Abstract
Background
Amlodipine is used for the treatment of hypertension, but reports on its use in early pregnancy are limited.
Methods and Results
In the present study, we recruited 231 women with chronic hypertension, including those who received amlodipine or other antihypertensives during early pregnancy, and investigated frequencies of morphologic abnormalities in their 231 offspring. Specifically, we evaluated 48 neonates exposed to amlodipine in the first trimester (amlodipine group, Group A), 54 neonates exposed to antihypertensives other than amlodipine (other antihypertensive group, Group O), and 129 neonates not exposed to antihypertensives (no‐antihypertensive group, Group N). The number of morphologic abnormalities of offspring in each group were 2 in Group A (4.2%; 95% CI, 0.51–14.25); 3 in Group O (5.6%; 95% CI, 1.16–15.39) and 6 in Group N (4.7%; 95% CI, 1.73–9.85). The odds ratio of the primary outcome comparing Group A and Group O was 0.74 (95% CI: 0.118–4.621) and Group A and Group N was 0.89 (95% CI: 0.174–4.575).
Conclusions
The odds of birth defects in Group A in the first trimester were not significantly different from those with or without other antihypertensives.
Keywords: amlodipine, chronic hypertension, first trimester, pregnancy
Subject Categories: High Blood Pressure, Hypertension, Pregnancy
Short abstract
See Editorial Malha and August
Clinical Perspective
What Is New?
The number of cases is greater than those in any previous study on amlodipine use in early pregnancy.
What Are the Clinical Implications?
The incidence of morphologic abnormalities in the offspring of hypertensive mothers treated with amlodipine in early pregnancy was not higher than in mothers treated with or without other antihypertensives.
Introduction
Pregnancies with chronic hypertension have become increasingly common, partly because of the upward shift of pregnancy ages as well as increasing rates of obesity. Chronic hypertension during pregnancy is a risk factor for various adverse outcomes,1, 2 including perinatal complications such as superimposed preeclampsia, premature birth, and low birth weight.
Amlodipine, a calcium channel blocker, is long‐acting and causes relatively few adverse drug reactions associated with vasodilation. Because of these advantages, amlodipine is frequently used for the treatment of hypertension, except for pregnant women. A few studies reported that calcium channel blockers as a group may not pose a significant teratogenic risk even in early pregnancy,3, 4, 5 although information on specific calcium channel blockers is limited.
It was recently reported that hypertension itself may be teratogenic.6, 7 In this study, therefore, we compared the pregnancy outcomes of 48 women with amlodipine exposure during the first trimester with those of hypertensive women who received nonamlodipine antihypertensives, as well as those who did not receive any antihypertensive drugs.
Methods
The authors declare that all supporting data are available within the article and its online supplementary files.
Study Subject
In this retrospective study, we examined birth outcomes of pregnant women with chronic hypertension whose deliveries resulted in live births from April 2008 to July 2016 at the National Center for Child Health and Development (NCCHD, Tokyo), Osaka Women's and Children's Hospital (OWCH, Osaka), and National Cerebral and Cardiovascular Center (NCCC, Osaka). We extracted the data of singletons whose mothers’ electronic health records were coded “Hypertension” during pregnancy and of those with “Chronic hypertension” documented in the delivery records. We then excluded those who did not meet the criteria for chronic hypertension8 and the remainder were included in the final analyses.
Ethics Committee Approval
This study has been approved by the research ethics boards of these hospitals (NCCHD: 1243, OWCH: 1010, NCCC: M29‐073). The requirement for informed consent was waived. All personal identifying data were removed from the study database so that the individuals could not be identified.
Use of Antihypertensives
The first trimester was defined in this study as the period from estimated conception to 11 weeks and 6 days’ gestation. We classified women and neonates exposed to amlodipine in the first trimester into the amlodipine group (Group A), those exposed to antihypertensives other than amlodipine (including other calcium channel blockers) into the other antihypertensive group (Group O), and those not exposed to antihypertensives into the no‐antihypertensive group (Group N).
Clinical Diagnosis
We confirmed the diagnosis of hypertension in pregnancy, according to the International Society for the Study of Hypertension in Pregnancy Classification, Diagnosis, and Management Recommendations for International Practice.8 In this study, therefore, hypertension in pregnancy was defined as “chronic hypertension” if the patient was diagnosed with hypertension before pregnancy, or if hypertension was noted before 20 weeks’ gestation. Hypertension was defined as a systolic blood pressure ≥140 mm Hg or a diastolic blood pressure ≥90 mm Hg. These measurements were made on at least 2 different occasions. Abnormal proteinuria in pregnancy was defined as the excretion of ≥300 mg of protein in 24 hours or a protein/creatinine ratio of ≥0.30 g/g·Cr. Superimposed preeclampsia was diagnosed if a woman with chronic hypertension developed new‐onset proteinuria in the setting of a rise in blood pressure or a sudden increase in pre‐existing proteinuria.
Data Analysis Framework
Primary outcome
In accordance with the European Surveillance of Congenital Anomalies Guide 1.4 and the Reference Documents9 developed by European Surveillance of Congenital Anomalies, neonates who exhibited “major anomalies” were considered to have morphologic abnormalities (Table S1).
Study Participant Characteristics
Clinical information, such as birth date, underlying disease, past medical history, previous pregnancy complications, family history, as well as information on the course of the index pregnancy and the newborn, were obtained from electronic medical records.
Statistical Analysis
A 95% CI was calculated for the incidence of malformations. P<0.05 was considered statistically significant. The χ2 test was used for analyzing primary outcome and discrete variables. Mean values of continuous variables were compared by 1‐way ANOVA. As a subgroup analysis, we repeated the comparison among the 3 groups after excluding 10 women with diabetes mellitus, which is a known risk factor for adverse pregnancy outcomes including congenital anomalies. All analyses were performed with SAS software, version 9.4 (SAS Institute, Cary, NC).
Results
There were 25 485 live births delivered in this period. Among them, there were 1624 singletons with “Hypertension” documented in their mothers’ electronic medical records or “Chronic hypertension” in the delivery records. We excluded preeclampsia and gestational hypertension (n=457), postpartum hypertension (n=683), white coat hypertension, and those who did not meet criteria of hypertension (n=244). We also excluded a case of pulmonary hypertension (n=1) and those without data on blood pressure before 20 weeks’ gestations (n=8). A total of 231 Japanese women met the definition of chronic hypertension, and they were included in the final analyses (Figure 1).
Figure 1.
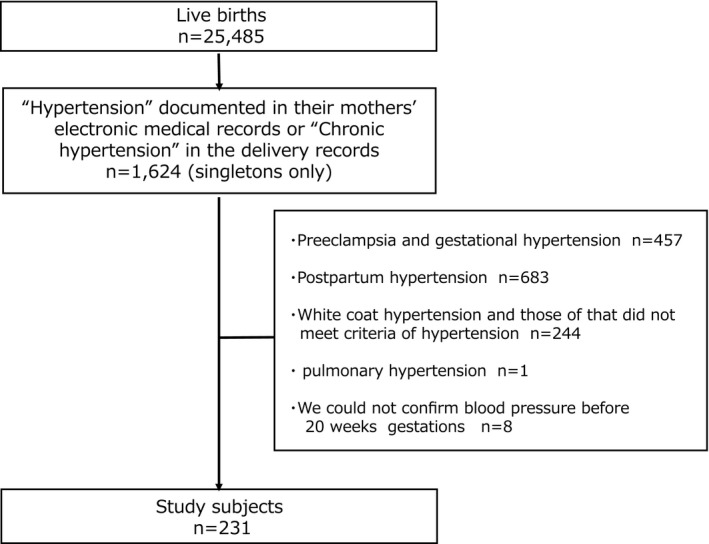
Flow chart representing the recruiting process of study subjects. There were 25 485 live births delivered in this period. Among them, there were 1624 singletons with “Hypertension” documented in their mothers’ electronic medical records or “Chronic hypertension” in the delivery records. After excluding those who did not meet criteria of chronic hypertension, a total of 231 neonates were included in the final analyses.
Forty‐eight neonates were classified into Group A, 54 neonates into Group O, and 129 neonates into Group N. No clear difference in patient background characteristics was observed between groups except that Group A showed a high proportion of women with thyroid disease as the underlying disease, a history of hypertensive disorders of pregnancy and those with a history of fetal growth restriction and that Group O showed a higher age at delivery than Group N (Table 1).
Table 1.
Maternal Baseline Characteristics
| Amlodipine (n=48) | Other Antihypertensives (n=54) | No Antihypertensives (n=129) | P Value | |
|---|---|---|---|---|
| Age at delivery (y)—mean (SD) | 37.5 (4.04) | 37.9 (5.04) | 36.3 (4.27) | 0.047 |
| Height, cm—mean (SD) | 158.3 (4.62) | 159.0 (4.64) | 159.0 (5.98) | 0.727 |
| Prepregnancy body weight, kg—mean (SD) | 62.2 (13.03) | 66.4 (14.92) | 67.9 (17.10) | 0.107 |
| Prepregnancy BMI, kg/m2—mean (SD) | 24.8 (5.02) | 26.4 (6.34) | 26.8 (6.42) | 0.153 |
| Nulliparous, N (%) | 20 (41.7) | 26 (48.1) | 70 (54.3) | 0.310 |
| Underlying disease | ||||
| Diabetes mellitus, N (%) | 3 (6.3) | 1 (1.9) | 6 (4.7) | 0.533 |
| Thyroid disease, N (%) | 7 (14.6) | 4 (7.4) | 5 (3.9) | 0.044 |
| Kidney disease, N (%) | 3 (6.3) | 0 (0.0) | 4 (3.1) | 0.184 |
| Collagen disease, N (%) | 4 (8.3) | 2 (3.7) | 2 (1.6) | 0.090 |
| Congenital heart disease, N (%) | 0 (0.0) | 0 (0.0) | 1 (0.8) | 0.672 |
| Previous pregnancy complications | ||||
| Hypertensive disorders of pregnancy, N (%) | 19 (39.6) | 10 (18.5) | 28 (21.7) | 0.024 |
| Fetal growth restriction, N (%) | 10 (20.8) | 5 (9.3) | 7 (5.4) | 0.008 |
| Smoking during pregnancy, N (%) | 0 (0.0) | 3 (5.6) | 5 (3.9) | 0.287 |
| Alcohol consumption during pregnancy, N (%) | 0 (0.0) | 1 (1.9) | 2 (1.6) | 0.662 |
BMI indicates body mass index.
There was no statistically significant difference between groups in all delivery outcomes (Table 2).
Table 2.
Delivery Outcomes
| Amlodipine (n=48) | Other Antihypertensives (n=54) | No Antihypertensives (n=129) | P Value | |
|---|---|---|---|---|
| Maternal outcomes | ||||
| Superimposed preeclampsia, N (%) | 15 (31.3) | 14 (25.9) | 43 (33.3) | 0.615 |
| Gestational diabetes mellitus, N (%) | 3 (6.3) | 8 (14.8) | 24 (18.6) | 0.125 |
| Newborn outcomes | ||||
| Gestational age, wks—mean (SD) | 37.7 (2.14) | 36.9 (3.43) | 37.1 (3.65) | 0.417 |
| Delivery weight, g—mean (SD) | 2778.4 (619.54) | 2520.1 (800.09) | 2536.0 (759.40) | 0.123 |
| Preterm birth (<37 wks), N (%) | 10 (20.8) | 12 (22.2) | 41 (31.8) | 0.221 |
| Low birth weight (<2500 g), N (%) | 12 (25.0) | 24 (44.4) | 49 (38.0) | 0.116 |
| Apgar score | ||||
| 1 min, mean (SD) | 8.0 (0.99) | 7.4 (1.97) | 7.6 (1.69) | 0.165 |
| 5 min, mean (SD) | 8.9 (0.43) | 8.5 (1.30) | 8.7 (1.22) | 0.203 |
| Birth defects, N (%) | 2 (4.2) | 3 (5.6) | 6 (4.7) | 0.944 |
There was no statistically significant difference among the groups.
Morphologic abnormalities were observed in 11 of the 231 neonates: 2 of 48 neonates in Group A (4.2%; 95% CI, 0.51–14.25), 3 of 54 neonates in Group O (5.6%; 95% CI, 1.16–15.39), and 6 of 129 neonates in Group N (4.7%; 95% CI, 1.73–9.85) (Table 2: P=0.944). The odds ratio of the primary outcome comparing Group A and Group O was 0.74 (95% CI: 0.118–4.621) and Group A and Group N was 0.89 (95% CI: 0.174–4.575). Details of observed birth defects are summarized in Table 3. We were unable to identify any specific pattern of birth defects in this group.
Table 3.
Details of Cases of Morphologic Abnormalities
| Case | Group | Birth Defects | Age at Delivery/Underlying Disease | Delivery Outcome | Superimposed Preeclampsia | Antihypertensive Agents | Other Drugs | |
|---|---|---|---|---|---|---|---|---|
| 1 | A | PVS |
41 y.o. Essential hypertension |
35w4d 1872 g |
− | Prepregnancy to 4w6d | Am | 12w0d to 28w0d: LDA |
| 34w5d to 35w0d | Me | |||||||
| 35w1d to delivery | Me, Nif | |||||||
| 2 | A | VSD |
36 y.o. Primary aldosteronism |
38w0d 3217 g |
− | Prepregnancy to 6w4d | Am | 8w3d to 28w0d: LDA |
| 6w5d to 8w0d | Hy | |||||||
| 8w1d to delivery | Am | |||||||
| 3 | O | Low‐lying conus medullaris/hypospadias/inguinal hernia |
42 y.o. Essential hypertension |
29w1d 521 g |
+ | Prepregnancy to 8w2d | La | 12w6d to delivery: LDA |
| 9w4d to 12w0d | Me | |||||||
| 12w1d to 12w5d | Me, La | |||||||
| 12w6d to 17w5d | Me, La, Am | |||||||
| 17w6d to delivery | Me, Am | |||||||
| 4 | O | VSD |
38 y.o. Essential hypertension RA |
25w6d 382 g |
− | Prepregnancy to delivery | Nic, La |
Prepregnancy to delivery: PSL 3w to delivery: LDA |
| 5 | O | Hypospadias |
39 y.o. Essential hypertension |
39w4d 2912 g |
+ | 8w4d to 30w2d | Me | None |
| 30w3d to delivery | Me, Am | |||||||
| 6 | N | Hypospadias |
33 y.o. Essential hypertension |
27w1d 506 g |
+ | 13w2d to 18w2d | Hy | 12w0d to delivery: LDA |
| 18w2d to delivery | La | |||||||
| 7 | N | Patent foramen ovale/low‐lying conus medullaris |
40 y.o. Essential hypertension |
35w4d 1351 g |
+ | 16w0d to 24w6d | Me | None |
| 25w0d to delivery | Me, Nif | |||||||
| 8 | N | Low anorectal anomaly/low‐lying conus medullaris |
42 y.o. Essential hypertension |
26w0d 379 g |
+ | 21w0d to 21w1d | Me | None |
| 21w2d to delivery | Me, Am | |||||||
| 9 | N | Low‐lying conus medullaris |
37 y.o. Essential hypertension |
34w3d 2108 g |
− | 16w4d to 28w5d | Me | None |
| 28w6d to delivery | Me, Am | |||||||
| 10 | N | Potter syndrome |
40 y.o. Essential hypertension |
33w6d 1836 g |
− | None | None | |
| 11 | N | Colpocephaly |
32 y.o. Essential hypertension |
40w4d 3396 g |
− | None | None | |
A indicates amlodipine group; Am, amlodipine; Hy, hydralazine; La, labetalol; LDA, low‐dose aspirin; Me, methyldopa; N, No antihypertensive group; Nic, nicardipine; Nif, nifedipine; O, Other antihypertensive group; PSL, prednisolone; PVS, pulmonary valve stenosis; RA, rheumatoid arthritis; VSD, ventricular septal defect; y.o., years old.
We have calculated total dose to quantify the exposure (Table S2). Total dose was obtained by amlodipine daily dose times the total number of days of amlodipine uses during the first trimester. The average of total dose±SD and the total number of days±SD of amlodipine use for all cases were 363.6±257.9 mg and 65.0±25.9 days. Those of cases with birth defects were 175 mg, 35 days (Case 1 in Table 3) and 740 mg, 74 days (Case 2 in Table 3).
Our study cohort included 10 women with diabetes mellitus, a known risk factor for congenital anomalies: 3 in group A, 1 in group O, and 6 in group N, although there were no birth defects among them. We performed a subgroup analysis after excluding these subjects, but group differences remained not statistically significant (P=0.960): morphologic abnormalities were observed in 11 of the 221 neonates: 2 of 45 neonates in Group A (4.4%; 95% CI, 0.54–15.15), 3 of 53 neonates in Group O (5.7%; 95% CI, 1.18–15.66), and 6 of 123 neonates in Group N (4.9%; 95% CI, 1.81–10.32) (Table S3). In this subgroup analysis, the odds ratio of the primary outcome comparing Group A and Group O was 0.78 (95% CI: 0.124–4.857) and Group A and Group N was 0.91 (95% CI: 0.176–4.666).
A pair of twins who were excluded from the final analysis were exposed to amlodipine in early pregnancy. We listed delivery outcomes including the twins in Group A in Table S4.
Discussion
In this study, the rate of chronic hypertension was 0.9% and the rate of preeclampsia and gestational hypertension was 1.8%. These rates are lower than those expected from previous data in Japan, which are 0.6% to 3.5% (0.6%; age 30–34, 1.2%; age 35–39, 2.0%; age 40–44, 3.5%; age ≥45)10 for chronic hypertension, 2.3% for preeclampsia, and 2.3% for gestational hypertension.11
Amlodipine Use and Morphologic Abnormalities
In this study, fetal morphologic abnormalities associated with exposure to amlodipine in the first trimester were investigated in pregnant women with chronic hypertension in Japan. Previous studies reported a total of 41 cases in which amlodipine was administered during the first trimester of pregnancy, and our dataset has added an additional 48 amlodipine‐exposed cases in the literature. In our study, morphologic abnormalities were observed in 2 neonates in Group A (4.2%). In this group, 26 women were exposed to only amlodipine in the first trimester. Our findings indicate that the point estimates of the odds of major malformations are not significantly different among the groups. However, the 95% CI was wide because of the small sample size. While our exploratory data are reassuring, further research effort is clearly needed.
Use of Antihypertensives and Morphologic Abnormalities
The use of antihypertensives in the first trimester has generally been found not to increase the risk of morphologic abnormalities in offspring,3, 4, 12, 13, 14, 15, 16 although studies exist showing potential associations with birth defects such as heart malformation,6, 17, 18, 19 hypospadias,20, 21 and central nervous system malformation.17 In our study, abnormalities were observed in 5 of 102 (4.9%) neonates whose mothers used antihypertensives in the first trimester (Group A+Group O) and 6 of 129 (4.7%) whose mothers did not use antihypertensives (Group N). Among the 5 offspring in the antihypertensive groups (Group A+Group O), 3 had heart malformations, and 2 had hypospadias. There was no increase in the risk of morphologic abnormalities in the exposed groups, compared with the group that did not take antihypertensives (Group N) (P=0.929), although the relatively frequent occurrence of heart malformations and hypospadias was consistent with previous reports.6, 17, 18, 19, 20, 21 In the present study, the women whose offspring had heart malformations or hypospadias did not use any nonantihypertensive drugs that are associated with heart malformations22 or hypospadias.23
Maternal Hypertension and Morphologic Abnormalities
As Shepard proposed,24 one of the essential criteria for a human teratogen is a specific phenotype(s) of the adverse effects. An association between maternal hypertension and specific birth defects was recently reported.6, 19, 21, 25, 26 Anomalies in the kidney, limbs, lips, and palate were frequently observed in the offspring of women with chronic hypertension.7 Maternal hypertension was also found to be associated with heart malformation,6, 19, 21, 25, 26, 27 hypospadias,28 and esophageal atresia or stenosis.29 In our study, consistent with the previous reports, the following abnormalities (including overlap) occurred in the offspring of women with chronic hypertension: heart malformation in 3 cases, hypospadias in 3, central nervous system abnormalities in 5, and renal abnormality (Potter syndrome) in 1. Possible teratogenicity of maternal hypertension itself cannot be ruled out or confirmed from the current study framework, mainly because of the absence of nonhypertensive control.
Importantly, 5 of 11 women in this study whose offspring showed morphologic abnormalities had superimposed preeclampsia (2 women in Group O and 3 women in Group N). Of these 5 women, 3 demonstrated hypospadias and 3 exhibited low‐lying conus medullaris. van Gelder et al reported a high risk of ventricular septal defect and atrial septal defect in chronic hypertension women who developed superimposed preeclampsia (antihypertensives were not used).21 Maternal hypertension is speculated to directly affect fetal growth via vascular disruption or teratogenic mechanisms.30 Physiological changes in early pregnancy that progress to preeclampsia or gestational hypertension in late pregnancy are also speculated to be associated with morphologic abnormalities in some cases.21 Whether these observations reflect the teratogenic nature of maternal hypertension requires further studies.
Limitations
We conducted a simulation about the unmeasured confounding factors that could have effects on birth defects in the comparison of Group A and Group N as a sensitivity analysis. We set a confounder‐birth defects odds ratio equal to 0.1, and the prevalence of confounding factors among Group N was equal to 0.05. We estimated the odds ratio of group‐birth defects depending on the prevalence of confounding factors among Group A. Within the condition of our sample size, when the prevalence of confounding factors among Group A was 0.95, the lower limit of 95% CI exceeds 1 (Figure 2). These results indicate that we cannot draw a conclusion regarding the difference in birth defects in this study. Because of this limitation, the effects of confounding maternal background factors such as age, body mass index, alcohol, smoking, underlying diabetes mellitus, and underlying congenital heart disease could not be examined.
Figure 2.
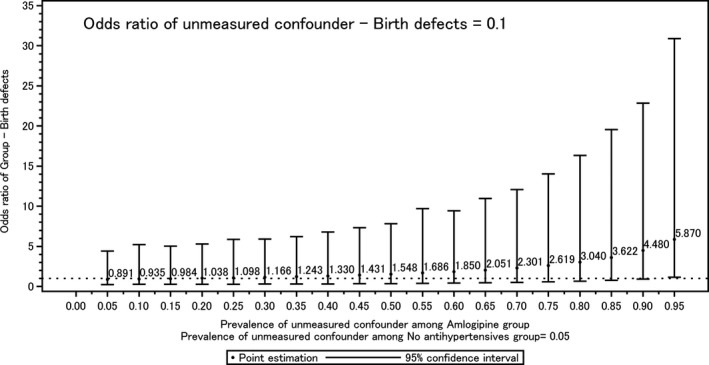
Unmeasured confounder‐birth defects odds ratio. The 95% CI was estimated by the range of 2.5 and 97.5 percentile points of exp (log [odds ratio]+error), which was calculated by Monte Carlo simulations. The error term was randomly sampled from the Normal distribution with mean 0 and the SD, which was substituted by the SE of unadjusted log odds ratio.
Sources of Funding
This research was supported by the Japan Agency for Medical Research and Development (AMED) under Grant Number JP17mk0101086.
Disclosures
None.
Supporting information
Table S1. All Anomalies
Table S2. Details of Cases Using Amlodipine During the First Trimester (Group A)
Table S3. Delivery Outcomes Excluding Diabetes Mellitus
Table S4. Delivery Outcomes Including a Pair of Twins in Group A
Acknowledgments
Our sincere thanks to Dr Toru Kobayashi for reviewing this report. Also, thanks to Dr Tadasu Shionoiri for collecting the cases presented in this article. Finally, thanks to Dr Rika Kosaki for her guidance in confirming the various cases.
(J Am Heart Assoc. 2019;8:e012093 DOI: 10.1161/JAHA.119.012093.)
References
- 1. Bramham K, Parnell B, Nelson‐Piercy C, Seed PT, Poston L, Chappell LC. Chronic hypertension and pregnancy outcomes: systematic review and meta‐analysis. BMJ. 2014;348:g2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seely EW, Ecker J. Chronic hypertension in pregnancy. Circulation. 2014;129:1254–1261. [DOI] [PubMed] [Google Scholar]
- 3. Magee LA, Schick B, Donnenfeld AE, Sage SR, Conover B, Cook L, McElhatton PR, Schmidt MA, Koren G. The safety of calcium channel blockers in human pregnancy: a prospective, multicenter cohort study. Am J Obstet Gynecol. 1996;174:823–828. [DOI] [PubMed] [Google Scholar]
- 4. Weber‐Schoendorfer C, Hannemann D, Meister R, Elefant E, Cuppers‐Maarschalkerweerd B, Arnon J, Vial T, Rodriguez‐Pinilla E, Clementi M, Robert‐Gnansia E, De Santis M, Malm H, Dolivo A, Schaefer C. The safety of calcium channel blockers during pregnancy: a prospective, multicenter, observational study. Reprod Toxicol. 2008;26:24–30. [DOI] [PubMed] [Google Scholar]
- 5. Ahn HK, Nava‐Ocampo AA, Han JY, Choi JS, Chung JH, Yang JH, Koong MK, Park CT. Exposure to amlodipine in the first trimester of pregnancy and during breastfeeding. Hypertens Pregnancy. 2007;26:179–187. [DOI] [PubMed] [Google Scholar]
- 6. Bateman BT, Huybrechts KF, Fischer MA, Seely EW, Ecker JL, Oberg AS, Franklin JM, Mogun H, Hernandez‐Diaz S. Chronic hypertension in pregnancy and the risk of congenital malformations: a cohort study. Am J Obstet Gynecol. 2015;212:337.e1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bellizzi S, Ali MM, Abalos E, Betran AP, Kapila J, Pileggi‐Castro C, Vogel JP, Merialdi M. Are hypertensive disorders in pregnancy associated with congenital malformations in offspring? Evidence from the WHO Multicountry cross sectional survey on maternal and newborn health. BMC Pregnancy Childbirth. 2016;16:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, Hall DR, Warren CE, Adoyi G, Ishaku S; International Society for the Study of Hypertension in Pregnancy (ISSHP) . Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72:24–43. [DOI] [PubMed] [Google Scholar]
- 9. EUROCAT (2013) . EUROCAT Guide 1.4: Instruction for the registration of congenital anomalies. EUROCAT Central Registry, University of Ulster. (last update version 28/12/2018). Available at: http://www.eurocat-network.eu/content/Full%20Guide%201%204%20version%2028_DEC2018.pdf. Accessed May 22, 2019.
- 10. Ogawa K, Urayama KY, Tanigaki S, Sago H, Sato S, Saito S, Morisaki N. Association between very advanced maternal age and adverse pregnancy outcomes: a cross sectional Japanese study. BMC Pregnancy Childbirth. 2017;17:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Umesawa M, Kobashi G. Epidemiology of hypertensive disorders in pregnancy: prevalence, risk factors, predictors and prognosis. Hypertens Res. 2017;40:213–220. [DOI] [PubMed] [Google Scholar]
- 12. Queisser‐Luft A, Eggers I, Stolz G, Kieninger‐Baum D, Schlaefer K. Serial examination of 20,248 newborn fetuses and infants: correlations between drug exposure and major malformations. Am J Med Genet. 1996;63:268–276. [DOI] [PubMed] [Google Scholar]
- 13. Nakhai‐Pour HR, Rey E, Berard A. Antihypertensive medication use during pregnancy and the risk of major congenital malformations or small‐for‐gestational‐age newborns. Birth Defects Res B Dev Reprod Toxicol. 2010;89:147–154. [DOI] [PubMed] [Google Scholar]
- 14. Diav‐Citrin O, Shechtman S, Halberstadt Y, Finkel‐Pekarsky V, Wajnberg R, Arnon J, Di Gianantonio E, Clementi M, Ornoy A. Pregnancy outcome after in utero exposure to angiotensin converting enzyme inhibitors or angiotensin receptor blockers. Reprod Toxicol. 2011;31:540–545. [DOI] [PubMed] [Google Scholar]
- 15. Hoeltzenbein M, Beck E, Fietz AK, Wernicke J, Zinke S, Kayser A, Padberg S, Weber‐Schoendorfer C, Meister R, Schaefer C. Pregnancy outcome after first trimester use of methyldopa: a prospective cohort study. Hypertension. 2017;70:201–208. [DOI] [PubMed] [Google Scholar]
- 16. Bateman BT, Patorno E, Desai RJ, Seely EW, Mogun H, Dejene SZ, Fischer MA, Friedman AM, Hernandez‐Diaz S, Huybrechts KF. Angiotensin‐converting enzyme inhibitors and the risk of congenital malformations. Obstet Gynecol. 2017;129:174–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cooper WO, Hernandez‐Diaz S, Arbogast PG, Dudley JA, Dyer S, Gideon PS, Hall K, Ray WA. Major congenital malformations after first‐trimester exposure to ACE inhibitors. N Engl J Med. 2006;354:2443–2451. [DOI] [PubMed] [Google Scholar]
- 18. Lennestal R, Otterblad Olausson P, Kallen B. Maternal use of antihypertensive drugs in early pregnancy and delivery outcome, notably the presence of congenital heart defects in the infants. Eur J Clin Pharmacol. 2009;65:615–625. [DOI] [PubMed] [Google Scholar]
- 19. Fisher SC, Van Zutphen AR, Werler MM, Lin AE, Romitti PA, Druschel CM, Browne ML; National Birth Defects Prevention Study . Maternal antihypertensive medication use and congenital heart defects: updated results from the National Birth Defects Prevention Study. Hypertension. 2017;69:798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Caton AR, Bell EM, Druschel CM, Werler MM, Mitchell AA, Browne ML, McNutt LA, Romitti PA, Olney RS, Correa A. Maternal hypertension, antihypertensive medication use, and the risk of severe hypospadias. Birth Defects Res A Clin Mol Teratol. 2008;82:34–40. [DOI] [PubMed] [Google Scholar]
- 21. van Gelder MM, Van Bennekom CM, Louik C, Werler MM, Roeleveld N, Mitchell AA. Maternal hypertensive disorders, antihypertensive medication use, and the risk of birth defects: a case‐control study. BJOG. 2015;122:1002–1009. [DOI] [PubMed] [Google Scholar]
- 22. Kallen BA, Otterblad Olausson P. Maternal drug use in early pregnancy and infant cardiovascular defect. Reprod Toxicol. 2003;17:255–261. [DOI] [PubMed] [Google Scholar]
- 23. Jentink J, Loane MA, Dolk H, Barisic I, Garne E, Morris JK, de Jong‐van den Berg LT. Valproic acid monotherapy in pregnancy and major congenital malformations. N Engl J Med. 2010;362:2185–2193. [DOI] [PubMed] [Google Scholar]
- 24. Shepard TH. “Proof” of human teratogenicity. Teratology. 1994;50:97–98. [DOI] [PubMed] [Google Scholar]
- 25. Caton AR, Bell EM, Druschel CM, Werler MM, Lin AE, Browne ML, McNutt LA, Romitti PA, Mitchell AA, Olney RS, Correa A; National Birth Defects Prevention Study . Antihypertensive medication use during pregnancy and the risk of cardiovascular malformations. Hypertension. 2009;54:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li DK, Yang C, Andrade S, Tavares V, Ferber JR. Maternal exposure to angiotensin converting enzyme inhibitors in the first trimester and risk of malformations in offspring: a retrospective cohort study. BMJ. 2011;343:d5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu S, Joseph KS, Lisonkova S, Rouleau J, Van den Hof M, Sauve R, Kramer MS. Association between maternal chronic conditions and congenital heart defects: a population‐based cohort study. Circulation. 2013;128:583–589. [DOI] [PubMed] [Google Scholar]
- 28. Van Zutphen AR, Werler MM, Browne MM, Romitti PA, Bell EM, McNutt LA, Druschel CM, Mitchell AA; National Birth Defects Prevention Study . Maternal hypertension, medication use, and hypospadias in the National Birth Defects Prevention Study. Obstet Gynecol. 2014;123:309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Banhidy F, Acs N, Puho EH, Czeizel AE. Chronic hypertension with related drug treatment of pregnant women and congenital abnormalities in their offspring: a population‐based study. Hypertens Res. 2011;34:257–263. [DOI] [PubMed] [Google Scholar]
- 30. van Gelder MM, van Rooij IA, Miller RK, Zielhuis GA, de Jong‐van den Berg LT, Roeleveld N. Teratogenic mechanisms of medical drugs. Hum Reprod Update. 2010;16:378–394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. All Anomalies
Table S2. Details of Cases Using Amlodipine During the First Trimester (Group A)
Table S3. Delivery Outcomes Excluding Diabetes Mellitus
Table S4. Delivery Outcomes Including a Pair of Twins in Group A


