Abstract
Background
Neprilysin is a metalloprotease involved in proteolysis of numerous peptides, including natriuretic peptides, and is of prognostic and therapeutic importance in heart failure with reduced ejection fraction. No studies have investigated circulating neprilysin in the community, its clinical correlates, or its relationship to cardiovascular disease in the general population.
Methods and Results
Plasma neprilysin was measured in 1536 participants from Olmsted County, Minnesota, using a commercially available sandwich ELISA assay. Clinical and echocardiographic correlates and subsequent outcomes were determined. Soluble neprilysin is non‐normally distributed in the community (median: 3.9 ng/mL; interquartile range: 1.0–43.0 ng/mL). There was no relationship between plasma neprilysin and age (Spearman correlation: −0.04, P=0.16); body mass index (Spearman correlation: −0.04, P=0.16); glomerular filtration rate (Spearman correlation: −0.007, P=0.8); or A‐, B‐, or C‐type natriuretic peptides (Spearman correlation: 0.03, P=0.22; −0.001, P=0.96; 0.01, P=0.67, respectively). Among tertiles of neprilysin, the lowest tertile group had the highest prevalence of smokers (P<0.001), hypertension (P=0.04), dyslipidemia (P=0.03), and diastolic dysfunction (P=0.02). Soluble neprilysin was not prospectively associated with death or heart failure over a median of 10.7 years.
Conclusions
In a large community‐based cohort, for the first time, we described the distribution of circulating neprilysin in the general community. We observed that neprilysin does not correlate with natriuretic peptide levels and is not independently associated with adverse outcomes. The novel associations observed between low soluble neprilysin levels and an adverse cardiometabolic and smoking profile requires further investigation.
Keywords: biomarker, diastolic dysfunction, neprilysin, smoking
Subject Categories: Biomarkers
Clinical Perspective
What Is New?
In a large community‐based cohort, for the first time, we described the distribution of circulating neprilysin in the general community.
We observed that neprilysin does not correlate with natriuretic peptide levels.
Low soluble neprilysin was paradoxically associated with an adverse cardiometabolic and smoking profile.
What Are the Clinical Implications
Contrary to the previously proposed inverse relationship between neprilysin and natriuretic peptides in humans, we observed no such interaction in a large community‐based cohort.
Our findings emphasize the complexity of the human neprilysin–natriuretic peptide system and suggest that low soluble neprilysin levels may be related to diastolic dysfunction and an adverse cardiometabolic profile.
Introduction
Since the publication of the landmark PARADIGM‐HF (Prospective Comparison of ARNI [Angiotensin Receptor‐Neprilysin Inhibitor] with ACEI [Angiotensin‐Converting‐Enzyme Inhibitor] to Determine Impact on Global Mortality and Morbidity in Heart Failure) trial demonstrating a reduction in mortality and heart failure (HF) hospitalization with neprilysin inhibition in HF with reduced ejection fraction (HFrEF),1 there has been renewed interest in the neprilysin pathway in HF. Neprilysin is a membrane‐bound and soluble metalloprotease that most notably breaks down brain natriuretic peptide (BNP), but it is rather nonspecific in substrate selectivity and breaks down both beneficial substances (A‐type natriuretic peptide [ANP], C‐type natriuretic peptide [CNP], adrenomedullin, bradykinin) and potentially deleterious substances (endothelin, angiotensin I, angiotensin II, substance P, antidiuretic hormone, oxytocin, IL1B [interleukin 1β], chemotactic peptide).2
Most research on neprilysin to date has focused on HFrEF,3 with very few data available on HF with preserved ejection fraction (HFpEF) and only 1 small study of 144 patients that showed no prognostic value for neprilysin as a biomarker in HFpEF.4 Importantly, no data are available on plasma neprilysin levels in community members who may be at risk for HFpEF. Consequently, we sought to better understand the clinical correlates of soluble circulating neprilysin as a biomarker and its relationship to diastolic dysfunction (DD) and incident HF in a cohort compiled from an age‐ and sex‐stratified random sample of the adult population of Olmsted County, Minnesota.5 We hypothesized (1) that interindividual variability in circulating neprilysin exists related to clinical factors and (2) that higher neprilysin levels would correlate with lower natriuretic peptide levels, worse DD, and subsequent clinical incident HFpEF.
Methods
Study Population
The design of this community‐based cohort study has been described previously.5, 6, 7, 8 From 1997 to 2000, a sex‐ and age‐stratified random sample (n=2042) of Olmsted County residents aged ≥45 years underwent physical examination, echocardiogram, and phlebotomy for biorepository samples. The study was approved by the institutional review board, and all participants gave informed consent. The data, analytic methods, and study materials will not be made available to other researchers for the purpose of reproducing the results. Trained nurse chart abstractors recorded risk factors or prevalent cardiovascular disease. The current analysis included the 1536 (75%) participants with remaining biorepository samples. Using the resources of the Rochester Epidemiology Project,9 electronic linkage for HF incidence (outpatient or hospitalized diagnosis) and all‐cause mortality were performed separately. Participants not known to have the outcome of interest were censored at the last known clinical follow‐up at the time of each linkage.
Soluble Neprilysin Assay
Plasma neprilysin was measured in previously unthawed EDTA plasma samples using a commercially available sandwich ELISA assay for soluble neprilysin (details in Data S1 and Table S1). The intra‐ and interassay variability was 7.5% and 9.5%, respectively. All measurements were taken in duplicate. A freeze–thaw analysis was performed on other control plasma samples and found that neprilysin levels were stable for up to 2 freeze–thaw cycles.
Natriuretic Peptide Assays
Plasma ANP and BNP were determined by immunoradiometric assay using antibody to human ANP and BNP, as described previously.8, 10, 11 Plasma NT‐proANP (N‐terminal pro‐ANP) levels were determined by radioimmunoassay, and plasma NT‐proBNP levels were measured using the Elecsys NT‐proBNP electrochemiluminescence immunoassay (Roche Diagnostics). Plasma CNP was measured from with a competitive radioimmunoassay using an antibody that detects human CNP‐22.12
Assessment of Ventricular Structure and Function
The echocardiographic methods have been described in detail5, 6 (details in Data S1). As a sensitivity analysis, DD and its relationship to neprilysin were assessed after excluding the 35 (2%) community participants with low ejection fraction (<45%).
Statistical Analysis
Continuous variables are reported as mean (standard deviation) or median (interquartile range [IQR]) depending on the data distribution as assessed by visual inspection of box plots. Categorical data are reported as number (percentage). For analyses utilizing neprilysin as a continuous variable, natural logarithm transformation was applied to approximate normality before analyses. Regression methods, linear or logistic, were used to test for trends in characteristics across neprilysin tertiles while controlling for age, sex, body mass index, and smoking history. Associations of neprilysin with outcomes were assessed using Cox proportional hazards regression with participants’ event data censored at last known clinical follow‐up. In these analyses, neprilysin was analyzed as both a trend across the tertiles and with the lowest tertile as the referent group, and results are presented as hazard ratios and associated confidence intervals after adjustment for age, sex, body mass index (BMI; kg/m2), and smoking history. The proportional hazards assumption was evaluated both visually, by plotting residuals versus time, and formally, by testing for a correlation between residual and time. No violations of the proportional hazards assumption were noted. In supplemental analyses, correlation among neprilysin levels and characteristics were repeated within the group of clinically normal nonsmoking individuals. Also in supplemental analyses, in an attempt to look at the phenotype of participants with extreme neprilysin levels, we analyzed participants below the median versus >100 times above the median and those with levels below the 5th percentile and above the 95th percentile. Similar analyses were conducted to compare participants across these extreme subgroups. To assess effect modification by smoking status, the association of clinical characteristics and neprilysin levels was evaluated within smoking subgroups. An interaction between smoking status and neprilysin was also evaluated as it related to clinical characteristics, and no strong interactions were noted (data not shown). All tests were 2‐sided, and P<0.05 was considered significant. All analyses were performed using SAS v9.4 (SAS Institute).
Results
Study Population and Baseline Characteristics
The overall cohort (n=1536) represented a middle aged to elderly cohort with a median age of 62 years (IQR: 54–70 years), and 97% were white (Table 1; Table S2). The population was generally overweight with a median BMI of 27.5 (IQR: 24.8–31.2). A third of patients (n=439, 29%) had hypertension, and 7% (n=114) had diabetes mellitus. Half the participants (n=774, 51%) were either active or prior smokers. Renal function was normal on average with a median creatinine level of 0.8 mg/dL (IQR: 0.7–1.0 mg/dL). In addition, 98% of the population had preserved ejection fraction, and 29% (n=402) of the overall population had DD with 8% (n=105) having moderate or severe DD. These values did not change appreciably after excluding the 35 participants with reduced ejection fraction (Table S2).
Table 1.
Baseline Characteristics of Study Population
| n | Result | |
|---|---|---|
| Age, y | 1536 | 61.6 (53.6–70.4) |
| Female, n (%) | 1536 | 803 (52) |
| White race, n (%) | 1536 | 1497 (97) |
| BMI, kg/m2 | 1535 | 27.5 (24.8–31.2) |
| BNP, pg/mL | 1535 | 15.0 (5.8–32.0) |
| NT‐proBNP, pg/mL | 1494 | 69.9 (28.6–142.3) |
| ANP | 1473 | 11.8 (7.2–16.4) |
| NT‐proANP | 1448 | 2236 (1428–3385) |
| CNP | 1413 | 13.0 (10.0–16.4) |
| Aldosterone | 1283 | 4.7 (2.5–8.0) |
| GFR, mL/min per 1.73 m2 | 1492 | 82.4 (71.6–89.2) |
| Adiponectin | 1529 | 9.7 (6.5–14.4) |
| Neprilysin, ng/mL | 1536 | 3.9 (1.0–43.0) |
Data are shown as median (interquartile range) except as noted. ANP indicates A‐type natriuretic peptide; BMI, body mass index; BNP, brain (B‐type) natriuretic peptide; CNP, C‐type natriuretic peptide; GFR, glomerular filtration rate (Modification of Diet in Renal Disease); NT‐pro, N‐terminal pro.
Soluble Neprilysin Levels in the Community
The distribution of soluble neprilysin levels was skewed with a median of 3.9 ng/mL (IQR: 1.0–43.0 ng/mL). There were no significant associations between soluble neprilysin levels and age by correlation (Figure 1) or for tertiles of soluble neprilysin with age, sex, or renal function (Tables 2 and 3). Similarly, there were no significant relationships between BMI, adiponectin, or aldosterone and soluble neprilysin.
Figure 1.
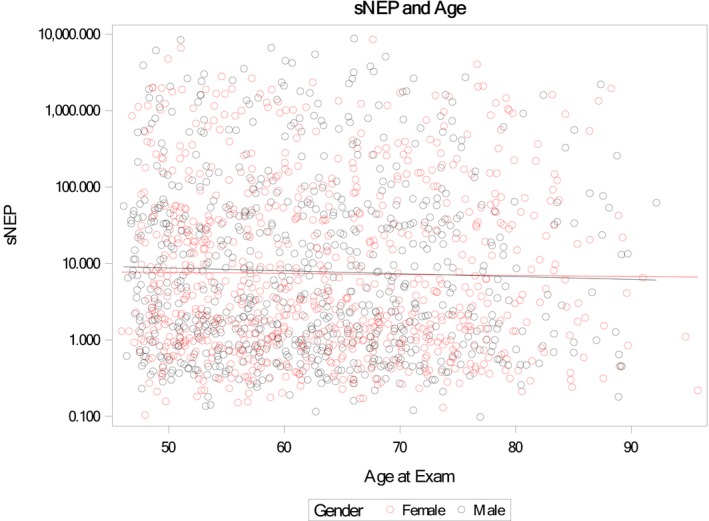
Scatter plot of age (x‐axis) vs soluble neprilysin (sNEP; y‐axis log scale) by sex. The Spearman correlation coefficients between age and neprilysin were −0.04 overall, −0.04 for male participants, and −0.03 for female participants.
Table 2.
Lack of Correlation of Neprilysin Levels With Age, BMI, Renal Function, Natriuretic Peptides, and Neurohormonal Activation
| Serum Neprilysin (Spearman correlation) | P Value | |
|---|---|---|
| Age, per year (n=1536) | −0.04 | 0.16 |
| BMI, per kg/m2 (n=1535) | −0.04 | 0.16 |
| SBP (n=1532) | −0.02 | 0.39 |
| DBP (n=1531) | −0.04 | 0.16 |
| BNP, pg/mL (n=1535) | −0.001 | 0.96 |
| NT‐proBNP (n=1535) | −0.02 | 0.47 |
| ANP (n=1473) | −0.03 | 0.22 |
| NT‐proANP (n=1448) | −0.02 | 0.42 |
| CNP (n=1413) | 0.01 | 0.67 |
| Aldosterone (n=1283) | 0.004 | 0.89 |
| GFR, mL/min/1.73 m2 (n=1492) | −0.007 | 0.80 |
| Adiponectin (n=1529) | −0.03 | 0.24 |
| Ejection fraction (n=1536) | 0.003 | 0.92 |
| E/A ratio (n=1489) | 0.03 | 0.25 |
| E/e′ (n=1293) | 0.01 | 0.62 |
| Left atrial size (n=1492) | −0.01 | 0.75 |
| Estimated PASP (n=1083) | −0.05 | 0.11 |
ANP indicates A‐type natriuretic peptide; BMI, body mass index; BNP, brain (B‐type) natriuretic peptide; CNP, C‐type natriuretic peptide; DBP, diastolic blood pressure; GFR, glomerular filtration rate; NT‐pro, N‐terminal pro; PASP, pulmonary artery systolic pressure; SBP, systolic blood pressure.
Table 3.
Clinical Characteristics and Biomarkers by Neprilysin Tertiles
| Variable | sNEP ≤1.4 (n=516) | sNEP 1.5–22.6 (n=514) | sNEP >22.6 (n=506) | Unadjusted Trend P Value | BMI‐Adjusted Trend P Value | Adjusted Trend P Valuea |
|---|---|---|---|---|---|---|
| Clinical characteristics | ||||||
| Age at exam, y | 63.52±10.35 | 62.16 ±10.41 | 62.79±10.83 | 0.27 | 0.25 | 0.16 |
| Female | 270 (52) | 275 (54) | 258 (51) | 0.67 | 0.59 | 0.11 |
| BMI, kg/m2 | 28.30±5.30 | 28.76±5.54 | 27.99±5.05 | 0.37 | … | 0.30 |
| BMI >30, kg/m2 | 166 (32) | 186 (36) | 139 (27) | 0.11 | … | 0.08 |
| Increased WCb | 165 (32) | 183 (36) | 158 (31) | 0.79 | … | 0.91 |
| Smoking status | <0.001 | <0.001 | <0.001 | |||
| Never | 217 (42) | 254 (50) | 286 (57) | |||
| Former | 234 (45) | 214 (42) | 192 (38) | |||
| Current | 64 (12) | 43 (8) | 27 (5) | |||
| Current/former smoker | 298 (58) | 257 (50) | 219 (43) | <0.001 | <0.001 | <0.001 |
| Diabetes mellitus | 41 (8) | 37 (7) | 36 (7) | 0.61 | 0.73 | 0.86 |
| Hypertension | 164 (32) | 144 (28) | 131 (26) | 0.04 | 0.05 | 0.09 |
| AF/flutter | 31 (6) | 25 (5) | 27 (5) | 0.63 | 0.62 | 0.82 |
| CAD | 60 (12) | 64 (12) | 58 (11) | 0.94 | 0.96 | 0.48 |
| Prior MI | 25 (5) | 23 (4) | 26 (5) | 0.83 | 0.80 | 0.50 |
| Prior stroke | 13 (3) | 4 (1) | 8 (2) | 0.24 | 0.24 | 0.34 |
| Metabolic syndrome | 103 (20) | 127 (25) | 97 (19) | 0.77 | 0.91 | 0.71 |
| SDP, mm Hg | 134.17±21.97 | 131.79±21.00 | 132.53±21.40 | 0.22 | 0.28 | 0.49 |
| DBP, mm Hg | 74.10±10.55 | 73.29±10.30 | 73.26±9.76 | 0.19 | 0.24 | 0.17 |
| Total cholesterol | 204.79±33.22 | 202.23±34.61 | 203.32±36.98 | 0.50 | 0.48 | 0.66 |
| Antilipemic therapy | 96 (20) | 88 (18) | 71 (15) | 0.03 | 0.04 | 0.07 |
| Creatinine | 0.80 (0.70–1.00) | 0.80 (0.70–0.90) | 0.80 (0.70–1.00) | 0.28 | 0.26 | 0.31 |
| Estimated GFR mL/minc | 85.01±24.79 | 85.38±24.38 | 82.85±21.65 | 0.25 | 0.25 | 0.17 |
| GFR <60 mL/min | 54 (10) | 57 (11) | 59 (12) | 0.54 | 0.55 | 0.45 |
| Insulin (μU/mL) | 4.80 (3.50–7.50) | 5.50 (3.80–8.40) | 5.00 (3.40–7.40) | 0.94 | 0.68 | 0.51 |
| Serum glucose | 95.00 (89.00–101.00) | 94.00 (89.00–102.00) | 93.00 (88.00–100.00) | 0.21 | 0.28 | 0.52 |
| Cardiac structure and function | ||||||
| EF | 62.76±7.21 | 62.94±7.19 | 62.65±7.25 | 0.80 | 0.80 | 0.63 |
| EF <45% | 14 (3) | 10 (2) | 11 (2) | 0.56 | 0.56 | 0.78 |
| MS DD | 42 (9) | 37 (8) | 26 (6) | 0.04 | 0.04 | 0.08 |
| Mild/MS DD | 151 (33) | 130 (28) | 121 (27) | 0.02 | 0.02 | 0.06 |
| MS DD except EF <45% | 38 (9) | 30 (7) | 24 (5) | 0.06 | 0.05 | 0.07 |
| Mild/MS DD except EF <45% | 141 (32) | 121 (27) | 115 (26) | 0.04 | 0.04 | 0.07 |
| LVH | 134 (35) | 128 (32) | 133 (35) | 0.28 | 0.32 | 0.52 |
| E/A ratio | 1.09±0.43 | 1.12±0.44 | 1.10±0.37 | 0.90 | 0.97 | 0.40 |
| E/e′ | 8.59±3.08 | 8.75±3.05 | 8.61±3.15 | 0.93 | 0.90 | 0.60 |
| Left atrial size | 3.94±0.67 | 3.89±0.63 | 3.93±0.72 | 0.68 | 0.93 | 0.79 |
| Estimated PASP | 23.03±5.20 | 22.60±5.11 | 22.52±5.09 | 0.18 | 0.21 | 0.39 |
| Serum biomarkers | ||||||
| Aldosterone | 4.90 (2.50–7.90) | 4.30 (2.50–7.80) | 4.90 (2.70–8.30) | 0.84 | 0.79 | 0.71 |
| NT‐proANP | 2284.0 (1469.0–3554.0) | 2077.0 (1329.0–3122.0) | 2375.0 (1436.0–3433.0) | 0.53 | 0.46 | 0.77 |
| NT‐proBNP | 75.36 (31.84–153.80) | 62.30 (24.59–133.80) | 68.52 (28.60–141.70) | 0.61 | 0.56 | 0.88 |
| BNP | 15.70 (6.30–33.80) | 13.70 (4.70–28.80) | 15.85 (6.70–32.30) | 0.85 | 0.86 | 0.57 |
| ANP | 11.60 (7.40–17.60) | 12.05 (7.10–16.50) | 11.65 (7.10–15.40) | 0.19 | 0.20 | 0.31 |
| sNEP | 0.65 (0.40–0.96) | 4.11 (2.13–8.80) | 119.00 (43.68–576.20) | … | … | … |
| Adiponectin | 9.80 (6.60–15.40) | 9.40 (6.40–14.30) | 9.60 (6.40–14.05) | 0.65 | 0.47 | 0.77 |
| Outcomesd | ||||||
| Death, K–M (no. of events) | 0.007 | 0.008 | 0.24 | |||
| 1 y | 0.99 (3) | 1.00 (2) | 0.99 (3) | |||
| 5 y | 0.94 (29) | 0.95 (27) | 0.95 (25) | |||
| 10 y | 0.86 (69) | 0.88 (60) | 0.87 (64) | |||
| HF, K–M (no. of events) | 0.80 | 0.85 | 0.28 | |||
| 1 y | 0.98 (12) | 0.98 (8) | 0.97 (13) | |||
| 5 y | 0.93 (34) | 0.94 (27) | 0.92 (36) | |||
| 10 y | 0.86 (62) | 0.88 (52) | 0.87 (58) | |||
Data are shown as mean±SD, number (percentage), or median (interquartile range). AF indicates atrial fibrillation; ANP, A‐type natriuretic peptide; BMI, body mass index; BNP, brain (B‐type) natriuretic peptide; CAD, coronary artery disease; DD indicates diastolic dysfunction; EF, ejection fraction; GFR, glomerular filtration rate; HF, heart failure; K–M, Kaplan–Meier; LVH, left ventricular hypertrophy; MI, myocardial infarction; MS, moderate or severe; NT‐pro, N‐terminal pro; PASP, pulmonary artery systolic pressure; sNEP, serum neprilysin; WC, waist circumference.
Adjusted for age, sex, BMI, and smoking.
Increased WC defined by as >102 cm in men, and >88 cm in women.
By Modification of Diet in Renal Disease.
Outcome data presented showing K–M survival estimates and number of observed events at 1, 5, and 10 y.
Relationship Between Soluble Neprilysin and Natriuretic Peptides in the Community
The median BNP was 15 pg/mL (IQR: 6–32 pg/mL) and NT‐proBNP was 70 pg/mL (IQR: 29–142 pg/mL; Table 1). ANP and CNP were similarly within the normal range. Contrary to our original hypothesis, no evidence showed an inverse relationship between plasma neprilsyin and ANP, BNP, or CNP (Table 2). Even when considering the subset of community participants without known cardiovascular disease, smoking, or risk factors (to remove potential of residual confounding), similar results were observed (Table S3).
Clinical and Neurohormonal Characteristics by Tertiles of Soluble Neprilysin Levels
To better explore nonlinear interactions between high and low soluble neprilysin levels, the cohort was split into tertiles of neprilysin, creating low, intermediate, and high groups (Table 3). There was a paradoxical inverse association of neprilysin with echocardiographic DD (P=0.02) in which participants with the lowest neprilysin levels had greater prevalence of DD (33%) compared with the intermediate (28%) and high (27%) groups. This relationship was unchanged with exclusion of the 35 participants with low ejection fraction. There was also a greater prevalence of hypertension (P=0.04) and dysplipidemia therapy (P=0.03) in the lowest tertile neprilysin group (Figure 2). These associations remained after adjustment for BMI but were attenuated with additional adjustment for smoking status, age, and sex (Table 3). All natriuretic peptide, aldosterone, and adiponectin levels were similar across neprilysin tertiles, consistent with our observation of a lack of correlation described earlier.
Figure 2.
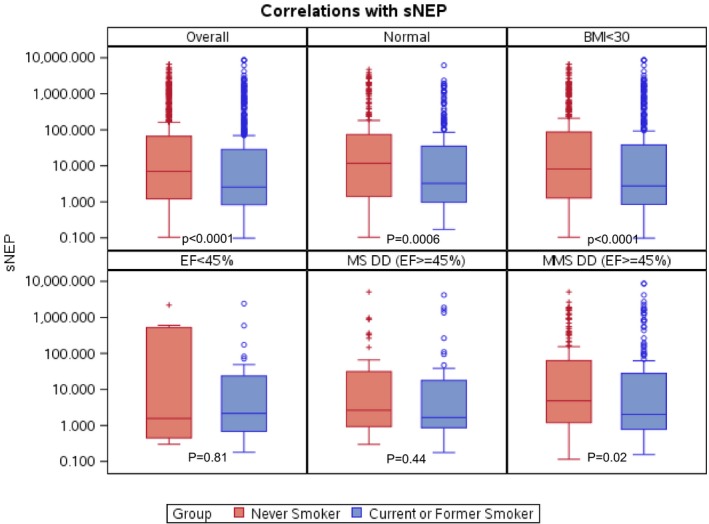
Median soluble neprilysin (sNEP) levels with interquartile ranges according to smoking status among participants. A, Overall. B, Those without any risk factors or cardiovascular disease (normal). C, Nonobese participants. D, Low ejection fraction (EF). E, Moderate/severe (MS) diastolic dysfunction (DD) with normal EF. F, Any DD with normal EF. MMS indicates mild/moderate/severe. P values are derived from the Wilcoxon rank sum test.
Again, no evidence showed association between BMI and neprilysin levels before or after adjusting for age, sex, and smoking status. In fact, there was a paradoxical trend toward less obesity in patients in the highest tertile of neprilysin levels (P=0.08). In sensitivity analyses, extreme elevations in plasma neprilysin were also associated with lower BMI and less obesity (Tables S4 and S5). The lack of a relationship of extreme elevation in neprilysin levels with BNP was also contradictory to a direct proteolytic effect of neprilysin on BNP, with paradoxically higher BNP levels in participants with extreme neprilysin elevation (Table S5).
Relationship Between Smoking and Neprilysin Levels
The strongest association of neprilysin levels was observed with prior or current smoking status (P<0.001), with smoking prevalence highest in the low neprilysin tertile (58%), intermediate in the middle tertile (50%), and lowest in the highest tertile (43%; Table 3; Figure 3). Similarly, the group with extreme neprilysin elevation had the lowest rates of smoking (Tables S4 and S5).
Figure 3.
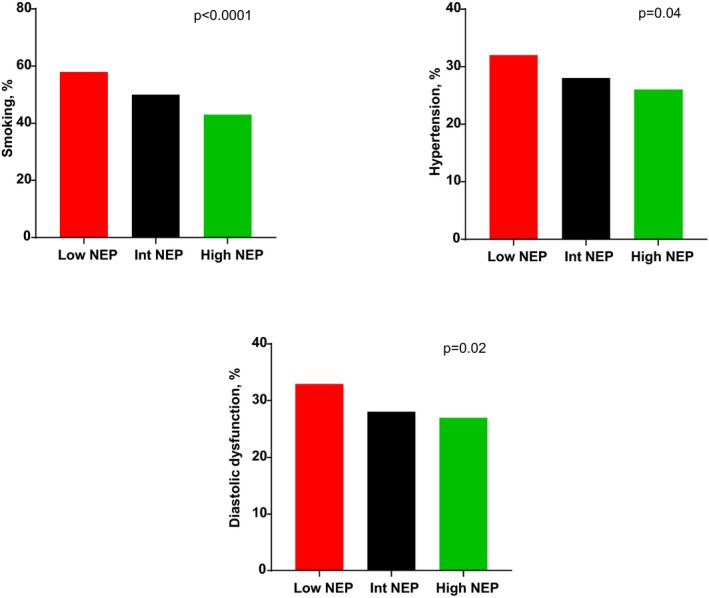
Prevalence of smoking, hypertension, and diastolic dysfunction across low, intermediate (Int), and high neprilysin (NEP) tertiles. P values are trend tests for proportions across NEP tertiles.
Plasma Neprilysin Levels as a Predictor of Incident Clinical HF and Death
At 10 years there were 193 deaths and 172 incident HF events. The group with low soluble neprilysin had the highest mortality (P=0.009 compared with those in the highest tertile), even after adjustment for BMI (P=0.01) but not after adjustment for other comorbidities (P=0.18). Consistent with the relatively modest association of plasma neprilysin with DD, there was no apparent relationship between baseline neprilysin levels and development of incident clinical HF over a median of 10.7 years (IQR: 7.8–11.5 years; Table 3; Table S2). The age‐, sex‐, BMI‐, and smoking‐adjusted hazard ratios for tertiles 2 and 3 of neprilysin compared with tertile 1 were 0.85 (95% CI, 0.64–1.01) and 0.85 (95% CI, 0.68–1.07) for mortality and 1.07 (95% CI, 0.76–1.51) and 1.21 (95% CI, 0.86–1.71) for HF.
Discussion
This study is the first to evaluate the distribution, clinical correlates, and outcomes of soluble circulating neprilysin levels in a well‐characterized, community‐based population. The major findings of our study are as follows: (1) smoking was strongly associated with lower soluble neprilysin levels; (2) low soluble neprilysin was paradoxically associated with worse DD, dyslipidemia, and hypertension; (3) soluble neprilysin levels did not independently predict subsequent HF hospitalization or death over a 10‐year period; and (4) contrary to the proposed proteolytic effect of soluble neprilysin on natriuretic peptides, we observed no relationship between soluble neprilysin and circulating ANP, BNP, or CNP in these community participants without HF. These data challenge our current understanding of the interaction and biological activity of circulating soluble neprilysin and natriuretic peptides in humans.
Role of Neprilysin Across the Spectrum of Asymptomatic Participants With Risk Factors for Clinical HF
Neprilysin is believed to be detrimental, given that inhibition in HFrEF demonstrated a marked reduction in mortality,1 and has been demonstrated in experimental studies to break down beneficial natriuretic peptides13, 14, 15; however, neprilysin is a nonspecific peptidase with numerous substrates (both beneficial and harmful) potentially affected by its proteolytic action.2 Consequently, the proposed construct of neprilysin inhibiting natriuretic peptides as the primary clinically relevant pathway may be overly simplistic and may differ based on whether the study population has HFrEF or HFpEF or comprises asymptomatic patients at risk for DD, as in our study. In a prior small experiment in asymptomatic patients, neprilysin was shown to have an important role in inhibiting vasoconstriction, which may explain the association of lower neprilysin with hypertension and DD in our cohort.13
Our study provides the first report of soluble neprilysin in a large‐scale, community‐based cohort, demonstrating both lack of association of natriuretic peptides with neprilysin and lack of independent prognostic value of neprilysin. These findings are similar to a recent report in which neprilysin did not demonstrate prognostic significance in HFpEF.4 The only randomized trial of neprilysin inhibition in HFpEF demonstrated a reduction in NT‐proBNP at 12 weeks that was not sustained through the duration of the trial at 36 weeks, suggesting a complex interaction between circulation natriuretic peptides and neprilysin.16 These data contrast with the established prognostic role of neprilysin in ambulatory HFrEF,3, 17 acute hospitalized HFrEF,18, 19 and the unequivocal beneficial impact of neprilysin inhibition in HFrEF.1 It is well established that extrapolating therapies from HFrEF to HFpEF have generally been unsuccessful,20 and further study of the role of soluble neprilysin in the development of HFpEF is required. In addition, it remains unclear whether tissue and soluble neprilysin have similar biological activity and tissue release in unselected participants.2 Our inability to demonstrate a correlation between active natriuretic peptides and soluble neprilysin questions this fundamental assumption and requires further investigation.
Prior Literature on Neprilysin–Natriuretic Peptide Interactions
The natriuretic peptides are processed by both natriuretic peptide C receptor and by neprilysin; in patients without marked elevations in natriuretic peptides, neprilysin has been proposed to play a lesser role in natriuretic peptide breakdown.21, 22 This description may partially explain the lack of interaction between neprilysin and natriuretic peptides in our participants, who did not have HF or marked elevation in natriuretic peptides at baseline. However, in sensitivity analyses of extreme neprilysin elevation, these patients paradoxically had higher and not lower BNP levels; again, this result refutes the existing simplistic paradigm of neprilysin breaking down natriuretic peptides in HFpEF. Although it has been proposed that higher levels of BNP (>916 pg/mL) can inhibit neprilysin activity, the levels of BNP in our cohort (even among those in the highest percentiles) were much lower and unlikely to cause such an effect in this range.23 Therefore, the mechanism for concordant elevation of soluble neprilysin and BNP in those with more prominent neprilysin levels is unclear and does not support the negative feedback hypothesis of elevated natriuretic peptides suppressing soluble neprilysin levels. Furthermore, the same study23 demonstrated that patients without HF had lower soluble neprilysin levels but paradoxically higher neprilysin activity than HF patients. In addition, correlation was poor between soluble neprilysin levels and biological activity, and this uncoupling may in part explain the lack of association between soluble neprilysin with natriuretic peptides in our study.
This existing paradigm of neprilysin–natriuretic peptide interactions derives from small acute studies of neprilysin inhibition13, 14, 15 or from populations with HFrEF, in which natriuretic peptides are in general elevated beyond the normal reference range and contribute to the pathophysiology of the HFrEF syndrome.24 In contrast, the centrality of BNP elevation in HFpEF and DD is not universal,25, 26 and BNP levels can be low in many patients with HFpEF and DD because of lower wall stress compared with HFrEF,27 particularly HFpEF of the obese28 and those with euvolemic exertional dyspnea.29, 30 Therefore, the large subset of HFpEF patients in whom natriuretic peptides are not elevated may not have an important neprilysin dependence with regard to their HF; this is supported by the results of our study in patients at risk for HFpEF and by a prior investigation in a small cohort with clinical HFpEF.4
Soluble Neprilysin, Smoking, and DD
One of the novel interactions observed in this study was the relationship between smoking and soluble neprilysin. Neprilysin is highly expressed in airways, pulmonary interstitium, and alveolar cells,31, 32, 33 which are susceptible to cigarette smoke–related epithelial injury and cellular loss. The observation of smoking being linked with low neprilysin levels is consistent with prior human lung tissue studies34 but, for the first time, was demonstrated in a general population–based cohort using circulating neprilysin levels. Smoking is a known risk factor for HFpEF35, 36, 37, 38 and is frequently associated with worse outcomes, DD,39 alveolar capillary remodeling, and pulmonary vascular disease in HFpEF,40, 41, 42 but the underlying pathophysiology driving these changes is unknown. Systemic inflammation linked to coronary microvascular and endothelial dysfunction has been proposed,43 but our study suggests another potential mechanism mediated by low neprilysin.
The association of low neprilysin levels with DD and hypertension suggests that there may be an increase in transforming growth factor β,44 endothelin, angiotensin, or other detrimental systemic mediators that may drive adverse left ventricular, aortic, pulmonary vascular,45 and alveolar capillary remodeling. In addition, absence of lung tissue neprilysin has been associated with exaggerated inflammation mediated by lack of clearance of tachykinins such as substance P.31, 46 Any or all of these mediators may underlie higher DD with low neprilysin, but we did not measure these substances in our study and this requires additional investigation. Smokers may also have reduced plasma neprilysin due to epigenetic mechanisms that affect MME (membrane metalloendopeptidase; the gene that encodes neprilysin) gene transcription and increased urinary neprilysin excretion.34, 47
Limitations
As with all transmembrane proteins, it is possible that circulating levels may not perfectly correlate with biologically active tissue levels, but this remains a limitation of all serological biomarker research. However, prior investigations have demonstrated low expression of neprilysin in lung tissue (at both protein and transcript levels) in smokers,34 which is concordant with our independent finding of low circulating neprilysin levels in smokers. This finding supports the validity of measured soluble neprilysin as a representation of tissue neprilysin levels. We previously demonstrated a linear correlation between neprilysin tissue expression and activity48, that is lower the tissue expression less is the enzymatic activity. Prior investigations have also confirmed that circulating neprilysin indeed retains biologically active catalytic activity,49, 50 but this has not been consistently observed. We did not measure neprilysin activity in this study, and further investigation of the relationship between soluble neprilysin levels with serum and tissue activity is required. Serum BNP assays as currently measured have cross‐reactivity between the precursor proBNP, inactive BNP subtypes, and biologically active BNP1‐32 subtypes.51, 52 Consequently, it has been proposed that current serological assays for BNP may not detect bioactive BNP1‐32 reliably.53 This may have affected our observed lack of interaction between neprilysin and serologically measured BNP. However, in subgroup analyses, we observed a significant but paradoxical higher BNP level in patients with extreme elevations of neprilysin, and levels of NT‐proBNP, ANP, CNP, and NT‐ANP similarly showed no interaction with neprilysin. These results suggest that our observation of the lack of an inverse association between BNP and soluble neprilysin is real and deserves further investigation. The event rate for clinical HF was low in this study over 10 years of follow‐up, emphasizing the long duration of follow‐up needed to transition from risk factors to DD to clinical HFpEF. It is possible that despite the observed associations between low neprilysin levels and DD, our study was underpowered to detect true associations between low neprilysin, incident HFpEF, and mortality. These associations require further study in large combined population data sets.
Conclusions
Contrary to the previously proposed inverse relationship between neprilysin and natriuretic peptides in humans, we observed no such interaction in a large community‐based cohort. Nevertheless, our findings emphasize the complexity of the human neprilysin–natriuretic peptide system and suggest that low soluble neprilysin levels may be modestly related to DD and an adverse cardiometabolic profile. Further study of the relationship among smoking, soluble and tissue neprilysin activity, and DD may help us better understand how smoking contributes to DD. Whether it relates to less neprilysin‐related proteolysis of harmful substances such as angiotensin and endothelin requires further study.
Sources of Funding
Dr Pereira is supported by National Institute on Aging grant R21AG53512, and Dr Reddy was supported by National Institutes of Health grant T32 HL007111.
Disclosures
None.
Supporting information
Data S1. Soluble Neprilysin Assay.
Table S1. Inter and Intra‐assay Variability for Neprilysin Analysis
Table S2. Overall Distribution
Table S3. Lack of Correlation of Neprilysin Levels With Age, Body Mass Index, Renal Function, Natriuretic Peptides, and Neurohormonal Activation Among Healthy Nonsmokers
Table S4. Clinical Characteristics and Biomarkers in Participants With Neprilysin Levels 100 Times the Median Versus Below the Median
Table S5. Clinical Characteristics and Biomarkers in Participants With Extreme Neprilysin Levels
Figure S1. Inter and intra‐assay variability for neprilysin analysis demonstrating good correlation.
Figure S2. Forest plot showing association of neprilysin tertiles with mortality and heart failure outcomes.
(J Am Heart Assoc. 2019;8:e012943 DOI: 10.1161/JAHA.119.012943.)
References
- 1. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K. Zile MR, Investigators P‐H and Committees. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 2. Bayes‐Genis A, Barallat J, Richards AM. A test in context: neprilysin: function, inhibition, and biomarker. J Am Coll Cardiol. 2016;68:639–653. [DOI] [PubMed] [Google Scholar]
- 3. Bayes‐Genis A, Barallat J, Galan A, de Antonio M, Domingo M, Zamora E, Urrutia A, Lupon J. Soluble neprilysin is predictive of cardiovascular death and heart failure hospitalization in heart failure patients. J Am Coll Cardiol. 2015;65:657–665. [DOI] [PubMed] [Google Scholar]
- 4. Goliasch G, Pavo N, Zotter‐Tufaro C, Kammerlander A, Duca F, Mascherbauer J, Bonderman D. Soluble neprilysin does not correlate with outcome in heart failure with preserved ejection fraction. Eur J Heart Fail. 2016;18:89–93. [DOI] [PubMed] [Google Scholar]
- 5. Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. [DOI] [PubMed] [Google Scholar]
- 6. Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC Jr. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol. 2002;40:976–982. [DOI] [PubMed] [Google Scholar]
- 7. Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC Jr, Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McKie PM, Cataliotti A, Lahr BD, Martin FL, Redfield MM, Bailey KR, Rodeheffer RJ, Burnett JC Jr. The prognostic value of N‐terminal pro‐B‐type natriuretic peptide for death and cardiovascular events in healthy normal and stage A/B heart failure subjects. J Am Coll Cardiol. 2010;55:2140–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Melton LJ III. History of the rochester epidemiology project. Mayo Clin Proc. 1996;71:266–274. [DOI] [PubMed] [Google Scholar]
- 10. Burnett JC Jr, Kao PC, Hu DC, Heser DW, Heublein D, Granger JP, Opgenorth TJ, Reeder GS. Atrial natriuretic peptide elevation in congestive heart failure in the human. Science. 1986;231:1145–1147. [DOI] [PubMed] [Google Scholar]
- 11. Cannone V, Boerrigter G, Cataliotti A, Costello‐Boerrigter LC, Olson TM, McKie PM, Heublein DM, Lahr BD, Bailey KR, Averna M, Redfield MM, Rodeheffer RJ, Burnett JC Jr. A genetic variant of the atrial natriuretic peptide gene is associated with cardiometabolic protection in the general community. J Am Coll Cardiol. 2011;58:629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sangaralingham SJ, McKie PM, Ichiki T, Scott CG, Heublein DM, Chen HH, Bailey KR, Redfield MM, Rodeheffer RJ, Burnett JC Jr. Circulating C‐type natriuretic peptide and its relationship to cardiovascular disease in the general population. Hypertension. 2015;65:1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Florkowski CM, Richards AM, Espiner EA, Yandle TG, Sybertz E, Frampton CM. Low‐dose brain natriuretic peptide infusion in normal men and the influence of endopeptidase inhibition. Clin Sci (Lond). 1997;92:255–260. [DOI] [PubMed] [Google Scholar]
- 14. Richards AM, Crozier IG, Espiner EA, Yandle TG, Nicholls MG. Plasma brain natriuretic peptide and endopeptidase 24.11 inhibition in hypertension. Hypertension. 1993;22:231–236. [DOI] [PubMed] [Google Scholar]
- 15. Richards AM, Wittert GA, Crozier IG, Espiner EA, Yandle TG, Ikram H, Frampton C. Chronic inhibition of endopeptidase 24.11 in essential hypertension: evidence for enhanced atrial natriuretic peptide and angiotensin II. J Hypertens. 1993;11:407–416. [DOI] [PubMed] [Google Scholar]
- 16. Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher‐Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, Lefkowitz M, Packer M, McMurray JJ; Prospective comparison of ACEI [Angiotensin‐Converting—Enzyme Inhibitor] . The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double‐blind randomised controlled trial. Lancet. 2012;380:1387–1395. [DOI] [PubMed] [Google Scholar]
- 17. Nunez J, Nunez E, Barallat J, Bodi V, Minana G, Pastor MC, Sanchis J, Lupon J, Serum Bayes‐Genis A. Neprilysin and recurrent admissions in patients with heart failure. J Am Heart Assoc. 2017; 6:e005712 DOI: 10.1161/JAHA.117.005712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bayes‐Genis A, Barallat J, Pascual‐Figal D, Nunez J, Minana G, Sanchez‐Mas J, Galan A, Sanchis J, Zamora E, Perez‐Martinez MT, Lupon J. Prognostic value and kinetics of soluble neprilysin in acute heart failure: a pilot study. JACC Heart Fail. 2015;3:641–644. [DOI] [PubMed] [Google Scholar]
- 19. Nunez J, Nunez E, Minana G, Carratala A, Sanchis J, Lupon J, Barallat J, Pastor MC, Pascual‐Figal D, Bayes‐Genis A. Serum neprilysin and recurrent hospitalizations after acute heart failure. Int J Cardiol. 2016;220:742–744. [DOI] [PubMed] [Google Scholar]
- 20. Borlaug BA, Redfield MM. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation. 2011;123:2006–2013; discussion 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Potter LR. Natriuretic peptide metabolism, clearance and degradation. FEBS J. 2011;278:1808–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hashimoto Y, Nakao K, Hama N, Imura H, Mori S, Yamaguchi M, Yasuhara M, Hori R. Clearance mechanisms of atrial and brain natriuretic peptides in rats. Pharm Res. 1994;11:60–64. [DOI] [PubMed] [Google Scholar]
- 23. Vodovar N, Seronde MF, Laribi S, Gayat E, Lassus J, Januzzi JL Jr, Boukef R, Nouira S, Manivet P, Samuel JL, Logeart D, Cohen‐Solal A, Richards AM, Launay JM, Mebazaa A, Network G. Elevated plasma B‐type natriuretic peptide concentrations directly inhibit circulating neprilysin activity in heart failure. JACC Heart Fail. 2015;3:629–636. [DOI] [PubMed] [Google Scholar]
- 24. Packer M, McMurray JJ, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile M, Andersen K, Arango JL, Arnold JM, Belohlavek J, Bohm M, Boytsov S, Burgess LJ, Cabrera W, Calvo C, Chen CH, Dukat A, Duarte YC, Erglis A, Fu M, Gomez E, Gonzalez‐Medina A, Hagege AA, Huang J, Katova T, Kiatchoosakun S, Kim KS, Kozan O, Llamas EB, Martinez F, Merkely B, Mendoza I, Mosterd A, Negrusz‐Kawecka M, Peuhkurinen K, Ramires FJ, Refsgaard J, Rosenthal A, Senni M, Sibulo AS Jr, Silva‐Cardoso J, Squire JB, Starling RC, Teerlink JR, Vanhaecke J, Vinereanu D, Wong RC; Investigators P‐H and Coordinators . Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015;131:54–61. [DOI] [PubMed] [Google Scholar]
- 25. Anjan VY, Loftus TM, Burke MA, Akhter N, Fonarow GC, Gheorghiade M, Shah SJ. Prevalence, clinical phenotype, and outcomes associated with normal B‐type natriuretic peptide levels in heart failure with preserved ejection fraction. Am J Cardiol. 2012;110:870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence‐based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138:861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Veldhuisen DJ, Linssen GC, Jaarsma T, van Gilst WH, Hoes AW, Tijssen JG, Paulus WJ, Voors AA, Hillege HL. B‐type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol. 2013;61:1498–1506. [DOI] [PubMed] [Google Scholar]
- 28. Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: a simultaneous invasive‐echocardiographic study. Circulation. 2017;135:825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nadel JA, Borson DB. Modulation of neurogenic inflammation by neutral endopeptidase. Am Rev Respir Dis. 1991;143:S33–S36. [DOI] [PubMed] [Google Scholar]
- 32. Johnson AR, Ashton J, Schulz WW, Erdos EG. Neutral metalloendopeptidase in human lung tissue and cultured cells. Am Rev Respir Dis. 1985;132:564–568. [DOI] [PubMed] [Google Scholar]
- 33. Roques BP, Noble F, Dauge V, Fournie‐Zaluski MC, Beaumont A. Neutral endopeptidase 24.11: structure, inhibition, and experimental and clinical pharmacology. Pharmacol Rev. 1993;45:87–146. [PubMed] [Google Scholar]
- 34. Wick MJ, Buesing EJ, Wehling CA, Loomis ZL, Cool CD, Zamora MR, Miller YE, Colgan SP, Hersh LB, Voelkel NF, Dempsey EC. Decreased neprilysin and pulmonary vascular remodeling in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183:330–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ho JE, Lyass A, Lee DS, Vasan RS, Kannel WB, Larson MG, Levy D. Predictors of new‐onset heart failure: differences in preserved versus reduced ejection fraction. Circ Heart Fail. 2013;6:279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ahmed AA, Patel K, Nyaku MA, Kheirbek RE, Bittner V, Fonarow GC, Filippatos GS, Morgan CJ, Aban IB, Mujib M, Desai RV, Allman RM, White M, Deedwania P, Howard G, Bonow RO, Fletcher RD, Aronow WS, Ahmed A. Risk of heart failure and death after prolonged smoking cessation: role of amount and duration of prior smoking. Circ Heart Fail. 2015;8:694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Eaton CB, Pettinger M, Rossouw J, Martin LW, Foraker R, Quddus A, Liu S, Wampler NS, Hank Wu WC, Manson JE, Margolis K, Johnson KC, Allison M, Corbie‐Smith G, Rosamond W, Breathett K, Klein L. Risk factors for incident hospitalized heart failure with preserved versus reduced ejection fraction in a multiracial cohort of postmenopausal women. Circ Heart Fail. 2016;9:e002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kamimura D, Cain LR, Mentz RJ, White WB, Blaha MJ, DeFilippis AP, Fox ER, Rodriguez CJ, Keith RJ, Benjamin EJ, Butler J, Bhatnagar A, Robertson RM, Winniford MD, Correa A, Hall ME. Cigarette smoking and incident heart failure: insights from the Jackson heart study. Circulation. 2018;137:2572–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nadruz W Jr, Claggett B, Goncalves A, Querejeta‐Roca G, Fernandes‐Silva MM, Shah AM, Cheng S, Tanaka H, Heiss G, Kitzman DW, Solomon SD. Smoking and cardiac structure and function in the elderly: the ARIC Study (Atherosclerosis Risk in Communities). Circ Cardiovasc Imaging. 2016;9:e004950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lam CS, Lyass A, Kraigher‐Krainer E, Massaro JM, Lee DS, Ho JE, Levy D, Redfield MM, Pieske BM, Benjamin EJ, Vasan RS. Cardiac dysfunction and noncardiac dysfunction as precursors of heart failure with reduced and preserved ejection fraction in the community. Circulation. 2011;124:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, Wehrens XH, Deswal A. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59:998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hoeper MM, Meyer K, Rademacher J, Fuge J, Welte T, Olsson KM. Diffusion capacity and mortality in patients with pulmonary hypertension due to heart failure with preserved ejection fraction. JACC Heart Fail. 2016;4:441–449. [DOI] [PubMed] [Google Scholar]
- 43. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. [DOI] [PubMed] [Google Scholar]
- 44. Suematsu Y, Miura S, Goto M, Matsuo Y, Arimura T, Kuwano T, Imaizumi S, Iwata A, Yahiro E, Saku K. LCZ696, an angiotensin receptor‐neprilysin inhibitor, improves cardiac function with the attenuation of fibrosis in heart failure with reduced ejection fraction in streptozotocin‐induced diabetic mice. Eur J Heart Fail. 2016;18:386–393. [DOI] [PubMed] [Google Scholar]
- 45. Dempsey EC, Wick MJ, Karoor V, Barr EJ, Tallman DW, Wehling CA, Walchak SJ, Laudi S, Le M, Oka M, Majka S, Cool CD, Fagan KA, Klemm DJ, Hersh LB, Gerard NP, Gerard C, Miller YE. Neprilysin null mice develop exaggerated pulmonary vascular remodeling in response to chronic hypoxia. Am J Pathol. 2009;174:782–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wong SS, Sun NN, Fastje CD, Witten ML, Lantz RC, Lu B, Sherrill DL, Gerard CJ, Burgess JL; Committee HEIHR . Role of neprilysin in airway inflammation induced by diesel exhaust emissions. Res Rep Health Eff Inst. 2011;159:3–40. [PMC free article] [PubMed] [Google Scholar]
- 47. Nortier J, Bernard A, Roels H, Deschodt‐Lanckman M, Gueuning C, Lauwerys R. Urinary neutral endopeptidase in workers exposed to cadmium: interaction with cigarette smoking. Occup Environ Med. 1997;54:432–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pereira NL, Aksoy P, Moon I, Peng Y, Redfield MM, Burnett JC Jr, Wieben ED, Yee VC, Weinshilboum RM. Natriuretic peptide pharmacogenetics: membrane metallo‐endopeptidase (MME): common gene sequence variation, functional characterization and degradation. J Mol Cell Cardiol. 2010;49:864–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yandle T, Richards M, Smith M, Charles C, Livesey J, Espiner E. Assay of endopeptidase‐24.11 activity in plasma applied to in vivo studies of endopeptidase inhibitors. Clin Chem. 1992;38:1785–1791. [PubMed] [Google Scholar]
- 50. Spillantini MG, Sicuteri F, Salmon S, Malfroy B. Characterization of endopeptidase 3.4.24.11 (“enkephalinase”) activity in human plasma and cerebrospinal fluid. Biochem Pharmacol. 1990;39:1353–1356. [DOI] [PubMed] [Google Scholar]
- 51. Hawkridge AM, Heublein DM, Bergen HR III, Cataliotti A, Burnett JC Jr, Muddiman DC. Quantitative mass spectral evidence for the absence of circulating brain natriuretic peptide (BNP‐32) in severe human heart failure. Proc Natl Acad Sci USA. 2005;102:17442–17447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Miller WL, Phelps MA, Wood CM, Schellenberger U, Van Le A, Perichon R, Jaffe AS. Comparison of mass spectrometry and clinical assay measurements of circulating fragments of B‐type natriuretic peptide in patients with chronic heart failure. Circ Heart Fail. 2011;4:355–360. [DOI] [PubMed] [Google Scholar]
- 53. Jaffe AS. Unwinding the interaction of natriuretic peptides and neprilysin. J Am Coll Cardiol. 2015;65:666–667. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Soluble Neprilysin Assay.
Table S1. Inter and Intra‐assay Variability for Neprilysin Analysis
Table S2. Overall Distribution
Table S3. Lack of Correlation of Neprilysin Levels With Age, Body Mass Index, Renal Function, Natriuretic Peptides, and Neurohormonal Activation Among Healthy Nonsmokers
Table S4. Clinical Characteristics and Biomarkers in Participants With Neprilysin Levels 100 Times the Median Versus Below the Median
Table S5. Clinical Characteristics and Biomarkers in Participants With Extreme Neprilysin Levels
Figure S1. Inter and intra‐assay variability for neprilysin analysis demonstrating good correlation.
Figure S2. Forest plot showing association of neprilysin tertiles with mortality and heart failure outcomes.


