Abstract
Background
Clinical characteristics and outcomes of takotsubo syndrome (TTS) patients with malignancy have not been fully elucidated. This study sought to explore differences in clinical characteristics and to investigate short‐ and long‐term outcomes in TTS patients with or without malignancy.
Methods and Results
TTS patients were enrolled from the International Takotsubo Registry. The TTS cohort was divided into patients with and without malignancy to investigate differences in clinical characteristics and to assess short‐ and long‐term mortality. A subanalysis was performed comparing long‐term mortality between a subset of TTS patients with or without malignancy and acute coronary syndrome (ACS) patients with or without malignancy. Malignancy was observed in 16.6% of 1604 TTS patients. Patients with malignancy were older and more likely to have physical triggers, but less likely to have emotional triggers compared with those without malignancy. Long‐term mortality was higher in patients with malignancy (P<0.001), while short‐term outcome was comparable (P=0.17). In a subanalysis, long‐term mortality was comparable between TTS patients with malignancies and ACS patients with malignancies (P=0.13). Malignancy emerged as an independent predictor of long‐term mortality.
Conclusions
A substantial number of TTS patients show an association with malignancy. History of malignancy might increase the risk for TTS, and therefore, appropriate screening for malignancy should be considered in these patients.
Clinical Trial Registration
URL: http://www.clinicaltrial.gov. Unique identifier: NCT01947621.
Keywords: acute coronary syndrome, broken heart syndrome, cancer, malignancy, outcome, takotsubo syndrome
Subject Categories: Heart Failure
Short abstract
See Editorial Angelini and Uribe
Clinical Perspective
What Is New?
A substantial number of takotsubo syndrome (TTS) patients show an association with malignancy.
The prevalence of malignancies in TTS is higher than in acute coronary syndrome.
TTS patients with malignancies have a different clinical profile including an older age at time of TTS onset and a higher prevalence of physical triggering factors.
What Are the Clinical Implications?
The findings of the present study suggest that specific malignancy‐related factors might be associated with the development of TTS.
Further research is required to investigate the interplay of malignancy and TTS, which might be helpful to elucidate the pathophysiological mechanism of TTS.
Introduction
Takotsubo syndrome (TTS) represents an acute heart failure condition, which can occur in the setting of severe psychological or physical stress.1, 2 While multiple theories have been postulated to explain the pathophysiology of TTS, the exact mechanism of this entity remains uncertain.3, 4, 5 Thus far, several case reports have described the occurrence of TTS in the setting of malignancy and chemotherapy administration.6, 7 However, it remains unclear what role malignancy or chemotherapy play in the development in TTS with regard to physical and emotional stress.8 It has been suggested that treatment of malignancy rather than malignancy itself is associated with the development of TTS.9 In recent publications using data from the International Takotsubo Registry (InterTAK Registry, www.takotsubo-registry.com), we found that outcomes of TTS in the acute setting carry high morbidity and mortality similar to that found in acute coronary syndrome (ACS).10, 11 This poses a challenging clinical scenario where the clinical course could potentially be affected by the triple hit of malignancy, treatment, and development of TTS. Enhancing our understanding of TTS as it pertains to malignancy may aid in improving our understanding of the diagnostic workup for potential triggers of TTS, particularly those associated with a worse outcome. There is only limited data on the impact of malignancy on clinical outcomes of patients with TTS. Small scale studies have reported that malignancy might affect long‐term outcomes of TTS.12, 13 Thus, the aim of the present study was to explore differences in clinical characteristics and to investigate both short‐ and long‐term outcomes in TTS patients with or without malignancy and to compare these outcomes to patients with ACS with or without malignancy.
Methods
Study Design
The InterTAK Registry is a multicenter collaboration comprised of 26 cardiovascular centers from 9 different countries (Austria, Finland, France, Germany, Italy, Poland, Switzerland, the United Kingdom, and United States). Data collected are observational and in part retrospective as well as prospective. The authors declare that all supporting data are available within the article. A detailed description of the rationale, design, and objectives of the InterTAK Registry has recently been published elsewhere.14 Diagnosis of TTS was based on modified Mayo Clinic Diagnostic Criteria.10, 15 Patients with evidence of myocarditis were excluded from the study. Additionally, the Zurich Acute Coronary Syndrome Registry,10 a single center database of ACS patients was used to compare TTS patients from the InterTAK Registry to age‐ and sex‐matched ACS patients. From these study populations patients were selected in whom information on malignancy was available. The study protocol was reviewed by the respective local ethics committees or investigational review boards at each collaboration site. Due to the partly retrospective nature of the study, ethics committees of most study centers waived the need for informed consent. At centers in which the ethics committees or investigational review boards required informed consent or in which patients were included prospectively, formal written consent was obtained from patients or surrogates.
Study Population and Data Collection
Of 1750 TTS patients in the InterTAK Registry, 1604 patients in whom comprehensive data on malignancy were available were included in the present analysis (Figure 1). Patients were categorized into 2 groups based on the presence or absence of malignancy documentation.
Figure 1.
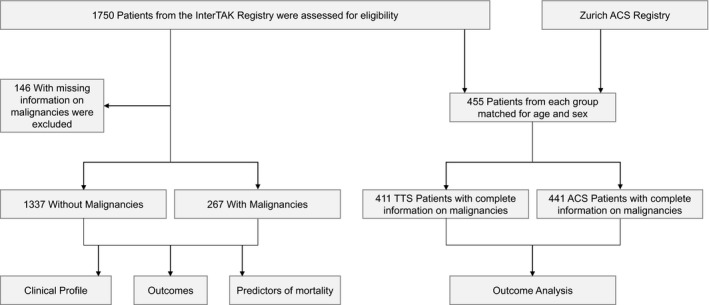
Study flowchart. Flowchart summarizes patients’ selection and respective analyses of the study. ACS indicates acute coronary syndrome; TTS, takotsubo syndrome.
For a subanalysis we included 455 TTS patients and 455 age‐ and sex‐matched ACS patients and selected those with available information on malignancy (n=411 for TTS patients and n=441 for ACS patients, Figure 1). TTS patients for the matching study were included from the University Hospital Zurich, Kantonsspital Lucerne, University Hospital Basel, Mayo Clinic Rochester, and Heidelberg University Hospital, as previously described.10
Characteristics for the comparison of TTS patients with and without malignancy included demographics, precipitating factors, cardiovascular risk factors, comorbidities, medication on admission and at discharge, laboratory values, electrocardiographic, echocardiographic, and cardiac catheterization data. Outcome data for TTS and ACS were obtained from hospital visits, medical charts, or telephone interviews. Analyses were performed within the TTS group as well as in comparison with the ACS group. In the first analysis, the TTS cohort was subdivided into patients with and without malignancy to investigate differences in patients’ characteristics and to assess short‐ and long‐term mortality. A separate analysis was performed comparing a subset of TTS patients with ACS to delineate the prevalence of malignancy and long‐term mortality (Figure 1).
Statistical Analyses
Descriptive statistics are given as counts with percentages for categorical variables and as mean±standard deviation or median with interquartile range (IQR) for continuous variables. Categorical variables were analyzed by using the Pearson Chi‐Square or Fisher exact test, if indicated. Student t test or Mann–Whitney U test were executed for continuous variables. Kaplan–Meier survival analysis was used to assess short‐ and long‐term mortality and the log‐rank test was applied for group comparison.
A multivariable Cox‐regression for long‐term mortality was performed with covariates, which were statistically significant in the univariable analysis. Factors controlled for in the multivariable analysis included: age >70 years, female sex, emotional trigger, physical trigger, chest pain on admission, atrial fibrillation, heart rate >70 bpm, systolic blood pressure >130 mm Hg, maximum troponin >10 times upper limit of the normal (ULN), maximum creatine kinase >10 times ULN, C‐reactive protein maximum, white blood cell count maximum, left ventricular ejection fraction (LVEF) <45%, malignancy, coronary artery disease, neurologic disorders, and psychiatric disorders. Data of covariates were 89.9% complete. Missing data were completed with multiple imputations before multivariable Cox‐regression. To address clustering within countries, we repeated the Cox‐regression with cluster robust standard errors adjusted for 9 countries. A 2‐sided P<0.05 was defined as statistical significance. IBM SPSS Statistics, version 22.0 and 23.0 (Armonk, NY: IBM Corp) and Stata software, version 13.1 (StataCorp) were used for statistical analyses. Figures were compiled with Prism 7.00 (GraphPad, La Jolla, CA).
Results
Study Population
Of the total 1604 TTS patients in the InterTAK Registry, malignancy was observed in 267 (16.6%) (Figure 1 and Figure 2, left column).10 The most frequent type of malignancy was breast cancer 26.2% (n=70), followed by tumors affecting the gastrointestinal system 16.1% (n=43), respiratory tract 15.4% (n=41), internal sex organs 14.6% (n=39), skin 13.1% (n=35), lymphatic system 7.1% (n=19), endocrine organs 6.7% (n=18), urinary tract 5.2% (n=14), and hematologic 3.0% (n=8) as well as the central nervous system 2.2% (n=6). In the subgroups, the overall prevalence of malignancy was 18.2% in the TTS cohort and 11.1% in the ACS cohort (Figure 2, middle and right column).
Figure 2.
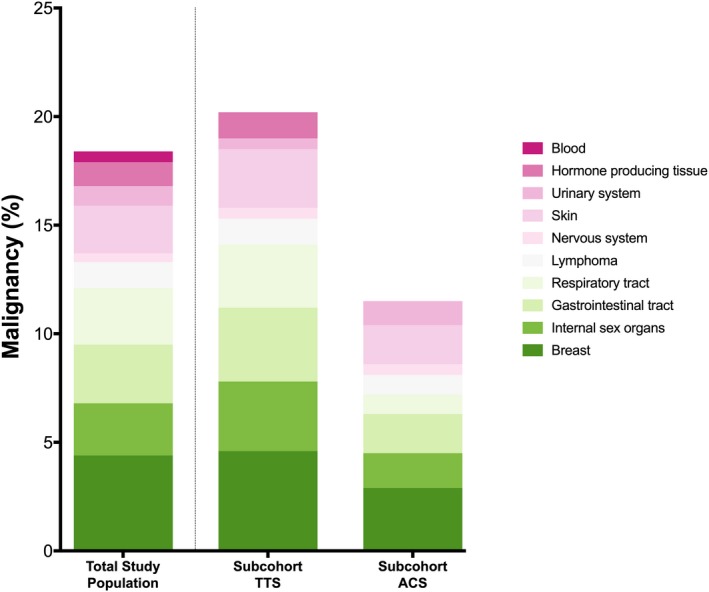
Prevalence of malignancy. Prevalence of malignancy in the total study cohort of TTS patients (left column), subcohort of TTS (middle column) and ACS (right column) shows an increased prevalence of malignancy in TTS when compared with ACS.10 If a patient had a history of >1 malignancy then the patient was categorized into all respective groups of malignancies. ACS indicates acute coronary syndrome; TTS, takotsubo syndrome.
Patients’ Characteristics
On admission, TTS patients with malignancy were older compared with those without malignancy (69.5±11.2 years versus 65.8±13.1 years, P<0.001), while no sex differences were observed (87.6% versus 89.8% women, P=0.29). TTS patients with malignancy on initial presentation experienced chest pain less frequently (67.8% versus 77.5%, P<0.001), but had dyspnea more often (53.5% versus 45.8%, P=0.029) (Table 1). Emotional stressors were less common in patients with malignancy compared with those without (18.0% versus 30.3%, P<0.001). In contrast, physical triggering factors were more often observed in TTS patients with malignancy than in those without malignancy (47.9% versus 34.2%, P<0.001). Of these, a substantial proportion of TTS patients with malignancy developed a TTS episode after a medical intervention or physical trauma (12.7% versus 5.5%, P<0.001 Table 2). No differences were observed with regard to admission systolic and diastolic blood pressure measurements between the 2 groups. Of note, LVEF was lower in TTS patients with malignancy than in TTS patients without malignancy (38.8%±11.8% versus 41.5%±11.9%, P=0.001), with no differences in left ventricular end‐diastolic pressure (21.5±8.1 mm Hg versus 21.5±8.0 mm Hg, P=0.97). Cardiac biomarkers on admission and their peak values did not significantly differ between TTS patients with or without malignancy (Table 1). TTS patients with malignancy had higher levels of C‐reactive protein on admission (6.50 mg/L, IQR 2.00–26.08 versus 3.56 mg/L, IQR 1.30–10.13, P<0.001) as well as peak levels during hospitalization (17.00 mg/L, IQR 4.90–71.60 versus 7.85 mg/L, IQR 2.56–33.00, P<0.001) compared with TTS patients without malignancy. White blood cell count showed no differences between the 2 groups (Table 1). Prevalence of preexisting cardiovascular risk factors was comparable between TTS patients with and without malignancy. TTS patients with malignancy tended to have an acute or chronic neurologic disorder more often (29.5% versus 23.5%, P=0.047).
Table 1.
Characteristics of Patients With and Without Malignancy
| Characteristics | TTS with Malignancy | TTS w/o Malignancy | P Value |
|---|---|---|---|
| n=267 | n=1337 | ||
| Demographics | |||
| Female sex | 234/267 (87.6) | 1201/1337 (89.8) | 0.29 |
| Age, y | 69.5±11.2 (n=267) | 65.8±13.1 (n=1337) | <0.001 |
| Body mass index, kg/m2 | 25.3±5.6 (n=206) | 25.1±5.4 (n=1031) | 0.59 |
| Triggers | |||
| Physical trigger | 128/267 (47.9) | 457/1337 (34.2) | <0.001 |
| Emotional trigger | 48/267 (18.0) | 405/1337 (30.3) | <0.001 |
| Both emotional and physical trigger | 26/267 (9.7) | 99/1337 (7.4) | 0.19 |
| No evident trigger | 65/267 (24.3) | 376/1337 (28.1) | 0.21 |
| TTS type | |||
| Apical type | 222/267 (83.1) | 1079/1337 (80.7) | 0.35 |
| Symptoms on admission | |||
| Chest pain | 166/245 (67.8) | 969/1250 (77.5) | 0.001 |
| Dyspnea | 131/245 (53.5) | 574/1252 (45.8) | 0.029 |
| Cardiac biomarkers | |||
| Troponin on admission—factor increase in ULN a | 7.50 (1.98–28.70) n=221 | 7.50 (2.36–22.00) n=1094 | 0.75 |
| Troponin maximum—factor increase in ULN a | 15.00 (4.34–50.13) n=226 | 12.55 (4.53–37.19) n=1104 | 0.49 |
| Creatine kinase on admission—factor increase in ULN | 0.81 (0.48–1.36) n=175 | 0.88 (0.54–1.50) n=925 | 0.11 |
| Creatine kinase maximum—factor increase in ULN | 1.14 (0.58–1.91) n=186 | 1.10 (0.64–2.17) n=938 | 0.49 |
| BNP on admission—factor increase in ULNb | 8.30 (3.55–18.86) n=66 | 5.52 (2.00–15.19) n=343 | 0.06 |
| BNP maximum—factor increase in ULNb | 12.60 (5.44–24.38) n=87 | 9.39 (3.73–22.91) n=449 | 0.11 |
| Inflammatory markers | |||
| CRP on admission, mg/L | 6.50 (2.00–26.08) n=152 | 3.56 (1.30–10.13) n=898 | <0.001 |
| CRP maximum, mg/L | 17.00 (4.90–71.60) n=167 | 7.85 (2.56–33.00) n=944 | <0.001 |
| WBC on admission, 103/μL | 9.70 (7.19–12.70) n=229 | 9.72 (7.54–12.80) n=1141 | 0.48 |
| WBC maximum, 103/μL | 10.70 (7.52–13.80) n=235 | 10.50 (8.20–13.62) n=1158 | 0.77 |
| ECG on admission | |||
| Atrial fibrillation | 17/244 (7.0) | 82/1215 (6.7) | 0.90 |
| ST‐segment elevation | 100/243 (41.2) | 532/1212 (43.9) | 0.43 |
| T‐wave inversion | 94/243 (38.7) | 510/1212 (42.1) | 0.33 |
| QTc, ms | 456.0±50.5 (n=193) | 457.9±49.5 (n=893) | 0.64 |
| Hemodynamics | |||
| Heart rate, beats/min | 90.8±23.0 (n=222) | 86.8±21.8 (n=1117) | 0.012 |
| Systolic blood pressure, mm Hg | 133.8±30.1 (n=224) | 130.3±28.5 (n=1116) | 0.10 |
| Diastolic blood pressure, mm Hg | 77.9±18.0 (n=223) | 76.8±16.9 (n=1069) | 0.36 |
| Left ventricular ejection fraction, %c | 38.8±11.8 (n=250) | 41.5±11.9 (n=1221) | 0.001 |
| Left ventricular end‐diastolic pressure, mm Hg | 21.5±8.1 (n=160) | 21.5±8.0 (n=801) | 0.97 |
| Cardiovascular risk factors/history | |||
| Hypertension | 176/262 (67.2) | 867/1328 (65.3) | 0.56 |
| Diabetes mellitus | 42/263 (16.0) | 194/1330 (14.6) | 0.56 |
| Current smoking | 44/258 (17.1) | 274/1290 (21.2) | 0.13 |
| Hypercholesterolemia | 77/262 (29.4) | 421/1325 (31.8) | 0.45 |
| Coexisting medical condition | |||
| Coronary artery diseased | 34/237 (14.3) | 193/1232 (15.7) | 0.61 |
| COPD or asthma | 56/260 (21.5) | 203/1332 (15.2) | 0.012 |
| Neurologic disorders (total)e | 74/251 (29.5) | 278/1181 (23.5) | 0.047 |
| Psychiatric disorders (total)e | 89/251 (35.5) | 376/1181 (31.8) | 0.27 |
| Medication on admission | |||
| ACE inhibitor or ARB | 77/215 (35.8) | 422/1088 (38.8) | 0.41 |
| Beta‐blocker | 79/215 (36.7) | 344/1088 (31.6) | 0.14 |
| Calcium‐channel antagonist | 21/209 (10.0) | 72/1080 (6.7) | 0.08 |
| Statin | 37/209 (17.7) | 196/1080 (18.1) | 0.89 |
| Medication at discharge | |||
| ACE inhibitor or ARB | 180/234 (76.9) | 978/1218 (80.3) | 0.24 |
| Beta‐blocker | 188/234 (80.3) | 945/1218 (77.6) | 0.35 |
| Calcium‐channel antagonist | 26/234 (11.1) | 98/1218 (8.0) | 0.12 |
| Statin | 109/234 (46.6) | 644/1218 (52.9) | 0.08 |
| In‐hospital complications | |||
| Cardiogenic shock | 31/266 (11.7) | 125/1328 (9.4) | 0.26 |
| Death | 18/267 (6.7) | 45/1337 (3.4) | 0.010 |
| Acute cardiac care treatment | 71/266 (26.7) | 258/1333 (19.4) | 0.007 |
| Intra‐aortic balloon pump | 8/266 (3.0) | 34/1333 (2.6) | 0.67 |
| Invasive or noninvasive ventilation | 63/266 (23.7) | 209/1333 (15.7) | 0.002 |
| Cardiopulmonary resuscitation | 26/266 (9.8) | 110/1333 (8.3) | 0.42 |
| Catecholamine use | 40/266 (15.0) | 151/1333 (11.3) | 0.09 |
Values are mean±SD, no./total n (%), or median (interquartile range). ACE indicates angiotensin‐converting‐enzyme; ARB, angiotensin‐receptor blocker; BNP, brain natriuretic peptide; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; ECG, electrocardiogram; QTc, QT interval corrected for heart rate; TTS, takotsubo syndrome; ULN, upper limit of the normal; WBC, white blood cell count.
Including upper limits of the normal range for troponin T, high‐sensitivity troponin T, and troponin I.
Including upper limits of the normal range for brain natriuretic peptide and the N‐terminal of prohormone brain natriuretic peptide.
Data obtained during catheterization or echocardiography if both results were available data from catheterization were used.
Coexisting coronary artery disease during acute hospitalization.
Category includes patients with either an acute as well as past or chronic disorder.
Table 2.
Triggering Factors of TTS Patients
| TTS with Malignancy | TTS w/o Malignancy | P Value | |
|---|---|---|---|
| Physical triggering factors | |||
| Acute respiratory failure | 6.5% | 7.6% | 0.47 |
| Central nervous system conditions | 7.5% | 5.1% | 0.12 |
| Malignancy | 3.0% | 0% | <0.001 |
| Infection | 5.2% | 2.5% | 0.014 |
| Post surgery/physical trauma | 12.7% | 5.5% | <0.001 |
| Others | 13.1% | 13.5% | 0.85 |
| Emotional triggering factors | |||
| Anger/frustration | 1.9% | 4.7% | 0.036 |
| Related to financial or employement problems | 0% | 2.5% | 0.008 |
| Grief/loss | 4.1% | 6.9% | 0.09 |
| Interpersonal conflict | 3.0% | 4.8% | 0.20 |
| Panic/fear/anxiety | 4.9% | 6.7% | 0.27 |
| Others | 4.1% | 4.7% | 0.67 |
TTS indicates takotsubo syndrome.
Outcomes
TTS patients with malignancy required acute cardiac care treatment more frequently (26.7% versus 19.4%, P=0.007), predominantly due to greater need for invasive or non‐invasive respiratory support (23.7% versus 15.7%, P=0.002) and had a higher in‐hospital mortality (6.7% versus 3.4%, P=0.010). While 30‐day mortality did not differ between TTS patients with and without malignancy (P=0.17; Figure 3, inset), long‐term survival analyses showed a higher mortality in TTS patients with malignancy (P<0.001; Figure 3). During the 5‐year follow‐up period, 47 patients with malignancies and 109 patients without malignancies deceased. In these 156 patients, the cause of death was known in 151 (96.7%) patients. 20 (46.5%) cardiovascular deaths and 23 (53.5%) non‐cardiovascular deaths were observed in the malignancy group, while there were 77 (71.3%) cardiovascular deaths and 31 (28.7%) non‐cardiovascular deaths in the non‐malignancy group. Interestingly, a subgroup survival analysis of TTS patients compared with ACS patients according to presence or absence of malignancy revealed that TTS patients with malignancy had a comparable long‐term outcome with ACS patients with malignancy (P=0.13, Figure 4) and TTS patients without malignancy also showed a comparable outcome with ACS patients without malignancy (P=0.54, Figure 4).
Figure 3.
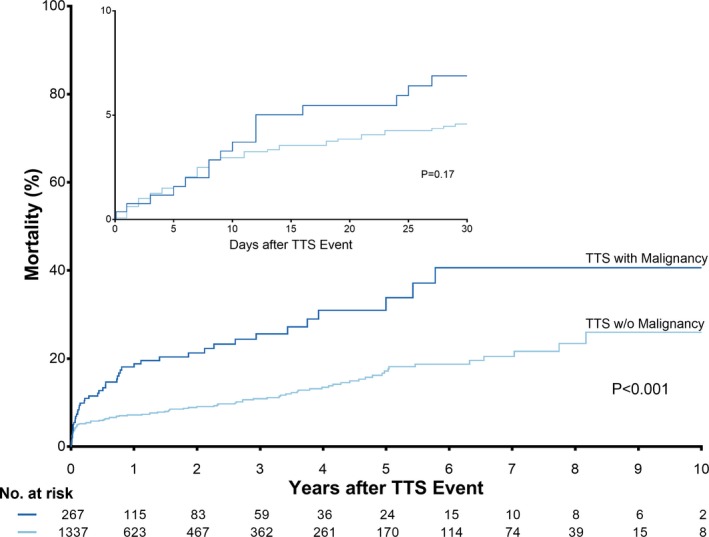
Short‐ and long‐term outcome in takotsubo patients with and without malignancy. Kaplan–Meier survival analysis demonstrated a comparable 30‐day survival of TTS patients with and without malignancy (P=0.17, inset), while long‐term mortality was significantly higher in TTS patients with malignancy than in TTS patients without malignancy (P<0.001). TTS indicates takotsubo syndrome.
Figure 4.
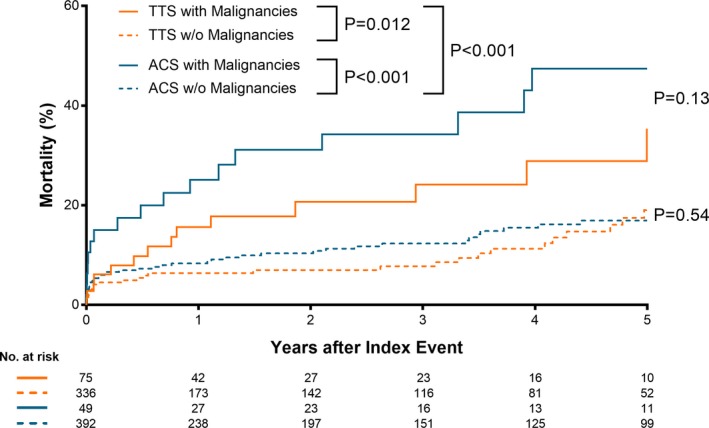
Long‐term outcome in takotsubo syndrome and acute coronary syndrome according to presence or absence of malignancy. Kaplan–Meier survival analysis showed that patients with malignancy had significantly worse outcome than those without malignancy both in patients with TTS and ACS. In addition, TTS patients with malignancy had a comparable long‐term outcome with ACS patients with malignancy (P=0.13) and TTS patients without malignancy also showed a comparable outcome with ACS patients without malignancy (P=0.54). ACS indicates acute coronary syndrome; TTS, takotsubo syndrome.
Results of multivariable analysis in the entire cohort of 1604 TTS patients demonstrated that age >70 years, atrial fibrillation, peak troponin >10x ULN, peak creatinine kinase >10x ULN, LVEF <45%, malignancy, neurologic conditions, and psychiatric disorders were independent predictors of long‐term mortality (Figure 5). Results remained similar when assessing clustering among countries (Figure S1).
Figure 5.
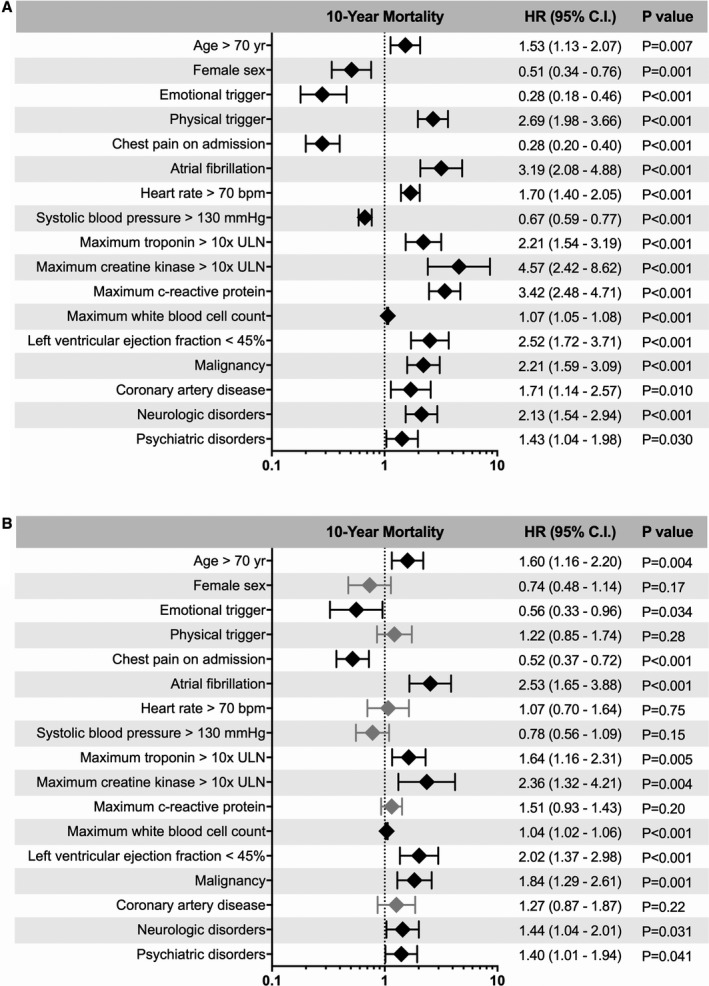
Univariable (A) and multivariable (B) predictors of long‐term mortality in takotsubo syndrome. Results of the multivariable Cox‐regression in the total cohort of TTS patients showed that age >70 years, atrial fibrillation, maximum troponin >10x ULN, maximum creatinine kinase >10x ULN, left ventricular ejection fraction <45%, malignancy, neurologic disorders, and psychiatric disorders are independent predictors of long‐term mortality. Error bars represent 95% CI. Black rhombi indicate statistical significance; grey rhombi not statistically significant. CI indicates confidence interval; HR, hazard ratio; ULN, upper limit of the normal range.
Discussion
Leveraging on the largest database of TTS patients published thus far, we have provided a more detailed analysis of prevalence and outcome of patients with malignancy and TTS. Here we show that TTS and ACS patients with malignancies have comparable long‐term outcomes. Furthermore, we found that in TTS patients with malignancy, the inciting event is an emotional trigger less frequently than in those without malignancy. Even though the psychological burden of a malignancy diagnosis could potentially increase sympathetic output, this was not the case in our analysis as emotional stressors were less common in patients with malignancy compared with those without. It is noteworthy that the prevalence of psychiatric disorders including anxiety and depression was not significantly different in TTS patients with malignancy compared to without malignancy. With regard to inflammatory markers, while the total number of white blood cell counts were not different between the groups, C‐reactive protein levels were significantly higher in the TTS patients with malignancy in those without malignancy, likely a marker of the greater comorbidity burden.
While multiple theories have been postulated to explain the pathophysiology of TTS, the exact mechanisms of this acute transient decrease in left ventricular function remain uncertain.1, 4, 5 The relationship between malignancy and TTS is particularly interesting from an epidemiologic, mechanistic, and outcome standpoint as malignancy and chemotherapy have been associated with TTS.9, 12, 16, 17, 18, 19 Endothelial dysfunction in epicardial and microvascular coronary arteries occurs frequently in patients with cancer,20 especially during and after systemic chemotherapy or radiotherapy of the heart region, which might be a predisposing factor for developing TTS. Therefore, assessment of endothelial function, such as acetylcholine spasm provocation testing, would probably be useful in further defining mechanisms in this patient population. Interestingly, the prevalence of malignancy in our TTS cohort exceeded the reported rates of the general population in Europe21 when stratified according to age and sex (female: 0–44 years [8.3% versus 0.4%], 45–64 years [12.8% versus 3.7%], >65 years [18.9% versus 7.9%]; male: 0–44 years [5.2% versus 0.3%], 45–64 years [21.9% versus 1.91%], >65 years [20.8% versus 7.8%]). Another investigation reported that underlying cardiac comorbidities were independent predictors of long‐term mortality in patients with TTS.22 Also in this cohort, malignancy was more common in those patients who did not survive long‐term. This is of particular interest since data from our registry further reveal a strong association between TTS and malignancy. Moreover, in a recent publication using data from the InterTAK Registry, we demonstrated that outcomes of TTS in the acute setting carry a high morbidity and mortality similar to ACS and that the prevalence of malignancy in TTS exceeds that of ACS.10, 23 This poses a challenging clinical scenario in the management of patients with malignancy. Not only does TTS carry a high rate of mortality similar to that of ACS,10 its concomitant diagnoses in malignancy patients may pose worse outcomes. As there are no standardized guidelines regarding screening or risk stratification for TTS in patients with malignancy, understanding potential underlying causes and the clinical importance of the association of TTS and malignancy may help improve clinical outcomes. As such, it is reasonable to consider malignancy as a potential triggering factor of TTS. Additionally, in certain patients TTS may be considered a paraneoplastic syndrome, a harbinger for the presence of underlying malignancy. Alternatively, metabolic and/or neurohumoral changes associated with malignancy may increase the likelihood of a TTS episode. Even if the relationship between developing TTS and malignancy cannot be deemed causative, screening for malignancy in those with acute episodes of TTS can perhaps prompt further therapy and improve overall outcomes.24
The numerous effects of malignancy and therapies on the heart are evidenced by the rapid evolution of the field of cardio‐oncology. As such the malignancy patient subset of TTS provides a unique milieu to investigate possible etiologic factors of the interaction between the 2 disease entities and their treatment. Furthermore, studies focusing on the time‐relationship of cancer therapies, such as chemotherapy, immunotherapy, and radiation treatment, and onset of TTS is essential to elucidate the relationship between TTS and cancer.
Limitations
To study the relationship between TTS and cancer is challenging as questions remain as to whether the worse prognosis is related to the presence and specific type, stage, and treatment for malignancy, the presence of TTS, or a combination of these factors. In addition, the different type and stage of cancer might affect the outcome, however, the number of patients with certain type or stage of cancer is too small for a meaningful comparison analysis in our cohort.
Conclusions
The findings of the present study have unraveled a high prevalence of malignancy in TTS patients. Additionally, our analyses illustrate the clinical course and unique characteristics in TTS patients with or without malignancy as compared to a cohort of ACS patients. Our findings suggest that specific malignancy‐associated factors impact the development and outcome of TTS. Therefore, to fully understand the pathophysiology of TTS and the role of malignancy in triggering or affecting outcomes, this subset of patients deserves further investigation. These factors may include analysis of specific malignancy types and metabolic and neurohumoral changes associated with it and/or the effects of distinct therapeutic molecules administered in such patients. Therefore, developing specific quantifiable metrics relevant to this unique patient population to improve outcomes is warranted.
Sources of Funding
V.L.C. has received a research grant “Kaltenbach scholarship” from the German Heart Foundation. C.T. has been supported by the H.H. Sheikh Khalifa bin Hamad Al‐Thani Research Programme and the Swiss Heart Foundation. The InterTAK Registry is supported by the Biss Davies Charitable Trust.
Disclosures
None.
Authors’ Affiliations
From the University Heart Center, Department of Cardiology, University Hospital Zurich, Zurich, Switzerland (V.L.C., K.J.D., K.K., D.D.V., K.A.S., S.G., S.J., B.B., J.M., A.H.F., F.R., J.R.G., C.T.); University of Southern California, Keck School of Medicine, Los Angeles, CA (A. Sarcon, J.S.); Division of Biostatistics, Epidemiology, Biostatistics and Prevention Institute (B.S.), University of Zurich, Switzerland; Centro Cardiologico Monzino, IRCCS, Milan, Italy (S.G.); Deutsches Herzzentrum München, Technische Universität München, Munich, Germany (A.H.F., W.K., H.S.); Department of Cardiology and Angiology, Hannover Medical School, Hannover, Germany (L.C.N., J.B.); First Department of Cardiology, Medical University of Gdansk, Poland (M.J.); Heart Department, University Hospital “San Giovanni di Dio e Ruggi d'Aragona”, Salerno, Italy (E.B., R.C.); Division of Cardiology, Department of Medical Sciences, AOU Città della Salute e della Scienza, University of Turin, Italy (F.D.); Department of Cardiology, Heidelberg University Hospital, Heidelberg, Germany (J.F., H.A.K.); Division of Cardiology, Angiology and Intensive Medical Care, Department of Internal Medicine III, University Hospital Halle, Martin‐Luther‐University Halle, Halle (Saale), Germany (M.N.); Cardiology 1, Center for Cardiology, University Medical Center Mainz, Mainz, Germany (M. Knorr, S.H., T.M.); Heart and Vascular Centre Bad Bevensen, Bad Bevensen, Germany (C.B.); DZHK (German Centre for Cardiovascular Research), partner site Munich Heart Alliance, Munich, Germany (W.K., H.S.); Department of Internal Medicine/Cardiology, Heart Center Leipzig – University Hospital, Leipzig, Germany (H.T.); Department of Cardiology, Charité, Campus Rudolf Virchow, Berlin, Germany (C. Tschöpe, B.M.P.); TJ Health Partners Heart and Vascular, Glasgow, Kentucky (L.R.); Department of Internal Medicine III, Heart Center University of Cologne, Germany (G.M., R.P.); Krankenhaus “Maria Hilf” Medizinische Klinik, Stadtlohn, Germany (A.C.); Clinic for Cardiology and Pneumology, Georg August University Goettingen, Goettingen, Germany (C.J., G.H.); Department of General and Interventional Cardiology, University Heart Center Hamburg, Hamburg, Germany (M. Karakas); DZHK (German Centre for Cardiovascular Research), partner site Hamburg/Kiel/Luebeck, Hamburg, Germany (M. Karakas); Department of Cardiology, John Radcliffe Hospital, Oxford University Hospitals, Oxford, United Kingdom (A.B.); Department of Cardiology, Kantonsspital Lucerne, Lucerne, Switzerland (F. Cuculi, R.K.); Department of Cardiology, Kantonsspital Winterthur, Winterthur, Switzerland (T.A.F.); Heart Center, Turku University Hospital and University of Turku, Finland (T.V., J.A.); Department of Cardiology, Kings College Hospital, Kings Health Partners, London, United Kingdom (R.D., P.M.); Department of Cardiology, University Hospital Basel, Basel, Switzerland (C.K., S.O.); Fondazione Policlinico Universitario A. Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome, Italy (L.G., F. Crea); University Hospital for Internal Medicine III (Cardiology and Angiology), Medical University Innsbruck, Innsbruck, Austria (W.D.); Department of Cardiology and Cardiac Imaging Center, University Hospital of Rangueil, Toulouse, France (C.D., O.L.); Department of Cardiology, Basil Hetzel Institute, Queen Elizabeth Hospital, University of Adelaide, Australia (J.D.H.); Charles University in Prague and University Hospital Kralovske Vinohrady, Prague, Czech Republic (M. Kozel, P.W., P.T.); Department of Medicine, College of Medicine, University of Florida, Gainesville, FL (D.E.W.); Intensive coronary care Unit, Moscow City Hospital # 1 named after N. Pirogov, Moscow, Russia (E.G., A. Shilova, M.G.); First Department of Medicine, Faculty of Medicine, University Medical Centre Mannheim (UMM) University of Heidelberg, Mannheim, Germany (I.E.‐B., I.A., M. Borggrefe); DZHK (German Center for Cardiovascular Research), partner site, Heidelberg‐Mannheim, Mannheim, Germany (I.E.‐B., I.A., M. Borggrefe); Klinik für Innere Medizin III, Universitätsklinikum des Saarlandes, Homburg/Saar, Germany (C.U., M. Böhm); Department of Internal Medicine II – Cardiology, University of Ulm, Medical Center, Ulm, Germany (W.R.); Internal Medicine/Cardiology, Angiology, and Pneumology, Magdeburg University, Magdeburg, Germany (R.C.B.‐D.); Department of Cardiology, Medical University of Warsaw, Poland (G.O.); Department of Internal Medicine B, University Medicine Greifswald, Greifswald, Germany (S.B.F.); DZHK (German Centre for Cardiovascular Research), partner site Greifswald, Greifswald, Germany (S.B.F.); Structural Interventional Cardiology, Careggi University Hospital, Florence, Italy (C.D.M.); Department of Cardiology, Leiden University Medical Centre, Leiden, The Netherlands (J.J.B.); Division of Cardiovascular Diseases, Mayo Clinic, Rochester, MN (A.P.); Cardiology, Royal Brompton & Harefield Hospital and Imperial College, London, United Kingdom (T.F.L.); Center for Molecular Cardiology, Schlieren Campus, University of Zurich, Zurich, Switzerland (T.F.L.).
Supporting information
Figure S1. Cox‐regression for long‐term mortality with cluster robust standard errors adjusted for 9 countries.
(J Am Heart Assoc. 2019;8:e010881 DOI: 10.1161/JAHA.118.010881.)
The authors' affiliations are listed at the end of the article.
References
- 1. Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, Cammann VL, Crea F, Galiuto L, Desmet W, Yoshida T, Manfredini R, Eitel I, Kosuge M, Nef HM, Deshmukh A, Lerman A, Bossone E, Citro R, Ueyama T, Corrado D, Kurisu S, Ruschitzka F, Winchester D, Lyon AR, Omerovic E, Bax JJ, Meimoun P, Tarantini G, Rihal C, Y‐Hassan S, Migliore F, Horowitz JD, Shimokawa H, Luscher TF, Templin C. International expert consensus document on takotsubo syndrome (Part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. 2018;39:2032–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Akashi YJ, Goldstein DS, Barbaro G, Ueyama T. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation. 2008;118:2754–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kato K, Lyon AR, Ghadri JR, Templin C. Takotsubo syndrome: aetiology, presentation and treatment. Heart. 2017;103:1461–1469. [DOI] [PubMed] [Google Scholar]
- 4. Ghadri JR, Ruschitzka F, Luscher TF, Templin C. Takotsubo cardiomyopathy: still much more to learn. Heart. 2014;100:1804–1812. [DOI] [PubMed] [Google Scholar]
- 5. Templin C, Napp LC, Ghadri JR. Takotsubo syndrome: underdiagnosed, underestimated, but understood? J Am Coll Cardiol. 2016;67:1937–1940. [DOI] [PubMed] [Google Scholar]
- 6. De Pasquale MD, Mastronuzzi A, De Sio L, Serra A, Grimaldi C, Chinali M, Giordano U. Transient global ventricular dysfunction in an adolescent affected by pancreatic adenocarcinoma. BMC Pediatr. 2016;16:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith SA, Auseon AJ. Chemotherapy‐induced takotsubo cardiomyopathy. Heart Fail Clin. 2013;9:233–242, x. [DOI] [PubMed] [Google Scholar]
- 8. Malley T, Watson E. A case of takotsubo cardiomyopathy after chemotherapy. Oxf Med Case Reports. 2016;2016:55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Munoz E, Iliescu G, Vejpongsa P, Charitakis K, Karimzad K, Lopez‐Mattei J, Yusuf SW, Marmagkiolis K, Iliescu C. Takotsubo stress cardiomyopathy: “Good news” in cancer patients? J Am Coll Cardiol. 2016;68:1143–1144. [DOI] [PubMed] [Google Scholar]
- 10. Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, Seifert B, Hellermann J, Schwyzer M, Eisenhardt K, Jenewein J, Franke J, Katus HA, Burgdorf C, Schunkert H, Moeller C, Thiele H, Bauersachs J, Tschope C, Schultheiss HP, Laney CA, Rajan L, Michels G, Pfister R, Ukena C, Bohm M, Erbel R, Cuneo A, Kuck KH, Jacobshagen C, Hasenfuss G, Karakas M, Koenig W, Rottbauer W, Said SM, Braun‐Dullaeus RC, Cuculi F, Banning A, Fischer TA, Vasankari T, Airaksinen KE, Fijalkowski M, Rynkiewicz A, Pawlak M, Opolski G, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Crea F, Dichtl W, Franz WM, Empen K, Felix SB, Delmas C, Lairez O, Erne P, Bax JJ, Ford I, Ruschitzka F, Prasad A, Luscher TF. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373:929–938. [DOI] [PubMed] [Google Scholar]
- 11. Ghadri JR, Kato K, Cammann VL, Gili S, Jurisic S, Di Vece D, Candreva A, Ding KJ, Micek J, Szawan KA, Bacchi B, Bianchi R, Levinson RA, Wischnewsky M, Seifert B, Schlossbauer SA, Citro R, Bossone E, Munzel T, Knorr M, Heiner S, D'Ascenzo F, Franke J, Sarcon A, Napp LC, Jaguszewski M, Noutsias M, Katus HA, Burgdorf C, Schunkert H, Thiele H, Bauersachs J, Tschope C, Pieske BM, Rajan L, Michels G, Pfister R, Cuneo A, Jacobshagen C, Hasenfuss G, Karakas M, Koenig W, Rottbauer W, Said SM, Braun‐Dullaeus RC, Banning A, Cuculi F, Kobza R, Fischer TA, Vasankari T, Airaksinen KEJ, Opolski G, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Crea F, Dichtl W, Empen K, Felix SB, Delmas C, Lairez O, El‐Battrawy I, Akin I, Borggrefe M, Horowitz J, Kozel M, Tousek P, Widimsky P, Gilyarova E, Shilova A, Gilyarov M, Winchester DE, Ukena C, Bax JJ, Prasad A, Bohm M, Luscher TF, Ruschitzka F, Templin C. Long‐term prognosis of patients with takotsubo syndrome. J Am Coll Cardiol. 2018;72:874–882. [DOI] [PubMed] [Google Scholar]
- 12. Burgdorf C, Kurowski V, Bonnemeier H, Schunkert H, Radke PW. Long‐term prognosis of the transient left ventricular dysfunction syndrome (tako‐tsubo cardiomyopathy): focus on malignancies. Eur J Heart Fail. 2008;10:1015–1019. [DOI] [PubMed] [Google Scholar]
- 13. Sattler K, El‐Battrawy I, Lang S, Zhou X, Schramm K, Tulumen E, Kronbach F, Roger S, Behnes M, Kuschyk J, Borggrefe M, Akin I. Prevalence of cancer in takotsubo cardiomyopathy: short and long‐term outcome. Int J Cardiol. 2017;238:159–165. [DOI] [PubMed] [Google Scholar]
- 14. Ghadri JR, Cammann VL, Templin C. The International Takotsubo Registry: rationale, design, objectives, and first results. Heart Fail Clin. 2016;12:597–603. [DOI] [PubMed] [Google Scholar]
- 15. Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (tako‐tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155:408–417. [DOI] [PubMed] [Google Scholar]
- 16. Girardey M, Jesel L, Campia U, Messas N, Hess S, Imperiale A, Blondet C, Trinh A, Ohlmann P, Morel O. Impact of malignancies in the early and late time course of takotsubo cardiomyopathy. Circ J. 2016;80:2192–2198. [DOI] [PubMed] [Google Scholar]
- 17. Burgdorf C, Kurowski V, Radke PW. Long‐term prognosis of transient left ventricular ballooning syndrome and cancer. Heart Lung. 2011;40:472. [DOI] [PubMed] [Google Scholar]
- 18. Song BG. Comment on “long‐term prognosis of transient left ventricular ballooning syndrome and cancer”. Heart Lung. 2011;40:270. [DOI] [PubMed] [Google Scholar]
- 19. Burgdorf C, Nef HM, Haghi D, Kurowski V, Radke PW. Tako‐tsubo (stress‐induced) cardiomyopathy and cancer. Ann Intern Med. 2010;152:830–831. [DOI] [PubMed] [Google Scholar]
- 20. Han X, Zhou Y, Liu W. Precision cardio‐oncology: understanding the cardiotoxicity of cancer therapy. NPJ Precis Oncol. 2017;1:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lutz JM, Francisci S, Mugno E, Usel M, Pompe‐Kirn V, Coebergh JW, Bieslka‐Lasota M; Group EW . Cancer prevalence in central Europe: the EUROPREVAL study. Ann Oncol. 2003;14:313–322. [DOI] [PubMed] [Google Scholar]
- 22. Song BG, Hahn JY, Cho SJ, Park YH, Choi SM, Park JH, Choi SH, Choi JH, Park SW, Lee SH, Gwon HC. Clinical characteristics, ballooning pattern, and long‐term prognosis of transient left ventricular ballooning syndrome. Heart Lung. 2010;39:188–195. [DOI] [PubMed] [Google Scholar]
- 23. Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, Cammann VL, Crea F, Galiuto L, Desmet W, Yoshida T, Manfredini R, Eitel I, Kosuge M, Nef HM, Deshmukh A, Lerman A, Bossone E, Citro R, Ueyama T, Corrado D, Kurisu S, Ruschitzka F, Winchester D, Lyon AR, Omerovic E, Bax JJ, Meimoun P, Tarantini G, Rihal C, Y‐Hassan S, Migliore F, Horowitz JD, Shimokawa H, Luscher TF, Templin C. International expert consensus document on takotsubo syndrome (Part II): diagnostic workup, outcome, and management. Eur Heart J. 2018;39:2047–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morel O, Messas N, Imperiale A, Blondet C, Jesel L. Cancer and takotsubo syndrome: a need to explore a very complex association‐ reply. Circ J. 2016;81:124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Cox‐regression for long‐term mortality with cluster robust standard errors adjusted for 9 countries.


