Abstract
Background
Ischemic heart disease continues to be a leading cause of mortality in patients. Extracellular vesicles (EVs) provide a potential for treatment that may induce collateral vessel growth to increase myocardial perfusion.
Methods and Results
Nineteen male Yorkshire pigs were given a high‐fat diet for 4 weeks, then underwent placement of an ameroid constrictor on the left circumflex artery to induce chronic myocardial ischemia. Two weeks later, the pigs received either intramyocardial vehicle (n=6), EVs (high‐fat diet with myocardial EV injection [HVM]; n=8), or HVM and calpain inhibition (n=5). Five weeks later, myocardial function, perfusion, coronary vascular density, and cell signaling were examined. Perfusion in the collateral‐dependent myocardium was increased during rapid ventricular pacing in the HVM group in both nonischemic (P=0.04) and ischemic areas of the ventricle (P=0.05). Cardiac output and stroke volume were significantly improved in the HVM group compared with the control group during ventricular pacing (P=0.006). Increased arteriolar density was seen in the HVM group in both nonischemic and ischemic myocardium (P=0.003 for both). However, no significant changes in the capillary density were observed between the control, HVM, and HVM and calpain inhibition groups (P=0.07). The group that received EVs with oral calpain inhibition had neither increased vessel density (P>0.99) nor improvement in blood flow or cardiac function (P=0.48) when compared with the control group.
Conclusions
These findings suggest that EVs promote angiogenesis in areas of chronic myocardial ischemia and improve cardiac function under conditions of diet‐induced metabolic syndrome.
Keywords: angiogenesis, cardiovascular disease, coronary vessels, extracellular vesicles
Subject Categories: Angiogenesis, Ischemia, Metabolism
Clinical Perspective
What Is New?
This article is the first to demonstrate that in the setting of metabolic syndrome and chronic myocardial ischemia, intramyocardial injection of human mesenchymal stem cell–derived extracellular vesicles increases vessel density, increases blood flow to ischemic myocardial tissue, and improves cardiac function in a large animal model.
What Are the Clinical Implications?
Our findings suggest that extracellular vesicles may be used in patients who are unsuitable candidates for invasive surgical procedures given heart failure and other comorbidities, history of revascularization, or diffuseness of coronary disease in the future.
Extracellular vesicles may improve coronary vessel density, myocardial blood flow, and cardiac function in these patients.
Introduction
Myocardial ischemia continues to be the leading cause of mortality in the United States.1 Although acute myocardial infarction is responsible for much of the disease burden, many patients have long‐standing chronic myocardial ischemia. Chronic ischemia may lead to apoptosis and myocardial necrosis, resulting in cardiac remodeling that, over time, may lead to heart failure. Interventions, such as coronary stenting and coronary artery bypass grafting, are often life saving. However, many patients are unsuitable candidates for invasive surgical procedures given heart failure and other comorbidities, or the diffuseness of coronary disease, prior revascularization, or other factors.
Metabolic syndrome affects a growing percentage of the population worldwide and includes a collection of clinical characteristics, such as obesity, hypertension, insulin resistance, and hypercholesterolemia.2 Collectively, these patients are both at higher risk of developing coronary artery disease and at higher operative risk, potentially even to the extent of not being surgical candidates.3, 4, 5 Therefore, there is an ever‐growing need for prevention and reversal of ventricular remodeling after ischemia.
In the past few decades, stem cell–based therapies have been an exciting approach to reversing left ventricular remodeling and improving function after ischemia and infarction. Cardiosphere‐derived cells have led the way, with both the ALLSTAR (Allogeneic Heart Stem Cells to Achieve Myocardial Regeneration) and CADUCEUS (Cardiosphere‐Derived Autologous Stem Cells to Reverse Ventricular Dysfunction) trials demonstrating preliminary safety and efficacy in translational human trials.6, 7, 8 However, despite initially promising results, it appears cell‐derived treatments only survive for ≈24 hours and fail to have long‐lasting clinical efficacy.9
An exciting new potential therapy derived from stem cells is the extracellular vesicles (EVs) they secrete. These EVs are surrounded by plasma membrane and contain various miRNA, siRNA, cytokines, and growth factors, without the DNA content of cell‐based therapies.10 They are small, ranging from around 50 to 1000 nm, and termed according to their size (ie, exosomes are 50‐200 nm, whereas microvesicles are >200 nm in diameter). Animal models show promising results in acute infarct models, particularly when delivered via coronary angiography catheters.11, 12, 13, 14 However, EV therapy in the setting of chronic myocardial ischemia is a little‐studied endeavor, yet likely the most clinically relevant. Furthermore, to date, there are no studies investigating the effects of EV therapy as a treatment for chronic myocardial ischemia in the setting of metabolic syndrome.
Calpains are a class of calcium‐dependent proteases with a wide variety of subtypes and functions in the body.15 They appear to function as a proteolytic unit for intracellular homeostasis and are often overexpressed as a response to ongoing stress, such as in metabolic syndrome and myocardial ischemia.16, 17 Calpain I and II are active in endothelial cells. Inhibition of calpain I and II by oral calpain inhibitors (CIs) has been previously shown by our laboratory to increase blood flow to areas of chronic myocardial ischemia, having their effect via downregulation of inflammatory protein expression in vessel walls.18, 19 In a previous study, we recently demonstrated that EVs may improve myocardial function and perfusion in a large animal model of chronic myocardial ischemia.20
In this study, we aim to determine the effects of EV therapy delivered by intramyocardial injection in the setting of diet‐induced (high‐fat fed) metabolic syndrome. This diet has been determined to interfere with the proangiogenic effects of growth factors and collateral vessel formation in general. Furthermore, we evaluate whether the addition of a CI exhibits a synergistic effect of revascularization via angiogenesis when given in conjunction with EVs.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Animal Model and Surgical Interventions
Nineteen intact male Yorkshire swine were started on a high‐fat diet at the age of 6 weeks to induce metabolic syndrome. The high‐fat diet consisted of 500 g/d high‐cholesterol diet consisting of 4% cholesterol, 17.2% coconut oil, 2.3% corn oil, 1.5% sodium cholate, and 75% regular chow (Sinclair Research, Columbia, MO), which has been previously shown by our laboratory to induce hypertension, hyperlipidemia, insulin resistance, and obesity.21 All animals underwent placement of an ameroid constrictor around the proximal left circumflex artery at 11 weeks of age. Two weeks later, the animals were subdivided into 3 groups: control group (high‐fat diet with myocardial vehicle injection) (CON; n=6), high‐fat diet with myocardial EV injection group (HVM; n=8), or HVM with CI (HVMC; n=5). The control group received a high‐fat diet along with an injection of 2 mL of 0.9% saline via intramyocardial injection at the age of 13 weeks. The HVM group received 50 μg of EVs suspended in 2 mL of 0.9% saline via intramyocardial injection at 13 weeks of age. The HVMC group received both 50 μg of EVs in 2 mL of 0.9% saline via intramyocardial injection at 13 weeks of age as well as oral CI starting at 13 weeks of age. The CI was given as an oral form at a high dose of 0.25 mg/kg (CI MDL28170; EMD Millipore, Danvers, MA). The high‐fat diet and oral CI were continued until the time of harvest. Five weeks after the start of treatment, animals underwent sedation and euthanasia, at which time the myocardium was harvested for analysis. All experiments were approved by the Institutional Animal Care and Use Committee of the Rhode Island Hospital, and animals were cared in coordination with the veterinary technicians at Rhode Island Hospital in compliance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals.22
Ameroid Constrictor Placement
General anesthesia was induced after the administration of antibiotic prophylaxis with cephalexin (30 mg/kg) and maintained with isoflurane throughout the procedure. Animals were prepared and draped in the usual sterile manner and a left minithoracotomy was made. The heart was exposed, and the left atrium was retracted to bring the left circumflex artery into view. The left circumflex artery was dissected free at the takeoff from the left main coronary artery and isolated. Heparin was injected intravenously (80 IU/kg). Blood was drawn from the left atrial appendage for metabolic analyses. The left circumflex artery was occluded for 2 minutes (determined by ST‐segment elevations on ECG tracings), whereas 5 mL of gold microspheres (BioPal, Worcester, MA) was injected into the left atrial appendage. Then, an ameroid constrictor, ranging from 1.5 to 2.5 mm, chosen by the surgical team to best fit the left circumflex diameter (Research Instruments SW, Escondido, CA), was placed on the left circumflex artery. Nitroglycerin (1 mL) was then administered over the vessel to induce relaxation from spasm. The incision was closed in a multilayered manner. Animals received buprenorphine (IM, 0.03 mg/kg) at the end of the operation as well as placement of a fentanyl patch (4 μg/kg) for pain control. To prevent thromboembolic events from manipulation of the left circumflex artery, all animals received 325 mg of aspirin 1 day before and 5 days after the operation.
EV Injection
Anesthesia and antibiotic prophylaxis were given in identical manner to the ameroid placement procedure. The left minithoracotomy incision was made one rib space below that used for the ameroid placement to avoid adhesions. The pericardium was opened and secured to expose the left ventricular myocardium inferior to the ameroid constrictor on the left circumflex artery. The HVM and HVMC groups received the EVs (50 μg) suspended in 2 mL of 0.9% saline, which were injected in 8 locations into the left ventricular myocardium deemed to be the area at risk of decreased perfusion and chronic myocardial ischemia distal to the location of the ameroid constrictor. The control group received 2 mL of 0.9% saline injected into the same location as the HVM and HVMC groups. The pericardium was reapproximated, and the incision was closed in a layered manner. For the group receiving the CI (HVMC), the oral dosing began the day after the EV injection operation.
Terminal Procedure: Analysis of Myocardial Function and Perfusion
After induction of general anesthesia, a pressure catheter (Millar, Colorado Springs, CO) was inserted into the right femoral artery and blood was collected for metabolic analyses. Then, a midline sternotomy was made to expose the heart. Lutetium‐ and europium‐labeled microspheres (5 mL; Biopal) were injected into the left atrial appendage at rest while blood was withdrawn from the femoral artery to assess blood flow. This was repeated with samarium‐labeled microspheres during pacing to 150 beats per minute. Microsphere content of dried sections of the left ventricle was analyzed by BioPal laboratories to determine ischemic and nonischemic tissue for each pig. The animal was euthanized via beating‐heart harvest, and the tissue was collected for analysis. Myocardium from the ischemic area in the left circumflex distribution as well as myocardial samples from nonischemic territory were collected and subdivided for further analysis.
Determination of Arteriolar and Capillary Density
A detailed description for tissue preparation and immunohistochemical staining for capillary and arteriolar density has been described previously.23 In brief, formalin‐fixed myocardial sections were incubated with antibodies against porcine endothelial marker cluster of differentiation 31 (CD31; R&D, Minneapolis, MN) and smooth muscle actin (Sigma‐Aldrich, St Louis, MO), followed by appropriate AlexaFluor‐conjugated antibody (Thermo Fisher Scientific, Waltham, MA). Images were examined at ×20 power magnification with a microscope (E800 Eclipse; Nikon, Tokyo, Japan) at the same exposure in x random fields. Capillaries were defined as structures 5 to 25 μm2 in a cross‐sectional area. Arterioles were defined by localization of smooth muscle actin and CD31 staining. Arteriolar and capillary densities were measured using Image J software (National Institutes of Health, Bethesda, MD) in a double‐blinded manner.23 The percentage of capillary and arteriolar density for each pig was averaged from 10 randomly selected myocardial tissue sections.
Isolation of EVs
Mesenchymal stem cells derived from human bone marrow (Lonza, Allendale, NJ) were cultured in growth media (MSCGM Bulletkit; Lonza, Allendale, NJ), grown to confluence, and passaged up to 6 times for expansion of the cell line. Medium was collected, and then replaced with serum‐free RPMI medium 1 day before isolation of EVs. Microsomes and exosomes were harvested by ultracentrifugation at 100 000g for 75 minutes, then washed with sterile PBS at 100 000g for an additional 30 minutes and resuspended in 10% dimethyl sulfoxide. Protein quantification was done using a radioimmunoprecipitation assay (Pierce BCA Protein Assay Kit; Thermo Fisher Scientific). A total of 50 μg of total protein was stored at −80°C in 10% dimethyl sulfoxide in PBS, then resuspended in 2 mL 0.9% sterile saline the day of administration.
Metabolic Parameters
To determine glucose tolerance, before each surgical procedure, animals received an IV injection of 50% dextrose (0.5 g/kg) and blood glucose measurements were taken before the injection and 30 and 60 minutes after the injection. Animals were fasted for a minimum of 8 hours before each procedure. Whole blood was collected at the time of the ameroid procedure and the harvest procedure and sent to the chemistry laboratory at Rhode Island Hospital for analysis.
Protein Analysis of Cell Signaling Molecules
Homogenization of flash‐frozen tissue from the ischemic area of the left ventricle was used to make lysates, from which protein concentration was determined using a radioimmunoprecipitation assay (Pierce BCA Protein Assay Kit). Western blot analysis was done using whole‐tissue lysates fractionated onto 4% to 15% Tris‐glycine Bio‐Rad gels (Life Science Research, Hercules, CA) and transferred onto polyvinylidene difluoride membranes (Millipore, Billerica, MA). Blocking buffer (Thermo Fisher Scientific) was applied for 1 hour before incubation with primary antibodies. Primary antibodies (against endothelial NO synthase [eNOS], phosphorylated eNOS [serine 1177], B‐cell lymphoma‐2 [BCL‐2], BCL‐2–associated death promoter, BCL‐2–associated X protein, transforming growth factor‐β, and vascular endothelial growth factor receptor 2, all from Cell Signaling, Danvers, MA) were applied at a 1:1000 dilution and incubated overnight at 4°C. Loading error was corrected using anti‐GAPDH on all membranes. A secondary anti‐rabbit antibody (Cell Signaling) was added at room temperature for 1 hour. Images were visualized using a digital camera to capture enhanced chemiluminescence images (G‐box; Syngene, Cambridge, England). Band densitometry was determined as arbitrary light units using Image J software. After normalization to GAPDH, data are presented as fold change compared with control±SEM.
Immunofluorescence
Tissue sections from the ischemic and nonischemic areas of the left ventricle were fixed in 10% formalin at the time of tissue harvest, then transferred after 24 hours to 70% ethanol. Sections (5 µm thick) were mounted onto slides and stained for both α‐smooth muscle actin (Cell Signaling) and CD31 (Abcam, Cambridge, UK), as previously described.20 Briefly, slides were paraffinized, then deparaffinized and rehydrated. Slides were blocked with peroxide, rinsed, and then reblocked. The antibody was incubated on the slides at a 1:50 dilution overnight at 4°C. After rinsing, a secondary antibody was added to incubate at room temperature for 1 hour. Slides were coverslipped, and images were captured using a Nikon Eclipse TE2000‐U microscope at 20× objective (Nikon Instruments, Melville, NY). Three images per slide were captured and analyzed using Image J software.
Statistical Analysis
All data were analyzed using either GraphPad Prism 7.04 Software (GraphPad Software Inc, San Diego, CA) or Microsoft Excel (Microsoft Corporation, Redmond, WA). Blood flow and Western blot data were analyzed using repeated‐measures ANOVA with Kruskal‐Wallis. Pressure‐volume loop analysis was done using simple linear regression. Metabolic parameters and hemodynamic parameters were analyzed using a Student t test. A probability value of <0.05 was considered significant, with all data reported as mean and SEM.
Sample Preparation and Liquid Chromatography–Tandem Mass Spectrometry Analysis
Briefly, EV pellets were subjected to 8 mol/L urea lysis buffer, followed by a 15‐second sonication in a water bath. Protein extraction, trypsin digestion, and tryptic peptide desaltation were performed as described previously.24 Tryptic peptides were desalted using C18 Sep‐Pak plus cartridges (Waters, Milford, MA) and were lyophilized for 48 hours to dryness. The dried eluted peptides were reconstituted in buffer A (0.1 mol/L acetic acid) and subjected for liquid chromatography–tandem mass spectrometry (MS/MS) analysis.
The liquid chromatography–MS/MS was performed on a fully automated proteomic technology platform that includes Agilent 1200 and 1260 Series Quaternary high‐performance liquid chromatography systems (Agilent Technologies, Santa Clara, CA) connected to a Q Exactive Plus mass spectrometer (Thermo Fisher Scientific). The liquid chromatography–MS/MS setup was used as described earlier.25, 26, 27, 28 The MS/MS spectra were acquired at a resolution of 17 500, with a targeted value of 2×104 ions or a maximum integration time of 200 milliseconds.
The MS raw file was searched against the Human UniProt database (UniProt; downloaded February 1, 2015) using MASCOT version 2.4 (Matrix Science, Ltd, London, UK). A concatenated database containing “target” and “decoy” sequences was used to estimate the false‐discovery rate.27 Msconvert from ProteoWizard (version 3.0.5047), using default parameters and with the MS2Deisotope filter on, was used to create peak lists for Mascot. The Mascot database search was performed with the following parameters: trypsin enzyme cleavage specificity, 2 possible missed cleavages, 10 ppm mass tolerance for precursor ions, and 20 mmu (milli mass unit) mass tolerance for fragment ions. Search parameters permitted variable modification of methionine oxidation (15.9949 Da) and static modification of carbamidomethylation (57.0215 Da) on cysteine. The resulting peptide spectrum matches were reduced to sets of unique peptide spectrum matches by eliminating lower‐scoring duplicates. To provide high confidence, the Mascot results were filtered for Mowse score (>20). Peptide assignments from the database search were filtered down to a 1% false‐discovery rate by a logistic spectral score, as previously described.27, 29
Results
Metabolic Parameters
The animals all had an increase in body mass index at both the second procedure as well as at the time of the terminal procedure and cardiac harvest. The HVM group's mean body mass index was higher throughout the study when compared with the other 2 groups; however, the percentage change in weight gain was not significantly different among groups (CON: 31.6±8.1%; HVM: 46.5±2.2% [P=0.11 compared with CON]; HVMC: 26.9±4.4% [P>0.99 compared with CON) (Table 1). Glucose tolerance tests were performed during each of the 3 surgical interventions, and no differences were seen between the groups at any time point (P>0.05 at every time point for all groups), although all were impaired, as defined by a glucose level >140 mg/dL at both 30 and 60 minutes (Figure 1 and Table 2). There were no significant differences in heart rate (heart rate for CON: 79±5.99 per minute, HVM: 83±11.2 per min, and HVMC: 82±12.78 per min; P=0.58) and blood pressure at the time of harvest between the groups, as measured by both blood pressure cuff and intra‐arterial pressure catheter (mean cuff mean arterial pressure for CON: 56.3±4.5 mm Hg, HVM: 64.6±8.8 mm Hg, HVMC 75.2±13.1 mm Hg; P=0.66; mean catheter mean arterial pressure for CON: 63.2±5.3 mm Hg, HVM: 72.6±6.1 mm Hg, HVMC: 61.2±6.6 mm Hg; P=0.35).
Table 1.
Mean BMI and Percentage Weight Change
| Variable | CON (n=6) | HVM (n=8) | CON vs HVM | HVMC (n=5) | CON vs HVMC | HVM vs HVMC | All 3 Groups |
|---|---|---|---|---|---|---|---|
| Ameroid | 45.5±3.9 | 48.8±2.2 | P=0.91 | 44.1±2.4 | P>0.99 | P=0.87 | P=0.48 |
| EV injection | 39.1±2.2 | 49.9±1.6 | P=0.01 | 49.0±1.6 | P=0.06 | P>0.99 | P<0.01 |
| Harvest | 46.6±4.4 | 53.6±1.7 | P=0.93 | 46.4±3.4 | P>0.99 | P=0.39 | P=0.30 |
| % Change in weight | 31.6±8.1 | 46.5±2.2 | P=0.11 | 26.9±4.4 | P>0.99 | P=0.04 | P=0.01 |
Data are given as mean±SEM BMI (mg/kg2). Last row demonstrates percentage weight change for each group from the ameroid to the harvest±SEM. Data were analyzed using ANOVA and Kruskal‐Wallis, with P<0.05 considered as significant. BMI indicates body mass index; CON, control group (high‐fat diet with myocardial vehicle injection); EV, extracellular vesicle; HVM, high‐fat diet with myocardial EV injection group; HVMC, HVM and calpain inhibition group.
Figure 1.
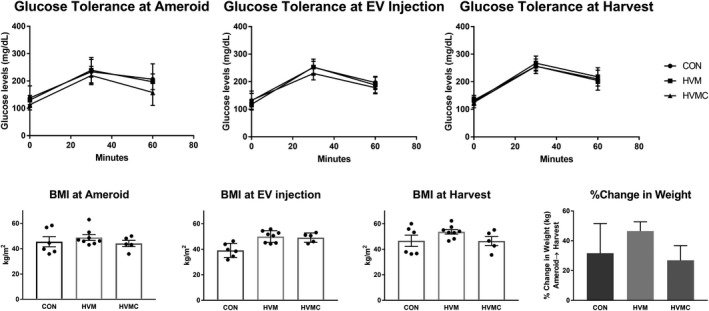
Glucose tolerance test and body mass index (BMI) values. There was no significant difference in glucose tolerance between any of the groups at any of the time points. The 30‐ and 60‐minute values at all time points in all groups reflect impaired glucose tolerance consistent with metabolic syndrome. The BMI levels were consistently higher in the high‐fat diet with myocardial extracellular vesicle (EV) injection group (HVM) when compared with the control group at all time points; however, there was no significant difference in percentage change of weight gain between the groups. Data were analyzed using Student t test. CON indicates control group (high‐fat diet with vehicle injection); HVMC, HVM and calpain inhibition group. *P<0.05 was considered significant.
Table 2.
Oral Glucose Tolerance Test
| Group | Ameroid, mg/dL | EV Injection, mg/dL | Harvest, mg/dL | |||
|---|---|---|---|---|---|---|
| Mean BG | SEM | Mean BG | SEM | Mean BG | SEM | |
| CON | ||||||
| Baseline | 138.17 | 16.34 | 131.50 | 12.83 | 128.00 | 5.45 |
| 30 min | 233.67 | 16.78 | 251.83 | 8.37 | 267.50 | 9.55 |
| 60 min | 206.33 | 21.02 | 196.67 | 8.40 | 217.83 | 8.97 |
| HVM | ||||||
| Baseline | 130.38 | 5.58 | 117.75 | 5.88 | 133.00 | 5.79 |
| 30 min | 239.63 | 15.34 | 253.00 | 9.57 | 256.63 | 5.84 |
| 60 min | 197.25 | 9.51 | 187.75 | 9.56 | 204.63 | 6.62 |
| HVMC | ||||||
| Baseline | 113.40 | 4.07 | 132.80 | 9.75 | 125.20 | 7.76 |
| 30 min | 220.00 | 12.94 | 229.80 | 9.37 | 256.00 | 10.57 |
| 60 min | 158.20 | 18.94 | 178.20 | 8.78 | 210.20 | 16.32 |
Data are given as BG level±SEM (mg/dL) after IV injection of 0.5 mg/kg of 50% dextrose. Data were analyzed using ANOVA and Kruskal‐Wallis, with P<0.05 considered as significant. BG indicates blood glucose; CON, control group (high‐fat diet with vehicle injection); EV, extracellular vesicle; HVM, high‐fat diet with myocardial EV injection group; HVMC, HVM and calpain inhibition group.
No differences were observed in aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, total bilirubin, direct bilirubin, total protein, albumin, CRP (C‐reactive protein), insulin, triglycerides, and high‐density lipoprotein between the control group and the HVM or HVMC groups at the time of harvest (data not shown). Total cholesterol levels were significantly elevated in all 3 groups at the time of harvest (CON: 973±265 mg/dL, HVM: 476±172 mg/dL, HVMC: 994±304 mg/dL). Low‐density lipoprotein levels were also significantly elevated in all 3 groups at the time of harvest (CON: 867±267 mg/dL, HVM: 379±166 mg/dL, HVMC: 880±305 mg/dL). Total cholesterol and low‐density lipoprotein levels were lower in the HVM group than the CON at both the time of ameroid placement and the time of harvest (ameroid: CON: 760.3±86.8 mg/dL, HVM: 534.8±38.6 mg/dL, P=0.034; harvest: CON: 973.5±108.2 mg/dL, HVM: 476.9±60.9 mg/dL, P=0.002). Fructosamine levels were observed to be significantly elevated in the HVM group compared with the control group at the time of ameroid placement (CON: 174.3±9.9 mg/dL, HVM: 206.6±4.8 mg/dL, P=0.01), but were then significantly lower than the control group at the time of harvest (CON: 229.3±10.2 mg/dL, HVM: 205.1±2.8 mg/dL, P=0.03).
Myocardial Perfusion
There was no significant difference in blood flow to the ischemic ventricle between groups at rest (CON: 0.41±0.07 ml/min/g, HVM: 0.58±0.10 ml/min/g, P>0.9; HVMC: 0.38±0.04 ml/min/g, P=0.82). However, under conditions of pacing to 150 beats per minute, blood flow was significantly increased in the HVM group when compared with the control group in both the ischemic ventricle (CON: 0.41±0.07 ml/min/g, HVM: 0.82±0.86 ml/min/g, P=0.05) as well as the nonischemic area (CON: 0.64±0.10 ml/min/g, HVM: 1.3±0.20 ml/min/g, P=0.04) (Figure 2). The group that received CI in addition to EVs (HVMC) did not show an increase in blood flow in the ischemic ventricle either at rest (HVMC: 0.28±0.49 ml/min/g, P=0.74) or during pacing (HVMC: 0.37±0.07 ml/min/g, P>0.99) compared with the control group. There was no difference in the nonischemic ventricle at rest between any of the groups.
Figure 2.

Blood flow is increased in the high‐fat diet with myocardial extracellular vesicle injection group (HVM) during pacing. Extracellular vesicle injection increased blood flow (mL/min per g) to both the ischemic and nonischemic areas of the left ventricle during paced conditions to 150 beats per minute in the HVM group. This was not recapitulated in the group that also received oral calpain inhibition (HVMC) nor was there any difference between groups when the heart was at rest. CON indicates control group (high‐fat diet with vehicle injection). *P<0.05 (Kruskal‐Wallis with Dunn's post hoc analysis test was used to compare the means among the 3 groups).
Cardiac Function
To examine whether EV injection and/or CI have any effect on cardiac function, pressure‐volume loop analysis was performed. There were no significant differences in cardiac function at rest between the groups, as determined by cardiac output at rest (CON: 1954±254 mL/min, HVM: 2800±838 mL/min, HVMC: 1716±99 mL/min; P=0.46), stroke volume (CON: 25.9±5.7 mL, HVM: 32.2±8.9 mL, HVMC: 21.62±4.7 mL; P=0.68), and other parameters, including dP/dt, stroke work, Tau, and Pmax (maximum pressure value) (data not shown). Interestingly, under conditions of pacing to 150 beats per minute, cardiac output and stroke volume were significantly increased in the HVM group compared with the control group (Figure 3). In addition, cardiac index was significantly increased in the HVM compared with the control and HVMC groups (CON: 2.93±0.14 L/min per m2, HVM: 6.91±1.69 L/min per m2, HVMC: 2.43±0.15 L/min per m2, P=0.008). However, there were no significant changes observed in stroke work, dP/dt, Tau, and Pmax between these 2 groups. Interestingly, there were no significant changes in cardiac function in HVMC animals compared with the control, as determined by cardiac output (CON: 2132±679 mL/min, HVMC: 2018±340 mL/min; P=0.48), CI (CON: 2.93±0.14 L/min per m2, HVMC: 2.43±0.15 L/min per m2, P=0.45), stroke volume (CON: 15.15±3.8 mL, HVMC:16.43±3.9 mL, P=0.68), and other parameters, including stroke work, dP/dt, Tau, and Pmax (data not shown). Together, these findings suggest that although EV treatment did not improve cardiac output and stroke volume at rest, EVs improved cardiac output and stroke work during pacing of the heart.
Figure 3.
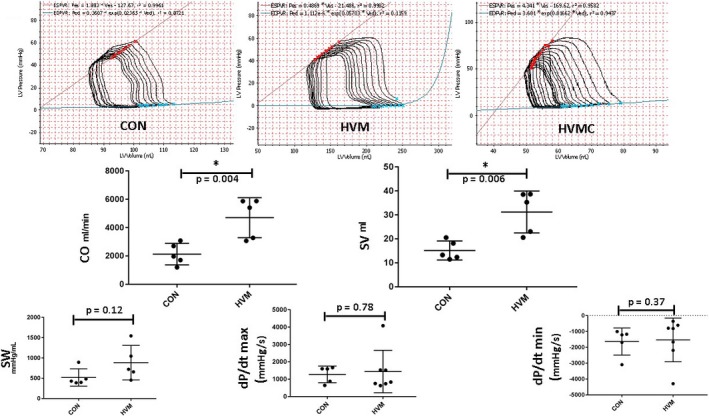
Cardiac output (CO) and stroke volume (SV), but not dP/dt, were increased in the high‐fat diet with myocardial extracellular vesicle (EV) injection group (HVM) during pacing. Pressure‐volume (PV) loop analyses were performed to determine cardiac function (top: a representative PV loop from each group). EV injection increased CO and SV during paced conditions to 150 beats per minute in the HVM group. This was not recapitulated in the group that also received oral calpain inhibition (HVMC) nor was there any difference between groups when the heart was at rest. dP/dt and stroke work (SW) are also shown. CON indicates control group (high‐fat diet with vehicle injection), LV, left ventricle. *P<0.05 (with linear regression used for analysis).
Myocardial Arteriolar and Capillary Density
The HVM group had significantly higher arteriolar density in both ischemic (CON: 1.0±0.06‐fold, HVM: 2.1±0.1 fold) and nonischemic (CON: 1.0±0.07 fold, HVM: 2.0±0.16 fold) areas of the left ventricle (P=0.003 for both), as determined by quantitative analysis of α‐smooth muscle actin staining (Figure 4A). The HVMC group did not differ from the control group in either the ischemic (HVMC: 1.03±0.04 fold, P>0.9) or nonischemic (HVMC: 1.05±0.08 fold, P>0.9) areas of the ventricle, suggesting abrogation of the effect of EV injection by inhibitory action of CI (Figure 4A and 4B). Interestingly, although there was a trend toward increased capillary density, there were no statistically significant differences between capillary density in either the ischemic or nonischemic areas of the CON and HVM groups (CON versus HVM: 17.913±3.529 versus 26.056±3.989 vessels per high‐power field; P=0.13), as determined by CD31 immunofluorescence study. Similarly, capillary density of the CI‐treated HVMC group in either the ischemic (HVMC: 19.03±3.04 vessels per high‐power field) or nonischemic (HVMC: 21.84±3.6 vessels per high‐power field) areas did not differ statistically from the CON (P=0.09) or HVM (P=0.14) groups.
Figure 4.
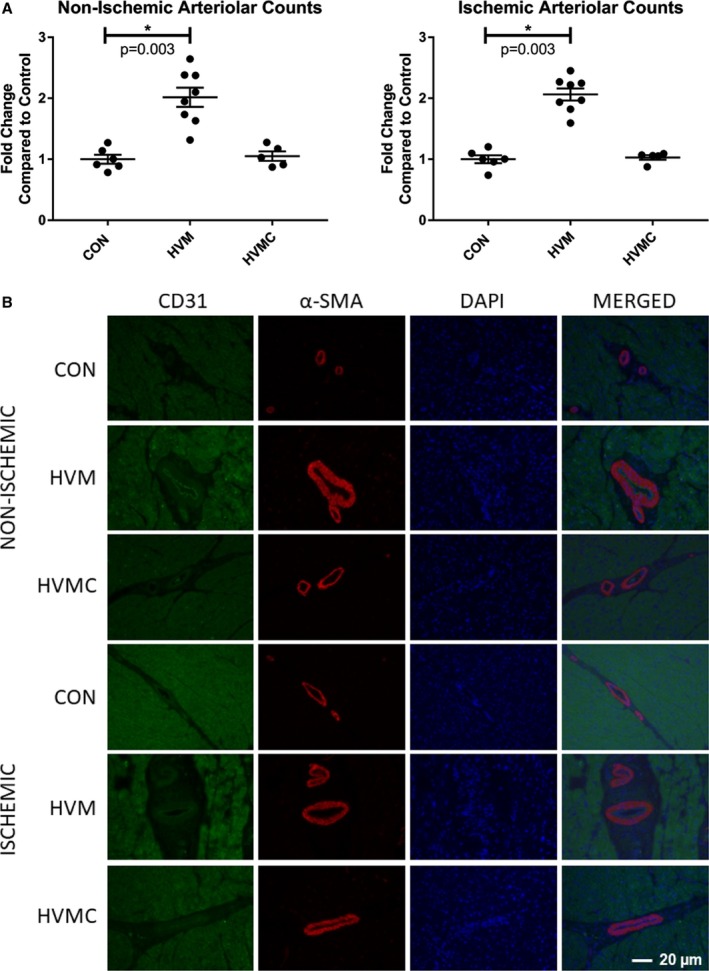
Extracellular vesicle (EV) injection improves arteriolar density in the myocardium of high‐fat fed animals. A, Arteriolar density, as determined by α‐smooth muscle actin (α‐SMA) staining, was significantly increased in the high‐fat diet with myocardial EV injection group (HVM) compared with the control group (high‐fat diet with vehicle injection) (CON) in both the nonischemic and the ischemic myocardium. There was no difference between the HVMC and calpain inhibition group (HVMC) and the CON in either the nonischemic or the ischemic ventricle. B, A ×20 high‐power field image representative of myocardial tissue with staining of α‐ in red, cluster of differentiation 31 (CD31) in green, and 4’,6‐diamidino‐2‐phenylindole (DAPI) in blue. *P<0.05 (Kruskal‐Wallis with Dunn's post hoc analysis test was used to compare the means among the 3 groups).
Protein Expression Analysis
There was no significant difference in expression of eNOS between either group (HVM and HVMC) compared with the control (CON: 1.0±0.11 fold, HVM: 0.9±0.10 fold, P>0.9; HVMC: 0.55±0.14 fold, P=0.17) or in phosphorylated eNOS/eNOS (CON: 0.94±0.21 fold, HVM: 1.4±0.34 fold, P>0.9; HVMC: 0.50±0.16 fold, P=0.56). There was also no difference between the control group and HVM group for phosphorylated eNOS (CON: 1.0±0.30 fold, HVM: 1.2±0.28 fold, P>0.9), although the HVMC group was significantly decreased compared with the control (0.2±0.04 fold, P=0.03), suggestive of inhibition of phosphorylated eNOS activation by CI. BCL‐2 was downregulated in both groups compared with the control; however, it did not approach significance (CON: 1.0±0.14 fold, HVM: 0.73±0.05 fold, P=0.42; HVMC: 0.69±0.08 fold, P=0.31). BCL‐2–associated X protein was unchanged between groups (CON: 1.0±0.19, HVM: 0.72±0.10 fold, P=0.65; HVMC: 0.88±0.06 fold, P>0.99). BCL‐2–associated death promoter, however, although not significantly different in the HVM group compared with the control (CON: 1.0±0.10 fold, HVM: 1.1±0.15 fold, P>0.9), was significantly downregulated in the HVMC group compared with the control (0.41±0.07 fold, P=0.04), suggestive of downregulation of apoptosis by the addition of CI to the EV treatment. Transforming growth factor‐β was not significantly different between groups (CON: 1.0±0.11 fold, HVM: 0.97±0.08 fold, P>0.9; HVMC: 1.2±0.06 fold, P=0.47), although the trend toward an increase in the HVMC group again suggests a protective effect against apoptosis and a decreased level of inflammation in the group that received CIs. Finally, vascular endothelial growth factor receptor 2 expression was not significantly different between groups (CON: 1.0±0.18 fold, HVM: 1.0±0.11 fold, P>0.9; HVMC: 0.55±0.14 fold, P=0.41), which may have likely contributed to the lack of differences in capillary densities and blood flow at rest seen in the HVM group (Figure 5). Taken together, these data suggest that in the setting of metabolic syndrome, although EVs improve arteriogenesis and cardiac function at pacing, EVs are unable to upregulate eNOS and vascular endothelial growth factor expression and have no significant effect on apoptosis. The EVs with CI do appear to downregulate apoptotic pathways; however, they may be limiting inflammatory signaling necessary to allow regeneration and restoration of cardiomyocyte activity.
Figure 5.
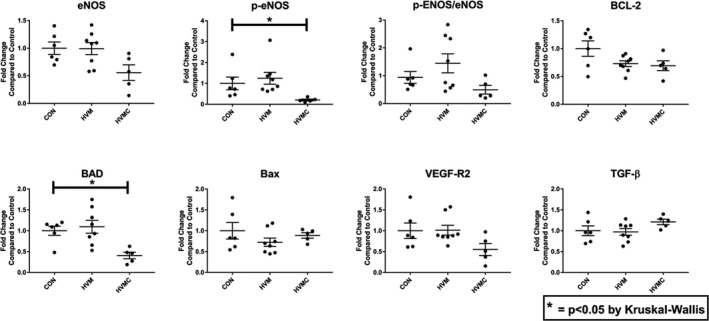
Calpain inhibition (CI) decreases apoptosis signaling in ischemic myocardium treated with extracellular vesicle (EV) injection in the setting of metabolic syndrome. There was no significant difference in expression of endothelial NO synthase (eNOS) or phosphorylated eNOS (p‐eNOS)/eNOS between either group compared with the control group (high‐fat diet with vehicle injection) (CON). The high‐fat diet with EV injection (HVM) and CI group (HVMC) had a significant decrease in p‐eNOS compared with CON, suggestive of inhibition of p‐eNOS activation by CI. B‐cell lymphoma‐2 (BCL‐2), BCL‐2–associated X protein (Bax), transforming growth factor‐β (TGF‐β), and vascular endothelial growth factor receptor 2 (VEGF‐R2) were not significantly different between groups. BCL‐2–associated death promoter (BAD) was significantly downregulated in the HVMC group compared with the control, suggesting a protective effect of CI against apoptosis. *P<0.05 (Kruskal‐Wallis with Dunn's post hoc analysis test was used to compare the means among the 3 groups).
Proteomic Profiling of EV Sample
To characterize the expressed proteins in the EV samples, a discovery‐based proteomic approach was used. Two sets of independent biological EV samples were collected in different time periods and subjected for proteomic analysis. From experiment sets 1 and 2, a total of 4566 peptides corresponding to 934 unique proteins and a total of 1801 peptides corresponding to 374 unique proteins were identified (Tables S1 and S2). Thus, a total of 1027 unique proteins were identified from the 2 sets of EV samples, wherein >25% (275) proteins were common in both sets of EV samples (Figure 6A). The proteins identified from each set of experiments are given as a supplementary data set (Table S3). Many of the known EV biomarker proteins were successfully identified in EV samples (Table S3), suggesting both samples were EV enriched. The low numbers of proteins identified from experiment 2 were caused by the initial sample volume subjected to proteomic analysis. We have focused on the common EV proteins that were identified in both sets of EV samples and were subject to further characterization. Gene Ontology analysis showed that proteins associated with focal adhesion and secretory granule lumen were mostly enriched in the cellular component category (Figure 6B). Among the biological process categories, the proteins involved in neutrophil degranulation, neutrophil activation, and neutrophil‐mediated immunity response were at high quantities among the EV proteins (Figure 6C). The gene ontology data are further supported by the Kyoto Encyclopedia of Genes and Genomes pathway analysis, wherein the regulation of actin cytoskeleton, ribosome, glycolysis/gluconeogenesis, and phagosome pathway–associated proteins was shown to be highly enriched (Figure 6D). The Online Mendelian Inheritance in Man disease pathway showed that myopathy, dilated cardiomyopathy, and cardiomyopathy‐associated proteins were enriched in EVs (Figure 6E). In addition, many known proteins, including tropomyosin α‐1 chain, tropomyosin β chain, tropomyosin α‐3 chain, vinculin, desmin, and filamin‐C, were also identified (Figure 6F).
Figure 6.
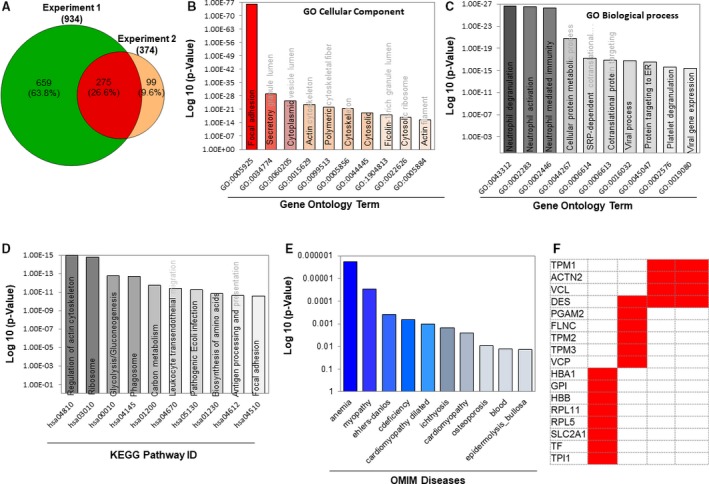
Proteomic footprint of the extracellular vesicle (EV) proteome overlapped in 2 different sets of EV samples. A, The Venn diagram analysis of the unique proteins identified from each EV sample. The Venn diagram was generated using 2 publicly available bio tools (namely, Venny 2.1 [http://bioinfogp.cnb.csic.es/tools/venny/] and Pacific Northwest National Laboratory [http://omics.pnl.gov/software/venn-diagram-plotter]). B through E, The Gene Ontology (GO) Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses of the overlapped proteins and the Online Mendelian Inheritance in Man (OMIM) analyses of the protein groups involved in disease/pathologies. GO and KEGG pathway analyses were done using Enrichr (http://amp.pharm.mssm.edu/Enrichr/), and OMIM analysis was performed using OMIM (https://omim.org/). F, Diseases that may result from dysfunction of the major proteins that are modulated in both groups of proteomics (taken from the common red area in the Venn diagram of A).
Discussion
As the prevalences of hypercholesterolemia, type 2 diabetes mellitus, metabolic syndrome, and coronary artery disease continue to increase, therapeutic modalities to combat left ventricular remodeling in chronic ischemia are of utmost importance. Although EVs demonstrate early promise for translational benefit in models of myocardial ischemia and infarction,30, 31 their use in the setting of underlying comorbidities, such as those of hypercholesterolemia and metabolic syndrome, has not previously been studied, to our knowledge. Recently, we demonstrated that EVs may improve myocardial function and perfusion in a large animal model of chronic myocardial ischemia.20 However, in that study, the animals were otherwise normal and received a normal diet. We and others have previously reported that diet‐induced (high‐fat fed) metabolic syndrome markedly diminishes the potential of growth factors, gene therapy, and cell therapy to improve myocardial perfusion and function.21, 32 Most clinical trials have been negative or have demonstrated small benefits to protein growth factor therapy, gene therapy, or cell therapy. Yet, nearly all preclinical studies have demonstrated robust improvements in myocardial perfusion and function in rodent models.
The current study demonstrated a significant improvement in myocardial perfusion in the chronically ischemic left circumflex artery territory, distal to the ameroid constrictor. Interestingly, myocardial perfusion in the nonischemic ventricular myocardium (left anterior descending territory) also increased with EV injection, suggesting that the local injection of EVs may have a systemic effect, being not limited to the area of myocardial ischemia. Rather, EVs could induce vessel growth and/or vessel dilatation in areas distant from the site of injury, resulting in better overall myocardial perfusion. These findings were further supported by the observation of increased arteriolar density in both the ischemic and nonischemic territory in the group treated with EVs alone, suggestive of increased angiogenesis both at the site of injection as well as surrounding areas of the left ventricle. Increased vessel density in and blood flow to the ischemic myocardium by EVs were accompanied by significant improvement in cardiac function, specifically in cardiac output and stroke volume, during pacing of the heart. Although the findings of increased blood flow and improved cardiac function were not recapitulated at rest, it is perhaps of even more clinical importance to have increased blood flow during tachycardia in a model of metabolic syndrome given the common limited exercise capacity of patients with metabolic syndrome. The negative impact of metabolic syndrome on cardiac function and recovery has been documented in children and, therefore, is of even higher clinical concern in adults with metabolic syndrome and coronary artery disease.33 Although we did observe a lower total cholesterol in the HVM group, the total cholesterol was still significantly elevated (mean, >300 mg/dL) in this group, which we believe would preclude the conclusion that a lower cholesterol level affects the EVs in this group. Furthermore, patients with metabolic syndrome even without coronary artery disease have had subclinical systolic and diastolic dysfunction and been at higher risk of inducible myocardial ischemia.34, 35 Therefore, the finding of increased blood flow during stress (pacing) may be an early sign of improvement after EV therapy, which has important clinical significance in the setting of metabolic syndrome. Interestingly, EVs did not induce improvement in the capillary densities of ischemic or nonischemic myocardium. Lack of improvement in blood flow at rest in the HVM group may plausibly be attributed to this finding. In previous studies from our group, the high‐fat feeding of pigs markedly diminished the angiogenic response of increased capillary development in the chronically ischemic territory.36, 37 The response to various growth factors, drugs, or dietary supplements has been variable, with some agents increasing both capillary and arteriolar density and others affecting one preferentially.38 In the current study, the injection of EVs increased only arteriolar density without any appreciable effect on capillary density. This is likely caused by the content of the EVs we used in the study. The increased arteriolar density in the HVM group is suggestive of increased vessel density as a result of angiogenesis after EV injection into the myocardium, which affects not only the region of injection (ischemic area), but also surrounding tissue (nonischemic area). It is possible that EVs could be manipulated to have a more favorable effect to improve vascular density and myocardial perfusion. We are currently investigating the effects of oxidative stress, hypoxia, and other manipulations on the differential expression of proteins within the vesicles.
An ideal translational therapy would not require a thoracotomy for intramyocardial administration as this would significantly limit its use in human applications. Our group has previously investigated the role of administration of EVs via intravenous injection in the setting of chronic myocardial ischemia. However, intravenous injection of EVs could not recapitulate the beneficial effects observed in the intramyocardial injection group.20 This may be because of a “dose effect” as intravenous route may require larger quantities of EV administration. The observed efficacy of EVs in increasing blood flow as well as arteriolar density in both the area of ischemia where they were administered as well as in the nonischemic myocardium is of interest given the suggested homing properties of EVs to sites of injury.39
Surprisingly, we did not observe any upregulation of protein expression in pathways and markers involved in angiogenesis, such as vascular endothelial growth factor receptor 2, with EV administration. Given the EVs contain RNAs, cytokines, and growth factors and our observation of increased arteriolar density and increased blood flow, we would have expected to find some elements of angiogenetic pathways to support these findings. Taken together, these findings suggest that although the HVM group has increased arterioles and increased blood flow under paced conditions, the mechanisms of angiogenesis may be via alternative pathways. Thus, proteomic profiling of the EV samples was deemed essential to have a better understanding of the molecules and/or signaling pathways that may have played critical roles in these processes. Our proteomic analysis data provide critical insights by providing novel information about the contents of EVs. The protein contents of EVs appear to be involved in important cardiovascular pathophysiological processes, including cardiomyopathy and tissue oxygenation (anemia) signaling pathways. The proteomic data also allow further investigation of the role of some target proteins, such as tropomyosin α‐1 chain, tropomyosin β chain, tropomyosin α‐3 chain, vinculin, desmin, and filamin‐C, and their roles in the regulation of coronary angiogenic processes. We speculate that it is not one single peptide or protein, but rather a group of proteins, in EV that may modulate the microenvironmental milieu of the tissue to improve angiogenesis and thus recovery of cardiac function in the setting of chronic myocardial ischemia. Further studies are required to elucidate the precise mechanisms by which EVs improved vessel growth and myocardial perfusion in chronic myocardial ischemia.
The EV injections did not seem to affect apoptotic pathways independently. Given our model is one of chronic ischemia and not acute infarct, the lack of significant changes in apoptosis is not surprising. On the other hand, the downregulation of BCL‐2–associated death promoter, a caspase that promotes apoptosis via mitochondrial and calcium‐dependent pathways, in the HVMC group (ie, EV injections followed by CI) suggests decreased apoptosis in response to CI. This supports previous findings of calpains having proapoptotic activity in stressed cardiomyocytes.40 CI has been shown to decrease apoptotic activity as well as inflammatory protein expression in the setting of chronic myocardial ischemia, which was in accordance with our findings in this study.19, 41 However, it has also been shown to increase collateral‐dependent perfusion, which we did not see in our study in combination with EV therapy.18 Perhaps the anti‐inflammatory properties of CI also function to inhibit the activity of EVs, thereby negating their efficacy rather than synchronizing to increase the beneficial effects.
One of the advantages of EV therapy over stem cell–based therapies include the lack of DNA transfer. Furthermore, EVs would ideally induce less of an immunosuppressive response than a cell‐based therapy; however, data about this appear to indicate some immunomodulatory effects of EVs.42 Our findings that the groups receiving the EV injections did not have any significant differences in metabolic parameters, such as CRP levels, suggest the lack of significant immunomodulatory activity by EVs in our model. Furthermore, the lack of differences between groups in liver function enzymes suggests no significant toxic effect of EV therapy when administered locally.
Limitations
The major limitations of this study in translational applications include the single dose of EVs, the intracardiac route of administration, and the limited time course between treatment and cardiac harvest. As chronic myocardial ischemia is often a multiyear development in humans, the short time frame of this study limits the applicability to the human disease model. Given the limitations of a large animal model, it was not feasible to perform a dose‐response curve; however, this would be ideal to determine in a small animal model. In the future, when the synthesis of EVs becomes more refined and large quantities can be readily created and manipulated, larger quantities can be injected and the contents of the EVs can be optimized. Similarly, only one dose of CI was used, determined to be a high dose on the basis of previous studies by our laboratory, and perhaps the overall effect was inhibitory on the EVs, whereas a lower dose may not have the same inhibitory effects on angiogenesis. Our study was also conducted entirely using young, male swine, which may have physiological sex‐ and age‐based differences that must be accounted for before translation to human studies. Another limitation is that the precise mechanisms by which EVs produce angiogenic effects are not known at this point. Ongoing studies in our laboratory will elucidate the mechanisms in the future.
Conclusions
In the setting of diet‐induced metabolic syndrome, intramyocardial injection of EVs results in increase in blood flow during stress under conditions of rapid pacing to all areas of the left ventricle and improvement in cardiac function, which resulted from the increased arteriolar formation in both ischemic and nonischemic myocardium. Interestingly, the addition of CI did not improve this effect and rather appeared to diminish the response, plausibly because of the anti‐inflammatory properties of CI. This is suggestive of the need of some level of inflammatory signaling to induce angiogenesis by EVs.
Sources of Funding
Funding for this research was provided by the National Heart, Lung, and Blood Institute (NHLBI) (R01HL46716 and R01HL128831‐01A1 [Dr Sellke]); NHLBI 1R01HL133624 and American Heart Association Grant‐in‐Aid 14GRNT20460291 (Dr Abid); and National Institutes of Health/National Institute of General Medical Sciences Training Grant 2T32 GM065085‐12 (Dr Potz) and 2T32 GM065085‐13 (Dr Scrimgeour).
Disclosures
None.
Supporting information
Table S1. List of Proteins Identified From Extracellular Vesicle Sample Biological Set 1 by LC‐MS/MS Analysis. Please See Excel File
Table S2. List of Proteins Identified From Extracellular Vesicle Sample Biological Set 2 by LC‐MS/MS Analysis. Please See Excel File
Table S3. List of Overlapping Proteins Identified From Two Biological Sets of Extracellular Vesicle Samples by LC‐MS/MS Analysis. Please See Excel File
Acknowledgments
We would like to thank the veterinary and animal care staff at Rhode Island Hospital for their excellent care of the animals used in this study. We would also like to thank Richard Clements, PhD, for his assistance in pressure‐volume loop analysis.
(J Am Heart Assoc. 2019;8:e012617 DOI: 10.1161/JAHA.119.012617.)
References
- 1. Roth GA, Huffman MD, Moran AE, Feigin V, Mensah GA, Naghavi M, Murray CJL. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation. 2015;132:1667–1678. [DOI] [PubMed] [Google Scholar]
- 2. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. [DOI] [PubMed] [Google Scholar]
- 3. Scott R, Donoghoe M, Watts GF, O'Brien R, Pardy C, Taskinen M‐R, Davis TME, Colman PG, Manning P, Fulcher G, Keech AC; FIELD Study Investigators . Impact of metabolic syndrome and its components on cardiovascular disease event rates in 4900 patients with type 2 diabetes assigned to placebo in the FIELD randomised trial. Cardiovasc Diabetol. 2011;10:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mente A, Yusuf S, Islam S, McQueen MJ, Tanomsup S, Onen CL, Rangarajan S, Gerstein HC, Anand SS; INTERHEART Investigators . Metabolic syndrome and risk of acute myocardial infarction a case‐control study of 26,903 subjects from 52 countries. J Am Coll Cardiol. 2010;55:2390–2398. [DOI] [PubMed] [Google Scholar]
- 5. Chuah LL, Papamargaritis D, Pillai D, Krishnamoorthy A, le Roux CW. Morbidity and mortality of diabetes with surgery. Nutr Hosp. 2013;28(suppl 2):47–52. [DOI] [PubMed] [Google Scholar]
- 6. Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marbán L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marbán E. Intracoronary cardiosphere‐derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Malliaras K, Makkar RR, Smith RR, Cheng K, Wu E, Bonow RO, Marbán L, Mendizabal A, Cingolani E, Johnston PV, Gerstenblith G, Schuleri KH, Lardo AC, Marbán E. Intracoronary cardiosphere‐derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1‐year results of the CADUCEUS trial (CArdiosphere‐Derived aUtologous stem CElls to reverse ventricUlar dySfunction). J Am Coll Cardiol. 2014;63:110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bartunek J, Terzic A, Davison BA, Filippatos GS, Radovanovic S, Beleslin B, Merkely B, Musialek P, Wojakowski W, Andreka P, Horvath IG, Katz A, Dolatabadi D, El Nakadi B, Arandjelovic A, Edes I, Seferovic PM, Obradovic S, Vanderheyden M, Jagic N, Petrov I, Atar S, Halabi M, Gelev VL, Shochat MK, Kasprzak JD, Sanz‐Ruiz R, Heyndrickx GR, Nyolczas N, Legrand V, Guédès A, Heyse A, Moccetti T, Fernandez‐Aviles F, Jimenez‐Quevedo P, Bayes‐Genis A, Hernandez‐Garcia JM, Ribichini F, Gruchala M, Waldman SA, Teerlink JR, Gersh BJ, Povsic TJ, Henry TD, Metra M, Hajjar RJ, Tendera M, Behfar A, Alexandre B, Seron A, Stough WG, Sherman W, Cotter G, Wijns W; CHART Program . Cardiopoietic cell therapy for advanced ischemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham‐controlled CHART‐1 clinical trial. Eur Heart J. 2016;38:ehw543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith RR, Marbán E, Marbán L. Enhancing retention and efficacy of cardiosphere‐derived cells administered after myocardial infarction using a hyaluronan‐gelatin hydrogel. Biomatter. 2013;3:e24490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. EL Andaloussi S, Mäger I, Breakefield XO, Wood MJA. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–357. [DOI] [PubMed] [Google Scholar]
- 11. Wang Y, Zhang L, Li Y, Chen L, Wang X, Guo W, Zhang X, Qin G, He S, Zimmerman A, Liu Y, Kim I, Weintraub NL, Tang Y. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int J Cardiol. 2015;192:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Timmers L, Lim SK, Arslan F, Armstrong JS, Hoefer IE, Doevendans PA, Piek JJ, Menshawe R, Oakley E, Choo A, Lee CN, Pasterkamp G, De Kleijn DP. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2008;1:129–137. [DOI] [PubMed] [Google Scholar]
- 13. Lai RC, Arslan F, Lee MM, Sze NSK, Choo A, Chen TS, Salto‐Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DPV, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. [DOI] [PubMed] [Google Scholar]
- 14. Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor ENE, Timmers L, Van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, De Kleijn DP. Mesenchymal stem cell‐derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10:301–312. [DOI] [PubMed] [Google Scholar]
- 15. Ono Y, Sorimachi H. Calpains: an elaborate proteolytic system. Biochim Biophys Acta. 2012;1824:224–236. [DOI] [PubMed] [Google Scholar]
- 16. Potz BA, Abid MR, Sellke FW. Role of calpain in pathogenesis of human disease processes. J Nat Sci. 2016;2:e218. [PMC free article] [PubMed] [Google Scholar]
- 17. Potz BA, Sabe AA, Abid MR, Sellke FW. Calpains and coronary vascular disease. Circ J. 2016;80:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sabe AA, Potz BA, Elmadhun NY, Liu Y, Feng J, Abid MR, Abbott JD, Senger DR, Sellke FW. Calpain inhibition improves collateral‐dependent perfusion in a hypercholesterolemic swine model of chronic myocardial ischemia. J Thorac Cardiovasc Surg. 2016;151:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Potz BA, Sabe AA, Elmadhun NY, Feng J. Calpain inhibition decreases myocardial apoptosis in a swine model of chronic myocardial ischemia. Surgery. 2016;158:445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Potz BA, Scrimgeour LA, Pavlov VI, Sodha NR, Abid MR, Sellke FW. Extracellular vesicle injection improves myocardial function and increases angiogenesis in a swine model of chronic ischemia. J Am Heart Assoc. 2018;7:e008344 DOI: 10.1161/JAHA.117.008344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lassaletta AD, Chu LM, Robich MP, Elmadhun NY, Feng J, Burgess TA, Laham RJ, Sturek M, Sellke FW. Overfed Ossabaw swine with early stage metabolic syndrome have normal coronary collateral development in response to chronic ischemia. Basic Res Cardiol. 2012;107:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National Research Council . Guide for the Care and Use of Laboratory Animals. 8th ed Washington, DC: The National Academies Press; 2011. [Google Scholar]
- 23. Elmadhun NY, Lassaletta AD, Chu LM, Liu Y, Feng J, Sellke FW. Atorvastatin increases oxidative stress and modulates angiogenesis in Ossabaw swine with the metabolic syndrome. J Thorac Cardiovasc Surg. 2012;144:1486–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ahsan N, Salomon AR. Quantitative phosphoproteomic analysis of T‐cell receptor signaling. Methods Mol Biol. 2017;1584:369–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kebling Yu, Salomon Arthur R. PeptideDepot: flexible relational database for visual analysis of quantitative proteomic data and integration of existing protein information. Proteomics. 2009;9:5350–5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu K, Salomon AR. HTAPP: high‐throughput autonomous proteomic pipeline. Proteomics. 2011;10:2113–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Elias JE, Gygi SP. Target‐decoy search strategy for increased confidence in large‐scale protein identifications by mass spectrometry. Nat Methods. 2007;4:2. [DOI] [PubMed] [Google Scholar]
- 28. Nagib A, Judson B, Zhuo C, James GC, Arthur RS. Highly reproducible improved label‐free quantitative analysis of cellular phosphoproteome by optimization of LC‐MS/MS gradient and analytical column construction. J Proteomics. 2017;165:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu K, Sabelli A, DeKeukelaere L, Park R, Sindi S, Gatsonis CA, Salomon A. Integrated platform for manual and high‐throughput statistical validation of tandem mass spectra. Proteomics. 2009;9:3115–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bolli R, Tang X‐L, Sanganalmath SK, Rimoldi O, Mosna F, Abdel‐Latif A, Jneid H, Rota M, Leri A, Kajstura J. Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation. 2013;128:122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liang X, Zhang L, Wang S, Han Q, Zhao RC. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR‐125a. J Cell Sci. 2016;129:2182–2189. [DOI] [PubMed] [Google Scholar]
- 32. Al‐Mashhadi AL, Poulsen CB, von Wachenfeldt K, Robertson A‐K, Bentzon JF, Nielsen LB, Thygesen J, Tolbod LP, Larsen JR, Moestrup SK, Frendéus B, Mortensen B, Drouet L, Al‐Mashhadi RH, Falk E. Diet‐induced abdominal obesity, metabolic changes, and atherosclerosis in hypercholesterolemic minipigs. J Diabetes Res. 2018;2018:6823193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bjelakovic L, Vukovic V, Jovic M, Bankovic S, Kostic T, Radovanovic D, Pantelic S, Zivkovic M, Stojanovic S, Bjelakovic B. Heart rate recovery time in metabolically healthy and metabolically unhealthy obese children. Phys Sportsmed. 2017;45:438–442. [DOI] [PubMed] [Google Scholar]
- 34. Samiei N, Bayat M, Firouzi A, Dehghani F, Parsaee M, Rahimi S, Ahmadi S, Pourmojib M, Ghaemmaghami Z, Rezaei Y, Peighambari MM. Subclinical systolic and diastolic dysfunctions in patients with metabolic syndrome and angiographically normal coronary arteries: an echocardiographic study. J Clin Ultrasound. 2018;46:195–201. [DOI] [PubMed] [Google Scholar]
- 35. Wong ND, Rozanski A, Gransar H, Miranda‐Peats R, Kang X, Hayes S, Shaw L, Friedman J, Polk D, Berman DS. Metabolic syndrome and diabetes are associated with an increased likelihood of inducible myocardial ischemia among patients with subclinical atherosclerosis. Diabetes Care. 2005;28:1445–1450. [DOI] [PubMed] [Google Scholar]
- 36. Ruel M, Sellke FW. Angiogenic protein therapy. Semin Thorac Cardiovasc Surg. 2003;15:222–235. [DOI] [PubMed] [Google Scholar]
- 37. Feng J, Chu LM, Robich MP, Clements RT, Khabbaz KR, Hagberg R, Liu Y, Osipov RM, Sellke FW. Effects of cardiopulmonary bypass on endothelin‐1‐induced contraction and signaling in human skeletal muscle microcirculation. Circulation. 2010;122:S150–S155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chu LM, Lassaletta AD, Robich MP, Liu Y, Burgess T, Laham RJ, Sweeney JD, Shen T‐L, Sellke FW. Effects of red wine and vodka on collateral‐dependent perfusion and cardiovascular function in hypercholesterolemic swine. Circulation. 2012;126:S65–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cesi G, Walbrecq G, Margue C, Kreis S. Transferring intercellular signals and traits between cancer cells: extracellular vesicles as “homing pigeons.” Cell Commun Signal. 2016;14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zheng D, Wang G, Li S, Fan G‐C, Peng T. Calpain‐1 induces endoplasmic reticulum stress in promoting cardiomyocyte apoptosis following hypoxia/reoxygenation. Biochim Biophys Acta. 2015;1852:882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Potz BA, Sabe AA, Elmadhun NY, Sabe SA, Braun BJV, Clements RT, Usheva A, Sellke FW. Calpain inhibition decreases inflammatory protein expression in vessel walls in a model of chronic myocardial ischemia. Surgery. 2017;161:1394–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of Proteins Identified From Extracellular Vesicle Sample Biological Set 1 by LC‐MS/MS Analysis. Please See Excel File
Table S2. List of Proteins Identified From Extracellular Vesicle Sample Biological Set 2 by LC‐MS/MS Analysis. Please See Excel File
Table S3. List of Overlapping Proteins Identified From Two Biological Sets of Extracellular Vesicle Samples by LC‐MS/MS Analysis. Please See Excel File


