the Cochran‐Mantel‐Haenszel χ2 test. The rates of stroke recurrence are reported per 100 patient‐years.
To enhance the visualization of the prevalence of PES and the degree of their overlap, we used UpSet (Caleydo, https://caleydo.org) to draw a matrix layout (Figure 1). The plot has 7 rows, each one of which corresponds to a specific PES, as described in the plot legend. Cells may be either empty (indicating absence of the specific PES) or filled (indicating presence of the specific PES). Each column corresponds to a specific combination of PES. The numbers in the plot correspond to the proportion of patients in the overall population with a specific PES (for the numbers shown at the rows) or with a specific combination of PES (for the numbers shown at the columns).
Figure 1.
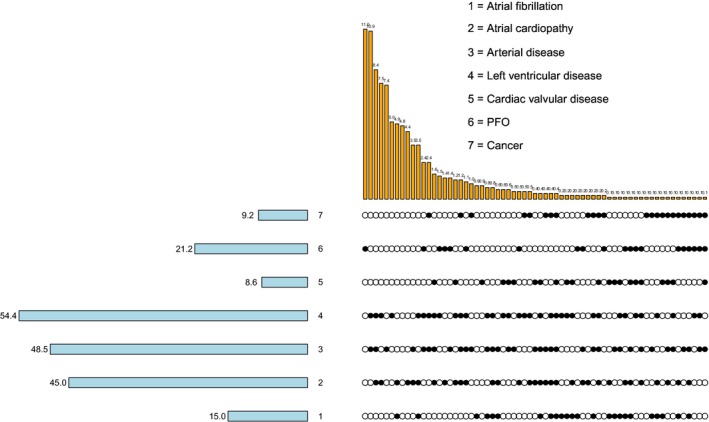
Prevalence of potential embolic sources (PES) and degree of their overlap. Each row corresponds to a specific PES, as described in the legend. Empty cells indicate absence of the specific PES, whereas filled cells correspond to the presence of the specific PES. Each column corresponds to a specific combination of PES. The numbers in the plot correspond to the proportion of patients in the overall population who have a specific PES (for the numbers shown at the rows) or a specific combination of PES (for the numbers shown at the columns). PFO indicates patent foramen ovale.
To assess the association between time to stroke recurrence and PES, which were fitted using a separate indicator, a Cox model was implemented. Adjustment was allowed for demographics (age and sex), medical history (hypertension, dyslipidemia, diabetes mellitus, smoking status, coronary artery disease, and prosthetic valve history), and National Institute of Health Stroke Scale score at admission. A similar model was used to quantify the relationship between time to stroke recurrence and number of PES per patient. In both models, stepwise methods were used to select significant covariates. Associations are presented as hazard ratios (HRs) with their corresponding 95% CIs, and the level of significance was set at 5%.
Graphical display of the results was produced using the Kaplan‐Meier product limit method, where adjusted estimation of the 10‐year cumulative probabilities of stroke recurrence for a specific PES or the number of PES per patient was derived. For patients lost during follow‐up, survival data were censored at the last time known to be alive. For patients who experienced >1 recurrence during the follow‐up period, the time of the first event was used in the analysis. Differences in Kaplan‐Meier curves were evaluated with the likelihood ratio test, and the level of significance was set at 5%. Missing data were not imputed. Statistical analyses were performed with the R package (version 3.5.1).
Results
Baseline Characteristics of the Overall Population
Among 839 consecutive patients with ESUS, 800 (43.1% women; median age, 67.0 years) with complete data were included in the final analysis. The baseline characteristics of patients are summarized in the Table. The prevalence of the classic cardiovascular risk factors did not have major differences across different PES, except for patients with PFO who were younger and had a lower prevalence of arterial hypertension, dyslipidemia, and previous stroke.
Table 1.
Baseline Characteristics and Outcomes of Patients per PES
| Variable | Atrial Fibrillation (n=120) | Atrial Cardiopathy (n=360) | Arterial Disease (n=388) | Left Ventricular Disease (n=435) | Cardiac Valvular Disease (n=69) | Patent Foramen Ovale (n=170) | Cancer (n=74) | P Value |
|---|---|---|---|---|---|---|---|---|
| Female sex | 57 (47.5) | 142 (39.4) | 178 (45.9) | 185 (42.5) | 35 (50.7) | 75 (44.1) | 36 (48.6) | 0.49 |
| Age, y | 73.7 (65.3–79.0) | 72.0 (63.3–79.3) | 72.2 (63.7–80.5) | 72.0 (63.0–79.5) | 74.2 (64.1–79.3) | 48.6 (37.2–63.9) | 74.5 (65.4–80.1) | 0.00 |
| NIHSS score | 5.0 (2.0–9.0) | 6.0 (3.0–13.0) | 7.0 (3.0–12.0) | 6.0 (3.0–12.0) | 7.0 (3.0–14.0) | 5.0 (2.0–11.8) | 7.0 (4.0–11.0) | 0.40 |
| Hypertension | 98 (81.7) | 269 (74.7) | 289 (74.5) | 332 (76.3) | 57 (82.6) | 53 (31.2) | 57 (77.0) | 0.00 |
| Dyslipidemia | 73 (60.8) | 241 (66.9) | 300 (77.3) | 307 (70.6) | 46 (66.7) | 96 (56.5) | 57 (77.0) | 0.00 |
| Diabetes mellitus | 32 (26.7) | 84 (23.3) | 68 (17.5) | 98 (22.5) | 9 (13.0) | 10 (5.9) | 16 (21.6) | 0.65 |
| Smoking | 41 (34.2) | 121 (33.6) | 179 (46.1) | 167 (38.4) | 28 (40.6) | 64 (37.6) | 31 (41.9) | 0.44 |
| Coronary artery disease | 27 (22.7) | 76 (21.2) | 53 (13.7) | 69 (15.9) | 9 (13.0) | 7 (4.1) | 10 (13.5) | 0.21 |
| Previous stroke | 21 (17.5) | 59 (16.4) | 89 (22.9) | 83 (19.1) | 19 (27.5) | 19 (11.2) | 14 (18.9) | 0.00 |
| Antiplatelet at discharge | 112 (94.9) | 323 (91.8) | 370 (96.1) | 396 (93.0) | 60 (89.6) | 154 (91.1) | 70 (94.6) | 0.36 |
| Anticoagulant at discharge | 5 (4.2) | 31 (8.8) | 19 (4.9) | 35 (8.2) | 6 (9.0) | 16 (9.5) | 5 (6.8) | 0.28 |
| Stroke recurrence* | 25.20 | 23.62 | 22.42 | 23.90 | 21.70 | 17.91 | 20.61 | 0.37 |
Data are given as number (percentage), unless otherwise indicated. Continuous covariates are summarized as median (interquartile range). NIHSS indicates National Institute of Health Stroke Scale; PES, potential embolic sources.
(Per 100 patient‐years). All comparisons were performed using Cochran‐Mantel‐Haenszel χ2 test.
Prevalence and Overlap of PES
The prevalence of each PES and the degree of their overlap is summarized in Figure 1. The 5 most prevalent PES were LV disease, arterial disease, AC, PFO, and AF, which were present in 54.4%, 48.5%, 45.0%, 21.3%, and 15.0% of patients, respectively. Most patients (65.5%) had >1 PES, whereas only 29.7% and 4.8% of patients had a single or no PES, respectively. In 31.1% of patients, there were ≥3 PES present. On average, each patient had 2 PES (median, 2).
Stroke Recurrence per Specific PES and Degree of PES Overlap
The median follow‐up was 3.7 years. Stroke recurrence occurred in 101 (12.6%) of patients in the overall population, corresponding to 23.3 recurrences per 100 patient‐years.
The rates of stroke recurrence according to PES are presented in the Table. In multivariate analysis, the risk of stroke recurrence was higher in the AF group compared with other PES groups (Figure 2). In Kaplan‐Meier analysis, the cumulative probabilities of stroke recurrence were borderline statistically different across PES groups (likelihood ratio test, 14.12; P=0.05) (Figure 3) because of the higher cumulative probability of recurrence in the AF group relatively to the other PES groups.
Figure 2.
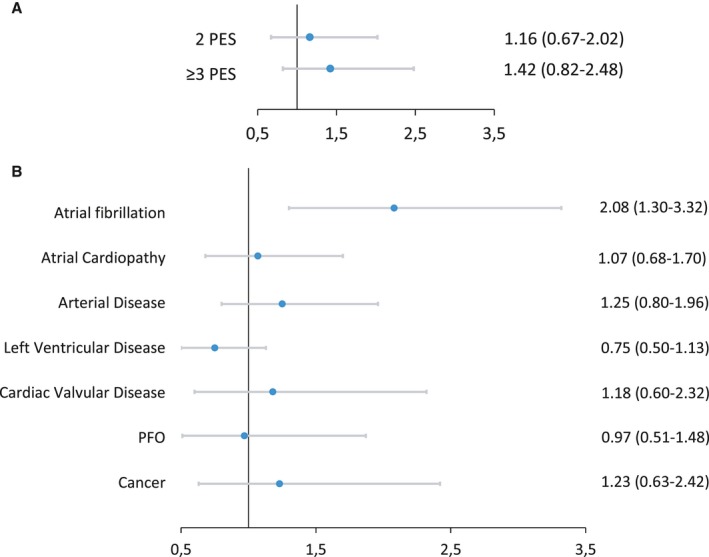
Top: Multivariable regression analysis of the association between the presence of each potential embolic source (PES) and stroke recurrence (for each PES, the comparison is made to patients without the specific PES). Bottom: Multivariable regression analysis of the association between the number of PES per patient and stroke recurrence (the comparisons are made to patients with 0 to 1 PES). Associations are presented as hazard ratios and 95% CIs. For both analyses, associations are adjusted for sex, age, hypertension, dyslipidemia, diabetes mellitus, smoking, coronary artery disease, and National Institute of Health Stroke Scale score at admission. For the top analysis, associations are also adjusted for other concomitant PES. PFO indicates patent foramen ovale.
Figure 3.
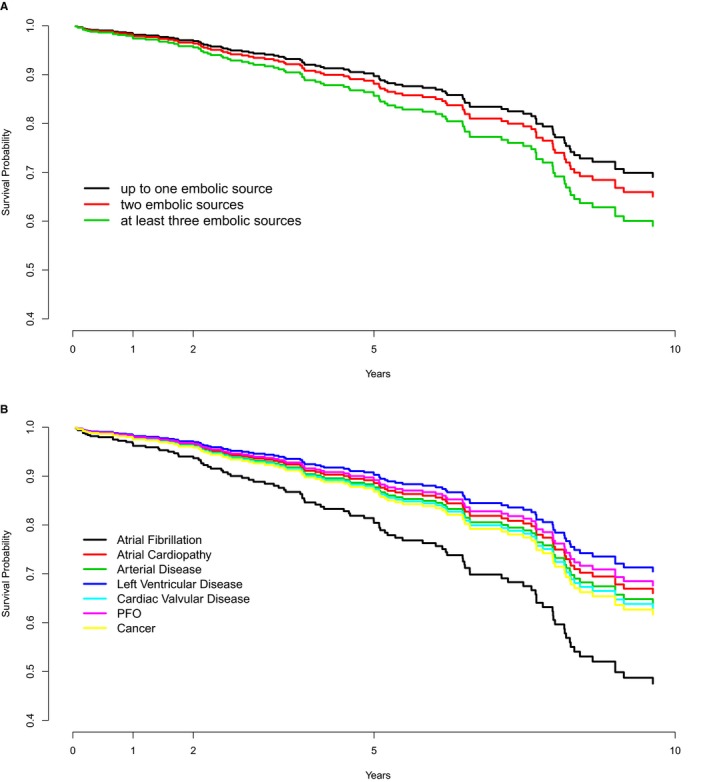
Ten‐year survival estimates of stroke recurrence in patients with embolic stroke of undetermined source, according to each potential embolic source (PES; top) and the number of PES per patient (bottom). PFO indicates patent foramen ovale.
The rates of stroke recurrence for patients with 0 to 1, 2, or ≥3 PES were 20.8, 27.6, and 21.9 per patient‐years, respectively. In multivariate analysis, the risk of stroke recurrence was not statistically different between patients with 0 to 1, 2, or ≥3 PES (Figure 2). In Kaplan‐Meier analysis, the cumulative probability of stroke recurrence was not statistically different between patients with 0 to 1, 2, or ≥3 PES (likelihood ratio test, 2.54; P=0.28) (Figure 3).
Discussion
The present analysis of a large multicenter data set of 800 consecutive patients shows that there was major overlap of PES in patients with ESUS: two thirds of patients with ESUS had at least 2 PES, whereas one third of patients had at least 3 PES. The most prevalent PES were LV disease, arterial disease, and AC, each one of which was present in nearly half of the study population.
It is likely that our estimate for the degree of overlap of PES is only an underestimate of the actual degree of overlap. Given the pragmatic nature of this study, the identification of PES relied on investigations that are routinely performed in clinical practice, like standard 12‐lead ECG, transthoracic echocardiogram, extracranial vascular imaging, and automated cardiac rhythm monitoring. It is likely that a larger number of PES might have been identified if all our patients had an exhaustive panel of investigations that are currently not routinely used in most patients with ESUS. For example, biomarkers (eg, NT‐proBNP [N‐terminal pro‐B‐type natriuretic peptide]17, 18 and high‐sensitivity cardiac troponin T19), cardiac magnetic resonance imaging (for the assessment of atrial fibrosis20), ECG indexes (eg, P‐wave terminal force in V121, 22, 23, 24), and transesophageal echocardiogram (for the assessment of spontaneous echocardiographic contrast25 and the morphological features of the left atrial appendage26) could have lead to more diagnoses of AC; intracranial vascular imaging and transesophageal echocardiogram could have lead to more diagnoses of intracranial and aortic atherosclerosis, respectively14, 15, 16; and transesophageal echocardiogram could have identified a larger number of patients with PFO, if performed in all patients regardless of their age. If patients were investigated with such an exhaustive diagnostic workup, it is highly likely that the degree of overlap of PES would be much higher and only a small minority of patients would be considered to have a single or no PES. However, such a diagnostic panel would be considered unrealistic to apply to the entire population with ESUS, even in high‐resource settings.
The ESUS concept has been criticized that it promotes the lumping approach (ie, a standardized, one‐size‐fits‐all diagnostic approach aiming to detect the major‐risk embolic sources for which there is strong evidence to guide secondary prevention).27 The results of the present study offer support to this strategy by showing that in a remarkable majority of patients, the strategy of an exhaustive diagnostic workup may be not only unrealistic in terms of availability of resources but also futile, as it would rarely lead to a single PES (for most of which there is low quality of evidence to guide management), but rather to multiple PES and subsequent frustration in the attempt to conclude on the causal one.5
The NAVIGATE ESUS and the RE‐SPECT ESUS (Dabigatran Etexilate for Secondary Stroke Prevention in Patients With ESUS) trials showed that anticoagulation is not superior to aspirin for prevention of stroke recurrence in patients with ESUS, indicating that the lumping therapeutic approach of oral anticoagulation for the unselected population with ESUS was not the optimal strategy and indirectly validating the ESUS concept as an etiologically heterogeneous entity. The heterogeneity of embolic sources and their remarkable overlap, as estimated in the present study, could possibly explain these negative results: for some of the embolic sources (like AC, AF, LV disease, PFO, and cancer), the main pathophysiologic mechanism for thrombogenesis is low blood flow, which predisposes to formation of red thrombi that may respond better to anticoagulation. On the other hand, for other embolic sources, like aortic and nonstenotic carotid atherosclerosis, the ulceration of a plaque triggers the formation of white thrombi that may respond better to aspirin. In this context, it may be hypothesized that treating an patients with ESUS with anticoagulation rather than aspirin would just result in exchanging red thrombi for white, with the overall burden remaining largely unchanged. If this hypothesis is correct, it would be rational to expect that simultaneous inhibition of red and white thrombi with a combination of anticoagulation and aspirin would be associated with a significant reduction of stroke recurrences in patients with ESUS. The COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) trial showed that a combination of low‐dose rivaroxaban and aspirin was associated with a large reduction of stroke risk compared with aspirin as monotherapy. These thoughts provide a rationale for a randomized controlled trial of combination of anticoagulation and aspirin for the prevention of stroke recurrence in the unselected population with ESUS. Also, this rationale does not apply to patients who are detected with AF who should be anticoagulated. Furthermore, it may not apply to patients with AC if, in the meanwhile, the ARCADIA (Atrial Cardiopathy and Antithrombotic Drugs in Prevention After Cryptogenic Stroke) trial, which is currently investigating whether oral anticoagulation with apixaban is a better strategy compared with aspirin in patients with AC, reports positive results.28
Among all potential PES, the AF group was associated with the highest risk of stroke recurrence. Although it is debateful how strong is the causative association between ESUS and episodes of AF detected during follow up, especially if they occur late or are of short duration,29 still it is important that these episodes are detected on time as they may warrant oral anticoagulation, which could reduce the risk of stroke recurrence. Given the large prevalence of ESUS and the restricted resources for prolonged cardiac monitoring, it is important to develop prognostic tools that may aid to the stratification of the likelihood of AF detection in patients with ESUS.
A limitation of the present study is that the estimated degree of PES overlap may be an underestimate of the actual overlap, as it might have been higher if a more extensive panel of diagnostic investigations had been performed, as argued above. However, at the same time, this strengthens further the conclusion of this study that there is remarkable overlap of PES in patients with ESUS. Other strengths of the study include the large number of consecutive, well‐defined patients with ESUS and its multicenter design. Limitations include the risk of registration bias within and between the participating registries, the retrospective design of the analysis, and differences in the workup of patients during the in‐hospital phase.
The present analysis concludes that there is major overlap of PES in patients with ESUS. This may possibly explain the negative results of the NAVIGATE ESUS and RE‐SPECT ESUS trials and offer a rationale for a randomized controlled trial of combination of anticoagulation and aspirin for the prevention of stroke recurrence in patients with ESUS.
Author Contributions
Dr Ntaios: study concept, study design, statistical analysis and interpretation, preparation of manuscript, study supervision. Dr Perlepe: data collection, study design, statistical analysis and interpretation, critical revision of the manuscript. Dr Lambrou: statistical analysis and interpretation, critical revision of the manuscript. Dr Sirimarco: data collection, critical revision of the manuscript. Dr Strambo: data collection, critical revision of the manuscript. Dr Eskandari: data collection, critical revision of the manuscript. E. Karagkiozi: data collection, critical revision of the manuscript. Dr Vemmou: data collection, critical revision of the manuscript. Dr Koroboki: data collection, critical revision of the manuscript. Dr Manios: data collection, critical revision of the manuscript. Dr Makaritsis: data collection, critical revision of the manuscript. Dr Vemmos: data collection, study design, statistical analysis and interpretation, critical revision of the manuscript. Dr Michel: data collection, study design, critical revision of the manuscript.
Sources of Funding
This work is part of the AF‐ESUS (Prediction of AF [Atrial Fibrillation] in ESUS [Embolic Stroke of Undetermined Source]) study (ClinicalTrials.gov Identifier: NCT02766205), which is an investigator‐initiated study supported by Pfizer through the BMS/Pfizer European Thrombosis Investigator Initiated Research Program. BMS/Pfizer was given the opportunity to comment on the draft of the manuscript but had no part in the collection, handling, analysis, or interpretation of the data or in the decision to submit the manuscript for publication. The Swiss Cardiology Foundation supported data collection in ASTRAL (Acute Stroke Registry and Analysis of Lausanne).
Disclosures
Dr Ntaios received through his institution a research grant for the AF‐ESUS (Prediction of AF [Atrial Fibrillation] in ESUS [Embolic Stroke of Undetermined Source]) study (Clinicaltrials.gov Identifier: NCT02766205), which is an investigator‐initiated study supported by Pfizer through the BMS/Pfizer European Thrombosis Investigator Initiated Research Program; was a member of the Steering Committee of the NAVIGATE ESUS (Rivaroxaban Versus Aspirin in Secondary Prevention of Stroke and Prevention of Systemic Embolism in Patients With Recent Embolic Stroke of Undetermined Source) trial; and received speaker fees/advisory board/research support from Amgen, Bayer, BMS/Pfizer, Boehringer‐Ingelheim, Elpen, Galenica, Sanofi, and Winmedica. All fees are paid to his institution (University of Thessaly). Dr Sirimarco received a research grant from Swiss Heart Foundation, received congress travel support from Bayer and Shire, and served on scientific advisory boards for Amgen and Daiichi‐Sankyo. All fees are paid to his institution (CHUV [Centre hospitalier universitaire vaudois]). Dr Korobok reports speaker fees/honoraria for advisory boards from Pfizer and Amgen. Dr Michel received within the past 2 years through his institution research grants from the Swiss Heart Foundation and BMS; speaker fees from Boehringer‐Ingelheim, Medtronic, and Amgen; consulting fees from Medtronic and Amgen; and honoraria from scientific advisory boards from Boehringer‐Ingelheim, Pfizer, and BMS. All this support is goes to his institution (CHUV) and is used for stroke education and research. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2019;8:e012858 DOI: 10.1161/JAHA.119.012858.)
References
- 1. Hart RG, Catanese L, Perera KS, Ntaios G, Connolly SJ. Embolic stroke of undetermined source: a systematic review and clinical update. Stroke. 2017;48:867–872. [DOI] [PubMed] [Google Scholar]
- 2. Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O'Donnell MJ, Sacco RL, Connolly SJ; Cryptogenic Stroke/ESUS International Working Group . Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429–438. [DOI] [PubMed] [Google Scholar]
- 3. Ntaios G, Papavasileiou V, Milionis H, Makaritsis K, Manios E, Spengos K, Michel P, Vemmos K. Embolic strokes of undetermined source in the Athens stroke registry: a descriptive analysis. Stroke. 2015;46:176–181. [DOI] [PubMed] [Google Scholar]
- 4. Ntaios G, Papavasileiou V, Milionis H, Makaritsis K, Vemmou A, Koroboki E, Manios E, Spengos K, Michel P, Vemmos K. Embolic strokes of undetermined source in the Athens stroke registry: an outcome analysis. Stroke. 2015;46:2087–2093. [DOI] [PubMed] [Google Scholar]
- 5. Ntaios G, Hart RG. Embolic stroke. Circulation. 2017;136:2403–2405. [DOI] [PubMed] [Google Scholar]
- 6. Michel P, Odier C, Rutgers M, Reichhart M, Maeder P, Meuli R, Wintermark M, Maghraoui A, Faouzi M, Croquelois A, Ntaios G. The Acute STroke Registry and Analysis of Lausanne (ASTRAL): design and baseline analysis of an ischemic stroke registry including acute multimodal imaging. Stroke. 2010;41:2491–2498. [DOI] [PubMed] [Google Scholar]
- 7. Vemmos K, Ntaios G, Spengos K, Savvari P, Vemmou A, Pappa T, Manios E, Georgiopoulos G, Alevizaki M. Association between obesity and mortality after acute first‐ever stroke: the obesity‐stroke paradox. Stroke. 2011;42:30–36. [DOI] [PubMed] [Google Scholar]
- 8. Ntaios G, Vemmos K, Lip GY, Koroboki E, Manios E, Vemmou A, Rodriguez‐Campello A, Cuadrado‐Godia E, Giralt‐Steinhauer E, Arnao V, Caso V, Paciaroni M, Diez‐Tejedor E, Fuentes B, Perez Lucas J, Arauz A, Ameriso SF, Hawkes MA, Pertierra L, Gomez‐Schneider M, Bandini F, Chavarria Cano B, Iglesias Mohedano AM, Garcia Pastor A, Gil‐Nunez A, Putaala J, Tatlisumak T, Barboza MA, Athanasakis G, Makaritsis K, Papavasileiou V. Risk stratification for recurrence and mortality in embolic stroke of undetermined source. Stroke. 2016;47:2278–2285. [DOI] [PubMed] [Google Scholar]
- 9. Ntaios G, Perlepe K, Sirimarco G, Strambo D, Eskandari A, Karagkiozi E, Vemmou A, Koroboki E, Manios E, Makaritsis K, Michel P, Vemmos K. Carotid plaques and detection of atrial fibrillation in embolic stroke of undetermined source. Neurology. 2019;92:e2644–e2652. [DOI] [PubMed] [Google Scholar]
- 10. Hart RG, Sharma M, Mundl H, Shoamanesh A, Kasner SE, Berkowitz SD, Pare G, Kirsch B, Pogue J, Pater C, Peters G, Davalos A, Lang W, Wang Y, Wang Y, Cunha L, Eckstein J, Tatlisumak T, Shamalov N, Mikulik R, Lavados P, Hankey GJ, Czlonkowska A, Toni D, Ameriso SF, Gagliardi RJ, Amarenco P, Bereczki D, Uchiyama S, Lindgren A, Endres M, Brouns R, Yoon B‐W, Ntaios G, Veltkamp R, Muir KW, Ozturk S, Arauz A, Bornstein N, Bryer A, O'Donnell MJ, Weitz J, Peacock F, Themeles E, Connolly SJ. Rivaroxaban for secondary stroke prevention in patients with embolic strokes of undetermined source: design of the NAVIGATE ESUS randomized trial. Eur Stroke J. 2016;1:146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yaghi S, Moon YP, Mora‐McLaughlin C, Willey JZ, Cheung K, Di Tullio MR, Homma S, Kamel H, Sacco RL, Elkind MS. Left atrial enlargement and stroke recurrence: the Northern Manhattan Stroke Study. Stroke. 2015;46:1488–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O'Neal WT, Kamel H, Kleindorfer D, Judd SE, Howard G, Howard VJ, Soliman EZ. Premature atrial contractions on the screening electrocardiogram and risk of ischemic stroke: the reasons for geographic and racial differences in stroke study. Neuroepidemiology. 2016;47:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bulwa Z, Gupta A. Embolic stroke of undetermined source: the role of the nonstenotic carotid plaque. J Neurol Sci. 2017;382:49–52. [DOI] [PubMed] [Google Scholar]
- 14. Amarenco P, Duyckaerts C, Tzourio C, Henin D, Bousser MG, Hauw JJ. The prevalence of ulcerated plaques in the aortic arch in patients with stroke. N Engl J Med. 1992;326:221–225. [DOI] [PubMed] [Google Scholar]
- 15. Amarenco P, Cohen A, Tzourio C, Bertrand B, Hommel M, Besson G, Chauvel C, Touboul PJ, Bousser MG. Atherosclerotic disease of the aortic arch and the risk of ischemic stroke. N Engl J Med. 1994;331:1474–1479. [DOI] [PubMed] [Google Scholar]
- 16. Lopes RD, Vora AN, Liaw D, Granger CB, Darius H, Goodman SG, Mehran R, Windecker S, Alexander JH. An open‐label, 2 x 2 factorial, randomized controlled trial to evaluate the safety of apixaban vs. vitamin K antagonist and aspirin vs. placebo in patients with atrial fibrillation and acute coronary syndrome and/or percutaneous coronary intervention: rationale and design of the AUGUSTUS trial. Am Heart J. 2018;200:17–23. [DOI] [PubMed] [Google Scholar]
- 17. Verma A, Ha ACT, Kirchhof P, Hindricks G, Healey JS, Hill MD, Sharma M, Wyse DG, Champagne J, Essebag V, Wells G, Gupta D, Heidbuchel H, Sanders P, Birnie DH. The optimal anti‐coagulation for enhanced‐risk patients post‐catheter ablation for atrial fibrillation (OCEAN) trial. Am Heart J. 2018;197:124–132. [DOI] [PubMed] [Google Scholar]
- 18. Anderson DR, Dunbar M, Murnaghan J, Kahn SR, Gross P, Forsythe M, Pelet S, Fisher W, Belzile E, Dolan S, Crowther M, Bohm E, MacDonald SJ, Gofton W, Kim P, Zukor D, Pleasance S, Andreou P, Doucette S, Theriault C, Abianui A, Carrier M, Kovacs MJ, Rodger MA, Coyle D, Wells PS, Vendittoli PA. Aspirin or rivaroxaban for VTE prophylaxis after hip or knee arthroplasty. N Engl J Med. 2018;378:699–707. [DOI] [PubMed] [Google Scholar]
- 19. Robertson L, Yeoh SE, Ramli A. Secondary prevention of recurrent venous thromboembolism after initial oral anticoagulation therapy in patients with unprovoked venous thromboembolism. Cochrane Database Syst Rev. 2017;12:CD011088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun Q, Chang S, Lu S, Zhang Y, Chang Y. The efficacy and safety of 3 types of interventions for stroke prevention in patients with cardiovascular and cerebrovascular diseases: a network meta‐analysis. Clin Ther. 2017;39:1291–1312.e1298. [DOI] [PubMed] [Google Scholar]
- 21. Alexopoulos D, Vlachakis P, Lekakis J. Triple antithrombotic therapy in atrial fibrillation patients undergoing PCI: a fading role. Cardiovasc Drugs Ther. 2017;31:319–324. [DOI] [PubMed] [Google Scholar]
- 22. Xu WW, Hu SJ, Wu T. Risk analysis of new oral anticoagulants for gastrointestinal bleeding and intracranial hemorrhage in atrial fibrillation patients: a systematic review and network meta‐analysis. J Zhejiang Univ Sci B. 2017;18:567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bosch J, Eikelboom JW, Connolly SJ, Bruns NC, Lanius V, Yuan F, Misselwitz F, Chen E, Diaz R, Alings M, Lonn EM, Widimsky P, Hori M, Avezum A, Piegas LS, Bhatt DL, Branch KRH, Probstfield JL, Liang Y, Liu L, Zhu J, Maggioni AP, Lopez‐Jaramillo P, O'Donnell M, Fox KAA, Kakkar A, Parkhomenko AN, Ertl G, Stork S, Keltai K, Keltai M, Ryden L, Dagenais GR, Pogosova N, Dans AL, Lanas F, Commerford PJ, Torp‐Pedersen C, Guzik TJ, Verhamme PB, Vinereanu D, Kim JH, Ha JW, Tonkin AM, Varigos JD, Lewis BS, Felix C, Yusoff K, Steg PG, Aboyans V, Metsarinne KP, Anand SS, Hart RG, Lamy A, Moayyedi P, Leong DP, Sharma M, Yusuf S. Rationale, design and baseline characteristics of participants in the cardiovascular outcomes for people using anticoagulation strategies (COMPASS) trial. Can J Cardiol. 2017;33:1027–1035. [DOI] [PubMed] [Google Scholar]
- 24. Kaymaz C. EINSTEIN CHOICE: comparison of rivaroxaban treatment and prophylactic doses with aspirin in the extended treatment of patients with venous thromboembolism [in Turkish]. Turk Kardiyol Dern Ars. 2017;45:1–7. [DOI] [PubMed] [Google Scholar]
- 25. Ng KH, Sharma M, Benavente O, Gioia L, Field TS, Hill MD, Coutts SB, Butcher K. Dabigatran following acute transient ischemic attack and minor stroke II (DATAS II). Int J Stroke. 2017;12:910–914. [DOI] [PubMed] [Google Scholar]
- 26. Suen K, Westh RN, Churilov L, Hardidge AJ. Low‐molecular‐weight heparin and the relative risk of surgical site bleeding complications: results of a systematic review and meta‐analysis of randomized controlled trials of venous thromboprophylaxis in patients after total joint arthroplasty. J Arthroplasty. 2017;32:2911–2919.e2916. [DOI] [PubMed] [Google Scholar]
- 27. Andreas M, Moayedifar R, Wieselthaler G, Wolzt M, Riebandt J, Haberl T, Angleitner P, Schloglhofer T, Wiedemann D, Schima H, Laufer G, Zimpfer D. Increased thromboembolic events with dabigatran compared with vitamin k antagonism in left ventricular assist device patients: a randomized controlled pilot trial. Circ Heart Fail. 2017;10:e003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kamel H, Longstreth WT Jr, Tirschwell DL, Kronmal RA, Broderick JP, Palesch YY, Meinzer C, Dillon C, Ewing I, Spilker JA, Di Tullio MR, Hod EA, Soliman EZ, Chaturvedi S, Moy CS, Janis S, Elkind MS. The atrial cardiopathy and antithrombotic drugs in prevention after cryptogenic stroke randomized trial: rationale and methods. Int J Stroke. 2019;14:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ntaios G, Papavasileiou V, Lip GY, Milionis H, Makaritsis K, Vemmou A, Koroboki E, Manios E, Spengos K, Michel P, Vemmos K. Embolic stroke of undetermined source and detection of atrial fibrillation on follow‐up: how much causality is there? J Stroke Cerebrovasc Dis. 2016;25:2975–2980. [DOI] [PubMed] [Google Scholar]


