Abstract
Background
Aging is associated with a modest decline in ankle‐brachial index (ABI); however, the underpinnings of this decline are not fully understood. The greater systolic ankle than brachial blood pressure, a normal ABI implies, is partially attributed to lower central than peripheral arterial stiffness. Hence, we examined the hypothesis that the age‐associated decline in ABI is associated with central arterial stiffening with aging, assessed by pulse wave velocity.
Methods and Results
We analyzed longitudinal data from 974 participants aged 27 to 95 years from the Baltimore Longitudinal Study of Aging who were free of clinically significant cardiovascular disease. Participants had an average of 4 visits with a 6.8‐year average follow‐up time. Linear mixed‐effects models showed that the average ABI decline beyond the age of 70 years was 0.03 per decade. In multiple regression analysis, the ABI rate of change was inversely associated with initial age (standardized β=−0.0711, P=0.0282), independent of peripheral disease factors and baseline ABI. After adjustment, the pulse wave velocity rate of change was inversely associated with ABI rate of change (standardized β=−0.0993, P=0.0040), rendering the association of the latter with initial age nonsignificant (standardized β=−0.0265, P=0.5418).
Conclusions
A modest longitudinal decline in ABI beyond the age of 70 years was shown to be independent of traditional risk factors for peripheral arterial disease but was accounted for by an increase in pulse wave velocity. A modest decline in ABI with aging might be a manifestation of changes in central hemodynamics and not necessarily attributable to peripheral flow–limiting factors.
Keywords: aging, arterial stiffness, epidemiology, hemodynamics, peripheral artery disease
Subject Categories: Peripheral Vascular Disease, Aging, Epidemiology, Hemodynamics
Clinical Perspective
What Is New?
This is the first study to show a longitudinal decline in ankle‐brachial index with aging in a healthy population over a broad age range and free of overt atherosclerotic disease.
These changes were independent of traditional risk factors of peripheral arterial disease and associated with central arterial stiffening.
What Are the Clinical Implications?
These findings suggest that modest reduction in ankle‐brachial index in older individuals represent exaggerated age‐associated hemodynamic changes rather than being exclusively attributed to flow‐limiting lesions.
Ankle‐brachial index (ABI), defined as the ratio of ankle systolic blood pressure (SBP) to that in the arm,1 has been considered to be a marker for peripheral arterial disease (PAD).2, 3 While ABI <0.90 is the traditional cutoff for a diagnosis of PAD, there is growing evidence that compared with patients with normal ABI (1.10–1.40), individuals with modestly reduced ABI (0.90–1.09) are more likely to have compromised walking endurance and lower thigh muscle oxidative capacity.4, 5, 6, 7
Previous studies have shown that aging is associated with a modest decline of ABI among older adults free of PAD, independent of other cardiovascular risk factors.8, 9 On the other hand, a study in younger adults has shown an initial increase in ABI in the 5th decade of life followed by plateauing of ABI by the 6th decade.10 The underpinnings of such modest declines in ABI have not been fully clarified. This information is essential to understand the mechanism of ABI changes with aging, elucidate mechanisms for the adverse health outcomes observed with modestly reduced ABI, and identify effective strategies for the treatment of this condition.
The physiologically higher ankle than brachial SBP is partially attributed to the relatively greater peripheral than central arterial stiffness11; specifically, the stiffer muscular artery result in increasing pulse pressure amplification with greater distance from the heart (ie, higher SBP at the ankle than the arm). Aging, however, is characterized by a marked increase in central but not peripheral arterial stiffness, hence attenuating pulse pressure amplification and subsequently shrinking the ankle‐brachial pressure difference.12 Current studies have yet to examine the association between the profound age‐associated changes in central hemodynamics and ABI.13
We hypothesized that the age‐associated increase in central arterial stiffness, assessed by central pulse wave velocity (cPWV), is associated with a decline in ABI. We tested this hypothesis in the BLSA (Baltimore Longitudinal Study of Aging), a prospective cohort study with repeated measures of ABI, cPWV, and other cardiovascular risk factors in addition to accompanying traditional cardiovascular risk factors.
We aimed to examine: (1) the patterns and rates of longitudinal trajectories in ABI over a broad age range, and (2) the association of ABI change with baseline values and rates of change of cPWV after adjusting for traditional cardiovascular risk factors.
Methods
Study Sample
Participants in the BLSA are community‐dwelling volunteers who are evaluated via medical, physiological, and psychological testing over 3 consecutive days at specified intervals.14 More specifically, the BLSA is an open‐entry, open‐exit study with irregularly spaced visits based on participants’ current ages. Individuals younger than 60 years return every 4 years, those between 60 and 79 years visit every 2 years, and participants 80 years and older return annually.
Between 2004 and 2017, a total of 3557 repeated measures of blood pressure, ABI, and other assessments were obtained from 974 participants. Participants were classified by race as being white or not. Twenty‐one participants were excluded for ever having an ABI <0.90. Ongoing approval from the National Institute of Environmental Health Sciences has been granted to the BLSA, and written informed consent was obtained from all study participants.
BLSA data are available based on requests made through the National Institutes of Health website,15 which will direct inquiries to the BLSA Data Sharing Proposal Review Committee. All data releases require approval of the institutional review board of the National Institute of Environmental Health Sciences. BLSA consent forms do not allow data publication in public domains.
Blood Pressures and ABI
Ankle and brachial SBPs and ABI were measured during each visit using an oscillometric device (Colin VP‐2000, Omron Healthcare Inc). The average of left and right appendage measurements was used in this analysis. At each visit, patients were categorized as having hypertension if their SBP was ≥140 mm Hg, diastolic blood pressure was ≥90 mm Hg, they were taking antihypertensive medications, or they self‐reported having hypertension, corresponding to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.16 Hypertension medication status during the study was categorized as never, initiated, terminated, and continuous based on medication usage reported at the first and last visits.
Pulse Wave Velocity
cPWV was assessed as carotid‐femoral pulse wave velocity as previously described.13 For all participants, pulse wave velocity was determined via commercial devices (Complior from 2004 to 2010 and SphygmoCor AtCor Medical from 2011 to 2017). Both devices computed carotid‐femoral pulse wave velocity as the distance traveled by the pulse wave over body surface from the carotid to the femoral artery, divided by the time delay in the pulse wave between sites as measured from the foot of each arterial wave and gated by ECG. Pulse wave velocity values were calibrated between devices as previously described.13 Patients were classified as having a change of instruments if different devices were used to assess their PWV during the study.
Clinical Variables
Height was measured for all participants. Patients were classified as ever versus never having been a smoker and ever versus never having PAD (ABI <0.90). Diabetes mellitus was defined per 2011 American Diabetes Association criteria17 (ie, fasting plasma glucose ≥126 mg/dL, 2‐hour glucose ≥200 mg/dL, or glycated hemoglobin ≥6.5%) or based on diabetic medication usage.
Laboratory Studies
After an 8‐hour overnight fast, morning blood samples were taken from the antecubital vein on each visit. Plasma triglyceride and total cholesterol concentrations were determined enzymatically by a standard clinical machine (Abbott Laboratories, ABA‐200 ATC Biochromatic Analyzer). HDL cholesterol was determined via an established precipitation procedure.18 Low‐density lipoprotein cholesterol concentrations were estimated using the Friedewald formula. The glucose oxidase method was employed by a standard instrument (Beckman Instruments, Inc) to measure glucose concentration.19
Statistical Analysis
For continuous variables, baseline values for men and women are reported as mean±SD and were compared via Student t test; proportions of categorical variables were reported and compared using chi‐square statistics. LME models, the best available analytical tools for unbalanced, unequally spaced observations such as those of the BLSA,20 were utilized to assess average longitudinal trajectories and estimate individual rates of change of indexed parameters. Population‐averaged longitudinal trajectories were generated with age expressed as initial age and follow‐up time to distinguish cross‐sectional differences (initial age) from longitudinal changes (initial age×follow‐up time), respectively.
The rates of change for ABI and PWV in each individual were determined by regressing the indexed variable against follow‐up time in years. To accommodate the varying number of follow‐up numbers and intervals, LME models were used to estimate regression coefficients. More specifically, separate LME models were fitted for both ABI and PWV with follow‐up time as a random effect. The rate of change (ROC) for each individual was calculated from the random effect coefficient estimated by the model. As a result of a phenomenon of shrinkage in regression models, a compensatory weighting procedure was performed factoring in individual‐specific shrinkage estimates (for additional details, see Data S1). Individual rates of change were indicated by the index variable with ROC as a subscript. Multiple linear regression analysis was used to examine the determinants of ABIROC, including, as covariates, baseline ABI, baseline and ROC of cPWV, and traditional cardiovascular risk factors, including hypertension, smoking, and diabetes mellitus. Given the established acceleration in PWV around the 6th decade,13 age‐stratified models were also constructed with the age of 70 as a cutoff point. All analyses were performed via SAS for Windows (version 9.4, SAS Institute).
Results
The average initial age of the cohort was 67 years with an average follow‐up time of 6.8 years and an average of 4 visits including repeated measures of PWV and ABI (Table 1). About 43% of participants were ever‐smokers and 48% had hypertension. The average ABI was 1.16±0.09 on the left and 1.17±0.09 on the right. The distributions of men and women by initial age and decade are shown in Table S1. Compared with women, men were older at baseline (68.5 versus 65.8 years, P=0.0007) with similar follow‐up time and number of follow‐ups (Table 1). Men were also taller than women and had higher glucose levels. Additionally, compared with women, men had lower levels of low‐density lipoprotein and HDL, higher baseline ankle and brachial SBPs, higher ABI with higher frequency of smoking and hypertension, and higher usage of hypertension medications.
Table 1.
Baseline Characteristics of the Study Population
| Variable (Units) | Total (N=974) | Men (n=473) | Women (n=501) | P Value |
|---|---|---|---|---|
| Initial age, y | 67.1±12.7 | 68.5±12.8 | 65.8±12.5 | 0.0007 |
| Follow‐up time, y | 6.8±3.3 | 6.7±3.2 | 6.9±3.3 | 0.2325 |
| No. of follow‐ups | 3.7±1.6 | 3.7±1.7 | 3.6±1.6 | 0.2207 |
| Smoking, ever | 406 (42.5) | 234 (49.5) | 172 (34.3) | <0.0001 |
| Hypertension | 470 (48.3) | 264 (55.8) | 206 (41.1) | <0.0001 |
| Diabetes mellitus | 175 (18.0) | 112 (23.7) | 63 (12.6) | <0.0001 |
| Hypertension medication use | 40 (4.1) | 36 (7.6) | 4 (0.8) | <0.0001 |
| Instrument, complior | 811 (83.3) | 397 (83.9) | 414 (82.6) | 0.6071 |
| White race | 659 (68.9) | 348 (73.6) | 311 (62.1) | 0.0002 |
| Height, cm | 168.7±9.2 | 175.3±7.1 | 162.5±6.2 | <0.0001 |
| HR, beats per min | 65.0±11.0 | 62.4±11.2 | 67.4±10.3 | <0.0001 |
| Left ABI | 1.16±0.09 | 1.17±0.09 | 1.15±0.09 | <0.0001 |
| Right ABI | 1.17±0.09 | 1.18±0.09 | 1.17±0.08 | 0.0012 |
| ABI | 1.17±0.08 | 1.18±0.09 | 1.16±0.08 | <0.0001 |
| Brachial SBP | 115.4±13.8 | 117.1±13.2 | 113.7±14.1 | 0.0001 |
| Left ankle SBP | 136.3±18.9 | 139.6±18.2 | 133.2±19.0 | <0.0001 |
| Right ankle SBP | 137.5±19.0 | 140.4±18.6 | 134.8±18.9 | <0.0001 |
| cPWV, m/s | 7.4±1.8 | 7.6±1.8 | 7.2±1.7 | <0.0001 |
Values are expressed as mean±SD or number (percentage). ABI indicates ankle‐brachial index; cPWV, central pulse wave velocity; HR, heart rate; SBP, systolic blood pressure.
An LME model predicting ABI showed significant (initial age×initial age) and (initial age×time) terms with negative coefficients indicating that cross‐sectional differences and longitudinal changes followed parabolic trends with an initial increase followed by a decline with advancing age (Table 2). While ABI was higher in men than women throughout the age spectrum (sex: β=0.0206, P<0.0001), there were no sex differences in age‐associated changes in ABI. To illustrate these complex changes, model‐predicted changes in ABI were plotted showing an initial increase in ABI, cross‐sectionally and longitudinally, up to the age of 70 years, after which ABI declined at greater rates with advancing age (Figure). These changes were similar between left and right ABI (Table S2); hence, average ABI was used for subsequent analysis.
Table 2.
Linear Mixed‐Effects Model Predicting ABI and Determining Cross‐Sectional Differences and Longitudinal Changes by Sex
| Variable (Units) | β | P Value |
|---|---|---|
| Intercept | 0.8948 | <0.0001 |
| Initial age, y | 0.0084 | <0.0001 |
| Initial age×initial age | −0.0001 | <0.0001 |
| Follow‐up time, y | 0.0080 | <0.0001 |
| Initial age×follow‐up time | −0.0001 | <0.0001 |
| Men | 0.0206 | <0.0001 |
| Instrument | 0.0037 | 0.2312 |
ABI indicates ankle‐brachial index.
Figure 1.
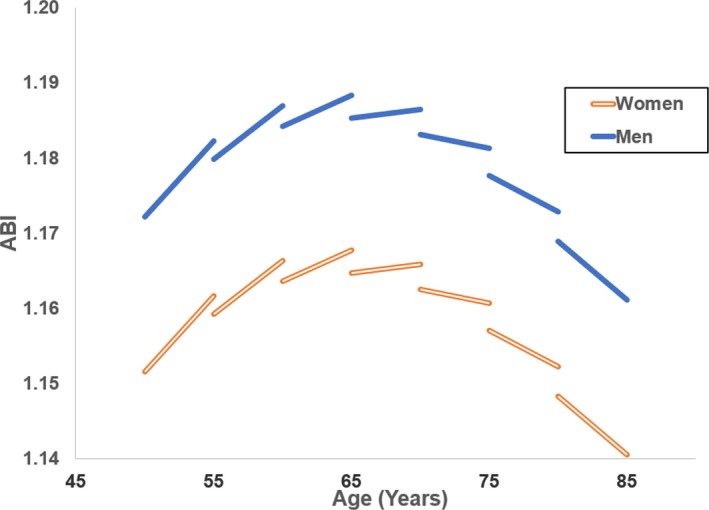
Model‐predicted cross‐sectional differences and longitudinal changes for men and women with age at 5‐year increments. ABI indicates ankle‐brachial index.
To investigate the role of traditional risk factors for PAD in ABI change with aging, multiple linear regression analysis showed that the inverse association of ABIROC with age (standardized β=−0.0711, P=0.0282) was independent of the effects of baseline ABI, sex, smoking, and diabetes mellitus status, with no significant effects of the latter 2 covariates (Table 3, model 1). To investigate the role of arterial stiffening in ABI change, we first included PWV as an independent variable in the LME model predicting ABI and showing an association between ABI and PWV as a time‐varying variable (Table S3). To further examine the association, baseline and ROC of PWV were regressed with ABIROC (Table 3, model 2). Interestingly, cPWVROC, but not baseline cPWV, was associated with ABIROC (standardized β=−0.0993, P=0.0040) and explained the association with aging, rendering initial age statistically nonsignificant (standardized β=−0.0265, P=0.5418) (Table 3, model 2). These changes were independent of change in PWV instrumentation while race and baseline HR, height, and right brachial MAP were not significant determinants of ABIROC. Among hypertension medication statuses, only initiating medication provided a significant effect, which was negative.
Table 3.
Random Effects Linear Regression Models Predicting ABIROC and Adjusting for Covariates
| Variable (Units) | Model 1 | Model 2 | ||
|---|---|---|---|---|
| STβ | P Value | STβ | P Value | |
| Initial age, y | −0.0711 | 0.0282 | −0.0265 | 0.5418 |
| Men | 0.0901 | 0.0061 | 0.0770 | 0.1107 |
| Smoking | ||||
| Never | Reference | |||
| Former | 0.0136 | 0.6756 | 0.0065 | 0.8443 |
| Current | −0.0053 | 0.8689 | −0.0095 | 0.7685 |
| Diabetes mellitus | 0.0091 | 0.7788 | 0.0201 | 0.5426 |
| Initial ABI | −0.4209 | <0.0001 | −0.4271 | <0.0001 |
| Hypertension medication | ||||
| Never | ··· | ··· | Reference | |
| Initiated | ··· | ··· | −0.0727 | 0.0230 |
| Terminated | ··· | ··· | 0.0294 | 0.3572 |
| Continuous | ··· | ··· | −0.0006 | 0.9861 |
| Instrument effect | ··· | ··· | 0.0264 | 0.4698 |
| White race | ··· | ··· | 0.0183 | 0.5938 |
| Height, cm | ··· | ··· | 0.0515 | 0.2825 |
| HR, beats per min | ··· | ··· | 0.0316 | 0.3441 |
| RB MAP, mm Hg | ··· | ··· | −0.0193 | 0.5627 |
| cPWV, m/s | ··· | ··· | −0.0194 | 0.6141 |
| cPWVROC, m/s per year | ··· | ··· | −0.0993 | 0.0040 |
cPWV indicates central pulse wave velocity refer to heart rate, HR, heart rate; RB MAP, right brachial mean arterial pressure; STβ, standardized beta coefficients. The ROC subscript denotes the rate of change and represents changes in each individual's ankle‐brachial index (ABI) and pulse wave velocity over time (ie, the random effects).
Given the parabolic nature of changes in ABI, an age‐stratified analysis was performed (Table 4) showing that the associations with PWVROC were observed in participants 70 years and older (PWVROC: standardized β=−0.1421, P=0.0068), but not among those who were younger than 70 years (PWVROC: standardized β=0.0010, P=0.9820). Additionally, among individuals younger than 70 years, former smoking status had a positive association with ABIROC (standardized β=0.1138, P=0.0066) compared with current and never smoking statuses. White race was also associated with greater ABIROC among younger but not older individuals. Similarly, starting hypertension medication was associated with a lower ABIROC, among participants older but not among those younger than 70 years. All other determinants in both age‐stratified models were nonsignificant.
Table 4.
Age‐Stratified Models Predicting ABIROC and Examining the Association With PWVROC
| Variable (Units) | Initial Age <70 y | Initial Age ≥70 y | ||
|---|---|---|---|---|
| STβ | P Value | STβ | P Value | |
| Initial age, y | −0.0136 | 0.7699 | −0.0342 | 0.5350 |
| Men | 0.0670 | 0.2691 | 0.1101 | 0.1316 |
| Smoking | ||||
| Never | Reference | |||
| Former | 0.1138 | 0.0066 | −0.0654 | 0.1889 |
| Current | 0.0553 | 0.1672 | −0.0653 | 0.1817 |
| Diabetes mellitus | 0.0085 | 0.8402 | 0.0167 | 0.7373 |
| Initial ABI | −0.5900 | <0.0001 | −0.3499 | <0.0001 |
| Hypertension medication | ||||
| Never | Reference | |||
| Initiated | −0.0087 | 0.8269 | −0.1307 | 0.0072 |
| Terminated | 0.0250 | 0.5311 | 0.0378 | 0.4357 |
| Continuous | −0.0027 | 0.9473 | −0.0057 | 0.9071 |
| Instrument effect | 0.0726 | 0.0697 | −0.0283 | 0.6065 |
| White race | 0.0852 | 0.0399 | −0.0398 | 0.4378 |
| Height, cm | 0.0051 | 0.9305 | 0.0632 | 0.3958 |
| HR, beats per min | −0.0349 | 0.4045 | 0.0684 | 0.1896 |
| RB MAP, mm Hg | −0.0190 | 0.6613 | −0.0331 | 0.5154 |
| cPWV, m/s | 0.0736 | 0.1008 | −0.0803 | 0.1466 |
| cPWVROC, m/s per year | 0.0010 | 0.9820 | −0.1421 | 0.0068 |
cPWV indicates central pulse wave velocity; HR, heart rate; RB MAP, right brachial mean arterial pressure; STβ, standardized beta coefficients. The ROC subscript denotes the rate of change and represents changes in each individual's ankle‐brachial index (ABI) and pulse wave velocity over time (ie, the random effects).
Discussion
This is the first study in a healthy population that examined the longitudinal changes in ABI over a broad age spectrum and explored its association with changes of central arterial stiffening. We found curvilinear cross‐sectional differences in ABI with advancing age and a longitudinal decline in ABI beyond the age of 70 years. These age‐associated changes were independent from traditional risk factors of PAD but could be explained by central arterial stiffening and rising PWV with advancing age.
Our analysis showed a consistently higher ABI in men than women across the age range studied. In addition, on average, ABI was slightly higher on the right than on the left side. These findings agree with previous study findings.21, 22, 23, 24, 25 However, our analysis showed no differences in the longitudinal changes between men and women, nor between left and right (data not shown), and therefore, bilateral ABIs were averaged (models predicting left and right ABI rates of change are presented in Table S2).
We report an increase in ABI in both men and women with advancing age until the age of 70 years, after which it plateaus and starts declining. These findings of curvilinear changes reconcile the few and contradicting reports showing different ABI trajectories in different cohorts with different age ranges. A longitudinal study of a younger Japanese population has shown a similar initial increase in ABI among participants younger than 50 years, followed by plateauing starting by the age of 50 years.10 A cross‐sectional analysis of the same cohort showed a maximum ABI in the 60‐ to 69‐year age group.24 On the older side of the age spectrum, a study using data from the Cardiovascular Health Study documented that a decline in ABI increases with advancing age.26
It is important to note that in other longitudinal studies, ABI rates of decline are 0.04,8 0.12,26 0.14,27 0.08,9 and 0.0528 per decade. These rates are up to more than 3 times greater than ours, which is potentially the result of the presence or progression of PAD in these populations. Also, these studies do not quantify the variation in ABIROC across the age spectrum studied, but instead present age‐averaged values.
In exploring the determinants of the longitudinal changes in ABI with aging, we found that traditional cardiovascular risk factors such as smoking and diabetes mellitus were not associated with accelerated decline in ABI at age older than 70 years. Interestingly, former smokers were more likely to have a more positive change compared with nonsmokers among those younger than 70 years. These findings are consistent with prior reports of improved ABI among individuals who stopped smoking.29, 30 ABIROC was also independent of common ABI covariates such as race, height, heart rate, and mean arterial pressure across the age spectrum. Therefore, our model suggests a mechanism for longitudinal ABI decline that is associated with arterial aging, in addition to the decline associated with PAD. Our findings that the association between rates of change of PWV and ABI are only evidenced in participants 70 years and older further advocates that the subclinical decline in ABI in this age group is associated with the dramatic increase in arterial stiffness beyond the age of 70 years. Alterations in the earlier stages of life are likely to be influenced by other changes such as wave reflection dynamics, which are currently not available for analysis. Further studies including these parameters will help elucidate earlier changes in ABI.
The mechanism that links changes in ABI and PWV are not fully examined in this analysis. However, we believe that one aspect of this association is related to the physiologic basis of the normal values of ABI. The higher ankle than brachial pressures is partially attributed to the greater peripheral pulse pressure amplification as a result of the relatively greater peripheral than central arterial stiffness. The increase in aortic but not peripheral stiffness, which accelerated beyond the age of 70 years,12 reduces the peripheral‐central stiffness gap and subsequently perhaps reduces the pressure gap, yielding a reduction in ABI.
Other Confounders
Other studies indicate that height31 and ethnicity31, 32 are factors that contribute to ABI variability. In our study, height and race were not significantly associated with ABIROC. However, it is important to note that our study is not powered to detect differences by race.
Our study showed that initiating hypertension medication resulted in more negative ABI changes in older individuals; however, this association did not alter the overall changes observed with aging. The mechanism by which hypertension medications change ABI is not clear, although it could potentially be caused by an alternation in the relationship between central and peripheral blood pressures.33, 34 Our analysis was not influenced by adjusting for these medications and their change over the study period.
Limitations
There are important limitations that must be taken into consideration when interpreting these findings. First, this cohort is selected to have high education and socioeconomic status and is relatively healthier than the general population; hence, rates of change might be smaller than observed in the community. Nonetheless, we believe this analysis sheds light on fundamental determinants of ABI changes that are likely accentuated among people with an increased health burden. Second, we did not have data on the proximal plaque that results in increased ABI. However, the low cardiovascular risk of this population and bilateral changes make such an effect less likely. Third, our study population has a complex array of classes, doses, and combinations of antihypertensive medications that makes analysis of these effects challenging and beyond the scope of this article. However, in light of the current study showing that the initiation of hypertension medication resulted in more negative ABI change in older individuals, a follow‐up study exploring detailed medication effects on ABI change with aging would be valuable. Fourth, the data do not explain the rise of ABI at younger age. While changes in PWV are known to occur later in life, other hemodynamic alterations such as those associated with wave reflection dynamics occur earlier and may partially explain the rise of ABI among younger adults. Further studies are needed to investigate the association between hemodynamics and ABI changes in early life. Fifth, this study evaluated cPWV; however, lower extremity PWV was not available. Prior research has shown much less age‐associated changes in peripheral PWV measured by carotid‐radial PWV.12 While one can infer that such change would apply to other muscular arterial beds, such as those of the legs, simultaneous measures of central and peripheral PWVs with ABI comprise a potentially valuable future study.
Conclusions
Aging is associated with a longitudinal decline in ABI beyond the age of 70 years in a healthy population free of clinical cardiovascular disease. The association is independent of cardiovascular risk factors and closely associated with increasing central arterial stiffness with aging. Hence, reduction in ABI could at least partially represent exaggerated age‐associated hemodynamic changes rather than being exclusively attributed to flow‐limiting lesions. Additional studies are necessary to identify other alterations in arterial properties that may underlie the subclinical reduction in ABI with aging.
Sources of Funding
This study was funded by the Intramural Research Program of the National Institute on Aging.
Disclosures
None.
Supporting information
Data S1. Supplemental methods.
Table S1. Sex of the Study Cohort by Decade (Number [Percentage])
Table S2. Linear‐Mixed Effects Models Predicting Left and Right ABI
Table S3. Linear Mixed‐Effects Model Predicting ABI and Determining Cross‐Sectional Differences and Longitudinal Changes by Sex Including PWV as an Independent Variable
(J Am Heart Assoc. 2019;8:e011650 DOI: 10.1161/JAHA.118.011650.)
References
- 1. Winsor T. Influence of arterial disease on the systolic blood pressure gradients of the extremity. Am J Med Sci. 1950;220:117–126. [DOI] [PubMed] [Google Scholar]
- 2. Carter SA. Indirect systolic pressures and pulse waves in arterial occlusive diseases of the lower extremities. Circulation. 1968;37:624–637. [DOI] [PubMed] [Google Scholar]
- 3. Yao ST, Hobbs JT, Irvine WT. Ankle systolic pressure measurements in arterial disease affecting the lower extremities. Br J Surg. 1969;56:676–679. [DOI] [PubMed] [Google Scholar]
- 4. Wang JC, Criqui MH, Denenberg JO, McDermott MM, Golomb BA, Fronek A. Exertional leg pain in patients with and without peripheral arterial disease. Circulation. 2005;112:3501–3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McDermott MMG, Liu K, Criqui MH, Ruth K, Goff D, Saad MF, Wu C, Homma S, Sharrett AR. Ankle‐brachial index and subclinical cardiac and carotid disease: the Multi‐Ethnic Study of Atherosclerosis. Am J Epidemiol. 2005;162:33–41. [DOI] [PubMed] [Google Scholar]
- 6. McDermott MM, Guralnik JM, Tian L, Liu K, Ferrucci L, Liao Y, Sharma L, Criqui MH. Associations of borderline and low normal ankle‐brachial index values with functional decline at 5‐year follow‐up. The WALCS (Walking and Leg Circulation Study). J Am Coll Cardiol. 2009;53:1056–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. AlGhatrif M, Zane A, Oberdier M, Canepa M, Studenski S, Simonsick E, Spencer RG, Fishbein K, Reiter D, Lakatta EG, McDermott MM, Ferrucci L. Lower mitochondrial energy production of the thigh muscles in patients with low‐normal ankle‐brachial index. J Am Heart Assoc. 2017;6:e006604 DOI: 10.1161/JAHA.117.006604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bird CE, Criqui MH, Fronek A, Denenberg JO, Klauber MR, Langer RD. Quantitative and qualitative progression of peripheral arterial disease by non‐invasive testing. Vasc Med. 1999;4:15–21. [DOI] [PubMed] [Google Scholar]
- 9. Smith FB, Lee AJ, Price JF, Van Wijk MCW, Fowkes FGR. Changes in ankle brachial index in symptomatic and asymptomatic subjects in the general population. J Vasc Surg. 2003;38:1323–1330. [DOI] [PubMed] [Google Scholar]
- 10. Toma Y, Ishida A, Kinjo K, Ohya Y. Change in ankle‐brachial index over time in a screened Japanese cohort—the Okinawa Peripheral Arterial Disease Study. Circ J. 2016;80:2004–2009. [DOI] [PubMed] [Google Scholar]
- 11. Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jönsson B, Lacroix P, Marin B, Mcdermott MM, Norgren L, Pande RL, Preux PM, Stoffers HE, Treat‐Jacobson D. Measurement and interpretation of the Ankle‐Brachial Index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–2909. [DOI] [PubMed] [Google Scholar]
- 12. Choi CU, Kim EJ, Kim SH, Shin SY, Choi UJ, Kim JW, Lim HE, Rha SW, Park CG, Seo HS, Oh DJ. Differing effects of aging on central and peripheral blood pressures and pulse wave velocity: a direct intraarterial study. J Hypertens. 2010;28:1252–1260. [DOI] [PubMed] [Google Scholar]
- 13. AlGhatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, Scuteri A, Najjar SS, Ferrucci L, Lakatta EG. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension. 2013;62:934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shock N, Greulich R, Andres R. Normal human aging: the Baltimore Longitudinal Study of Aging. 1984.
- 15. BLSA data use. Available at: https://www.blsa.nih.gov/. Accessed July 1, 2019.
- 16. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Roccella EJ; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee . The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560. [DOI] [PubMed] [Google Scholar]
- 17. American Diabetes Association . Standards of medical care in diabetes—2011. Diabetes Care. 2011;34:S11–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Warnick GR, Benderson J, Albers JJ. Dextran sulfate‐Mg2+ precipitation procedure for quantitation of high‐density‐lipoprotein cholesterol. Clin Chem. 1982;28:1379–1388. [PubMed] [Google Scholar]
- 19. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 20. Morrell CH, Brant LJ, Ferrucci L. Model choice can obscure results in longitudinal studies. J Gerontol A Biol Sci Med Sci. 2009;64A:215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hiatt WR, Hoag S, Hamman RF. Effect of diagnostic criteria on the prevalence of peripheral arterial disease. Circulation. 1995;91:1472–1479. [DOI] [PubMed] [Google Scholar]
- 22. Zheng ZJ, Sharrett AR, Chambless LE, Rosamond WD, Nieto FJ, Sheps DS, Dobs A, Evans GW, Heiss G. Associations of ankle‐brachial index with clinical coronary heart disease, stroke and preclinical carotid and popliteal atherosclerosis: the Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis. 1997;131:115–125. [DOI] [PubMed] [Google Scholar]
- 23. Meijer WT, Hoes AW, Rutgers D, Bots ML, Hofman A, Grobbee DE. Peripheral arterial disease in the elderly: the Rotterdam Study. Arterioscler Thromb Vasc Biol. 1998;18:185–192. [DOI] [PubMed] [Google Scholar]
- 24. Ishida A, Miyagi M, Kinjo K, Ohya Y. Age‐ and sex‐related effects on ankle–brachial index in a screened cohort of Japanese: the Okinawa Peripheral Arterial Disease Study (OPADS). Eur J Prev Cardiol. 2014;21:712–718. [DOI] [PubMed] [Google Scholar]
- 25. Stoffers HE, Rinkens PE, Kester AD, Kaiser V, Knottnerus JA. The prevalence of asymptomatic and unrecognized peripheral arterial occlusive disease. Int J Epidemiol. 1996;25:282–290. [DOI] [PubMed] [Google Scholar]
- 26. Kennedy M, Solomon C, Manolio TA, Criqui MH, Newman AB, Polak JF, Burke GL, Enright P, Cushman M. Risk factors for declining ankle‐brachial index in men and women 65 years or older. Arch Intern Med. 2005;165:1896. [DOI] [PubMed] [Google Scholar]
- 27. Aquino R, Johnnides C, Makaroun M, Whittle JC, Muluk VS, Kelley ME, Muluk SC. Natural history of claudication: long‐term serial follow‐up study of 1244 claudicants. J Vasc Surg. 2001;34:962–970. [DOI] [PubMed] [Google Scholar]
- 28. Lahoz C, Garcia‐Fernandez T, Barrionuevo M, Vicente I, Gonzalez‐Alegre T, Mostaza JM. Differences in the ankle‐brachial index in the general population after 4 years of follow‐up. Vasa. 2013;42:112–119. [DOI] [PubMed] [Google Scholar]
- 29. Lee YH, Shin MH, Kweon SS, Choi JS, Rhee JA, Ahn HR, Yun WJ, Ryu SY, Kim BH, Nam HS, Jeong SK, Park KS. Cumulative smoking exposure, duration of smoking cessation, and peripheral arterial disease in middle‐aged and older Korean men. BMC Public Health. 2011;11:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wei Y, Liu H, Liu B. Impact of smoking and smoking cessation on arterial stiffness in healthy individuals. Heart. 2011;97:A107. [Google Scholar]
- 31. Aboyans V, Criqui MH, McClelland RL, Allison MA, McDermott MM, Goff DC, Manolio TA. Intrinsic contribution of gender and ethnicity to normal ankle‐brachial index values: the Multi‐Ethnic Study of Atherosclerosis (MESA). J Vasc Surg. 2007;45:319–327. [DOI] [PubMed] [Google Scholar]
- 32. Allison MA, Peralta CA, Wassel CL, Aboyans V, Arnett DK, Cushman M, Eng J, Ix J, Rich SS, Criqui MH. Genetic ancestry and lower extremity peripheral artery disease in the Multi‐Ethnic Study of Atherosclerosis. Vasc Med. 2010;15:351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Protogerou AD, Papaioannou TG, Lekakis JP, Blacher J, Safar ME. The effect of antihypertensive drugs on central blood pressure beyond peripheral blood pressure. Part I: (Patho)‐physiology, rationale and perspective on pulse pressure amplification. Curr Pharm Des. 2009;15:267–271. [DOI] [PubMed] [Google Scholar]
- 34. Protogerou AD, Stergiou GS, Vlachopoulos C, Blacher J, Achimastos A. The effect of antihypertensive drugs on central blood pressure beyond peripheral blood pressure. Part II: evidence for specific class‐effects of antihypertensive drugs on pressure amplification. Curr Pharm Des. 2009;15:272–289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental methods.
Table S1. Sex of the Study Cohort by Decade (Number [Percentage])
Table S2. Linear‐Mixed Effects Models Predicting Left and Right ABI
Table S3. Linear Mixed‐Effects Model Predicting ABI and Determining Cross‐Sectional Differences and Longitudinal Changes by Sex Including PWV as an Independent Variable


