Abstract
Background
Fat deposition (FD) is part of the healing process after myocardial infarction. The characteristics of FD and its impact on the outcome in patients undergoing ventricular tachycardia (VT) ablation have not been thoroughly studied.
Methods and Results
We studied consecutive patients undergoing post–myocardial infarction VT ablation with pre‐procedural cardiac computed tomography. FD was defined as intra‐myocardial attenuation ≤ −30 HU on computed tomography. Clinical, anatomical, and post‐procedural outcome was assessed in the overall population. Electrophysiological characteristics were assessed is a subgroup of patients with high‐density electro‐anatomical maps. Sixty‐nine patients were included (66±12 years). FD was detected in 44 (64%) patients. The presence of FD related to scar age (odds ratio [OR]: 1.14 per year; P=0.001) and scar extent (OR: 1.27 per segment; P=0.02). On electro‐anatomical maps, FD was characterized by lower bipolar amplitude (P<0.001) and prolonged electrogram duration (P<0.001). Although the proportion of local abnormal ventricular activation was similar (P=0.22), local abnormal ventricular activation showed lower amplitude (P<0.001) and were more delayed (P<0.001) in scars with FD. After a mean follow‐up of 26 months, patients with FD experienced a worse outcome including all‐cause mortality and VT recurrence (70% versus 28%, P log rank=0.009). On multivariate analysis, FD (hazard ratio=2.69; 95% CI, 1.12–6.46; P=0.027) and left ventricular systolic dysfunction (hazard ratio=2.57; 95% CI, 1.13–5.85; P=0.024) were independent predictors of adverse outcomes.
Conclusions
FD in patients with post–myocardial infarction VT undergoing catheter ablation relates to scar age and size and may be a marker of adverse outcomes including all‐cause mortality and VT recurrence.
Keywords: catheter ablation, computed tomography, fat deposition, myocardial infarction, ventricular tachycardia
Subject Categories: Computerized Tomography (CT), Arrhythmias, Catheter Ablation and Implantable Cardioverter-Defibrillator, Mortality/Survival
Clinical Perspective
What Is New?
Fat deposition is extremely prevalent within the scar in patients referred for post–myocardial infarction ventricular tachycardia; it relates to scar age and size, and typically progresses as a patchy process.
Scars with fat deposition show distinctive electrophysiological properties including lower voltage, more delayed local abnormal ventricular activation of lower amplitude, and possible colocalization with critical ventricular tachycardia isthmuses.
Fat deposition is potentially associated with a worse outcome after ventricular tachycardia ablation including all‐cause mortality and ventricular tachycardia recurrence.
What Are the Clinical Implications?
Subendocardial fat deposition is an important parameter that should be assessed prior to ablation of ischemic ventricular tachycardia.
Ventricular tachycardia (VT) is a major complication of myocardial infarction (MI) as it carries a substantial risk of death. VT can occur late after the MI as a result of scar remodeling, with areas of slow conduction promoting electrical reentry.1 At the chronic stage, myocardial scar is a partly viable tissue where multiple cells can coexist.2, 3, 4 The dynamic process of myocardial healing increases the complexity of the substrate underlying VT. Sub‐endocardial fat deposition (FD) within the scar is part of this healing process after MI, and has been reported in about two‐thirds of the patients on imaging (computed tomography [CT] or magnetic resonance imaging (MRI))5, 6 and up to 84% on histology.7 Catheter ablation was shown to reduce adverse outcomes in post–MI VT.8 However, many patients still experience VT recurrence after ablation, and multiple shocks significantly impact their quality of life and mortality.9, 10 Part of this failure rate is attributable to the complexity of the VT substrate. The impact of FD on scar electrophysiological properties, arrhythmogenicity, and outcomes after ablation has not been thoroughly studied. We sought to investigate the clinical, anatomical, and electrophysiological characteristics of FD in patients with ventricular tachycardia, and its relationship with post‐ablation outcome.
Methods
The authors declare that all data supporting the findings of this study are available within the article. Interested investigators can contact the authors if further information is needed.
Study Population and Design
In this pilot study, we included consecutive patients with a history of MI who underwent catheter ablation for recurrent sustained VT preceded by a CT scan in our institution between March 2012 and March 2015. Exclusion criteria included presence of a left ventricular assist device, history of prior catheter ablation or LV surgery, severe renal failure or history of severe allergic reaction to iodine contrast media precluding contrast‐enhanced CT, and failure to obtain patient consent. Patients with inconclusive CT imaging were excluded. Based on the analysis of CT images, 2 groups were identified according to the presence of FD within scar. Clinical characteristics, anatomical features, and post‐VT ablation outcome were compared between the 2 groups. In a subset of patients with available electro‐anatomical maps (EAM) acquired during sinus rhythm at high density using a multipolar high‐density mapping catheter (PentaRay; Biosense Webster, Diamond Bar, CA), CT data were registered to characterize the electrophysiological properties in scars with versus without FD. The study was approved by the local Institutional Ethics Committee, and all patients provided informed consent before inclusion.
CT Image Acquisition and Processing
Cardiac CT was performed using a contrast‐enhanced cardiac‐gated method on a 64‐slice scanner (Somatom Definition; Siemens, Forchheim, Germany). Images were acquired during the first pass of 120‐mL iodine contrast media injected using a biphasic protocol (ie, 60 mL of iodine contrast at the rate of 5 mL/s followed by 120 mL of a 50:50 mixture of saline and contrast media, also at the rate of 5 mL/s). Images were reconstructed using a soft tissue convolution filter at end‐diastole in a series of contiguous 1‐mm‐thick short‐axis slices encompassing the whole left ventricle (LV) from base to apex. Typical in‐plane resolution was 0.4×0.4 mm. Mean X‐ray exposure was 3.3±1.1 mSv. The LV was divided into 17 segments using the American Heart Association model.11 In each segment, the following criteria were assessed: minimum thickness, presence of fat defined as areas with attenuation value ≤ −30 HU,12 transmural extent of fat, and the presence of calcifications. Patients with multiple scars were included in the FD group if any scar contained fat. Total scar area was defined as the area with wall thickness ≤5 mm. In the subset of patients with available high‐density EAM during sinus rhythm, images were segmented to derive 3‐dimensional meshes suitable for integration in mapping systems. Image segmentation was performed using MUSIC software (IHU Liryc, Bordeaux and Inria Sophia Antipolis, France), and included all cardiac chambers, coronary vessels, as well as LV wall thickness maps, myocardial fat (ie, pixels with attenuation ≤ −30 HU), and calcification.
Electrophysiological Mapping and Ablation Procedure
All anti‐arrhythmic drugs except amiodarone were discontinued for at least 5 half‐lives before the ablation. EAM was performed during sinus rhythm using Carto V3 or V4 (Biosense‐Webster, Diamond Bar, CA). After the acquisition of endocardial geometry, the 3D‐EAM geometry was registered with the imported CT model. Identifiable anatomic reference points (coronary sinus, left atrium, right atrium, the aorta and mitral annulus) were used as landmarks for alignment and orientation of the 3D EAM and imaging models. Mapping was performed with a multipolar high‐density mapping catheter (PentaRayNav; Biosense Webster). We defined a peak‐to‐peak bipolar amplitude of <1.5 mV as the bipolar low‐voltage zone and unipolar amplitude <8.3 mV as the low unipolar voltage. During sinus rhythm, local abnormal ventricular activities (LAVA) were annotated as defined in reference.13 Additional ventricular pacing maneuvers were performed to confirm LAVA potentials. For each LAVA potential, the bipolar amplitude and the delay to the local farfield were measured manually. VT inducibility was tested in all patients before ablation. In patients with inducible, stable, and hemodynamically tolerated VT, a VT map was acquired to identify the critical isthmus of the VT circuit.
Catheter Ablation
Ablation was performed using an irrigated 4‐mm tip‐catheter (Thermocool, Biosense Webster), with point by point radiofrequency applications up to 50 W. In sinus rhythm, ablation targeted LAVA potentials. In patients with induced VT, ablation was performed during VT by targeting the critical isthmus, aiming at VT termination, and additional ablation was performed during sinus rhythm to eliminate LAVA potentials. Complete LAVA elimination was the main procedural end point. The loss of VT inducibility was also a procedural end point, but inducibility was not tested in case the VT induced before ablation had required direct‐current shock.
Statistical Analysis
Statistical analyses were performed using SPSS 24 (SPSS Inc., Chicago, IL). Continuous variables are presented as the mean±SD or median (25th percentile–75th percentile) as appropriate. Differences between patient groups were tested using the Student t‐test or the Mann–Whitney U test, as appropriate. The Chi‐square test was applied for non‐continuous variables. The correlation between continuous variables was analyzed using Pearson or Spearman tests as appropriate. A multivariate stepwise logistic regression model was built to identify independent clinical and anatomical association with FD, the model's entry criteria being a P<0.1 on univariate analysis. Post‐VT ablation outcome was assessed on a combined end point including all‐cause mortality and VT recurrence. For all time‐to‐event analyses, rates were estimated by the method of Kaplan and Meier and were compared by the log‐rank test. Fat deposition, scar age defined as the delay between the MI and CT scan, left ventricular ejection fraction, the scar extent defined as the number of segments with wall thickness ≤5 mm, the presence of 3‐vessel disease, diabetes mellitus, and smoking were used to construct a multivariate Cox proportional‐hazards survival model with a stepwise analysis. A P≤0.05 was considered statistically significant. All tests were 2‐tailed.
Results
Clinical Characteristics
A flow diagram of the study is shown in Figure 1. Out of 72 patients fulfilling the inclusion criteria, 3 were excluded because of inconclusive CT imaging (2 because of massive implantable cardioverter defibrillator lead artifacts covering the scar area, and 1 because of issues with post‐contrast delay resulting in insufficient enhancement). Thus, 69 patients were analyzed (aged 66±12 years, 7% women). The clinical characteristics of the study population are shown in Table 1. Fat was detected on CT in 44 (64%) patients. Examples of scars with and without fat are shown in Figure 2. Patient characteristics with and without FD are shown in Table 1. The presence of fat did not relate to clinical characteristics (age, sex, risk factors, vascular territory, revascularization method, ventricular function) except for time since infarction which was longer in patients with FD (20±10 versus 10±8 years, P<0.001). The relationship between scar age and presence of fat is illustrated in Figure 3A. By 10 years post–MI, 75% of scars had FD. In addition, 83% of the scars with FD were >10 years. In a stepwise multivariate analysis including 3‐vessel disease, scar age, coronary artery bypass graft (CABG), and left ventricular ejection fraction (LVEF), scar age was the only predictor of FD. The probability of FD increased by 14% per year post–MI (OR: 1.14; 95% CI, 1.06, 1.24; P=0.001).
Figure 1.
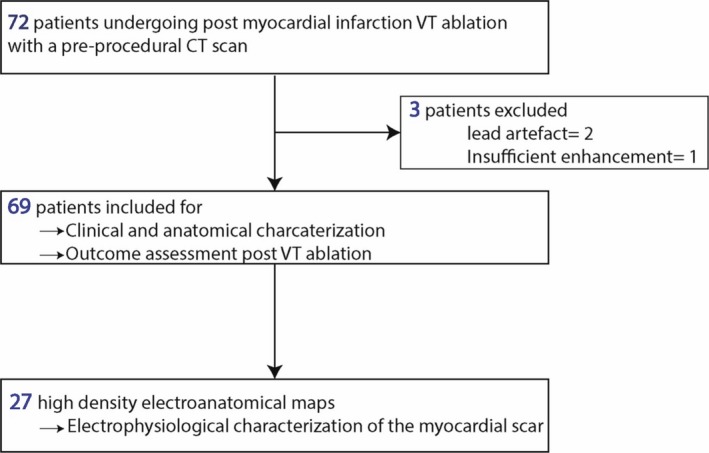
Flow diagram. CT indicates computed tomography; VT, ventricular tachycardia.
Table 1.
Patient Characteristics
| Overall Population n=69 | Fat Deposition (+) 44 (64%) | Fat Deposition (−) 25 (35%) | P Value | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, y | 66±12 | 66±11 | 65±12 | 0.58 |
| Men | 64 (93%) | 41 (93%) | 23 (92%) | 0.86 |
| Hypertenstion | 36 (52%) | 23 (53%) | 13 (52%) | 0.16 |
| Diabetes mellitus | 15 (23%) | 10 (23%) | 5 (20%) | 0.83 |
| Dyslipidemia | 39 (57%) | 25 (58%) | 14 (56%) | 0.54 |
| Smoking | 18 (26%) | 10 (24%) | 8 (32%) | 0.74 |
| BMI, kg/m2 | 28±6 | 28±5 | 29±6 | 0.43 |
| Total cholesterol, mmol/L | 3.9±1.0 | 3.7±0.9 | 4.2±1.0 | 0.09 |
| HDL cholesterol, mmol/L | 1.0±0.4 | 1.0±0.3 | 1.1±0.4 | 0.23 |
| LDL cholesterol, mmol/L | 2.2±0.8 | 2.1±0.7 | 2.4±0.9 | 0.28 |
| Triglyceride, mmol/L | 1.6±0.7 | 1.5±0.6 | 1.6±0.8 | 0.59 |
| Scar age, y | 17±10 | 20±10 | 10±8 | <0.001a |
| 3‐vessel disease | 16 (23%) | 13 (29%) | 3 (12%) | 0.09 |
| CABG | 17 (25%) | 9 (20%) | 8 (32%) | 0.07 |
| PCI | 42 (61%) | 26 (59%) | 16 (64%) | 0.12 |
| ICD | 63 (91%) | 41 (93%) | 22 (88%) | 0.46 |
| CRT | 17 (25%) | 12 (27%) | 5 (21%) | 0.56 |
| Imaging characteristics | ||||
| Scar vascular territory | ||||
| LAD | 51 (74%) | 31 (70%) | 20 (80%) | 0.65 |
| RCA | 30 (44%) | 20 (45%) | 8 (32%) | |
| Cx | 11 (16%) | 9 (20%) | 2 (8%) | |
| Number of scars | ||||
| 1 scar | 48 (70%) | 28 (64%) | 20 (80%) | 0.16 |
| ≥2 scars | 21 (31%) | 16 (36%) | 5 (20%) | |
| Scar extent (n segments) | 5.7±3.2 | 6.5±2.8 | 4.4±3.4 | 0.01a |
| Calcifications | 21 (30%) | 17 (39%) | 4 (16%) | 0.05a |
| LVEDD, mm | 63±10 | 63±10 | 62±9 | 0.61 |
| LVEF, % | 37±11 | 35±10 | 40±11 | 0.09 |
| LVEF <35% | 40 (58%) | 28 (64%) | 12 (48%) | 0.21 |
BMI indicates body mass index; CABG, coronary artery bypass graft; CRT, cardiac resynchronization therapy; Cx, circumflex; HDL, high‐density lipoprotein; ICD, implantable cardioverter defibrillator; LAD, left anterior descending artery; LBBB, left bundle branch block; LDL, low‐density lipoprotein; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; PCI, primary coronary intervention; RBBB, right bundle branch block; RCA, right coronary artery.
P≤0.05.
Figure 2.
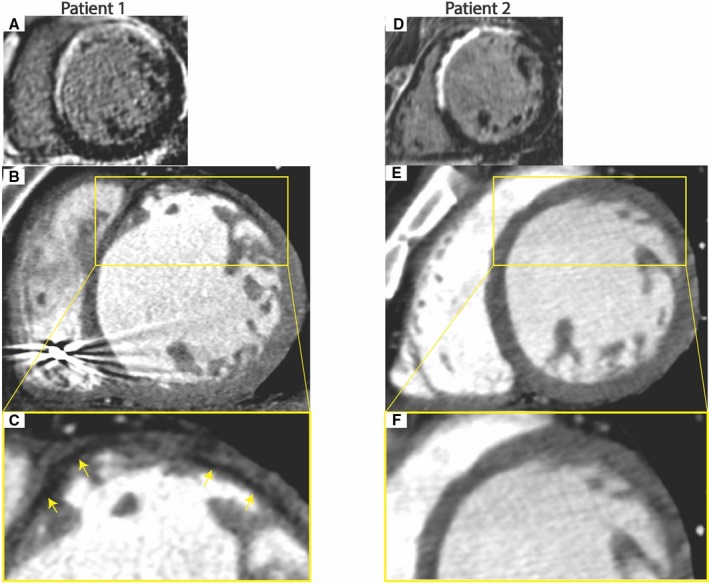
Examples of CT findings in patients with and without fat deposition with scar. A through C, Fifty‐eight‐year‐old man with VT 19 years after anterior infarction. Late gadolinium‐enhanced magnetic resonance imaging (MRI) shows antero‐septal scar (A). Arterial‐enhanced CT shows antero‐septal wall thinning with subendocardial fat deposition (yellow arrows) (B and C). D through F, 73‐year‐old man with VT 9 years after anterior infarction. Late gadolinium‐enhanced MRI shows antero‐septal scar (D). Arterial‐enhanced CT shows antero‐septal wall thinning with no fat deposition (E and F).
Figure 3.
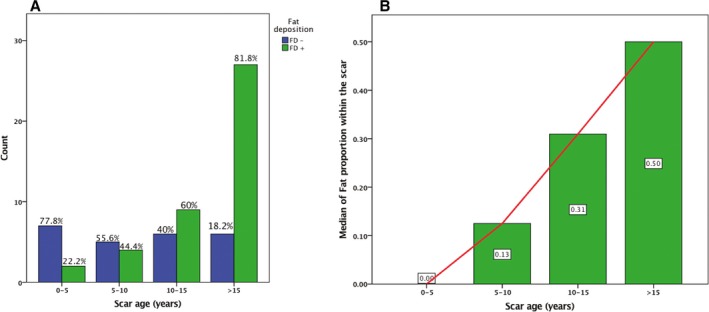
Relationship between fat deposition within scar and time since myocardial infarction. A, Prevalence of fat deposition according to scar age. B, Extent of fat within scar according to scar age. FD+ indicates fat deposition; FD−, no fat deposition.
Anatomical Characteristics of Fat on CT
In the 69 patients, a total of 1173 ventricular segments were analyzed. Out of the 748 segments analyzed in patients with FD, FD was identified in 158 (21%) segments, with a median extent per patient of 4.5 [3–6] segments. Fat was present in larger scars as attested by a higher number of segments with <5‐mm wall thickness on CT (6.5±2.8 versus 4.4±3.4 segments, P=0.01). On multivariate analysis, the extent of myocardial scars was an independent correlate of FD, whose probability increased by 27% per scar segment (OR: 1.27; 95% CI, 1.04, 1.56; P=0.02). The extent of fat according to scar age is illustrated in Figure 3B. The proportion of fat within the scar increased with scar age, being absent before 5 years and covering up to 50% of the scar after 15 years. Examples of fat distributions are shown in Figure 4. Areas with FD were typically patchy, with interposition of non‐fatty areas between several islets of fat. Fat was located in the sub‐endocardial layer in all cases and had a variable transmural extent, ie, transmural in 50% of segments showing fat. In addition, there was an inverse correlation between the presence of fat and the local wall thickness (R=−0.36, P<0.001). Calcification was more frequently present in patients with FD (39% versus 16%, P=0.05) and was also significantly related to scar age (21±11 versus 15±9 years in scars with versus without calcification, P=0.04).
Figure 4.
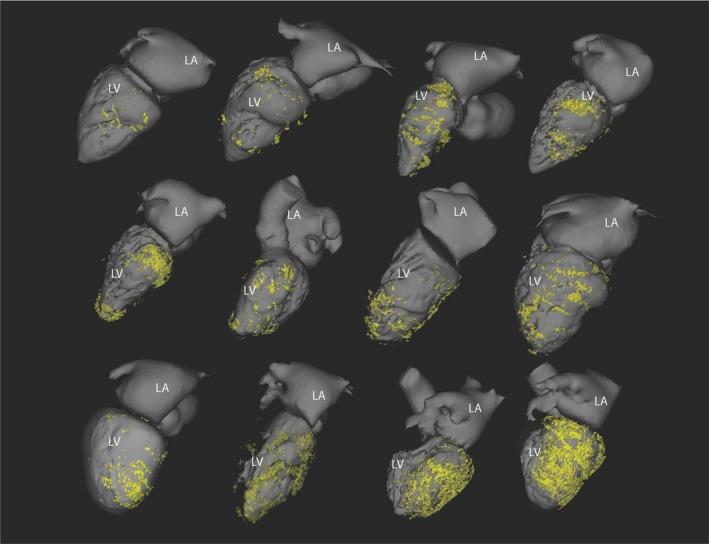
Variability of fat distribution within chronic myocardial infarction. Fat assessed from CT is mapped over left chamber geometry in 12 patients with post‐infarction ventricular tachycardia. LA indicates left atrium; LV, left ventricle.
Electrophysiological Characteristics
Electrophysiological characteristics of scars with FD were studied in a subset of 27 patients with available high density EAM acquired during sinus rhythm using a Pentaray catheter. Of these, 14 patients showed FD on CT, while 13 did not. An example of fat distribution registered on an EAM geometry is shown in Figure 5. The characteristics of electrograms recorded within scars with and without FD are shown in Table 2. Fatty scars showed significantly lower bipolar voltage (0.5 versus 0.8 mV, P<0.001) and prolonged electrogram duration (99 versus 89 ms, P<0.001). While the prevalence of LAVA within the scar did not differ between the 2 groups (23% versus 34% of all electrograms within scar; P=0.22), LAVA in fatty scars showed lower bipolar amplitude (0.22 versus 0.29 mV; P<0.001) and longer delay from the local farfield (75 versus 52 ms; P<0.001). The critical VT isthmus was identified in 6 patients with FD, and colocalized with fat in 4 (75%) cases. An example of a critical isthmus mapped in a fatty area is shown in Figure 5.
Figure 5.
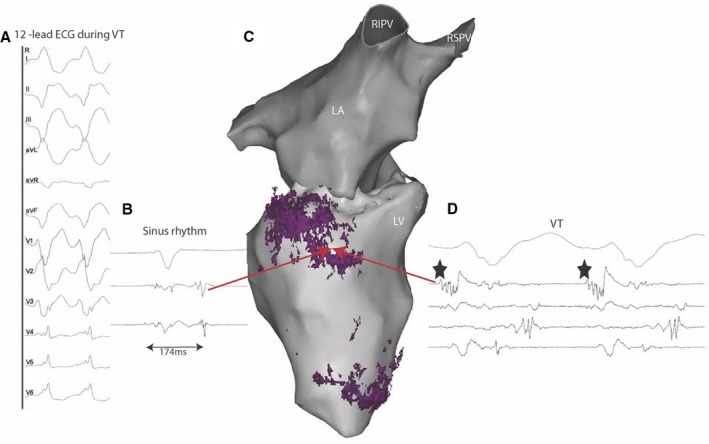
Registration of fat distribution on electro‐anatomical mapping data. Sixty‐one‐year‐old man with history of prior myocardial infarction in right coronary artery and distal left anterior descending artery territories. The 12‐lead recording of his clinical VT indicated an infero‐septal exit (A). The distribution of fat assessed on CT was registered on the electro‐anatomical geometry (C, inferior view of left chambers with fat deposition shown in purple). During sinus rhythm, late potentials were identified on the basal fatty area (B, red arrow in C). During VT, the same fat site hosted the exit part of the VT circuit (black stars in D). LA indicates left atrial; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; VT, ventricular tachycardia.
Table 2.
Electrophysiological Characteristics
| Fat Deposition (+) 1072 Points | Fat Deposition (−) 768 Points | P Value | |
|---|---|---|---|
| LV volume Carto, mL | 297±115 | 284±105 | 0.76 |
| Total mapped area, cm2 | 272±68 | 284±64 | 0.64 |
| Unipolar low voltage area <8.3 mV, cm2 | 109 [60–125] | 85 [55–109] | 0.48 |
| Bipolar low voltage area <1.5 mV, cm2 | 75 [45–104] | 60 [33–82] | 0.26 |
| Bipolar low voltage area <0.5 mV, cm2 | 22 [13–46] | 14 [9–34] | 0.11 |
| Bipolar voltage, mV | 0.5 [0.3–0.9] | 0.8 [0.4–1.4] | <0.001a |
| Unipolar voltage, mV | 3.5 [2.3–4.8] | 3.6 [2.3–5.4] | 0.12 |
| Median EGM duration, ms | 99 [83–120] | 89 [70–111] | <0.001a |
| LAVA prevalence (% of scar EGMs) | 23±18 | 34±22 | 0.22 |
| LAVA delay to local EGM, ms | 75 [45–106] | 52 [22–90] | <0.001a |
| LAVA bipolar amplitude, mV | 0.22 [0.14–0.35] | 0.29 [0.16–0.66] | <0.001a |
Data expressed as mean±SD when following a normal distribution, and median [Q1–Q3] otherwise. EGM indicates electrogram; LAVA, local abnormal ventricular activity; LV, left ventricle.
P≤0.05.
Post‐VT Ablation Outcome
Complete LAVA elimination was equally achieved in the 2 groups (58% versus 54%, P=0.73). The radiofrequency time to obtain complete LAVA elimination did not differ between the 2 groups (44±19 versus 37±23 minutes, P=0.24). Inducibility was tested at the end of the procedure in 47 cases (30 with and 17 without FD). Patients with FD showed a trend to remain inducible (40% versus 12%, P=0.09). After a mean follow‐up of 26±17 months, a total of 38 (55%) patients met the primary end point including all‐cause mortality and VT recurrence. This included VT recurrence in 26 (38%), and death in 13 (19%), including 10 (14%) from a cardiac cause. A total of 11 (16%) patients underwent a repeat ablation procedure. The characteristics of patients with versus without adverse outcome at follow‐up are compared in Table 3. On univariate analysis, adverse outcome was related to fat deposition, LV systolic dysfunction, presence of 3‐vessel disease and scar age. Patients with fat deposition experienced a markedly worse outcome including all‐cause mortality and VT recurrence (70% versus 28%, P log rank=0.009). On multivariate cox regression analysis (Table 4), the presence of fat was an independent predictive factor of worse outcome (hazard ratio=2.69; 95% CI, 1.12–6.46; P=0.027) along with left ventricular dysfunction (hazard ratio=2.57; 95% CI, 1.13–5.85; P=0.024). Kaplan–Meier survival curves illustrating outcomes in patients with and without FD are shown in Figure 6.
Table 3.
Characteristics of Patients With Adverse Outcome
| Adverse Outcome n=38 (55%) | Favorable Outcome n=31 (45%) | P Value | |
|---|---|---|---|
| Clinical characteristics | |||
| Age, y | 65±12 | 67±12 | 0.34 |
| Men | 37 (97%) | 27 (87%) | 0.18 |
| Hypertension | 18 (47%) | 18 (58%) | 0.28 |
| Diabetes mellitus | 9 (24%) | 6 (19%) | 0.89 |
| Smoking | 7 (18%) | 11 (35%) | 0.78 |
| BMI, kg/m2 | 29±6 | 27±5 | 0.19 |
| Scar age, y | 20±11 | 13±8 | 0.02a |
| 3‐vessel disease | 12 (32%) | 4 (13%) | 0.04a |
| CABG | 9 (24%) | 8 (26%) | 0.92 |
| PCI | 21 (55%) | 21 (68%) | 0.24 |
| Imaging characteristics | |||
| Scar extent (N segments) | 6 [4–8] | 6 [3–8] | 0.41 |
| Calcifications | 14 (37%) | 7 (23%) | 0.22 |
| Fat deposition | 31 (82%) | 13 (42%) | 0.009a |
| LVEDD, mm | 65±10 | 61±10 | 0.3 |
| LVEF <35% | 27 (71%) | 13 (42%) | 0.02a |
| Procedural outcomes | |||
| Radiofrequency duration, min | 44±19 | 39±23 | 0.23 |
| Incomplete LAVA elimination | 16 (43%) | 12 (41%) | 0.28 |
| Post‐ablation VT inducibility | 9 (33%) | 5 (25%) | 0.7 |
BMI indicates body mass index; CABG, coronary artery bypass graft; CRT, cardiac resynchronization therapy; Cx, circumflex; ICD, implantable cardioverter defibrillator; LAD, left anterior descending artery; LAVA, local abnormal ventricular activity; LBBB, left bundle branch block; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; PCI, primary coronary intervention; RBBB, right bundle branch block; RCA, right coronary artery; VT, ventricular tachycardia.
P≤0.05.
Table 4.
Variables With Significant Correlation to Worse Outcome Included in Univariate and Multivariate Analysis
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Combined events=all‐cause mortality and VT recurrence | ||||
| Fat deposition | 2.85 (1.25–6.5) | 0.013a | 2.69 (1.12–6.46) | 0.027a |
| 3‐vessel disease | 2.02 (1.02–4.01) | 0.044a | 2.03 (0.98–4.18) | 0.056 |
| LVEF <35% | 2.23 (1.10–4.53) | 0.026a | 2.57 (1.13–5.86) | 0.024a |
| Scar extent | 1.04 (0.94–1.15) | 0.45 | 0.59 (0.32–1.11) | 0.10 |
| Smoking | 1.05 (0.76–1.45) | 0.78 | 1.13 (0.79–1.16) | 0.50 |
| Diabetes mellitus | 1.03 (0.62–1.38) | 0.90 | 1.00 (0.57–1.78) | 0.99 |
| Redo procedure | 1.43 (0.83–2.84) | 0.20 | 1.45 (0.8–2.63) | 0.22 |
| Age | 0.99 (0.96–1.02) | 0.48 | ||
| Scar age | 1.25 (0.70–2.22) | 0.45 | ||
| VT recurrence | ||||
| Fat deposition | 2.64 (1.06–6.6) | 0.037 | 2.91 (1.10–7.75) | 0.033a |
| LVEF <35% | 2.38 (1.03–5.50) | 0.042 | 2.05 (0.82–5.13) | 0.12 |
| Inducible after ablation | 1.65 (1.03–2.66) | 0.038 | 1.49 (0.89–2.50) | 0.13 |
| Scar extent | 1.12 (0.63–1.99) | 0.70 | 1.00 (0.86–1.16) | 0.97 |
| Scar age | 1.34 (0.90–2.00) | 0.15 | 1.02 (0.63–1.64) | 0.94 |
| Diabetes mellitus | 1.05 (0.58–1.90) | 0.87 | ||
| 3‐vessel disease | 1.36 (0.52–3.60) | 0.53 | ||
| Age | 0.98 (0.96–1.01) | 0.31 | ||
| Acute procedural complication | 0.97 (0.28–3.34) | 0.96 | ||
HR indicates hazard ratio; LVEF, left ventricular ejection fraction; VT, ventricular tachycardia.
P≤0.05.
Figure 6.
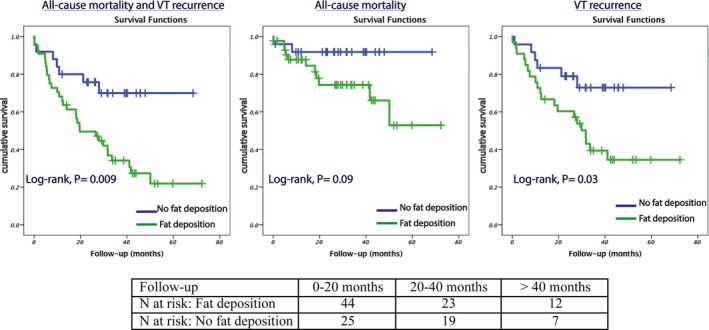
Post‐ablation outcomes in patients with vs without fat deposition within scar on CT scan. VT indicates ventricular tachycardia.
Discussion
This study is to our knowledge the first to consistently analyze clinical, anatomical, and electrophysiological characteristics of fat deposition after MI in patients experiencing VT, including its impact on patient outcomes after catheter ablation. Its main findings are that (1) fat is extremely prevalent within the scar in patients referred for post–MI VT, (2) it relates to scar age and size, and typically progresses as a patchy process, (3) fatty scars show distinctive electrophysiological properties including lower voltage, more delayed LAVA of lower amplitude, and possible colocalization with critical VT isthmuses, and (4) FD is potentially associated with a worse outcome after VT ablation including all‐cause mortality and VT recurrence.
Prevalence of Fat in Post–MI Scars
The prevalence of fat in post–MI scars may differ according to the investigating technique. In the present study, the prevalence of fat was higher than usually reported in CT series after MI.5, 14 This may be because of the specificity of the post–MI population experiencing VT, and may reflect the arrhythmogenic role of fat deposition, although this aspect should be studied prospectively in a non‐selected population. Histological studies have demonstrated an even higher prevalence of fat after MI (up to 84% of explanted hearts with a history of MI).7 This discrepancy outlines the spatial resolution limitations of current imaging techniques, and one should keep in mind that in contrast to histology, fat detected on CT corresponds to macroscopic fat. In the present study we chose to apply a strict attenuation threshold < −30 HU to define intra‐myocardial fat, while other groups have used substantially higher thresholds.15 Although we acknowledge that this may have led to missing less macroscopic fat deposition, this threshold was based on prior validation on cadavers16 and is the one routinely used to quantify body fat content.12 This strict threshold was also chosen so as not to mistake as fat other confounding scar components such as hypoperfused dense fibrotic scars, also known to lower CT attenuation as compared with the normal myocardium on arterial‐enhanced CT images.17
Clinical and Anatomical Characteristics of Fat Deposition
The relationship between fat and scar age and size suggests a progressive and dynamic process. In addition, the patchy appearance of the fat and the presence of areas with fat separated by non‐fatty areas suggests independent signaling pathways occurring at different myocardial sites. Interestingly, the duration of the fatty replacement process parallels the delay usually required to observe VT after MI. This also supports a role of fat deposition in the arrhythmogenicity of chronic MI. The highest proportion of fat was found 10 years after the index event. This correlation has been reported by previous studies that included patients with prior MI regardless of the presence of VT.5, 14 In concordance with our results, previous studies showed that the areas with fat deposition are typically located in the scar area and frequently involve the subendocardium with variable transmural extent.5, 18, 19 Except for scar age and size, no additional clinical factors were correlated with the presence of fat within the MI scar in our population. Different factors have variously been reported to correlate with the presence of fat in other studies. An initial study found that increasing age, male sex, previous bypass surgery and infarcts involving the anterior, septal, and lateral walls of the apex were correlated to the presence of fat20; however, these factors have not been confirmed in other studies.5, 6
Electrophysiological Characteristics of Scars With Fat Deposition
We demonstrated that the VT substrate has different electrophysiological properties according to the presence of fat. The latter was associated with lower bipolar amplitude, and although the proportion of LAVA electrograms was similar, these showed lower bipolar amplitude and were more delayed in fatty scars. Pouliopoulos et al21 performed left ventricular plunge‐needle and non‐contact mapping in 8 sheep with prior MI and without VT (MI+VT−), 7 sheep with prior MI and VT (MI+VT+), and 5 sheep without MI. Inside the scar areas, the fat was abundant with an intramyocardial adipose/collagen ratio of ≈0.8. Fat was less abundant in areas remote from the infarct borders. The presence of fat correlated inversely with myocardial viability. It was also related to gap junction remodeling marked by an increase in Cx43 lateralization (80.5%, 81.1,% and 93.6% when the scar contains collagen, adipose, and collagen‐adipose, respectively). The group MI+VT+ showed significantly higher adipose content and myocardial discontinuity. It also had lower voltage amplitude, reduced electrogram dV/dt, slower conduction velocities and a shorter corrected and uncorrected wavelength of excitation (λ). Sasaki et al15 performed a pilot study to investigate the impact of fat on the local electrograms and the VT circuit. The study included 22 patients with a history of MI and VT that were compared with 20 patients with previous MI and without a history of VT. Fat was documented using a CT scan in all patients with VT. The presence of fat was associated with lower bipolar and unipolar amplitude, and longer electrogram duration. Fragmented and isolated electrograms were more frequently observed in areas with fat. VT circuits were identified in 32 cases; three‐fourths of them were located either in areas of FD (50%) or adjacent to them (25%). The VT circuit sites in areas with FD had thinner LV wall thickness. The role of fat in reducing the electrograms amplitude inside the scar area was also reported in a recent animal study.22
Relationship Between Fat and Post‐Ablation Outcome
A prior study reported on the outcome of patients with FD. In a population of 316 patients with a history of MI, regardless of the presence of VT,23 fat was associated with a worse outcome including all‐cause mortality, sustained ventricular arrhythmia, and heart failure hospitalizations. Our study also suggests this worse outcome in patients undergoing VT ablation. The worse outcome and the independent predictive role of fat deposition suggested by our study raise some hypotheses. Fat is potentially arrhythmogenic. In fact, it potentially stabilizes the VT circuit by creating areas of slow conduction (as attested by more prolonged electrograms and delayed LAVA). In addition, critical VT isthmuses may be located in the mid‐wall or epicardial layers and might be protected by the surrounding fat, as they have lower amplitude and may not be effectively treated by catheter ablation.
Study Limitations
This study has significant limitations, including the small number of patients and the inclusion of unmatched groups. This may alter the power of the statistical analysis and the conclusion of the study. Many confounding parameters could potentially interact with the results. Scar age in the FD group was almost double that of the non‐FD group. Thus, higher mortality in the FD group could be explained if the non‐FD group had insufficient time to develop FD, and mortality might become similar if the non‐FD group was followed for a longer period. In addition, an association between diabetes mellitus, smoking, size of scar, procedural complications and worse outcome cannot be excluded even if no statistical association could be identified. Our results need to be validated in a larger and prospective study involving matched groups. An additional limitation is the absence of longitudinal patient follow‐up with CT to further detail the progression of fat during the scar healing process. This was practically not possible given the long time course of this process. Another limitation is the limited number of critical VT isthmuses. As a consequence, as mentioned above, it remains unclear whether worse outcomes after ablation are because of increased arrhythmogenicity or to inefficacy of endocardial RF ablation because of fat interposition.
Conclusions
Fat deposition is extremely prevalent within the scar in patients referred for post–MI VT. It relates to scar age and size, and typically progresses as a patchy process. Fatty scars show distinctive electrophysiological properties including lower voltage, more delayed LAVA of lower amplitude, and possible co‐localization with critical VT isthmuses. Fat deposition may be a marker of worse outcome after VT ablation including all‐cause mortality and VT recurrence.
Sources of Funding
The research leading to these results has received funding from l'Agence Nationale de la Recherche (ANR) under Grant Agreements Equipex MUSIC ANR‐11‐EQPX‐0030 and LIRYC ANR‐10‐IAHU‐04, and the European Research Council under Grant Agreement ERC no. 715093.
Disclosures
None.
(J Am Heart Assoc. 2019;8:e012482 DOI: 10.1161/JAHA.119.012482.)
References
- 1. Stevenson WG, Khan H, Sager P, Saxon LA, Middlekauff HR, Natterson PD, Wierner I. Identification of reentry circuit sites during catheter mapping and radiofrequency ablation of ventricular tachycardia late after myocardial infarction. Circulation. 1993;88:1647–1670. [DOI] [PubMed] [Google Scholar]
- 2. Sun Y, Kiani MF, Postlethwaite AE, Weber KT. Infarct scar as living tissue. Basic Res Cardiol. 2002;97:343–347. [DOI] [PubMed] [Google Scholar]
- 3. Sun Y, Weber KT. Infarct scar: a dynamic tissue. Cardiovasc Res. 2000;46:250–256. [DOI] [PubMed] [Google Scholar]
- 4. Rog‐Zielinska EA, Norris RA, Kohl P, Markwald R. The living scar‐cardiac fibroblasts and the injured heart. Trends Mol Med. 2016;22:99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ichikawa Y, Kitagawa K, Chino S, Ishida M, Matsuoka K, Tanigawa T, Nakamura T, Hirano T, Takeda K, Sakuma H. Adipose tissue detected by multislice computed tomography in patients after myocardial infarction. JACC Cardiovasc Imaging. 2009;2:548–555. [DOI] [PubMed] [Google Scholar]
- 6. Goldfarb JW, Roth M, Han J. Myocardial fat deposition after left ventricular myocardial infarction: assessment by using MR water‐fat separation imaging. Radiology. 2009;253:65–73. [DOI] [PubMed] [Google Scholar]
- 7. Su L, Siegel JE, Fishbein MC. Adipose tissue in myocardial infarction. Cardiovasc Pathol. 2004;13:98–102. [DOI] [PubMed] [Google Scholar]
- 8. Sapp JL, Wells GA, Parkash R, Stevenson WG, Blier L, Sarrazin JF, Thibault B, Rivard L, Gula L, Leong‐Sit P, Essebag V, Nery PB, Tung SK, Raymond JM, Sterns LD, Veenhuyzen GD, Healey JS, Redfearn D, Roux JF, Tang AS. Ventricular tachycardia ablation versus escalation of antiarrhythmic drugs. N Engl J Med. 2016;375:111–121. [DOI] [PubMed] [Google Scholar]
- 9. Maskoun W, Saad M, Abualsuod A, Nairooz R, Miller JM. Outcome of catheter ablation for ventricular tachycardia in patients with ischemic cardiomyopathy: a systematic review and meta‐analysis of randomized clinical trials. Int J Cardiol. 2018;267:107–113. [DOI] [PubMed] [Google Scholar]
- 10. Wissner E, Stevenson WG, Kuck K‐H. Catheter ablation of ventricular tachycardia in ischaemic and non‐ischaemic cardiomyopathy: where are we today? A clinical review Eur Heart J. 2012;33:1440–1450. [DOI] [PubMed] [Google Scholar]
- 11. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the American Heart Association. Circulation. 2002;105:539–542. [DOI] [PubMed] [Google Scholar]
- 12. Yoshizumi T, Nakamura T, Yamane M, Islam AH, Menju M, Yamasaki K, Arai T, Kotani K, Funahashi T, Yamashita S, Matsuzawa Y. Abdominal fat: standardized technique for measurement at CT. Radiology. 1999;211:283–286. [DOI] [PubMed] [Google Scholar]
- 13. Jais P, Maury P, Khairy P, Sacher F, Nault I, Komatsu Y, Hocini M, Forclaz A, Jadidi AS, Weerasooryia R, Shah A, Derval N, Cochet H, Knecht S, Miyazaki S, Linton N, Rivard L, Wright M, Wilton SB, Scherr D, Pascale P, Roten L, Pederson M, Bordachar P, Laurent F, Kim SJ, Ritter P, Clementy J, Haïssaguerre M. Elimination of local abnormal ventricular activities: a new end point for substrate modification in patients with scar‐related ventricular tachycardia. Circulation. 2012;125:2184–2196. [DOI] [PubMed] [Google Scholar]
- 14. Ahn SS, Kim YJ, Hur J, Lee HJ, Kim TH, Choe KO, Choi BW. CT detection of subendocardial fat in myocardial infarction. AJR Am J Roentgenol. 2009;192:532–537. [DOI] [PubMed] [Google Scholar]
- 15. Sasaki T, Calkins H, Miller CF, Zviman MM, Zipunnikov V, Arai T, Sawabe M, Terashima M, Marine JE, Berger RD, Nazarian S, Zimmerman SL. New insight into scar‐related ventricular tachycardia circuits in ischemic cardiomyopathy: fat deposition after myocardial infarction on computed tomography: a pilot study. Heart Rhythm. 2015;12:1508–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rossner S, Bo WJ, Hiltbrandt E, Hinson W, Karstaedt N, Santago P, Sobot WT, Crouse JR. Adipose tissue determinations in cadavers–a comparison between cross‐sectional planimetry and computed tomography. Int J Obes. 1990;14:893–902. [PubMed] [Google Scholar]
- 17. Nieman K, Cury RC, Ferencik M, Nomura CH, Abbara S, Hoffmann U, Hk Gold, Jang IK, Brady TJ. Differentiation of recent and chronic myocardial infarction by cardiac computed tomography. Am J Cardiol. 2006;98:303–308. [DOI] [PubMed] [Google Scholar]
- 18. Kimura F, Matsuo Y, Nakajima T, Nishikawa T, Kawamura S, Sannohe S, Hagiwara N, Sakai F. Myocardial fat at cardiac imaging: how can we differentiate pathologic from physiologic fatty infiltration? Radiographics. 2010;30:1587–1602. [DOI] [PubMed] [Google Scholar]
- 19. Zafar HM, Litt HI, Torigian DA. CT imaging features and frequency of left ventricular myocardial fat in patients with CT findings of chronic left ventricular myocardial infarction. Clin Radiol. 2008;63:256–262. [DOI] [PubMed] [Google Scholar]
- 20. Baroldi G, Silver MD, De Maria R, Parodi O, Pellegrini A. Lipomatous metaplasia in left ventricular scar. Can J Cardiol. 1997;13:65–71. [PubMed] [Google Scholar]
- 21. Pouliopoulos J, Chik WW, Kanthan A, Sivagangabalan G, Barry MA, Fahmy PN, Midekin C, Lu J, Kizana E, Thomas SP, Thiagalingam A, Kovoor P. Intramyocardial adiposity after myocardial infarction: new implications of a substrate for ventricular tachycardia. Circulation. 2013;128:2296–2308. [DOI] [PubMed] [Google Scholar]
- 22. Samanta R, Kumar S, Chik W, Qian P, Barry MA, Al Raisi S, Bhaskaran A, Farraha M, Nadri F, Kizana E, Thiagalingam A, Kovoor P, Pouliopoulos J. Influence of intramyocardial adipose tissue on the accuracy of endocardial contact mapping of the chronic myocardial infarction substrate. Circ Arrhythm Electrophysiol. 2017;10:e004998. [DOI] [PubMed] [Google Scholar]
- 23. Mordi I, Radjenovic A, Stanton T, Gardner RS, McPhaden A, Carrick D, Berry C, Tzemos N. Prevalence and prognostic significance of lipomatous metaplasia in patients with prior myocardial infarction. JACC Cardiovasc Imaging. 2015;8:1111–1112. [DOI] [PubMed] [Google Scholar]


